CHAPTER 125 Management of Parathyroid Disorders
The recorded history of hyperparathyroidism in modern medicine is relatively recent. Owen, a renowned British anatomist and curator, is generally acknowledged as being the first to describe the existence of the parathyroid glands in 1852.1 The discovery occurred subsequent to the death of the Zoological Society of London’s Indian rhinoceros, the postmortem of which was conducted by Owen and subsequently reported to the Zoological Society. In 1877, the Swedish histologist Sandstrom reported the existence of distinct glandular tissue adjacent to the thyroid in a dog.2 Over the subsequent 2 years, similar findings in other small mammals led to the search for, and ultimate discovery of, a similar organ in humans (glandulae parathyroideae), which Sandstrom reported on in 1880.
The earliest reports of clinical hyperparathyroidism involved bone disease, or osteitis fibrosa cystica, as termed by von Recklinghausen.3 These reports did not associate the characteristic changes in bone from hyperparathyroidism with parathyroid gland abnormalities, however. In 1903, Askanazy4 reported on an autopsy performed on a patient with osteomalacia and nonfusing long bone fractures in whom a large (>4 cm) tumor was seen adjacent to the thyroid gland, noting that it might represent a parathyroid tumor.
When Erdheim,5 a Viennese pathologist, discovered parathyroid gland morphologic and histologic abnormalities in patients with bone disease, an association between osteomalacia and parathyroid gland function was suspected. Erdheim studied the parathyroid glands by autopsy on all patients who died with bone disease, and noted that many patients with osteomalacia and osteofibrosis cystica showed enlarged parathyroid glands. He postulated that these glandular enlargements were secondary to compensatory hyperplasia, and that the bone disease was the primary initiating factor. Following up on initial experiments performed in rats by Gley, a French physiologist, Erdheim showed that cautery destruction of the parathyroid glands in rats produced not only tetany, as shown by Gley, but also the typical dental changes consistent with calcium not being laid down.5
Numerous reports of large parathyroid glands and bone disease followed until Schlagenhaufer6 suggested, at a meeting in Vienna in 1915, that if only a single parathyroid gland was thought to be enlarged, it should be excised. The event that followed this suggestion years later would usher in the future treatment of parathyroid disease. von Eiselberg,7 a pupil of Billroth, is noted as having performed the first parathyroid transplant. After performing total thyroidectomy in cats, von Eiselberg autografted the thyroid gland and a parathyroid gland into the animal’s abdominal wall. Postoperatively, the animals showed no sign of tetany, and, when subjected to histologic examination, these grafts showed evidence of neovascularization.7
Halsted’s experience with chronic hypocalcemia in thyroidectomy prompted him to study parathyroid transplantation experimentally in dogs.8 He showed that even very small portions of surviving parathyroid tissue autograft could be lifesaving in these animals, and tetany and death would result after removal. In addition, Halsted used intravenous calcium gluconate solution to treat animals after experimental thyroidectomy. These experiments and others sparked his ever-present mandate to perform thyroidectomy carefully and meticulously avoiding injury to the parathyroid glands and their blood supply. Halsted worked with Evans, a medical student at Johns Hopkins, to define the blood supply to the parathyroid glands using vascular casting technique, and emphasized that tetany after thyroidectomy was caused more by interruption of the vascular supply to the parathyroid glands than by their inadvertent removal.8
From a clinical standpoint, the treatment of parathyroid disease was to change significantly with the work of Mandle.9 Albert Gahne, a tram conductor, lived in Vienna after a bout with tuberculosis, which he acquired while serving in the Army during the years 1914-1918. He subsequently developed bone pain and muscle fatigue in 1921 from which he became disabled. In 1924, after a fall that resulted in a fractured femur, he came under the treatment of Mandle, who recognized these events as being consistent with parathyroid abnormality. Believing that this case might represent compensatory parathyroid hyperplasia as postulated by Erdheim, Mandle administered fresh parathyroid extract to Gahne without improvement. Subsequently, Mandle transplanted fresh parathyroid glands obtained from the victim of a street accident into Gahne, which again did not result in any resolution of symptoms. Recalling Schlagenhaufer’s suggestion of nearly 10 years earlier, Mandel explored the neck of the now severely crippled tram conductor and removed a parathyroid tumor.9 Gahne experienced a remarkable and immediate improvement in bone pain, and nearly 4 years later was walking with a cane free of pain. The disease recurred, and the patient was subsequently re-explored, but died after the procedure.10 This experience illustrated several important issues that would come to influence future work with surgical parathyroid disease, including clinical use of parathyroid transplantation in humans and the treatment of recurrent disease by re-exploration.
In 1924, Hanson developed a potent extract of the parathyroid glands, which when injected into animals led to increased serum calcium, decreased phosphate, and elevated output of calcium in the urine. When used chronically, this extract would produce osteoporosis in the animals.11
The association of elevated blood calcium levels and parathyroid dysfunction was well acknowledged when Charles Martell, a sea captain, was evaluated at the Massachusetts General Hospital in 1927, and found to have hypercalcemia and generalized demineralization of the skeleton believed to be caused by hyperparathyroidism. The first two of a total of six operations performed on Martell were done by Richardson, the Chief of Surgery at Massachusetts General Hospital. These first two neck explorations yielded only a single normal parathyroid gland on each side without identification of abnormal tissue.12 A third neck exploration was performed in New York in 1929 by Patterson without success. As renal function began to deteriorate with increasing symptoms of parathyroidism, Martell returned to Massachusetts General Hospital under the care of Albright and Cope. Cope had experience in several parathyroid explorations under the supervision of Churchill, and began cadaver dissection in preparation for re-exploration of Martell, which he did on three occasions in 1932 without success. At the urging of Martell, who had read extensively about his own disease and the potential locations of ectopic parathyroid tissue, Churchill planned a mediastinal exploration, the seventh surgical procedure on Martell. With Cope assisting, Churchill identified and removed most of a 3-cm tumor from the mediastinum, leaving an attached remnant portion with its vascular pedicle intact to avoid profound hypocalcemia. Despite these measures, tetany developed postoperatively, which required treatment with calcium supplementation. Several weeks after surgery, Martell experienced renal colic from an impacted ureteral stone, which required surgery. Martell died from laryngospasm after an operation to relieve obstruction from the impacted stone.
Although the series of procedures performed on Martell predated it and received more notoriety, the first successful parathyroidectomy performed in the United States was performed by Olsch in 1928 at Barnes Hospital of Washington University. In this instance, a large adenoma was removed precipitating a profound decline in serum calcium, which required massive doses of parathyroid extract and intravenous calcium to save the patient.12,13
Etiology and Pathogenesis of Hyperparathyroidism
Parathyroid adenomas apparently are monoclonal or oligoclonal neoplasms, whereby the mechanism of propagation is believed to be clonal expansion of cells that have an altered sensitivity to calcium.14 The work of Arnold and colleagues14 indicates that the molecular events that seem to trigger clonal propagation are heterogeneous. The genetic mutational events that occur in hyperparathyroidism have been characterized in a few tumors. Among the events identified are genetic rearrangements of PRAD1, or parathyroid adenomatosis 1 oncogene, which is also known as cyclin D1. This proto-oncogene is located in the vicinity of the regulatory region of the gene for PTH production (Fig. 125-1).15,16 Subsequent realignment of DNA in this event now combines a growth promoter (PRAD1) with a regulatory region that would ordinarily control only PTH synthesis. This genetic realignment has not been uniformly shown in most parathyroid adenomas, with only a few having been shown to manifest rearrangement.
A more common molecular event, postulated to occur in parathyroid neoplasia, is alteration in tumor suppressor gene expression (Fig. 125-2). For this gene to be inactivated and product deficient, both alleles on the gene must be affected by the mutational event. Tumorigenesis occurs as a sequential event by inactivation of both copies of the suppressor gene.17 The most well known of these is the MEN1 tumor suppressor gene, which shows somatic mutations in both gene copies in 20% of patients with primary hyperparathyroidism.18 This gene was initially recognized in patients with multiple endocrine neoplasia type I (MEN-I) syndrome.19 Evidence of loss of suppressor gene function on chromosome 1p has been postulated as being an even more common event in the development of sporadic parathyroid adenomas. It has been suggested that patients with this chromosomal abnormality may be subject to developing the same constellation of endocrine changes found in MEN-I syndrome and perhaps at an earlier age.17 Suspected loss of tumor suppressor gene function has been identified in other chromosomal loci in patients with parathyroid adenomas including sites 15q, 9p, 6q, and 1q.17
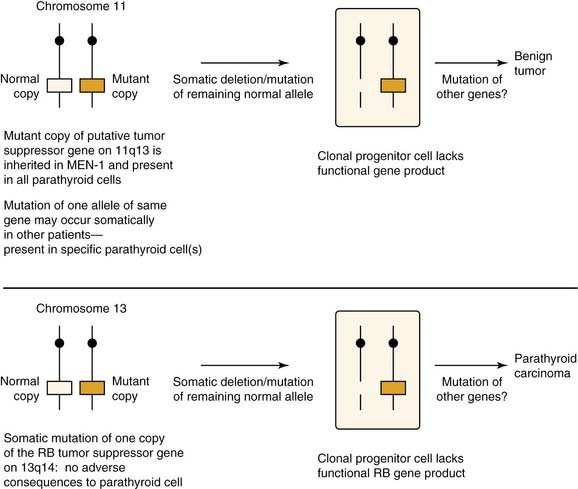
Figure 125-2. Hypothesized roles of inactivated tumor suppressor genes in the development of parathyroid neoplasia.
(From Arnold A. Genetic basis of endocrine disease 5: molecular genetics of parathyroid gland neoplasia. J Clin Endocrinol Metab. 1993;77:1108-1112. The Endocrine Society; reprinted with permission.)
Point mutations in the calcium-sensing receptor gene that reduced the activity of this gene have been elucidated as the basis for familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism.20 This gene seems to represent a likely candidate for molecular rearrangement and altered calcium sensing function in patients with hyperparathyroidism. To date, however, a calcium-sensing receptor gene mutation or allelic inactivation has not been shown in these patients. It has been postulated that alterations in the calcium-sensing function in primary hyperparathyroidism may represent postgenomic events related to reduced RNA transcript or the actual protein receptor in the parathyroid cell clone.21 Whether these events may represent the primary cause for, or secondary effect of, hypercalcemia remains to be determined. Abnormalities in the parathyroid cells vitamin D receptor similarly may represent changes occurring secondary to hypercalcemia and not a primary genetic event. An inactivating mutation in the gene coding for this receptor has been postulated in primary hyperparathyroidism, but results from these investigations are conflicting.22
Firm evidence supports the theory that ionizing radiation can represent the etiologic factor in several solid and humoral human cancers. Tisell and colleagues23 observed that there seems to be an association between exposure to ionizing radiation to the head and neck at an early age and the late development of hyperparathyroidism. This finding is supported by other independent observations where hyperparathyroidism has developed, presumably as a late complication of radiation therapy to the head and neck, similar to the finding noted with differentiated thyroid cancer.
Calcium Homeostasis and Parathyroid Hormone Secretion and Regulation
Calcium homeostasis is maintained by the complex interrelationship of PTH, vitamin D and its derivatives, and calcitonin. The polypeptide PTH contains 84 amino acids. When secreted by the parathyroid glands, PTH undergoes immediate degradation into the amino- and carboxyl-terminal fragments. The amino-terminal fragment is biologically active, but is rapidly cleared from the circulation, whereas the carboxyl-terminal fragment is biologically inert, is predominately cleared from the circulation by the kidney, and persists for a longer time particularly in patients with renal failure.24,25 The intact 1-84 molecule is the major circulating form of biologically active PTH; assays that measure the intact hormone more clearly reflect dynamic changes in PTH metabolism compared with the previous polyvalent assays, which measured a predominately inactive section of the PTH molecule.26
The major target end organs for PTH action are the kidneys, skeletal system, and intestine.27 PTH functions by binding to receptor sites in bone and kidney, which results in stimulation of the production of cyclic adenosine monophosphate (cAMP), which acts to carry out the cellular response of that specific target tissue to PTH.
The primary response to PTH by the kidney is to increase the tubular resorption of calcium and decrease the tubular resorption of phosphorus.28,29 The action of PTH on bone to regulate serum calcium is through the remodeling effect of osteoclast and osteoblast activity. The osteoblasts and their precursor cells in bone have a PTH receptor site, and binding to this site results in the production of cAMP. The osteoclasts do not have a PTH receptor site, but are stimulated indirectly through the cAMP response in the osteoblasts.30 The last important function of PTH occurs indirectly by increasing the rate of conversion in the kidney of 25-hydroxyvitamin D3 (calcifediol) to 1,25-dihydroxyvitamin D3 (calcitriol).31 The coordinated actions of PTH on bone, kidney, and intestine increase the flow of calcium into the extracellular fluid and as a consequence increase the serum calcium levels.
PTH is the primary regulator of rapid changes of extracellular calcium levels. The action of vitamin D affects delayed changes in calcium balance as opposed to the more immediate direct action of PTH.27 Calcitonin plays a much smaller role in calcium homeostasis. Calcitonin is secreted by the parafollicular cells of the thyroid gland and inhibits bone resorption. Calcitonin tends to be decreased in postmenopausal women, and may be increased by estrogen administration in these patients. Extremely high levels of calcitonin found in medullary carcinoma of the thyroid gland do not result in hypocalcemia.32
Several endogenous substances, including peptides, steroid hormones, and amines, have been found to influence PTH release.33,34 Calcium represents the most potent regulator of PTH secretion, however. Minor alterations within the physiologic calcium concentration range can induce considerable secretory responses—reduction of ionized plasma calcium by 0.04 mmol/L may elevate serum PTH by 100% or more. The rapid effect of extracellular calcium on PTH release suggests that calcium directly interferes with the release process, but the nature of this interference has been only partly clarified. It has been shown that external calcium mainly regulates secretion of newly synthesized hormone, which may bypass the few secretory granules in the parathyroid’s chief cells.35 Intracellular degradation with release of carboxy terminal PTH fragments occurs especially at high extracellular calcium concentrations; this attenuates the biologic activity of the secretory product because the calcium-regulating properties of PTH reside predominantly in its amino terminal portion.
Messenger RNA (mRNA) levels for PTH are increased within hours by low extracellular calcium, consistent with the effects of calcium on PTH secretion.36,37 Calcitriol (1,25-dihydroxyvitamin D3) reduces PTH mRNA levels and inhibits PTH secretion.38
Abnormalities in calcium-controlled PTH release represent a fundamental characteristic of the pathologic parathyroid tissue from hypercalcemic patients with parathyroid adenoma and hyperplasia of varying etiologies.39,40 The representative disturbance is characterized by variable calcium insensitivity of PTH secretion. The calcium insensitivity of ionized calcium and PTH release correlate to each other and to the degree of hypercalcemia in the patient.39
Intact PTH 1-84 is rapidly cleared from the human circulation and has a half-life of only a few minutes.41 Evidence does not exist to support dynamic regulation of peripheral PTH metabolism. PTH clearance mainly depends on high capacity uptake of Kupffer’s cells in the liver and on glomerular filtration. A small amount of PTH appears in the urine, however, because of tubular resorption and proteolysis. Circulating PTH is heterogeneous and contains various carboxy-terminal peptide sequences arising by cleavage, mainly at residues 33 to 43. Although approximately 15% of intact PTH 1-84 is metabolized to such circulating fragments, they make up at least half, and sometimes substantially more, of the immunoactive PTH in the circulation.42 The metabolism of such fragments depends virtually only on an intact renal function; consequently, they are accumulated to considerable degrees in renal insufficiency. In contrast, very few amino terminal PTH fragments exist in the circulation of euparathyroid individuals, although these fragments may become appreciable in primary and secondary hyperparathyroidism.
The diagnosis of primary hyperparathyroidism and evaluation of the extent or severity of secondary hyperparathyroidism have been facilitated by the development of immunometric “sandwich” assays. These assays use a pair of antibodies that recognize different regions of the PTH protein sequence.43–45 Because of cooperative effort of the antibodies, such a sandwich assay is more sensitive than either of the antibodies alone in displacement radioimmunoassays. With careful selection of antibodies, the immunometric assays are specific and sensitive for intact PTH, which allows identification of insufficient PTH secretion of hypoparathyroidism and a wide range of PTH levels without sample dilution. The immunometric analyzing process is swifter than the radioimmunoassays. With a reduction in the incubation times, the analysis may be accomplished in 15 to 30 minutes and, consequently, may be applicable intraoperatively.46,47
Clinical analysis with immunometric PTH assays usually discriminates hypercalcemic patients with hyperparathyroidism from patients with other causes of hypercalcemia. This discrimination is particularly evident with regard to malignancies of nonparathyroid origin, although approximately 5% to 10% of these patients show intact serum PTH in the low-normal range. Nonparathyroid tumors that produce intact PTH are exceptionally rare. These include ovarian and small cell carcinomas and thymoma.48–50
Parathyroid Anatomy and Histopathology
Normal Parathyroid Gland
The usual weight, size, and fat content of a normal parathyroid gland vary. The weight of a normal gland has been recorded to be as low as 40 mg, and a limit of 50 to 60 mg has been suggested.51 One study showed that the weights of normal parathyroid glands have a skewed distribution.51 The mean total weight in the study was 29.5 ± 17.8 mg, with an upper limit of 65 mg. The actual value for the 98th percentile was 75 mg, and this correlates with the operative findings typically noted in primary hyperparathyroidism.
