Chapter 150 Management of Intracranial Aneurysms Caused by Infection
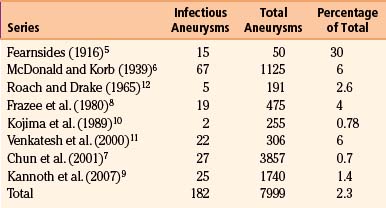
Infectious intracranial aneurysms are a heterogeneous group of rare lesions that develop from inflammation-induced weakening of arterial walls. Their first description was provided by Church, who in 1869 described a 13-year-old male with endocarditis who died from rupture of a middle cerebral artery (MCA) aneurysm.1 Osler then coined the term mycotic aneurysm to describe an aortic aneurysm that arose in the setting of endocarditis. This term has since been employed as a misnomer to describe all aneurysms of infectious origin.2 By 1923, 34 cases of intracranial bacterial aneurysms were reported.3 Combined with 16 cases reported in 1954,4 only 4 patients survived. Fortunately, as time has progressed, the prevalence of infectious intracranial aneurysms has been on an overall decline as rheumatic heart disease and endocarditis wane (Table 150-1).5–11 Across series reported in recent years, 0.7% to 6% (mean 2.3%, 182 of 7999 patients across all series) of aneurysms are infectious.7–11 However, the true incidence of infectious intracranial aneurysms remains unknown given their ability to grow and regress while remaining clinically silent. We comprehensively review their natural history, pathogenesis, diagnosis, treatment modalities, and management algorithms.
Natural History and Presentation
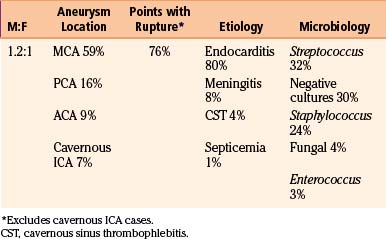
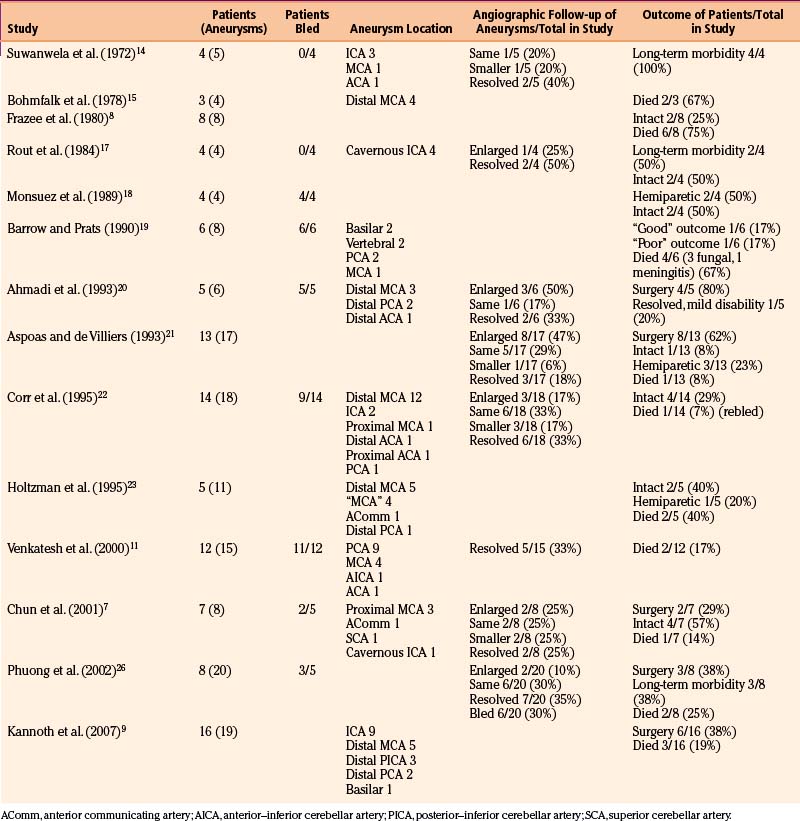
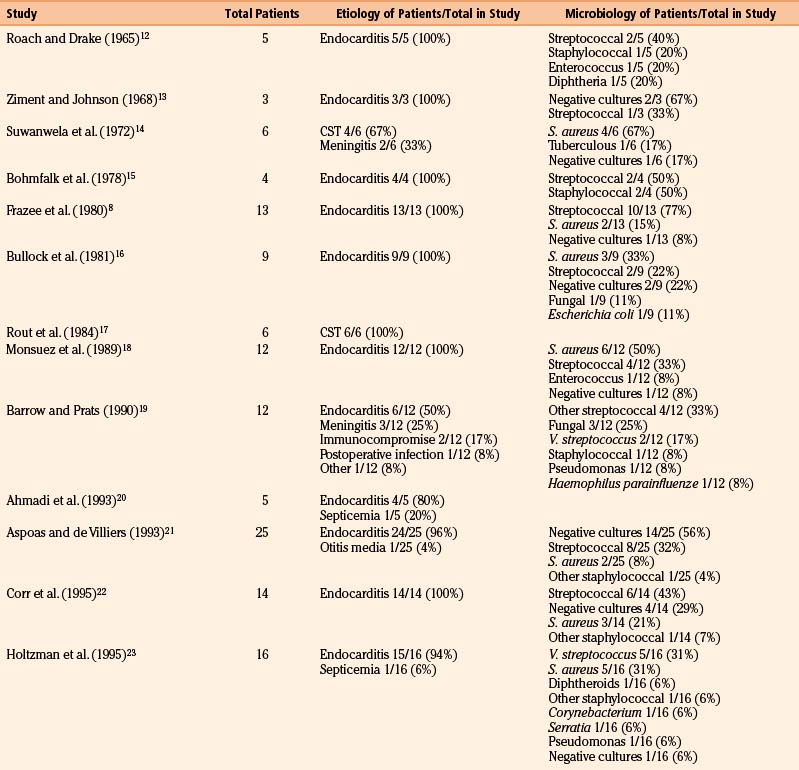
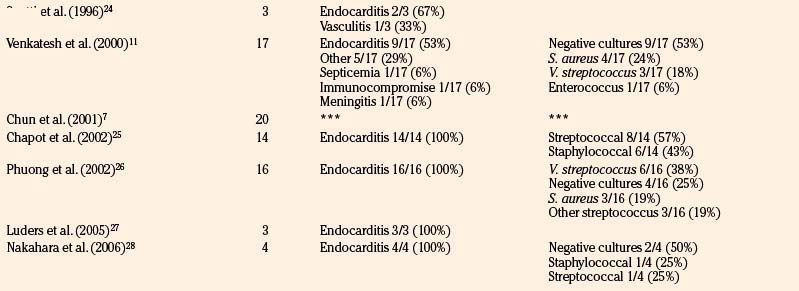
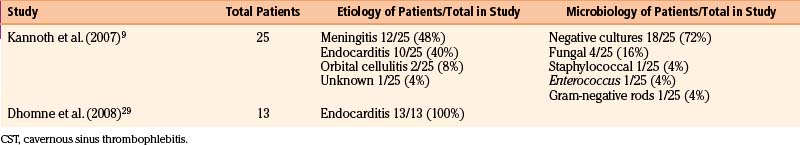
We reviewed 22 original series with 245 patients harboring 316 infectious aneurysms (see the Appendix, summarized in Table 150-A).7-,11–29 The overall male-to-female ratio was 1.2:1; 59% of aneurysms were in the MCA territory, 16% were in the posterior cerebral artery (PCA) territory, 9% were in the anterior cerebral artery (ACA) territory, and 7% were along the cavernous internal cerebral artery (ICA) (Table 150-2). Among aneurysms in the MCA territory, 55% were specified as being distal MCA while only 8% were specified as being proximal MCA (the remainder were unspecified). This is reinforced by an earlier review of 81 patients with infectious aneurysms—69% were in the distal MCA territory, while only 9% were in the proximal cerebral vasculature.30
Table 150-B Review of Etiologies and Offending Organisms Where Specified Among Modern Series of Infectious Aneurysms
Presenting symptoms include seizures or those referable to either ischemic or hemorrhagic strokes, such as hemiparesis, dysphasia, visual field deficits, and change in level of consciousness. Patients with infectious intracranial aneurysms of the cavernous carotid segment (most frequently secondary to cavernous sinus thrombophlebitis) often present with persistent ophthalmoplegia and often have accompanying meningitis.14,17 Given that infectious aneurysms may form from septic emboli, it is not surprising that patients with these lesions can present with ischemic symptoms that may precede aneurysm formation. However, Salgado et al. reviewed 68 patients with infective endocarditis and infectious aneurysms and found that only 43% of patients had neurologic prodromes prior to their diagnosis (median 17 days prior).31 In comparing this group of patients to those with endocarditis without infectious aneurysms, there were no particular neurologic symptoms distinguishing this particular group. Across three of the larger series, 40 of 59 patients (68%) presented with hemorrhage while 5 of 59 patients (8%) presented with only ischemic infarction; 7 of 59 patients (12%) presented with seizure.7,21,22 Excluding patients with cavernous aneurysms, across 17 series with 202 patients, as shown in Table 150-2, 153 patients (76%) had ruptures of at least one infectious aneurysm. The bleeding pattern may involve any combination of subarachnoid, intraparenchymal, and intraventricular hemorrhage. Epidural hemorrhage from middle meningeal mycotic aneurysm rupture has also been reported,21 as has subdural hemorrhage.27,32,33
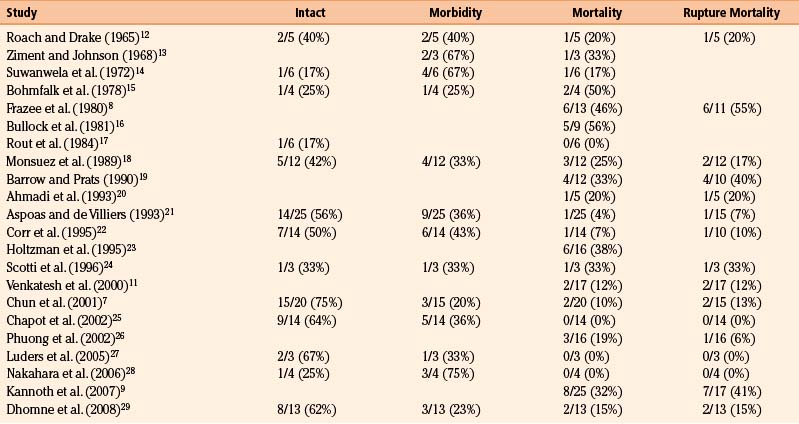
