Chapter 88 Management of Lead Systems for Implantable Devices
Techniques and Interventions
Pacemaker and implantable cardioverter-defibrillator (ICD) systems have undergone enormous changes and innovation since the initial invention of the permanent implanted pacemaker.1 Leads have had to keep pace with the constant demand for improvement, and novel products and patients are surviving longer. It is hardly surprising that this rapid innovation and expanded usage increases the risk of dysfunction. This chapter describes the normal and abnormal functions of implanted leads. The incidence, mode of presentation, and treatment of the common lead-related complications, including lead fracture, insulation failure, perforation, dislodgment, and lead infection, are discussed. Finally, the indications, methods, and complications associated with lead extraction are reviewed.
Lead Design
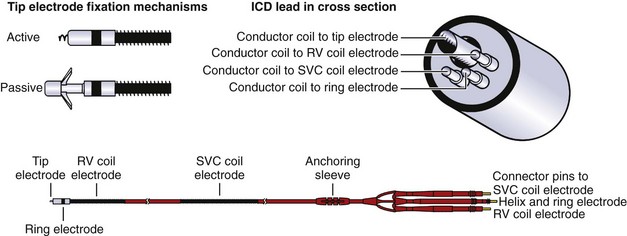
Pacemaker and ICD leads are designed to transmit current from the generator to the myocardium as well as from the myocardium to the generator. The connection is made through a terminal pin electrically continuous with one or more conductor coils, which, in turn, are continuous with the electrodes making contact with the myocardium (Figure 88-1). The lead tip is stabilized at the myocardial interface by an active fixation mechanism such as a helix or a passive mechanism with tines to reduce the risk of lead dislodgment. The number of conductor coils within each lead varies with the function and complexity of the lead. For example, simple unipolar and bipolar pacemaker leads have one and two conductor coils, respectively.
Implant and Related Complications
Perforation
The incidence of symptomatic perforation is 0.1% to 0.8% for pacemakers and 0.6% to 5.2% for ICDs.2,3 However, the incidence of asymptomatic perforation rates found incidentally by computed tomography (CT) scan can be much higher: up to 15% for atrial leads and 6% for ventricular leads.4 Most perforations occur within the first month after implantation; however, they can occur up to 10 months after implantation.5 This risk appears to be higher when leads are inserted into the right ventricular apex or atrial free wall and with smaller diameter leads.2,3,5
Patients with lead perforation may present with symptoms of pleuritic chest pain, diaphragmatic stimulation, and, less frequently, hypotension caused by cardiac tamponade. Interrogation often reveals poor sensing and a high capture threshold with a variable effect on impedance.6 Imaging often reveals a change in the lead tip position compared with the initial chest radiograph. Lead perforation requires emergent lead revision. The authors of this chapter prefer to perform lead revision in the operating room, where access to urgent pericardiocentesis or thoracotomy, if necessary, in the event of tamponade is available. This risk is low, however, and many centers perform lead revision in an implant room without on-site surgical backup.
Dislodgment
Lead dislodgment can occur in up to 2% of ventricular permanent pacemaker leads, 5% of atrial leads and 5% of ICD leads.3,7–9 Dislodgment should be suspected if a significant change in sensed amplitude, lead impedance, or capture threshold occurs, particularly within the first month after lead insertion. Subsequent imaging may show that the lead tip has changed compared with the postprocedure radiograph.10 Most cases of lead dislodgment require surgical revision. In rare cases, the risk of lead revision is prohibitive, and the risk of the lead causing mechanical injury is low. In these cases, programming may satisfactorily resolve abnormal interrogation parameters.
High Defibrillation Threshold
Defibrillation threshold (DFT) testing is performed to assess the minimum energy needed for the device to successfully terminate ventricular fibrillation. The need for DFT testing is still being debated. The authors of this chapter perform DFT testing routinely in patients who have had a cardiac arrest and in cases where circumstances that may impair defibrillation efficacy (e.g., the lead tip is in a nonapical lead position, the generator is on the right side, or the patient is on medications known to increase defibrillation threshold) are present. In the remaining cases, the decision to test is operator dependent and performed in approximately 50% of cases. During the era when DFT testing was routinely performed, DFT was typically less than 10 J.11 An increase in DFT can be caused by lead factors (Table 88-1) or other factors such as patient factors, medications, and recreational drugs.12–24 Lead factors that may increase DFT are lead fracture, reversal of the coil terminal pins in a dual-coil system, and all causes that increase the high-voltage lead impedance.
| LEAD CAUSES |
Lead Performance Monitoring
Lead performance monitoring is essential to ensuring appropriate device function and helps be prepared for lead failure. This begins at device implantation by the documentation of specific lead, patient, and implantation factors (Table 88-2). These factors provide a baseline to compare subsequent interrogations.
Clinical Follow-up
Long-term monitoring of lead performance occurs simultaneously with ICD and permanent pacemaker generator follow-up. The recommended frequency of follow-up varies between ICDs and permanent pacemakers (Table 88-3).25–27 At the authors’ center, patients are seen in the clinic 1 week and 3 months after implantation, regardless of device type, and then every year for pacemakers and every 6 months for ICDs. In addition to in-clinic visits, trans-telephonic monitoring and remote monitoring systems facilitate lead follow-up by transmitting interrogation parameters to the device clinic at scheduled times. This allows the clinician to review the trends in lead performance and to be notified should lead performance deviate from predetermined normal values. Furthermore, should the interrogation parameters deviate from their expected values, the remote monitoring system can automatically transmit this information to the device follow-up team. The authors use remote monitoring systems for patients with leads or generators on advisory, in patients living remote from ready access to a follow-up clinic, and increasingly for all patients as capacity permits.
| PERMANENT PACEMAKER FOLLOW-UP | ICD FOLLOW-UP |
|---|---|
| At implantation | At implantation |
| Within 72 hours of implantation | Within 72 hours of implantation |
| 2–12 weeks following implantation | 2–12 weeks following implantation |
| Every 3–12 months | Every 3–6 months |
ICD, Implantable cardioverter-defibrillator.
Many manufacturers have warning systems that alert the patient to system malfunction in the form of an auditory or vibratory stimulus emitted from the device. These warning systems alert the patient to aberrations in the system function of the device. The primary lead parameter that these alerts monitor is lead impedance. If lead impedance deviates outside the programmable range, the alert is triggered, prompting the patient to seek medical attention.28 At each in-person visit, the pacing or ICD system (generator and lead) is reviewed to ensure normal function. This includes a focused patient history, physical examination, and device interrogation.
Measured Data
In their current iterations, many devices routinely assess lead performance by performing impedance, sensing, and capture threshold tests (Figures 88-2 through 88-4). These tests are typically performed daily and allow interrogation parameters to be tracked over time. At each clinic follow-up, lead performance is confirmed manually to ensure that the values measured daily by the device are valid and not discrepant from those measured automatically. Specifically, evaluation of lead function includes a pacing capture threshold test, impedance measurement, and P-wave and R-wave sensing.
