63 Management of Emergencies Related to Implanted Cardiac Devices
• Chest compressions should not be performed on patients with an implanted left ventricular assist device who are in cardiac arrest because the device may shear the aorta.
• Major failure of an implanted left ventricular assist device is extremely serious but not catastrophic; patients’ minimal but present cardiac function gives time for temporizing maneuvers.
• Patients who have had a cardioverter-defibrillator implanted and experience multiple shocks per day are deemed to be in electrical storm and should be managed very aggressively for ischemia and electrolyte abnormalities.
• A magnet changes most pacemakers to a continuous, preset rate, usually about 80 beats/min. When a magnet is moved away from a pacemaker, the device returns to the previously programmed, baseline function. Implantable cardioverter-defibrillators may need to be reprogrammed after exposure to a magnet.
Introduction
The use of implantable devices to support the electrical function of the heart has become widespread since they were first described in the early 1950s.1 Most recently, the use of ventricular assist devices has become more common in the scenario of an increasing number of patients with severe congestive heart failure (CHF).2
Currently, millions of patients in the United States have such implants, and they seek care in emergency departments (EDs) because of complications related to these devices and the conditions that led to the implantation.3
Implantable Cardioverter-Defibrillators
An ICD is the first line of therapy for prevention of sudden cardiac death, which is commonly the result of ventricular fibrillation (VF) or ventricular tachycardia (VT).4 The first internal defibrillator was implanted in 1980 by Mirowski and Mower. Since then, the technology and indications have grown enormously. More than 120,000 devices are implanted in the United States every year.5 Given this scenario, emergency physicians (EPs) face a growing number of patients with increasing complexity who are arriving at EDs with ICD-related complaints.
General Concepts of Function
Current ICDs correspond to third-generation devices, which are small (40 mL) and reliable and contain sophisticated electrophysiologic analysis algorithms. They can store and report a large number of variables, such as electrocardiograms (ECGs), defibrillation logs, energies, lead impedance, and battery charge.6 ICDs are usually implanted in the left infraclavicular area via a transvenous technique.
The ICD unit consists of a case containing the battery, circuitry, and pulse generator; a right ventricular apex lead for sensing and defibrillation; and an atrial lead. Current devices for biventricular pacing can be equipped with a coronary venous lead. The diagnostic and treatment functions are configured during placement of the device, along with determination of the defibrillatory threshold (DFT) necessary for the specific patient.6,7 Typically, the ICD is set to deliver energies 5 to 10 J above the DFT. According to these specifications, the battery life of the modern lithium-silver-vanadium device is approximately 8 years but depends largely on the frequency of the shocks delivered. Most of the devices are equipped with a patient alert system that prevents clinically significant battery-related complications.7
• Antitachycardia pacing, generally for monomorphic tachycardias
• Low-energy defibrillation (±2 J synchronized with the R wave)
• High-energy defibrillation in which the shocks are delivered as a biphasic wave
These treatments can be felt by the patient as sensations varying from discomfort to frank pain.6
Approximately 50% of the patients will experience an ICD discharge in the first 2 years of use. To lower the incidence of ventricular and supraventricular arrhythmias, a considerable proportion of these patients receive adjunctive pharmacologic therapy, usually with amiodarone, sotalol, and statins.8
After the ICD delivers a shock, three scenarios are possible: successful defibrillation, continuation of the VT or VF, or conversion to another rhythm—usually pulseless electrical activity (PEA) or asystole. After an efficacious shock, the patient’s heart returns to a previous stable rhythm. If the patient continues in VT or VF, the device delivers five more rescue shocks, after which it reanalyzes the waveform.6,7 In case of postdefibrillation (or primary) bradycardia or asystole, the ICD can display antibradycardiac features similar to those of a VVI PM.