Table 150-3 summarizes outcomes across our 22 reviewed series. Overall mortality was 20% (50 of 245 patients). This is an underestimate, because patients with catastrophic/lethal hemorrhage may not even come to neurosurgical attention, may not obtain an actual diagnosis of infectious aneurysm because they do not undergo angiograms, or are explicitly excluded from certain series.26 Long-term neurologic morbidity, where reported, was seen in 36% of patients, and 52% of patients (67 of 129 patients) were neurologically intact at follow-up. These data are promising, because more dated, oft-cited reviews quote significantly more pessimistic outcome figures. A detailed literature review of 85 cases in 1978 cited an overall mortality rate of 45% and a mortality rate of 42% from initial rupture (8 of 19 patients).15 It also found that 80% of patients with aneurysmal subarachnoid hemorrhage during hospitalization for endocarditis died (a frequently cited figure based on 15 patients), while 3 of 10 patients (30%) with unruptured mycotic aneurysms treated for endocarditis died. In another literature review of 45 patients from 1977, excluding patients who were “inevitably” going to die, 29% of patients died of aneurysmal rupture (20% overall).34
Pathogenesis
Infectious intracranial aneurysms can develop in the setting of (1) septic emboli (e.g., endocarditis) or (2) contiguous spread (e.g., meningitis or cavernous sinus thrombophlebitis).14,35,36 Alternative sources for contiguous spread described in the literature include cerebral abscesses,9,37,38 osteomyelitis,39 sinusitis,40 and otitis media.21,41 Across 21 series with 225 patients specifying infectious aneurysm etiology (Table 150-B), 180 cases (80%) were due to endocarditis, 18 cases (8%) were from meningitis, and 10 cases (4%) from cavernous sinus thrombophlebitis. The pattern of aneurysm formation is defined by the mechanism by which it arises—those from septic emboli are more likely to occur in multiples and tend to occur distally at branch points in the MCA territory, while those from contiguous spread tend to develop along the proximal circulation.19 A recent case series suggested a relatively better prognosis for patients with infectious aneurysms due to endocarditis compared to for those with infectious aneurysms from “other” causes—10% of patients with endocarditis and infectious aneurysms died, compared to 47% with infectious aneurysms from other etiologies.9
Endocarditis
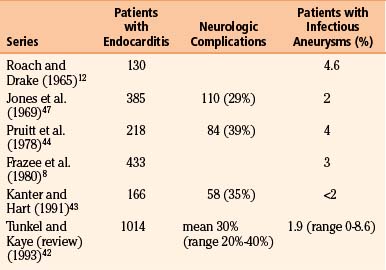
Despite medical advances, the persistence of endocarditis in populations is likely attributable to continued intravenous drug abuse and rheumatic heart disease in underdeveloped countries. Neurologic sequelae are seen in 20% to 40% (average 30%) of cases42 (Table 150-4). Several series have noted that neurologic complications are more likely to occur and occur more quickly18 with Staphylococcus aureus infection than with streptococcal. In Kanter and Hart’s series, 67% of patients with S. aureus endocarditis had neurologic complications compared to 22% of patients with streptococcal infections.43 Tunkel and Kaye reviewed 1014 patients with endocarditis and found central nervous system involvement in 53% to 71% of cases of S. aureus endocarditis.42
The most common neurologic complication of endocarditis is embolic infarction, afflicting as many as 31% of patients with this disease.42 As reinforced by several case series,43,44 Hart et al.’s literature review of 2119 patients with endocarditis found an overall 5% rate of intracerebral hemorrhage.45 Interestingly, in an autopsy study of 16 patients with intracranial hemorrhage and endocarditis, 9 cases (56%) were hemorrhagic transformations of infarcts, 4 cases (25%) were arterial ruptures from pyogenic arteritis, and only 3 cases (19%) were from infectious aneurysm rupture.46 In Hart et al.’s series of 209 patients with endocarditis, 17 patients (8%) had intracerebral hemorrhages, 4 patients (24%) attributed endocarditis to hemorrhagic infarcts, 4 patients (24%) attributed it to pyogenic arteritis, and only 2 patients (12%) attributed it to infectious aneurysm rupture.45 In their larger review of 2119 patients with endocarditis, only 1.7% had infectious aneurysms.45 Table 150-4 summarizes the prevalence of infectious aneurysms in several large series of patients with endocarditis, underscoring that infectious aneurysms are an overall rare complication of this disease.8,12,43,44,47
Molinari et al. developed an elegant model that continues to be cited as the likely mechanism of infectious aneurysm formation in the setting of septic emboli.48 They demonstrated that septic particles embolize into the vasa vasorum, leading to inflammation of the adventitia first, followed by centripetal progression into the muscularis and then the internal elastic lamina. Early-developing aneurysms were first noted at the proximal or distal end of an occluded segment as quickly as 24 hours after embolization. Enlargement then occurred via pulsations against the necrotic wall in some cases. This model also demonstrated the efficacy of antibiotic treatment, because partially treated aneurysms, as opposed to untreated aneurysms, had indurated, intact fibrotic walls.
