Chapter 67 Management of Dissections of the Carotid and Vertebral Arteries
Arterial dissection is an important cause of stroke. The symptomatology, natural history, pathology, and management of extracranial and intracranial arterial dissections differ. The majority of dissections affect the extracranial carotid and vertebral arteries and result in ischemic strokes. Intracranial dissections mainly affect the vertebral and basilar arteries and present most commonly with subarachnoid hemorrhage. The treatment of carotid and vertebral artery dissections has evolved significantly since the early diagnosis and management descriptions by Fisher and colleagues in the 1970s.1 Modern treatment of extracranial carotid and vertebral dissections is prolonged anticoagulation or antiplatelet therapy with surgical or endovascular intervention being reserved for refractory luminal irregularities or worsening neurologic status despite medical therapy. With recent innovations in neuroendovascular therapies, intracranial dissections are commonly treated with stents and/or coil embolization of associated pseudoaneurysms. This chapter reviews the presentation, treatment, and clinical outcomes for patients with extracranial and intracranial carotid and vertebral artery dissections.
Arterial Histology and Surrounding Milieu
Differences in arterial histology reflect the pathophysiologic dissimilarities between extracranial and intracranial arterial dissections. Craniocervical artery dissections (CAD) arise from a tear in the vessel lumen. Depending upon the extent of the tear, associated hemodynamic forces, and strength of the various arterial layers, a dissection may result in stenosis or aneurysmal dilatation of the artery. Subintimal dissections tend to cause stenosis of the parent artery, while subadventitial dissections may result in pseudoaneurysm formation and compression of surrounding soft tissue structures. Compared to extracranial arteries, intradural arteries have a thinner tunica media and adventitia with a relative paucity of elastic fibers making them more susceptible to subadventitial dissection resulting in pseudoaneurysm formation and potential subarachnoid hemorrhage.2 Extradural arteries are more likely to have subintimal dissections presenting with arterial stenosis or thromboembolic complications.1,3 The relatively weak milieu consisting of cerebrospinal fluid and brain parenchyma that surrounds the intracranial arteries predisposes patients to hemorrhagic complications in addition to potential thromboembolic phenomena.
Epidemiology
Craniocervical artery dissection accounts for approximately 2% of all ischemic strokes, but 10% to 25% of strokes in young and middle-aged patients.3 Extracranial arterial dissection is more common than intracranial dissection. Specifically, the extracranial carotid artery is the most common site of arterial dissection, with an incidence estimated at 2.5 to 3.0 per 100,000 people.3–5 Extracranial vertebral artery dissection occurs less frequently with an estimated incidence of 1.0 to 1.5 per 100,000 people.3–7 Intracranial arterial dissection is relatively rare, with the intradural vertebral artery (V4 segment) being the most commonly affected vessel. In Yamaura’s series of 338 patients with intracranial arterial dissections, 60% involved the intradural vertebral artery while only 9% affected the internal carotid artery.8