Cardiac Resynchronization Therapy–Specific Follow-up
Cardiac resynchronization therapy (CRT) follow-up is similar to pacemaker and ICD follow-ups. In addition, left ventricular lead threshold should be assessed, anodal stimulation should be excluded, and right/left ventricular timing optimization should be evaluated. In newer devices, one can perform a capture threshold on both right and left ventricular leads individually. The authors find that it is helpful, and often necessary, to perform the capture threshold test with simultaneous 12-lead rhythm strips to determine when each lead fails to capture. Unipolar left ventricular leads use the lead electrode tip as the cathode and the right ventricular proximal ring or right ventricular coil electrode as the anode, depending on whether the lead is a true bipolar lead or an integrated bipolar lead. Occasionally, the anode will also capture the myocardium, as is more frequently seen at higher pacing outputs or with right ventricular leads that are true bipolar or pace/sense leads. This can result in unwanted right ventricular capture in the case of programmed left ventricular only capture or triple-site capture (left ventricular cathodal and right ventricular cathodal and anodal captures) with biventricular pacing. Performing a left ventricular lead capture threshold test with a 12-lead rhythm strip will aid in distinguishing anodal stimulation, pure left ventricular capture, and loss of capture.29
Lead Failure and Related Lead Problems
The incidence of pacemaker lead failure is highly variable. At one end of the spectrum, optimally performing leads fail at a rate of 0.2% to 1.0% per year after 5 to 10 years. In contrast, some leads fail at an unexpectedly high rate of 15% to 35% at 5 years, as high as 5.7% per year.30–32 However, it is reasonable to expect the annual failure rate of modern pacing leads to be less than 0.5%.30,33–35 ICD leads are much more complex, are larger, and thus have an attendant higher “expected” failure rate. In the current generation of ICD leads, the reported average annual failure rate ranges from 0.58% to 3.75%.36–38 The failure rate increases as leads age and can be as high as 20% per year in leads more than 10 years old.38 The estimated overall survival of ICD leads at 2, 5, and 8 years is approximately 91% to 99%, 81% to 95%, and 60% to 95%, respectively (Table 88-4).36–43 Lead design directly affects lead performance. For example, the type of insulation material correlates well with the risk of insulation failure. Although the median time to insulation failure is 7.2 years, this is significantly shorter with polyurethane insulation than with silicone insulation (5.7 years vs. 9.4 years).44
In addition, certain alterations in lead design have adversely affected lead safety and performance. The Accufix (Telectronics Pacing Systems, Engelwood, CO) atrial lead incorporated a preformed J-curved wire into the distal portion of the lead to allow for ease of positioning of the lead tip within the right atrial appendage. Over time, this wire protruded from the insulation resulting in laceration of the right atrium and, rarely, fatalities. In other cases, the J-wire fractured and embolized into the pulmonary circulation.45 Ironically, more patients died during interventions to remove the leads than from consequences of lead malfunction.45
More recently, an apparent improvement in reducing the size of an ICD lead resulted in suboptimal performance with the Sprint Fidelis ICD lead. This lead is a 6.6-F bipolar high-voltage lead, whose distribution was suspended in 2007 because of a higher-than-normal fracture rate.46 Although implantation technique and patient factors (age and ejection fraction) may contribute to lead fracture, the lead’s reduced diameter and construction are presumed to be the primary reasons for the increased risk of fracture.46
The rates and causes of lead failure are estimated on the basis of clinical variables and interrogation parameters. For example, lead fracture is often diagnosed with significant increases in lead impedance in the absence of other causes. Confirmation of the precise cause of lead failure is often not possible, as many leads that fail are either abandoned or damaged during extraction. These inferential observations, however, are often inaccurate in predicting the cause of lead failure and likely overestimate the incidence. When the leads with suspected failure are returned to the manufacturer for analysis, a substantial portion are found not to have the proposed malfunction.36
Mode of Presentation
Most (68%) of the pacemaker lead malfunctions are detected at routine follow-up or during scheduled remote follow-up monitoring. The remaining lead malfunctions are detected at unscheduled clinic visits (21%) or at the time of pulse generator replacement (9.1%).44 The indicators of lead failure include failure to capture on ECG (33%), high pacing threshold (14% to 30%), undersensing (13%), oversensing (12%), high impedance (5%), low impedance (12%), and a combination of capture and sensing abnormalities (13% to 15%).31,44
Patients with ICD lead malfunction most commonly present with inappropriate shocks (33% to 76%) caused by oversensing of intracardiac signals, particularly T-wave oversensing or sensing of artifact.38 The remainder of the patients are found to have an abnormally high-voltage lead impedance (56%), an increased capture threshold (22%), or noise on the lead (11%). These findings are typically discovered at routine testing (24% to 61% of lead problems) or at the time of elective generator replacement (2%).36,38,40,42
Lead Impedance Abnormalities
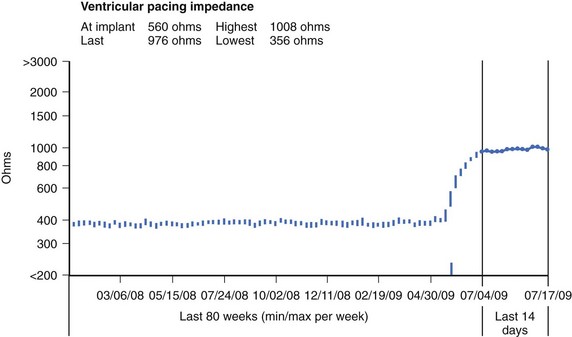
Lead impedance is an estimate of the combined effects of capacitance, resistance, and inductance in opposition to current flow. In simpler terms, it is a measure of stimulation resistance. Lead impedance is affected by lead electrode design (size, configuration, and materials). For example, smaller-diameter leads and bipolar leads tend to have higher impedance. The normal trend in pacemaker or pace/sense lead impedance is to slowly decrease over lead lifespan.47,48 However, lead impedance should not change significantly from visit to visit. A marked change from the previous measurement (i.e., over 200 ohms) is abnormal and warrants careful assessment.
Up until recently, high-voltage ICD lead impedance could only be tested following a shock or at device replacement or implantation. Presently, the high-voltage lead impedance can be measured during routine follow-up interrogation in all ICDs at regular intervals and through remote monitoring systems. This has provided insight into the normal trend and fluctuation in lead impedance of high-voltage leads. Normal high-voltage lead impedance falls slightly in the first few months after lead implantation, after which it can be expected to rise to baseline values within a year of implantation. From visit to visit, fluctuations in ICD lead impedance up to 6 ohms are often seen. However, a change in impedance of over 12 ohms should prompt an investigation, as this is unusual in normally functioning leads.49 Large fluctuations in impedance or aberrations in lead impedance, which are induced by provocative maneuvers at the time of device interrogation, may be caused by problems that manifest intermittently, such as conductor fracture, insulation break, or a loose set screw.
A marked increase in lead impedance in the short term after lead implantation is most likely caused by lead dislodgment, perforation, or an incomplete circuit caused by a header-connector problem, such as a loose connection between the lead and the pulse generator or when the lead is not fully inserted into the header. A rise in lead impedance beyond the early postimplant period may be caused by lead conductor fracture. A marked reduction in lead impedance after the early implant phase is most often caused by an insulation break (see below).41
Output Failure
Failed output occurs when an expected current is not generated at the conductor tip. This can be the result of generator or lead malfunction or more often erroneously suspected in normally functioning devices (Table 88-5). Lead-associated problems that result in failure to output include conductor fracture or a header connector problem, such as incomplete insertion of the lead pin into the connector block, incompatible lead and pulse generator, or a loose set screw.7
| NORMAL DEVICE FUNCTION (PSEUDOFAILURE) |
PVARP, Postventricular atrial refractory period; PVC, premature ventricular contraction; ECG, electrocardiogram.
Abnormalities in Capture Threshold
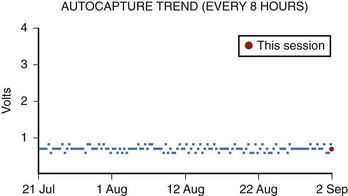
Capture threshold is the minimum amount of current necessary to elicit an evoked potential. The long-term trend in capture threshold varies from lead to lead. Historically, capture threshold would often rise after implantation because of the inflammatory response at the distal electrode. Current leads elute steroid from the distal electrode, typically eliminating this problem. As such, capture threshold should not deviate significantly from baseline over the lead’s lifetime.47,48
Failure to capture may or may not be lead related (Table 88-6). Unrelated causes include intrinsic cardiac disease, metabolic or electrolyte derangement, or medications.7,21,50,51 The most common causes of early lead-related failure to capture are lead dislodgment and lead perforation.52,53 Lead insulation break and conductor fracture are the most common causes of failure to capture later in the follow-up period.7 Local exit block is an uncommon cause of failure to capture and can occur at any time after lead insertion. In the acute setting, this is caused by inflammation at the lead-electrode interface. In the long term, fibrosis develops at the lead tip–myocardium interface, resulting in an associated increase in capture threshold.
