Chapter 221 Management of Ossification of the Posterior Longitudinal Ligament
Cervical Laminoplasty: Open Door
Ossification of the posterior longitudinal ligament (OPLL) is a pathologic condition in which ectopic bone formation within the posterior longitudinal ligament (PLL) causes progressive narrowing of the spinal canal. First described by Key in 1838,1 an autopsy report by Tsukimoto2 in 1960 in a Japanese patient is credited with redirecting attention on this pathologic process as a cause of cervical myelopathy in this population.3 Since then, there has been a growing body of evidence that has helped to define its pathogenesis, underlying genetics, and natural history. A clear understanding of these processes is critical to provide the best neurosurgical management for these patients.
Etiology and Epidemiology
Evidence suggests that OPLL is a multifactorial disease with underlying genetic, environmental, and biomechanical contributions.4 Early epidemiologic and more recent genetic studies, for example, have identified numerous genes that may have causal associations with OPLL, including the genes for collagen 11,5,6 nucleotide pyrophosphate,7 and transforming growth factor-β.8 Likewise, histochemical studies of OPLL show possible involvement of a number of cytokines and growth factors, such as bone morphogenetic proteins9,10 and transforming growth factor-β.8,11 It is proposed that these proteins may function by inducing osteogenesis in the PLL of patients with this condition.12
Other factors, such as diet and comorbidities, may also be related to the induction or progression of OPLL in individual patients. Vegetarian diets and diets high in salt and vitamin A have all been reported to be associated with OPLL,13–17 although data are limited and further studies are necessary to clarify these associations. In addition, a higher incidence of OPLL has been reported in patients with endocrinologic comorbidities such as glucose metabolism disturbances, hypoparathyroidism, and acromegaly.18–21
Epidemiologic studies have identified a modest radiographic frequency (1.9–4.3%) of OPLL in the Japanese population.22 Although similar ranges have been reported in other Asian populations, the incidence in the European and North America populations remains much lower (0.1–1.3%).22 Proportionally, however, patients with OPLL may represent up to one fourth of those presenting with signs and symptoms of myelopathy, regardless of their ethnic background.23,24 Most studies also support a sex difference in the prevalence of OPLL, with a reported male-to-female ratio of 2 to 3:1.22,25
Radiographic and Clinical Presentation
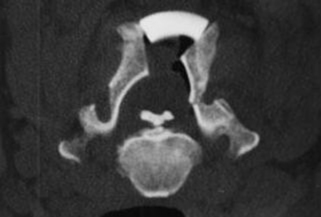
The diagnosis of OPLL is established by its pathognomonic radiographic characteristics. Most commonly, but not exclusively, identified in the cervical spine, a dense calcified strip of variable thickness (1–5 mm) can be found along the dorsal margins of the vertebral bodies and the intervertebral discs. The intervertebral discs in the involved area usually are well preserved, without evidence of disc space narrowing. The shape and volume of OPLL can be quite varied, compromising anywhere from a small fraction to greater than 50% of the spinal canal. Radiographically, OPLL has been classified into four groups: segmental (one or more separate lesions behind the vertebral body); continuous (a continuous lesion spanning several vertebral bodies); mixed (a combination of the two aforementioned types); and circumscribed (located primarily behind the intervertebral disc spaces).23
Anatomically, the PLL consists of two layers, a deep and a superficial one. The deep layer fuses with the periosteum of the dorsal corner of the vertebral body.26 This point of attachment is believed to be the site of initiation of OPLL in the segmental subtype. In the continuous form, the development is less clear. Although some authors advocate that it represents a continuous extension of the segmental subtype, others maintain that it results from progression of multiple scattered OPLLs.26
The clinical presentation of patients with OPLL can be varied, largely because of the differing forms and extent of the pathologic process, as described previously. Approximately half the patients are asymptomatic when the diagnosis is made.23 Typically, it presents in the fourth and fifth decades of life with an insidious onset of symptoms of cervical radiculopathy or myelopathy.24 More commonly, up to two thirds of patients complain of neck pain or stiffness and/or pain or numbness in the upper extremities, with fewer (40%) complaining of motor symptoms in the arms.27 Motor and sensory changes in the lower extremities are less common (40%), although the majority of patients (57%) are found to be hyper-reflexic. Less than 20% have bowel or bladder symptoms on presentation.27
Natural History
An understanding of the natural history of OPLL is essential in managing patients, particularly in the subset of patients for whom the diagnosis was found incidentally. Not unexpectedly, reviewing recent studies on OPLL patients demonstrates a natural radiographic progression of the disease in the majority of patients regardless of treatment approach.4 In one recent study, Matsunaga et al.28 observed an increase in PLL thickness in 70 (42%) of 167 patients managed conservatively; in 32 of these patients (19%) this increase was greater than 2 mm (marked development) over a mean follow-up of 11.2 years. There was a longitudinal increase in 144 (86%) in this same group, with 59 patients (35%) demonstrating ossification behind more than one vertebral level (marked development).
More recent studies by this same group examined the symptomatic progression of patients with OPLL in an effort to better delineate the clinical course of the disease as well as the radiographic predictors that might correlate to this course.29,30 Following a small group of patients (N = 36) who presented with myelopathy, Matsunaga et al.30 reported that 64% progressed over the study’s more than 10-year follow-up. Of the 323 patients who were not myelopathic on presentation, myelopathy developed in only 55 (17%). The Kaplan-Meier estimate of the myelopathy-free rate in the latter group was 71% at 30-year follow-up.
A severely narrowed spinal canal has been identified as a risk factor in the development of myelopathy in this condition, with 100% of patients with a canal diameter of less than 6 mm28 or greater than 60% stenosis30 experiencing this neurologic debility. In patients with canal diameters reduced by OPLL but not to such critical levels, presence of myelopathy does not appear to be correlated with diameter size per se.28 Such findings have led authors to consider other factors that may contribute to the development of neurologic symptomatology in these patients. Instead, the pathology of cervical myelopathy in patients with OPLL can be explained in two different ways. In patients with significant canal compromise (>60%), the ossified ligament acts as a source of ongoing mechanical or static compression. In others with more moderate compression, cervical motion between adjacent ossified segments plays a role in the development of symptoms as a form of dynamic compression.29,31,32
Management Strategies
The primary indication for surgical management in a patient with OPLL is cervical myelopathy. Variable combinations of motor, sensory, and reflex changes are usually present. Prophylactic surgery in mildly symptomatic or asymptomatic patients is controversial. As indicated previously, only a small proportion of these patients have been found to progress clinically,30 suggesting that in the absence of known risk factors such as extreme canal compromise or evidence of increased cervical motion, conservative treatment may be preferable. And although trauma is a known risk factor for development of neurologic symptomatology in this patient population, this risk appears to be relatively low; in a recent prospective study only 6 of 368 asymptomatic patients with OPLL (2%) went on to develop trauma-induced myelopathy during a minimum 10-year follow-up period.33 As a result, most authors do not recommend prophylactic surgery in this group, although a frank discussion with patients is warranted to involve them in the decision-making process.
Conservative management of OPLL is largely empirical. There exist no well-designed clinical studies systematically evaluating the efficacy of common treatment modalities. The mainstay of conservative therapy is immobilization together with anti-inflammatory medications, in an attempt to address modifiable sources of static and dynamic cord compression. Cervical traction has also been suggested by some based on their overall experience with patients with cervical spondylitic myelopathy.34 In theory, immobilization of the grossly or microscopically unstable spine prevents ongoing dynamic compression and may facilitate resorption of degenerative osteophytes.
The goal of surgical intervention in patients with OPLL is to decompress the neural structures while stabilizing the spine: in effect, to eliminate the static and dynamic factors that are thought to be responsible for the clinical disease. Once the decision for surgical intervention is taken, the principal decision regarding surgical management is whether a ventral or dorsal approach should be used because comparable results have been achieved with both.35,36 In general, a ventral decompressive procedure and fusion is the most appropriate surgical procedure in the presence of ventral compressive factors, such as OPLL, limited to three or fewer vertebral bodies. This procedure, combined with resectioning or floating of the ossified PLL, directly addresses the pathologic process and facilitates fusion with the use of bone graft. For many, it is the procedure of choice in patients with OPLL with significant canal stenosis (>60%), particularly if the lesion is focal or kyphotic angulation of the cervical spine is present. Compared with a dorsal approach, it may carry increased risk of surgical complications, including risk of cerebrospinal fluid leak as well as adjacent-segment disease due to progression of OPLL.35,37 At 5-year follow-up, good to excellent clinical results have been achieved in as much as 80% of patients who underwent a ventral procedure, although 8% of these patients ultimately required further dorsal surgery.35
Sir Victor Horsley is the neurosurgeon first credited with decompressing the cervical spine of a patient with progressive cervical spondylotic myelopathy using a dorsal approach.38 This was once the procedure of choice for patients with cervical OPLL.39 Although this procedure effectively enlarges the functional spinal canal area, thus allowing the spinal cord to move away from compressive elements and expand, it does so at the expense of dorsal stabilizing structures. Depending on preoperative spinal alignment and time since surgery, up to 50% of patients with OPLL who undergo a laminectomy for treatment develop spinal instability and a gradual kyphotic deformity.39 Given these concerns, together with a considerable population suffering from a multilevel compressive pathologic process necessitating dorsal decompression, Asian surgeons developed the laminoplasty as a “tissue-sparing” procedure in the late 1970s to effectively remodel the spinal canal.40,41 In theory, one can successfully enlarge the spinal canal while largely preserving dorsal stabilizing structures, thus reducing postoperative deformity or instability and alleviating the need to perform additional fusions.
Initial laminoplasty procedures were cumbersome and lengthy, requiring complex reconstruction of the dorsal arch.41 Numerous subsequent modifications have resulted in simplified, faster procedures with improved stability.40,42–46 In particular, preservation of posterior elements was recognized significantly to increase the closing force on the elevated laminae, resulting in a “sinkage” effect with associated restenosis of the spinal canal.47 To combat this problem, a variety of techniques have been used. These primarily involve the use of spacers to buttress the created gap or the use of sutures or titanium plates to “attach” the remaining spinous process(es) to the facet or soft tissue structures of the hinge side.40,46,48–50 We prefer the former, using fibular allograft, with the “closing forces” maintaining firm positioning of the graft and facilitating fusion. Further stability may be obtained, in addition to or in lieu of the aforementioned techniques, using onlay bone grafting at the hinge site(s).51
Animal studies have supported the presumed benefits of laminoplasty over laminectomy. In one study, laminectomies or laminoplasties were performed from C3-5 in goats, with monthly radiographic follow-up for 6 months. Radiographic results were compared with control animals, confirming that laminoplasties were biomechanically superior in maintaining alignment.52 An additional study in rabbits found that although postoperative range of motion was similar between groups, laminectomy was associated with increased angle deformity and poorer outcome.53 Similarly, a biomechanical advantage of laminoplasty has been found in a retrospective study comparing different cohorts of patients.54
Surgical Technique
Perioperative Considerations
Neurophysiologic monitoring options include somatosensory evoked potentials, motor evoked potentials, and electromyography. The value of routine neurophysiologic monitoring is often questioned because it has been difficult to demonstrate that the information provided can actually change what the surgeon does during the surgery, making it safer. However, some retrospective studies have demonstrated the positive predictive value of such tests in determining outcome.55,56 The stimulating and recording electrodes are placed and secured and baseline recordings are obtained before turning the patient. A number of options exist for holding the head during surgery in the prone position. We use the Mayfield three-pin head holder, which allows the surgeon to easily control the degree of cervical spine flexion and extension and reduces the possibility of pressure being exerted on the patient’s eyes. The patient is then transferred onto the operating table in the prone position, with the head secured in a slightly flexed position. Tape can be applied to the superior and dorsolateral aspects of both shoulders and secured to the caudal region of the operating table to assist with intraoperative radiographic visualization of the lower cervical levels.
