Chapter 62 Management of Pediatric Severe Traumatic Brain Injury
Traumatic brain injury (TBI) still remains a leading cause of injury-related morbidity and mortality among the pediatric population of the United States. The impact on the individual child as well as the injured child’s family provides a potent stimulus for improving management techniques within the neurosurgical community. While the exact financial cost of pediatric TBI to the family, society, and the medical system is not known, it has been estimated to be in excess of $35 billion annually in direct costs with additional indirect costs to the families of these children including lost wages, other nonmedical expenditures, and so on. It is known, however, that chronically disabled children require approximately four times the medical expenditures compared to their nondisabled cohorts.1 This burden along with the relative lack of defined standards of care for pediatric TBI serves to create new methods for prevention and innovative intervention for pediatric neurotrauma.
Approximately 600,000 children in the United States visit the emergency department (ED) for TBI yearly and results in 60,000 hospitalizations and 7400 deaths per year. Children less than 4 years old visit the emergency department most frequently while adolescents more commonly are hospitalized and have the highest death rate of 24.3 per 100,000 secondary to TBI.2,3 In those areas where it has been instituted, regionalization of pediatric trauma centers has taken a large step in reducing the morbidity and mortality of TBI among this population.4 It has been demonstrated that injured children with moderate or severe TBI are more likely to undergo neurosurgical intervention and have improved outcomes when treated at a pediatric trauma center as opposed to adult trauma centers. With the likely future shortages in pediatric specialists, future steps to maintain and improve these outcomes may require regionalization to ensure volume and expertise.
Imaging in Pediatric Neurotrauma
Diagnostic imaging protocols and technology in the setting of acute pediatric TBI has received much attention in recent years. A general trend toward minimizing some imaging modalities, in particular the use of computed tomography (CT), has been due to concerns of potential delayed radiation injury.4–6 In turn, the use of other modalities as well as the improvement in technology and further refinement of CT protocols have lessened the radiation exposure of these children yet still provide the requisite early, accurate, clinically significant information.
Plain Radiographs
Plain skull films are rarely used today in pediatric trauma centers. While previously useful for evaluation of fractures, as well as for pneumocephalus, evaluation of bony fragments in depressed, comminuted fractures, and for evaluation of diastatic sutural fractures has been offset by CT, which provides soft tissue assessment as well as excellent cranial vault imaging especially with the more routine use of three-dimensional (3D) reconstruction algorithms. Today the role of the skull radiograph is used primarily as a map for identification of foreign bodies, or to document child abuse.7 Plain films, however, still remain helpful to rule out spinal column injury while minimizing radiation exposure to the developing child that would come from a full-body, spine CT study. Since children more often suffer ligamentous injury, a screen with plain x-rays followed by magnetic resonance imaging (MRI) has provided an alternative to the radiation incurred with a full-body scan with mixed efficacy. If the child is awake and particularly uncomfortable due to either limitation to care in the intensive care unit setting or anxiety associated with wearing a rigid cervical collar, flexion/extension radiographs or dynamic fluoroscopic films may be used. Patients with diminished responsiveness are challenging, as actual clearance and collar removal requires radiologic in combination with clinical clearance, and thus there is little indication for cervical clearance in the early period in these patients. MRI for evaluation of ligamentous injury within the first 48 hours of the event can be performed if removal of the collar is necessary for the care of the patient, such as in operative intervention requiring manipulation of the neck. Unfortunately, MRI use has become increasingly popular as a screen, although it may be an unnecessary expense or risk due to frequent need for sedation and limited monitoring ability to obtain the requisite images. Based on extrapolation from recent studies in adult trauma patients, the risk of missing an occult unstable cervical injury in the teenage group with adequate prior static radiographs is less than 1%;8–11 these data may not apply to the very young pediatric population. In pediatric spine injury, since there is a predominance of upper cervical and occipitocervical pathology in the younger pediatric population, we prefer imaging the cervical spine alone with CT including 3D reconstructions as necessary for initial radiologic clearance. Due to the low incidence of injury, the thoracic and lumbar spine can be imaged by plain radiograph unless a CT is desired for an area of pain or deformity, or the mechanism of injury is significantly violent, such as motor vehicle collision.
Computed Tomography
For TBI, the noncontrasted axial head CT is the imaging modality of choice in pediatric neurotrauma. The scan can be performed very rapidly providing immediate information regarding cranial injury, intra- and extra-axial blood, fractures, ventriculomegaly associated with TBI, and to a less-specific degree, ischemia. The progression of intra- and extra-axial hemorrhagic lesions has been well documented. A repeat CT scan may be obtained within twelve hours if significant blood is present or there is a change in neurologic status. Data have failed to reconcile personal practice bias into standard protocols and practice regarding early repeat CT scanning. Repeat imaging should be conservatively considered given the “trauma” of transport and potential for worsening of hemodynamic instability. In adults, Oertel and colleagues evaluated 142 cases and described hemorrhagic progression by hematoma type as follows: 51% in parenchymal contusions, 22% in epidural hematomas, and 11% of subdural hematomas on 24-hour, follow-up CT scanning.12 Unique to the pediatric population is the usual absence of anticoagulant use for comorbid conditions; this likely decreases the development of a delayed insult on CT from 85% to 31%.13 These results can be applied to the pediatric TBI patient as a general guide in assessing the need for repeat CT evaluation.