Contiguous Spread: Meningitis and Cavernous Sinus Thrombophlebitis
Cavernous carotid infectious aneurysms are special cases that most often develop from cavernous sinus thrombophlebitis. These cases have the overall best prognosis of infectious intracranial aneurysms. A recent case series of 8 patients with 9 cavernous carotid infectious aneurysms reported that all were “cured” at follow-up.9 In a comprehensive literature review of 36 cases of cavernous carotid infectious aneurysms (7 bilateral, primarily from case reports), 24 patients (67%) were improved at follow-up.49 Although 6 patients (17%) died, 2 patients had fungal aneurysms and 1 patient never received antibiotic treatment. The pathogen was unknown in 17 cases (47%) but was staphylococcal in 12 (33%), streptococcal in 3 (8%), and fungal in 3 (8%).
Microbiology
Although most commonly due to bacteria, infectious intracranial aneurysms have been attributed to fungal (true mycotic),50 spirochetal,51 amebic,52 and viral pathogens, including the human immunodeficiency virus (HIV).53,54 A recent review of the literature of HIV-associated mycotic aneurysms found 17 cases in children and 6 cases in adults.54 The aneurysms had a tendency to occur in younger patients and in the anterior circulation, and some resolved following antiretroviral therapy.55,56 Across reviewed series specifying microbiology in 195 cases (see the Appendix, Table 150-A), 32% were due to streptococcus (8% specified as Viridans streptococcus), 24% were from staphylococcus (19% specified as S. aureus), 4% were fungal, and 3% were due to enterococci. In 30% of these cases, cultures were negative. This is similar to the results of a review of 81 cases in the literature that found 44% due to streptococcal species, 19% due to S. aureus, no growth from cultures in 12% of cases.30
Fungal cases deserve particular attention given their exceedingly poor prognosis. Among the first 16 cases reported in the literature, none survived.19,57 A recent review of the literature consisting primarily of case reports identified 35 cases of fungal (“true mycotic”) aneurysms.58 Although 2 of 35 cases (6%) had favorable outcomes, the vast majority died; 18 cases were seen in immunocompetent patients, most commonly after surgery (11 cases) but also following sinusitis in 3 cases and trauma in 2 cases. The most common organism seen, potentially from its angioinvasive nature, given its ability to produce elastase, is Aspergillus. Aneurysms from aspergilli typically arise from local intracranial disease. The second tier of common fungal organisms includes Candida (typically from endocarditis or systemic infection) and Phycomycetes (associated with diabetes).
Diagnosis
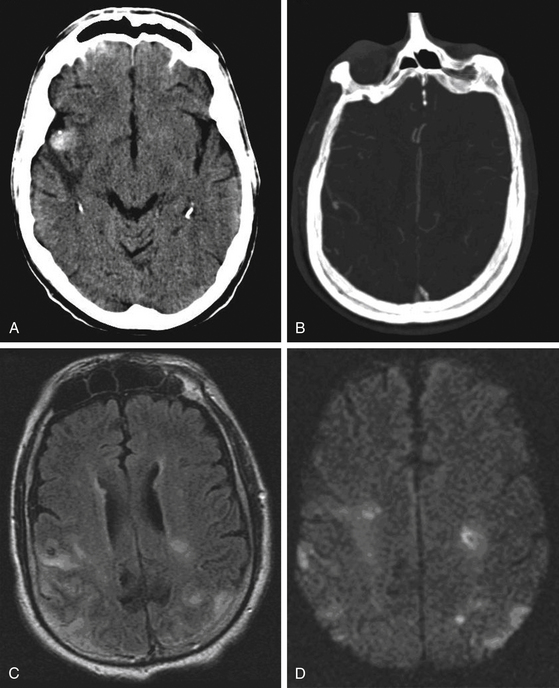
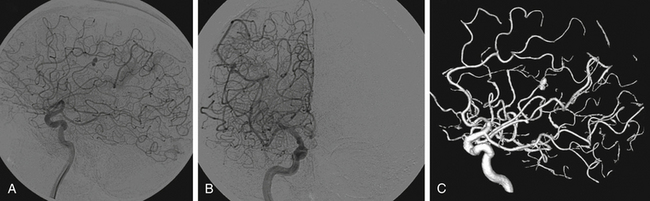
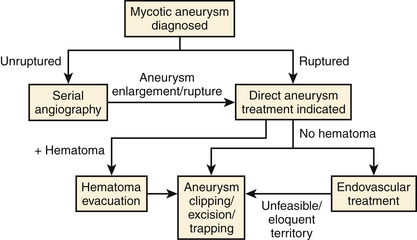
Patients with endocarditis and new neurologic symptoms should undergo computed tomography (CT) angiography followed by digital subtraction angiography (Figs. 150-1 and 150-2). Van der Meulen et al., however, demonstrated that surveillance angiography in neurologically asymptomatic patients with endocarditis should not be performed because it does not confer a survival benefit overall.59 Patients with meningitis, cavernous sinus thrombophlebitis, or other potential contiguous infectious processes with persistent or new neurologic symptoms should also undergo CT angiography followed by digital subtraction angiography. The diagnosis of an infectious aneurysm is then rendered in the event of a positive angiogram. Proximal saccular aneurysms discovered in the setting of endocarditis should be viewed with scrutiny as potential true hemodynamic berry aneurysms, though the treatment algorithm (Fig. 150-3) does not need modification—ruptured aneurysms should be treated, and unruptured, stable aneurysms should be observed. Kannoth et al. reported a sensitivity of 96% and specificity of 100% for a diagnosis of infectious intracranial aneurysms in patients with a cerebral aneurysm and a diagnosis of infective endocarditis, meningitis, orbital cellulitis, or cavernous sinus thrombophlebitis.60 Further specific, supportive features from their study include distal location of the aneurysm, multiplicity, fusiform shape, change in size or appearance, patient age less than 45 years, recent fever, recent lumbar puncture, and intraparenchymal hemorrhage on imaging. However, several studies have demonstrated that lumbar puncture is not particularly correlated with the presence of infectious aneurysms in patients with endocarditis.26,31,44
Treatment
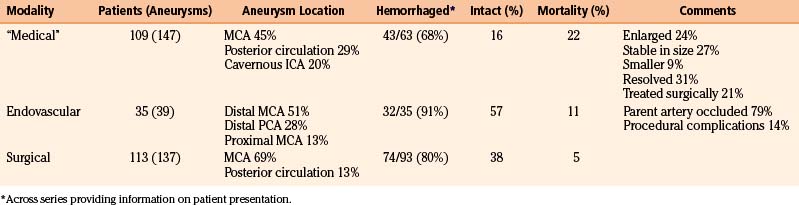