Up to 25% of patients can experience an inappropriate shock, defined as a shock delivered for a rhythm different from sustained VT or VF. The most common causes of inappropriate shocks are supraventricular tachycardias (e.g., atrial fibrillation, paroxysmal supraventricular tachycardia, sinus tachycardia), which are read as VT.6 Another common cause is misreading of T waves as part of the QRS complex, thereby duplicating the sensed rate. Occasionally, the leads can suffer mechanical damage, such as insulation defects or lead fracture, and such damage can cause electronic noise to be mistakenly detected as VT or VF. These problems are partially solved in modern “noncommitted” apparatuses, which can reanalyze the appropriateness of the rhythm after charging but before shocking.7
Currently, no evidence has shown that the electromagnetic fields from daily life artifacts can interfere significantly with defibrillators. However, it is recommended that patients with ICDs avoid placing their cell phones closer than 15 cm to the device and avoid long exposure to metal detectors or antitheft devices.7 Some medically related sources of interference such as electrocauteries can cause significant malfunction; therefore, interrogation of the device after exposure is recommended.9 Magnetic resonance imaging (MRI) is contraindicated in patients with ICDs given the risk for mechanical torque, thermal injury, and deprogramming.
Indications for Placement of an Implantable Cardioverter-Defibrillator
The major and commonly accepted indications for use of an ICD are summarized in Box 63.1. In recent years the indications and uses have expanded such that the current published guidelines have been outpaced.10 Multiple trials showing a significant reduction in sudden cardiac death have been followed by publications with similar results regarding primary prevention in selected populations with structural heart disease and a low ejection fraction, as well as in other populations with specific cardiac abnormalities.
Box 63.1 Commonly Accepted Indications for Placement of an Implantable Cardioverter-Defibrillator
Class I
Structural heart disease and episode of sustained VT
Syncope of unknown source with inducible VT or VF in electrophysiologic studies
Patients with LVEF < 35% (NYHA class II or III)
Patients with LVEF < 40% because of previous myocardial infarction with inducible VT or VF in electrophysiologic studies
Class II
Unexplained syncope with LV dysfunction
Hypertrophic cardiomyopathy with associated with risk factors for sudden cardiac death
Arrhythmogenic right ventricular dysplasia with associated risk factors for sudden cardiac death
Patients with long QT syndrome and syncope or VT while receiving treatment
Nonhospitalized patients waiting for a heart transplant
Patients with Brugada syndrome and an episode of syncope or VT
Patients with catecholaminergic polymorphic VT and an episode of syncope or VT while receiving treatment
Patients with cardiac sarcoidosis, giant cell myocarditis, or Chagas disease
Approach to Complications Related to the Implantation Procedure
During the early days of ICDs, with their large abdominal cases and pericardial leads, the morbidity and mortality associated with the procedure were considerable. With later use of the transvenous technique, the perioperative mortality rate for ICD placement is less than 0.8%.4 Nevertheless, infectious, lead-related, thromboembolic, and mechanical complications can occur.
The rate of pocket or lead infection has been reported to be between 2% and 7%.11 The most common pathogens are cutaneous flora, usually Staphylococcus aureus and Staphylococcus epidermidis. During the first year after ICD implantation, infections related to the device are primarily due to the procedure; after that period they are probably due to secondary seeding. From a clinical perspective, common infectious signs and symptoms in patients with ICDs are notoriously absent and patients may have only vague complaints. Infections of the case and leads have a wide incidence of about 1% to 12%. Diagnosis of a delayed hardware infection requires a high index of suspicion given the absence of a confirmatory ancillary test. Almost without exception, suspected or proven hardware infections require contact with the patient’s primary electrophysiologist, hospital admission, long-term intravenous antibiotic therapy, and potential removal of the device.7,11
Modern lead systems are extremely reliable but are still prone to fracture, malposition, dislodgment, and damage to the insulation. These defects commonly lead to electrical noise that can precipitate inappropriate shocks. In the evaluation of a patient with suspected lead malfunction, a chest radiograph is required for confirmation of proper positioning and integrity of the leads.4,7,11 Contacting the implant team for replacement of the lead is the only alternative possible for hardware failure. Problems related to the battery, pulse generator, and circuitry are extraordinarily rare.