Concern has been raised about the effect that ionizing radiation has on the immature central nervous system. Prediction models for the use of CT in mild TBI (Glasgow Coma Scale (GCS) 14 to 15) for the pediatric population have recently been investigated. The estimated rate of lethal malignancies from CT is 1 per 1000 to 1 per 5000 scans with increased risk with younger age.4,5 Of 14,969 pediatric patients who underwent CT-scanning of the head for suspected TBI and met study parameters, 376 (0.9%) had clinically important TBI (defined as requiring acute intervention including neurosurgery), and only 60 (0.1%) underwent neurosurgery. The negative predictive value is 100% if the following criteria were met: (1) normal mental status, (2) no scalp hematoma except frontal, (3) no loss of consciousness or loss of consciousness for less than 5 seconds, (4) non-severe injury mechanism, (5) no palpable skull fracture, and (6) acting normally per parents. The negative predictive value is equivalent in children over 2 years of age with normal mental status, no loss of consciousness, no vomiting, no severe headache, no evidence of basilar skull fracture, and non-severe injury mechanism. It can be concluded from these data that CT scanning in the low risk TBI pediatric population may be avoided based on provider preference and likelihood of surgery.6 Even in the higher risk categories, the authors’ preference is not to repeat imaging unless there is consideration for a change in management strategy, that is, decision making for surgical intervention.
Recently there has been an increased utilization of the so-called “pan scan” including head, cervical spine, chest, abdomen, and pelvis. Tillou and colleagues reported on the effectiveness of the “pan CT scan” in an adult cohort indicating that if any study was omitted, from 311 CT scans, 17 injuries (5.4%) requiring immediate attention would have been missed.14 We recommend caution and careful consideration of each patient’s mechanism of injury, neurologic status, and age prior to undertaking a “pan scan” to limit the potential radiation exposure; however, this is determined in large part by trauma surgery. With the advent of “pediatric” protocols developed to lower the radiation load without compromising image quality, these studies, especially if limited to the head and cervical spine, can facilitate the care of the patient, reducing time in the radiology department and providing a wealth of information useful for clinical decision making.
Magnetic Resonance Imaging
Magnetic resonance imaging in the pediatric trauma population is problematic primarily due to time constraints. The time for examination is significantly longer than the CT and frequently requires sedation in the young to ensure adequate image quality. If the patient is intubated in the field or on arrival to the ED, MRI becomes a more practical modality with extension of sedation, although the decision should be based on a specific question particularly as it relates to the cervical spine. In emergent and urgent settings, the potential benefits of subtler imaging seldom outweigh the screening achieved by CT alone for cranial trauma. This may differ in the cervical spine where bony abnormalities are less common and soft tissue injury may be better imaged by MR. Following the emergent acute phase, it must be recognized that certain implants such as intracranial pressure (ICP) sensors or cranial bolts have to be removed or disconnected to ensure safety from potential further injury or artifact,15 such as inducible radio frequency heating, movement in the magnetic field leading to further parenchymal damage, or metallic artifact from skin staples, and thus, at this time MR in the acute setting has little efficacy.
In contrast, cervical spine or other spinal injury in a child is often best assessed by MR, although it is often impractical to image the entire spine in the acute setting. The initial screening of patients with clinical exam and CT usually provide a target area for more focused regional imaging. A patient with a spinal fracture and correlating neurologic findings can be better assessed for surgical pathology such as a hematoma or disc protrusion into the canal. As mentioned, since in children most often it is the extent of the ligamentous injury that is being evaluated, T2, FLAIR, or FIESTA sequences provide more information as to the extent of significant ligamentous injury, the potential for instability, and need for surgical intervention.
Technology for Management of Intracranial Hypertension in Pediatric Head Trauma
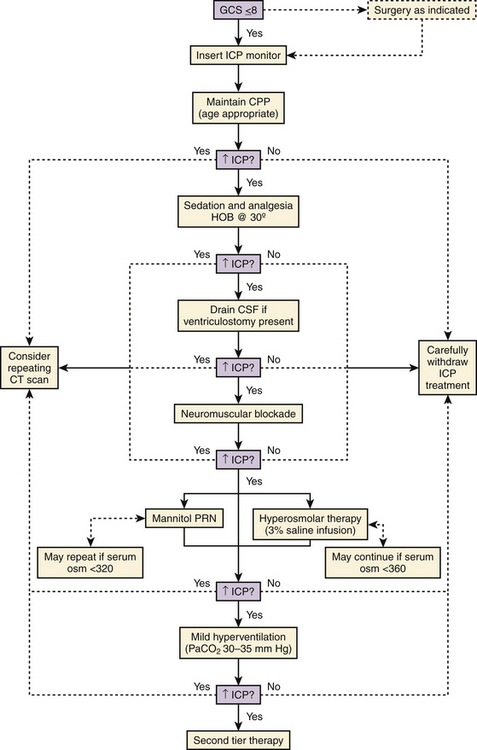
The detection of intracranial hypertension and prompt treatment are typically the primary focus of the neurosurgeon. In those patients with moderate and severe TBI, the potential for intracranial hypertension or its evolution is sufficiently high enough that recommendations for monitoring have been established.16 Despite these recommendations and algorithms for treatment as delineated in the pediatric guidelines, implementation and compliance in children remain modest except in tertiary academic and neurosurgical centers. While clearly ICP monitoring is not the optimal mode of understanding real-time neurophysiology, it is the most established and understood means of providing insight to the injured brain. Standard methods of ICP transduction are broadly available and frequently although inconsistently used, particularly in children. Keenan and Bratton2 surprisingly reported in 2006 that only 33% of infants and young toddlers (<2 years of age) with severe TBI, defined as GCS score of less than 8, received ICP monitoring in the state of North Carolina. While newer monitoring systems, including efforts at noninvasive ICP monitoring, brain compliance monitoring, oximetry, local and regional cerebral blood flow, electroencephalography, and cerebrospinal fluid (CSF) biomarkers are emerging into protocols or research trials, their utilization is still infrequent and inconsistent from center to center. At a minimum, and despite the lack of a level I recommendation, the evaluation for increased ICP must be strongly considered in the setting of a GCS score ≤8. The overall assessment of the patient needs to be considered. As many patients arrive intubated, sedated, or with pharmacologic paralytics, careful examiner reassessment at post-CT scan screening and postresuscitation once medication has been metabolized, is essential to ensure that the data are consistent. Even in the setting of a “normal” CT, or in the infant with a normal head circumference and fontanel assessment, elevated ICP should still be considered in children. While a normal fontanelle does not indicate normal ICP, an elevated convex fontanelle most often does indicate ICP.