Open-Door Expansile Cervical Laminoplasty
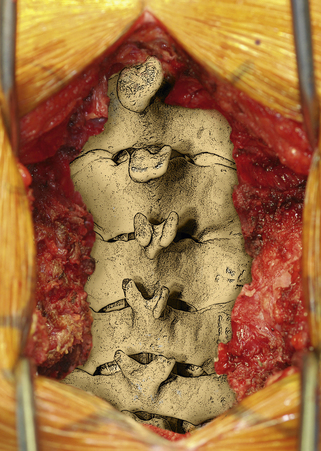
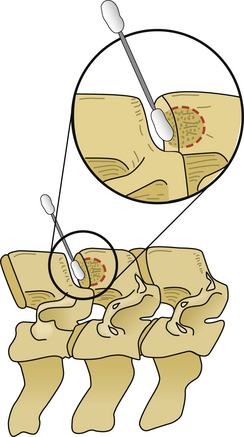
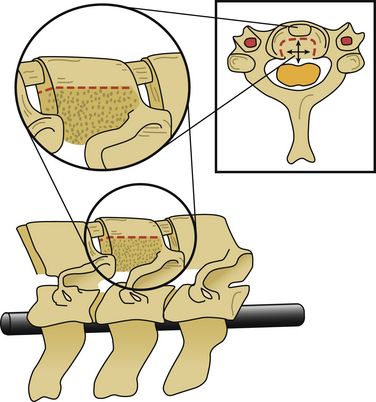
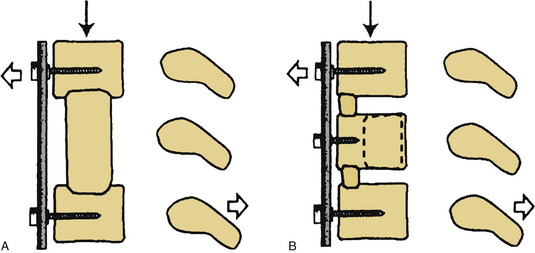
After the operative field is prepared and draped, the midline is infiltrated with commercially available 1% lidocaine with epinephrine to minimize skin bleeding. A midline incision is made and monopolar or bipolar electrocautery is used to control soft tissue bleeding. The midline fascia is then incised with monopolar electrocautery, and subperiosteal dissection is used to reflect the extensor cervical muscle groups, exposing the cervical lamina and mesial facets from the caudal portion of C2 to the rostral limit of T1, taking care to preserve the facet capsules (Fig. 221-1). The caudal one third of the C2 lamina and the rostral one third of the T1 lamina are removed, using a combination of a high-speed air drill and a 2-mm Kerrison punch, to visualize the underlying dura at this level. We also remove the spinous processes of C3 to C7 inclusively with Stille-Horsley bone-cutting forceps and morselize the bone for subsequent autografting.
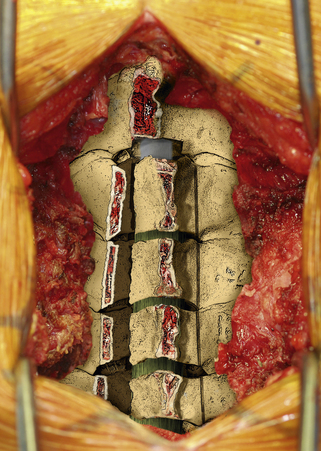
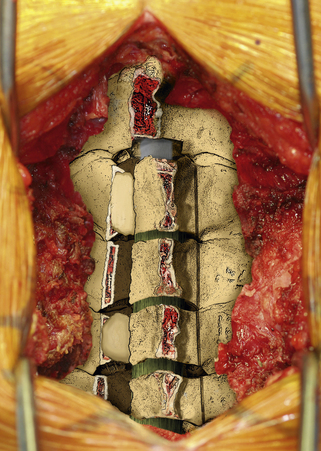
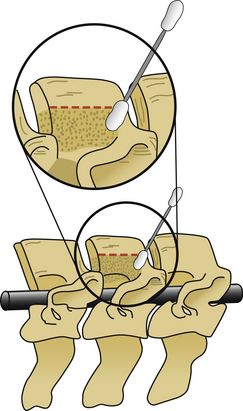
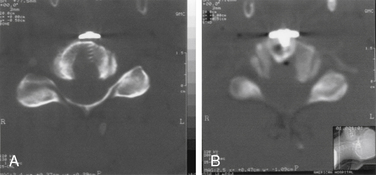
The next phase of the procedure involves performing osteotomies of the C3-7 laminae. In so doing, one creates an “open” side and a “hinged” side of these laminae (Figs. 221-2 and 221-3). In general, the side with the greatest compression or the most clinically symptomatic side is the open side. If one is planning to perform foraminotomies in addition to the laminoplasty, the open side is best placed on the side of the intended foraminotomies. A high-speed air drill with a small bit is used to create troughs at the level of the laminofacet junction from C3 to C7. Drilling proceeds through the outer and inner cortical margins of the lamina on the side to be opened. On the hinge side, drilling proceeds through the outer cortical margin and cancellous bone; however, the inner cortex is not violated.
After the grafts have been prepared, attention is turned to “opening the door.” Initially, two small curettes are introduced into the gap produced by drilling the laminae and advanced just deep to the outer cortex. By pulling the curettes upward, the laminar facet gap on the open side is slowly enlarged, and this creates a greenstick fracture along the previously created trough on the hinged side. Minimal advances are made before moving to other laminae in an effort to open all the involved laminae as a functional unit. The goal is to expand the anteroposterior diameter of the canal by approximately 4 mm (see Fig. 221-3). Great care must be taken to achieve this goal without fracturing the inner cortex of the hinge side.
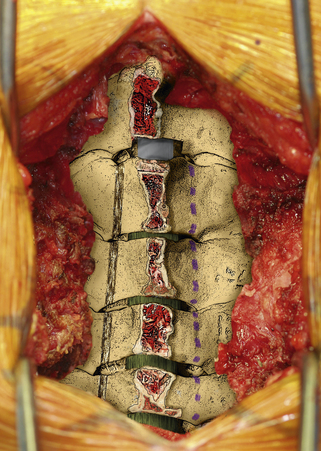
Once this is accomplished, the rib allografts are placed in the gap that has been created at the C3, C5, and C7 levels, with the cut edges of the laminae resting in the cut grooves of the rib grafts (Fig. 221-4). If done properly, the grafts should fit snugly in the gap, there should be a slight “closing” force securing the grafts in position, and the inner cortex of the hinge side should be intact. We then use the morselized spinous process autograft and place it over the decorticated bone surfaces of the facet and lamina on the hinged side to promote intersegment fusion.
Should rigid stabilization be required, facet cables with or without rib allograft can be inserted. Lateral mass screws attached to a plate or a rod can also be applied. It is sometimes difficult to position the rib allografts to hold open the laminae once additional hardware is placed, but it can be done. In this case, we perform the drilling and “opening the door” first, but no graft is inserted until the instrumentation is in place. Variations to the approach include use of the spinous process autograft instead of the rib allograft to hold open the lamina. Some surgeons prefer to use miniplates or sutures to stabilize the rib allograft to the adjacent lamina and facet on the open side.50 The lamina can be split in the midline with a T-handled “Gigli-like” saw and the allograft spacers positioned between the greenstick-fractured hemi-lamina.57
In our experience, an open-door expansile cervical laminoplasty (without additional stabilization procedures) takes approximately 90 minutes to complete, with an average blood loss of 200 mL. In general, the complication rate is low (see next section), particularly compared with decompressive procedures that attempt to achieve the same number of levels of decompression and stabilization from a ventral approach.58
Complications
The surgical complication rate for dorsal decompressive procedures is low,48 and includes but is not limited to infection, cerebrospinal fluid leak, hemorrhage, spinal cord injury, nerve root injury, and the risk associated with the general anesthetic. Among them, long-tract paralysis, although rare, can often be attributed to definite causes such as traumatic surgical technique, reclosure of the opened laminae, or postoperative hematoma, all of which are theoretically preventable.59
Specific longer-term complications historically associated with laminoplasty itself include postoperative neck pain, reduced range of motion (ROM), canal restenosis, loss of cervical lordosis, and segmental motor paralysis/nerve root palsy.49,60–63 Axial neck and shoulder girdle pain can be problematic and several papers have highlighted an increased incidence of neck pain when comparing dorsal (40–60%) and ventral (15–19%) surgery groups.64,65 Neck pain usually responds to an aggressive course of physical therapy.
Both clinically and radiographically, limited ROM is frequently observed after laminoplasty. Studies suggest that approximately 50% of ROM is lost after laminoplasty, particularly in extension.36,57,61,66 This correlates well with radiographic evidence of spontaneous bony fusion.67 It has been proposed that this is actually beneficial in that it ameliorates ongoing mechanical stress or injury without being “rigid” and inducing stress and degeneration in adjacent levels. Numerous studies have shown no correlation between limited ROM and recovery rates or outcome, including neck pain.36,66 However, it is important to remember that most of the literature pertains to patients undergoing decompression for OPLL, which is itself associated with increased rigidity, and thus may overestimate the restricted ROM attributable to the laminoplasty procedure.
Despite the theoretical biomechanical advantage conferred by laminoplasty, up to 50% of patients experience deteriorating cervical alignment after the procedure, with 2% to 15% developing new-onset kyphosis.66 The clinical implication of these radiographic changes remains unclear because both axial neck pain and malalignment have little impact on the ultimate outcome and Japanese Orthopaedic Association (JOA) scores,49,68,69 unless associated with a decrease of greater than 10 in the cervical curvature index.44
Segmental motor paralysis involving primarily C5 is another neurologic complication occasionally seen in patients undergoing laminoplasty. Delayed C5 nerve root weakness occurs at a reported rate of 4.6% to 13.3%.44,68 Although it is transient in most cases, recovery may require up to 6 years.44 Traditionally, it has been attributed to either direct damage to exiting nerve roots by suboptimal surgical techniques, or a tethering effect on the nerve root secondary to posterior shift of the spinal cord after decompression.44,47,61 The prevalence of C5 injury in particular was thought to result from the most significant “migration” of the cord at this level because it frequently represents the apex of the lordotic curve. However, more recent studies with improved imaging quality suggest that it may, in fact, represent intrinsic perioperative damage to the spinal cord itself at that level.70,71
An additional concern that stems from the laminectomy literature is the development of the “postlaminectomy membrane.”72 This entity has been implicated in arachnoiditis and restenosis, and may theoretically result in clinical deterioration.50 To date, these findings have not been reported after laminoplasty.
Neurologic Outcomes
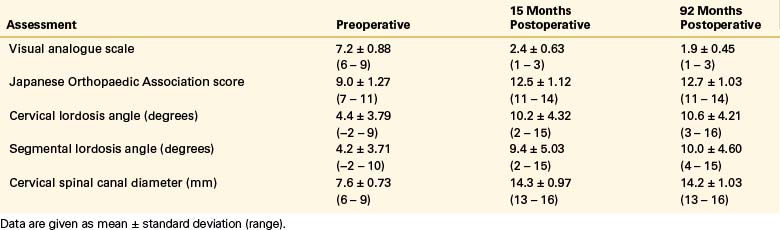
Overall, the recovery rate in patients with OPLL after cervical laminoplasty, typically assessed by the JOA scoring system, has been reported to be approximately 60% at 5-year follow-up.44,73–75 In these series, a subsequent decline in patient scores appears to develop between the 5- and 10-year follow-ups. The cause for this late deterioration is not well understood. Although radiographic progression of OPLL was cited as a cause of deterioration in at least one study,75 it was not found to correlate with clinical deterioration among the 53 patients followed by Chiba et al.73 Several factors have been implicated as predictors of poor outcome in these patients, including severity of preoperative myelopathy, increasing age, history of trauma, and duration of symptoms.36,39,44,69,74 Likewise, a recent study highlights the need properly to assess patients’ preoperative imaging before deciding on surgical approach. Based on both the degree of stenosis secondary to OPLL and the spinal curvature, Fujiyoshi et al.76 proposed an index to predict if the amount of posterior shift of the spinal cord after laminoplasty would be adequate for improvement in neurologic status.