The dimensions of normal glands are rarely mentioned in the literature. Normal dimensions of 3 to 6 mm in length, 2 to 4 mm in width, and 0.5 to 2 mm in thickness, and an average of three dimensions of 5 mm × 3 mm × 1 mm have been proposed.52 Normal glands measuring 12 mm and greater have been reported.53
The stromal fat content of parathyroid glands is the hallmark in the evaluation of their functional status. Detailed studies of normal glands have shown wide variations in fat content.54,55 The accepted percentage of normal fat content is approximately 50%. One study indicated that more than 75% of normal parathyroid glands had less than 30% stromal fat, 50% had less than 10%, and only a few had 40%.54 The variability of fat content reported by different studies suggests that measurement of stromal fat within parathyroid glands has become nearly useless as an indicator of function.54,55 In children and adolescents, parathyroid glands contain very sparse amounts of fat. After adolescence, stromal fat progressively increases until 25 to 30 years of age; subsequently fat content is largely determined by constitutional factors. Women seem to have a tendency to have higher glandular fat content, which may be related to total body fat concentrations.56
Four parathyroid glands is the usual number found in humans. In dissection studies of 428 human subjects by Gilmour,57 four parathyroid glands were found in 87% of all patients, and three glands were found in 6.3%. Akerstrom and coworkers58 reported comparable rates in an autopsy study of 503 individuals. In this study, four parathyroid glands were found in 84% of patients, and three parathyroid glands were found in 3%. The presence of supernumerary parathyroid glands is rare, and may have important clinical consequences, especially with respect to patients with hyperparathyroidism resulting from multiple-gland disease.
In a series of 2015 patients who were operated on for primary hyperparathyroidism, a hyperfunctioning supernumerary fifth parathyroid gland was the source of hypercalcemia in 15 patients (0.7%).59 Nine of these patients required reoperation to remove the supernumerary gland representing the parathyroid tumor. Most of these fifth gland tumors were located in the mediastinum, either in the thymus (seven tumors) or related to the aortic arch (three tumors). Edis and Levitt60 reported a rate of persistent hyperparathyroidism of 10% resulting from an enlarged supernumerary parathyroid gland in patients with secondary hyperparathyroidism. In a series of 762 patients with primary hyperparathyroidism, Wang and colleagues61 documented 6 patients with persistent hyperparathyroidism caused by hyperfunctional supernumerary glands (0.6%), all of which were located in or in close association with the thymus.
In the previously mentioned study by Gilmour,57 supernumerary parathyroid glands were observed in 29 of 428 specimens (6.7%). Five parathyroid glands were observed in 25 specimens (5.8%), 6 parathyroids were observed in 2 specimens (0.05%), 8 parathyroids were observed in 1 specimen, and 12 parathyroids were observed in 1 other specimen. From the studies by Gilmour57 and Akerstrom and coworkers,58 it is evident that supernumerary parathyroids are most commonly found within the thymus or in relation to the thyrothymic tract.
Parathyroid Gland Location
The location of parathyroid glands may vary, as a consequence of the variation in degree of migratory descent during development. Additional influences on these variable locations involves displacement of enlarged parathyroid glands during the development of hyperparathyroidism. Enlarged parathyroid glands tend to migrate in a fibroareolar plane, which offers little resistance as a result of gravity and the action of swallowing and variations in intrathoracic pressure.62
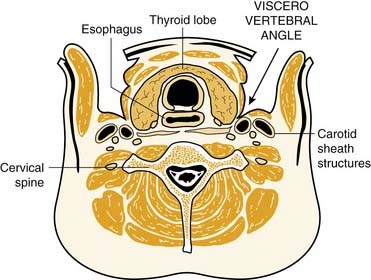
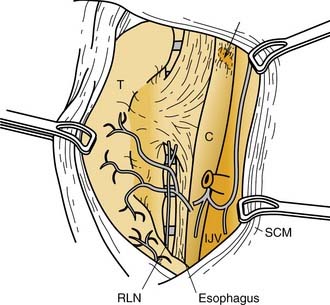
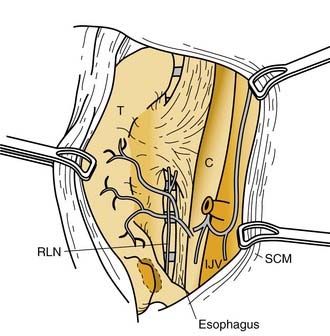
Eighty percent of the superior parathyroid glands are found at the cricothyroid junction approximately 1 cm cranial to the juxtaposition of the recurrent laryngeal nerve and the inferior thyroid artery.58 The superior parathyroids, which are intimately associated with the posterior capsule of the superior thyroid pole, are usually covered by an extension of the pretracheal fascia that envelopes the thyroid gland and connects it to the hypopharynx and esophagus and the carotid sheath. The relationship of these superior parathyroid glands with the pretracheal fascia is such that the glands themselves are allowed freedom of movement under this “pseudocapsule.” This feature discriminates parathyroid glands from thyroid nodules, which cannot move freely because they are enveloped by the true capsule of the thyroid gland.
Normal superior parathyroid glands may be found in the retroesophageal or paraesophageal space in approximately 1% of all instances.63 These spaces represent sites where enlarged superior parathyroid glands potentially descend to the superior and posterior mediastinum.
The incidence of intrathyroidal parathyroid glands is controversial. Akerstrom noted true superior intrathyroidal parathyroid glands in 3 instances (0.2%) among 503 autopsy specimens.58 Wang64 considered the superior parathyroid gland the most likely to be intrathyroidal primarily because of the close embryologic relationship of the primordium of the superior parathyroid gland with the lateral complex of the thyroid. In a series by Wheeler64a in which 8 intrathyroidal parathyroid tumors were noted in 7 of 200 patients (3.5%) undergoing neck exploration, 7 of these intrathyroidal glands were considered to originate from the inferior position. The overall incidence of intrathyroidal parathyroid glands ranges from approximately 0.5% to 3% as reported in the literature.65–68
Morphologic Characteristics of Parathyroid Glands
The visible discrimination between normal and abnormal hyperfunctioning parathyroid glands is essential to successful parathyroid surgery. The appearance of normal and abnormally functioning parathyroid glands varies and depends on the anatomic position and relationship to the thyroid capsule. Parathyroid glands that are located in loose connective tissue generally are more characteristically oval-shaped, bean-shaped, or teardrop-shaped. When parathyroid glands are closely juxtaposed to the thyroid capsule compressed by the pretracheal fascia, their appearance tends to be more conforming resulting in a flat shape with well-defined edges. The color of normal parathyroid glands ranges from yellowish brown to reddish brown. Generally, the color may depend on the amount of stromal fat, oxyphil cell concentration, and degree of vascularity.69 Normal glands tend to be more reddish brown or rust-colored in younger patients, whereas older individuals have parathyroid glands of a more yellow-brown or tobacco color. Enlarged hyperfunctional parathyroid glands have a color variation from dark brown to light yellow. Enlarged glands occurring in either secondary or tertiary hyperparathyroidism may have a lighter gray tone to the coloration. Parathyroid carcinoma can also show a mottled gray-to-white surface appearance.
Vascular Anatomy of the Parathyroid Glands
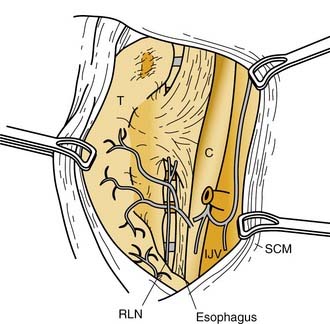
Normal parathyroid glands most commonly are supplied by a single dominant artery (80%).70 The length of the dominant artery supplying the parathyroid gland may vary from 1 to 40 mm. In most instances, the superior and inferior parathyroid glands derive their dominant arterial blood supply from the inferior thyroid artery. Ligation of the inferior thyroid artery during thyroid surgery may not always compromise the blood supply to the superior parathyroid gland, however. Abundant arterial anastomoses exist between the parathyroid glands and include anastomoses with thyroid arteries and dominant arteries of the larynx, pharynx, esophagus, and trachea. Of the superior parathyroid glands, 20% or more may be vascularized solely by the superior thyroid artery. In an autopsy study by Delattre and associates,70 10% of the inferior parathyroid glands derived their dominant arterial supply from a branch of the superior thyroid artery. In most of these instances, the inferior thyroid artery was noted to be absent. Primary mediastinal parathyroid glands have shown an arterial supply that represents a thymic branch of the internal mammary artery.71
Histopathology of the Parathyroid Glands
The parathyroid glands are enveloped in their own thin collagenous connective tissue capsule. This capsule extends septa into the gland, which separate the parenchyma into elongated chords or clusters of functional secretory cells. Blood vessels, lymphatics, and nerves travel along the septa to reach the interior of the gland.72
The major functional parenchymal cells of the parathyroid glands are the chief cells, which are slightly eosinophilic staining and measure 5 to 8 millimicrons in diameter. The chief cells contain many cytoplasmic granules (200 to 400 nm in diameter), which arise from the Golgi complex and represent the secretory granules.72 These granules contain PTH, which is synthesized from a precursor of prepro-PTH. With increasing age, the secretory cells of the parathyroid glands may be replaced by adipose cells, which may make up 50% to 60% of the gland in older individuals.
The second cell type making up parathyroid glandular parenchyma is the oxyphil cell. Although their function is unknown, it is believed that oxyphil cells and a third cell type, sometimes described as intermediate cells, may represent inactive phases of a single cell type.73 Oxyphil cells are less numerous, are larger (6 to 10 millimicrons in diameter), and stain more deeply with eosin than chief cells. Oxyphil cells tend to be more mitochondrial-rich, which may explain the increased ability of abnormal parathyroid glands with high oxyphil cell concentrations to concentrate technetium 99m (Tc 99m) sestamibi.74
By electron microscopy, chief cells show Golgi apparatus among dispersed granular endoplasmic reticulum and few secretory granules. The resting chief cells contain abundant lipid and glycogen, whereas during active phase the chief cells are smaller in size and contain decreased amounts of glycogen and lipid.75 The oxyphil cells are characterized by their large size and numerous cytoplasmic mitochondria.75
Single Glandular Enlargement or Parathyroid Adenoma
Single glandular enlargement, or adenoma, is the most common cause of hyperparathyroidism. Because of the variation in pathologic interpretation and patient population, however, the reported incidence of parathyroid adenoma ranges from 30% to 90%.76–80 In larger series of patients, where more uniformly accepted pathologic criteria were followed, approximately 80% to 85% of patients with primary hyperparathyroidism were found to have solitary parathyroid adenoma.76–78 Parathyroid adenoma may occur in any of the four parathyroid glands, but tends to involve inferior glands more commonly than superior glands.74
The gross appearance of parathyroid adenomas varies, but adenomas generally are oval-shaped or bean-shaped, red-brown in color, and soft in consistency.81,82 Adenomas may be bilobed or multilobulated in conformation. In 70% of adenomas, a rim of normal parathyroid tissue may be found around the hypercellular portion of the replaced normal gland. The absence of this characteristic does not exclude the presence of a parathyroid adenoma, however. The incised surface of an adenoma may appear smooth or nodular, or may show obvious areas of cystic change. Under light microscopy, adenomas appear similar to normal parathyroid glands, exhibiting a thin fibrous capsule with a cellular framework arranged in nests and cords invested by a rich capillary network. Other growth patterns include follicular, pseudopapillary, and acinar patterns.
Chief cells are the dominant cell types in most parathyroid adenomas. Oxyphil cells and transitional oxyphil cells are usually seen in varying proportions interspersed between the collections of chief cells.80–82 The chief cells in adenomas may be larger than found in normal glands, and may exhibit a greater degree of nuclear pleomorphism and giant cell formation.83,84 Nuclear atypia is of limited value, however, in distinguishing between parathyroid adenoma and carcinoma. Mitotic figures are uncommon in adenomas; however, they may be seen in a small percentage of cases.85
Variations in single glandular enlargement representative of parathyroid adenoma may occur and include the following subtypes: oncocytic adenoma, lipoadenoma, large clear cell adenoma, water clear cell adenoma, and atypical adenoma. Oncocytic adenoma is a rare subtype of parathyroid adenoma (4.4% to 8.4% of adenomas) that is composed predominantly (>80% to 90%) or exclusively of oxyphil cells.86,87 Previously, adenomas of the oncocytic variety were believed to be nonfunctional; however, oxyphil adenomas associated with hyperparathyroidism have been reported.88–90 Similar to typical adenomas, oncocytic tumors occur more frequently in women and are found most often in the sixth or seventh decade.89,90 Grossly, the tumors tend to be large, reported to range in size from 0.2 to 61 g; they are soft, spherical, ellipsoid, lobulated, or nodular, and range in color from light tan to dark orange-brown or mahogany.89,91 Microscopically, adenomas are composed predominantly of polygonal cells with abundant brightly eosinophilic granular cytoplasm and small round central hyperchromatic nuclei. Fat stain shows reduce cytoplasmic fat as per typical adenomas. Numerous mitochondria are densely packed throughout the cytoplasm on ultrastructural examination.
Lipoadenoma is another rare subtype of adenoma that was first described in 1958 as a parathyroid hamartoma.92 The initial description was that of a nonfunctioning mass; subsequent reports documented that these lesions can be responsible for hyperparathyroidism.93–95 The tumor comprises a lobulated yellow-tan mass composed of nests, acini, and cords of chief cells and occasional oxyphil and clear cells, intimately associated with large areas of adipose tissue or myxoid stroma or both. A rim of normal parathyroid tissue may be present at the periphery.
Water clear cell adenomas have been described, although their existence was initially doubted.96 In contrast to the large clear cell adenomas that accumulate glycogen, true water clear cell adenomas show a glycogen-free cytoplasm that is filled with membrane bound vesicles.97
Atypical adenoma is the term used to describe parathyroid adenomas that exhibit atypical cytologic features without definite evidence of malignancy (i.e., vascular or soft tissue invasion or both or metastases).98 It is important to distinguish these benign lesions from parathyroid carcinoma. The malignant potential of atypical adenomas in terms of recurrent or metastatic behavior is uncertain. These lesions may exhibit conspicuous mitoses, adherence to surrounding tissues, trabecular cellular arrangements, capsular invasion, or broad fibrous bands.99 One such lesion described consisted of a multifocal spindle cell proliferation that was mitotically active, averaging eight mitoses per 10 high-power fields within an otherwise typical parathyroid adenoma.100
Multiple Enlarged Glands or Parathyroid Gland Hyperplasia
Primary parathyroid hyperplasia is defined as proliferation of the parenchymal cells leading to increase in gland weight in multiple parathyroid glands in the absence of a known stimulus for PTH secretion. Two types of parathyroid hyperplasia are seen: the common chief cell hyperplasia and the rare water cell or clear cell hyperplasia.76,77
Chief Cell Hyperplasia
In 1958, Cope and associates101 first showed chief cell hyperplasia as a cause of primary hyperparathyroidism. It accounts for approximately 15% of hyperparathyroidism in reported series; however, some reports have indicated that about half of primary hyperparathyroidism may be produced by hyperplasia. A variation in this reporting is generally attributable to discrepancies in pathologic interpretation of abnormal parathyroid glands. The stimulus for this disorder is unknown; some studies have indicated a role of a possible circulating factor that can induce proliferation of parathyroid cells in culture. Approximately 30% of patients with chief cell hyperplasia have some type of familial hyperparathyroidism or one of the MEN syndromes.76,77,102,103 Molecular studies have shown that hyperplasias are monoclonal proliferations.15,16
Grossly, there is enlargement of all four glands. The glands may be of variable size, or they may be uniformly enlarged. By light microscopy, the dominant cell types are chief cells; however, one may also observe intermixed oxyphil cells and transitional oxyphil cells. The cellular proliferations may also give rise to nodular formation, and this can cause asymmetric gland enlargement.76,77
The amount of cytoplasmic fat in the chief cells is either reduced or absent.76,77 The chief cell in the nodular areas may be totally devoid of any fat, whereas the cells between the nodules may contain fat. Abnormal nuclei or mitoses are distinctly rare.77
Water Clear Cell Hyperplasia
Water clear cell hyperplasia is rare and is characterized by a proliferation of vacuolated water clear cells in multiple parathyroid glands. It shows a female predilection and leads to pronounced hypercalcemia and severe clinical disease. This represents the only parathyroid disorder in which the superior glands are larger than the inferior parathyroid glands. The glands affected by water clear cell hyperplasia tend to be larger and more irregular in shape with lobular extensions to surrounding soft tissue. By light microscopy, the glands show diffuse proliferations of clear cells characterized by clear cytoplasm and small dense nuclei. On high-power magnification, the cytoplasm is shown to be filled with small vacuoles. Cytoplasmic lipid is generally not present; however, moderate amounts of glycogen may be identified. The histologic appearance of water clear cell hyperplasia bears a resemblance to that of renal cell carcinoma.104
Secondary parathyroid hyperplasia as a consequence of renal failure cannot be distinguished from primary hyperplasia with the exception that early in the disorder there seems to be a greater tendency for the glands to be more uniform in size.105 As the disease progresses, asymmetry becomes more evident in renal failure–induced disease. The degree of glandular enlargement tends to reflect the severity of the underlying renal disorder.106 The largest glands are noted in patients whose renal disease began in childhood.107
Parathyroid Carcinoma
Parathyroid carcinoma is a rare malignant neoplasm derived from the parenchymal cells of the parathyroid glands. It has been reported to be responsible for 0.1% to 5% of cases of primary hyperparathyroidism.108–112 It is uncertain whether parathyroid carcinoma actually begins within preexisting benign parathyroid lesions.112,113 Carcinoma has been postulated to arise in the setting of primary parathyroid hyperplasia, notably familial hyperplasia.114–116 Only rare patients with parathyroid carcinoma have a history of prior neck irradiation.110,112
Morphologic features diagnostic of parathyroid malignancy are difficult in terms of definition and practical application during surgery. In one series of 40 patients with metastatic parathyroid cancer, 50% were thought to have benign disease by the operating surgeon and consulting pathologist during the time of initial exploration.117 Metastases are the only certain sign of malignancy. Metastatic behavior at the time of presentation is distinctly rare, however.108
Parathyroid carcinomas are characteristically large tumors, with 30% to 50% being palpable at the time of presentation.108,109 The tumors may measure 6 cm in diameter with a mean of approximately 3 cm.108 Although the average weights of carcinomas are reported to be greater than those of adenomas, there seems to be great overlap, indicating that weight alone may not be a major distinguishing characteristic between benign and malignant lesions. Carcinomas generally arise in the usual parathyroid locations, although they have been described in ectopic supernumerary glands within the mediastinum.118 Most parathyroid carcinomas are firm or hard in consistency and have a gray-to-white surface color as opposed to adenomas, which tend to be soft and tan in appearance. Adherence of the lesion to surrounding tissues is common, and these glands may be noted to extend to involve the soft tissues around the thyroid gland or the thyroid parenchyma itself. This may not prove to be a valuable differentiating feature because previous hemorrhage into a benign adenoma may be associated with fibrosis and adherence to adjacent structures in benign disease.112
Metastases at the time of presentation are unusual, but may rarely be found in regional lymph node basins.117 As opposed to regional metastases, parathyroid carcinoma is more often associated with widespread local infiltration, with invasion into contiguous structures, such as thyroid gland, strap muscles, trachea, and recurrent laryngeal nerve. Advanced metastases may occur and may be found in the lungs, bone, cervical and mediastinal lymph nodes, liver, occasionally kidney, and adrenal glands.117,119 Pulmonary metastases are the most common distant metastasis noted.117
Microscopic diagnosis of parathyroid carcinoma is a difficult task. The entire gland is traversed by broad fibrous bands that seem to originate from the capsule and extend into the substance of tumor leading to a lobulated appearance. The cells may be clear or rarely oxyphilic, and are arranged in nests and trabeculae.120 The cell may be uniformly bland or may show metaplasia, but the cases with minimal atypical findings may be difficult to distinguish from an adenoma.121–123
Mitosis can be seen in most instances and has been suggested as a primary factor in diagnosing parathyroid carcinoma.124 Mitotic figures may also be seen in parathyroid adenoma and hyperplasia, however, and their absence does not rule out a diagnosis of carcinoma.125,126 It is generally acknowledged that mitoses in parathyroid lesions should be of concern, especially because the follow-up in reported benign cases of parathyroid tumor exhibiting mitoses has been limited. Increased mitotic activity in unequivocal parathyroid carcinoma is an indicator of poor prognosis.127
The only reliable indicators of malignancy in parathyroid carcinoma are invasion of the surrounding structures, metastases, or both.127 Features such as desmoplastic reaction, mitotic activity, nuclear atypia, and necrosis may be more common in carcinoma than in benign lesions, but do not constitute criteria sufficient for a diagnosis of malignancy.127 In the absence of an infiltrative growth pattern, the parathyroid lesion showing some other feature of malignancy including mitoses may be designated as atypical adenoma. 128 Nonfunctioning parathyroid carcinomas have been rarely described, tending to be large and consisting of clear or oxyphil cells.129,130
Parathyroid carcinoma usually grows slowly and can be an indolent tumor. Multiple recurrences after surgical resection are common and may occur over a 15- to 20-year period.121,122 Patients with parathyroid carcinoma often die as a result of the effects of excessive PTH secretion and uncontrolled hypercalcemia, rather than growth of the tumor mass. Surgical excision of recurrence or metastases may provide excellent palliation by reducing tumor burden and consequently hormone production.131,132
Surgical Embryo-Anatomic Relationships in the Central Neck
Parathyroid tissue originates from primordial pharyngeal endoderm formed in the third and fourth pharyngeal pouches during the fifth week of embryologic development (Fig. 125-3). The epithelial lining of the dorsal wing of the third pharyngeal pouch differentiates into primordial parathyroid glandular tissue, whereas the ventral portion of the pouch differentiates into the thymus. As the thymus migrates medially and inferiorly, it pulls the inferior parathyroid gland (parathymus) with it into the thymic tail. Eventually, the main portion of the thymus migrates to its final position in the upper thoracic region, and its tail involutes, leaving the developing parathyroid gland to come to its position on the dorsal surface of the inferior pole of the thyroid gland. This glandular tissue eventually forms the inferior parathyroid gland. Simultaneously, the epithelium of the dorsal wing of the fourth pharyngeal pouch begins to differentiate into parathyroid glandular tissue. After separation from the regressing pouch, it becomes associated with the lateral portion of the caudally migrating thyroid, and is carried a short distance medially and inferiorly until it resides posterior to the superior pole of the thyroid gland. This tissue eventually develops into the superior parathyroid gland.