Thromboembolic complications can be seen in as many as 30% of patients with ICDs; they usually involve the cephalic and subclavian veins and do not generally lead to ICD malfunction. Affected patients exhibit unilateral arm swelling, pain, discoloration, and paresthesias, which require evaluation with ultrasonography, venography, or computed tomography. Standard treatment with heparin and warfarin usually results in a good outcome.11
The many mechanical complications related to placement may be manifested early or in delayed fashion. A considerable number of patients experience some degree of tricuspid regurgitation, with approximately 10% of cases being clinically significant. In addition, there is a theoretic risk for fibrosis of the apical lead, which could increase the DFT and make the shocks ineffective. Later manifestations with life-threatening mechanical complications, such as cardiac perforation, cardiac tamponade, hemothorax, pneumothorax, and air embolism, are very rare.11
Approach to Problems Related to Function and Dysfunction
In a patient with complaints related to functioning of the ICD, the EP must consider that the majority of such patients have severe structural heart disease with a poor ejection fraction and that most of them are in end-stage CHF.7 They can have a myriad of symptoms; however, these symptoms can be approached systematically (Box 63.2). Evaluation of a patient with an ICD must start by placing the patient in a monitored setting with external defibrillator capacity.8,12
Cardiac Arrest
Causes of death in this population are PEA after VT or VF (29%), defibrillation failure (26%), primary PEA (16%), and refractory VT or VF (13%).12 When a patient is seen in VT- or VF-related cardiac arrest, the most likely scenario is that VT or VF occurred and the ICD correctly sensed and delivered the shocks but failed to achieve defibrillation. It is critical for the EP to recognize and treat correctable causes of VT and VF. Common causes in this population are ongoing ischemia, electrolyte disturbances (especially hypokalemia and hypomagnesemia), and the arrhythmic effect of drugs.4 Many such patients have non–VT/VF-associated cardiac arrest in the context of end-stage CHF; in these cases, disabling the device could be helpful in the resuscitative efforts. Disabling can be accomplished by placing a magnet over the surface of the case pocket. It is very important to remember that after a magnet is placed over the device, it must be assumed that the device is permanently disabled and reprogramming is needed. Standard advanced cardiac life support (ACLS) protocols must be followed both for VT/VF- and non–VT/VF-related causes of cardiac arrest, the only difference being that the external defibrillator paddles and patches should not be placed directly over the ICD case.
Unstable with Ongoing Shocks
• Inappropriate shocks leading to hemodynamic compromise
• Refractory VT with a pulse and delivery of multiple appropriate shocks
Patients can experience cardiovascular collapse caused by repetitive inappropriate shocks secondary to true or misdiagnosed supraventricular tachycardias. In such cases, treatment must start by decreasing the rate of the tachycardia with the usual pharmacologic treatment (e.g., beta-blockers, calcium channel blockers). If these measures fail, the next step is to disable the ICD. A considerable proportion of patients arrive at the ED in unstable bradycardia; in this setting the VVI function of the device will be helpful, but prompt recognition of the cause (commonly end-stage CHF) and standard ACLS treatment are mandatory.7
Those with sustained VT with a pulse and appropriately shocked by the device represent an in extremis population. The most critical intervention is the delivery of higher-energy shocks with an external defibrillator (regardless of whether the ICD is disabled) and rapid correction of the cause of the VT.7,8
Both populations require mandatory electrophysiologic consultation and interrogation of the device.
Electrical Storm
Electrical storm is commonly defined as more than two therapies (antitachycardia pacing or shocks) delivered in a 24-hour period.13 It is believed to affect about 10% of patients with ICDs7 and is a common complaint in these patients.8 Classically, such patients are rather stable but complain of several shocks delivered in the preceding hours. The significance of electrical storm is that it is usually a herald of life-threatening acute pathology, commonly acute cardiac ischemia, hyperkalemia, and decompensated CHF, thus placing these patients at immediate high risk for death.
Stable with a Recent Isolated Shock
The most important first step is to determine whether the shocks were appropriate.4 The patient should be placed in a monitored setting where the heart rhythm can be recorded, followed by a chest radiograph to evaluate for possible hardware failure (e.g., lead fracture) and basic laboratory tests to look for ischemia or electrolyte disturbances. Any signs or symptoms around the moment of the shock (e.g., chest pain, shortness of breath, chest trauma) should be noted. It is also important to inquire about new drugs or changes in dosage (especially for amiodarone). Arrhythmias discovered during monitoring, as well as metabolic causes of VT or VF, must be treated in the usual fashion.