The etiology of CAD is multifactorial and appears to be linked to both environmental and genetic factors. Trauma has been proposed as an inciting factor of dissections, although in many cases there is no clear antecedent injury. The severity of trauma may range from a seemingly trivial injury (i.e., vigorous nose blowing or chiropractic maneuvers) to severe high-impact motor vehicle collisions resulting in blunt or direct injury to the craniocervical arteries.9 Genetic factors have also been associated with CAD. In a population-based study by Schievink etal., approximately 5% of patients with CAD had a positive family history of spontaneous arterial dissections.10 Patients with heritable connective tissue disorders such as Ehlers-Danlos syndrome type IV, Marfan’s disease, autosomal dominant polycystic kidney disease, osteogenesis imperfecta type I, and α1-antitrypsin deficiency have an increased risk of spontaneous extra- or intra-cranial artery dissection.11–13 Recently, hyperhomocysteinemia has been linked to CAD. A significant reduction in methylenetetrahydrofolate reductase (MTHFRT) levels due to a mutation in the coding region of this gene results in increased serum levels of homocysteine.14 Respiratory infections have also been associated with spontaneous CAD in a case-controlled study.15
Radiographic Diagnosis
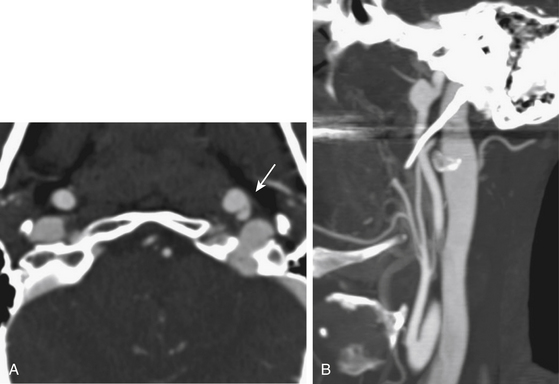
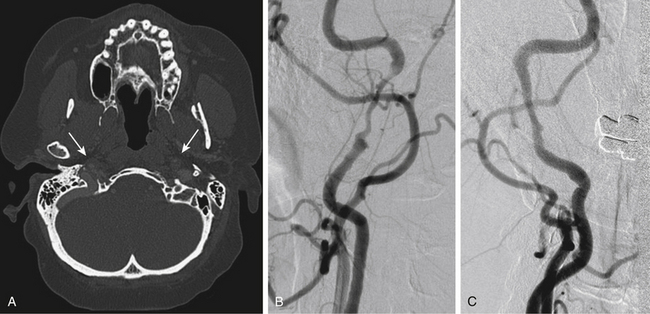
Pathognomonic radiographic features of CAD include intramural hematoma, the presence of a double lumen, and/or intimal flap. Digital subtraction angiography (DSA) is the gold standard method for diagnosing CAD. The “string sign,” a long arterial segment with a narrowed lumen, is the most common angiographic finding diagnostic of an arterial dissection. Intimal flaps and/or double lumens are appreciated in less than 10% of CAD cases diagnosed using DSA.16 DSA is an invasive test with an estimated iatrogenic stroke rate of 0.5% to 1%.17 Other risks of DSA include potential contrast-induced nephropathy and symptomatic hemorrhage at the arteriotomy site. Doppler ultrasonography (DUS) is a safe and effective method of diagnosing arterial dissections with reported sensitivity rates estimated at 90% when used in combination with hemodynamic signs and direct ultrasonic findings.18 However, DUS is limited by bony regions that cannot be accurately insonated (i.e., skull base and/or carotid canal) and may overestimate the degree of stenosis.19,20
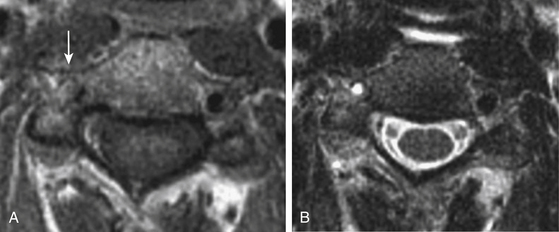
Magnetic resonance (MR) and computed tomographic (CT) angiography have replaced conventional angiography at many institutions as the primary diagnostic modalities for CAD. Advances in MR and CT angiography techniques have improved overall sensitivity and specificity rates at detecting CAD.21 Intramural hematomas can be readily identified on T1-weighted MR series as hyperintense signals due to methemoglobin accumulation with a characteristic crescent shape adjacent to the arterial lumen. Fat suppression techniques may accurately differentiate small intramural hematomas from surrounding soft tissues in the acute period. MR angiography can accurately display luminal stenosis and/or occlusion. The temporal relationship between dissection occurrence and MRI/MRA imaging is a potential limitation to this diagnostic modality as the sensitivity is highest within the first 2 days following the dissection.
Multidetector row CT angiography provides improved spatial resolution of less than 1 mm sections with relatively short acquisition times and reduced doses of contrast material.22 CT angiography may be superior to MR angiography at detecting acute intramural hematoma.23 Complete hyperintense signal in the affected artery may be difficult to distinguish between intramural hematoma and vessel occlusion on MRI. In addition, CT angiography more accurately depicts near complete arterial occlusions and pseudoaneurysms than time-of-flight MR angiography, which is not as sensitive in slow flow arterial segments.24 For these reasons, CT angiography is the primary diagnostic modality used at our institution in the rapid diagnosis of CAD. Recent public and governmental concerns regarding patient and healthcare worker radiation exposure during diagnostic testing have tempered our institutional use of CT angiography for interval follow-up imaging in favor of MRI/MRA especially in young patients.25,26 Figs. 67-1 to 67-3 illustrate classic radiographic findings associated with extracranial carotid and vertebral artery dissections.