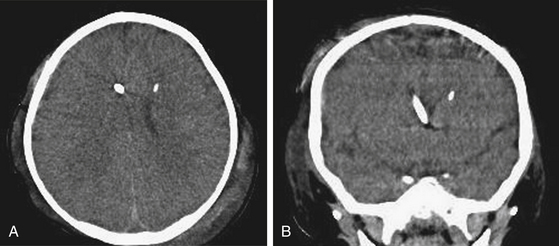
Three methods for monitoring ICP have been widely adopted:1 the use of an inserted external ventricular drain (EVD) coupled with an adjustable drain bag and external saline column strain gauge;2 parenchymal pressure sensors, which work by strain gauge or fiber optic methods; and3 combination sensors integrating the transducer to the implanted EVD. Older technologies including epidural and subarachnoid sensors are rarely used due to concerns about accuracy. Among all of these, a key concern for accuracy rests on high-fidelity transduction of the ICP waveform with its systolic/diastolic peaks. If the waveform morphology cannot be discerned, accurate pressure transduction cannot be assumed. Further, the experienced neurosurgeon will observe in that waveform morphology features of risk for poor compliance, such as more vertical or rapid rise time or elevated P2 segment. Various commercial kits have been developed that simplify bedside placement of the EVD or ICP transducer. The authors use a combination of the EVD as a treatment modality with continuous CSF drainage and a concurrent strain gauge catheter for continuous monitoring of ICP (Fig. 62-1).
External Ventricular Drain Placement
Many institutions have developed protocols to be followed when an EVD is inserted. The major potential risks of ventriculostomy placement are ventriculostomy-related infection (VRI) or insertional hemorrhage. A recent review of VRI indicated that a body of retrospective studies was limited by nonuniform definitions of infection versus colonization versus contamination. It lists, however, the rate of VRI from 0% to 22% among 23 studies with 5733 EVD insertions. The cumulative rate of positive cultures was 8.08% per EVD placed. With an earlier, more stringent definition of VRI in 1988, the incidence of VRI among the 5733 EVD insertions dropped to 6.1%.17 Most studies have defined VRI as a single positive CSF culture obtained from the ventricular catheter or from CSF drawn via lumbar puncture. To limit this potential complication, prophylactic intravenous antibiotics or antibiotic impregnated catheters have been used. A controlled multicenter, prospective, randomized trial performed by Zabramski et al., showed striking results when antibiotic-impregnated catheters were used. In adult patients randomized to minocycline and rifampin-impregnated versus nonimpregnated drains, the rate of positive CSF cultures dropped from 9.4% to 1.3%, and the colonization rate of the drain dropped from 37% to 18%.18 In contrast, another prospective, randomized, controlled trial in a single institution established equivalent infection rates among 116 patients comparing antibiotic-impregnated catheters to that of nonimpregnated drains with systemic antibiotics. These investigators concluded that impregnated catheters may diminish the cutaneous opportunistic infections associated with systemic antibiotics without tradeoff of VRI.19 Technical aspects of insertion have been investigated; it has been showed that extended tunneling of the catheter has no effect on infection rate.20 Risk factors for VRI development have been established in multiple reports, including subarachnoid hemorrhage (SAH), intraventricular hemorrhage (IVH), craniotomy, cranial fracture with CSF leak, ventriculostomy irrigation, concomitant systemic infection, and duration of placement.21,22 Factors not associated with VRI include hydrocephalus, closed head trauma, tumor, CSF drainage, multiple catheters, and concomitant ICP sensors.22 Most agree that sterile placement with some prophylactic antibiotic coverage whether impregnated or systemic can reduce the potential for VRI.