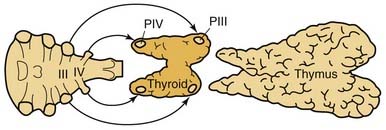
Figure 125-3. Embryologic derivation and subsequent descent of the parathyroid glands with associated structures.
This embryologic pattern of development has significant implications for the identification of ectopic or normal glandular variance during the course of parathyroidectomy. The longer embryologic migration results in an extensive area of potential dispersal for the normal inferior parathyroid gland. In 61% of cases, the glands are situated at the level of the inferior poles of the thyroid lobes on the posterior, lateral, or anterior aspects. In 26% of cases, they are situated in the thyrothymic ligament or on the upper, cervical portion of the thymus. More rarely, in 7% of cases, they are situated higher up, at the level of the middle third of the posterior aspect of the thyroid lobes, and may then be confused with the superior parathyroid gland. Because the embryonic descent of the thymus extends from the angle of the mandible to the pericardium, anomalies of migration of the parathymus, whether excessive or insufficient, are responsible for high or low ectopic locations of the inferior parathyroid gland. The incidence of higher ectopia along the carotid sheath, from the angle of the mandible to the lower pole of the thyroid, does not seem to exceed 1% to 2%.133–135 Alternatively, if the separation from the thymus is delayed, the inferior parathyroid gland may be pulled inferiorly into the anterior mediastinum to a varying degree. In this circumstance, they are usually within the thymus, at the posterior aspect of its capsule, or still in contact with the great vessels of the mediastinum. Lower ectopic regions such as these are noted in 3.9% to 5% of instances.134,136
The superior parathyroid glands follow the thyroid migration of the ultimobranchial bodies, which travel toward the lateral part of the main medial thyroid rudiment. In contradistinction to the inferior glands, the superior parathyroids have a limited descent within the neck. They remain in contact with the posterior part of the middle third of the thyroid lobes. This limited course of embryonic migration explains why they remain stable in their regional distribution when not pathologic. In most instances, they are grouped at the posterior aspect of the thyroid lobes, in an area 2 cm in diameter, whose center is situated about 1 cm above the crossing of the inferior thyroid artery and the recurrent laryngeal nerve.134,137 Because of the more extensive descent of the inferior parathyroid gland, the descent of the parathymus result in the inferior and superior glands crossing during development. This embryonic crossing of the glands explains why their grouping at the level of the inferior thyroid artery, at the junction of the middle and inferior thirds of thyroid lobe, is in many respects quite close, depending on the degree of migration of the inferior parathyroid gland.
Because of the short migratory descent of the superior parathyroid gland, the area of dispersal of these glands is limited, and congenital ectopic positions of the superior gland are unusual. In 13% of instances, the glands are on the posterior aspect of the superior pole of the thyroid lobe in a laterocricoid, lateropharyngeal, or intercricothyroid position, and, exceptionally, in less than 1% of instances, they are above the upper pole of the lobe. In 1% to 4% of instances, they are clearly posteriorly based behind the pharynx or esophagus. Parathyroid glands that are found in the posterior superior mediastinum are usually neoplastic superior parathyroid glands that have migrated as a consequence of gravity and changes in intrathoracic pressure.137
Clinical Features of Primary Hyperparathyroidism
Incidence
The incidence of hyperparathyroidism has changed dramatically over the past 3 decades. In the early 1970s, before the widespread use of multichannel autoanalysis of blood chemistry, Heath and colleagues138 reported an annual incidence of 7.8 cases/100,000 in Rochester, Minnesota. After the introduction of routine serum calcium assessment later in the 1970s, the incidence rate increased to 51 cases/100,000/year. After the most prevalent or clinically significant cases were managed, the incidence declined to 27 cases/100,000 annually, and more recent reports indicate that within the population associated with this area of Minnesota, a steady decline in the incidence of hyperparathyroidism has been noted since the late 1970s. This decline cannot be explained by the more limited routine use of multichannel autoanalyzing of blood, which has become part of cost-saving measures since the late 1990s; also, this declining incidence has not been similarly experienced in other populations nationally or internationally.
A higher rate of incidence was noted in the Stockholm study, which examined more than 15,000 subjects over a 2-year period (1971-1973) with a follow-up at 10 years. The early rate was assessed at 6 cases/1000, which at the 10-year follow-up was verified to be 4.4 cases/1000. This rate has not changed appreciably over a 20-year period, in contrast to the Rochester experience.139 One may postulate that the Rochester experience is unique in that after obtaining a very high incidence rate promulgated by routine calcium screening, higher prevalence cases were eliminated to reveal a lower base incidence rate in a population that receives its treatment at a single major center, explaining the steady decline in this fixed population base.
There seems to be a distinct predilection for a higher incidence of hyperparathyroidism in women, especially women beyond menopause. The highest prevalence rate among women in the Stockholm experience confirmed at the 10-year follow-up was approximately 13/1000, which represented a female-to-male ratio of about 4 : 1. This experience is similar to that of other published reports.138,140–143 Several of these studies based on serum calcium screening have shown that the prevalence of primary hyperparathyroidism in the general population is 0.1% to 0.3%, and in women older than 60 years, the prevalence is more than 1%.138,139,144 The clear female preponderance points to the fact that every woman has a 1% risk of experiencing primary hyperparathyroidism during her lifetime. It has been estimated, however, that about 90% of individuals with primary hyperparathyroidism remain undiagnosed.145 The screening of serum calcium has been a particularly important factor leading to the detection of patients with mild symptoms or no symptoms, especially among postmenopausal women.
Presentation
The concept of clinical features associated with primary hyperparathyroidism has changed during recent years. So-called classic, specific symptoms (i.e., bone disease, renal stones, and hypercalcemic crisis) represent obvious manifestations of the disease. The relative proportion of patients with osteitis fibrosis cystica, nephrolithiasis, and hypercalcemic crisis has continuously decreased in clinical series because of the increase in the number of patients with nonspecific or nonapparent symptoms. Currently, osteitis fibrosis occurs in about 1% of patients, and only 10% to 20% of patients have renal stones.146–148 Nonspecific symptoms include malaise, fatigue, depression and other psychiatric symptoms, sleep disturbance, weight loss, abdominal pains, constipation, vague musculoskeletal pains in the extremities, and muscular weakness.
The term asymptomatic hyperparathyroidism has been commonly applied when the disorder is detected during health screenings and population studies, or coincidentally during medical examinations. The usual presenting abnormality in these patients is an abnormally elevated serum calcium detected on routine blood chemistry screening. Despite the lack of obvious abnormalities noted at the time of diagnosis, caution should be exercised before declaring that a patient is asymptomatic. Many seemingly asymptomatic patients may manifest subtle or even “silent” sequelae of hyperparathyroidism at the time of presentation, and these patients may be more appropriately described as minimally symptomatic because nonspecific symptoms and unidentified complications of hyperparathyroidism would be eliminated by parathyroidectomy.149 Some of these nonspecific sequelae include emotional complaints, muscular fatigue, constipation, bone and joint pain, and silent objective findings such as asymptomatic renal calculi and decreased bone mineral density. In most patients with asymptomatic or minimally symptomatic hyperparathyroidism, these symptoms are subtle and may be so common in the general population that they preclude establishment of a causal relationship to primary hyperparathyroidism.
Kidney and Urinary Tract
Historically, greater than 50% of patients with hyperparathyroidism develop renal symptoms manifested by nephrolithiasis and nephrocalcinosis. This percentage decreased significantly to approximately 4% after the widespread use of screening tests for serum calcium levels.150 Most stones are composed of calcium oxalate; however, calcium phosphate stones may also occur. The symptoms associated with urolithiasis include renal colic, hematuria, and pyuria. Metabolic acidosis may also be a part of the clinical syndrome.
Skeletal System
Bone loss in hyperparathyroidism occurs at cortical bone sites generally sparing trabecular bone.151 Because of this finding, the role of hyperparathyroidism in osteoporosis is unclear, especially for patients in whom symptoms are minimal or absent, and in whom the disease is mild. Postmenopausal women with primary hyperparathyroidism exhibiting early signs of osteoporosis seem to be at significant risk for developing more severe bone disease and resultant sequelae (i.e., vertebral and hip fractures). The benefit of parathyroidectomy is most apparent in these patients.152
Neuromuscular System
Muscle weakness, particularly in the proximal extremity muscle groups, together with progressive fatigue and malaise, may occur in symptomatic primary hyperparathyroidism. Electromyography changes may be seen in these patients together with atrophy of the skeletal muscle on biopsy specimens.153–155 Although the severe symptoms are rarely encountered, some signs of muscle fatigue and weakness may be present in 40% of patients with mild primary hyperparathyroidism.156 Usually these subtle symptoms are manifested as muscle aches and fatigue on rising from a chair or climbing stairs. Progression of the disease may ultimately result in weakness that limits activity and ambulation over weeks to months. This neuromuscular syndrome is noted to improve after parathyroidectomy in 80% to 90% of the patients affected.157,158
Neurologic System
Neurologic manifestations of primary hyperparathyroidism are represented by a spectrum of symptoms ranging from anxiety and mild emotional disturbance to frank psychosis. Depression, nervousness, and cognitive dysfunction may commonly occur to varying degrees in primary hyperparathyroidism. Cerebral dysfunction characterized by organic brain syndrome is more common in elderly patients with an underlying mild cognitive abnormality exposed to hypercalcemia. Other neurologic changes occasionally seen in patients with hyperparathyroidism include deafness, dysphagia, dysosmia, and dysesthesia.159 Many of the psychiatric symptoms in patients with primary hyperparathyroidism are improved after parathyroidectomy.158,160 Fifty percent of patients with depression or anxiety, or both, improve after surgery. A favorable effect has also been shown in about 50% of patients with organic brain syndrome and dementia. Some older patients experience dramatic improvement; however, it is impossible to predict whether any specific patient will improve after surgery.160
Cardiovascular System
Hypertension may occur in 50% of patients with hyperparathyroidism.161 Convincing evidence of a pathogenic mechanism does not exist, however, and parathyroidectomy results in a reduction in blood pressure in a few of these patients.162 Swedish investigators reported an association with myocardial ischemia and left ventricular dysfunction in patients with hyperparathyroidism and various symptoms, which exhibited reversibility after parathyroidectomy.163
Hypercalcemic Abnormalities
Hypercalcemic syndrome occurring as a result of hyperparathyroidism includes polydipsia and polyuria, anorexia, vomiting, constipation, muscle weakness and fatigue, mental status changes, and skin abnormalities. Patients developing markedly elevated serum calcium levels approaching 15 mg/dL may present with severe mental status changes or coma, a so-called hypercalcemic crisis. If untreated, this condition may progress to acute renal failure and the onset of dysrhythmias, which may precipitate sudden death.164 Other abnormalities include metastatic calcifications at the corneal-scleral junction (so-called band keratopathy), shortened Q-T interval on electrocardiogram, ectopic calcium deposits in various organs, and pruritus. Some patients also may present with a nonspecific debility manifested by anorexia, fatigue, anemia, weight loss, and advancing osteitis, all of which are reversible after parathyroidectomy.
Clinical Course of Untreated Hyperparathyroidism
Several 8- to 10-year prospective studies have shown a benign course of untreated mild hyperparathyroidism in most patients, with no significant progression of symptoms, hypercalcemia, bone marrow density loss, or renal function impairment. No pathologic fractures or kidney stones developed during these observation periods.165,166 There was evidence, however, of disease progression in 27% of patients, including marked hypercalcemia, hypercalciuria, or loss of bone mineral density in 10%.167
Osteitis fibrosis cystica is currently uncommon in patients with primary hyperparathyroidism, and is most often seen in patients with severe hypercalcemia. Bone density measurements have shown an average reduction in cortical bone of 17% among current patients with primary hyperparathyroidism, however, and the bone loss tends to be the most pronounced in postmenopausal women.167–169 Total and trabecular bone mass is often significantly but less markedly reduced.170 No bone loss has been detected in postmenopausal women with borderline hypercalcemia, but losses were significant when the serum calcium level was greater than 2.74 mmol/L.168
Clinically evident renal failure is currently an unusual complication of primary hyperparathyroidism. Reduction of creatinine clearance and urinary concentrating capacity occur in more than one third of patients with mild hypercalcemia, however, indicating that impairment of glomerular and tubular function may occur silently.168 Serum creatinine measurements are crude estimates, and increase only after creatinine clearance is substantially reduced. Serum creatinine levels also decrease with declining muscle mass of aging.168
Rare patients with initially mild hypercalcemia but rapidly advancing disease may have parathyroid carcinoma.171 In addition, stepwise “clinical progression” may occur during observation in patients with primary hyperparathyroidism, possibly representing development of secondary mutations that cause accelerated growth of the tumor. Bleeding in parathyroid tumor may also cause an abrupt increase in serum calcium levels. A history of mild primary hyperparathyroidism has been reported in one third of patients with hypercalcemic crisis. Because it is impossible to predict whether progressive disease will occur in any patient, extended follow-up is crucial if surgery is deferred in primary hyperparathyroidism.172–174 Younger patients seem to be at greater risk for progressive disease. Medical surveillance is inappropriate in younger patients and in patients with more marked hypercalcemia. The indications for surgical intervention and the alternative of medical surveillance are discussed later.
Diagnosis of Hyperparathyroidism
Evaluation of Hypercalcemia
Hypercalcemia has been reported to occur in 1% to 3.9% of the general adult population and 0.2% to 2.9% of hospitalized patients.175 These patients present with widely variable clinical symptoms, depending on the severity of the elevated serum calcium. In most situations, mild hypercalcemia is asymptomatic, but severe hypercalcemia may become life-threatening, especially when the serum calcium is greater than 14 mg/dL.
Several factors may influence measured serum total or ionized calcium. Alterations in serum albumin level increase or decrease serum total calcium without affecting the ionized calcium level. Of the circulating calcium that is bound to protein, 70% is albumin bound, having 12 calcium binding regions per molecule. Under normal circumstances, only about 20% of these specific calcium binding sites are actually occupied by serum calcium. Decreases in serum albumin less than 4 g/dL decrease total calcium by 0.8 mg/dL for each 1-g/dL decrease in serum albumin. Increases in serum albumin greater than 4 g/dL increase serum total calcium by 0.8 mg/dL for each 1-g/dL increase in serum albumin. Dehydration may increase total serum total calcium because of the resulting hemoconcentration. Acidosis increases serum ionized calcium by decreasing binding of calcium to albumin, and alkalosis decreases ionized calcium by increasing binding of calcium to albumin, without affecting serum total calcium. Under most clinical circumstances, it is adequate and appropriate to measure serum total calcium, but serum ionized calcium should be measured in clinical situations that may be associated with changes in albumin concentration or serum pH.176,177
The differential diagnosis of hypercalcemia is varied and extensive. The most common etiology of hypercalcemia in nonhospitalized patients is primary hyperparathyroidism, and the most common cause of hypercalcemia in hospitalized patients is malignancy. In most circumstances, the differential diagnosis of hypercalcemia may be categorically divided into causes that are mediated by PTH and causes that are not (Tables 125-1, 125-2 and 125-3). Hypercalcemia that is mediated by PTH is most frequently caused by primary hyperparathyroidism (see Table 125-1). Additionally, physiologic secondary hyperparathyroidism, which is defined as hyperparathyroidism caused by a physiologic source without associated renal insufficiency, or pathologic secondary hyperparathyroidism with associated renal failure may result in PTH-mediated hypercalcemia.