Abnormalities in Sensing
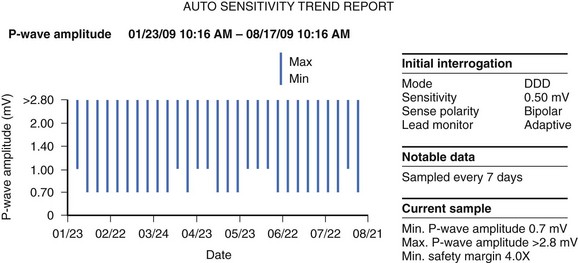
Sensing is the ability of the device to detect intrinsic myocardial depolarization. The long-term R-wave amplitude is predicted by the R wave at first follow-up.47,48,54 Sensing abnormalities can be in the form of undersensing when intrinsic myocardial depolarization is not detected or oversensing when signals that do not represent myocardial depolarization are sensed.
Undersensing can result in inappropriate pacing in both pacemakers and ICDs and in the inappropriate withholding of therapy in ICDs (anti-tachycardia [ATP] and shocks). Undersensing can be caused by patient factors, generator factors, or lead factors or can be erroneously suspected in a normally functioning device (Table 88-7).7,51 Lead causes of undersensing include lead dislodgment, fibrosis at the tip electrode, poor initial lead position, lead insulation defect, and lead conductor coil fracture.7
| LOW-AMPLITUDE ELECTROGRAM |
Oversensing can result in inhibition of pacing in both pacemakers and ICDs and in inappropriate therapy in ICDs (ATP and shocks).10,55 Oversensing can be caused by the inappropriate sensing of physiological signals or artifacts; both can be associated with lead malfunctions (Table 88-8).7,56,57 For example, atrial depolarizations can be sensed on a ventricular lead in cases of insulation failure in the segment of the lead that is within the atrium. Often, the sensing of physiological signals such as P waves, R waves, T waves, or diaphragmatic myopotentials occurs because of lead electrode position and necessitates lead repositioning or replacement. However, certain processes such as hyperkalemia, hyperglycemia, Brugada syndrome, ischemia, and exercise can result in T-wave oversensing that improves with resolution of the underlying cause. Artifact can often be the result of lead malfunction.58–61 For example, a loose set screw, conductor coil fracture, failed insulation between anode and cathode conductors in a bipolar system, interaction between active and abandoned pacing electrodes, and a loose distal fixation helix or screw can all result in sensed make-break signals.62–66
Table 88-8 Causes of Oversensing
| PHYSIOLOGICAL SIGNALS |
| MAKE-BREAK SIGNALS |
The clinical scenario determines the appropriate course of action. After correcting any non–device-related causes of abnormal sensing, such as metabolic abnormalities, and avoiding external sources of artifacts, one can consider lead repositioning or replacement or programming changes. Adjustment of the sensitivity will resolve the sensing issue in many cases. In addition, altering the RV-LV timing may resolve T-wave oversensing in biventricular devices.61,67 In ICD leads, oversensing is particularly troublesome, as decreasing the sensitivity may result in underdetection of ventricular arrhythmias. However, performing defibrillator threshold testing after decreasing the sensitivity will test the detection ability of ventricular arrhythmias and provide a surrogate for the detection ability of clinical arrhythmias. If adjustment of the sensitivity does not provide a sufficient safety margin to ensure appropriate sensing, lead repositioning or replacement may be necessary. Furthermore, lead replacement or revision is necessary if a lead fracture, insulation failure, lead perforation, or lead dislodgment is suspected. Occasionally, the risk of lead repositioning or replacement is prohibitive, and programming does not resolve the sensing problem. Satisfactory noninvasive alternatives may include changing the mode to a triggered or asynchronous mode, particularly if the ventricular lead is affected. Changing to a ventricular-based mode may be a satisfactory solution when atrial leads have abnormal sensing.
Nonphysiological Interval Detection
Modern ICDs have a measure that documents the presence and frequency of nonphysiologically short V-V intervals (typically sensed cycle lengths shorter than 130 ms). This is designed to provide early warning of lead failure and should prompt an investigation or more frequent follow-up. Alerts are typically triggered if frequent short intervals are detected over a brief period.68,69 The cause of nonphysiologically short V-V intervals includes lead fracture, header-connector problem, T wave oversensing followed by a premature beat, and lead dislodgment.26
Lead Fracture
The term lead fracture refers to a fracture in the lead’s conductor coil. Lead fracture rates are typically less than 2% per year, although the incidence with certain leads can be as high as 4%.7,70 Although lead fractures can occur at any time following implantation, the risk increases with age, with the median time to conductor fracture being 5.2 years.44 Lead fractures often occur at stress points such as near the pulse generator, at the venous access site, or at the lead tip, where repetitive motion places stress on the conductor coil.7,44 Certain patient and anatomic factors increase the risk of lead fracture. For example, the risk of lead fracture at the proximal portion of the lead appears to be higher if the angle of the lead is acute, either at the entry point to the header or at the point of venous access.44 A medial venipuncture may trap the lead between the first rib and clavicle, costoclavicular ligament, or subclavius muscle, resulting in subclavian crush.71 Other risk factors for lead fracture include stress on the distal tip at the time of implantation when maneuvering the lead tip to its final position, younger patient age, and lead trauma.7,44,70
Lead fracture may result in undersensing, oversensing, failure to output, or all.7 In ICD leads, lead fractures may also result in inappropriate shocks from sensed artifacts from the fractured lead, often with nonphysiologically short sensed intervals.70,72 If a fracture occurs within the high-voltage conductor coil, the ability to deliver therapy may be compromised.44 In most cases of lead fracture, lead interrogation will show an increase in lead impedance, which may rise transiently or abruptly.72–75 In cases of lead fracture, a new lead must be inserted, unless the lead can be abandoned. Whether the fractured lead should be removed in addition to the insertion of a new lead is still being debated. Replacing a lead carries the risk of lead extraction but may decrease the risk of worsening tricuspid valve regurgitation, oversensing caused by lead-lead interaction, lead infection, and venous thrombosis.76,77 Although cases of inappropriate shocks from oversensing noise caused by lead-lead interaction when the ICD lead is abandoned have been reported, the rate of inappropriate shocks does not appear to be higher than baseline.78 Furthermore, the theoretical increase in DFT caused by shunting of energy to the abandoned ICD lead does not appear to be a common occurrence in clinical practice.78
In ICD leads, the pace or sense conductor coil, the high-voltage conductor coil, or both coils can fail. Opinions regarding the appropriate response to the failure of one component of an ICD lead vary widely. The concern is that other components of the lead may also be at higher risk of failure over time. At present, data to support or refute this concern are insufficient. However, it appears that the risk of high-voltage conductor coil fracture is low in leads with pace or sense conductor coil fracture when only a pace or sense lead was inserted.79
Insulation Failure
Insulation failure accounts for up to 56% of PPM and ICD lead failures.9,36,38,40,42,44 The incidence of insulation failure increases with lead age, with a median time to failure of 9.4 years for polyurethane leads and 5.7 years for silicone leads.44 Breakdown of insulation can occur because of friction that may occur between the generator and the lead within the device pocket or between two leads.13,80 In addition, polyurethane insulation is subject to deterioration caused by metal ion oxidation (MIO), which typically occurs at areas of high mechanical stress, such as the costoclavicular space.7,41
The clinical manifestations of insulation failure depend on the location of the insulation failure and lead design. In unipolar leads, where insulation surrounds the only conductor coil, insulation failure can manifest as complete or intermittent failure of myocardial capture caused by reduced myocardial current delivery as a result of dissipation of current from the conductor coil into the surrounding tissues at the site of failure.7 In addition, insulation break can result in a reduction in the sensed amplitude of myocardial depolarization and, as a result, undersensing. Oversensing of both physiological and nonphysiological events can occur at the site of the insulation failure.7 Lead diagnostics may also show a reduction in lead impedance. The clinical manifestations of insulation failure, however, may be exacerbated or ameliorated by lead orientation. As such, all of these clinical manifestations, including impedance reduction, may be transient and not manifest at the time of the device interrogation.