Less frequent risks of placement include malposition, hemorrhage, and neurologic injury. Almost all of the above studies were performed in the adult population. Generalization to the pediatric population seems appropriate but warrants further study. A recent retrospective study of 96 EVD placements in pediatric patients reported complication rates of infection (9.4%), malposition (6.3%), hemorrhage (4.2%), and obstruction (3.1%).23 The senior author in a database of 147 consecutive EVD placements using nonantibiotic-impregnated catheters has had zero culture positive infections utilizing a culture screening protocol while the EVD was in place and prophylactic antibiotics with a second- or third-generation cephalosporin in each case (PD Adelson, unpublished data). As studies proceed in the future, we can expect that an optimal catheter, the ideal tunneling distance, the use of periprocedural antibiotics, and catheter indwelling time may be clarified.
Multimodality and Other Sensor Types
In addition, indwelling sensors which incorporate temperature, pH, and pO2 measurements have become commercially available. Local tissue intraparenchymal oxygen sensors (LICOX, Integra Neuroscience) and regional noninvasive brain oxygen sensors using near-infrared technology (Foresite, CASmed, INVOS, Somanetics) are available and can be integrated into various monitoring protocols in an effort to further refine clinical decision making toward a better outcome. The LICOX oxygen sensor is placed through a bolt into the cranium, although free catheter placement is also possible (Fig. 62-2). Concerns as to its value have been raised due to the limited sensing of the surrounding area and the known regional effects of evolving brain edema, contusion, or traumatic infarction. Recent literature on both adult24 and pediatric25 patients, however, has shown the potential efficacy of this type of modality in conjunction with ICP/cerebral perfusion pressure (CPP)-directed therapy. While the transcutaneous near-infrared monitoring of frontal lobe oxygenation may be of limited benefit depending on the distribution of the regional insult, children more commonly suffer diffuse types of injuries, and at present, the potential to monitor one region with adequate generalizability is preferable to no monitoring. Preliminary data coupled with ICP monitoring is emerging. Although it is unclear at this time whether focal oxygenation is representative of global oxygenation in the damaged cerebrum, it is believed that it assists in the avoidance of second insults and further secondary injury. Similarly, the use of microdialysis and cerebral blood flow measurement may contribute in the future, although presently they are minimally utilized in only limited settings and have not been shown to be efficacious.
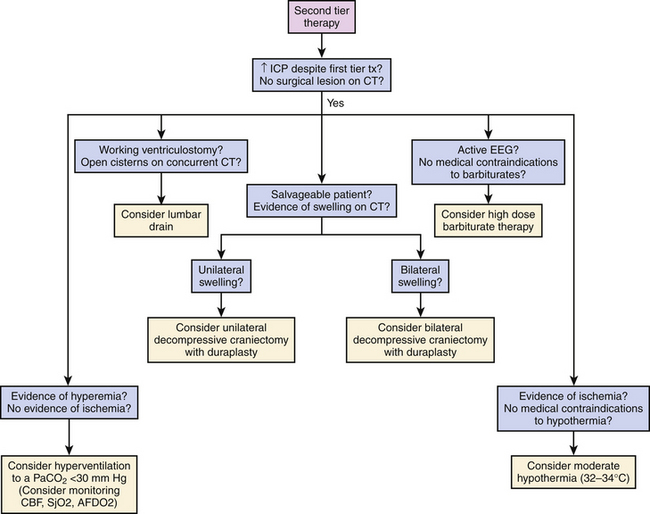
Current Pediatric ICP Management Guidelines
Repeat CT imaging may help to guide second-tier management and is dependent on the findings on the first scan and risk benefit of transporting a critically ill child to the scanner. The increasing use of portable CT scanners may help to obviate this concern. Focal mass lesions, especially with documented expansion, should be evacuated if deemed appropriate. If parenchymal swelling is unilateral or diffuse, the unilateral decompressive craniectomy (DC) or bifrontal craniectomy may be performed, respectively, and is discussed in more detail below. The addition of barbiturate therapy with escalation to 90% burst suppression, or moderate (32–34°C) hypothermia may also prove beneficial if all other measures fail16 (Figs. 62-3 and 62-4).
Surgical Management of Pediatric Neurotrauma
Pediatric head trauma differs from adult brain injury in that the pediatric brain less often has an acute significant surgical lesion (i.e., epidural hematoma). In contrast, the pediatric brain post-TBI more frequently results in a diffuse type of response with resultant cytotoxic and vasogenic cerebral edema. This difference impacts surgical management of the pathophysiology in that acute unilateral evacuation of hematomas and decompression are infrequent especially relative to adult patients. As a result, most management to date has been medical with surgical interventions for hematomas less common. Similarly, in children decompressive craniectomy has not been as widely utilized as compared to adults. DC, a useful, albeit controversial intervention, has been shown to decrease mortality,26–31 but is only at a class III level through a number of small case series and will likely have a more prominent role in the next edition of the Pediatric Guidelines.
One question is the timing (early vs. delayed) of surgical intervention after failed medical therapy as it relates to controlling elevated ICP. Survival benefit in many retrospective series of DC in children has been well-documented.26–31 The results of a 10-year, single-institution retrospective study appear encouraging, in that survivors benefited from immediate postoperative ICP control (83%) with a 30% perioperative mortality rate. Eventually, 81% returned to school and 18% were dependent on a caregiver.31 Further studies and particularly randomized controlled trials regarding early versus delayed treatment will hopefully refine indications and timing in the future.