Treatment options upon diagnosis of infectious intracranial aneurysms include a prolonged course of aggressive antibiotics (medical), endovascular therapy, and open microsurgery (overall results summarized in Table 150-5). Our treatment paradigm is presented in Figure 150-3. Upon initial diagnosis, if not already done, all patients are pan cultured and undergo echocardiography. After obtaining cultures, aggressive broad-spectrum antibiotic therapy is initiated and continued for at least 6 weeks, with tailoring of antibiotics based on culture susceptibility. Patients with infectious aneurysms, regardless of etiology, should not be anticoagulated.44 In a review of 218 patients with cerebral embolic events and endocarditis, 3 of 7 patients (43%) who underwent anticoagulation had major intracranial hemorrhage as opposed to 10 of 211 patients (4.7%) who were not anticoagulated.44
Unruptured infectious aneurysms are followed with serial angiography at 1 week, 2 weeks, 4 weeks, 3 months, 6 months, and 1 year, if needed. Aneurysm enlargement or rupture is an indication for endovascular or microsurgical treatment. We do not advocate surveillance with CT or magnetic resonance imaging alone, because subtle morphologic changes or new aneurysms can be missed and the usage of digital subtraction angiography potentially allows for both diagnosis and treatment in one sitting. Early intervention should be done for any ruptured aneurysm given the risk of early rerupture. Although treatment delay to allow for fibrosis is sometimes considered, this should be reserved for aneurysms that are extremely risky to treat or for patients who are too medically unstable for intervention. As Ojemann demonstrated in his early review, outcomes are significantly better when interventions for infectious aneurysms are performed electively (5% mortality) as opposed to urgently (52% mortality).30
Medical
Unruptured infectious aneurysms should initially be treated “medically” with antibiotics and supportive therapy for their predisposing condition without anticoagulation. These aneurysms may spontaneously resolve, diminish in size, thrombose, enlarge, or rupture in the interim.18,61–63 New aneurysms may also develop following therapy,14,34,64 though fortunately this is rare—0 of 121 patients followed for a mean of 40 months after treatment of endocarditis developed new aneurysms or suffered new hemorrhages.31
Patients should initially be observed in an intensive care unit setting with close serial neurologic examinations, an arterial line for close blood pressure monitoring (with a systolic blood pressure goal of less than 140 mm Hg), and aggressive deep venous thrombosis prophylaxis. At our institution, we also use prophylactic antiepileptics such as levetiracetam in these patients. Serial digital subtraction angiography is performed at 1 week, 2 weeks, 1 month, 3 months, 6 months, and 1 year following diagnosis, if needed. In a literature review of 131 patients with endocarditis and infectious aneurysms, Kojima et al. noted that the interval from aneurysm formation to resolution ranged from 2 days to 2 years (mean 14.4 weeks); increases or decreases in size were noted after an average of 4.6 weeks from aneurysm formation (range 2 days to 4 months).10 Aneurysm enlargement becomes an indication for direct intervention. Fortunately, since the inflammatory process in these dynamic aneurysms is by definition at least subacute or chronic at that point, the aneurysm becomes less friable and more easily handled by intervention—which further reinforces the logic of initially observing unruptured infectious aneurysms.
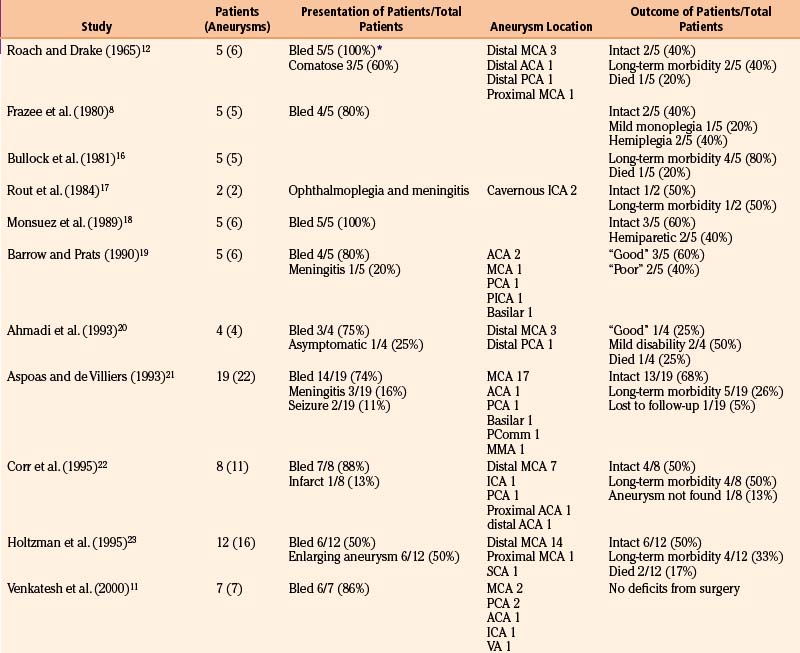
Table 150-6 summarizes results from 14 series of 109 patients with 147 aneurysms initially managed “medically.” As expected, the distribution of aneurysm location differs, because the classical distal MCA aneurysm is more likely to be treated surgically—45% of aneurysms were in the MCA territory (31% specified as distal MCA), while 29% were in the posterior circulation and 20% were cavernous ICA aneurysms. Across 7 series with 78 aneurysms followed angiographically, 19 (24%) enlarged, 21 (27%) were stable in size, 7 (9%) were smaller, and 24 (31%) resolved.7,14,17,20–22,26 Resolution, potentially via thrombosis of the parent vessel, is generally likely to be well tolerated given that initial aneurysm formation likely entailed an initial thromboembolic event.11,17
Across the 14 series of 109 patients in Table 150-6, the mortality rate was 22% (24 of 109 patients), and 23 patients (21%) ultimately underwent surgery (see Table 105-5). Notably, 17 patients (16%) were neurologically intact at follow-up. These figures demonstrate the significant improvement in prognosis for patients with mycotic aneurysms in modern series compared to a literature review from 1978 that describes 44 patients managed “conservatively.”15 Of 27 with ruptured aneurysms, 22 patients (81%) died, while 3 of 17 patients (18%) with unruptured aneurysms managed conservatively died.
