25 Management of end-stage liver disease and liver transplantation
Case
A 57-year-old female with primary biliary cirrhosis presents with new onset peripheral oedema and abdominal swelling. Her husband has noticed increasing jaundice and mild attention and concentration changes. Vital signs reveal a mild fever of 37.3° Celsius. The examination is notable for mild confusion, scleral icterus, asterixis, peripheral oedema and a distended abdomen with shifting dullness. Initial laboratory work reveals a total bilirubin of 9.5 mg/dL (normal range 0.1 –1.0), alanine amino-transferase of 68 U/L (7–45), aspartate amino-transferase of 87 U/L (8–43) and alkaline phosphatase of 322. The International Normalized Ratio (INR) is elevated at 1.3 (0.9–1.2) as is the creatinine at 1.5 mg/dL (0.6–1.1), both of which had been previously normal.
End-stage Liver Disease
Compensated cirrhosis
The most common severity assessment tools in use are the Child-Turcotte-Pugh (CTP) score and the Model for End Stage Liver Disease (MELD) score. The CTP score was originally devised to risk stratify cirrhotic patients in need of portocaval shunt surgery due to oesophageal variceal bleeding and incorporates bilirubin, albumin and prothrombin time as well as encephalopathy and ascites measurements (see Table 24.6).
The MELD score was first developed to predict short-term prognosis in patients undergoing TIPS. The MELD score (http://www.unos.org/resources/meldpeldcalculator.asp) is based on three continuous, objective variables: bilirubin, creatinine and the INR of prothrombin time. Patients are assigned a score based on these three variables, from 6 through 40, corresponding to a 3-month survival of nearly zero and over 80% respectively. The MELD score has been validated as an accurate predictor of survival in acute liver failure, alcoholic hepatitis, a variety of chronic liver diseases and variceal bleeding, amongst others.
A patient with cirrhosis who has not developed jaundice or portal hypertensive complications of hepatic encephalopathy, ascites or variceal bleeding is ‘well compensated’, meaning he or she has adequate hepatic reserve. These patients are often categorised as Child-Turcotte-Pugh class (CTP) A. The median survival age in this group is 9–12 years. Since there are no available medical treatments to reverse cirrhosis, most management strategies are directed towards prevention.
Medication counselling
Paracetamol (acetaminophen) can be hepatotoxic above a dose of 4 g/day. Certainly, patients with concurrent alcohol use or malnutrition can experience additional liver injury at lower doses of paracetamol. In this subset of patients, it is probably safest to avoid chronic dosing of paracetamol, but a low dose (2 g/day) one time or infrequent dosing appears safe. In other cirrhotic patients, paracetamol at doses under 4 g/day for short periods of time appear safe, but less than 2.6 g/day is now the standard of care. The chronic use of paracetamol has not been studied in cirrhotic patients, thus most hepatologists will recommend dose reduction (2 g/day in divided doses) for longer term dosing. Many over-the-counter herbal medications can be hepatotoxic and can cause acute liver failure and thus should be avoided.
Hepatocellular carcinoma
Several treatment options are available for HCC including resection, locoregional therapy such as transarterial chemoembolisation (TACE), radiofrequency ablation (RFA) and liver transplantation. Radiofrequency ablation technique utilises a probe inserted percutaneously into the tumour with ultrasound or CT guidance and induces coagulative necrosis from heat generated by electromagnetic radiation. TACE delivers small embolic particles and a chemotherapeutic agent (cisplatin or doxorubicin commonly) to deprive the tumour of its vascular supply and concomitantly deliver cytotoxic therapy, resulting in tumour hypoxaemia and necrosis.
Hepatic encephalopathy
Hepatic encephalopathy is a set of potentially reversible neuropsychiatric symptoms seen in patients with liver dysfunction. The symptoms can range from mild inattention and disorientation to coma. Hepatic encephalopathy may also be accompanied by an elevation in venous ammonia values, but these values have not been found to correlate well with the presence, absence or grade of encephalopathy. Treatment requires correction of precipitating factors such as gastrointestinal haemorrhage, uraemia, hypoxia, use of psychoactive medication, infection, constipation, electrolyte disturbance and, very rarely, high protein intake. Treatment should include lactulose (oral, nasogastric or rectal) until the bowels begin to move, and then titrated to 2–3 loose stools per day. An alternative therapy is neomycin, but this is rarely used due to the risk of ototoxicity and nephrotoxicity. For lactulose refractory cases, rifaximin has been used with some success. Rifaximin is not superior to lactulose, and is much more expensive. Nonetheless, some data suggest that use of rifaximin (when added to lactulose) is effective for secondary prophylaxis of acute hepatic encephalopathy and may prevent recurrent hospitalisation for hepatic encephalopathy. L-ornithine L-aspartate (LOLA) is another option for lactulose refractory hepatic encephalopathy, and stimulates the urea cycle, leading to decreased ammonia levels. Administration of LOLA for treatment of overt hepatic encephalopathy has been shown to be superior to placebo although the beneficial effects when combined with lactulose remain unknown. Diet is thought to play an important role in hepatic encephalopathy, although it is rare to see a high-protein meal trigger worsening encephalopathy. Skeletal muscle is important for removing ammonia from the blood stream and low protein intake may result in muscle mass loss. Vegetable proteins produce less ammonia than animal proteins and may be preferred. As a rule, protein restriction is rarely required.