Studies have shown that clinical improvement directly correlates with the degree of canal expansion. However, excessive expansion as well as an irregular canal area may be associated with additional problems.77,78 It appears that the optimal canal expansion approximates 4 to 5 mm in the sagittal anteroposterior diameter,40 correlating with approximately a 50% increase in canal area79,80 and facilitating a 3-mm dorsal shift of the spinal cord.81 However, decreased lordosis correlates with decreased volume expansion after laminoplasty, as well as with decreased dorsal migration of the cord.80 Far more critical than canal expansion is subsequent cord expansion, with studies showing a direct correlation between JOA scores and spinal cord area.82
Epstein N. Ossification of the cervical posterior longitudinal ligament: a review. Neurosurg Focus. 13(2), 2002. ECP1, 2002
Inamasu J., Guiot B.H., Sachs D.C. Ossification of the posterior longitudinal ligament: an update on its biology, epidemiology, and natural history. Neurosurgery. 2006;58:1027-1039. discussion 1039
Iwasaki M., Okuda S., Miyauchi A., et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament. Part 1: clinical results and limitations of laminoplasty. Spine (Phila Pa 1976). 2007;32:647-653.
Matsunaga S., Nakamura K., Seichi A., et al. Radiographic predictors for the development of myelopathy in patients with ossification of the posterior longitudinal ligament: a multicenter cohort study. Spine (Phila Pa 1976). 2008;33:2648-2650.
Nakamura K. History of research. In: Yonenobu K., Nakamura K., Toyama Y., editors. OPPL: ossification of the posterior longitudinal ligament. ed 2. Tokyo: Springer; 2006:3-6.
1. Key C.A. Paraplegia depending upon ligament of the spine. Guys Hosp Rep. 1838;3:173-174.
2. Tsukimoto H. A case report: autopsy of the syndrome of compression of the spinal cord owing to ossification within the spinal canal of the cervical spine. Nihon Geka Hokan. 1960;29:1003-1007.
3. Nakamura K. History of research. In: Yonenobu K., Nakamura K., Toyama Y., editors. OPPL: ossification of the posterior longitudinal ligament. ed 2. Tokyo: Springer; 2006:3-6.
4. Inamasu J., Guiot B.H., Sachs D.C. Ossification of the posterior longitudinal ligament: an update on its biology, epidemiology, and natural history. Neurosurgery. 2006;58:1027-1039. discussion 1039
5. Koga H., Hayashi K., Taketomi E., et al. Restriction fragment length polymorphism of genes of the alpha 2(XI) collagen, bone morphogenetic protein-2, alkaline phosphatase, and tumor necrosis factor-alpha among patients with ossification of posterior longitudinal ligament and controls from the Japanese population. Spine (Phila Pa 1976). 1996;21:469-473.
6. Maeda S., Ishidou Y., Koga H., et al. Functional impact of human collagen alpha2(XI) gene polymorphism in pathogenesis of ossification of the posterior longitudinal ligament of the spine. J Bone Miner Res. 2001;16:948-957.
7. Tahara M., Aiba A., Yamazaki M., et al. The extent of ossification of posterior longitudinal ligament of the spine associated with nucleotide pyrophosphatase gene and leptin receptor gene polymorphisms. Spine (Phila Pa 1976). 2005;30:877-880. discussion 881
8. Kawaguchi Y., Furushima K., Sugimori K., et al. Association between polymorphism of the transforming growth factor-beta1 gene with the radiologic characteristic and ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 2003;28:1424-1426.
9. Hayashi K., Ishidou Y., Yonemori K., et al. Expression and localization of bone morphogenetic proteins (BMPs) and BMP receptors in ossification of the ligamentum flavum. Bone. 1997;21:23-30.
10. Tanaka H., Nagai E., Murata H., et al. Involvement of bone morphogenic protein-2 (BMP-2) in the pathological ossification process of the spinal ligament. Rheumatology (Oxford). 2001;40:1163-1168.
11. Inaba K., Matsunaga S., Ishidou Y., et al. Effect of transforming growth factor-beta on fibroblasts in ossification of the posterior longitudinal ligament. In Vivo. 1996;10:445-449.
12. Epstein N.E., Grande D.A., Breitbart A.S. In vitro characteristics of cultured posterior longitudinal ligament tissue. Spine (Phila Pa 1976). 2002;27:56-58.
13. Imamura K., Sakou T., Taktomi E., et al. Retinoid induced ossification of the spinal ligament. Orthop Traumatol. 1993;42:1540-1542.
14. Morisu M. Influence of foods on the posterior longitudinal ligament of the cervical spine and serum sex hormones [in Japanese]. Nippon Seikeigeka Gakkai Zasshi. 1994;68:1056-1067.
15. Okamoto K., Kobashi G., Washio M., et al. Dietary habits and risk of ossification of the posterior longitudinal ligaments of the spine (OPLL): findings from a case-control study in Japan. J Bone Miner Metab. 2004;22:612-617.
16. Washio M., Kobashi G., Okamoto K., et al. Sleeping habit and other life styles in the prime of life and risk for ossification of the posterior longitudinal ligament of the spine (OPLL): a case-control study in Japan. J Epidemiol. 2004;14:168-173.
17. Wang P.N., Chen S.S., Liu H.C., et al. Ossification of the posterior longitudinal ligament of the spine: a case-control risk factor study. Spine (Phila Pa 1976). 1999;24:142-144. discussion 145
18. Akune T., Ogata N., Seichi A., et al. Insulin secretory response is positively associated with the extent of ossification of the posterior longitudinal ligament of the spine. J Bone Joint Surg [Am]. 2001;83:1537-1544.
19. Goto K., Yamazaki M., Tagawa M., et al. Involvement of insulin-like growth factor I in development of ossification of the posterior longitudinal ligament of the spine. Calcif Tissue Int. 1998;62:158-165.
20. Okazaki T., Takuwa Y., Yamamoto M., et al. Ossification of the paravertebral ligaments: a frequent complication of hypoparathyroidism. Metabolism. 1984;33:710-713.
21. Shingyouchi Y., Nagahama A., Niida M. Ligamentous ossification of the cervical spine in the late middle-aged Japanese men: its relation to body mass index and glucose metabolism. Spine (Phila Pa 1976). 1996;21:2474-2478.
22. Matsunaga S., Sakou T. OPLL: disease entity, incidence, literature search and prognosis. In: Yonenobu K., Nakamura K., Toyama Y., editors. OPPL: ossification of the posterior longitudinal ligament. ed 2. Tokyo: Springer; 2006:11-18.
23. Tsuyama N. Ossification of the posterior longitudinal ligament of the spine. Clin Orthop Relat Res. 1984;184:71-84.
24. Epstein N. Ossification of the cervical posterior longitudinal ligament: a review. Neurosurg Focus. 13(2), 2002. ECP1, 2002
25. Matsunaga S., Sakou T. Overview of epidemiology and genetics. In: Yonenobu K., Nakamura K., Toyama Y., editors. OPPL: ossification of the posterior longitudinal ligament. ed 2. Tokyo: Springer; 2006:7-12.
26. Tsukuki N. Review of histopathological studies on OPLL of the cervical spine, with insights into mechanism. In: Yonenobu K., Nakamura K., Toyama Y., editors. OPPL: ossification of the posterior longitudinal ligament. ed 2. Tokyo: Springer; 2006:41-48.
27. Kaneko K. Clinical manifestations of cervical OPLL. In: Yonenobu K., Nakamura K., Toyama Y., editors. OPPL: ossification of the posterior longitudinal ligament. ed 2. Tokyo: Springer; 2006:115-120.
28. Matsunaga S., Kukita M., Hayashi K., et al. Pathogenesis of myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg. 2002;96(Suppl 2):168-172.
29. Matsunaga S., Nakamura K., Seichi A., et al. Radiographic predictors for the development of myelopathy in patients with ossification of the posterior longitudinal ligament: a multicenter cohort study. Spine (Phila Pa 1976). 2008;33:2648-2650.
30. Matsunaga S., Sakou T., Taketomi E., et al. Clinical course of patients with ossification of the posterior longitudinal ligament: a minimum 10-year cohort study. J Neurosurg. 2004;100(Suppl 3 Spine):245-248.
31. Mochizuki M., Aiba A., Hashimoto M., et al. Cervical myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg Spine. 2009;10:122-128.
32. Morio Y., Nagashima H., Teshima R., et al. Radiological pathogenesis of cervical myelopathy in 60 consecutive patients with cervical ossification of the posterior longitudinal ligament. Spinal Cord. 1999;37:853-857.
33. Matsunaga S., Sakou T., Hayashi K., et al. Trauma-induced myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg. 2002;97(Suppl 2):172-175.
34. Fukui K., Kataoka O., Sho T., et al. Pathomechanism, pathogenesis, and results of treatment in cervical spondylotic myelopathy caused by dynamic canal stenosis. Spine (Phila Pa 1976). 1990;15:1148-1152.
35. Matsuoka T., Yamaura I., Kurosa Y., et al. Long-term results of the anterior floating method for cervical myelopathy caused by ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 2001;26:241-248.
36. Iwasaki M., Kawaguchi Y., Kimura T., et al. Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine: more than 10 years follow up. J Neurosurg. 2002;96(Suppl 2):180-189.
37. Iwasaki M., Yonenobu K. Choice of surgical procedure. In: Yonenobu K., Nakamura K., Toyama Y., editors. OPPL: ossification of the posterior longitudinal ligament. ed 2. Tokyo: Springer; 2006:181-185.
38. Theodore N., Sonntag V.K. Spinal surgery: the past century and the next. Neurosurgery. 2000;46:767-777.
39. Kato Y., Iwasaki M., Fuji T., et al. Long-term follow-up results of laminectomy for cervical myelopathy caused by ossification of the posterior longitudinal ligament. J Neurosurg. 1998;89:217-223.
40. Hirabayashi K., Watanabe K., Wakano K., et al. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine (Phila Pa 1976). 1983;8:693-699.
41. Oyama M., Hattori S., Moriwaki N., et al. A new method of posterior decompression [in Japanese]. Cent Jpn J Orthop Traumat Surg. 1973;16:792-794.
42. Tomita K., Nomura S., Umeda S., et al. Cervical laminoplasty to enlarge the spinal canal in multilevel ossification of the posterior longitudinal ligament with myelopathy. Arch Orthop Trauma Surg. 1988;107:148-153.
43. Kurokawa T. Enlargement of the spinal canal by sagittal splitting of spinal processes. Bessatsu Seikeigeka. 1982;2:234-240.
44. Satomi K., Nishu Y., Kohno T., et al. Long-term follow-up studies of open-door expansive laminoplasty for cervical stenotic myelopathy. Spine (Phila Pa 1976). 1994;19:507-510.
45. Nakano N., Nakano T., Nakano K. Comparison of the results of laminectomy and open-door laminoplasty for cervical spondylotic myeloradiculopathy and ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 1988;13:792-794.
46. Itoh T., Tsuji H. Technical improvements and results of laminoplasty for compressive myelopathy in the cervical spine. Spine (Phila Pa 1976). 1985;10:729-736.
47. Tsuzuki N., Abe R., Saiki K., et al. Extradural tethering effect as one mechanism of radiculopathy complicating posterior decompression of the cervical spinal cord. Spine (Phila Pa 1976). 1996;21:203-211.