The best prognosis is seen for patients with cavernous ICA aneurysms that can be treated conservatively. Kannoth et al. reported as “cured” all 8 cases of infectious cavernous ICA aneurysms in their series—5 were managed “medically.”9 In a review of 36 cases reported in the literature, 12 (33%) were managed “conservatively”—9 patients (75%) improved while 3 patients (25%) died, though 1 case was fungal and another did not receive antibiotic treatment; 8 of 12 aneurysms (67%) disappeared or thrombosed.49
Endovascular
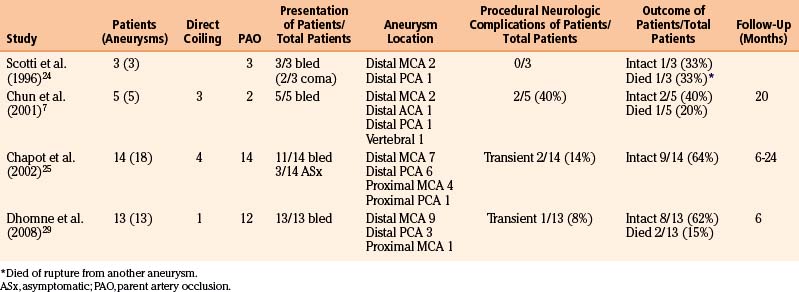
The proclivity toward a fusiform morphology coupled with their distal location often makes mycotic aneurysms poor candidates for direct endovascular coiling. Instead, parent vessel occlusion with glue, such as n-butyl cyanoacrylate (n-BCA), after hyperselective catheterization with a microcatheter is the most commonly employed therapeutic option.7,25,29 Favorable results have also recently been obtained with Onyx embolization.65 Two cases of patients with bacterial endocarditis and intracranial hemorrhage underwent Onyx embolization without procedural morbidity. Advantages of Onyx include the ability to release the polymer more proximally in a controlled fashion and the ability to perform multiple injections, in contrast to n-BCA. Alternatively, hemodynamic inflow reduction and resultant aneurysm thrombosis via the placement of a stent have been reported,66 as has stent coiling. A potential shortcoming is the possibility of Onyx migration distally, causing infarction by occlusion of small distal branches.
Advantages of endovascular therapy compared to microsurgical treatment include overall greater tolerance for medically ill patients, the ability to treat multiple aneurysms in one sitting, the ability to treat potentially surgically inaccessible lesions, and the ability to more rapidly anticoagulate post-therapy.66,67 It is particularly attractive for the treatment of enlarging, symptomatic cavernous ICA aneurysms. Shortcomings include the danger of parent artery occlusion of aneurysms in eloquent locations and the potential added need for surgical evacuation of a hematoma.24 Although stenting materials, coiling materials, or both can theoretically serve as added nidi for persistent infection, this has not yet been reported.49 This technique is particularly favorable for cavernous ICA aneurysms.49 We thus advocate endovascular therapy for most ruptured aneurysms that are in a location that does not potentially sacrifice eloquent territory. As seen in Table 150-7, however, ischemic complications are fewer than might be expected. This is likely because of the mechanism of infectious aneurysm formation. Initial parent vessel occlusion with a septic embolus is likely to either cause the infarction initially or serve as an indication of tolerance of parent vessel occlusion when mycotic aneurysm formation is clinically silent.25 Thus, if this event is asymptomatic, it is plausible that subsequent occlusion is likely to be asymptomatic, potentially due to ample pial collateralization. When in doubt, prior to parent artery occlusion, Amytal testing or balloon-test occlusion may be performed.7
Table 150-A Male-to-Female Ratios, Rupture Rates, and Aneurysm Location from Series of Infectious Intracranial Aneurysms
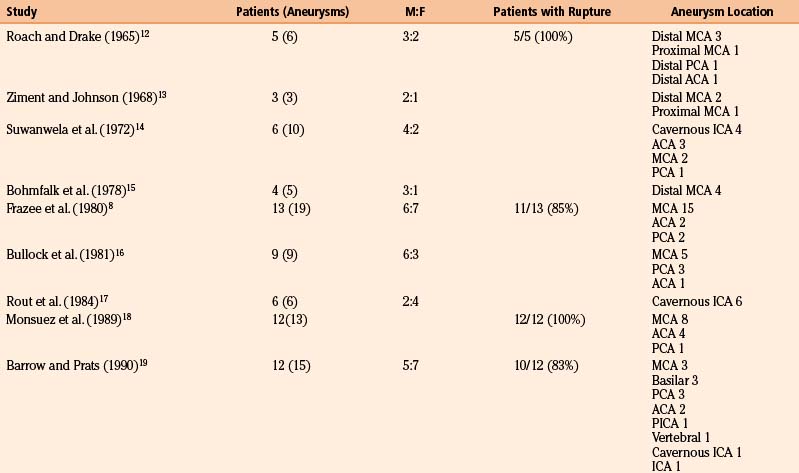
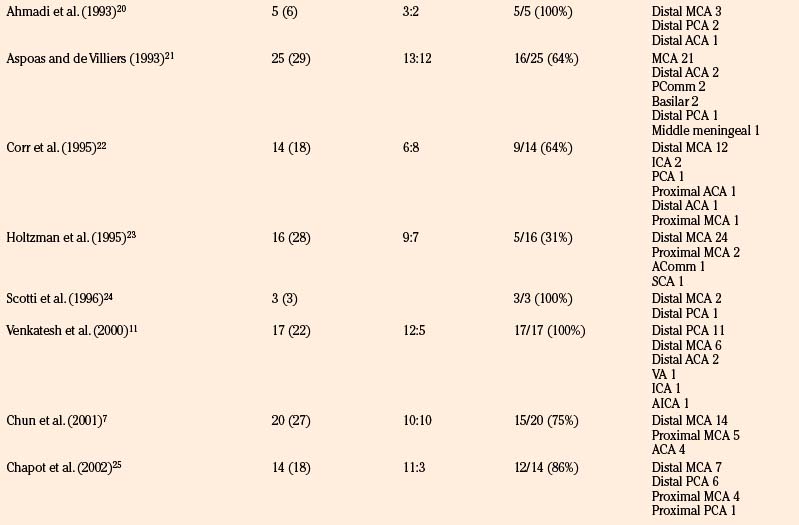
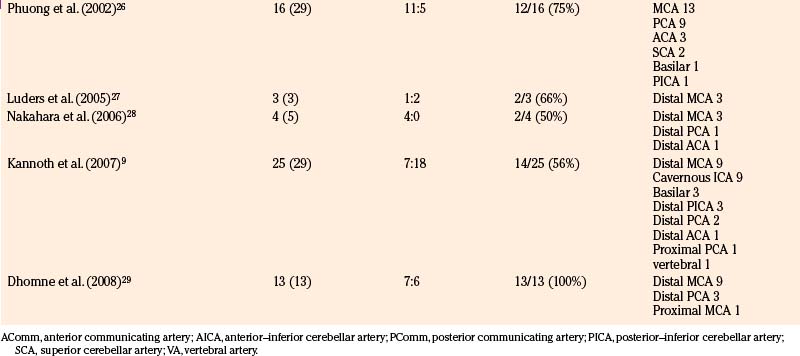
Table 150-7 reviews results from four endovascular series with 35 patients harboring 39 aneurysms.7,24,25,29 Of the 39 aneurysms, 31 (79%) were treated with parent artery occlusion. In addition, 91% of patients presented with hemorrhage, and 51% of aneurysms treated were of distal MCA branches; however, 13% were proximal MCA aneurysms and 28% of aneurysms treated were of the distal PCA. Procedural neurologic complications were seen in 5 of 35 cases (14%), though they were often transient. Chapot et al. required second embolization sessions in two cases—in one, the parent artery recanalized after balloon deflation, and in another, collateral vessels subsequently filled the aneurysm.25 At follow-up, 20 of 35 patients (57%) were neurologically intact, and 4 of 35 patients (11%) had died; 1 case was from rupture of another, initially unseen aneurysm.