Occasionally, patients complain of hearing a beep from the device. Some models can emit a beep in the event of battery discharge or another cause of malfunction. Stable patients with an isolated shock require interrogation of the ICD so that the underlying rhythms can be evaluated and the ICD can be reprogrammed.4 The decision for hospital admission is usually made jointly by the EP and the electrophysiologist based on the appropriateness of the shock, the availability of follow-up, and the overall clinical status of the patient. Patients who have experienced isolated appropriate shocks but have no change in cardiopulmonary status, no evidence of ischemia, and no electrolyte abnormalities can be discharged from the ED for follow-up with an electrophysiologist.14
Stable with Other Cardiorespiratory Symptoms
![]() Priority Actions
Priority Actions
Implantable Cardioverter-Defibrillator
Patients with ICD-related complaints must have prompt transport by emergency medical services to the closest emergency department.
Always place a patient with an ICD in a monitored bed.
In the case of a disabled or malfunctioning ICD, the defibrillator patches must be placed in the anteroposterior position during monitoring and away from the ICD pocket.
Gather focused clinical and technical information about the device, and contact the electrophysiologist taking care of the patient.
Obtain an anteroposterior chest radiograph and measurements of cardiac biomarkers and serum electrolytes.
The patient will most probably need admission to a unit with a monitored bed.
![]() Red Flags
Red Flags
Implantable Cardioverter-Defibrillator
• Multiple shocks probably represent electrical storm, which confers high risk for an adverse outcome in the patient.
• Chest pain before ICD discharge can be a sign of ischemia leading to ventricular tachycardia or ventricular fibrillation.
• Amiodarone and class I antidysrhythmic agents raise the defibrillatory threshold, and recent changes in the dose or interactions with other medications can cause failure of ICD defibrillation.
• A history of syncope may represent successfully treated ventricular tachycardia or fibrillation.
Tips and Tricks
Implantable Cardioverter-Defibrillator
The ICD does not prevent but only treats ventricular fibrillation or tachycardia. Always look for reversible causes: ischemia, hypokalemia, hypomagnesemia, proarrhythmic drugs.
If the ICD does not deactivate when a magnet is placed over it, try to place the magnet in the opposite corner of the ICD, or if the patient is very obese, use two magnets.
Always assume that the ICD is permanently disabled after being exposed to a magnet.
The most common cause of death in a patient with an ICD is pulseless electrical activity.
Always look for mechanical lead complications (fracture or dislodgement) on the chest radiograph, especially after the patient has experienced trauma or has undergone cardiopulmonary resuscitation.
Patients receiving multiple shocks may require at least mild anxiolysis.
ST abnormalities on the electrocardiogram are attributable to ICD shocks only if they occur soon after the shock event.
In patients with cardiorespiratory symptoms, it is always advisable to interrogate the device to obtain information regarding dysrhythmias, both supraventricular and ventricular.
![]() Patient Teaching Tips
Patient Teaching Tips
Implantable Cardioverter-Defibrillator
The occurrence of more than one shock makes urgent medical attention mandatory; the patient should call 911.
If the ICD is firing, the patient must avoid driving.
The patient should always carry the identification card for the ICD, as well as contact information (emergency phone number) for the treating cardiologist.
Pain or swelling in the pocket area could be a sign of infection; the patient should seek medical care as soon as possible.
It is recommended that a cell phone not be placed closer than 15 cm from the ICD and that the patient not linger in metal detectors or antitheft devices.