48. Lee T.T., Green B.A., Gromelski E.B. Safety and stability of open-door cervical expansive laminoplasty. J Spinal Disord. 1998;11:12-15.
49. Mochida J., Nomura T., Chiba M., et al. Modified expansive open-door laminoplasty in cervical myelopathy. J Spinal Disord. 1999;12:386-391.
50. O’Brien M.F., Peterson D., Casey A.T., et al. A novel technique for laminoplasty augmentation of spinal canal area using titanium miniplate stabilization: a computerized morphometric analysis. Spine (Phila Pa 1976). 1996;21:474-483. discussion 484
51. Shikata J., Yamamuro T., Shimizu K., et al. Combined laminoplasty and posterolateral fusion for spinal canal surgery in children and adolescents. Clin Orthop Relat Res. 1990;259:92-99.
52. Baisden J., Voo L.M., Cusick J.F., et al. Evaluation of cervical laminectomy and laminoplasty: a longitudinal study in the goat model. Spine (Phila Pa 1976). 1999;24:1283-1288. discussion 1288-1289
53. Fields M.J., Hoshijima K., Feng A.H., et al. A biomechanical, radiologic, and clinical comparison of outcome after multilevel cervical laminectomy or laminoplasty in the rabbit. Spine (Phila Pa 1976). 2000;25:2925-2931.
54. Matsunaga S., Sakou T., Nakanisi K. Analysis of the cervical spine alignment following laminoplasty and laminectomy. Spinal Cord. 1999;37:20-24.
55. Bouchard J.A., Bohlman H.H., Biro C. Intraoperative improvements of somatosensory evoked potentials: correlation to clinical outcome in surgery for cervical spondylitic myelopathy. Spine (Phila Pa 1976). 1996;21:589-594.
56. Calancie B., Harris W., Broton J.G., et al. “Threshold-level” multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tracts: description of method and comparison to somatosensory evoked potential monitoring. J Neurosurg. 1998;88:457-470.
57. Edwards C.C.2nd, Heller J.G., Silcox D.H.3rd. T-saw laminoplasty for the management of cervical spondylotic myelopathy: clinical and radiographic outcome. Spine (Phila Pa 1976). 2000;25:1788-1794.
58. Macdonald R.L., Fehlings M.G., Tator C.H., et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86:990-997.
59. Yonenobu K., Hosono N., Iwasaki M., et al. Neurologic complications of surgery for cervical compression myelopathy. Spine (Phila Pa 1976). 1991;16:1277-1282.
60. Baba H., Maezawa Y., Furusawa N., et al. Flexibility and alignment of the cervical spine after laminoplasty for spondylotic myelopathy: a radiographic study. Int Orthop. 1995;19:116-121.
61. Hirabayashi K., Toyama Y., Chiba K. Expansive laminoplasty for myelopathy in ossification of the longitudinal ligament. Clin Orthop Relat Res. 1999;359:35-48.
62. Kawaguchi Y., Kanamori M., Ishihara H., et al. Minimum 10-year followup after en bloc cervical laminoplasty. Clin Orthop Relat Res. 2003;411:129-139.
63. Kimura I., Shingu H., Nasu Y. Long-term follow-up of cervical spondylotic myelopathy treated by canal-expansive laminoplasty. J Bone Joint Surg [Br]. 1995;77:956-961.
64. Wada E., Suzuki S., Kanazawa A., et al. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy: a long-term follow-up study over 10 years. Spine (Phila Pa 1976). 2001;26:1443-1447. discussion 1448
65. Hosono N., Yonenobu K., Ono K. Neck and shoulder pain after laminoplasty: a noticeable complication. Spine (Phila Pa 1976). 1996;21:1969-1973.
66. Ratliff J.K., Cooper P.R. Cervical laminoplasty: a critical review. J Neurosurg. 2003;98(Suppl 3):230-238.
67. Seichi A., Takeshita K., Ohishi I., et al. Long-term results of double-door laminoplasty for cervical stenotic myelopathy. Spine (Phila Pa 1976). 2001;26:479-487.
68. Hidai Y., Ebara S., Kamimura M., et al. Treatment of cervical compressive myelopathy with a new dorsolateral decompressive procedure. J Neurosurg. 1999;90(Suppl 2):178-185.
69. Fujimura Y., Nishi Y., Chiba K., et al. Multiple regression analysis of the factors influencing the results of expansive open-door laminoplasty for cervical myelopathy due to ossification of the posterior longitudinal ligament. Arch Orthop Trauma Surg. 1998;117:471-474.
70. Chiba K., Toyama Y., Matsumoto M., et al. Segmental motor paralysis after expansive open-door laminoplasty. Spine (Phila Pa 1976). 2002;27:2108-2115.
71. Sakaura H., Hosono N., Mukai Y., et al. Segmental motor paralysis after cervical laminoplasty: a prospective study. Spine (Phila Pa 1976). 2006;31:2684-2688.
72. Morimoto T., Okuno S., Nakase H., et al. Cervical myelopathy due to dynamic compression by the laminectomy membrane: dynamic MR imaging study. J Spinal Disord. 1999;12:172-173.
73. Chiba K., Ogawa Y., Ishii K., et al. Long-term results of expansive open-door laminoplasty for cervical myelopathy: average 14-year follow-up study. Spine (Phila Pa 1976). 2006;31:2998-3005.
74. Iwasaki M., Okuda S., Miyauchi A., et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament. Part 1: clinical results and limitations of laminoplasty. Spine (Phila Pa 1976). 2007;32:647-653.
75. Ogawa Y., Toyama Y., Chiba K., et al. Long-term results of expansive open-door laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine. J Neurosurg Spine. 2004;1:168-174.
76. Fujiyoshi T., Yamazaki M., Kawabe J., et al. A new concept for making decisions regarding the surgical approach for cervical ossification of the posterior longitudinal ligament: the K-line. Spine (Phila Pa 1976). 2008;33:E990-E993.
77. Kimura S., Homma T., Uchiyama S., et al. Posterior migration of cervical spinal cord between split laminae as a complication of laminoplasty. Spine (Phila Pa 1976). 1995;20:1284-1288.
78. Shiozaki T., Otsuka H., Nakata Y., et al. Spinal cord shift on magnetic resonance imaging at 24 hours after cervical laminoplasty. Spine (Phila Pa 1976). 2009;34:274-279.
79. Shaffrey C.I., Wiggins G.C., Piccirilli C.B., et al. Modified open-door laminoplasty for treatment of neurological deficits in younger patients with congenital spinal stenosis: analysis of clinical and radiographic data. J Neurosurg. 1999;90(4 Suppl):170-177.
80. Baba H., Uchida K., Maezawa Y., et al. Three-dimensional computed tomography for evaluation of cervical spinal canal enlargement after en bloc open-door laminoplasty. Spinal Cord. 1997;35:674-679.
81. Sodeyama T., Goto S., Mochizuki M., et al. Effect of decompression enlargement laminoplasty for posterior shifting of the spinal cord. Spine (Phila Pa 1976). 1999;24:1527-1531. discussion 1531–1532
82. Morio Y., Yamamoto K., Teshima R., et al. Clinicoradiologic study of cervical laminoplasty with posterolateral fusion or bone graft. Spine (Phila Pa 1976). 2000;25:190-196.
Laminoplasty: French Door
Cervical laminoplasty has become the treatment of choice for CSM in many regions. This procedure has been recommended for CSM and ossification of the posterior longitudinal ligament. The rationale behind laminoplasty is related to its ability to prevent kyphosis and instability, postlaminectomy membrane formation, arachnoiditis, and restenosis.1 The main goal of laminoplasty is to enlarge the spinal canal and, in turn, increase the cross-sectional area of the spinal cord, while maintaining stability. In 1972, Hattori introduced Z-plasty, which has not been widely accepted because of the technical difficulties of the operation.2 Open-door laminoplasty was first described in 1978.3 Laminoplasty by splitting the spinous processes (French-door laminoplasty) was described in 1982.4
Today, three fundamental laminoplasty procedures are commonly used: Z-plasty; hemilateral open-door laminoplasty; and bilateral open-door laminoplasty (mid-dorsal laminoplasty or French-door laminoplasty; Fig. 221-5). Several additional modifications have been reported. In one study comparing three modifications of laminoplasty, no difference in outcomes between the modifications was demonstrated.5
This section describes laminoplasty by midline enlargement, also termed Kurokawa’s method or French-door laminoplasty. Kurokawa et al.4 reported their experience in 19 patients with cervical myelopathy. The clinical results were assessed as very effective in six patients, effective in nine, improved in two, and unchanged in two; none was worse. Kyphotic changes were found in six patients in whom interlaminar fusion was not performed.
Discussion
In the presence of an effective lordosis, cervical laminoplasty may be indicated in multilevel OPLL, congenital spinal canal stenosis, multilevel cervical stenosis, and dorsal ligamentous hypertrophy, and as part of a staged ventral/dorsal spinal canal expansion procedure.6 Most studies of laminoplasty for CSM and ossification of the posterior longitudinal ligament have reported postoperative enlargement of the spinal canal and an improved neurologic outcome. However, several recent studies suggest that there may be no significant postoperative difference between laminectomy and laminoplasty regarding decompression, neurologic recovery, kyphosis, and instability.7,8 Laminoplasty was found to provide increased stability, with less translation, tilting, and range of motion, compared with laminectomy.9 Comparing the results of 28 cases (10 laminectomy and 18 laminoplasty with 5-year follow-up), Hukuda et al.7 did not find laminoplasty to be superior to laminectomy in CSM for functional recovery and enlargement of the epidural space. There was no difference in the occurrence of kyphosis or instability. They reported a reduction in neck extension in patients who underwent laminoplasty. Naito et al.5 compared the results of hemilateral open-door laminoplasty, bilateral open-door laminoplasty, and Z-plasty and found no difference among the three modifications. Yoshida et al.10 reported 50% limitation in flexion and extension of the neck after laminoplasty. On the other hand, Tsuzuki et al.11 reported radiculopathies after laminoplasty procedures. They hypothesized that this was caused by extradural nerve root tethering. Sakaura et al.,12 in a review of the incidence of C5 nerve root palsy after decompression surgery for CSM, found that C5 palsy occurred in 4.6% of patients after surgery for CSM, with no statistical differences between unilateral hinge laminoplasty and French-door laminoplasty. Patients with postoperative C5 palsy generally have a good prognosis for functional recovery, but the severely paralyzed cases required significantly longer recovery times than the mild cases.
Takeuchi and Yasuhiro13 evaluated the importance of preserving the C7 spinous process and attached nuchal ligament in French-door laminoplasty to reduce postoperative axial symptoms. They studied 41 patients and divided them into 2 groups, those in whom the whole structure of the C7 spinous process and nuchal ligament was preserved, and those in whom it was not. Significant differences were noted in the visual analogue pain scale questionnaire at 1- and 2-year follow-up, indicating the presence of fewer postoperative axial symptoms in the group in whom these structures were preserved intraoperatively. Recently, Okada et al.14 directly compared clinical outcomes in patients treated with open-door versus French-door laminoplasty and found perioperative complications occurred more frequently in the former than in the latter procedure. In this study, Japanese Orthopaedic Association (JOA) scores and recovery rates suggested that both procedures could be similarly effective in decompressing the spinal cord; however, axial pain was improved in French-door laminoplasty but became worse in open-door laminoplasty, suggesting that French-door laminoplasty could be more beneficial than open-door laminoplasty for patients with CSM. Although all laminoplasty techniques have been used for the prevention of kyphosis and instability, several authors have reported a rate of kyphosis development of up to 28% with the various different types of laminoplasty procedures.7 Hence, in view of the foregoing, French-door laminoplasty as a treatment for cervical stenosis continues to be controversial.