Table 125-1 Causes of Parathyroid Hormone–Mediated Hypercalcemia
| Primary hyperparathyroidism |
| Parathyroid adenoma |
| Parathyroid lipoadenoma |
| Parathyroid hyperplasia |
| Parathyroid carcinoma |
| Neck or mediastinal parathyroid cyst |
| Secondary hyperparathyroidism |
| Tertiary hyperparathyroidism |
Table 125-2 Causes of Hypercalcemia of Malignancy
| Parathyroid hormone–related protein secretion by lung, esophagus, head and neck, renal cell, ovary, bladder, and pancreatic cancers; thymic carcinoma; islet cell carcinoma; carcinoid; sclerosing hepatic carcinoma |
| Ectopic parathyroid hormone secretion by small cell lung cancer, small cell ovarian carcinoma, squamous cell lung carcinoma, ovarian adenocarcinoma, thymoma, papillary thyroid carcinoma, hepatocellular carcinoma, undifferentiated neuroendocrine tumor |
| Ectopic 1,25-dihydroxyvitamin D production by B-cell lymphoma, Hodgkin’s disease, lymphomatoid granulomatosis |
| Lytic bone metastases caused by multiple myeloma, lymphomas, breast cancer, invasive sarcoma |
| Tumor production of other cytokines by T-cell lymphomas/leukemias, non-Hodgkin’s lymphoma, other hematologic malignancies |
Table 125-3 Causes of Non–Parathyroid Hormone–Mediated, Nonmalignant Hypercalcemia
| Benign Tumors |
| Parathyroid hormone–resecreting ovarian dermoid cyst or uterine fibroid |
| Endocrine Disease |
| Thyrotoxicosis |
| Pheochromocytoma |
| Addison’s disease |
| Islet cell pancreatic tumors |
| VIPoma |
| Granulomatous Disorders |
| Sarcoidosis |
| Wegener’s granulomatosis |
| Berylliosis |
| Silicone-induced and paraffin-induced granulomatosis |
| Eosinophilic granuloma |
| Tuberculosis (focal, disseminated, Mycobacterium avium complex in AIDS) |
| Histoplasmosis |
| Coccidioidomycosis |
| Candidiasis |
| Leprosy |
| Cat-scratch disease |
| Drugs |
| Vitamin D excess (oral or topical) |
| Vitamin A excess |
| Thiazide diuretics |
| Lithium |
| Estrogens and antiestrogens |
| Androgens |
| Aminophylline, theophylline |
| Ganciclovir |
| Recombinant growth hormone treatment of AIDS patients |
| Foscarnet |
| 8-Chloro-cyclic adenosine monophosphate |
| Miscellaneous |
| Familial hypocalciuric hypercalcemia |
| Immobilization with or without Paget’s disease of bone |
| End-stage liver failure |
| Total parenteral nutrition |
| Milk-alkali syndrome |
| Hypophosphatasia |
| Systemic lupus erythematosus |
| Juvenile rheumatoid arthritis |
| Recent hepatitis B vaccination |
| Gaucher’s disease with acute pneumonia |
| Aluminum intoxication (long-term hemodialysis) |
| Manganese intoxication |
| Primary oxalosis |
AIDS, acquired immunodeficiency syndrome; VIPoma, vasoactive intestinal polypeptide–secreting tumor.
An important entity that must be considered in the differential diagnosis of hypercalcemia is familial hypocalciuric hypercalcemia.178,179 This disorder has the ability to mimic the serum biochemical appearance of primary hyperparathyroidism, but is not treated surgically. Familial hypocalciuric hypercalcemia is an autosomal dominant disorder that is linked to chromosomes 3q, 19p, and 19q, and is largely caused by inactivating mutations in the parathyroid cell calcium-sensing receptor.180 Patients with familial hypocalciuric hypercalcemia have low 24-hour urinary calcium excretion relative to their hypercalcemia. Presently, the most useful study to distinguish familial hypocalciuric hypercalcemia from primary hyperparathyroidism is the 24-hour urinary calcium-to-creatinine clearance ratio.178 Patients with familial hypocalciuric hypercalcemia typically have ratios of less than 0.01, whereas patients with primary hyperparathyroidism have ratios greater than 0.01.
The second most common etiology of hypercalcemia is malignancy (see Table 125-2). Humoral hypercalcemia of malignancy, which is caused by excessive PTH-related protein secretion by tumors of various types, represents the most common source of malignancy-associated hypercalcemia.181 Numerous solid tumors have been reported to secrete excessive PTH-related protein, including cancers of the lung, esophagus, head and neck, kidney, ovary, bladder, breast, and pancreas. Other tumors that have been variably noted to secrete PTH-related protein include thymic carcinoma, islet cell carcinoma, malignant carcinoid, and hepatic carcinomas. Patients with adult T-cell leukemia or lymphoma or B-cell lymphoma have also been variably shown to produce excess PTH-related protein.182,183
Ectopic PTH secretion has been shown in small cell lung cancer, small cell ovarian carcinoma, squamous cell lung carcinoma, ovarian adenocarcinoma, thymoma, papillary thyroid carcinoma, hepatocellular carcinoma, and undifferentiated neuroendocrine tumor.184,185 Ectopic 1,25-dihydroxyvitamin D has been found to be secreted by B-cell lymphomas, Hodgkin’s disease, and lymphomatoid granulomatosis.186–190 Excessive cytokine production that may result in hypercalcemia has been associated with T-cell lymphomas and leukemias, non-Hodgkin’s lymphomas, and other hematologic malignancies. Another source of hypercalcemia resulting from malignancy is that of calcium released by extensive lytic bone metastasis as a consequence of multiple myeloma, lymphomas, breast cancer, or invasive sarcomas.191
Table 125-3 is an extensive list of causes of non–PTH-mediated hypercalcemia. Nine tumors, including ovarian dermoid cysts or uterine fibroids, may occasionally secrete PTH-related protein or other bone-resorbing cytokines.192 Endocrine disorders, including thyrotoxicosis (resulting from increased bone resorption), pheochromocytoma (caused by coexisting primary hyperparathyroidism in MEN syndrome), adrenal insufficiency or crisis (caused by volume depletion or hemoconcentration), and VIPomas (resulting from excess secretion of vasoactive intestinal peptide causing dehydration and metabolic acidosis), may also cause non–PTH-mediated hypercalcemia.193–197 Granulomatous disease in the absence of malignancy or endocrine disorder may be a source of hypercalcemia, especially in younger and middle-aged patients, and may manifest with elevated serum calcium levels. The source of this hypercalcemia is generally increased production of 1,25-dihydroxyvitamin D, and has been shown in patients with sarcoidosis, Wegener’s granulomatosis, berylliosis, silicone-induced or paraffin-induced granulomatosis, eosinophilic granuloma, focal or disseminated tuberculosis, histoplasmosis, coccidioidomycosis, candidiasis, leprosy, and cat-scratch disease.198–210
Medications may cause hypercalcemia via various mechanisms. Excessive vitamin D intake, or hypervitaminosis D, may stimulate intestinal calcium absorption, and thiazide diuretics may directly inhibit renal calcium excretion.211,212 Lithium compounds may interfere with the ability of calcium to interact with parathyroid and renal calcium-sensing receptors, increasing PTH secretion by the parathyroid glands.213 Numerous other agents, including estrogens, antiestrogens, androgens, aminophylline, theophylline, ganciclovir, and recombinant growth hormone in acquired immunodeficiency syndrome (AIDS) patients, and hypervitaminosis A may affect other physiologic mechanisms resulting in hypercalcemia.214–220 Miscellaneous disorders that can also be associated with hypercalcemia are listed in Table 125-3.
Localization Studies and Their Application
Although a comprehensive bilateral exploration of all appropriate cardinal parathyroid locations in the surgical management of hyperparathyroidism remains the gold standard, and must be fundamental to the armamentarium of all parathyroid surgeons, the development of more effective imaging methods or parathyroid localization has permitted surgeons to employ more limited procedures in neck exploration, while achieving the same, or improved, surgical outcomes as achieved by four-gland exploration.74,221 Aside from the application of these imaging techniques for parathyroid localization in more limited, focused explorations of the neck for primary hyperparathyroidism, the use of localization studies before re-exploration for persistent or recurrent hyperparathyroidism has been universally accepted.222–224 Localization methods may be operationally classified as preoperative (invasive or noninvasive) and intraoperative (Table 125-4).
| Noninvasive Preoperative Methods |
| Ultrasonography |
| Radioiodine or technetium thyroid scan |
| Thallium-technetium scintigraphy |
| Technetium 99m sestamibi scintigraphy |
| Computed tomography scan |
| Magnetic resonance imaging |
| Invasive Preoperative Methods |
| Fine-needle aspiration |
| Selective arteriography or digital subtraction angiography |
| Selective venous sampling for parathyroid hormone assay |
| Arterial injection of selenium-ethionine |
| Intraoperative Methods |
| Intraoperative ultrasonography |
| Toluidine blue or methylene blue |
| Urinary adenosine monophosphate |
| Quick parathyroid hormone intraoperative |
Noninvasive Preoperative Localization
Pertechnetate (Technetium 99m) Thallous Chloride (Thallium 201) Imaging
Thallium uptake by parathyroid adenomas was initially reported by Fukunaga and others.225–232 Subsequently, Ferlin and Young and their colleagues226,227 performed the clinical application using Tc 99m together with thallium 201 (Tl 201). Subtraction of the resulting two images helped to locate the abnormal parathyroid gland or glands. This technique required prolonged patient immobilization for obtaining images as the patient remained in the same position for both radionuclide administrations. Results of this localization technology have been variable, with sensitivity rates ranging from 27% to 82% reported.228,229 Sensitivity has been reported to be improved by the application of single-photon emission computed tomography (SPECT) techniques with three-dimensional images.230 The previously noted advantages of this imaging technique are widespread availability, minimal irradiation, and low risk. False-positive results, attributable to patient motion during the examination or as a result of concurrent thyroid abnormalities, have resulted in poor acceptance of this technique as a reliable preoperative localization method for limited parathyroid exploration.
Technetium 99m Sestamibi Scintigraphy
In 1989, Coakley and colleagues231 reported using Tc 99m sestamibi for cardiac function studies. Chiu and coworkers232 showed the incorporation of Tc 99m into the cytoplasm and mitochondria of mouse fibroblasts in response to certain stimuli. Parathyroid cells have large numbers of mitochondria that enable sestamibi to enter parathyroid tissue more intensely than into the neighboring thyroid parenchyma.233 O’Doherty and associates234 compared radionuclide uptake in parathyroid tissue, and noted a greater uptake per gram for sestamibi than for Tl 201.
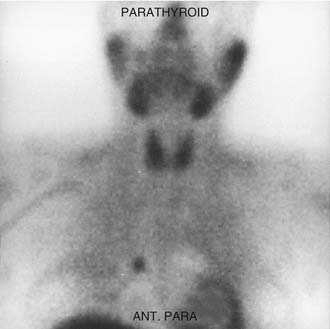
In 1992, Taillefor and colleagues235 proposed a dual-phase scan using Tc 99m sestamibi and cervicothoracic planar parathyroid scintigraphy. In this method, the patient is intravenously injected with 20 to 25 mCi of Tc 99m sestamibi. Subsequent images are obtained at 10 to 15 minutes and then at 2 to 3 hours after the injection. Late phase is usually preferable for detecting parathyroid adenomas because the thyroid and thyroid nodules clear of uptake faster than do parathyroid neoplasms. Jofre and associates236 showed that 70% of delayed images are best seen at 2 hours, and only 15% are best seen at 4 hours. In certain circumstances, oblique or lateral images can be obtained to attempt to add a third dimension to the study. The advantages of the technique include the ability to use this imaging method without patient immobilization between images.
The sensitivity reported for solitary adenomas is 100%, with a specificity of approximately 90%.237–239 Few false-positive results have been reported using this imaging technique, and are predominantly due to solid thyroid nodules, predominantly adenomas.240 Hürthle cell carcinoma and malignant thyroid lymph node metastases have also been noted to result in false-positive sestamibi imaging results; however, cystic lesions of the thyroid gland have not.74,241
False-negative results may be attributable to smaller parathyroid adenoma size or suboptimal dosing of Tc 99m sestamibi.74 Intrathyroid, mediastinal, and deep cervical parathyroid glands have been located with Tc 99m sestamibi; location seems to be independent of its efficiency (representing an advantage over technetium/thallium subtraction scanning). This technique has shown the presence of multiple-gland disease in the form of double parathyroid adenomas (Fig. 125-4).74 Sestamibi, similar to Tl 201, is inaccurate in patients with diffuse four-gland parathyroid hyperplasia.
Tc 99m sestamibi has been combined with iodine 123 and recorded simultaneously in nonoverlapping windows and subtraction. In a series by Hindie and colleagues,242 25 of 27 solitary adenomas were identified with a sensitivity of 94%. In the same population, examination with Tc 99m sestamibi as the sole radionuclide identified 22 of these 27 patients correctly for a sensitivity of 79%. Neumann and associates243 used Tc 99m sestamibi/iodine 123 subtraction and SPECT in patients with secondary hyperparathyroidism with a sensitivity of 77%. In this series of 13 patients with recurrent secondary hyperparathyroidism, all hyperplastic parathyroids were predicted and subsequently correctly identified at surgery.
Technetium 99m Sestamibi with Single-Photon Emission Computed Tomography
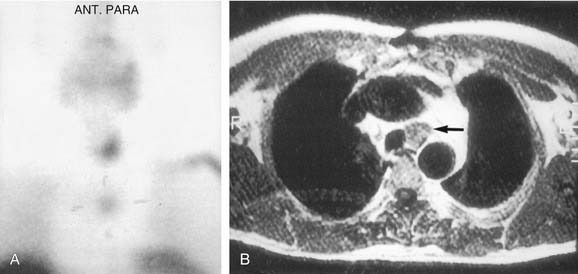
The application of SPECT uses a camera collimator that rotates 360 degrees around the patient in the axial plane, and in essence portrays a three-dimensional image as the sequence progresses from mandible through the thorax. Some investigators have shown a superior sensitivity and localization in the early phase compared with cervicothoracic planar images, whereas the delayed images are approximately equivalent.244 The images appear to have a higher resolution and have two definite recognized advantages over planar sestamibi images: (1) anatomic localization in ectopic adenomas within the carotid sheath, and (2) localization of mediastinal lesions to the anterior or posterior compartment, allowing planning of surgery by lateral thoracotomy versus median sternotomy.245
Computed Tomography–Sestamibi Fusion
Although the regional anatomic localization provided by MIBI-SPECT imaging has been shown to be superior over that of planar imaging, the anatomic relationship of imaged parathyroid tissue with other cervical structures (bony, vascular, visceral) is not clearly defined by these physiologic imaging techniques. Computed tomography (CT)–sestamibi fusion is a combined imaging modality whereby anatomic and physiologic images are combined (fused) to create a single image providing concise anatomic localization of physiologically hyperactive parathyroid tissue (Fig. 125-5). Images are created after the acquisition of contrast-enhanced CT scans of the neck and mediastinum and a standard Tc 99m sestamibi scan with planar and SPECT imaging. The delayed (3-hour) SPECT image is “fused” with the CT scan using specialized software, which provides an automated alignment using a matrix of coordinates. The radiopharmaceutical uptake resolution within the fused image is fine-tuned manually by the radiologist to produce the final localized region. Images are depicted in three dimensions (axial, coronal, and sagittal) in 3-mm slices to provide precise anatomic localization and correlation with other central neck structures in each plane of orientation.
One of the authors (P.K.P.) has accrued experience with this technique in more than 300 patients; the analysis of this experience has yielded specific clinical settings for which this modality has shown clear superiority over planar and SPECT imaging. These settings include refining the directed “minimally” invasive approach (Fig. 125-6); the accurate localization of ectopic glands (i.e., thymic, retroesophageal, mediastinal, intrathyroid) (Figs. 125-7, 125-8, and 125-9); and for patients in whom re-exploration is being carried out in a previously operated region with significant fibrovascular change (Figs. 125-10 and 125-11), to focus the approach and limit dissection in hopes of minimizing the risk of complications. In contrast, early experience suggests that CT-MIBI fusion has limited advantage in distinctly localizing solitary parathyroid glands that are tightly juxtaposed to the thyroid capsule, especially inferior glands. Although CT-MIBI fusion has shown superiority over conventional sestamibi imaging, selection criteria for routine implementation have not been defined and require further investigation.
Figure 125-9. Intraoperative photograph showing thyroid lobotomy to expose intrathyroid parathyroid gland in the patient from Figure 125-8.