Left Ventricular Assist Devices
Currently, more than 5 million Americans are estimated to suffer from heart failure with an aggregate 5-year survival rate of 50%.15 The high mortality and poor quality of life in patients with late-stage heart failure has led to further study and proposed uses for LVADs; the device has now been approved as a therapy for candidates waiting for a heart transplant (bridge therapy) and for patients with end-stage heart failure who are not candidates for heart transplantation because of age or other medical comorbid conditions, known as destination therapy.16 Currently, nearly a thousand patients have undergone implantation of an LVAD as destination therapy for severe heart failure,17 but an estimated 30,000 to 60,000 patients per year could be eligible for the procedure.18
General Concepts of Function
Originally, most models consisted of mechanical pumps, which were diaphragm oscillators that generated pulsatile blow flow through the outflow tract. Current devices work on axial pump flow in which electromagnetic centrifugal or rotatory engines generate nonpulsatile flow; this translates to no pulse or a very faint pulse when examining such patients. These newer axial flow pumps confer several benefits such smaller pumps and drivelines, fewer moving parts, and more efficient use of energy. Among their disadvantages are the requirement for systemic anticoagulation and the lack of a backup method (e.g., the hand-operated pump in older models) when device failure occurs.19
Indications for Placement of a Left Ventricular Assist Device
Indications for LVAD placement can be categorized according to therapeutic goals (Box 63.3). Patients awaiting cardiac transplantation and those with New York Heart Association class IV heart failure (and who are ineligible for transplantation) are candidates for LVAD implantation. The Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial compared patients with optimal medical management alone and those with medical management and implantation of a HeartMate VE LVAD. The study, conducted over a period of 2 years, showed a relatively lower risk for death (48%) during the follow-up period in the LVAD group than in the medical management group.16 The Food and Drug Administration approved the LVAD device in 2002 as destination therapy for patients with heart failure. Further follow-up has continued to show better survival in LVAD recipients, as well as improved quality of life.20
Approach to Problems Related to Function and Dysfunction
As growing numbers of patients are undergoing LVAD implantation, it will be important for EPs to understand the major complications of these devices. Problems with LVADs can be divided into four broad categories (Box 63.4).
Hardware malfunction is not uncommon and can occur at a combined rate of about 0.87 events per patient per year.21 The incidence of life-threatening hardware failure can be as high as 7.8% during the implantation period, often related to pump or inflow valve problems.22,23 Because these patients are in extremis, it is paramount for the EP to know that chest compressions should almost never be performed in patients with an LVAD because the trauma from the compressions can shear the outflow tract from the aorta and cause massive hemorrhage. ACLS protocols can otherwise be followed during resuscitation of patients with an LVAD.
Infections
Infectious complications of the LVAD components are common, about 6 per 1000 device-days.24 The signs and symptoms can be deceiving given the paucity of classic infections. Pain located near the case or driveline is often a sign of hardware infection. Patients in whom such infections are suspected must be admitted to the hospital for intravenous antibiotic therapy under the care of the implantation team. Frequently, débridement and mobilization of the components (e.g., the driveline) must be performed.
Minor Device Failure
Minor device failure is a broad category related to malfunction of the hardware apart from the pump. Commonly, the patient has a nonfunctioning device secondary to a discharged battery or an external circuitry error.22 In older mechanical models, the pumping function of the LVAD can be supported manually until the battery or the control panel can be replaced. Before discharge from the hospital all patients undergoing LVAD implantation and their family members are taught how to disengage the device and attach a hand pump to continue blood flow through the LVAD. The hand pump should be carried with the patient at all times. Information about how to engage the hand pump system can be found on websites for each of the devices.17
Major Device Failure
A life-threatening emergency, major device failure is caused by outflow or inflow disconnections, flow valve problems, or pump failure, and patients are commonly initially seen in cardiogenic shock or cardiac arrest.22 The catastrophic nature of such an event requires prompt and aggressive cardiac life support and emergency consultation with the cardiovascular surgical team. The use of vasoactive drugs and intraaortic balloon pump support constitute the basis of management.
Tips and Tricks
Left Ventricular Assist Device
Assume that the patient has severe right ventricular failure and almost no left ventricular reserve.
Be aware of the type-specific devices common in the local population.
Stock troubleshooting manuals and batteries in the emergency department for devices common in the local population.
Exercise caution with the amount of fluid used for volume resuscitation.
In patients with axial flow pump devices, absence of a peripheral pulse is expected.
Pacemakers
In 1952 a PM became the first electronic implantable device when it was used to treat a patient with a high-degree block. A few years later, PM use became widespread for treating myriad conditions, from sick sinus rhythm to cardiac resynchronization.1 Now, millions of Americans have an implanted PM, with an estimated 425 new PMs per 100,000 people per year.25
General Concepts of Function
PMs have evolved and become more complex in recent years. A five-digit code system to describe function was developed by North American Society of Pacing and Electrophysiology and the British Pacing and Electrophysiology Group.26 It explains the sensing and pacing abilities and the expected actions (Table 63.1). Currently, the most common pacing modes are AAI, VVI, and DDD.