Hirabayashi H., Satomi K. Expansive open-door laminoplasty. In: Denaro V., editor. Stenosis of the cervical spine: causes, diagnosis and treatment. Berlin: Springer-Verlag; 1991:264-278.
Koshu K., Tominaga T., Yoshimoto T. Spinous process splitting laminoplasty with an extended foraminotomy for cervical myelopathy. Neurosurgery. 1995;17:430-435.
Kurokawa T., Tsuyama N., Tanaka H., et al. Enlargement of the spinal canal by sagittal splitting of the spinous processes [in Japanese]. Bessatsu Seikeigeka. 1982;2:234-240.
Naito M., Ogata K., Kurose S., et al. Canal-expansive laminoplasty in 83 patients with cervical myelopathy: a comparative study of three different procedures. Int Orthop. 1994;18:347-351.
Nakano N., Nakano T., Nakano K. Comparison of the results of laminectomy and open-door laminoplasty for cervical spondylotic myeloradiculopathy and ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 1988;13:792-794.
Okada M., Minamide A., Endo T., et al. A prospective randomized study of clinical outcomes in patients with cervical compressive myelopathy treated with open-door or French-door laminoplasty. Spine (Phila Pa 1976). 2009;34:1119-1126.
1. Koshu K., Tominaga T., Yoshimoto T. Spinous process splitting laminoplasty with an extended foraminotomy for cervical myelopathy. Neurosurgery. 1995;17:430-435.
2. Raynor R.B., Pugh J., Shapiro I. Cervical facetectomy and its effect on spine strength. J Neurosurg. 1985;63:278-282.
3. Hirabayashi H., Satomi K. Expansive open-door laminoplasty. In: Denaro V., editor. Stenosis of the cervical spine: causes, diagnosis and treatment. Berlin: Springer-Verlag; 1991:264-278.
4. Kurokawa T., Tsuyama N., Tanaka H., et al. Enlargement of the spinal canal by sagittal splitting of the spinous processes [in Japanese]. Bessatsu Seikeigeka. 1982;2:234-240.
5. Naito M., Ogata K., Kurose S., et al. Canal-expansive laminoplasty in 83 patients with cervical myelopathy: a comparative study of three different procedures. Int Orthop. 1994;18:347-351.
6. O’Brien M.F., Peterson D., Casey A.T.H., et al. A novel technique for laminoplasty augmentation of spinal canal area using titanium miniplate stabilization: a computerized morphometric analysis. Spine (Phila Pa 1976). 1996;21:474-484.
7. Hukuda S., Ogata M., Mochizuki T., et al. Laminectomy versus laminoplasty for cervical myelopathy: brief report. J Bone Joint Surg [Br]. 1988;70:325-326.
8. Nakano N., Nakano T., Nakano K. Comparison of the results of laminectomy and open-door laminoplasty for cervical spondylotic myeloradiculopathy and ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 1988;13:792-794.
9. Zdeblick T.A., Abitbol J.J., Kunz D.N., et al. Cervical stability after sequential capsule resection. Spine (Phila Pa 1976). 1993;18:2005-2008.
10. Yoshida M., Otani K., Shibasaki K., et al. Expansive laminoplasty with reattachment of spinous process and extensor musculature for cervical myelopathy. Spine (Phila Pa 1976). 1992;17:491-497.
11. Tsuzuki N., Abe R., Saiki K., et al. Extradural tethering effect as one mechanism of radiculopathy complicating posterior decompression of the cervical spinal cord. Spine (Phila Pa 1976). 1996;21:203-211.
12. Sakaura H., Hosono N., Mukai Y., et al. C5 palsy after decompression surgery for cervical myelopathy: review of the literature. Spine (Phila Pa 1976). 2003;28:2447-2451.
13. Takeuchi T., Yasuhiro S. Importance of preserving the C7 spinous process and attached nuchal ligament in French-door laminoplasty to reduce postoperative axial symptoms. Eur Spine J. 2007;16:1417-1422.
14. Okada M., Minamide A., Endo T., et al. A prospective randomized study of clinical outcomes in patients with cervical compressive myelopathy treated with open-door or French-door laminoplasty. Spine (Phila Pa 1976). 2009;34:1119-1126.
Laminectomy
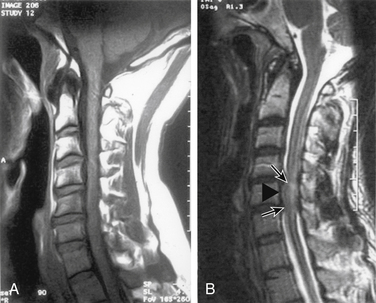
Tsukimoto first reported ossification of the posterior longitudinal ligament (OPLL) in 1960.1 The disease process begins as calcification followed by ossification of the posterior longitudinal ligament, beginning rostrally and extending caudally.2 The disease extends across vertebral bodies, spanning the intervening disc spaces.3,4
Initially, there is a proliferation of cartilaginous cells in the periosteum.5 Rostral and caudal expansion occurs and incorporates bony maturation and posterior longitudinal ligament vascular fibrosis. Calcified foci coalesce and the ossification process further matures with the production of active marrow.4,5
OPLL has been classified according to imaging characteristics.6 The classifications include continuous, segmental, mixed, and other. Continuous OPLL includes those cases in which the process extends across intervening vertebral bodies and includes the disc spaces. Segmental OPLL includes those cases in which the process is solely behind the involved vertebral body or bodies and does not extend across the intervertebral disc. Mixed OPLL includes elements of both continuous and segmental OPLL. At times, the disease process is located dorsal to the disc space, similar to a calcified herniated disc; such cases may be classified in the “other” category.
Initially, patients may be asymptomatic. As the disease progresses, myelopathy or radiculopathy develops, with myelopathy seen much more often than radiculopathy. Sphincter disturbances are rarely seen.4,7 It is rare for a patient to present with cervical pain alone.
Patients who are asymptomatic or have mild symptoms and nonprogressive disease may be initially managed conservatively. Those with myelopathy, myeloradiculopathy, or progressive disease should be treated with surgery. Better results have been observed in those patients operated on at a younger age and in those with a better myelopathy score.1 In general, surgical options include ventral cervical corpectomy, ventral cervical discectomy, laminectomy with or without fusion, and the multiple variants of laminoplasty. Each operation has its own relative indications, contraindications, and complications.
Indications
The indications for laminectomy are relative and may be surgeon specific. Most important is the preoperative spinal alignment. Laminectomy is indicated for those patients with an effective cervical lordosis. It may also be used cautiously for those with a straightened spine (see later discussion). Patients with multisegment involvement (i.e., three or more levels) may be more effectively managed with laminectomy than with a ventral approach.4 Older patients or those with medical comorbidities may benefit more from laminectomy compared with an extensive ventral procedure. This is related to the shorter operative time and lower morbidity of laminectomy compared with extensive ventral corpectomy. Finally, in patients with severe spinal stenosis accompanying OPLL, laminectomy may be indicated before performing ventral surgery.8
Defining Cervical Alignment
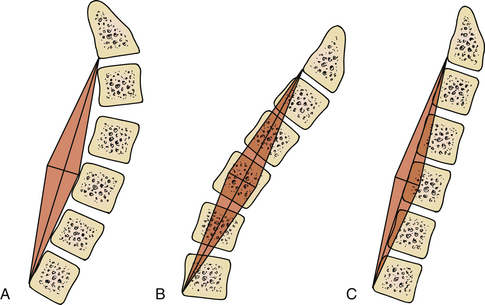
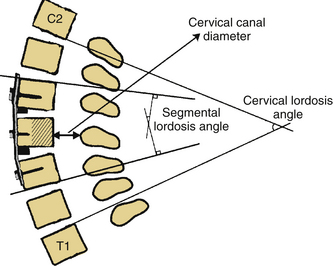
As previously mentioned, in our opinion the most important consideration for laminectomy is spinal alignment. Those patients with an effective cervical lordosis should undergo laminectomy, those with an effective cervical kyphosis should undergo ventral corpectomy or discectomy, whereas those with a straightened spine may be decompressed with either laminectomy or a ventral decompressive procedure. A midline lateral cervical radiograph or sagittal MRI may be used to determine spinal alignment.9 A vertical line is drawn from the dorsocaudal aspect of the C2 vertebral body to the dorsocaudal aspect of C7. A horizontal line is then drawn at the midpoint of the vertical line. The extent of this horizontal line depends on the surgeon’s preferences and biases. The lines drawn describe a shape similar to a kite, and will be referred to as such. If the dorsal aspects of the cervical vertebral bodies lie ventral to the kite, then the spine is in effective cervical lordosis. If the dorsal aspects of the cervical vertebral bodies lie dorsal to the kite, the spine is in effective cervical kyphosis. A “gray zone” exists when the dorsal aspects of the cervical vertebral bodies lie within the kite. In this circumstance, the spine is straight, and either a ventral or dorsal procedure may be performed (Fig. 221-6). Gwinn et al.10 recently evaluated this simple straight-line method of determining effective cervical lordosis and found it was superior to traditional methods used to make angular measurements of sagittal cervical alignment (e.g., Cobb angle). The traditional methods often failed to take into account ventral obstructions such as dorsally projecting osteophytes or spondylolisthesis, which may force the approach to be altered to a ventral decompression.
Procedure
A few features of the procedure specific to OPLL should be mentioned. Ossification frequently spreads; it was seen in 70% of cases in the series reported by Kato et al.1 In their series, deterioration due to spread of ossification was seen in paucilevel laminectomies (e.g., three-level laminectomy). Therefore, more extensive laminectomy is recommended in the management of OPLL, even when the disease is somewhat limited. The decompression should usually include C3-7, or at a minimum one to one-and-one-half segments above and below the radiographically abnormal segments. Because of the risk of postlaminectomy kyphosis, lateral resection should not incorporate more than the medial 25% of the facets on either side.11
Sectioning of the dentate ligaments has been advocated by some,12 but not by others.13 The benefit of sectioning the dentate ligaments is unclear and it necessitates opening the dura, which exposes the patient to the inherent risks of this procedure, such as cerebrospinal fluid leak and spinal cord injury. Therefore, we do not recommend this procedure.
Outcome
Outcome is adversely affected by older age and more severe myelopathy at the time of decompression.1 Therefore, we recommend earlier surgery for patients with OPLL.
Miyazaki and Kirita12 performed laminectomy on 155 patients with OPLL with at least 1 year of follow-up . Three patients developed quadriplegia after the procedure, whereas six demonstrated only transient quadriplegia during the first 24 hours after surgery. Functional outcome was evaluated by the Japanese Orthopaedic Association (JOA) score. Overall, 81.9% of the patients showed some improvement. Specifically, 36.8% were rated as excellent, 18.1% good, 27.1% fair, 7.1% unchanged, and 11% poor. Seventeen percent of the patients demonstrated postoperative kyphosis or a worsening of their kyphotic deformity. It was not reported if the new or worsened spinal alignment negatively affected their neurologic status.
Similar results were reported by Nakano et al.14 The authors performed laminectomy on 14 patients with OPLL and measured their outcomes using the JOA score. Overall, 81.1% of the patients demonstrated some improvement.
Complications
Spinal cord injury may be seen in up to 10% of cases, whereas nerve root injury has been seen in up to 12.8% of cases.15–17 Spinal cord injury is most often directly related to surgery and should be minimized by meticulous attention to detail. Most important, perioperative and intraoperative hypotension should be avoided. This may result is cord ischemia and quadriplegia. The spinal cord may also be injured during surgical decompression, that is, when placing instruments under the laminae. This risk is mitigated by careful choice and usage of instruments. The C5 nerve root may be injured during the extensive cervical laminectomy required for decompression of OPLL. This is most likely due to tethering of the root during dorsal migration of the spinal cord after decompression,15,16 and the deficit is usually temporary.