Ultrasonography
High-resolution ultrasonography (10 or 12 MHz) was introduced by Edis and Evans in 1979.246 High-resolution ultrasonography provides for sonographic exploration of the thyroid, carotid, and jugular areas and the cervical area between the thyroid cartilage and the sternal margin. The advantages of this technique are that it is easy to perform, it is well tolerated by the patient, it does not require the injection of a radiotracer, and it can be performed rapidly and at low cost. Disadvantages include localization of enlarged parathyroid glands in the retroesophageal, retrotracheal, retrosternal, and deep cervicothoracic inlet regions. Intrathyroid parathyroid adenomas have reportedly been localized more efficiently with ultrasonography than with other techniques, although they may be confused with cystic thyroid lesions.247 The sensitivity of ultrasonography in identifying abnormal parathyroid glands varies according to the ultrasonographer’s experience, the frequency of the transducer, the resolution of the image, and parathyroid gland size.248
Gray-scale ultrasound with high-resolution linear array transducers is the ideal modality for parathyroid study. Transverse and longitudinal scans are required. Transverse scans are useful in identifying adenomas, whereas longitudinal scans better show the relationship of the adenoma to the thyroid and its internal architecture and vascularity. A parathyroid adenoma typically appears as a hypoechoic mass posterior or inferior to the thyroid gland in its usual extracapsular location. Retrothyroid lesions tend to be oval or flat, whereas infrathyroid lesions are usually spherical. Some parathyroid adenomas may show cystic portions within, but calcification is rare. Given that the most common false-positive image with a Tc 99m sestamibi image is that caused by a thyroid adenoma (Fig. 125-12), this particular entity may be reconciled with adjunctive ultrasonography. A discrete blood supply to the mass within the thyroid parenchyma identified by color Doppler suggests a diagnosis of intrathyroidal parathyroid adenoma, rather than a lesion of the thyroid itself (Fig. 125-13).
False-positive ultrasound studies have resulted from thyroid nodules, adenopathy, and esophageal lesions.248 The presence of surgical clips can also make interpretation more difficult. The overall false-positive rare has been reported to be approximately 15% to 20%.247,249,250
Computed Tomography
CT is a less sensitive method than magnetic resonance imaging (MRI). It is relatively expensive, exposes the patient to radiation, and requires the administration of contrast material to obtain optimal imaging. It may be useful for ectopic parathyroid glands (retrotracheal, retroesophageal, and mediastinal), but is less effective for parathyroid adenomas that reside in a normal anatomic location. Metal clips distort the image of CT. Perithyroidal lymph nodes and ectopic vasculature may make identification of adenoma difficult.251 This technique does not selectively image endocrine tissue, and is not physiologically based, supplying only anatomic information. False-positive results are usually more frequent than with other techniques and may be 50%, making this technique impractical for most clinical situations.252,253
Magnetic Resonance Imaging
MRI is generally believed to be superior to CT because it does not require the administration of radioiodinated contrast material, and there is no interference from surgical clips left in the neck after initial exploration. A parathyroid neoplasm usually has a low signal intensity in T1-weighted imaging (similar to muscle or thyroid) and a high signal intensity (more than or the same as fat) in T2-weighted imaging (Fig. 125-14).254
Figure 125-14. MR image showing a mass in the left submandibular triangle consistent with an undescended superior parathyroid adenoma.
MRI may be more useful for identifying ectopic parathyroid tissue. In an investigation evaluating patients undergoing reoperation for recalcitrant hyperparathyroidism, Jeelos and colleagues255 noted that MRI localized 79% of ectopic glands and only 59% of enlarged glands situated in a normal position. Parathyroid adenomas located in a superior position have been noted to be difficult to locate posterior to the thyroid at the level of the cricoid cartilage.256
The sensitivity for MRI has been reported to be superior than that for CT, ranging from 50% to 80%.252,255 MRI, similar to Tl 201 scanning, shows false-positive findings as a consequence of enlarged lymph nodes.257 Thyroid adenomas may also lead to false-positive results with MRI.
Invasive Preoperative Localization
Parathyroid Arteriography
Appropriate parathyroid arteriography includes examination of both thyrocervical trunks (search for glands in the superior mediastinum, tracheoesophageal sulcus, or intrathyroid or juxtathyroid glands), the internal mammary arteries (glands in the thymus and the anterior mediastinum) and carotids (juxtathyroid or undescended glands), and sometimes the selected catheterization of the superior thyroid artery. Parathyroid adenomas appear highly vascularized and oval or round in shape. Conventional arteriography has a reported sensitivity of about 60%, although the results obtained with digital subtraction arteriography are slightly better.258–260
Selective Venous Sampling for Parathyroid Hormone
Angiography is performed primarily to outline the venous drainage, facilitating sampling for PTH assay. This investigation is expensive and technically difficult. Sampling must be obtained as selectively as possible from the smallest venous branch to document the exact location of the parathyroid tumor.261 Published reports suggest, however, that sampling from large veins such as the internal jugular vein, innominate vein, and superior vena cava may yield optimal results.262 A twofold gradient between the PTH concentration in peripheral blood and that in the selectively sampled venous tributary establishes the site of the venous drainage from the tumor.
This technique is generally believed to be the most sensitive, lateralizing about 80% of the parathyroid tumors.255,259,263,264 It seems to be just as effective in localizing mediastinal glands as it is for cervical glands, and depends on physiologic properties of the parathyroid gland rather than size. It may be helpful in multiple-gland disease, and has the ability to reconcile an equivocal noninvasive study so that localization may be achieved.
Ultrasound-Guided Fine-Needle Aspiration
Fine-needle aspiration of a parathyroid tumor performed under ultrasound guidance may offer an improvement over the results obtained with ultrasound. Fine-needle aspiration may provide for direct cytologic examination, or it may facilitate the use of a bioassay of the aspirate to determine PTH level. When the aspirate is positive for PTH, it confirms the presence of parathyroid tissue within the enlarged gland.265 PTH determination is generally more helpful than cytology examination for diagnosing parathyroid lesions because of the difficulty in differentiating between parathyroid and thyroid tissue in such a limited sample.266 In one study reported by Tikkakosky and associates,267 100% of abnormal parathyroid glands were diagnosed by bioassay, but only 60% were diagnosed by cytologic examination.
Intraoperative Localization Methods
High-resolution intraoperative ultrasonography may be useful in many operative settings, including primarily re-exploration for hyperparathyroidism in a neck that shows significant surgical fibrosis. The injection of methylene blue or toluidine blue is of little value because the pathologic gland has to be identified.268 Methylene blue has been used in the setting of reoperation within 1 to 2 days after initial parathyroidectomy for persistent hyperparathyroidism. Because postoperative edema and seroma fluid makes re-exploration difficult in this circumstance, the blue color may be helpful in identifying parathyroid tissue.
Norman and colleagues269,270 have advocated radiation-guided parathyroid gland identification and removal. Hand-held gamma probes developed for operating room use are used to detect the gland concentrating Tc 99m sestamibi, which is injected on the day of operation usually within approximately 2 hours of the operation. The initial scan provides information regarding localization of putative adenomas and the presence of delayed uptake of nuclear material within the thyroid gland. If excessive delayed activity is present in the thyroid, the patient is subjected to thyroid suppression for 6 to 8 weeks before operation to reduce background radiation in the thyroid bed, and to increase the accuracy of the probe. This is the usual circumstance in reoperative settings. After identification and removal of the abnormal gland or glands, the final abnormal gland removed is checked for the degree of radioactivity ex vivo against background tissues in the surgical bed. Based on previous reported data, excised glandular tissue emitting radiation greater than 20% of that found in tissues in the surgical bed was confirmed as the hyperfunctional parathyroid tissue implicated in disease.269
Surgical Management
Indications for Exploration
The severity of hypercalcemia represents a consideration in the decision to perform surgery. Although no absolute level of serum calcium provides stringent criteria for surgery, most endocrine surgeons consider a serum calcium level of 11.5 mg/dL or greater as an absolute indication for surgery. Surgery in postmenopausal women should be given special consideration independent of the severity of hypercalcemia or absence of symptoms. Women in this population are at greater risk for development of long term skeletal complications from generalized demineralization and osteopenia (i.e., hip and vertebral fractures).271
A major factor to be considered in determining the need for surgery is the potential for long-term benefits and prospects for cure. In 85% to 90% of patients, hyperparathyroidism occurs as a result of a single adenoma. Exploration and removal of the adenoma is curative in greater than 95% of patients, and the long-term benefit and potential for cure is high. Primary hyperplasia occurs in approximately 10% to 12% of patients with hyperparathyroidism. Surgery in these patients involves subtotal parathyroidectomy, with the amount of tissue left ultimately determining the long-term benefit of the surgery. Because of the variable amount of parathyroid tissue left in the neck, and potential for variable activity, the prospect for cure is less reliable than for patients with adenoma. These patients have cure rates significantly reduced compared with patients in whom an adenoma is removed.272
Because of the considerations mentioned previously, the decision to perform surgery on patients who seem asymptomatic and who have no obvious metabolic complications is problematic. Although early surgical intervention seems to be favored, stringent criteria as to whether or not these patients should undergo surgery have not been clearly defined. About 50% of asymptomatic patients go on to develop metabolic complications from hyperparathyroidism within 5 to 7 years of the onset of hypercalcemia.273
Because of the uncertainties about the indications for surgery in asymptomatic patients, a consensus conference held by the National Institutes of Health in 1990 addressed the question of management in this clinical situation.274 Recommendations for surgical management or surveillance or both for patients with primary hyperparathyroidism derived from that conference predated much of what has evolved over the course of the last decade with respect to clinical experience with asymptomatic primary hyperparathyroidism, the evolution of contemporary surgical technique, and the development of newer technologies in preoperative localization. This conference was reconvened in early 2002 in an effort to re-evaluate the original recommendations from the 1990 symposium and contemporize recommendations on the management of asymptomatic primary hyperparathyroidism.275 The indications for surgery as suggested by the conference are as follows:
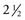 standard deviations (by T-score).
standard deviations (by T-score).These recommended indications are conservative; they provide a framework for surgical decision making, but are not absolute or universal. The decision to perform surgery on a patient with primary hyperparathyroidism and metabolic complications is straightforward. The decision is less clear in asymptomatic patients and must be guided by the potential benefits of surgery, the patient’s risk of developing complications from disease, the wishes of the patient, and, importantly, the experience of the surgeon. The success rate of surgery and the incidence of complications after parathyroidectomy have been documented to vary greatly depending on the surgeon’s experience. In one study, experienced Swedish surgeons achieved normal calcium in greater than 90% of patients with recurrent laryngeal nerve complications in less than 1%. Surgeons who perform fewer than 10 parathyroidectomies per year had a success rate of 70%, but with 15% of patients remaining hypercalcemic and 14% becoming permanently hypocalcemic.276 In weighing potential benefits of surgery against risks for patients with asymptomatic hyperparathyroidism, the experience of the surgeon should be a primary consideration.
Although patients with mild to moderate hypercalcemia seldom experience rapid elevation in serum calcium level, hypercalcemia and the risk of developing occult symptoms and risks for potential end organ damage increase with time. As a result, patients with untreated primary hyperparathyroidism are at risk for major morbidity and mortality from cardiovascular disease.277,278 The benefits derived from parathyroidectomy in patients with occult or minimally symptomatic disease were discussed earlier in the section on clinical manifestations of hyperparathyroidism. In addition, parathyroidectomy seems to offer a distinct and measurable advantage in patients with mild asymptomatic primary hyperparathyroidism, as indicated by the results of a randomized trial in which patients were subjectively surveyed using a standardized health survey instrument.279 In this investigation, patients with mild symptomatic hyperparathyroidism were randomly assigned to surgery or observation, and assessed every 6 months after randomization for 2 years using the SF-36 Health Survey, an instrument that measures wellness.280 Significantly improved function was noted in patients after parathyroidectomy compared with patients who were observed.
Technique of Parathyroidectomy (see Key Indicator Video on website)
Anesthesia and Preparation
Exploration of the neck in patients with hyperparathyroidism is generally performed under general anesthesia with endotracheal intubation. In patients who are medically unfit for general anesthesia, parathyroid exploration for removal of a solitary adenoma localized preoperatively may be performed under local anesthesia supplemented by intravenous sedation.281,282
Exploration of the Neck
After this region has been accessed, blunt dissection of fibroareolar tissue facilitates evaluation of the area where normal and abnormal parathyroid glands generally reside. This dissection permits visualization or palpation, or both, of enlarged parathyroid tissue with minimal dissection to prevent staining of tissues with blood. It is essential to maintain anteromedial retraction of the thyroid gland to expose the angle of visualization and subsequent exploration (Fig. 125-15); this is facilitated further by opening the fascial sheath (pretracheal fascia) connecting the carotid sheath and the thyroid gland. This maneuver provides access to the paraesophageal and retroesophageal spaces. Although identification of the recurrent laryngeal nerve is not generally mandatory, dissection in either the retroesophageal or paraesophageal space requires visualization of the nerve to prevent injury.
Postoperative Care
Successful parathyroid exploration with removal of a solitary adenoma results in a decrease in total serum calcium level, which usually reaches a nadir at approximately 48 hours after the operation. Postoperative hypocalcemia may be seen in patients with severe skeletal depletion of calcium, resulting in “bone hunger.” In some patients, symptoms of hypocalcemia or tetany may develop while the serum calcium level is normal. This phenomenon may be due to a rapid decrease in serum calcium after removal of the parathyroid tumor, causing an increased neural excitability, but may also persist after calcium replacement because of accompanying hypomagnesemia.283
Surgical Strategy and Approach to Exploration
Traditionally, the argument for a bilateral cervical exploration in patients with suspected adenoma has been the high success rate in achieving normocalcemia with a conventional bilateral approach in experienced hands (>95% cure), the inability to predict accurately which side to explore selectively, and the potential risk of missing unsuspected multiple-gland disease such as double adenoma or unsuspected hyperplasia.284–289 More recent technologic advances have helped address these areas of concern, and there is now growing consensus for a less extensive, directed exploration in the approach to primary hyperparathyroidism. The first impetus to this shift in surgical philosophy was provided by improvements in preoperative imaging for the accurate localization of hyperfunctioning solitary adenomas through the use of Tc 99m sestamibi imaging (see section on noninvasive preoperative localization). The second major advance involved the quest for a more precise and timely means of judging surgical success, which led to the development of assays to measure the intact PTH molecule. With the ability to confirm biochemically removal of all hyperfunctioning parathyroid tissue intraoperatively, the theoretical advantages of a directed unilateral cervical approach have increasingly become reality.290–292
Single-Gland Disease
Directed Unilateral Cervical Exploration
If the scan is inconclusive or equivocal, as judged by the surgeon and nuclear medicine specialist, a standard bilateral cervical exploration is planned and carried out, whether or not an enlarged gland is found on the side explored first. Failure to localize with an optimally performed Tc 99m sestamibi scan, in the absence of significant thyroid disease, is strongly suggestive of sporadic diffuse hyperplasia.293,294 If the nuclear scan identifies a discrete nuclear focus on delayed imaging, suggestive of adenoma, a directed exploration to the side localized is performed, and biochemical confirmation of removal of all hyperfunctioning parathyroid tissue is obtained through the use of intraoperative assay for PTH determination.
It has been previously shown that the most precipitous decrease in PTH levels occurs 5 minutes after removal of all hyperfunctioning parathyroid tissue.295 The short half-life of PTH (roughly 2 to 5 minutes) allows peripheral blood samples to be obtained intraoperatively for rapid PTH testing 7 to 10 minutes after excision of all suspected hyperfunctioning parathyroid tissue.293 A peripheral blood sample for rapid PTH assays is drawn at the time of abnormal gland identification (baseline) and subsequently at 10 minutes after removal of all suspected hyperfunctioning parathyroid tissue. A degradation in the serum PTH level exceeding 50%, noted in the postexcision PTH level compared with the pre-excision level, provides biochemical confirmation of removal of all hyperfunctioning parathyroid tissue, allowing the procedure to be concluded without identification or biopsy of any other parathyroid glands. If the postexcision PTH level is greater than 50% of the baseline pre-excision level, suggesting the presence of residual hyperfunctioning parathyroid tissue, a standard bilateral cervical exploration is performed.
Intraoperative PTH determination may be carried out by many methodologies. One method uses a radioimmunoassay developed through a simple, previously described modification of an intact PTH overnight assay method.294 The intact PTH assay is a two-antibody sandwich system. One antibody (the capture antibody) is fixed to a plastic bead; the second is conjugated with a measurable marker. The amount of intact PTH present in a plasma sample can be determined by measuring the amount of the marker material remaining after all unbound solutions have been removed. Results of this rapid PTH assay are generally available within 8 to 10 minutes after submission to the radioimmunoassay laboratory.
To overcome problems associated with sensitivity of the rapid immunoradiometric assay, researchers began conjugating the labeling antibody with chemiluminescent tracers to avoid the handling issues of their radioactive predecessors.291,292,295 Since the introduction of the immunochemiluminometric assay in 1993, researchers have experimented with several different variations on this theme, and some manufacturers now offer so-called quick kits. Experience with this assay is consistent among the investigators in that a decrease of 50% or greater between the postexcision and baseline pre-excision PTH values is indicative of removal of all hyperfunctioning parathyroid tissue.293–301 The major drawback of these kits is their high cost. Because the assay negates the need for frozen section analysis in most cases, however, the overall cost is only slightly more expensive than the traditional unilateral approach.291 These modifications can provide a cost-effective alternative to preformed kits, especially when weighed against the potential costs of a missed ectopic adenoma.74
The kinetics of postadenoma resection are important to understand if logical management strategies are based on these objective data. Libuttis302 showed that the half-life of PTH varies within a brief window—0.42 to 3.81 minutes. Randolph303 showed that the lowest values occur within 1 to 3 days. In 38% of patients with successful resection of a single parathyroid adenoma and return to normocalcemia, intact PTH levels may remain elevated at 1 month. Suggested mechanisms are cortical bone remineralization, increase in calcium receptor set-point, and a relative secondary hyperparathyroidism.
Minimally Invasive Techniques
Surgical techniques that derive from the basic cervical exploration described previously have been termed minimally invasive techniques primarily because of modifications resulting from advances in technology. Scanned directed surgery under local or regional anesthesia has been advocated by many investigators reporting excellent surgical results.302,303 These techniques capitalize on the ability to administer effective regional anesthesia to an awake, sedated patient to perform directed unilateral focused exploration with removal of a localized solitary parathyroid adenoma. The technique employs preoperative scanning with Tc 99m sestamibi using planar and SPECT imaging and intraoperative PTH assay for biochemical confirmation of removal of all hyperfunctioning parathyroid tissue.
Advantages of this technique have been reported as improved patient comfort postoperatively, the performance of ambulatory procedures, and reduced cost.302 Disadvantages of the technique include the potential for conversion to a comprehensive bilateral dissection if the adenoma is not found in the area indicated by the scan (necessitating general anesthesia), and the potential for patient anxiety or discomfort or both requiring conversion to general anesthesia.