Table 63.1 North American Society of Pacing and Electrophysiology and the British Pacing and Electrophysiology Group Pacemaker Code
| Position | Action | Description |
| I | Chamber(s) paced | A = Atrium V = Ventricle D = Dual O = None |
| II | Chamber(s) sensed | A = Atrium V = Ventricle D = Dual O = None |
| III | Response to sensing | T = Triggered I = Inhibited D = Dual O = None |
| IV | Programmability | P = Simple M = Multi C = Communicating R = Rate modulation O = None |
| V | Antitachydysrhythmia functions | P = Pacing S = Shock D = Dual O = None |
Indications for Placement of a Pacemaker
There are many indications for PM placement, some of which are widely accepted whereas others are more controversial. Common indications include patients with high-degree atrioventricular block, symptomatic blocks, and sick sinus rhythm. Recent years have seen an increase in the use of dual-chamber PMs for cardiac resynchronization in patients with heart failure. Box 63.5 contains a summary of class I indications for PM placement.27
Box 63.5 Class I Indications for Placement of a Pacemaker
1. Third-degree and advanced second-degree atrioventricular (AV) block at any anatomic level and associated with any of the following conditions:
2. Second-degree AV block regardless of the type or site of block and with associated symptomatic bradycardia (level of evidence: B)
Approach to Complications Related to the Implantation Procedure
Infections have an incidence of about 1.9 events per 1000 device-years.28 Signs and symptoms such as malaise, pocket pain, or swelling may indicate infection. It is recommended that blood be collected for culture and broad-spectrum antibiotics be initiated with a focus on cutaneous flora. Admission and consultation with the primary electrophysiologist are recommended.
Thromboembolic disease is manifested similar to other causes, including pain, swelling, vein distention, and shortness of breath. Treatment does not differ from that for standard venous thromboembolism and rests on the use of anticoagulants. The decision to perform lead change or explantation is not an emergency, and it is usually done at the discretion of the implantation team.29
The incidence of tricuspid regurgitation after PM placement is relatively low. Fifty percent of these patients will have right heart failure, whereas others may remain asymptomatic. Valve replacement or annuloplasty is sometimes required.30
Approach to Problems Related to Function and Dysfunction
PM emergencies can be broadly divided into the following categories (Box 63.6):
1. Failure to capture, in which the pulse generator is sending an appropriate electrical impulse but it is not resulting in a heartbeat
2. Failure to pace, in which the device is not generating an electrical impulse
3. Failure to sense, in which the pacemaker is firing inappropriately despite a normal cardiac rhythm
Failure to Capture
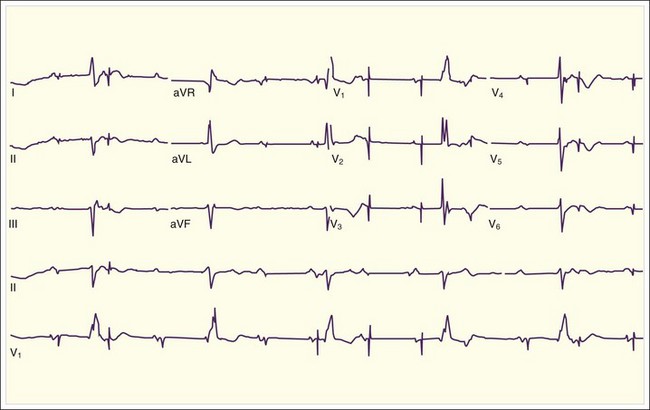
Failure to capture is defined as the PM being able to sense and deliver an electric stimulus but the electric current fails to elicit myocardial depolarization. Classically, PM spikes are present, but no atrial or ventricular activity follows (Fig. 63.1). Potential causes include lead dislodgment or malposition31 and inflammation at the electrode tip; as mentioned previously, a chest radiograph can assist in assessing lead location and damage. Similar to the previous scenario, the patient will have symptoms of the underlying disease. Standard ACLS management is recommended, in addition to consideration of a transcutaneous PM in patients dependent on a PM.