Ascites
The term ‘refractory ascites’ encompasses diuretic resistant and diuretic intractable (intolerable) ascites. Diuretic resistant ascites fails to resolve despite maximal medical therapy whereas diuretic intractable ascites refers to failure to control ascites due to medication side effects. In this setting, intermittent large volume paracenteses are the next step. Diuretics, if tolerated, should be continued if urinary excretion of sodium is more than 30 mEq/L. A transjugular intrahepatic portal systemic shunt procedure is second-line therapy for refractory ascites, but should be considered in patients requiring over one or two large volume paracenteses per month and is more likely to be effective in the setting of normal renal function. Contraindications for transjugular intrahepatic portal systemic shunt are shown in Table 25.1.
Spontaneous bacterial peritonitis
Spontaneous bacterial peritonitis (SPB) can complicate ascites and historically resulted in mortality greater than or equal to 80%. With heightened clinical suspicion for SBP and early antibiotic therapy, mortality can be reduced drastically. Blood cultures may be positive in about 50% of cases and should be obtained around the time of paracentesis. The diagnosis of SBP is made if the cell count reveals more than 250 polymorphonuclear cells per mm3 or the patient has positive ascitic fluid cultures in addition to clinical symptoms/signs of SBP. Symptoms and signs of SBP include worsening hepatic encephalopathy, fever, elevated/reduced white count, abdominal pain or tenderness, and renal dysfunction. It is important to note that patients with this infection may also be asymptomatic. Once the diagnosis is made, treatment should be initiated with intravenous cefotaxime or the equivalent. Intravenous albumin infusions should be administered at 1.5 g/kg on the day of diagnosis followed by 1.0 g/kg on day 3 to prevent further renal dysfunction. Reassess after 5 days of therapy. If symptoms or white count persist, repeating paracentesis and the cell count will determine whether or not the infection has been eradicated. Primary antibiotic prophylaxis for patients with low ascitic fluid total protein (under 1.5 g) is recommended. Secondary prophylaxis (after first episode) is recommended for all patients due to the high (about 70%) 1-year recurrence rate. Norfloxacin 400 mg/day is the most studied in this setting, but other quinolones (ciprofloxacin) or sulfamethoxazole/trimethoprim can be used.
Renal failure
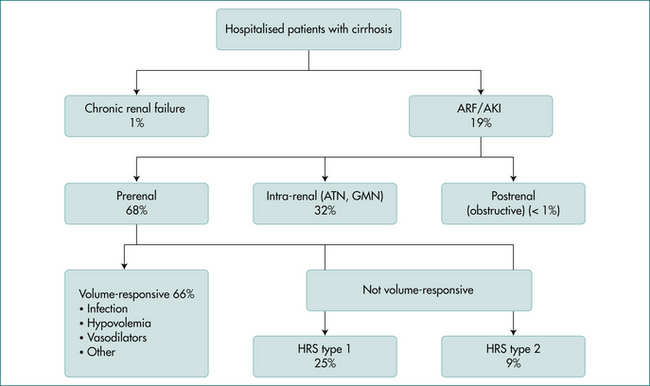
Renal failure in the cirrhotic patient is an important prognostic sign. Evidence consistently shows that renal failure, now called acute kidney injury, predicts early mortality in cirrhotic patients and is one of three variables used in the Model for End Stage Liver Disease (MELD) score. Aetiologies of acute kidney injury include hypovolaemia, sepsis, acute tubular necrosis, nephrotoxic drugs, and hepatorenal syndrome (Fig 25.1). Evaluation of acute kidney injury should begin by ruling out infection, and urinalysis and urine sodium measurement should also be performed. In all forms of acute kidney injury, the clinician should discontinue diuretics, lactulose and nephrotoxic agents.