The ability to localize solitary parathyroid adenomas accurately using Tc 99m sestamibi has allowed numerous investigators to pursue intraoperative nuclear mapping to isolate and recover the enlarged parathyroid gland representing the adenoma. This procedure has been termed radioguided parathyroidectomy, using preoperative administration of Tc 99m sestamibi immediately before operation combined with intraoperative use of the hand-held gamma detection device (gamma probe).269,304,305 In this technique, the patient is administered approximately 18 to 20 mCi of Tc 99m sestamibi approximately 2 hours before surgery. The timing of the preoperative injection may be modified by the visualized washout sequence shown by the solitary parathyroid gland on review of delayed images.
When the adenoma is found, it is carefully dissected close to its capsule; its pedicle is identified, clipped, and divided insuring first that the recurrent laryngeal nerve is not within the dissected field. Radioactivity of the removed parathyroid adenoma (ex vivo) and the operative basin is measured, the sum of which should roughly equal the radioactivity in the corresponding quadrant of the neck before making the incision. In most cases, frozen section analysis is unnecessary, but a permanent histopathologic assessment on the specimen is performed. As experience with this procedure has increased, the technique has been applied more selectively to exclude the following clinical situations: deep retroesophageal lesions, ectopic glands, superior adenomas in men, and persistent or recurrent hyperparathyroidism.305
Endoscopic Parathyroid Exploration
Parathyroid exploration performed in humans was first described by Gagner in 1996.306 With the advent of improved technology and video-assisted endoscopic technology, several pioneering investigators have evaluated this technique in the management of hyperparathyroidism.307–310 This procedure is generally done under general anesthesia on patients who have shown focal areas of nuclear uptake on delayed imaging using Tc 99m sestamibi scanning for parathyroid localization. The instrumentation allows for minimal access surgery through several very small incisions placed in the cervical region, the upper chest wall, or the axilla.308–310 Miccoli and colleagues310 reported a larger series of video-assisted parathyroidectomy with limited use of insufflation in 137 patients.
Potential advantages of the endoscopic approach to parathyroid removal include excellent cosmesis, negligible discomfort, and minimal problems with cervical motion. Investigators advocating this technique have indicated that the selection process in determining which patients are candidates for the procedure is a crucial aspect with regard to surgical success. Generally, the criteria for consideration of the procedure include initial explorations, single-gland disease, and the absence of significant thyroid enlargement or multinodularity. All patients must have a preoperative localization study performed with unequivocal focus of nuclear uptake on delayed imaging. In addition, all patients should have PTH levels assessed intraoperatively using the intraoperative PTH assay to confirm biochemically removal of the suspected hyperfunctional parathyroid gland. Although endoscopic cervical exploration for parathyroid disease represents the least invasive and most focused of the minimally invasive surgical techniques, the time required to perform this procedure may be extensive, and in some ways counterproductive to limiting anesthesia time and cost.311 At this point, although this procedure shows promise, the role of endoscopic parathyroid exploration remains undefined.
Multiple-Gland Disease
True Double Adenoma
The incidence of double parathyroid adenomas seems to increase with age. Synchronous double adenomas have been variably reported at rates ranging from 1% to 2% to 10% in patients older than 60 years of age.312,313 Approximately 50% of true double adenomas image accurately to each of the two locations (see Fig. 125-4). Bilateral exploration is required despite high suspicion on localization because of the possibility of asymmetric hyperplasia. Intraoperative PTH assay provides biochemical confirmation that all hyperfunctional parathyroid tissue has been removed: a sequential decrease in PTH levels is noted after successive excision of the first and then second enlarged gland (Fig. 125-16). At least one other normal gland should be identified, but surgical biopsy and histologic analysis of additional glands is unnecessary, provided that the final postexcision PTH level ultimately decreases beyond 50% of the pre-excision level intraoperatively to within a normal range.
Sporadic Hyperplasia
A scan that does not indicate an unequivocal area of nuclear uptake on delayed images raises the surgeon’s suspicion of diffuse hyperplasia. In a series performed by one of the authors (P.K.P.), 40% of equivocal scans were associated with the ultimate finding of multiglandular hyperplasia at exploration (Fig. 125-17). An equivocal scan showing the absence of a distinct focus of delayed uptake of nuclear material mandates the performance of a bilateral exploration with histologic identification of at least one abnormal and one normal gland, and the assurance that no additional grossly enlarged glands exist on either side. After removal of all enlarged glands (either single or multiple), and the demonstration of a histologically normal gland (in the event of less than four gland disease), rapid PTH assessment (Fig. 125-18) is performed to confirm the removal of all hyperfunctional parathyroid tissue, per the same protocol used in the directed exploration strategy. Failure to achieve this degree of PTH degradation mandates further exploration, for either an ectopically located gland or, uncommonly, a supernumerary gland.
When all hyperplastic glands have been located in situ, the three largest glands are removed and histologically confirmed. Subtotal excision of the remaining gland follows, leaving at least one third to one half of the gland as a viable vascularized remnant. Titration of PTH levels is helpful using rapid PTH assay. If the postexcision level declines to less than 10 pg/mL, consideration should be given to cryopreservation of parathyroid tissue excised from the fourth gland. Although this is rarely necessary, this approach is favored over routine forearm autotransplantation of parathyroid tissue because additional surgery is avoided, and because a measurable PTH level detected intraoperatively is generally predictive of euparathyroid function and normocalcemia.74,314
Familial Hyperparathyroidism
Familial hyperparathyroidism accounts for less than 5% of all cases of hyperparathyroidism.315 Familial hyperparathyroidism comprises a spectrum of autosomal dominant inherited diseases that include MEN-I, MEN-IIA, non-MEN, and familial neonatal hyperparathyroidism. In contrast to sporadic hyperparathyroidism, patients with familial hyperparathyroidism are younger and more likely to have multiglandular disease, and persistent or recurrent hyperparathyroidism after parathyroidectomy. Subtotal or total parathyroidectomy in combination with bilateral transcervical thymectomy is more frequently necessary for definitive treatment, rather than simple excision of an adenoma, which is all that is required for approximately 80% of patients with sporadic primary hyperparathyroidism. It is imperative that long-term follow-up of patients with familial hyperparathyroidism be performed for early detection and treatment of other endocrine neoplasms associated with these disorders, and for the diagnosis of recurrent hyperparathyroidism. In addition, screening of family members represents an important aspect of the overall management of familial hyperparathyroidism. As a result of the identification of causative genetic components in these disorders, genetic screening of family members to allow for a global treatment plan for members identified as gene carriers is now possible.
Multiple Endocrine Neoplasia Type I
MEN-I is an autosomal dominant inherited syndrome characterized by the presence of neoplastic lesions involving the parathyroid glands, the anterior pituitary, the pancreas, and the duodenum. Patients may also have carcinoid tumors of the bronchus or thymus; tumors of the ovaries, thyroid gland, or adrenal glands; or multiple lipomas. MEN-I is an uncommon disorder, with an incidence of 2 to 20/100,000.316 Not all patients with MEN-I present with the complete syndrome indicating a variable degree of penetrance. Primary hyperparathyroidism is the most common manifestation of MEN-I, occurring in greater than 95% of patients, usually before age 30 and as the initial manifestation of the syndrome.317–319 Pancreatic endocrine tumors are most often multiple and are distributed throughout the pancreas. Nonfunctioning tumors, gastroma, and insulinoma are the islet cell tumors that are most often associated with MEN-I. A pituitary tumor is diagnosed in 30% to 40% of patients and is most commonly a prolactinoma.
The syndrome of hyperparathyroidism may occur 10 years before the onset of other endocrine disorders, and MEN-I should be considered in any patient diagnosed with primary hyperparathyroidism at an early age or with multiglandular disease.318 The hyperparathyroidism in MEN-I is due to diffuse four-gland parathyroid hyperplasia.318–323
Generally, the clinical manifestations of primary hyperparathyroidism in patients with MEN-I are similar to manifestations found in patients with sporadic primary hyperparathyroidism. Some of the symptoms in primary hyperparathyroidism associated with MEN-I may be masked by Zollinger-Ellison syndrome or insulinoma.318 Alternatively, the symptoms associated with hyperparathyroidism may also aggravate the clinical manifestations of Zollinger-Ellison syndrome as a result of calcium stimulation of gastrin secretion.318
Biochemical findings (serum calcium and PTH levels) in MEN-I patients are similar to findings in patients with sporadic primary hyperparathyroidism, and the diagnosis is made by documenting hypercalcemia associated with an inappropriately elevated PTH level.324 If a patient with hyperparathyroidism has a family history of the same disorder or other endocrinopathies, further screening for tumors of the pituitary gland and pancreas is warranted.319 This screening should consist of serum prolactin levels, glucose, basal serum gastrin, and pancreatic polypeptide levels.
Werner325 noted that 50% of the offspring of individuals with MEN-I inherited the disorder (without gender differentiation), and that this inheritance did not skip generations. This pattern of inheritance is characteristic of an autosomal dominant trait with a high degree of penetrance.319,326
The MEN1 locus has been mapped to a section of chromosome 11327; this was further shown to involve a mutation at the 11q13 locus.327 Subsequently, the MEN1 gene has been identified as a tumor suppressor gene that encodes the protein menin.328 Greater than 90% of MEN-I patients have germline menin gene mutations, and most MEN-I families have their own unique mutation.329 The predisposition to MEN-I is a heterozygotic mutation.324
The finding of multiple abnormal parathyroid glands and, commonly, supernumerary glands in patients with MEN-I represents a formidable management dilemma in these patients. The inability to recognize a supernumerary gland at the time of initial exploration for this disorder is a well-documented cause of persistent disease.318,330–332
The surgical approach should consist of a routine bilateral neck exploration with identification of all four parathyroid glands. The extensiveness of surgical resection of the parathyroid glands, after they have been identified, is controversial with advocates of subtotal and total parathyroidectomy. Numerous authors have reported their experience with subtotal parathyroidectomy in patients with MEN-I and hyperparathyroidism with differing degrees of surgical success.318,320,321,330 Edis and colleagues331 reported that 82% of patients with chief cell hyperplasia had normal parathyroid function at least 1 year after subtotal parathyroidectomy. Of these, only 6 of 55 had hyperparathyroidism secondary to MEN-I syndrome, however. In 12 patients undergoing subtotal parathyroidectomy for hyperparathyroidism of MEN-I as reported by Prinz and colleagues,330 only 5 ultimately achieved normocalcemia.
It has been postulated that the difference in success rates between hyperparathyroidism attributable to sporadic four-gland hyperplasia and hyperparathyroidism associated with MEN-I may be due to persistent exposure of a trophic factor. A potential parathyroid mitogenic humoral factor was identified by Brandi and coworkers333 in 1986 from the serum of patients with familial MEN-I syndrome. The mitogenic activity of this humoral factor persisted in the patient’s plasma for 4 years after total parathyroidectomy.333 As a consequence, any parathyroid remnant remaining after subtotal parathyroidectomy would be exposed to this humeral factor, potentially increasing the chance of recurrence. This theory was supported by a finding by Prinz and colleagues,330 who reported a patient with persistent hypoparathyroidism requiring calcium supplementation for 10 years after subtotal parathyroidectomy before development of recurrent disease. The re-exploration of this patient showed that remnant parathyroid hyperplasia was the etiology of recurrence.
Total parathyroidectomy with autotransplantation of fragmented parathyroid tissue has been advocated by other investigators as the procedure of choice for the initial operation for patients with primary parathyroid hyperplasia caused by MEN-I syndrome.334,335 In this procedure, four glands are identified and removed with sectioning of the most morphologically normal-appearing gland into cubic centimeter fragments. These fragments are implanted into the brachioradialis muscle of the nondominant forearm. Sections from this gland may also be cryopreserved and successfully autotransplanted if the primary autograft does not function. Wells and associates335 pioneered this approach, and reported that 30% of their patients developed recurrent, graft-dependent hyperparathyroidism. Autotransplantation of cryopreserved parathyroid autografts has been successful in only 50% of patients resulting in permanent hypoparathyroidism.336
The concept that a humoral factor may be initiating growth in the remnant parathyroid tissue is supported further by the finding of Mallete and colleagues,322 who reported a higher incidence of graft-dependent recurrence in MEN-I patients treated with total parathyroidectomy with autotransplantation than in patients with sporadic hyperplasia undergoing the same operation. Independent of a humoral stimulating factor for parathyroid cell proliferation is the finding that MEN-I–associated hyperparathyroidism is associated with the finding of supernumerary parathyroid glands. This factor contributes to the potential for missed glands at the time of initial operation, increasing the risk of recurrence. The implementation of intraoperative assay for PTH determination has been helpful in reconciling this problem in patients with hyperparathyroidism associated with MEN-I.74
Primary surgical explorations in patients with MEN-I and hyperparathyroidism may result in cure rates greater than 90%. The requisite for identifying all four parathyroid glands in these patients is emphasized in a report by O’Riordan and colleagues.320 In this series, immediate cure was noted in 94% of patients; however, hypercalcemia was noted to be persistent in 19% of patients in whom fewer than four glands were visualized at initial operation compared with 3% of patients in whom four glands or more were noted at initial surgery. Generally, persistent and recurrent disease rates are higher in patients who have less than total parathyroidectomy performed and in whom fewer than four glands are found at the time of initial operation.319,320
Multiple Endocrine Neoplasia Type IIA
MEN-IIA is a syndrome characterized by medullary thyroid carcinoma, pheochromocytoma, hyperparathyroidism, lichen planus amyloidosis, and Hirschsprung’s disease. In 1932, Eisenberg and Wallerstein337 first reported a pheochromocytoma and concurrent thyroid carcinoma in a patient at autopsy. In 1961, Sipple338 estimated that the incidence of thyroid cancer in patients with pheochromocytoma was 14 times higher than that of the normal population; Cushman339 subsequently reported a family with hereditary thyroid carcinoma and pheochromocytoma in which one affected member had a parathyroid tumor. These reports resulted in the description and characterization of the syndrome, formerly known as Sipple’s syndrome, now known as MEN-IIA.340
The penetrance of the independent entities for patients with MEN-IIA syndrome is variable, with the exception of medullary thyroid carcinoma, which is seen in essentially all affected individuals. Pheochromocytoma and hyperparathyroidism show variable penetrance with pheochromocytoma occurring in 70% of individuals affected, and hyperparathyroidism reported least commonly in approximately 20% to 35% of affected patients.341–345
Hyperparathyroidism is usually diagnosed as a result of screening patients or family members with MEN-IIA, or incidentally during thyroidectomy for C cell hyperplasia or medullary thyroid carcinoma.346 In a review of 67 patients with hyperparathyroidism associated with MEN-IIA syndrome, Raue and coworkers345 noted that 75% were diagnosed at the time of thyroidectomy for either C cell hyperplasia or medullary thyroid carcinoma. These patients had normal serum calcium and PTH levels preoperatively, and the diagnosis was based on intraoperative morphology or histology. Uncommonly, a diagnosis of hyperparathyroidism is made because of the development of clinical symptoms that are similar to those encountered in sporadic primary hyperparathyroidism.344
MEN-IIA–associated hyperparathyroidism usually develops after the third decade of life in the form of mild hypercalcemia with rare hypercalcemic crises.343,345 It is generally acknowledged that the hyperparathyroidism associated with MEN-IIA is less aggressive than its counterpart noted for MEN-I or non-MEN syndromes.320,344 Patients with MEN-IIA generally have lower serum calcium levels, fewer symptoms or complications of hypercalcemia, less frequent multiple-gland involvement, and a lower incidence of persistent or recurrent disease after surgical treatment than patients with MEN-I or non-MEN hyperparathyroidism. A circulating humeral factor that stimulates parathyroid cell proliferation has not been postulated to exist in association with the hyperparathyroidism associated with MEN-IIA.
Similar to MEN-I, MEN-IIA is a genetic disease that is transmitted in an autosomal dominant fashion with a high degree of penetrance but with variable expression. In the late 1980s, the inherited defect of the MEN-II syndrome was mapped to the pericentromeric region of chromosome 10.347,348 Subsequent work identified the ret proto-oncogene as a segment on chromosome 10 that encodes for a specific cell surface receptor complex, the exact function of which is poorly characterized. Mutations in the segment of the ret proto-oncogene coding for the extracellular domain of the tyrosine kinase receptor protein are responsible for producing the MEN-IIA phenotype.349 Although the mutation has been well characterized for its association with medullary thyroid carcinoma, the exact relationship to parathyroid disease is unknown.
Surgical exploration for patients with MEN-IIA–associated hyperparathyroidism is generally quite effective. Cance and Wells341 reported a 100% surgical success rate and 3% recurrence rate treating patients with primary hyperparathyroidism associated with MEN-IIA. In contrast to MEN-I, in which the extensiveness of parathyroid gland resection is important in defining operative success, the degree of parathyroid gland resection does not seem to influence the success rate significantly in patients operated for hyperparathyroidism associated with MEN-IIA. O’Riordan and colleagues320 reported a 100% cure rate with no recurrences whether total parathyroidectomy, subtotal parathyroidectomy, or excision of enlarged glands independently was performed.
Non–Multiple Endocrine Neoplasia Familial Hyperparathyroidism
Non-MEN familial hyperparathyroidism, also known as familial isolated hyperparathyroidism, refers to hyperparathyroidism occurring in the absence of other endocrinopathies in patients with at least one first-degree relative with surgically proven hyperparathyroidism and no personal or family history of MEN. Familial hyperparathyroidism occurs in young patients with a mean age at diagnosis of approximately 36 years. Some of these patients experience this disorder as children, although it is rare before age 10 years.350 In contradistinction, patients with sporadic primary hyperparathyroidism typically present during the fifth or sixth decades of life.315 Familial hyperparathyroidism seems to be more aggressive than sporadic or MEN-related hyperparathyroidism; frequently, patients with this disorder manifest profound hypercalcemia and more frequently present with hypercalcemic crisis.350,351 Renal lithiasis occurs in one third to one half of patients reported with familial hyperparathyroidism; patients commonly also present with other nonspecific signs and symptoms, such as fatigue, weakness, hypertension, and peptic ulcer disease.350 It has been postulated that familial hyperparathyroidism may be associated with an increased risk for the development of parathyroid cancer.350,352,353
Either subtotal or total parathyroidectomy together with bilateral cervical thymectomy is generally performed with autotransplantation of parathyroid tissue as necessary depending on the extent of parathyroid gland resection. If only one or two parathyroid glands are morphologically abnormal and enlarged, the goal of therapy is to resect all abnormal parathyroid tissue from one side of the neck, and leave existing remnant parathyroid tissue in only one side.350 Intraoperative PTH determination is helpful in determining the degree of hyperfunctional parathyroid tissue remaining after subtotal parathyroidectomy in these patients, and may serve as an indicator of remnant parathyroid function after abnormally enlarged glands have been removed. The visualization and subsequent removal of abnormally enlarged glands and the intraoperative PTH findings guide the extensiveness of surgery in patients who manifest this disorder and have fewer than four glands appearing abnormal. It is important that these patients be followed long-term to recognize if and when recalcitrant disease occurs.