Postlaminectomy scar formation and kyphosis may develop, which has led to the development and widespread use of laminoplasty. The exact incidence of postlaminectomy kyphosis is unknown, but may be as high as 21%.18 Miyazaki and Kirita12 noted that 17% of their patients developed either postoperative kyphosis or a worsening of their existing deformity. Although kyphosis and scar formation have been demonstrated after laminectomy, their clinical sequelae are unknown and often do not have a negative effect on the patient’s neurologic status.12
Conclusions
Laminoplasty has been advocated as an alternative to laminectomy. The incidences of postoperative cervical deformity and scar formation with laminoplasty are claimed to be lower than with laminectomy. Despite these claims, there are some downsides to laminoplasty worth mentioning. Adequate decompression may be not achieved on the “hinged side” of the laminoplasty. Furthermore, the hinged laminae may fracture through the inner cortex, actually worsening the compression.13 Although increased kyphosis may be seen after laminectomy, it does not appear to have a negative impact on patient neurologic status. Nakano et al.14 compared laminectomy with laminoplasty for the management of OPLL and found that the outcome as measured on the JOA scale was no different between the two groups.
Gwinn D.E., Iannotti C.A., Benzel E.C., et al. Effective lordosis: analysis of sagittal spinal canal alignment in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:667-672.
Hirabayashi K., Watanabe K., Wakano K., et al. Extensive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine (Phila Pa 1976). 1983;8:693-699.
Miyazaki K., Kirita Y. Extensive simultaneous multisegment laminectomy for myelopathy due to ossification of the posterior longitudinal ligament in the cervical region. Spine (Phila Pa 1976). 1986;11:31-42.
Yonenobu K., Hosono N., Iwasaki M., et al. Neurologic complications of surgery for cervical compression myelopathy. Spine (Phila Pa 1976). 1991;16:1277-1282.
1. Kato Y., Iwasaki M., Fuji T., et al. Long-term follow-up results of laminectomy for cervical myelopathy caused by ossification of the posterior longitudinal ligament. J Neurosurg. 1998;89:217-223.
2. Epstein N. Ossification of the posterior longitudinal ligament. In: Benzel E.C., editor. Spine surgery: techniques, complication avoidance, and management. New York: Churchill Livingstone; 1999:489-502.
3. Hiramatsu Y., Nobechi T. Calcification of the posterior longitudinal ligament of the spine among Japanese. Radiology. 1971;100:307-312.
4. McAfee P.C., Regan J.J., Bohlman H.H. Cervical cord compression from ossification of the posterior longitudinal ligament in non-Orientals. J Bone Joint Surg [Br]. 1987;69:569-575.
5. Kojima T., Waga S., Kubo Y., et al. Anterior cervical vertebrectomy and interbody fusion for multi-level spondylosis and ossification of the posterior longitudinal ligament. Neurosurgery. 1989;24:864-872.
6. Hirabayashi K., Watanabe K., Wakano K., et al. Extensive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine (Phila Pa 1976). 1983;8:693-699.
7. Lee T., Chacha P.B., Khoo J. Ossification of posterior longitudinal ligament of the cervical spine in non-Japanese Asians. Surg Neurol. 1991;35:40-44.
8. Itoh T., Tsuji H. Technical improvements and results of laminoplasty for compressive myelopathy in the cervical spine. Spine (Phila Pa 1976). 1985;10:729-736.
9. Benzel E.C. Degenerative and inflammatory diseases of the spine. In Biomechanics of spine stabilization, ed 2. Rolling Meadows, IL: American Association of Neurologic Surgeons; 2001. pp 45–60
10. Gwinn D.E., Iannotti C.A., Benzel E.C., et al. Effective lordosis: analysis of sagittal spinal canal alignment in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:667-672.
11. Raynor R.B., Pugh J., Shapiro L. Cervical facetectomy and its effect on spine strength. J Neurosurg. 1985;63:278-282.
12. Miyazaki K., Kirita Y. Extensive simultaneous multisegment laminectomy for myelopathy due to ossification of the posterior longitudinal ligament in the cervical region. Spine (Phila Pa 1976). 1986;11:31-42.
13. Epstein N. Posterior approaches in the management of cervical spondylosis and ossification of the posterior longitudinal ligament. Surg Neurol. 2002;58:194-208.
14. Nakano N., Nakano T., Nakano K. Comparison of the results of laminectomy and open-door laminoplasty for cervical spondylotic myeloradiculopathy and ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 1988;13:92-94.
15. Dai L., Ni B., Yuan W., et al. Radiculopathy after laminectomy for cervical compression myelopathy. J Bone Joint Surg [Br]. 1998;80:846-849.
16. Saunders R.L., Pikus H.J., Ball P. Four-level cervical corpectomy. Spine (Phila Pa 1976). 1998;23:2455-2461.
17. Yonenobu K., Hosono N., Iwasaki M., et al. Neurologic complications of surgery for cervical compression myelopathy. Spine (Phila Pa 1976). 1991;16:1277-1282.
18. Kaptain G.J., Simmons N., Replogle R.E., et al. Incidence and outcome of kyphotic deformity following laminectomy for cervical spondylotic myelopathy. J Neurosurg. 2000;93(Suppl 2):199-204.
Ventral Approach: Smith-Robinson Technique
Rationale for Performing a Ventral Approach
A ventral approach to the cervical spine is favored by many surgeons because it provides excellent exposure, has a good cosmetic result, and has low rates of complication and discomfort. The ventral discectomy and fusion procedure done initially by Smith and Robinson involves removal of the cervical disc and placement of a tricortical iliac crest graft. Their approach focused on fusion and stabilization rather than decompression of the spinal cord. Modifications of the Smith-Robinson approach have since come to include decompression of the disc (discectomy) or vertebral body (corpectomy, which includes the removal of the vertebral body ventrally, as well as the complete removal of the discs above and below the offending level).1–3 Complete excision of the vertebral body provides exposure of the posterior longitudinal ligament (PLL) for decompression along the length of the vertebral body and disc spaces, but the longer the corpectomy, the greater the risk of instability of the construct. Ultimately, the patient’s pathologic process and anatomy should influence selection of the optimal approach.
If a discectomy is performed, a spacer is recommended to maintain disc height, prevent deformity, and maintain foraminal height.4–6 The likelihood of obtaining fusion in the cervical spine increases when using the ventral rather than dorsal approach.7–9 Another benefit of the ventral over the dorsal approach is a potential decrease in blood loss because the surgeon uses the natural dissection planes in ventral loose connective tissue, compared with performing a dorsal subperiosteal dissection of paraspinal muscles.10,11
Of the three surgical procedures (i.e., ventral, dorsal, and ventral combined with dorsal) for patients with ossification of the PLL (OPLL), corpectomy and resection of the OPLL is the most direct approach. Corpectomy is usually indicated for patients who present with less than 50% narrowing of the spinal canal and with three or fewer ossified segments. This is because the bone graft and plate construct becomes more susceptible to rotational torque as the number of levels increases. A dorsal approach such as a laminectomy or laminoplasty can also be considered if a longer multilevel procedure is necessary. Patients with bulky OPLL do not always present with cervical myelopathy.12 However, in cases where OPLL causes spinal cord compression and myelopathy, surgical treatment may dictate ventral removal of the offending structures (osteophytes or the calcified PLL); otherwise, the cord may remain draped over the ventral structure even if a dorsal decompression is performed. Therefore, cases with prominent ventral impingement on the cord that spans over three levels may need a ventral approach for decompression combined with a dorsal fusion for stabilization of the construct.13,14
A ventral approach is favorable in certain situations. Sakai et al. performed a prospective study of 44 patients, half of whom had a ventral decompression and fusion and half a French-door laminoplasty, with 5-year follow-up.15 The ventral group had improved Japanese Orthopaedic Association (JOA) scores and better recovery rates compared with the laminoplasty group, especially for cases with greater than 50% preoperative spinal canal compromise by OPLL or kyphotic alignment of the cervical spine. Progression of OPLL was observed in 5% of the ventral group compared with 50% of the laminoplasty group.
In cases limited to compression at the disc spaces, anterior cervical discectomy and fusion is the standard treatment. This includes treating cases with degenerative cervical disc disease associated with radiculopathy or myelopathy.16,17 The original Smith-Robinson technique for achieving arthrodesis in the cervical spine after discectomy is a successful anterior cervical discectomy and fusion technique for managing OPLL, and remains quite popular.18–21
Smith-Robinson Surgical Technique
After acquisition of intravenous access, induction of general anesthesia, and intubation, the patient is positioned in the supine position with the head in slight extension. At the time of original publication, nasal intubation was used to avoid neck hyperextension. Today, awake fiberoptic intubation, awake positioning, and neuromonitoring, including continuous intraoperative somatosensory evoked potential (SSEP) monitoring, are adjunct techniques that can be used if cord compression is particularly worrisome.22
There is controversy as to which side to approach the ventral cervical spine.23 Arguments supporting a left approach are based primarily on the theoretically increased risk of recurrent laryngeal nerve injury (which could lead to dysphagia and dysphonia) or the presence of a variant nonrecurrent right inferior laryngeal nerve. Arguments supporting a right approach are based on optimized surgeon comfort for right-handed surgeons, less risk of thoracic duct injury, and less esophageal retraction (i.e., the esophagus lies slightly to the left of midline). Although it remains a theoretical risk for operating on the right, there is as yet no clear evidence that the right recurrent laryngeal nerve is at greater risk than the left.24
Modifications to the Smith-Robinson Surgical Technique
Today, there are also several alternatives to the iliac crest bone graft used in the Smith-Robinson technique. Several studies have noted morbidity with harvest of iliac crest graft, including a 29% incidence of chronic pain, with other complications including arterial injury, ureteral injury, herniation, nerve injury, infection, fracture, pelvic instability, cosmetic defects, hematoma, and tumor seeding.25 Alternatives today include but are not limited to fibular ring allograft, a milled bone graft, or a polyetheretherketone (PEEK) cage.
A significant depression or loss of SSEPs can indicate that the segment has been overdistracted and mandates removal and revision of the graft. The ideal graft size is determined as a function of preoperative disc space height, with typical sizes ranging between 6 and 10 mm, which is less than the original Smith-Robinson graft.7 After spacer placement, fixation is usually performed using a plate-and-screw system. This helps limit motion to facilitate fusion. The plate also helps prevent ventral displacement of the spacer.
Bohlman H.H., Emery S.E., Goodfellow D.B., et al. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy: long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg [Am]. 1993;75:1298-1307.
Epstein N.E. The surgical management of ossification of the posterior longitudinal ligament in 43 North Americans. Spine (Phila Pa 1976). 1994;19:664-672.
Iwasaki M., Okuda S., Miyauchi A., et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament. Part 2: advantages of anterior decompression and fusion over laminoplasty. Spine (Phila Pa 1976). 2007;32:654-660.
Macdonald R.L., Fehlings M.G., Tator C.H., et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86:990-997.
Nakanishi T., Mannen T., Toyokura Y., et al. Symptomatic ossification of the posterior longitudinal ligament of the cervical spine: clinical findings. Neurology. 1974;24:1139-1143.
Robinson R.A., Smith G.W. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome [abstract]. Johns Hopkins Hosp Bull. 1955;96:223-224.
1. Macdonald R.L., Fehlings M.G., Tator C.H., et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86:990-997.
2. Mayr M.T., Subach B.R., Comey C.H., et al. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg. 2002;96:10-16.
3. Okada K., Shirasaki N., Hayashi H., et al. Treatment of cervical spondylotic myelopathy by enlargement of the spinal canal anteriorly, followed by arthrodesis. J Bone Joint Surg [Am]. 1991;73:352-364.