Lead Infection
Lead infection is difficult to differentiate from generator infection or pocket infection, as the specificity of infectious signs and symptoms for localizing the site of infection is poor. Leads may become infected as a result of extension from pocket infection or when intravascular seeding results in lead colonization. The risk of device infection is 0.68% in the first year and can occur in up to 12.6% of all patients.81,82 Although the majority of device infections occur within 1 year of device implantation or reimplantation (median 52 days), a substantial portion of cases can occur later.81–84 The clinical presentation of device infection manifests as either localized symptoms (69%), systemic symptoms (11%), or both (20%).83 These include fever (29% to 78%), local symptoms including pocket erythema (55%), pocket pain (55%), pocket warmth (23%), draining pocket sinus (42%), pocket swelling (36%), chills (22%), sepsis (fever and tachycardia; 11.5%), malaise (21%), anorexia (11.5%), and nausea (8%).82–84
Positive blood cultures are detected in 81% to 93% of patients with device infection, with the highest yield with tissue biopsy found in pocket infection, or lead cultures with lead infection (63% to 93%), in contrast to blood cultures (51% to 68%).82,83,85–87 The most common organisms cultured are the Staphylococcus species (mostly S. epidermidis and S. aureus).82,85,87,88 Other implicated pathogens include Streptococcus bovis, S. viridens, S. mitis, Enterobacter cloacae, and Klebsiella oxytoca, with a small number of infections being polymicrobial (13%).82,83
Several factors appear to increase the risk of device-related infections. These include immunosuppression (cancer, glucocorticoid therapy, or diabetes mellitus), fever within the 24 hours preceding implantation, temporary pacing before implantation, early reintervention (e.g., for hematoma or lead repositioning), repeated pocket procedures (i.e., replacement or revision), pocket hematoma, and absence of prophylactic antibiotics.81,82 The impact of the number of leads on risk infection is being debated.81,89
Complications associated with lead infection include lead vegetation, pulmonary embolism, and death. Lead vegetations are a rare complication of device-related infection and are often only detected by trans-esophageal echocardiograpy.82 It is essential to distinguish a vegetation, a sterile thrombus, stranding, and a fibrous mass when there is a mass on a lead, as their treatments vary markedly. However, this is often difficult, and the etiology is presumed according to clinical factors. In the authors’ group, it is presumed that a mass is a vegetation in a patient who has clinically suspected pacemaker infection (septicemia, noncardiac manifestations of endocarditis, pocket infection). In contrast, a mass is presumed to be a sterile thrombus, stranding, or a fibrous mass in the absence of infectious symptoms, particularly in patients with a reduced ejection fraction or atrial fibrillation.90 If the etiology of the mass is unclear, typically periodic clinical evaluation and serially imaging with either transthoracic or trans-esophageal echocardiography are conducted and treatment is determined on a case-by-case basis.
Although the risk of pulmonary embolism with lead-related endocarditis is low, it appears higher in patients with vegetations greater than 10 mm in diameter.82 Device-related mortality rates are variable but can be as high as 18% at 6 months.85 Risk factors for mortality in patients with device-related infection are renal insufficiency, positive blood cultures, significant atrioventricular valve regurgitation, right ventricular dysfunction, pulmonary hypertension, and pulmonary or systemic emboli.85
Treatment of Infection
The initial treatment of cellulitis over the device pocket as well as pocket infection is with antibiotics. However, a substantial portion of patients with only local symptoms will have bacteremia or will eventually be found to have intravascular lead infection.83,87 As such, most cases will require complete extraction of the generator and all leads. In clear cases of device infection, attempts to avoid lead extraction by removing the device and débriding the pocket with lead abandonment, cutting leads, and allowing them to retract into the vein or long-term antibiotics have a substantially increased risk of recurrent infection (50%) compared with complete device removal (1%).84,87 An incomplete extraction should only be considered when the risk of lead extraction is higher than that of recurrent infection.
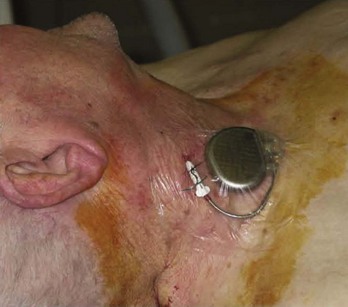
The total duration of therapy and the type of antibiotics will depend on the etiology and severity of infection and usually requires the expertise of an infectious disease specialist. Ideally, device reimplantation can be postponed until no further signs or symptoms of local or systemic infection are present. Following this, the new device should be implanted at a site remote from the original infected pocket to decrease the risk of recurrent infection.84 At the authors’ institution, a semi-permanent pacing system with an externalized active fixation permanent pacing lead attached to a pacemaker is used in dependent patients as a bridge to reimplantation (Figure 88-5).91 This allows the patient freedom of movement and reduces the risk of dislodgment.
Lead Extraction
The number of lead extractions is increasing in parallel with the increased number of device implants and, to some degree, because of expanding indications in patients at increased risks of lead malfunction and infection associated with increased lead age and multiple device replacements.71 The reader is directed to a recent comprehensive review and extraction guideline by the Heart Rhythm Society.92,93 Lead extraction is defined as the removal of a lead more than 1 year after implantation, where the lead removal requires specialized extraction tools or where the lead is removed by a route other than the implanting access site.92 The indications for lead extraction are described in Table 88-9.92 The most common reasons for extraction are infection (54% to 60%), removal of a nonfunctioning or incompatible leads (29% to 40%), and to facilitate device upgrade (8.8%).89,94
| CLASS I INDICATIONS |
The risk of major complications associated with lead extraction is 0.6% to 5.6%.44,95 These include death, superior vena cava (SVC) laceration, tamponade, septic shock, pulmonary or air embolism, tricuspid valve injury, pneumothorax, hemothorax, and hematoma at the pocket type.96 The risk factors for complications are described in Table 88-10.96–99 The presence of intracardiac vegetations increases the risk of pulmonary embolism associated with lead extraction. Most operators will perform transvenous lead extraction when vegetations are <2 cm in diameter.71 Transvenous extraction of vegetations <4 cm in diameter has been performed.98,100
Table 88-10 Factors that Increase the Risk of Complications with Lead Extraction
| PATIENT FACTORS |
| LEAD FACTORS |
Lead extractions are most often performed in the operating room or in the procedure room, where access to emergency cardiovascular surgical backup, extracorporeal membrane oxygenation perfusion, and adequate fluoroscopy are readily available.71 A pre-extraction checklist is provided in Table 88-11. In the operating room or the procedure room, the patient is connected to pads capable of defibrillation and pacing. The authors’ group routinely performs lead extractions with general anesthesia and with arterial line access for continuous blood pressure monitoring, although some operators perform extraction with neuroleptic sedation and local anesthesia. Intravenous access is obtained with a large-bore catheter for urgent fluid resuscitation, as necessary, and the chest is prepared with sterile solution to prepare for urgent thoracotomy, if needed.98,101 The authors obtain femoral vein access and place a long wire into the internal jugular vein contralateral to the extraction site to permit deployment of a 22-Fr SVC dilating balloon to tamponade bleeding in case of major vessel laceration.102 This also allows for an access site for urgent circulatory support.71 Continuous monitoring with trans-esophageal echocardiography is often performed to monitor for complications such as tricuspid valve regurgitation, pericardial effusion or tamponade, and embolization of vegetations.