Surgical
Open microsurgery with stereotactic guidance68,69 is an option for ruptured aneurysms with associated hematomas, those in locations that place eloquent territory at risk for ischemia, and those inaccessible to endovascular means. Surgery for these aneurysms can be quite challenging—particularly in the absence of a hemorrhage to guide localization of the aneurysm, because small lesions can be elusive to stereotactic guidance (at least two cases in the literature19,20). Furthermore, the morphology is often fusiform, with poorly defined necks and friable walls, making these lesions frequently unamenable to direct clip reconstruction. Trapping with or without bypass is often needed. Fortunately, hematomas can facilitate aneurysm localization and provide generous corridors for potential arterial reconstructions or bypass once evacuated. As mentioned previously, the distal MCA branches are the most common locations for infectious aneurysms—an attractive milieu for open surgical approaches and for potential bypass. Indeed, aneurysms in eloquent territory that cannot undergo direct clip reconstruction may be treated with trapping and bypass,70–72 preserving perfusion to eloquent territory in a fashion that endovascular therapy cannot. Although only applicable to a small subset of healthier, cooperative patients, Luders et al. described the utility of awake stereotactic craniotomy to assess for tolerance of parent vessel occlusion during surgery.27 If perforator branches emanate from the aneurysm, wrapping may be necessary.7
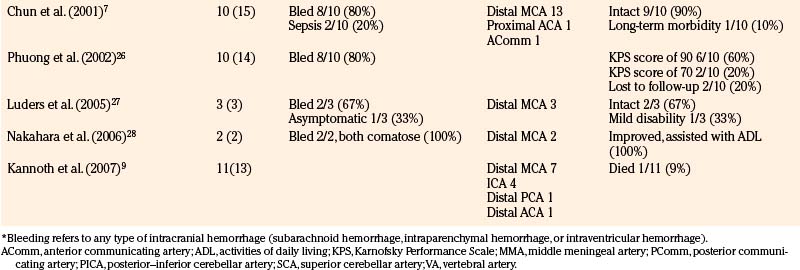
Table 150-8 reviews 16 surgical series including 113 patients with 137 aneurysms. Of those reported, 74 of 93 patients (80%) presented with hemorrhage; only 2 patients were neurologically intact. Of all cases, 69% were in the MCA territory (49% specified as distal MCA), and only 13% were in the posterior circulation. Of 113 patients, 43 (38%) were reported neurologically intact at follow-up; 6 of 113 patients (5%) died. The vast majority of neurologic morbidity in these series was primarily attributed to the initial ischemic/hemorrhagic event that led to diagnosis of the aneurysm. Surgical morbidity was generally quite low—in the series of Aspoas and de Villiers, 2 of 19 patients (11%) had new transient postoperative neurologic worsening.21 The overall observed improved outcomes after surgery as compared to medical management may in one sense be skewed by exceedingly sick patients deemed poor candidates for surgery and managed medically, though the degree of improvement is quite compelling. Even in a literature review from 1978, patients undergoing intervention fared better than those managed medically.15 The mortality rate was 13% for patients with unruptured aneurysms managed surgically, and while the mortality rate was 36% for patients with ruptured aneurysms managed surgically, all deaths were from cases taken to the operating room emergently.
Aspoas A.R., de Villiers J.C. Bacterial intracranial aneurysms. Br J Neurosurg. 1993;7:367-376.
Barrow D.L., Prats A.R. Infectious intracranial aneurysms: comparison of groups with and without endocarditis. Neurosurgery. 1990;27:562-573.
Bingham W.F. Treatment of mycotic intracranial aneurysms. J Neurosurg. 1977;46:428-437.
Bohmfalk G.L., Story J.L., Wissinger J.P., et al. Bacterial intracranial aneurysms. J Neurosurg. 1978;48:369-382.
Bullock R., van Dellen J.R., van den Heever C.M. Intracranial mycotic aneurysms. S Afr Med J. 1981;60:970-973.
Chapot R., Houdart E., Saint-Maurice J.P., et al. Endovascular treatment of cerebral mycotic aneurysms. Radiology. 2002;222:389.
Chun J.Y., Smith W., Halbach V.V., et al. Current multimodality management of infectious intracranial aneurysms. Neurosurgery. 2001;48:1203-1214.
Corr P., Wright M., Handler L.C. Endocarditis-related cerebral aneurysms: radiologic changes with treatment. AJNR Am J Neuroradiol. 1995;16:745-748.
Dhomne S., Rao C., Shrivastava M., et al. Endovascular management of ruptured cerebral mycotic aneurysms. Br J Neurosurg. 2008;22:46-52.
Eddelman C.S., Surdell D., DiPatri A., et al. Infectious intracranial aneurysms in the pediatric population: endovascular treatment with Onyx. Childs Nerv Syst. 2008;24:909-915.
Frazee J.G., Cahan L.D., Winter J. Bacterial intracranial aneurysms. J Neurosurg. 1980;53:633-641.
Kannoth S., Iyer R., Thomas S.V., et al. Intracranial infectious aneurysm: presentation, management and outcome. J Neurol Sci. 2007;256:3-9.
Kannoth S., Thomas S.V., Sarma P.S. Proposed diagnostic criteria for intracranial infectious aneurysms. J Neurol Neurosurg Psychiatry. 2008;79:943-946.
Kanter M., Hart R. Neurologic complications of infective endocarditis. Neurology. 1991;41:1015-1020.
Kojima Y., Saito A., Kim I. The role of serial angiography in the management of bacterial and fungal intracranial aneurysms. Neurol Med Chir (Tokyo). 1989;29:202-216.
Modi G., Ranchod K., Modi M., et al. Human immunodeficiency virus associated intracranial aneurysms: report of three adult patients with an overview of the literature. J Neurol Neurosurg Psychiatry. 2008;79:44-46.
Molinari G.F., Smith L., Goldstein M.N., et al. Pathogenesis of cerebral mycotic aneurysms. Neurology. 1973;23:325-332.
Monsuez J.J., Vittecoq D., Rosenbaum A., et al. Prognosis of ruptured intracranial mycotic aneurysms: a review of 12 cases. Eur Heart J. 1989;10:821-825.
Phuong L.K., Link M., Wijdicks E. Management of intracranial infectious aneurysms: a series of 16 cases. Neurosurgery. 2002;51:1145-1151.
Pruitt A.A., Rubin R.H., Karchmer A.W., et al. Neurologic complications of bacterial endocarditis. Medicine (Baltimore). 1978;57:329-343.
Roach M.R., Drake C.G. Ruptured cerebral aneurysms caused by microaneurysms. N Engl J Med. 1965;273:240-244.