4. Bohlman H.H., Emery S.E., Goodfellow D.B., et al. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy: long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg [Am]. 1993;75:1298-1307.
5. DePalma A.F., Rothman R.H., Lewinnek G.E., et al. Anterior interbody fusion for severe cervical disc degeneration. Surg Gynecol Obstet. 1972;134:755-758.
6. Emery S.E., Bohlman H.H., Bolesta M.J., et al. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy: two to seventeen-year follow-up. J Bone Joint Surg [Am]. 1998;80:941-951.
7. An H.S., Evanich C.J., Nowicki B.H., et al. Ideal thickness of Smith-Robinson graft for anterior cervical fusion: a cadaveric study with computed tomographic correlation. Spine (Phila Pa 1976). 1993;18:2043-2047.
8. Robinson R.A., Southwick W.O. Surgical approaches to the cervical spine. Instr Course Lect. 1960;17:299-330.
9. Whitesides T.E.J., Kelly R.P. Lateral approach to the upper cervical spine for anterior fusion. South Med J. 1966;59:879-883.
10. An H.S., Xu R. Posterior cervical spine procedures. In: An H.S., Riley L.H.III, editors. An atlas of surgery of the spine. London: Martin Dunitz; 1998:13-54.
11. Maurice-Williams R.S., Dorward N.L. Extended anterior cervical discectomy without fusion: a simple and sufficient operation for most cases of cervical degenerative disease. Br J Neurosurg. 1996;10:261-266.
12. Nakanishi T., Mannen T., Toyokura Y., et al. Symptomatic ossification of the posterior longitudinal ligament of the cervical spine: clinical findings. Neurology. 1974;24:1139-1143.
13. Iwasaki M., Okuda S., Miyauchi A., et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament. Part 2: advantages of anterior decompression and fusion over laminoplasty. Spine (Phila Pa 1976). 2007;32:654-660.
14. Mizuno J., Nakagawa H. Ossified posterior longitudinal ligament: management strategies and outcomes. Spine J. 2006;6(Suppl 6):S282-S288.
15. Sakai K., Okawa A., Takahashi M., et al. 5-year follow-up evaluation of surgical treatment for cervical myelopathy caused by ossification of the posterior longitudinal ligament: a prospective comparative study of anterior decompression and fusion with floating method versus laminoplasty. Spine (Phila Pa 1976). May 2, 2011. [Epub ahead of print]
16. Demircan M.N., Kutlay A.M., Colak A., et al. Multilevel cervical fusion without plates, screws or autogenous iliac crest bone graft. J Clin Neurosci. 2007;14:723-728.
17. Kulkarni A.G., Hee H.T., Wong H.K. Solis cage (PEEK) for anterior cervical fusion: preliminary radiological results with emphasis on fusion and subsidence. Spine J. 2007;7:205-209.
18. Aronson N., Filtzer D.L., Bagan M. Anterior cervical fusion by the Smith-Robinson approach. J Neurosurg. 1968;29:396-404.
19. Robinson R.A. Anterior and posterior cervical spine fusions. Clin Orthop Relat Res. 1964;35:34-62.
20. Robinson R.A., Smith G.W. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome [abstract]. Johns Hopkins Hosp Bull. 1955;96:223-224.
21. Smith G.W., Robinson R.A. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg [Am]. 1958;40:607-624.
22. Epstein N.E. The surgical management of ossification of the posterior longitudinal ligament in 43 North Americans. Spine (Phila Pa 1976). 1994;19:664-672.
23. Kilburg C., Sullivan H.G., Mathiason M.A. Effect of approach side during anterior cervical discectomy and fusion on the incidence of recurrent laryngeal nerve injury. J Neurosurg Spine. 2006;4:273-277.
24. Ghanayem A.J. Dysphagia and dysphonia after anterior cervical spine surgery. Contemp Spine Surg. 2003;4:89-93.
25. Fernyhough J.C., Schimandle J.J., Weigel M.C., et al. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine (Phila Pa 1976). 1992;17:1474-1480.
Ventral Approach: Open-Window Corpectomy
Compressive myelopathy is the most clinically significant problem associated with ossification of the posterior longitudinal ligament (OPLL) and advanced cervical spondylosis. There remains a lack of agreement about the most appropriate method of treatment of such cases. Extensive cervical laminectomy is a frequently used treatment. However, there is an ongoing search for better techniques because many complications, such as spinal instability, progression of spondylotic changes, spinal deformities, and constriction of the dura, are frequently reported.1–6 Researchers, particularly the Japanese, have developed laminoplasty techniques to prevent these complications.7–14 However, such dorsal approaches are often inadequate. This is due to many factors, including the nature of the compression (usually from the ventral side), anchoring effects of the dentate ligaments, and calcification or immobilization of the dura by attachment to the OPLL. Therefore, it has been suggested that a ventral approach may be preferable.15–25
Surgical Technique
Patients are operated under general anesthesia in the supine position. Using a ventral approach, the posterior longitudinal ligament (PLL) is reached after performance of ventral discectomies. A variety of retractors may be used. The retractor I use is a practical and useful alternative to the existing self-retaining retractors designed especially for this operation, particularly for Smith-Robinson fusions.26,27
A high-speed drill is used to drill upward from the connection of the PLL and the caudal surface of the vertebral body, under a surgical microscope. The hole is broadened with the help of the drill, and the OPLL is removed (Fig. 221-7). The same method is then applied to the upper discectomized intervertebral space in the downward direction (Fig. 221-8). Generally, the removal of the dorsal half of the vertebral body is sufficient to achieve an effective decompression. As a result, a space is formed at the dorsal aspect of the intervertebral discs and vertebral body that broadens the spinal canal (to approximately 15 mm; see Fig. 221-8). The removal of the intervertebral discs and the cartilaginous end plates and the creation of a mortise for graft material on both the rostral and caudal surfaces of the vertebral body result in diminished vertebral body height. However, this shortening of the vertebral body height does not influence graft placement. After obtaining an effective decompression, a Smith-Robinson fusion is applied to the intervertebral spaces (Fig. 221-9). In the surgical technique presented here, allograft fibula was used as the graft material. It is also possible to use autogenous iliac crest bone as a graft material. To minimize the incidence of graft dislodgment, the grafts are placed after distraction of the vertebral bodies, so that graft compression is achieved upon relaxation of the distraction. In addition, the grafts are shaped because the ventral height of the graft is a few millimeters (1–3 mm) greater than the height of dorsal surface. This minimizes dorsal dislodgment. Ventral spinal fixation is routinely used to increase the fusion rate.
This OWC technique is particularly useful for moderately advanced cervical spondylosis or OPLL (Fig. 221-10). In general, excellent results are obtained with this technique.28
Rationale
Laminectomy has been accepted as the most appropriate surgical approach to achieve these goals. However, instability and constriction of the dura mater after laminectomy are common complications.1–6
Compression is generally from the ventral aspect of spinal cord in advanced spondylosis and OPLL, except for patients with a congenitally narrow spinal canal. The dura and the PLL are attached and even ossified in severe cases. The dentate ligaments, which connect the spinal cord to the ventral side of the spinal canal, and the nerve roots that bind the dura and the spinal cord ventrally, limit dorsal spinal cord movement. For these reasons, dorsal approaches are usually insufficient to relieve compression from the ventral side of the spinal column.15,17–25
Total OPLL extirpation is currently one of the best surgical techniques to achieve a satisfactory outcome while avoiding the complications secondary to a dorsal approach. With this technique, the ossified annulus, the ossified cartilaginous end plates, and OPLL are totally extirpated after anterior corpectomy.15,17–20,22
The ventral floating technique is another commonly used surgical approach for such cases. This technique frees the OPLL over the dura after corpectomy. Incisions are made to the level of the OPLL at the upper and lower aspects of the affected levels. Thus, the expanded dura pushes the attached OPLL segment inward to the removed vertebral body.20,21,27
Both of these techniques are associated with pseudarthrosis, instability, and dislodgment owing to the use of anterior strut grafts, especially when three or more disc spaces are included in the operation.21,27,29
Biomechanics
The technique described here removes only the dorsal aspect of the vertebral corpus after performance of appropriate ventral microdiscectomies. This provides a wide exposure of the spinal canal, along with the OPLL. After removal of the OPLL, the spinal cord is decompressed. This technique allows the ventral aspect and both lateral portions of the vertebral body to remain intact (Fig. 221-11). The importance of this is obvious with regard to the biomechanics of the spine. Biomechanically, the extent and location of ventral spinal decompression significantly affects spine instability.4 The ventral vertebral body cortex provides a significant advantage with regard to its buttressing effect; it bears axial loads much more effectively than the softer cancellous bone. The OWC technique allows the bone graft to contact the three cortical margins of the vertebral body (two lateral and ventral cortical margins).
Graft Placement
Other advantages of this technique include the ease of appropriate bone graft placement. The location of the ventral interbody bone graft significantly affects the biomechanical efficacy of the construct. Biomechanically, the optimal location of interbody bone graft placement is inline or slightly ventral to the instantaneous axis of rotation if the dorsal elements of the spine are intact.30 The instantaneous axis of rotation is usually located at the junction of the anterior and middle columns of Denis in the nonpathologic spine. The OWC technique thus allows the placement of the interbody bone graft in an optimal location. Obviously, this should result in a substantial increase in axial load-bearing ability.
Multilevel Fixation
Finally, in cases where ventral cervical fixation constructs are applied, this technique provides the option of multilevel fixation (Fig. 221-12). Removal of only the dorsal aspect of the vertebral body permits the surgeon to place screws ventrally (Fig. 221-13). Multilevel fixation distributes the fixation forces over multiple segmental levels. In other words, multilevel fixation applies the “load-sharing” principle.31 Although it is not yet proven experimentally, on a theoretical basis, the distribution of forces decreases the stresses applied to the metal-bone interface at each segmental level. As a result, this minimizes the incidence of construct failure.32 In addition, a rigid ventral construct can act as a fixed moment arm cantilever. In such an application, where two screws are used at each terminus of the implant (Fig. 221-14A), the construct bears axial loads relatively well. However, it does not resist translational forces well. The addition of intermediate points of fixation (Fig. 221-14B) allows the implant to resist these translational forces by using three-point bending forces. Therefore, the additional fixation points not only allow axial loads to be borne more effectively by adding additional points of fixation, but allow for resistance of translational forces. Moreover, a more stable construct is expected to provide a higher fusion rate. In my report,28 a constrained construct with cancellous bone screws was used because this was the only implant available at the time. However, I believe that use of a dynamic construct may be more appropriate because it permits settling.
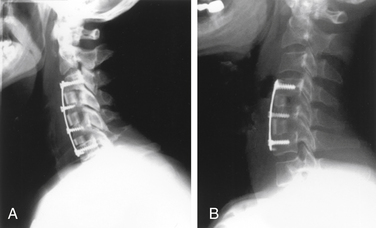
FIGURE 221-12 Case 1 (A) and case 2 (B): Plain lateral radiographs after surgery. Note the construct’s multiple points of fixation.
Controversies
The use of multiple small grafts instead of a long strut graft is controversial. It is well known that an increase in the number of fusion sites decreases the fusion rates.29,33 It has been also reported that placing an internal fixation implant significantly increases the fusion rate.34–36 However, there are no data comparing plated multilevel, multigraft fusion rates with plated multilevel, single-graft rates. The OWC and similar techniques provide improved load sharing between the implant and the vertebral bodies, and a more stable construct (by using three-point bending fixation). Therefore, multiple grafts may be expected to be associated with a high union rate. As a result, problems such as dislodgment, pseudarthrosis, and construct failure may be infrequent with this method. The OWC technique provides a wide decompression (see Fig. 221-13) in a biomechanically sound manner.