Patients with hepatorenal syndrome also do not respond well to intravascular volume expansion. This syndrome is unique to liver disease patients and occurs due to renal vasoconstriction in reaction to systemic vasodilation (decreased effective circulating volume). Type 1 hepatorenal syndrome occurs in patients with advanced liver disease and ascites. It is by definition rapidly progressive and results in a median survival of approximately 4 weeks without liver transplantation. Diagnostic criteria are listed in Box 25.1. Type 2 hepatorenal syndrome is a milder form of renal failure that occurs over a period of months. Hepatorenal syndrome is potentially reversible. There is usually a precipitating event (spontaneous bacterial peritonitis, gastrointestinal bleeding). If this inciting factor is addressed, and adequate intravenous albumin has not resulted in improvement in the creatinine (and other causes are ruled out), then hepatorenal syndrome treatments should be initiated. Terlipressin or the combination of octreotide plus midodrine is initiated in conjunction with intravenous albumin infusions. The goal is to increase the mean arterial pressure by 15 mmHg. Treatment should be continued for 7–14 days or until liver transplantation. Liver transplantation is the only definitive treatment for type 1 hepatorenal syndrome.
Box 25.1 Diagnostic criteria for hepatorenal syndrome type 1
IV = intravenous; max = maximum; RBC = red blood cell; hpf = high power field.
Adapted from Salerno et al. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 2007; 56(9):1310–1318.
Orthotopic Liver Transplantation
After a liver transplant, patients are generally followed closely by the transplant centre, particularly with respect to immunosuppression and graft-related issues. Most patients are discharged home between 2 and 4 weeks after transplant, depending on the distance they must travel. Patients have regular blood work at least weekly for the first 4–8 weeks until liver and renal functions are stable and immunosuppression levels are satisfactory. After the first 8 weeks, blood work may be performed less frequently with longer intervals between blood draws as per transplant centre protocols. Most patients will return to the transplant centre between 3 and 6 months for a follow-up appointment. Many centres will see the patient at least annually, but every centre may be different. Most transplant programs will adjust all transplant immunosuppression medications. Transplant programs will follow the patient for many of their ongoing medical issues, but most transplant centres rely heavily on the family practitioner to manage the patient’s chronic care issues. These issues include diabetes, hypertension, hyperlipidaemia, bone disease and other chronic ailments, as well as acute issues such as infections or even pregnancy. Treatment of all of these issues is generally similar to that of the general population, but with careful choices for medications, as many drugs interact with immunosuppression medications (Box 25.2). If there is any doubt, discussing the case with the transplant centre physicians is the best choice.
Box 25.2
| Increase levels of calcineurin inhibitor | ||
|---|---|---|
| Antimicrobial | Calcium channel blockers | Others |
| Caspofungin | Diltiazem | Danazol |
| Azoles∗ | Verapamil | Grapefruit juice |
| Terbinafine Macrolides† | Amlodipine (less) | †Amlodipine (less)Diazepam, alprazolam |
| ChloroquineProtease inhibitors | Felodipine (less)Nicardipine | AllopurinolSertraline, nefazodone |
| Decrease levels of calcineurin inhibitor | ||
|---|---|---|
| Antimicrobials | Anticonvulsants | Others |
| Rifampin | Carbamazepine | Orlistat |
| Rifabutin | PhenobarbitalPhenytoin | St John’s wort |
∗ Azoles = ketoconazole, fluconazole, miconazole, voriconazole
† Macrolides = clarithromycin, erythromycin, azithromycin (less so)
Key Points
Bass N.M., Mullen K.D., Sanyal A., et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
Blei A.T., Cordoba J. Hepatic encephalopathy. Am J Gastroenterol. 2001;96:1968-1976.
Boyer T.D., Haskal Z.J. American Association for the Study of Liver Diseases practice guidelines: the role of transjugular intrahepatic portosystemic shunt creation in the management of portal hypertension. J Vasc Interv Radiol. 2005;16:615-629.
Boyer T.D., Haskal Z.J. The role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology. 2009;51:306.
Bruix J., Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236.
D’Amico G., Garcia-Tsao G., Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231.
Fernandez J., Ruiz del Arbol L., Gomez C., et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology. 2006;131:1049-1056.
Garcia-Tsao G., Lim J.K. Management and treatment of patients with cirrhosis and portal hypertension: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program. Am J Gastroenterol. 2009;104:1802-1829.
Garcia-Tsao G., Parikh C.R., Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064-2077.
Gonzalez R., Zamora J., Gomez-Camarero J., et al. Meta-analysis: combination endoscopic and drug therapy to prevent variceal rebleeding in cirrhosis. Ann Intern Med. 2008;149:109-122.
Hanje A.J., Patel T. Preoperative evaluation of patients with liver disease. Nat Clin Pract Gastroenterol Hepatol. 2007;4:266-276.
Kamath P.S., Kim W.R. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797-805.
Korenblat K.M., Mazariegos G.V., Moonka D., et al. Long-term management of the liver transplant patient: recommendations for the primary care doctor. Am J Transplant. 2009;9:1988-2003.
McGuire B.M., Rosenthal P., Brown C.C., et al. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-1318.
Murray K.F., Carithers R.L.Jr. AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407-1432.