Rout D., Sharma A., Mohan P.K., et al. Bacterial aneurysms of the intracavernous carotid artery. J Neurosurg. 1984;60:1236-1242.
Salgado A.V., Furlan A.J., Keys T.F. Mycotic aneurysm, subarachnoid hemorrhage and indications for cerebral angiography in infective endocarditis. Stroke. 1987;18:1057-1067.
Scotti G., Li M.H., Righi C., et al. Endovascular treatment of bacterial intracranial aneurysms. Neuroradiology. 1996;38:186-189.
Sundaram C., Goel D., Uppin S.G., et al. Intracranial mycotic aneurysm due to Aspergillus species. J Clin Neurosci. 2007;14:882-886.
Suwanwela C., Suwanwela N., Charuchinda S., et al. Intracranial mycotic aneurysms of extravascular origin. J Neurosurg. 1972;36:552-559.
Tunkel A.R., Kaye D. Neurologic complications of infective endocarditis. Neurol Clin. 1993;11:419-440.
Venkatesh S.K., Phadke R.V., Kalode R.R., et al. Intracranial infective aneurysms presenting with hemorrhage: an analysis of angiographic findings, management and outcome. Clin Radiol. 2000;55:946-953.
1. Church W.S. Aneurysm of the right cerebral artery in a boy of thirteen. Trans Pathol Soc Lond. 1869;20:109-110.
2. Osler W. Gulstonian lectures on malignant endocarditis. Lancet. 1885;1:415-418. 459-464, 505–508
3. Stengel A., Wolferth C.C. Mycotic (bacterial) aneurysms of intravascular origin. Arch Intern Med. 1923;31:527-554.
4. Shnider B.I., Cotsonas N.J.Jr. Embolic mycotic aneurysms: a complication of bacterial endocarditis. Am J Med. 1954;16:246-255.
5. Fearnsides E.G. Intracranial aneurysms. Brain. 1916;39:224-296.
6. McDonald C.A., Korb M. Intracranial aneurysms. Arch Neurol Psychiatry. 1939;42:298-328.
7. Chun J.Y., Smith W., Halbach V.V., et al. Current multimodality management of infectious intracranial aneurysms. Neurosurgery. 2001;48:1203-1214.
8. Frazee J.G., Cahan L.D., Winter J. Bacterial intracranial aneurysms. J Neurosurg. 1980;53:633-641.
9. Kannoth S., Iyer R., Thomas S.V., et al. Intracranial infectious aneurysm: presentation, management and outcome. J Neurol Sci. 2007;256:3-9.
10. Kojima Y., Saito A., Kim I. The role of serial angiography in the management of bacterial and fungal intracranial aneurysms. Neurol Med Chir (Tokyo). 1989;29:202-216.
11. Venkatesh S.K., Phadke R.V., Kalode R.R., et al. Intracranial infective aneurysms presenting with hemorrhage: an analysis of angiographic findings, management and outcome. Clin Radiol. 2000;55:946-953.
12. Roach M.R., Drake C.G. Ruptured cerebral aneurysms caused by microaneurysms. N Engl J Med. 1965;273:240-244.
13. Ziment I., Johnson B.L.Jr. Angiography in the management of intracranial mycotic aneurysms. Arch Intern Med. 1968;122:349-352.
14. Suwanwela C., Suwanwela N., Charuchinda S., et al. Intracranial mycotic aneurysms of extravascular origin. J Neurosurg. 1972;36:552-559.
15. Bohmfalk G.L., Story J.L., Wissinger J.P., et al. Bacterial intracranial aneurysms. J Neurosurg. 1978;48:369-382.
16. Bullock R., van Dellen J.R., van den Heever C.M. Intracranial mycotic aneurysms. S Afr Med J. 1981;60:970-973.
17. Rout D., Sharma A., Mohan P.K., et al. Bacterial aneurysms of the intracavernous carotid artery. J Neurosurg. 1984;60:1236-1242.
18. Monsuez J.J., Vittecoq D., Rosenbaum A., et al. Prognosis of ruptured intracranial mycotic aneurysms: a review of 12 cases. Eur Heart J. 1989;10:821-825.
19. Barrow D.L., Prats A.R. Infectious intracranial aneurysms: comparison of groups with and without endocarditis. Neurosurgery. 1990;27:562-573.
20. Ahmadi J., Tung H., Giannotta S.L., et al. Monitoring of infectious intracranial aneurysms by sequential computed tomography/magnetic resonance imaging studies. Neurosurgery. 1993;32:45-50.
21. Aspoas A.R., de Villiers J.C. Bacterial intracranial aneurysms. Br J Neurosurg. 1993;7:367-376.
22. Corr P., Wright M., Handler L.C. Endocarditis-related cerebral aneurysms: radiologic changes with treatment. AJNR Am Jour Neuroradiol. 1995;16:745-748.
23. Holtzman R.N.N., Brust J.C.M., Hughes J.E.O., et al. Surgical management of infected intracranial aneurysms, in Operative Neurosurgical Techniques, Schmidek HH and Sweet WH. Philadelphia: WB Saunders; 1995. 1133-1154
24. Scotti G., Li M.H., Righi C., et al. Endovascular treatment of bacterial intracranial aneurysms. Neuroradiology. 1996;38:186-189.
25. Chapot R., Houdart E., Saint-Maurice J.P., et al. Endovascular treatment of cerebral mycotic aneurysms. Radiology. 2002;222:389.
26. Phuong L.K., Link M., Wijdicks E. Management of intracranial infectious aneurysms: a series of 16 cases. Neurosurgery. 2002;51:1145-1151.
27. Luders J.C., Steinmetz M.P., Mayberg M.R. Awake craniotomy for microsurgical obliteration of mycotic aneurysms: technical report of three cases. Neurosurgery. 2005;56:E201.
28. Nakahara I., Taha M.M., Higashi T., et al. Different modalities of treatment of intracranial mycotic aneurysms: report of 4 cases. Surg Neurol. 2006;66:405-409.
29. Dhomne S., Rao C., Shrivastava M., et al. Endovascular management of ruptured cerebral mycotic aneurysms. Br J Neurosurg. 2008;22:46-52.
30. Ojemann R.F. Surgical management of bacterial intracranial aneurysms. In: Schmidek H.H., Sweet W.H. Operative Neurosurgical Techniques. Orlando: Grune & Stratton; 1988:997-1001.
31. Salgado A.V., Furlan A.J., Keys T.F. Mycotic aneurysm, subarachnoid hemorrhage and indications for cerebral angiography in infective endocarditis. Stroke. 1987;18:1057-1067.
32. Bandoh K., Sugimura J., Hosaka K., et al. Ruptured intracranial mycotic aneurysm associated with acute subdural hematoma: case report. Neurol Med Chir (Tokyo). 1987;27:56-59.
33. Ho K.L. Acute subdural hematoma and intracerebral hemorrhage: rare complications of rhinocerebral mucormycosis. Arch Otolaryngol Head Neck Surg. 1979;105:279-281.
34. Bingham W.F. Treatment of mycotic intracranial aneurysms. J Neurosurg. 1977;46:428-437.
35. Tomita T., McClone D., Naidich T.P. Mycotic aneurysm of the intracavernous portion of the carotid artery in childhood: case report. J Neurosurg. 1981;54:681-684.