Failure to Pace
This type of failure is characterized as the PM being able to adequately sense but does not deliver an electric stimulus when it should do so. In failure to pace, PM spikes are absent from the ECG despite an abnormal or slow native rhythm. Causes include lead fracture, battery depletion, failure of the pulse generator, and most commonly, oversensing.31,32 Frequently, the scenario is defined as the sensing element of the PM being confused by other electrical signals such as muscular electrical activity, electrical noise, or ventricular signals. The device inappropriately recognizes these signals as atrial in nature and decides to inhibit the delivery of an electric impulse. Failure of the pulse generator can result from an internal malfunction, blunt trauma, and a number of iatrogenic causes, including MRI, radiation therapy, and electrocautery.31,33
Patients will have signs and symptoms related to the underlying disease, commonly with bradycardic rhythms and high atrioventricular blocks. Management consists of standards ACLS bradycardia management followed by interrogation and reprogramming of the PM.31,32 Use of a magnet may be helpful in converting the device into an asynchronous mode, thereby potentially bypassing the effect of oversensing.
Failure to Sense
Patients will have the signs and symptoms of CHF. In patients with no obvious trigger to CHF exacerbation, the possibility of PM undersensing must be considered. Device interrogation is highly recommended because information will be obtained about functioning of the device and will potentially be helpful in reprogramming the PM with the necessary new settings.32
Pacemaker-Induced Tachycardia
![]() Priority Actions
Priority Actions
Pacemaker
![]() Red Flags
Red Flags
Pacemaker
Look for neck and upper extremity venous distention as a sign of superior vena cava syndrome.
Look for the classic findings of the Beck triad—distended neck veins, muffled heart sounds, and hypotension—in a patient with cardiac tamponade caused by perforation of the myocardium by a lead, especially with recently placed devices.
Pacemaker-induced tachycardia is a rare complication that may respond to placement of a magnet over the pacemaker.
Tips and Tricks
Pacemaker
Understand pacemaker nomenclature (see Table 63.1).
Look for the presence of pacemaker spikes on the electrocardiogram; they can be very subtle or, rarely, not visible.
The presence of a bundle branch block pattern on the electrocardiogram should alert the clinician to the possibility of a paced rhythm.
Chan TC, Cardall TY. Electronic pacemakers. Emerg Med Clin North Am. 2006;24:179–194. vii
DiMarco JP. Implantable cardioverter-defibrillators. N Engl J Med. 2003;349:1836–1847.
Gehi AK, Mehta D, Gomes JA. Evaluation and management of patients after implantable cardioverter-defibrillator shock. JAMA. 2006;296:2839–2847.
John R. Current axial-flow devices—the HeartMate II and Jarvik 2000 left ventricular assist devices. Semin Thorac Cardiovasc Surg. 2008;20:264–272.
1 Jeffrey K, Parsonnet V. Cardiac pacing, 1960–1985: a quarter century of medical and industrial innovation. Circulation. 1998;97:1978–1991.
2 Copeland JG, Smith RG, Arabia FA, et al. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med. 2004;351:859–867.
3 Bernstein AD, Parsonnet V. Survey of cardiac pacing and implanted defibrillator practice patterns in the United States in 1997. Pacing Clin Electrophysiol. 2001;24:842–855.
4 Stevenson WG, Chaitman BR, Ellenbogen KA, et al. for the Subcommittee on Electrocardiography and Arrhythmias of the American Heart Association Council on Clinical Cardiology; Heart Rhythm Society. Clinical assessment and management of patients with implanted cardioverter-defibrillators presenting to nonelectrophysiologists. Circulation. 2004;110:3866–3869.
5 Mond HG, Irwin M, Ector H, et al. The world survey of cardiac pacing and cardioverter-defibrillators: calendar year 2005. An International Cardiac Pacing and Electrophysiology Society (ICPES) project. Pacing Clin Electrophysiol. 2008;31:1202–1212.