Table 88-11 Pre-extraction Checklist
Extraction can be performed transvenously; with a parasternal, subxiphoid, or intercostal transthoracic approach; or with an open thoracotomy.98 The latter two approaches are used only when a transvenous approach is not feasible, such as with very large vegetations. Transvenous extraction initially begins with incising and débriding of the pocket and freeing the leads from fibrosis through their extrathoracic course.98 Electrocautery is advised for pocket dissection to reduce lead damage. The suture on the suture sleeve is dissected and removed, and the lead’s retractable helix is retracted, if applicable. If the helix does not retract fully, counterclockwise rotation of the lead may free the tip from the endocardial surface. In potentially minimally fibrosed leads, the lead may be removed at this stage with constant, gentle traction. Caution should be exercised, as excessive force may damage the lead, thus limiting the ability to deploy a proper locking stylet, and may also result in chest pain, myocardial or SVC perforation, right ventricular collapse, and ventricular arrhythmias.71 Extracting the most recently implanted lead first will often increase the success of the extraction of subsequent leads.98,100
Manual traction is more likely to be successful in leads that have been inserted for a short duration, but success has been reported even in cases where the lead had been in place up to 4 years. Manual extraction with traction alone is facilitated by leads with more tensile strength, such as those with silicone insulation or coaxially aligned conductor coils.71,89
If traction alone is unsuccessful, then a locking stylet should be inserted. Typically, lead preparation includes cutting the lead to remove the proximal connector component and cutting the insulation to expose the proximal inner conductor coil lumen. The lumen’s inner diameter is sized with a sizing stylet to direct and lock the appropriately sized locking stylet. Recent universal locking stylets make sizing the lumen unnecessary. A suture is then tied from the insulation to the locking stylet such that manual traction can be applied to both the insulation and conductor coil via the locking stylet.71,98 Manual traction with the use of a locking stylet results in successful lead removal in up to 46% of cases.86,96 This appears to be more likely in leads that have been in place for a short duration, those extracted because of systemic infection, and pacemaker leads as opposed to ICD leads.89,96,98,103
The inability to remove a lead with manual traction with or without a locking stylet is primarily caused by fibrosis around the lead. Fibrosis is more likely to occur in young people and at the venous entry site, at curves within the veins, and in the region from the anodal ring to the lead tip or ICD coils.71 Patients at the extremes of age appear to have an increase in the degree of fibrotic calcification, a finding that also makes manual traction less successful.71
Sheaths can increase the likelihood of success of lead extraction as they disrupt, dilate, and cut through fibrous tissue when they track the lead within the vessel wall.71 Sheaths are either nonpowered sheaths such as those made of steel, Teflon, and polypropylene or powered sheaths such as laser or electrosurgical dissection sheaths. The laser sheath cuts an oblique ring to a depth of 100 µm, whereas the electrosurgical dissection sheath uses radiofrequency energy at its tip to dissect through fibrous tissue.71 Neither of the powered sheaths can cut through bone, though they may pass through calcified fibrotic tissue. Powered sheaths are more successful at disrupting fibrous tissue than are nonpowered sheaths.71 Steel sheaths are used primarily for fibrosis at the venous access site, as they cannot follow the venous curves. Both Teflon and polypropylene sheaths are flexible and can track the lead as it courses within the venous system. Traction is placed on the lead and locking stylet as the sheath is advanced to allow it to track the lead within the vessel lumen. When advancing a powered sheath, the leading edge of the bevel should be kept toward the inside of the curve so that the vessel is not lacerated. Furthermore, when crossing the tricuspid valve, the leading edge of the bevel should be kept on the nonvalvular side (most often on the medial-superior portion of the tricuspid valve) to decrease the risk of valvular injury.
As the sheath is advanced, it may create a “snow plowing” effect by pushing fibrous tissue or the components of the lead forward. If this occurs, upsizing the sheath or withdrawing and redirecting the sheath with the bevel rotated in a different direction may allow the sheath to advance. Powered sheaths should not be used at the interface between the lead and the endocardium because of the expected effect of perforation. The blunt end of a nonpowered sheath should be used in a countertraction maneuver to advance the endocardium away from the lead while providing traction on the lead.71,100
Leads may be extracted to create a conduit for the insertion of a new lead.71,95,104 If the lead dislodges from the endocardial surface prior to sheath insertion, the distal lead can be snared with a snare system inserted via the femoral vein. This allows countertraction to be placed on the distal portion of the lead while the sheath is inserted.105
Leads inserted into the thin-walled coronary sinus also appear to develop significant fibrosis.71 These leads can also be extracted both by manual traction and the use of laser and other sheaths.71 The technique is similar in that used for noncoronary sinus leads. However, powered sheaths should not be used within the coronary sinus because of the excessively high risk of tamponade. Additional care is necessary when using nonpowered sheaths within the coronary sinus, as it can be easily lacerated.106
Complete transvenous lead extraction is successful in 90% of cases.96 Predictors of unsuccessful lead extraction include advanced lead age, multiple leads, ventricular and tined leads, and younger age of the patient.71,96 Following transvenous lead extraction, it is recommended that the patient be observed in the hospital for 24 hours and have a chest radiograph and transthoracic echocardiogram performed to ensure no late complications are present.102 In the authors’ experience, local venous thrombosis is sufficiently common, anticoagulation is routinely started within 24 hours and continued for 4 weeks following extraction to prevent subclavian vein thrombosis, particularly if there has been significant endovascular manipulation.92,98,100,107
Approach to a Lead Advisory
The reader is directed to the recently published comprehensive Heart Rhythm Society Guidelines on lead monitoring and advisories.43 A description of the function and response system of the Canadian Heart Rhythm Society’s Device Advisory Committee is also summarized in two recent papers.108,109 The course of action in the event of a lead advisory is determined by balancing the risk of death or serious harm with close follow-up alone with the risk of operative treatment such as the placement of a new lead with or without extraction of the advisory lead. These risks depend on patient factors such as pacemaker dependence, comorbid illnesses, risk of surgical revision or replacement, anxiety about lead failure, and the risk of future ventricular arrhythmia in patients with ICDs. Lead factors that alter these risks include the rate and predictability of lead failure, clinical consequences of lead failure, and the availability of systems that warn or mitigate this risk.110 Recent device advisories have resulted in early device replacement, arguably a procedure with less risk than lead extraction. This operative intervention has resulted in unexpectedly high complication rates, which emphasizes the importance of careful risk assessment.111–113 Currently, it is recommended that a noninvasive approach be used in patients with pacemakers who are not pacemaker dependent, in patients with ICDs who have never had a ventricular arrhythmia (both before and after ICD insertion), and in patients whose operative risk is high.30,43
Key References
Ellenbogen KA, Wood MA, Shepard RK, et al. Detection and management of an implantable cardioverter defibrillator lead failure: Incidence and clinical implications. J Am Coll Cardiol. 2003;41:73-80.
Field ME, Jones SO, Epstein LM. How to select patients for lead extraction. Heart Rhythm. 2007;4:978-985.
Gould PA, Gula LJ, Champagne J, et al. Outcome of advisory implantable cardioverter-defibrillator replacement: One-year follow-up. Heart Rhythm. 2008;5:1675-1681.
Hauser RG, Hayes DL, Kallinen LM, et al. Clinical experience with pacemaker pulse generators and transvenous leads: An 8-year prospective multicenter study. Heart Rhythm. 2007;4:154-160.
Hayes DL, Vlietstra RE. Pacemaker malfunction. Ann Intern Med. 1993;119:828-835.
Kleemann T, Becker T, Doenges K, et al. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of >10 years. Circulation. 2007;115:2474-2480.
Love CJ. Lead extraction. Heart Rhythm. 2007;4:1238-1243.
Love CJ, Wilkoff BL, Byrd CL, et al. Recommendations for extraction of chronically implanted transvenous pacing and defibrillator leads: Indications, facilities, training. North American Society of Pacing and Electrophysiology Lead Extraction Conference Faculty. Pacing Clin Electrophysiol. 2000;23:544-551.