While some have advocated early DC for certain subgroups without an acute surgical lesion, the literature currently supports instituting medical therapy first and only if secondary swelling due to regionalized cerebral edema with contusion, should a unilateral decompression be considered, especially if the source of swelling is not within the temporal lobes. As frontotemporal brain trauma is so common in the adult population, unilateral hemicraniectomy with emphasis on sufficiently low decompression of the temporal lobe is most frequently employed. In children, however, diffuse global cerebral edema is more commonly managed with bifrontal decompression. The Pediatric Guidelines provide upper limits of utilization of medical therapy, that is, serum osmolality of 360 osm/ml when utilizing hypertonic saline, and burst suppression with pentobarbital. In the setting of rapidly failing first-tier and even second-tier therapy or the drift toward higher levels of therapies with diminishing efficacy, a DC should be considered as a next approach.
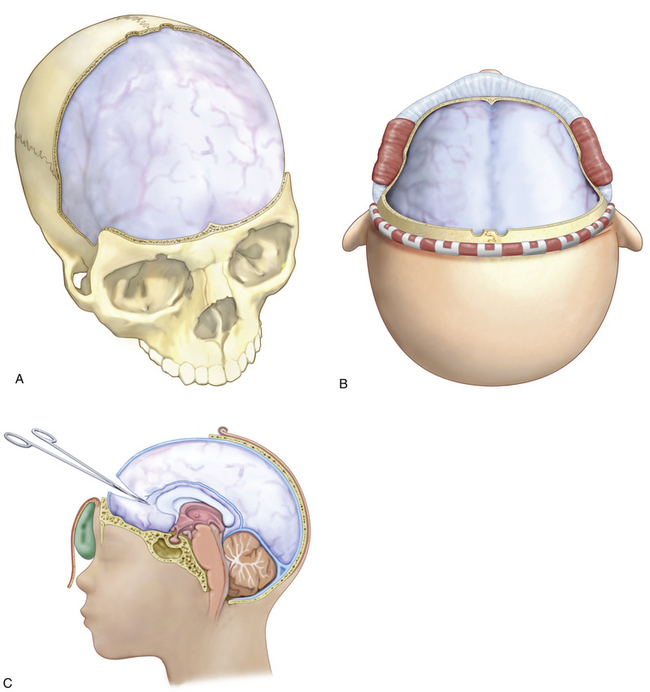
There are various techniques of hemicraniectomy and bifrontal craniectomy as described in an evolving, small literature, which for the most part, would seem fairly standard. If the patient presents with a surgical lesion requiring immediate surgical intervention for evacuation or hemostasis, the decision to leave the bone flap out is dependent on clinical judgment secondary to the intraoperative findings. The extent of damage, tightness of the dura/brain, etc. represents the degree of swelling, the physiologic state, and the expectation of secondary injury development. While most techniques for DC emphasize width and breadth of decompression including low temporal exposure to allow for adequate swelling of the brain and avoidance of herniation, differences in technique include leaving midline bony and/or dural bridges, opening of the dura, inclusion of dural substitutes, dural substitutes and closure, incising across the superior and inferior sagittal sinuses and take down of the falx, storing or not of the bone flap, various types of storage (i.e., abdominal vs. freezer), and various methods for cranial reconstruction such as autologous graft, titanium mesh, or customized implants.
Technique: Decompressive Bifrontal Craniectomy
Once the dura is exposed, preemptive planning is important in regard to brain swelling as well as risk of bleeding. The patient should likely have received maximal medical therapy with mannitol or hypertonic saline, and brief hyperventilation to ensure the lowest possible ICP when the durotomy is made. The senior author creates the durotomy along the frontal polar region and then carries the incisions posteriorly parallel along the lateral bone incisions creating a U-shaped dural flap hinged medially by the superior sagittal sinus. The most anterior midline incision includes suture and coagulation ligation of the superior sagittal sinus at its anterior origin from the ethmoidal emissary vein, and division of the falx to release the dura and accommodate greater frontal swelling. The flow volume at the origin of the sagittal sinus is minimal. The falx is incised parallel to the frontal fossa base and does not necessarily include the inferior sagittal sinus (Fig. 62-5). As mentioned, care should be exercised when handling bridging veins in this region to prevent stretching or tearing of the veins and resultant bleeding directly from the sinus. The pericranium can be harvested, trimmed into lenticular form, and grafted into the dural decompression site in watertight fashion or left as vascularized flap sutured in multiple locations for brain coverage. Alternatively, dural collagen substitutes can be cut to size and sutured to the dural margins for a watertight or coverage-only closure. Deep galeal stitches are followed by skin closure. A subgaleal drain may be employed as scalp serum and CSF leakage across the dural closure can contribute to constrictive tightness despite wide decompression, although the senior author does not use a drain to avoid creating a CSF fistula, preferring to drain CSF through an EVD. If an EVD is still necessary after the decompression has been performed, this can be placed easily with the use of ultrasound and then tunneled posteriorly over the bone edge.