6 DiMarco JP. Implantable cardioverter-defibrillators. N Engl J. Med 2003;349:1836–1847.
7 Kowalski M, Huizar JF, Kaszala K, et al. Problems with implantable cardiac device therapy. Cardiol Clin. 2008;26:441–458. vii
8 Klein RC, Raitt MH, Wilkoff BL, et al. Analysis of implantable cardioverter defibrillator therapy in the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial. J Cardiovasc Electrophysiol. 2003;14:940–948.
9 Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120(21):e169–e276.
10 Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51(21):e1–62.
11 Pavia S, Wilkoff B. The management of surgical complications of pacemaker and implantable cardioverter-defibrillators. Curr Opin Cardiol. 2001;16:66–71.
12 Mitchell LB, Pineda EA, Titus JL, et al. Sudden death in patients with implantable cardioverter defibrillators: the importance of post-shock electromechanical dissociation. J Am Coll Cardiol. 2002;39:1323–1328.
13 Israel CW, Barold SS. Electrical storm in patients with an implanted defibrillator: a matter of definition. Ann Noninvasive Electrocardiol. 2007;12:375–382.
14 Gehi AK, Mehta D, Gomes JA. Evaluation and management of patients after implantable cardioverter-defibrillator shock. JAMA. 2006;296:2839–2847.
15 Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018.
16 Rose ER, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443.
17 Deng MC, Edwards LB, Hertz MI, et al. Mechanical circulatory support device database of the International Society for Heart and Lung Transplantation: third annual report—2005. J Heart Lung Transplant. 2005;24:1182–1187.
18 Copeland JG. Multicenter bridge to transplantation with the HeartMate assist device: evaluation from another perspective. J Thorac Cardiovasc Surg. 2003;125:228–230.
19 John R. Current axial-flow devices—the HeartMate II and Jarvik 2000 left ventricular assist devices. Semin Thorac Cardiovasc Surg. 2008;20:264–272.
20 Park SJ, Tector A, Piccioni W, et al. Left ventricular assist devices as destination therapy: a new look at survival. J Thorac Cardiovasc Surg. 2005;129:9–17.
21 Dembitsky WP, Tector AJ, Park S, et al. Left ventricular assist device performance with long-term circulatory support: lessons from the REMATCH trial. Ann Thorac Surg. 2004;78:2123–2130.
22 Shinn JA. Implantable left ventricular assist devices. J Cardiovasc Nurs. 2005;20(Suppl):S22–S30.
23 Birks EJ, Tansley PD, Yacoub MH, et al. Incidence and clinical management of life-threatening left ventricular assist device failure. J Heart Lung Transplant. 2004;23:964–969.
24 Gordon SM, Schmitt SK, Jacobs M, et al. Nosocomial bloodstream infections in patients with implantable left ventricular assist devices. Ann Thorac Surg. 2001;72:725.
25 Birnie D, Williams K, Guo A, et al. Reasons for escalating pacemaker implants. Am J Cardiol. 2006;98:93–97.
26 Bernstein AD, Camm AJ, Fisher JD, et al. North American Society of Pacing and Electrophysiology policy statement. The NASPE/BPEG defibrillator code. Pacing Clin Electrophysiol. 1993;16:1776–1780.
27 Gregoratos G, Abrahms J, Epstien AE, et al. for the American College of Cardiology/American Heart Association Task Force on Practice Guidelines/North American Society for Pacing and Electrophysiology Committee to Update the 1998 Pacemaker Guidelines. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines). Circulation. 2002;106:2145–2161.
28 Uslan DZ, Sohail MR, St Sauver JL, et al. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med. 2007;167:669–675.
29 Ferguson R, McCaughan B, May J, et al. Venous occlusion: a rare complication of transvenous cardiac pacing. Aust N Z J Surg. 1992;62:977–980.
30 Lin G, Nishimura RA, Connolly HM, et al. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. J Am Coll Cardiol. 2005;45:1672–1675.
31 Hayes DL, Vlietstra RE. Pacemaker malfunction. Ann Intern Med. 1993;119:828–835.
32 Chan TC, Cardall TY. Electronic pacemakers. Emerg Med Clin North Am. 2006;24:179–194. vii
33 McCann WJ. Pacemaker malfunction associated with blunt trauma. N Y State Med J. 1978;78:645.