Familial Neonatal Hyperparathyroidism
Neonatal hyperparathyroidism is a rare condition characterized by severe hypercalcemia occurring in association with severe hypotonia, poor feeding, constipation, failure to thrive, and respiratory distress. The clinical manifestations of this disorder become evident during the first week of life; however, the disorder may not become manifest until age 3 months or older.350 Most patients with familial neonatal hyperparathyroidism have been part of families with a known history of benign familial hypercalciuric hypercalcemia. The disease locus for familial hypercalciuric hypercalcemia has been identified on the long arm of chromosome 3, and patients with familial hypercalciuric hypercalcemia are heterozygous for the mutation, with one affected allele.354,355 The occurrence of two defective alleles is believed to cause severe neonatal hyperparathyroidism.356 Most patients with this disorder require urgent parathyroid exploration and resection of all four glands. Total parathyroidectomy is advocated together with parathyroid autotransplantation, bilateral transcervical thymectomy, and cryopreservation of parathyroid tissue because of the high recalcitrance rate for this disorder.
Renal Failure–Induced Hyperparathyroidism
The indications for parathyroid exploration in patients with renal failure–induced hyperparathyroidism may be characterized as those manifested before and after renal transplantation. Surgery is generally indicated when medical therapy fails to control progressive secondary hyperparathyroidism.357–359 The clinical manifestations occurring in this disorder include persistent or worsening skeletal symptoms, intractable pruritus, and soft tissue calcifications.360 Additional indications for a parathyroidectomy include the presence of biopsy-proven, high-turnover bone disease, calciphylaxis, or both in a patient with chronic renal failure and secondary hyperparathyroidism.361–363 Parathyroidectomy may be indicated in some patients after successful renal transplantation because of the development of clinical manifestations similar to the manifestations of primary hyperparathyroidism, including hypercalcemia with nephrolithiasis, pancreatitis, central nervous system manifestations, and overt bone demineralization.364 The presence of mild hypercalcemia in and of itself does not seem to be a serious threat to a patient after renal transplantation, but impaired kidney function in the presence of high PTH levels and hypercalcemia represents an indication for parathyroidectomy, as is the association of kidney stones and long-standing hypercalcemia.365–368
If only three parathyroid glands are found after a comprehensive exploration, all three are removed. This circumstance has been noted to result in approximately 30% of patients with persistent hyperparathyroidism.369 If total parathyroidectomy and autotransplantation with or without cryopreservation is planned, all four glands are removed after discovery with the most normal-appearing gland selected as the autograft.370 Because hemorrhage into the implant beds may result in poor “take” of the grafts, 10 to 15 cubic millimeter fragments of the selected autotransplant gland are placed into intramuscular pockets in the brachioradialis muscle of the nondominant forearm in a hemostatic fashion.335 Cryopreservation of the remaining portion of this autotransplant gland should be performed if sufficient parathyroid function does not develop after revascularization of the implants.
Numerous reports supporting one procedure versus the other are noted in the literature, but objective controlled trials dealing specifically with renal failure–induced hyperparathyroidism are rare. Of the few trials conducted, the only prospective randomized series was reported by Rothmund and colleagues,371 who found that parathyroidectomy with autotransplantation was superior to subtotal thyroidectomy in controlling symptoms in a group of 40 patients with renal failure–induced hyperparathyroidism. In this series where follow-up was noted over a mean of 4 years, four patients who were randomly assigned to the subtotal parathyroidectomy cohort experienced recurrent disease. The elimination of bone pain was significantly improved proportionally in patients who underwent total parathyroidectomy with autotransplantation. Three independent reports compared both techniques in a retrospective analysis and found that both procedures resulted in similar outcomes.372–374 In assessing the merit of each technique, one should keep in mind that most surgeons find the procedure they routinely use and have more experience with to be the most applicable and appropriate in dealing with patients with this disorder.
The success of subtotal parathyroidectomy depends mainly on the size and viability of the remnant parathyroid gland left in situ. Remnants that are nodular are more likely to grow and result in recurrent hyperparathyroidism. This procedure has a theoretical advantage of producing less postoperative hypocalcemia because the remnant continues to function. The main disadvantage is that if these remnants become hyperfunctional, the reoperations often are tedious, are difficult technically, and carry increased risk of complications such as injury to the recurrent laryngeal nerve. Generally, successful subtotal parathyroidectomy alleviates bone pain to a lesser degree than total parathyroidectomies with autotransplantation, but carries less risk of postoperative low-turnover bone disease.375
The success of total parathyroidectomy with autotransplantation depends mainly on the nodularity of the gland from which the graft is obtained, and number and weight of the fragments that are implanted. As in recurrent disease within a nodular remnant after subtotal parathyroidectomy, graft-dependent recurrence is three times higher when implanting a nodular gland instead of a diffusely hyperplastic one.376 The advantage of autotransplantation is that if hyperparathyroidism recurs in the graft remnant, this may be partially resected under local anesthesia with minimal potential complications. Several studies show a 5% to 38% rate of postoperative hypercalcemia attributable to a hyperfunctional graft with a 2% to 6% chance of recurrence requiring graft resection, and a 5% to 30% chance of hypocalcemia lasting more than 12 months secondary to poor graft viability and function.377–385
Re-exploration for Recalcitrant Hyperparathyroidism
The distinction between persistent (hypercalcemia persisting or recurring within 6 months after initial operation) and recurrent (hypercalcemia recurring after 6 months of normocalcemia following the initial surgery) hyperparathyroidism after a prior cervical or mediastinal exploration for hypercalcemia has been applied loosely, but poses an equitable management dilemma and technical challenge to the surgeon. It has been estimated that 2% to 10% of surgical failures may be attributed to an incorrect diagnosis.386,387 The first requirement for success in reoperative parathyroid surgery is proper diagnosis. By definition, hyperparathyroidism (primary or secondary) must be proven by elevation of serum calcium levels associated with high or inappropriately elevated PTH levels. Elevated serum chloride and decreased serum phosphate levels are frequently noted. In addition, urinary calcium should be appropriately elevated to exclude a diagnosis of familial hypocalciuric hypercalcemia. If all these parameters are not present, other causes of hypercalcemia must be considered because a repeat surgical procedure is almost guaranteed to be unsuccessful.388 The diagnosis of hyperparathyroidism is currently more straightforward with immunoradiometric and immunochemiluminescent assays of PTH levels. Diagnostic errors in hyperparathyroidism can result from medications (calcium, vitamin D, furosemide, thiazide diuretics, calcitonin, lithium), familial hypocalciuric hypercalcemia, malignancy (bone metastasis or humoral hypercalcemia), granulomatous disease, acute renal failure, bone disease (Paget’s disease, immobilization), hyperthyroidism, or adrenal insufficiency.
Causes of Failed Exploration
The most common finding on parathyroid re-exploration by an experienced parathyroid surgeon is a missed single adenoma. Akerstrom and colleagues389 reported on 84 parathyroid re-explorations in 69 patients with primary hyperparathyroidism. Of these patients, 37 had missed adenomas; 4 of these patients had double “adenomas” with only one adenoma being resected on the initial exploration. Most of the remaining patients had persistent hyperparathyroidism secondary to inadequate resection of parathyroid hyperplasia, with only four patients showing recurrent “single” adenomas. Rotstein and colleagues390 analyzed their series of 28 reoperations for primary hyperparathyroidism. They identified solitary adenomas in 24 patients, with 2 patients each having hyperplasia and carcinoma. Norman and Denham391 have used the technique of minimally invasive radiation-guided parathyroidectomy for reoperative disease and resected 23 solitary adenomas from 24 patients. Jaskowiak and associates392 reviewed their experience at the National Institutes of Health with 288 patients with persistent or recurrent hyperparathyroidism. Of these patients, 222 (77%) were ultimately shown to have solitary adenomas.
In patients with secondary hyperparathyroidism, hyperplasia is the expected histopathology. Cattan and colleagues393 explored 89 patients for persistent or recurrent secondary hyperparathyroidism; 53 of these patients had undergone subtotal parathyroidectomy, whereas 36 had prior total parathyroidectomy with autotransplantation. They identified hypertrophy of the remnant as the principal cause of recurrence in the subtotal group. In the group receiving total parathyroidectomy, recurrence was located in the autotransplant in half; hyperplastic disease was identified in the neck or mediastinum in the other half.
Equally as important is the finding that the cause of recalcitrant hyperparathyroidism is a solitary adenoma in most patients, through the observation that in most of these cases the adenoma is in a “standard” location. In the report by Akerstrom and colleagues,389 only eight patients required sternotomy for ectopic adenomas identified in the mediastinum, despite 17 sternotomies being performed. In 5 of the 17 patients who had sternotomy, the offending lesion was ultimately identified in a normal location in the neck. Of the more unusual ectopic locations, one was ventral to the left atrium, one was ventral to the aortic root, and one was in the aortopulmonary window. Only one gland was truly intrathyroid, despite 19 thyroid lobectomies performed as part of the re-exploration.
In the series by Norman and Denham,391 only one gland was in the mediastinum (just anterior to the right atrium), whereas two were intrathyroid. Jaskowiak and associates392 identified adenomas in the posterior superior mediastinum, specifically in the tracheoesophageal groove in 27% of their patients (59 of 215). This was the most common location of the adenoma in the failed first exploration. This site is easily explored transcervically, and should be considered as an inferior extension of the normal superior gland position. The authors point out that these adenomas were almost always in direct apposition to the recurrent laryngeal nerve, perhaps suggesting that inadequate dissection around the nerve initially contributed to the failure. Another 24.3% of patients had their adenomas in the normal positions adjacent to the thyroid gland.394
In the National Institutes of Health series, the most common ectopic site was within the thymus or mediastinum, accounting for 16.7%.394 This value is lower than other reports of 22% intrathymic tumors at the initial operation and 38% for reoperation.393 An intrathyroidal lesion was noted in 10% (22 patients) of their study population. A similar percentage of patients had undescended parathyroid glands. These so-called parathymus lesions are located at the bifurcation of the carotid artery, high in the neck, and represent an inferior gland that is arrested in the descent from the third branchial pouch. Other “typical” ectopic locations included lesions within the carotid sheath and the retroesophageal space. Unusual ectopic locations include the aortopulmonary window in two patients, the hypopharynx at the base of the tongue in one patient, the wall of the nasopharynx near the nasal septum in one patient, and within the vagus nerve high in the neck at the level of C1-C2 vertebrae. Three patients had lesions “seeded” within the strap muscles, most likely because of the first exploration.394
Operative Risk in Re-exploration
When a decision has been made to pursue re-exploration for persistent or recurrent hyperparathyroidism, the patient and surgeon must review and mutually understand the inherent risks of such surgery. The risk of injury to the recurrent laryngeal nerve, a potentially very problematic complication, is greater in re-explored necks than the risk for initial exploration. In two large studies, the incidence of vocal paralysis exceeded 6% after parathyroid re-exploration, in sharp contrast to the exceedingly low rate (<1%) after initial bilateral exploration.395 Although more limited approaches to parathyroid re-exploration (e.g., targeted exploration, minimally invasive radiation-guided surgery) are likely to result in a reduced incidence of nerve injury after reoperation, surgical treatment of the operated neck will always be associated with increased risk. In addition to an incidence of recurrent nerve injury, re-exploration carries with it a greater likelihood for postoperative temporary and permanent hypocalcemia.396 Finally, although the success rate for reoperative parathyroid surgery may exceed 90% in experienced hands, this remains substantially less than the near-perfect success rate for initial surgery. In addition to reconfirmation of the diagnosis and careful patient preparation, it is important that the risks associated with reoperation be discussed candidly with the patient.
Operative Strategy
This approach exploits the concept of the viscerovertebral angle as described by Tenta and Keyes.397 This potential anatomic space is defined as the area bordered laterally by the carotid sheath structures, medially by the trachea and esophagus, anteriorly by the thyroid, and posteriorly by the cervical spine (Fig. 125-19). In accessing this region, the surgeon may take advantage of a tissue plane with relatively little vascularity and fibrosis. This area allows the surgeon to examine the superior mediastinum inferiorly, the retroesophageal compartment medially, and as far superiorly as the hyoid bone, all within planes of dissection that separate with relative freedom. Although unnecessary in most cases, the recurrent laryngeal nerve may be identified and isolated for protection by following this approach because dense fibrosis is infrequently encountered over the prevertebral space, even after a thorough initial exploration.
The order in which regions are approached may vary according to the surgeon; however, it is important that all potential areas be accessed to increase the chance of success and avoid a failed re-exploration. The preferred approach is to explore each side through the viscerovertebral angle via a lateral-to-medial orientation. Regions of dissection are addressed in the following manner: The anterior superior mediastinum is dissected first, with careful attention to the thyrothymic ligament and tracheoesophageal groove region adjacent to the recurrent nerve (Fig. 125-20). Cervical thymectomy, if not performed during initial surgery, is completed at this time (Fig. 125-21). Dissection then turns to the retropharyngeal, retroesophageal region where blunt dissection within the prevertebral space allows for digital exploration superiorly above the cricoid larynx and inferiorly into the posterior mediastinum. Enlarged glands in this anatomic plane may often be felt before they are seen using these techniques. Next, the thyroid lobe is mobilized, possibly truncating the superior vascular pedicle to rotate the gland anteromedially so that the posterior thyroid capsule may be closely examined for a folded, lobulated parathyroid gland under the capsular fascia (Fig. 125-22). Using this maneuver, the thyroid lobe is palpated for any nodular densities that may be suspicious for an intrathyroidal parathyroid gland. The carotid sheath is opened from the superior mediastinum to the hyoid bone, inspecting and palpating for nodular structures within the sheath (Fig. 125-23).
Special Circumstances
Parathyroid Hormone Level Considerations
The interpretation of intraoperative PTH levels may be confounded by unrecognized PTH “spikes,” whereby pre-excision values may variably exceed baseline or preincision levels. These acute differences in PTH levels have been postulated to occur from various etiologies, including technical problems with the assay performance, acute changes in renal status, pharmacologic influences, and pre-excision gland manipulation. In most instances, when technical assay problems are excluded, manipulation of the hyperfunctional gland before resection would seem to present the most plausible explanation for these “spikes.” Riss and colleagues398 noted that a substantial delay in PTH degradation beyond 10 minutes post resection in patients shows PTH “spikes” thought to be due to gland manipulation before excision. This study and the authors’ own experience acknowledge the importance of recognizing PTH “spikes” and reacting to them by delaying postexcision PTH measurements 20 to 25 minutes. A subthreshold decline (<50%) beyond this interval should prompt further exploration. PTH “spikes” may best be recognized by comparing the pre-excision value with the baseline obtained before surgery, provided that the same laboratory was used for both determinations.
A finding that may result in considerable frustration and anxiety is an elevated PTH level in a normocalcemic patient after curative parathyroid surgery. Approximately 20% (and perhaps more) of patients achieving normocalcemia after parathyroid surgery, during which intraoperative PTH determination predicted curative resection, have persistently elevated PTH levels postoperatively. This phenomenon has been postulated to occur as a result of the development of secondary hyperparathyroidism in response to a rapidly changing serum calcium level, variably explained by vitamin D deficiency, changes in renal function, or peripheral resistance to PTH.399,400 In a setting of normocalcemia after curative parathyroid surgery, PTH levels obtained postoperatively do not seem to improve on the predictive value of intraoperative PTH levels. Further elevations in PTH levels in these patients postoperatively do not indicate operative failure in most patients in whom intraoperative PTH levels predicted curative resection.399
The concept that time-indexed PTH decay curve kinetics held potential for discriminating between single-gland and multiple-gland disease, and that this may be influenced by baseline PTH levels, was investigated by Miller and associates.401 Two groups of patients were investigated: patients noted to have indexed PTH levels greater than (high baseline) and less than (low baseline) 100 pg/mL. Using PTH decay curve slope analysis and two time points for each group individually and combined, data were generated and correlated with pathologic findings (i.e., single-gland versus multiple-gland disease), and then compared with the standard (50% rule) arithmetic results. This study yielded two important findings with reference to the patients with low baseline (<100 pg/mL) hormone levels: (1) 50% degradation was less valid in confirming single-gland disease, and (2) multiple-gland disease was more prevalent in low baseline patients.
Unsuccessful Localization
In most situations, failure to localize to a discrete area of the neck after sestamibi imaging occurs as a result of unrecognized multinodular thyroid disease (Fig. 125-24). In the absence of a nodular thyroid gland, one must suspect diffuse hyperplasia or, less likely, an ectopically located cervical or mediastinal gland. One must be careful that an appropriately dosed (minimum 23 to 26 mCi) and correctly conducted nuclear scan has been carried out in all studies. SPECT Tc 99m sestamibi imaging may be helpful in reconciling equivocal planar images.402,403 A failure to localize on delayed nuclear imaging mandates the performance of a standard bilateral exploration.
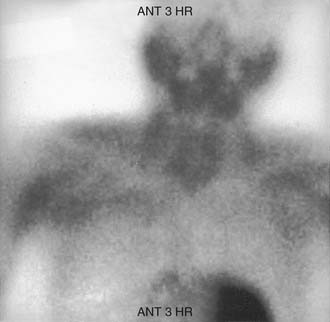
Figure 125-24. Tc 99m sestamibi nuclear scan in a patient with hyperparathyroidism and nodular thyroid disease.