Long-Term Results
Long-term results were investigated in 15 patients using both clinical and radiologic outcomes after OWC in cases of advanced cervical spondylosis and/or OPLL. The visual analogue scale scores (pain) and Japanese Orthopaedic Association (JOA) scores (upper extremity function) revealed significant clinical improvement from before surgery to the 15-month time point, and no significant changes from 15 to 92 months.37 In accord with this, MRI and CT assessments revealed improvements in cervical lordosis, segmental lordosis, and cervical spinal canal diameter from before surgery to short-term follow-up, and no significant changes from 15 to 92 months (Fig. 221-15 and Table 221-1).
Ozer et al. also used the method developed by Hilibrand et al.38 to assess the spinal segments adjacent to the operative site in long-term follow-up. All 15 of the patients underwent multilevel surgeries involving OWC, and 5 patients developed adjacent-segment disease (grade II, 4 patients below and 1 patient above the operated region) in the long term. This result is concordant with literature data.38 Patient age was not part of the decision-making process with respect to performing OWC; however, only patients who had anterior compressive cervical spondylosis and/or OPLL were chosen.
Total and conventional corpectomy procedures may lead to complications such as graft or instrumentation failure, pseudarthrosis, neurologic deficits, vocal cord paralysis, and respiratory problems.39,40 On the other hand, OWC is less destructive over the long term than other cervical corpectomy techniques. We also believe that the three-point fixation in OWC (the bone graft contacts both lateral portions and the ventral portion of the intact vertebral body) provides significant support for axial loads. Our long-term findings suggest that OWC is as safe and effective as anterior conventional corpectomy for patients with advanced cervical spondylosis and/or OPLL (Fig. 221-16).
As mentioned, in OWC the inserted bone graft material contacts all three intact cortical margins of the vertebral body (both lateral portions and the ventral portion).28 This is very important with respect to spinal biomechanics because the site and extent of anterior decompression can have a significant impact on spine instability.15 Three-point fixation and the small bone grafts used in OWC mean that there is lower risk of pseudarthrosis and infection compared with conventional corpectomy with strut-graft placement. None of the 15 OWC patients developed problems with pseudarthrosis, infection, chronic inflammation, screw loosening, screw avulsion, or plate breakage in long-term follow-up. However, a single screw broke in two cases and was attributed to incorrect plate selection. A single-screwed plate for each vertebra had been used, but after this problem occurred double-screwed plates were used.
Conclusion
Open-window corpectomy is a safe and effective technique for removing compressive spurs, osteophytes, and OPLL anterior to the cervical spinal cord. Currently, there are no data allowing fusion rates for plated, multilevel, multigraft placement to be compared with fusion rates for plated, multilevel, single-graft placement in patients with advanced cervical spondylosis and/or OPLL. However, OWC creates a more stable construct with three-point fixation and results in better load sharing between implants and healthy vertebrae. Our observations in long-term follow-up after OWC suggest that this is a good surgical option for patients with anterior compressive cervical spondylosis and/or OPLL.
Benzel E.C. Destabilizing effects of spinal surgery. In Biomechanics of spine stabilization: principles and clinical practice. New York: McGraw-Hill; 1995. pp 97–102
Herkowitz H.N. A comparison of anterior cervical fusion, cervical laminectomy and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine (Phila Pa 1976). 1988;13:774-780.
Hilibrand A.S., Carlson G.D., Palumbo M.A., et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg [Am]. 1999;81:519-528.
Kamikozuru M. Significance of the anterior floating method for cervical myelopathy due to the ossification of the posterior longitudinal ligament [in Japanese]. Nippon Seikeigeka Gakkai Zesshi. 1991;65:431-440.
Ozer A.F., Oktenoglu T., Cosar M., et al. Long-term follow-up after open-window corpectomy in patients with advanced cervical spondylosis and/or ossification of the posterior longitudinal ligament. J Spinal Disord Tech. 2009;22:14-20.
Ozer A.F., Oktenoglu T., Sarioglu A.C. A new surgical technique: open window corpectomy in the treatment of ossification of the posterior longitudinal ligament and advanced cervical spondylosis: technical note. Neurosurgery. 1999;45:1481-1486.
Wada E., Suzuki S., Kanazawa A., et al. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy: a long-term follow-up study over 10 years. Spine (Phila Pa 1976). 2001;26:1443-1447.
1. Adams C.B., Logue V. Studies in cervical spondylotic myelopathy: functional effects of operation for cervical spondylotic myelopathy. Brain. 1971;94:587-596.
2. Alvisi C., Borromei A., Cerisoli M., et al. Long-term evaluation of cervical spine disorders following laminectomy. J Neurosurg Sci. 1988;32:109-112.
3. Butler J.C., Whitecloud T.S.3rd. Postlaminectomy kyphosis: causes and surgical management. Orthop Clin North Am. 1992;23:505-511.
4. Cybulski G.R., D’Angelo C.M. Neurological deterioration after laminectomy for spondylotic cervical myoradiculopathy: the putative role of spinal cord ischaemia. J Neurol Neurosurg Psychiatry. 1988;51:717-718.
5. Mikawa Y., Shikata J., Yamamuro T. Spinal deformity and instability after multilevel cervical laminectomy. Spine (Phila Pa 1976). 1987;12:6-11.
6. Miyazaki K., Kirita Y. Extensive simultaneous multisegment laminectomy for myelopathy due to the ossification of the posterior longitudinal ligament in the cervical region. Spine (Phila Pa 1976). 1986;11:531-542.
7. Edwards C.C.2nd, Heller J.G., Silcox D.H.3rd. T-saw laminoplasty for the management of cervical spondylotic myelopathy: clinical and radiographic outcome. Spine (Phila Pa 1976). 2000;25:1788-1794.
8. Hirabayashi K., Satomi K. Operative procedure and results of expansive open door laminoplasty. Spine (Phila Pa 1976). 1988;13:870-876.
9. Hirabayashi K., Watanabe K., Wakano K., et al. Expansive open door laminoplasty for cervical spinal stenotic myelopathy. Spine (Phila Pa 1976). 1983;8:693-699.
10. Koyama T., Handa J. Cervical laminoplasty using apatite beads as implants: experience in 31 patients with compressive myelopathy due to developmental canal stenosis. Surg Neurol. 1985;24:663-667.
11. Nakano K., Harata S., Suetsuna F., et al. Spinous process-splitting laminoplasty using hydroxyapatite spinous process spacer. Spine (Phila Pa 1976). 1992;17:41-43.
12. O’Brien M.F., Peterson D., Casey A.T., et al. A novel technique for laminoplasty augmentation of spinal canal area using titanium miniplate stabilization: a computerized morphometric analysis. Spine (Phila Pa 1976). 1996;21:474-484.
13. Ranawat C.S., O’Leary P., Pellicci P., et al. Cervical spine fusion in rheumatoid arthritis. J Bone Joint Surg [Am]. 1979;61:1003-1010.
14. Saruhashi Y., Hukuda S., Katsuura A., et al. A long term follow-up study of cervical spondylotic myelopathy treated by “French window” laminoplasty. J Spinal Disord. 1999;12:99-101.
15. Baba H., Furusawa N., Tanaka Y., et al. Anterior decompression and fusion for cervical myeloradiculopathy secondary to ossification of posterior ligament. Int Orthop. 1994;18:204-209.
16. Born J.D. Evaluation and treatment of cervical spondylotic myelopathy by subtotal corpectomy without grafting [in French]. Bull Mem Acad R Med Belg. 2000;155:171-179.
17. Cheng W.C., Chang C.N., Lui T.N., et al. Surgical treatment for ossification of the posterior longitudinal ligament of the cervical spine. Surg Neurol. 1994;41:90-97.
18. Epstein N. The surgical management of ossification of the posterior longitudinal ligament in 51 patients. J Spinal Disord. 1993;6:432-455.
19. Hanai K., Fujiyoski F., Kamei K. Subtotal vertebrectomy and spinal fusion for cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1986;11:310-315.
20. Herkowitz H.N. A comparison of anterior cervical fusion, cervical laminectomy and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine (Phila Pa 1976). 1988;13:774-780.
21. Kamikozuru M. Significance of the anterior floating method for cervical myelopathy due to the ossification of the posterior longitudinal ligament [in Japanese]. Nippon Seikeigeka Gakkai Zesshi. 1991;65:431-440.
22. Kojima T., Waga S., Kubo Y., et al. Anterior cervical vertebrectomy and interbody fusion for multi-level spondylosis and ossification of the posterior longitudinal ligament. Neurosurgery. 1989;24:864-872.
23. McAfee P.C., Regan J.J., Bohlman H.H. Cervical cord compression from ossification of the posterior longitudinal ligament in non-Orientals. J Bone Joint Surg [Br]. 1987;69:569-575.
24. Seichi A., Takeshita K., Ohishi I., et al. Long-term results of double-door laminoplasty for cervical stenotic myelopathy. Spine (Phila Pa 1976). 2001;1(26):479-487.
25. Smith G.W., Robinson R.A. The treatment of certain cervical spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg [Am]. 1958;40:607-624.
26. Ozer A.F. A novel retractor for the anterior approach to the cervical spine. Neurol Res. 1999;21:43-44.
27. Shinomiya K., Okamoto A., Kamikozuru M., et al. An analysis of failures in primary cervical anterior spinal cord decompression and fusion. J Spinal Disord. 1993;6:277-288.
28. Ozer A.F., Oktenoglu T., Sarioglu A.C. A new surgical technique: open window corpectomy in the treatment of ossification of the posterior longitudinal ligament and advanced cervical spondylosis: technical note. Neurosurgery. 1999;45:1481-1486.
29. Yamaura I. Anterior decompression for cervical myelopathy caused by ossification of the posterior longitudinal ligament: anterior floating method of OPLL [in Japanese]. Nippon Seikeigeka Gakkai Zesshi. 1996;70:296-310.
30. Benzel E.C. Destabilizing effects of spinal surgery. In Biomechanics of spine stabilization: principles and clinical practice. New York: McGraw-Hill; 1995. pp 97–102
31. Benzel E.C. Qualitative attributes of spinal implants. In Biomechanics of spine stabilization: principles and clinical practice. New York: McGraw-Hill; 1995. pp 135–150
32. Benzel E.C. Spinal fusion. In In Biomechanics of spine stabilization: principles and clinical practice. New York: McGraw-Hill; 1995. pp 103–108
33. Yonenobu K., Fuji T., Ono K., et al. Choice of surgical treatment for multisegmental cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1985;10:710-716.
34. Apfelbaum R.I. Ventral and upper cervical spine fixation techniques. In: Benzel E.C., editor. Spinal instrumentation: neurosurgical topics. Rolling Meadows, IL: American Association of Neurological Surgeons; 1994:63-96.
35. Connolly P.J., Esses S.I., Kostuik J.P. Anterior cervical fusion: outcome analysis of patients fused with and without anterior cervical plates. J Spinal Disord. 1996;9:202-206.
36. O’Shea J.F., Sunderasan N. Use of instrumentation in degenerative disease of the cervical spine. Mt Sinai J Med. 1994;61:248-256.
37. Ozer A.F., Oktenoglu T., Cosar M., et al. Long-term follow-up after open-window corpectomy in patients with advanced cervical spondylosis and/or ossification of the posterior longitudinal ligament. J Spinal Disord Tech. 2009;22:14-20.
38. Hilibrand A.S., Carlson G.D., Palumbo M.A., et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg [Am]. 1999;81:519-528.
39. Wada E., Suzuki S., Kanazawa A., et al. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy: a long-term follow-up study over 10 years. Spine (Phila Pa 1976). 2001;26:1443-1447.
40. Fessler R.G., Steck J.C., Giovanini M.A. Anterior cervical corpectomy for cervical spondylotic myelopathy. Neurosurgery. 1998;43:257-265.