Replacement of Bone Flap
The bone is not replaced until the brain relaxation is sufficient. This allows replacement without exerting pressure upon the brain surface and is typically 4 to 6 weeks after head injury. This time frame also allows for adequate skin healing, improved nutrition, and freedom from systemic infection as well as assurance that there is no evidence of delayed post-traumatic hydrocephalus. Protective head gear may be necessary to shield the brain until replacement if the patient has recovered well and has begun the rehabilitation process. If only a few months have passed, banked bone can commonly be replaced utilizing sutures or permanent or absorbable plates (Fig. 62-6). In the latter instance, it is essential to obtain a follow-up CT scan 6 months later to ensure the expected healing and fixation. If banked bone is not suitable or was discarded, an excellent alternative material is titanium mesh, which can be shaped to an acceptable conforming calvarial contour on the surgical field and may be coated with a bone substitute as a matrix for bone reconstitution if desired (Fig. 62-7). In the instance of extensive replacement or reconstruction of a cosmetically sensitive area, computer-generated, customized synthetic materials or titanium are available.
Considerations for Temperature Regulation in Optimizing Outcome
Hyperpyrexia
Cerebral metabolism is known to increase in the setting of fever. It is also known that the autoregulatory capacity of the prearteriolar sphincter is overcome in TBI causing mismatch of cerebral blood flow and the oxygen requirement of brain. In order to prevent secondary insults and further secondary injury due to this mismatch, fever should be aggressively treated with antipyretics and cooling blankets. Most studies of temperature regulation in TBI were performed in adults and have established that fever is a negative predictor of outcome.32 Attempts at therapeutic intervention for fever include newer methods of localized cooling, specifically to the head. One such device delivers vaporized substances to the nasal cavity creating a cerebral cooling effect. Intravenous cooling devices are likewise in use and provide very reliable cooling but are plagued by complications (e.g., deep vein thrombosis) and not likely efficacious in young children due to the large bore size of the catheters placed in the femoral vein. It should be stressed that, prior to initiating any treatment modalities aimed at cooling, a febrile patient must be worked up for potential infectious etiologies and treated appropriately.
Hypothermia
Hypothermia for neurotrauma has been considered as a therapeutic option for quite some time. One of the first TBI studies in the modern era focused on moderate hypothermia as a neuroprotective management strategy in TBI33 and showed initial positive results in a single-institution trial. Nevertheless, these results were not substantiated by a multicenter, prospective, randomized, controlled trial performed by Clifton et al.34 Despite inconsistent results, it appears that with early cooling, younger patients did trend toward better outcomes than adults. Still, the level III recommendations in Adult Guidelines for the use of hypothermia in adults with TBI was based on a meta-analysis of eight randomized controlled trials (n = 781) that showed a deceased mortality and improved global function (relative risk [RR] = 0.51) with hypothermia at the possible expense of increased risk for pneumonia (RR = 2.37). They also concluded that the neurologic outcome was more likely to be favorable (RR = 1.91) if hypothermia was maintained for at least 48 hours.35 The best times for initiation and total duration of treatment have not been established.
In the 2003 Pediatric TBI Guidelines, Adelson et al.16 again highlighted a lack of clinical trials specifically addressing hypothermia in children but there was a level III recommendation for the use of hypothermia as a second-tier therapy. Biswas et al. noted in a single-institution, randomized study that moderate hypothermia significantly decreased intracranial hypertension.37 More recently, a phase II clinical trial of moderate hypothermia after severe TBI by the senior author (PDA) showed no significant increase in complications including arrhythmias, infection, or coagulopathy36 and preliminarily showed improved mortality and global functional outcome. A phase-III, multicenter, international randomized controlled trial of a moderate hypothermia protocol (32.5 ± 0.5°C) initiated within 8 hours and continued for 24 hours among children and adolescents actually showed a trend toward worsened mortality at 6 months and possibly increased morbidity. There has been some criticism of the study due to issues with the screening process that may have included sicker patients in the hypothermia treatment arm (higher number of GCS 3)38,39 as well as increased hypotension and hypoxia in the hypothermia study arm, lack of uniform clinical management (ICP monitoring was not required), variability of time to initiation of cooling, and a significant number of children lost to follow-up. The protocol also included rapid rewarming of 0.5°C every 2 hours paralleled by significant increases in ICP. These results substantiated the findings of Adelson et al., (2005) who found a significant decrease in ICP during cooling but rebound intracranial hypertension with rewarming.36 Recognizing the potential for worsened secondary injury with rapid rewarming, more recent methodology includes a much slower rewarming of 0.5°C to 1.0°C every 12 to 24 hours. Using this methodology, the Pediatric Traumatic Brain Injury Consortium: Hypothermia or the “CoolKids Trial,” was initiated as a phase-III trial investigating moderate hypothermia (32°C to 33°C) beginning within 6 hours of injury and maintained for 48 hours with a slow rewarm for children to evaluate efficacy versus mortality. This trial includes the less than 18-year-old age group with postresuscitation GCS scores of 4 to 8, who appeared to derive improved mortality benefits from moderate hypothermia.
Acknowledgment
Medical illustrations were drafted and refined by Dr. Shih Liu, USF Department of Neurosurgery.
Adelson P.D., Ragheb J., Kanev P., et al. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56:740-754. discussion 740–754
Adelson P.D., Bratton S.L., Carney N.A., et al. Chapter 17. Critical pathway for the treatment of established intracranial hypertension in pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(Suppl 3):S65-S67.