Maisel WH, Hauser RG, Hammill SC, et al. Heart Rhythm Society Task Force on Lead Performance Policies and Guidelines; American College of Cardiology (ACC); American Heart Association (AHA): Recommendations from the Heart Rhythm Society Task Force on Lead Performance Policies and Guidelines: Developed in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Heart Rhythm. 2009;6:869-885.
Santini M, Ricci R. News from the XIII World Congress on Cardiac Pacing and Electrophysiology focus on implantable device performance and recalls. Pacing Clin Electrophysiol. 2008;31(5):613-616.
Smith MC, Love CJ. Extraction of transvenous pacing and ICD leads. Pacing Clin Electrophysiol. 2008;31:736-752.
Transvenous lead extraction. Heart Rhythm Society Expert consensus on facilities, training, indications, and patient management: This document was endorsed by the American Heart Association (AHA). Heart Rhythm. 2009;6:1085-1104.
Wilkoff BL. How to treat and identify device infections. Heart Rhythm. 2007;4:1467-1470.
Wilkoff BL, Auricchio A, Brugada J, et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): Description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. 2008;5:907-925.
1 Lagergren H. How it happened: My recollection of early pacing. Pacing Clin Electrophysiol. 1978;1:140-143.
2 Corbisiero R, Armbruster R. Does size really matter? A comparison of the Riata lead family based on size and its relation to performance. Pacing Clin Electrophysiol. 2008;31:722-726.
3 Ellis CR, Rottman JN. Increased rate of subacute lead complications with small-caliber implantable cardioverter-defibrillator leads. Heart Rhythm. 2009;6:619-624.
4 Hirschl DA, Jain VR, Spindola-Franco H, et al. Prevalence and characterization of asymptomatic pacemaker and ICD lead perforation on CT. Pacing Clin Electrophysiol. 2007;30:28-32.
5 Khan MN, Joseph G, Khaykin Y, et al. Delayed lead perforation: A disturbing trend. Pacing Clin Electrophysiol. 2005;28:251-253.
6 Danik SB, Mansour M, Heist EK, et al. Timing of delayed perforation with the St. Jude Riata lead: A single-center experience and a review of the literature. Heart Rhythm. 2008;5:1667-1672.
7 Hayes DL, Vlietstra RE. Pacemaker malfunction. Ann Intern Med. 1993;119:828-835.
8 Alter P, Waldhans S, Plachta E, et al. Complications of implantable cardioverter defibrillator therapy in 440 consecutive patients. Pacing Clin Electrophysiol. 2005;28:926-932.
9 Epstein AE, Baker JH2nd, Beau SL, et al. Performance of the St. Jude Medical Riata leads. Heart Rhythm. 2009;6:204-209.
10 Veltmann C, Borggrefe M, Schimpf R, Wolpert C. Fatal inappropriate ICD shock. J Cardiovasc Electrophysiol. 2007;18:326-328.
11 Tokano T, Pelosi F, Flemming M, et al. Long-term evaluation of the ventricular defibrillation energy requirement. J Cardiovasc Electrophysiol. 1998;9:916-920.
12 Brooks R, Garan H, Torchiana D, et al. Determinants of successful nonthoracotomy cardioverter-defibrillator implantation: Experience in 101 patients using two different lead systems. J Am Coll Cardiol. 1993;22:1835-1842.
13 Chen J, Naseem RH, Obel O, Joglar JA. Habitual cocaine use is associated with high defibrillation threshold during ICD implantation. J Cardiovasc Electrophysiol. 2007;18:722-725.
14 Echt DS, Black JN, Barbey JT, et al. Evaluation of antiarrhythmic drugs on defibrillation energy requirements in dogs. Sodium channel block and action potential prolongation. Circulation. 1989;79:1106-1117.
15 Gold MR, Khalighi K, Kavesh NG, et al. Clinical predictors of transvenous biphasic defibrillation thresholds. Am J Cardiol. 1997;79:1623-1627.
16 Guarnieri T, Levine JH, Veltri EP, et al. Success of chronic defibrillation and the role of antiarrhythmic drugs with the automatic implantable cardioverter/defibrillator. Am J Cardiol. 1987;60:1061-1064.
17 Horton RP, Canby RC, Román CA, et al. Determinants of nonthoracotomy biphasic defibrillation. Pacing Clin Electrophysiol. 1997;20:60-64.
18 Jones DL, Kim YH, Natale A, et al. Bretylium decreases and verapamil increases defibrillation threshold in pigs. Pacing Clin Electrophysiol. 1994;17:1380-1390.
19 Khalighi K, Daly B, Leino EV, et al. Clinical predictors of transvenous defibrillation energy requirements. Am J Cardiol. 1997;79:150-153.
20 Leitch JW, Yee R. Predictors of defibrillation efficacy in patients undergoing epicardial defibrillator implantation. The Multicenter Pacemaker-Cardioverter-Defibrillator (PCD) Investigators Group. J Am Coll Cardiol. 1993;21:1632-1637.
21 Reiffel JA, Coromilas J, Zimmerman JM, Spotnitz HM. Drug-device interactions: Clinical considerations. Pacing Clin Electrophysiol. 1985;8:369-373.
22 Shinlapawittayatorn K, Sungnoon R, Chattipakorn S, Chattipakorn N. Effects of sildenafil citrate on defibrillation efficacy. J Cardiovasc Electrophysiol. 2006;17:292-295.
23 Simon RD, Sturdivant JL, Leman RB, et al. The effect of dofetilide on ventricular defibrillation thresholds. Pacing Clin Electrophysiol. 2009;32:24-28.
24 Strickberger SA, Brownstein SL, Wilkoff BL, Zinner AJ. Clinical predictors of defibrillation energy requirements in patients treated with a nonthoracotomy defibrillator system. The ResQ Investigators. Am Heart J. 1996;131:257-260.
25 Schoenfeld MH. Contemporary pacemaker and defibrillator device therapy: Challenges confronting the general cardiologist. Circulation. 2007;115:638-653.
26 Theuns DA, Rivero-Ayerza M, Knops P, et al. Analysis of 57,148 transmissions by remote monitoring of implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2009;32(Suppl 1):S63-S65.
27 Wilkoff BL, Auricchio A, Brugada J, et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): Description of techniques, indications, personnel, frequency and ethical considerations, Heart Rhythm Society, European Heart Rhythm Association, American College of Cardiology, American Heart Association, European Society of Cardiology, Heart Failure Association of ESC, Heart Failure Society of America. Heart Rhythm. 2008;5:907-925.
28 Becker R, Ruf-Richter J, Senges-Becker JC, et al. Patient alert in implantable cardioverter defibrillators: Toy or tool? J Am Coll Cardiol. 2004;44:95-98.
29 Thibault B, Roy D, Guerra PG, et al. Anodal right ventricular capture during left ventricular stimulation in CRT-implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2005;28:613-619.
30 Santini M, Ricci R. News from the XIII World Congress on Cardiac Pacing and Electrophysiology focus on implantable device performance and recalls. Pacing Clin Electrophysiol. 2008;31:613-616.
31 Dreifus LS, Zinberg A, Hurzeler P, et al. Transtelephonic monitoring of 25,919 implanted pacemakers. Pacing Clin Electrophysiol. 1986;9:371-378.
32 Griffin JC, Schuenemeyer TD, Hess KR, et al. Pacemaker follow-up: Its role in the detection and correction of pacemaker system malfunction. Pacing Clin Electrophysiol. 1986;9:387-391.
33 Arnsbo P, Moller M. Updated appraisal of pacing lead performance from the Danish Pacemaker Register: The reliability of bipolar pacing leads has improved. Pacing Clin Electrophysiol. 2000;23:1401-1406.