This finding suggests the importance of performing high-resolution ultrasound evaluation in patients with nodular thyroid disease because the finding of a cystic nodule would decrease suspicion that the localization result is a false-positive result. Even in the presence of solid nodular thyroid disease, subcapsular and intrathyroidal abnormal parathyroid glands have been encountered that have imaged correctly. This finding emphasizes the importance of evaluating all nodularity within or associated with the thyroid gland in the event that these abnormal parathyroid glands be located in a “hidden” region either between thyroid nodules or tightly held under the pretracheal fascia representing the “pseudocapsule” over the thyroid gland (Fig. 125-25). More likely, however, is that solid thyroid nodularity with a false-positive sestamibi image is consistent with an adenomatous thyroid nodule, mandating bilateral cervical exploration.
Mediastinal Exploration
Initial exploration or re-exploration for parathyroid disease may require exploration of the mediastinum. Ectopic parathyroid glands located within the mediastinum and below the level of the thymus account for a small percentage (0.2%) of all abnormally located glands.404 In contrast, Wang405 and Norton406 have shown that a more substantial proportion, 18% (Wang405) and 20% (Norton406), of ectopically located adenomas reside in the mediastinum accessible only through a mediastinal approach. These inferior parathyroid glands are associated in almost all circumstances with the thymus with which they descend during embryonic development, having arisen with the thymus as a third pharyngeal pouch derivative.
Several approaches to the mediastinum are available for exploration. The choice of approach depends on the location of the putative adenoma. Localization studies that, in combination, corroborate and specify the mediastinal location are required before undertaking exploration. In the experience of most investigators Tc 99m sestamibi imaging together with MRI represents the optimal combination of physiologic and anatomic imaging for localization (Fig. 125-26).
The techniques available for approaching the mediastinum include transcervical substernal with thymectomy using anterior retraction of the sternum for superior mediastinal glands; median sternotomy with direct approach to the anterior middle and caudal mediastinal compartments; posterolateral thoracotomy for selective, posteriorly based glands in the lower mediastinal compartment; and endoscopic, minimally invasive mediastinal dissection for selectively focused exploration.407 Most glands are approached via a median sternotomy, owing to this technique’s capability to address safely numerous areas within the mediastinum and the lower cervical region immediately posterior to the clavicular heads and manubrium. This technique also allows uninterrupted visualization of both recurrent laryngeal nerves, preventing inadvertent injury to these structures within the mediastinum. Surgical adjuncts that may aid in the intraoperative localization of adenomas, and that may be employed after median sternotomy include intraoperative ultrasound and gamma probe after preoperative sestamibi injection.
Gamma Radiation Detection Device
Minimally invasive surgical exploration using a gamma radiation detection device has been developed and advocated by Norman and Chheda269 to assist actively and expedite initial surgery for parathyroid adenoma (see the section on intraoperative localization).404 This methodology exploits the nuclear uptake characteristics of parathyroid adenoma with respect to Tc 99m sestamibi, and the ability of the gamma probe to localize these glands in the neck intraoperatively after preoperative sestamibi injection.
This technique has been applied by one of the authors (P.K.P.) in mediastinal exploration after sternotomy with satisfactory results (Fig. 125-27). Despite uptake of sestamibi by myocardial tissue, background radioactivity within the mediastinum seems to be less than that of thyroid tissue, facilitating a focused dissection with minimal disturbance of surrounding mediastinal structures. Reoperation for multiple-gland disease in secondary and tertiary hyperparathyroidism has been aided with the use of the gamma probe when supernumerary glands are present. In one notable instance in this series, three additional parathyroid glands (two cervical and one mediastinal) were recovered in a patient with tertiary hyperparathyroidism who previously underwent documented four-gland parathyroidectomy during preparation for cadaveric renal transplantation. The gamma probe was instrumental in identifying one of the cervical glands and one mediastinal gland, which was the only parathyroid gland that was localized accurately on initial sestamibi imaging. The gamma probe may also be applied for removal of hyperfunctional parathyroid tissue autotransplanted to the forearm in the setting of secondary or tertiary hyperparathyroidism.
Parathyroid Carcinoma
Parathyroid carcinoma is reported to be the cause of primary hyperparathyroidism in only 0.1% to 4% of individuals affected.404–407 Parathyroid carcinoma occurs with equal frequency in male and female patients; in contrast, parathyroid adenomas occur more frequently in female patients.408 The epidemiology of parathyroid carcinoma offers few clues about its etiology and pathogenesis. The development of cancer of the parathyroid glands has been linked to chronic renal failure and dialysis,409–411 and familial hyperparathyroidism syndromes including MEN-I and MEN-IIA and the hereditary hyperparathyroidism–jaw tumor syndrome.412 The hyperparathyroidism–jaw tumor syndrome is characterized by recurrent parathyroid adenomas, fibroosseous tumors of the mandible, and Wilms’ tumors.412
Generally, patients with parathyroid cancer have higher serum calcium levels, higher levels of intact PTH, and more profound metabolic abnormalities than patients with parathyroid adenoma or hyperplasia. Approximately 70% of patients with parathyroid carcinoma have serum calcium levels greater than 14 mg/dL and intact PTH levels at least five times the upper limit of normal.413–417 Approximately 80% of patients with parathyroid cancer are either symptomatic or have some metabolic abnormality associated with their disease, and 40% were found to have a palpable neck mass.413,415 This finding contrasts with the presentation in patients with benign causes of hyperparathyroidism; 50% of that cohort are asymptomatic in diagnosis, and the presence of a neck mass is rare.
A definitive preoperative diagnosis of parathyroid carcinoma is impossible to make; metabolic manifestations of parathyroid cancer overlap with the manifestations of parathyroid adenoma. A high index of suspicion of parathyroid carcinoma should be maintained especially in patients with serum calcium levels greater than 14 mg/dL and a palpable neck mass.413,415,417 Recurrent laryngeal nerve palsy manifesting in a patient with hyperparathyroidism is also highly suggestive of parathyroid cancer.418
The definitive treatment of parathyroid cancer is en bloc resection of the tumor and areas of potential local invasion or regional metastasis or both. Parathyroid cancer frequently recurs in the central neck compartment, and typically exhibits a natural history marked by recurrent hypercalcemia. Performance of the appropriate surgical procedure during the initial operation is crucial, and is one of the most important prognostic factors in parathyroid cancer.419 The integrity of the parathyroid capsule should be maintained during dissection using an en bloc resection of the ipsilateral central neck contents, including the thyroid lobe and tracheoesophageal soft tissues and lymphatics.420 Structures such as the recurrent laryngeal nerve, esophageal wall, or strap muscles may require sacrifice if the tumor adheres to them; this reduces the risk of tumor spillage and local recurrence. The increased local control achieved with resection of the recurrent laryngeal nerve outweighs the complication of vocal cord paralysis, which may be managed by phonosurgical rehabilitative procedures.
A central compartment (level 6 and possibly level 7) neck dissection together with resection of soft tissues within the superior anterior mediastinum is important to stage appropriately any lymph node involvement that is not directly palpable during the initial surgery. Lymph node metastasis lateral to the jugular vein is rare in parathyroid cancer during the initial presentation.413 Prophylactic modified radical or selective dissection of levels 1 through 5 is not generally recommended. Neck dissection is reserved for patients with lymph node metastasis detected radiographically or by clinical examination in the jugular distribution, or for patients with massive soft tissue invasion of lateral neck structures.
Although cure after resection of recurrent parathyroid carcinoma is rare, aggressive resection of local recurrence is recommended to control severe hypercalcemia. Selected patients achieve prolonged disease-free intervals after one or more surgical procedures for recurrence in the neck or anterior superior mediastinum.421
In addition to the approach for initial surgery of parathyroid carcinoma and local recurrence, an aggressive surgical approach to metastatic parathyroid cancer has been advocated to control marked hypercalcemia. Obara and colleagues420 reported on lung resection for metastasis from parathyroid cancer in which 32% of patients resected achieved a significant reduction in total serum calcium levels, and 14% achieved long-term survival ranging from 9 to 30 years. Surgery for locally recurrent parathyroid cancer may be guided by preoperative localization studies to define better the extent and location of recurrent parathyroid cancer (Fig. 125-28). Localization studies should be interpreted with caution, however, because not all tumor foci may be detected, and in patients with very high intact PTH levels, benign lesions of bone (brown tumors) may mimic metastases.422–426
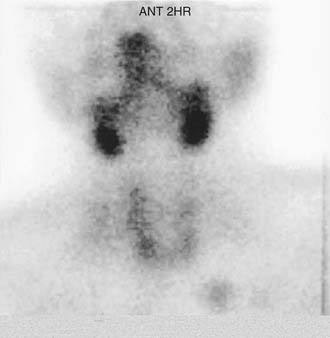
Figure 125-28. Tc 99m sestamibi image showing delayed uptake in the left superior mediastinum in a patient with recurrent parathyroid carcinoma.
The low incidence of parathyroid cancer has made it difficult to study the roles of radiation and chemotherapy. A report issued by the National Cancer Database described the use of radiation therapy in combination with surgery in less than 7% of the 286 cases included.408 Some reports have advocated the importance of complete en bloc resection followed by radiation therapy to increase local control.427,428 The results of these reports must be tempered, however, by the finding that patients in these series may not have had advanced poorly resectable disease, a finding that characterized investigations in which radiation therapy was believed to be a factor that worsened prognosis for parathyroid cancer.
Chemotherapy has a very limited role in the management of parathyroid cancer. Some response to therapy has been noted with a combination of fluorouracil, cyclophosphamide, and dacarbazine, and the combination of methotrexate, doxorubicin, cyclophosphamide, and lomustine. These agents were not administered in a controlled study environment, and patient numbers were exceedingly few.429,430
Hyperparathyroidism during Pregnancy
Hyperparathyroidism during pregnancy is rare, and pregnant women usually represent a very small fraction of the total number of patients treated for this disease.431 As in nonpregnant patients, a single parathyroid adenoma is the most likely to be the cause of hyperparathyroidism, although hyperplasia and carcinoma have been reported.432–434
During pregnancy, there is a degree of protection in the mother against hypercalcemia provided by calcium transport across the placenta.432 Large amounts of calcium depress the fetal parathyroid function and result in fetal hypoparathyroidism. After delivery, when maternal calcium is no longer available, neonatal tetany develops as a consequence of hypercalcemia; this is the most common presenting sign for maternal hyperparathyroidism in pregnancy.434 Additional fetal complications described in mothers with hyperparathyroidism include spontaneous abortion, prematurity, intrauterine growth restriction, and stillbirth.431 Although many pregnant women are asymptomatic, presenting symptoms of hyperparathyroidism during pregnancy may include muscle weakness, abdominal symptoms, disorientation, coma, and death.433
The risk of obstetric complications has been shown to be significantly greater in women who do not undergo surgery for hyperparathyroidism while pregnant.433 Some investigators believe that neonatal hypocalcemia is transient and treatable, and maternal disease can be successfully controlled medically when the diagnosis is already known.434,435 The opposing opinion is that regardless of symptom complex severity, all pregnant patients with hyperparathyroidism should undergo neck exploration to prevent the array of potential maternal and fetal complications.436
There seems to be uniform agreement that in severely symptomatic patients, surgery should not be postponed until after delivery. The ideal safe period for parathyroidectomy is the second trimester, when chances of pregnancy loss or premature labor as a consequence of surgical intervention are minimal.436 If the diagnosis is made during the third trimester, parathyroid exploration usually can be postponed until after labor and delivery. Preoperative hypercalcemia may be successfully controlled with fluids, diuretics, and orally administered phosphate. Plicamycin, an antineoplastic agent, should be avoided during pregnancy, however, because it is highly toxic to the fetal bone marrow, liver, and kidney. The most severe cases of hypercalcemia may require hemodialysis for appropriate control and stabilization.432

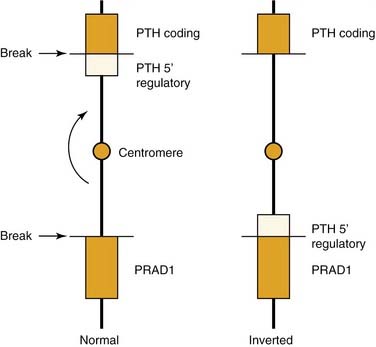
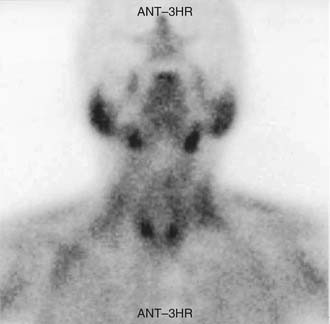
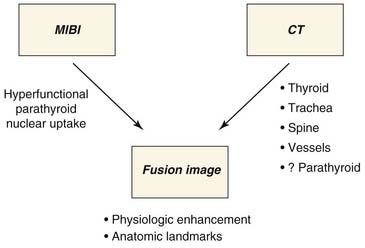
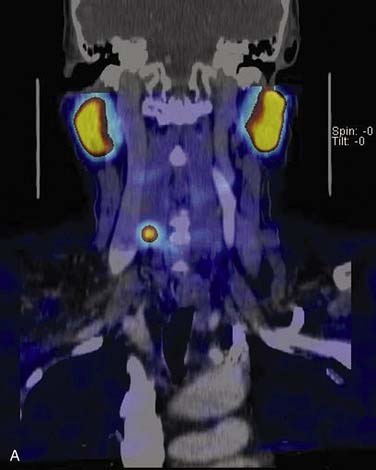
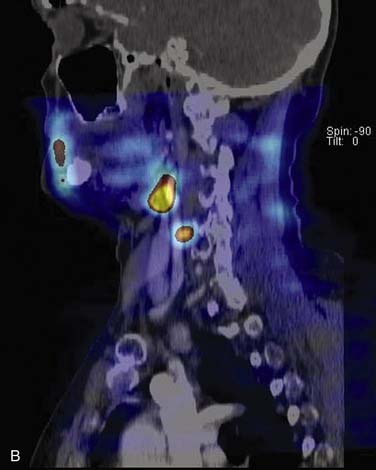
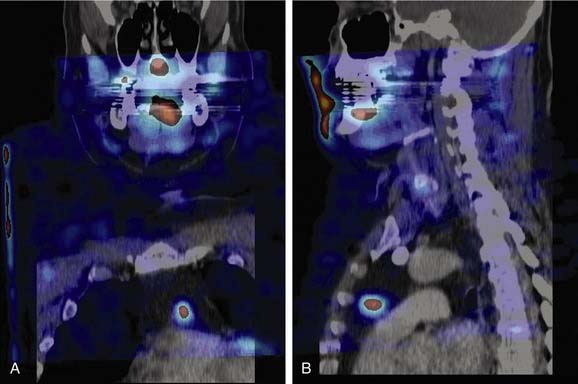
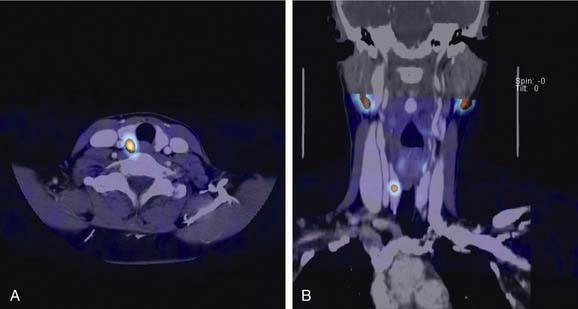
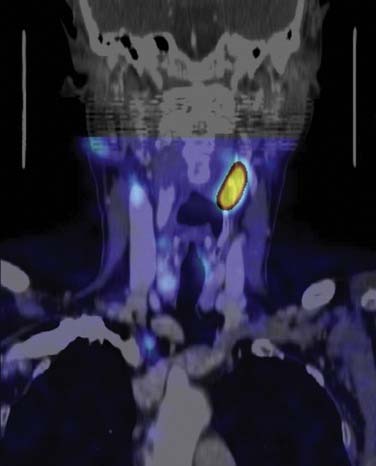
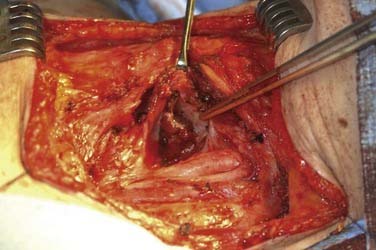
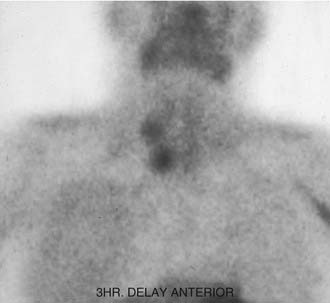
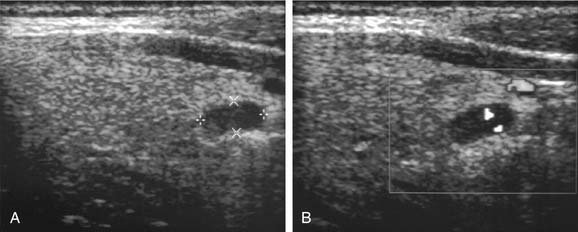
 gland) parathyroidectomy. The distinction between adenoma and hyperplastic parathyroid tissue is difficult or impossible on frozen section analysis; the surgeon cannot rely on histologic analysis of a single gland for definitive therapy.
gland) parathyroidectomy. The distinction between adenoma and hyperplastic parathyroid tissue is difficult or impossible on frozen section analysis; the surgeon cannot rely on histologic analysis of a single gland for definitive therapy.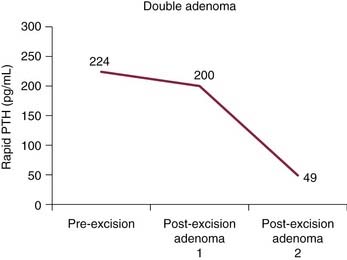
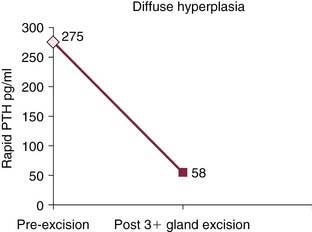
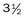 -gland subtotal parathyroidectomy in a patient with diffuse 4-gland hyperplasia.
-gland subtotal parathyroidectomy in a patient with diffuse 4-gland hyperplasia.