36. Weisman A.D. Cavernous-sinus thrombophlebitis: report of a case with multiple cerebral infarcts and necrosis of the pituitary body. N Engl J Med. 1944;231:118-122.
37. Funakoshi T., Tsuchiya J., Sakai N., et al. Peripheral arterial aneurysm of the brain occurring after brain abscess extirpation and healing spontaneously: report of a case and review of the literature. No Shinkei Geka Apr. 1976;4:405-410.
38. Sato T., Sakuta Y., Suzuki J., et al. Successful surgical treatment of intracranial mycotic aneurysm with brain abscess: report of a case. Acta Neurochir (Wien). 1979;47:53-61.
39. Heydenrych J.J. Mycotic aneurysm: a rare complication of acute osteomyelitis in a child. S Afr Med J. 1985;68:609-610.
40. Morriss F.H.Jr., Spock A. Intracranial aneurysm secondary to mycotic orbital and sinus infection: report of a case implicating Penicillium as an opportunistic fungus. Am J Dis Child. 1970;119:357-362.
41. Du Plessis J.J., van Rensburg M.J. Bacterial aneurysm of the intracavernous carotid artery secondary to suppurative otitis media. S Afr Med J. 1988;73:185-186.
42. Tunkel A.R., Kaye D. Neurologic complications of infective endocarditis. Neurol Clin. 1993;11:419-440.
43. Kanter M., Hart R. Neurologic complications of infective endocarditis. Neurology. 1991;41:1015-1020.
44. Pruitt A.A., Rubin R.H., Karchmer A.W., et al. Neurologic complications of bacterial endocarditis. Medicine (Baltimore). 1978;57:329-343.
45. Hart R.G., Kagan-Hallet, Joerns S.E. Mechanisms of intracranial hemorrhage in infective endocarditis. Stroke. 1987;18:1048-1056.
46. Masuda J., Yutani C., Waki R., et al. Histopathological analysis of the mechanisms of intracranial hemorrhage complicating infective endocarditis. Stroke. 1992;23:843-850.
47. Jones H.R.Jr., Siekert R.G., Geraci J.E. Neurologic manifestations of bacterial endocarditis. Ann Intern Med. 1969;71:21-28.
48. Molinari G.F., Smith L., Goldstein M.N., et al. Pathogenesis of cerebral mycotic aneurysms. Neurology. 1973;23:325-332.
49. Yen P.S., Teo B.T., Chen S.C., et al. Endovascular treatment for bilateral mycotic intracavernous carotid aneurysms. Case report and review of the literature. J Neurosurg. 2007;107:868-872.
50. Ahuja G.K., Jain N., Vijayaraghaven M., et al. Cerebral mycotic aneurysm of fungal origin. J Neurosurg. 1978;49:107.
51. Stehbens W.E. Subarachnoid hemorrhage. In: Pathology of the Cerebral Blood Vessels. St Louis: CV Mosby; 1972:252-283.
52. Martinez A.J., Sotelo-Avila C., Alcala H., et al. Granulomatous encephalitis, intracranial arteritis and mycotic aneurysm due to a free-living ameba. Acta Neuropathol (Berl). 1980;49:7-12.
53. O’Donohue J.M., Enzmann D.R. Mycotic aneurysm in angiitis associated with herpes zoster ophthalmicus. AJNR Am J Neuroradiol. 1987;8:615-619.
54. Modi G., Ranchod K., Modi M., et al. Human immunodeficiency virus associated intracranial aneurysms: report of three adult patients with an overview of the literature. J Neurol Neurosurg Psychiatry. 2008;79:44-46.
55. Martinez-Longoria C.A., Morales-Aguirre J.J., Villalobos-Acosta C.P., et al. Occurrence of intracerebral aneurysm in an HIV-infected child: a case report. Pediatr Neurol. 2004;31:130-132.
56. Mazzoni P., Chiriboga C.A., Millar W.S., et al. Intracerebral aneurysms in human immunodeficiency virus infection: case report and literature review. Pediatr Neurol. 2000;23:252-255.
57. Mielke B., Weir B., Oldring D., et al. Fungal aneurysms: case report and review of the literature. Neurosurgery. 1981;9:578-582.
58. Sundaram C., Goel D., Uppin S.G., et al. Intracranial mycotic aneurysm due to Aspergillus species. J Clin Neurosci. 2007;14:882-886.
59. Van der Meulen J.H.P., Weststrate W., Gijn J., et al. Is cerebral angiography indicated in infective endocarditis? Stroke. 1992;23:1662-1667.
60. Kannoth S., Thomas S.V., Sarma P.S. Proposed diagnostic criteria for intracranial infectious aneurysms. J Neurol Neurosurg Psychiatry. 2008;79:943-946.
61. Cantu R.C., LeMay M., Wilkinson H.A. The importance of repeated angiography in the treatment of mycotic–embolic intracranial aneurysms. J Neurosurg. 1966;25:189-193.
62. Morawetz R.B., Karp R.B. Evolution and resolution of intracranial bacterial (mycotic) aneurysms. Neurosurgery. 1984;15:43-49.
63. Moskowitz M.A., Rosenbaum A.E., Tyler H.R. Angiographically monitored resolution of cerebral mycotic aneurysms. Neurology. 1974;24:1103-1108.
64. Venger B.H., Aldama A.E. Mycotic vasculitis with repeated intracranial aneurysmal hemorrhage. Case report. J Neurosurg. 1988;69:775-779.
65. Eddelman C.S., Surdell D., DiPatri A., et al. Infectious intracranial aneurysms in the pediatric population: endovascular treatment with Onyx. Childs Nerv Syst. 2008;24:909-915.
66. Sugg R.M., Weir R., Vollmer D.G., Cacayorin E.D. Cerebral mycotic aneurysms treated with a neuroform stent: technical case report. Neurosurgery. 2006;58:E381.
67. Asai T., Usui A., Miyachi S., Ueda Y. Endovascular treatment for intracranial mycotic aneurysms prior to cardiac surgery. Eur J Cardiothorac Surg. 2002;21:948-950.
68. Origitano T.C., Anderson D.E. CT angiographic–guided frameless stereotactic assisted clipping of a distal posterior inferior cerebellar artery aneurysm: technical case report. Surg Neurol. 1996;46:450-453.
69. Steinberg G.K., Guppy K.H., Adler J.R.Jr., Silverberg G.D. Stereotactic, angiographic-guided clipping of a distal, mycotic intracranial aneurysm using the Cosman-Roberts-Wells system: technical note. Neurosurgery. 1992;30:408-411.
70. Day A.L. Extracranial intracranial bypass grafting in the surgical treatment of bacterial aneurysms: report of 2 cases. Neurosurgery. 1981;9:583-588.
71. Eguchi T., Nakagomi T., Teraoka A. Treatment of bilateral mycotic intracavernous carotid aneurysms. Case report. J Neurosurg. 1982;56:443-447.
72. Hadley M.N., Spetzler R.F., Martin N.A., et al. Middle cerebral artery aneurysm due to Nocardia asteroides: case report of aneurysm excision and extracranial–intracranial bypass. Neurosurgery. 1988;22:923-928.