34 Hayes DL, Holmes DRJr, Merideth J, et al. Bipolar tined polyurethane ventricular lead: A four-year experience. Pacing Clin Electrophysiol. 1985;8:192-196.
35 Maisel WH, Sweeney MO, Stevenson WG, et al. Recalls and safety alerts involving pacemakers and implantable cardioverter-defibrillator generators. JAMA. 2001;286:793-799.
36 Hauser RG, Hayes DL. Increasing hazard of Sprint Fidelis implantable cardioverter-defibrillator lead failure. Heart Rhythm. 2009;6:605-610.
37 Hauser RG, Maron BJ, Marine JE, et al. Safety and efficacy of transvenous high-voltage implantable cardioverter-defibrillator leads in high-risk hypertrophic cardiomyopathy patients. Heart Rhythm. 2008;5:1517-1522.
38 Kleemann T, Becker T, Doenges K, et al. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of >10 years. Circulation. 2007;115:2474-2480.
39 Dorwarth U, Frey B, Dugas M, et al. Transvenous defibrillation leads: High incidence of failure during long-term follow-up. J Cardiovasc Electrophysiol. 2003;14:38-43.
40 Eckstein J, Koller MT, Zabel M, et al. Necessity for surgical revision of defibrillator leads implanted long-term: Causes and management. Circulation. 2008;117:2727-2733.
41 Ellenbogen KA, Wood MA, Shepard RK, et al. Detection and management of an implantable cardioverter defibrillator lead failure: Incidence and clinical implications. J Am Coll Cardiol. 2003;41:73-80.
42 Luria D, Glikson M, Brady PA, et al. Predictors and mode of detection of transvenous lead malfunction in implantable defibrillators. Am J Cardiol. 2001;87:901-904.
43 Maisel WH, Hauser RG, Hammill SC, et al. Recommendations from the Heart Rhythm Society Task Force on Lead Performance Policies and Guidelines: Developed in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA), Heart Rhythm Society Task Force on Lead Performance Policies and Guidelines; American College of Cardiology (ACC); American Heart Association (AHA). Heart Rhythm. 2009;6:869-885.
44 Hauser RG, Hayes DL, Kallinen LM, et al. Clinical experience with pacemaker pulse generators and transvenous leads: An 8-year prospective multicenter study. Heart Rhythm. 2007;4:154-160.
45 Kay GN, Brinker JA, Kawanishi DT, et al. Risks of spontaneous injury and extraction of an active fixation pacemaker lead: Report of the Accufix Multicenter Clinical Study and Worldwide Registry. Circulation. 1999;100:2344-2352.
46 Farwell D, Green MS, Lemery R, et al. Accelerating risk of Fidelis lead fracture. Heart Rhythm. 2008;5:1375-1379.
47 Medi C, Mond HG. Right ventricular outflow tract septal pacing: Long-term follow-up of ventricular lead performance. Pacing Clin Electrophysiol. 2009;32:172-176.
48 Yeh KH, Wang CC, Wen MS, et al. Long-term performance of transvenous, steroid-eluting, high impedance, passive-fixation ventricular pacing leads. Pacing Clin Electrophysiol. 2004;27:1399-1404.
49 Val-Mejias JE, Janet DR, Ramadas S. Long-term changes in high-voltage impedance of defibrillating leads. Pacing Clin Electrophysiol. 2009;32(Suppl 1):S151-S154.
50 Goldschlager N, Epstein A, Friedman P, et al. Environmental and drug effects on patients with pacemakers and implantable cardioverter/defibrillators: A practical guide to patient treatment. Arch Intern Med. 2001;161:649-655.
51 Lloyd MS, El Chami MF, Langberg JJ. Pacing features that mimic malfunction: A review of current programmable and automated device functions that cause confusion in the clinical setting. J Cardiovasc Electrophysiol. 2009;20:453-460.
52 Freedman RA, Petrakian A, Boyce K, et al. Performance of dedicated versus integrated bipolar defibrillator leads with CRT-defibrillators: Results from a Prospective Multicenter Study. Pacing Clin Electrophysiol. 2009;32:157-165.
53 Iuliano A, Shopova G, De Simone A, et al. Long-term performance of coronary sinus leads used for cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2007;30(Suppl 1):S47-S49.
54 Kenigsberg DN, Mirchandani S, Dover AN, et al. Sensing failure associated with the Medtronic Sprint Fidelis defibrillator lead. J Cardiovasc Electrophysiol. 2008;19:270-274.
55 DiMarco JP. Implantable cardioverter-defibrillators. N Engl J Med. 2003;349:1836-1847.
56 Scher DL, Man DC, Edery T, Lu S. Isoelectric PVCs diagnosed with a monitor with simultaneous ECG, and ICD intracardiac electrogram and marker channels. Pacing Clin Electrophysiol. 2009;32:137-139.
57 Thaker JP, Patel MB, Jongnarangsin K, et al. Electromagnetic interference with pacemakers caused by portable media players. Heart Rhythm. 2008;5:538-544.
58 Koul AK, Keller S, Clancy JF, et al. Hyperkalemia induced T wave oversensing leading to loss of biventricular pacing and inappropriate ICD shocks. Pacing Clin Electrophysiol. 2004;27:681-683.
59 Krishen A, Shepard RK, Leffler JA, et al. Implantable cardioverter defibrillator T wave oversensing caused by hyperglycemia. Pacing Clin Electrophysiol. 2001;24:1701-1703.
60 Porres JM, Brugada J, Marco P, et al. T wave oversensing by a cardioverter defibrillator implanted in a patient with the Brugada syndrome. Pacing Clin Electrophysiol. 2004;27:1563-1565.
61 Silver JS, Gray ME, John RM. Strategy to eliminate inappropriate shocks secondary to T-wave oversensing in a biventricular ICD. Pacing Clin Electrophysiol. 2009;32:134-136.
62 Bracke FA, Meijer A, Van Gelder LM. Malfunction of endocardial defibrillator leads and lead extraction: Where do they meet? Europace. 2002;4:19-24.
63 Karanam S, John RM. Inappropriate sensing of pectoral myopotentials by an implantable cardioverter defibrillator system. Pacing Clin Electrophysiol. 2004;27:688-689.
64 Roelke M, O’Nunain SS, Osswald S, et al. Subclavian crush syndrome complicating transvenous cardioverter defibrillator systems. Pacing Clin Electrophysiol. 1995;18:973-979.
65 Seifert T, Block M, Borggrefe M, Breithardt G. Erroneous discharge of an implantable cardioverter defibrillator caused by an electric razor. Pacing Clin Electrophysiol. 1995;18:1592-1594.
66 Takahashi T, Bhandari AK, Watanuki M, et al. High incidence of device-related and lead-related complications in the dual-chamber implantable cardioverter defibrillator compared with the single-chamber version. Circ J. 2002;66:746-750.
67 Gilliam FR3rd. T-wave oversensing in implantable cardiac defibrillators is due to technical failure of device sensing. J Cardiovasc Electrophysiol. 2006;17:553-556.
68 Nguyen BL, Swerdlow C, Gaudio C, Gang ES. High sensing integrity counter in a patient with a Sprint Fidelis defibrillation lead: What is the cause? Heart Rhythm. 2009;6:579-581.
69 Swerdlow CD, Gunderson BD, Ousdigian KT, et al. Downloadable algorithm to reduce inappropriate shocks caused by fractures of implantable cardioverter-defibrillator leads. Circulation. 2008;118:2122-2129.
70 Krahn AD, Champagne J, Healey JS, et al. Canadian Heart Rhythm Society Device Advisory Committee: Outcome of the Fidelis implantable cardioverter-defibrillator lead advisory: A report from the Canadian Heart Rhythm Society Device Advisory Committee. Heart Rhythm. 2008;5:639-642.
71 Smith MC, Love CJ. Extraction of transvenous pacing and ICD leads. Pacing Clin Electrophysiol. 2008;31:736-752.
72 Swerdlow CD. Small-diameter defibrillation electrodes: Can they take a licking and keep hearts ticking? Heart Rhythm. 2007;4:900-903.