Biswas A.K., Bruce D.A., Sklar F.H., et al. Treatment of acute traumatic brain injury in children with moderate hypothermia improves intracranial hypertension. Crit Care Med. 2002;30:2742-2751.
Bota D.P., Lefranc F., Vilallobos H.R., et al. Ventriculostomy-related infections in critically ill patients: a 6-year experience. J Neurosurg. 2005;103:468-472.
Brenner D.J. Estimating cancer risks from pediatric CT: going from the qualitative to the quantitative. Pediatr Radiol. 2002;32:228-231.
Clifton G.L., Miller E.R., Choi S.C., et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556-563.
Figaji A.A., Fieggen A.G., Argent A.C. Intracranial pressure and cerebral oxygenation changes after decompressive craniectomy in children with severe traumatic brain injury. Acta Neurochirg Suppl. 2008;102:77-80.
Figaji A.A., Fieggen A.G., Peter J.C. Early decompressive craniotomy in children with severe traumatic brain injury. Childs Nerv Syst. 2003;19:666-673.
Harris O.A., Moure F.C., Chappell E.T. Use of modified EAST practice parameters in clearing the cervical spine in the obtunded trauma patient: a prospective study. J Neurosurg. 2001;94:413A-414A.
Hutchinson P.J., Kirkpatrick P.J. Decompressive craniectomy in head injury. Curr Opin Crit Care. 2004;10:101-104.
Hutchison J.S., Ward R., Lacroix J., et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358:2447-2456.
Jagannathan J., Okonkwo D.O., Dumont A.S. Outcome following decompressive craniectomy in children with severe traumatic brain injury: a 10-year single-center experience with long-term follow-up. J Neurosurg Pediatr. 2007;106(4):268-275.
Keenan H.T., Bratton S.L. Epidemiology and outcomes of pediatric traumatic brain injury. Dev Neurosci. 2006;28(4-5):256-263.
Lozier A.P., Sciacca R.R., Romagnoli M.F. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. 2002;51:170-182.
Lozier A.P., Sciacca R.R., Romagnoli M.F., et al. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. 2002;51(1):170-181. discussion 181–182
Marciano F.F., Apostolides P.J., Vishteh A.G., et al. Cervical spine management in severe, nonpenetrating closed head injury: a prospective study. Neurosurgery. 1997;41:740-741.
Ngo Q.N., Ranger A., Singh R.N., et al. External ventricular drains in pediatric patients. Pediatr Crit Care Med. 2009;10(3):346-351.
Oertel M., Kelly D.F., McArthur D., et al. Progressive hemorrhage after head trauma: predictors and consequences of the evolving injury. J Neurosurg. 1980;96:109-116.
Peterson K., Carson S., Carney N. Hypothermia treatment for traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma. 2008;25:62-71.
Ruf B., Heckmann M., Schroth I., et al. Early decompressive craniectomy and duraplasty for refractory intracranial hypertension in children: results of a pilot study. Crit Care. 2003;7:133-138.
Rutigliano D., Egnor M.R., Priebe C.J., et al. Decompressive craniectomy in pediatric patients with traumatic brain injury with intractable elevated intracranial pressure. J Pediatr Surg. 2006;41:83-87.
Stiefel M.F., Spiotta A., Gracias V.H., et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103(5):805-811.
Tillou A., Gupta M., Baraff L.J., et al. Is the use of pan-computed tomography for blunt trauma justified? A prospective evaluation. J Trauma. 2009;67(4):779-787.
Wong G.K., Poon W.S., Ng S.C., et al. The impact of ventricular catheter impregnated with antimicrobial agents on infections in patients with ventricular catheter: interim report. Acta Neurochir Suppl. 2008;102:53-55.
Zabramski J.M., Whiting D., Darouiche R.O., et al. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J Neurosurg. 2003;98(4):725-730.
1. Newacheck P.W., Inkelas M., Kim S.E. Health services use and health care expenditures for children with disabilities. Pediatrics. 2004;114:79-85.
2. Keenan H.T., Bratton S.L. Epidemiology and outcomes of pediatric traumatic brain injury. Dev Neurosci. 2006;28(4-5):256-263.
3. Potoka D.A., Schall L.C., Gardner M.J. Impact of pediatric trauma centers on mortality in a statewide system. J Trauma. 2000;49(2):237-245.
4. Brenner D.J. Estimating cancer risks from pediatric CT: going from the qualitative to the quantitative. Pediatr Radiol. 2002;32:228-231.
5. Brenner D.J., Hall E.J. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277-2284.
6. Kupperman N., Holmes J.F., Dayan P.F., et al. Identification of children at very low risk of clinically-important traumatic brain injuries after head trauma: a prospective cohort study. Lancet. 2009;374:1160-1170.
7. Grossman R., Yousem D. Neuroradiology, 2nd ed. New York: Mosby; 2003.
8. Sees D.W., Rodriguez Cruz L.R., Glaherty S.F., et al. The use of bedside fluoroscopy to evaluate the cervical spine in obtunded trauma patients. J Trauma. 1998;45:768-771.
9. Marciano F.F., Apostolides P.J., Vishteh A.G., et al. Cervical spine management in severe, nonpenetrating closed head injury: a prospective study. Neurosurgery. 1997;41:740-741.