73 Chung EH, Casavant D, John RM. Analysis of pacing/defibrillator lead failure using device diagnostics and pacing maneuvers. Pacing Clin Electrophysiol. 2009;32:547-549.
74 Donaldson DM, Singh JP, Heist EK, et al. Multiple ICD discharges associated with lead fracture without triggering of high impedance alert. Pacing Clin Electrophysiol. 2009;32:543-546.
75 Kallinen LM, Hauser RG, Lee KW, et al. Failure of impedance monitoring to prevent adverse clinical events caused by fracture of a recalled high-voltage implantable cardioverter-defibrillator lead. Heart Rhythm. 2008;5:775-779.
76 Silvetti MS, Drago F. Outcome of young patients with abandoned, nonfunctional endocardial leads. Pacing Clin Electrophysiol. 2008;31:473-479.
77 Suga C, Hayes DL, Hyberger LK, Lloyd MA. Is there an adverse outcome from abandoned pacing leads? J Interv Card Electrophysiol. 2000;4:493-499.
78 Glikson M, Suleiman M, Luria DM, et al. Do abandoned leads pose risk to implantable cardioverter-defibrillator patients? Heart Rhythm. 2009;6:6568.
79 Wollmann CG, Böcker D, Löher A, et al. Incidence of complications in patients with implantable cardioverter/defibrillator who receive additional transvenous pace/sense leads. Pacing Clin Electrophysiol. 2005;28:795-800.
80 Fyke FE3rd. Simultaneous insulation deterioration associated with side-by-side subclavian placement of two polyurethane leads. Pacing Clin Electrophysiol. 1988;11:1571-1574.
81 Klug D, Balde M, Pavin D, et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: Results of a large prospective study. Circulation. 2007;116:1349-1355.
82 Massoure PL, Reuter S, Lafitte S, et al. Pacemaker endocarditis: Clinical features and management of 60 consecutive cases. Pacing Clin Electrophysiol. 2007;30:12-19.
83 Chua JD, Wilkoff BL, Lee I, et al. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med. 2000;133:604-608.
84 Wilkoff BL. How to treat and identify device infections. Heart Rhythm. 2007;4:1467-1470.
85 Baman TS, Gupta SK, Valle JA, Yamada E. Risk factors for mortality in patients with cardiac device-related infection. Circulation Arrhythmia electrophysiol. 2009:129-134.
86 Golzio PG, Vinci M, Anselmino M, et al. Accuracy of swabs, tissue specimens, and lead samples in diagnosis of cardiac rhythm management device infections. Pacing Clin Electrophysiol. 2009;32(Suppl 1):S76-S80.
87 Klug D, Wallet F, Lacroix D, et al. Local symptoms at the site of pacemaker implantation indicate latent systemic infection. Heart. 2004;90:882-886.
88 Jones SOT, Eckart RE, Albert CM, Epstein LM. Large, single-center, single-operator experience with transvenous lead extraction: Outcomes and changing indications. Heart Rhythm. 2008;5:520-525.
89 Chauhan A, Grace AA, Newell SA, et al. Early complications after dual chamber versus single chamber pacemaker implantation. Pacing Clin Electrophysiol. 1994;17:2012-2015.
90 Lauck G, Hagendorff A, Omran H. Sessile structures on implanted leads of cardiac pacemakers [abstract]. Eur Heart J. 1994;15:58.
91 Chihrin SM, Mohammed U, Yee R, et al. Utility and cost effectiveness of temporary pacing using active fixation leads and an externally placed reusable permanent pacemaker. Am J Cardiol. 2006;98:1613-1615.
92 Love CJ. Lead extraction. Heart Rhythm. 2007;4:1238-1243.
93 Wilkoff BL, Love CJ, Byrd CL, et al. Transvenous lead extraction: Heart Rhythm Society Expert consensus on facilities, training, indications, and patient management: This document was endorsed by the American Heart Association (AHA), Heart Rhythm Society; American Heart Association. Heart Rhythm. 2009;6:1085-1104.
94 Smith HJ, Fearnot NE, Byrd CL, et al. Five-years experience with intravascular lead extraction. U.S. Lead Extraction Database. Pacing Clin Electrophysiol. 1994;17:2016-2020.
95 Field ME, Jones SO, Epstein LM. How to select patients for lead extraction. Heart Rhythm. 2007;4:978-985.
96 Marijon E, Boveda S, De Guillebon M, et al. Contributions of advanced techniques to the success and safety of transvenous leads extraction. Pacing Clin Electrophysiol. 2009;32(Suppl 1):S38-S41.
97 Agarwal SK, Kamireddy S, Nemec J, et al. Predictors of complications of endovascular chronic lead extractions from pacemakers and defibrillators: A single-operator experience. J Cardiovasc Electrophysiol. 2009;20:171-175.
98 Kennergren C. A European perspective on lead extraction: Part I. Heart Rhythm. 2008;5:160-162.
99 Roux JF, Pagé P, Dubuc M, et al. Laser lead extraction: Predictors of success and complications. Pacing Clin Electrophysiol. 2007;30:214-220.
100 Kennergren C. European perspective on lead extraction: Part II. Heart Rhythm. 2008;5:320-323.
101 Love CJ, Wilkoff BL, Byrd CL, et al. Recommendations for extraction of chronically implanted transvenous pacing and defibrillator leads: Indications, facilities, training. North American Society of Pacing and Electrophysiology Lead Extraction Conference Faculty. Pacing Clin Electrophysiol. 2000;23:544-551.
102 Henrikson CA, Brinker JA. How to prevent, recognize, and manage complications of lead extraction. Part III: Procedural factors. Heart Rhythm. 2008;5:1352-1354.
103 Gula LJ, Krahn AD, Yee R, et al. Arrhythmia device lead extraction: Factors that necessitate laser assistance. Can J Cardiol. 2008;24:767-770.
104 Gula LJ, Ames A, Woodburn A, et al. Central venous occlusion is not an obstacle to device upgrade with the assistance of laser extraction. Pacing Clin Electrophysiol. 2005;28:661-666.
105 Fischer A, Love B, Hansalia R, Mehta D. Transfemoral snaring and stabilization of pacemaker and defibrillator leads to maintain vascular access during lead extraction. Pacing Clin Electrophysiol. 2009;32:336-339.
106 Burke MC. Percutaneous extraction of coronary sinus vein and branch leads. Heart Rhythm. 2008;5:491-495.
107 Henrikson CA, Brinker JA. How to prevent, recognize, and manage complications of lead extraction. Part I: Avoiding lead extraction—infectious issues. Heart Rhythm. 2008;5:1083-1087.
108 Krahn AD, Simpson CS, Parkash R, et al. Utilization of a national network for rapid response to the Medtronic Fidelis lead advisory: The Canadian Heart Rhythm Society Device Advisory Committee. Heart Rhythm. 2009;6:474-477.
109 Krahn AD, Simpson CS, Parkash R, et al. Formation of a national network for rapid response to device and lead advisories: The Canadian Heart Rhythm Society Device Advisory Committee. Can J Cardiol. 2009;25:403-405.
110 Varma N. Remote monitoring for advisories: Automatic early detection of silent lead failure. Pacing Clin Electrophysiol. 2009;32:525-527.
111 Costea A, Rardon DP, Padanilam BJ, et al. Complications associated with generator replacement in response to device advisories. J Cardiovasc Electrophysiol. 2008;19:266-269.
112 Gould PA, Gula LJ, Champagne J, et al. Outcome of advisory implantable cardioverter-defibrillator replacement: One-year follow-up. Heart Rhythm. 2008;5:1675-1681.
113 Gould PA, Krahn AD. Complications associated with implantable cardioverter-defibrillator replacement in response to device advisories. JAMA. 2006;295:1907-1911.