10. Harris O.A., Moure F.C., Chappell E.T. Use of modified EAST practice parameters in clearing the cervical spine in the obtunded trauma patient: a prospective study. J Neurosurg. 2001;94:413A-414A.
11. Davis J.W., Kaups K.L., Dunningham M.A., et al. Routine evaluation of the cervical spine in head-injured patients with dynamic fluoroscopy: a reappraisal. J Trauma. 2001;50:1044-1047.
12. Oertel M., Kelly D.F., McArthur D., et al. Progressive hemorrhage after head trauma: predictors and consequences of the evolving injury. J Neurosurg. 1980;96:109-116.
13. Rivas J.J., Lobato R.D., Sarabia R., et al. Extradural hematoma analysis of factors influencing the courses of 161 patients. Neurosurgery. 1988;23:44-51.
14. Tillou A., Gupta M., Baraff L.J., et al. Is the use of pan-computed tomography for blunt trauma justified? A prospective evaluation. J Trauma. 2009;67(4):779-787.
15. MR Safety Document: codman ICP CODMAN Microsensor Products. Revision August 1, 2004.
16. Adelson P.D., Bratton S.L., Carney N.A., et al. Chapter 17. Critical pathway for the treatment of established intracranial hypertension in pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(Suppl 3):S65-S67.
17. Lozier A.P., Sciacca R.R., Romagnoli M.F. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. 2002;51:170-182.
18. Zabramski J.M., Whiting D., Darouiche R.O., et al. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J Neurosurg. 2003;98(4):725-730.
19. Wong G.K., Poon W.S., Ng S.C., Ip M. The impact of ventricular catheter impregnated with antimicrobial agents on infections in patients with ventricular catheter: interim report. Acta Neurochir Suppl. 2008;102:53-55.
20. Leung G.K., Ng K.B., Taw B.B., Fan Y.W. Extended subcutaneous tunnelling technique for external ventricular drainage. Br J Neurosurg. 2007;21(4):359-364.
21. Bota D.P., Lefranc F., Vilallobos H.R., et al. Ventriculostomy-related infections in critically ill patients: a 6-year experience. J Neurosurg. 2005;103:468-472.
22. Lozier A.P., Sciacca R.R., Romagnoli M.F., et al. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. 2002;51(1):170-181. discussion 181–182
23. Ngo Q.N., Ranger A., Singh R.N., et al. External ventricular drains in pediatric patients. Pediatr Crit Care Med. 2009;10(3):346-351.
24. Stiefel M.F., Spiotta A., Gracias V.H., et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103(5):805-811.
25. Figaji A.A., Fieggen A.G., Argent A.C. Intracranial pressure and cerebral oxygenation changes after decompressive craniectomy in children with severe traumatic brain injury. Acta Neurochirg Suppl. 2008;102:77-80.
26. Hutchinson P.J., Kirkpatrick P.J. Decompressive craniectomy in head injury. Curr Opin Crit Care. 2004;10:101-104.
27. Ruf B., Heckmann M., Schroth I., et al. Early decompressive craniectomy and duraplasty for refractory intracranial hypertension in children: results of a pilot study. Crit Care. 2003;7:133-138.
28. Figaji A.A., Fieggen A.G., Peter J.C. Early decompressive craniotomy in children with severe traumatic brain injury. Childs Nerv Syst. 2003;19:666-673.
29. Bell M.J., Kochanek P.M. Traumatic brain injury in children: recent advances in management. Ind J Pediatr. 2008;75(11):1159-1165.
30. Rutigliano D., Egnor M.R., Priebe C.J., et al. Decompressive craniectomy in pediatric patients with traumatic brain injury with intractable elevated intracranial pressure. J Pediatr Surg. 2006;41:83-87.
31. Jagannathan J., Okonkwo D.O., Dumont A.S. Outcome following decompressive craniectomy in children with severe traumatic brain injury: a 10-year single-center experience with long-term follow-up. J Neurosurg Pediatr. 2007;106(4):268-275.
32. Jones P.A., Andrews P.J., Midgley S., et al. Measuring the burden of secondary insults in head-injured patients during intensive care. J Neurosurg Anesthesiol. 1994;6:4-14.
33. Marion D.W., Obrist W.D., Carlier P.M., et al. The use of moderate therapeutic hypothermia for patients with severe head injuries: a preliminary report. J Neurosurg. 1993;79:354-362.
34. Clifton G.L., Allen S., Barrodale P., et al. A phase II study of moderate hypothermia in severe brain injury. J Neurotrauma. 1993;10:263-271.
35. Clifton G.L., Miller E.R., Choi S.C., et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556-563.
36. Peterson K., Carson S., Carney N. Hypothermia treatment for traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma. 2008;25:62-71.
37. Biswas A.K., Bruce D.A., Sklar F.H., et al. Treatment of acute traumatic brain injury in children with moderate hypothermia improves intracranial hypertension. Crit Care Med. 2002;30:2742-2751.
38. Adelson P.D., Ragheb J., Kanev P., et al. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56:740-754. discussion 740-754
39. Hutchison J., Ward R., Lacroix J., et al. Hypothermia pediatric head injury trial: the value of a pretrial clinical evaluation phase. Dev Neurosci. 2006;28:291-301.
40. Hutchison J., Ward R., Lacroix J., et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358:2447-2456.