Extracranial Carotid Artery Dissection
The extracranial carotid artery is the most common site of CAD, with an estimated incidence of 2.5 to 3.0 cases per 100,000 people.3–5 Cervical internal carotid artery dissection occurs approximately 2 cm distal to the artery’s origin and usually terminates proximal to its entry into the petrous bone.1,27,28 The classic clinical triad for presentation of extracranial carotid dissection is ipsilateral pain in the head, face, or neck,1 post-ganglionic partial Horner’s syndrome characterized by ipsilateral miosis and ptosis without anhidrosis,2 and cerebral or retinal ischemic symptoms.3 All three components are found in less than one third of patients with extracranial carotid artery dissection.3 Dissection-induced ischemia may manifest as transient ischemic attacks (TIAs) or cerebral infarctions. Fisher coined the term “carotid allegro” to describe dissection-associated TIAs as they tend to occur more frequently than ischemic episodes due to atherosclerotic disease.1 Ipsilateral lower cranial nerve palsies may result from extracranial carotid artery dissection. In a retrospective series of 190 consecutive adult patients diagnosed with spontaneous dissection of the internal carotid artery, 23 (12%) presented with cranial nerve palsies.29 The hypoglossal nerve was most frequently affected (5.2% of patients), with or without involvement of cranial nerves IX, X, and XI. Oculomotor and trigeminal nerve palsies were also observed in this series, possibly due to indirect interruption of nutrient vessels supplying the respective cranial nerves. Taste disturbance and tongue weakness are the most common manifestations of hypoglossal nerve compression due to its proximity to the carotid sheath. Pulsatile tinnitus may be experienced in up to one third of patients.30
Treatment of extracranial carotid artery dissections is medical, with neurointerventional and surgical procedures being reserved for dissections refractory to medical therapy and/or symptomatic associated pseudoaneurysms or flow-limiting stenosis. Anticoagulation with intravenous heparin followed by conversion to oral warfarin has been recommended as first-line treatment of patients with extracranial carotid artery dissections to prevent the risk of thromboembolic complications.3,31,32 After 3 to 6 months of anticoagulation with a goal international normalized ratio of 2.0 to 3.0, patients should undergo reimaging to evaluate for dissection resolution. Arterial recanalization rates range from 50% to 70% with an estimated 10% of patients suffering a delayed thromboembolic event during medical therapy.33–35 Patients should be reimaged following completion of anticoagulant therapy. No randomized controlled trial has investigated the optimal medical treatment of CAD. A 2003 Cochrane Database Review found no significant differences in mortality or clinical outcomes among 327 patients in 27 studies with carotid artery dissections who were treated with either anticoagulation or antiplatelet therapy.36 A small, non–statistically significant increased incidence of intracranial hemorrhage was observed in patients treated with anticoagulation (0.5%). A prospective multicenter nonrandomized study conducted by the Canadian Stroke Consortium found a non–statistically significant increased risk of recurrent stroke in patients with CAD treated with aspirin (12.4%) versus anticoagulation (8.3%).37 The Cervical Artery Dissection in Stroke Study (CADISS) is an ongoing multicenter, prospective study investigating the medical treatment of extracranial arterial dissections.38 Patients diagnosed with acute extracranial carotid and/or vertebral artery dissection are randomized to antiplatelet or anticoagulation therapy. The primary end points are ipsilateral stroke or death within 3 months of randomization. The results of this trial should be available in the next several years.
Endovascular and surgical treatments are typically reserved for cases of anticoagulation treatment failure defined by persistent luminal irregularities in the setting of recurrent thromboembolic disease and/or enlarging associated pseudoaneurysms. Endoluminal stenting and/or coil embolization of associated dissecting aneurysms are effective therapies for the treatment of extracranial carotid artery dissections.39–44 Multiple stents may be deployed in a telescoping fashion for treatment of long segment arterial dissections.40 In a 2008 review article that analyzed 13 clinical studies on 62 patients with 63 extracranial carotid artery dissections, endoluminal stents were successfully deployed in all patients with primary and 1-year stent patency rates of 100%.45 Seven postprocedural strokes occurred (11%) without any mortalities within 30days of treatment. One case of delayed asymptomatic in-stent stenosis occurred 22 months following initial treatment. Associated pseudoaneurysms may be effectively treated through coil embolization or deployment of covered stents. Yi etal. published a recent series of 10 patients with extracranial carotid (n = 7) or vertebral artery (n = 1) dissections and associated pseudoaneurysms successfully treated with covered stents.46 Systemic intravenous and intra-arterial thrombolytic therapies have also shown promise in the treatment of dissection-induced extracranial carotid artery occlusion with a low incidence of intracranial hemorrhage.31,47–49 Stent-assisted intra-arterial thrombolysis for the treatment of tandem carotid artery dissection or occlusion in the setting of acute stroke has been reported in several small case series.50–52
Surgical management of extracranial carotid artery dissections includes endarterectomy of the involved segment,1 carotid ligation with or without external-to-internal carotid artery bypass contingent upon the patient’s tolerance of balloon test occlusion,2 and pseudoaneurysm wrapping or resection.3 The major risks of surgical treatment include stroke and lower cranial nerve deficits. In Schievink’s series of surgically treated extracranial carotid artery dissections, 9% of patients suffered a postoperative stroke within 30days of surgery and nearly one third experienced transient lower cranial nerve palsies.53
Extracranial Vertebral Artery Dissection
Extracranial vertebral artery dissection incidence is estimated to be 1.0 to 1.5 per 100,000 people.3,6,7 Traumatic injury is the main etiologic factor associated with extracranial vertebral artery dissection, with spontaneous dissections accounting for an estimated 4% of cases among middle-aged adults.54 Chiropractic therapy of the cervical spine carries a risk of extracranial vertebral artery dissection and resultant stroke estimated at 1 in 20,000 manipulations.55 The relative increased mobility of the extracranial vertebral artery at the atlanto-axial segments makes this region particular vulnerable to dissection.9 Clinical manifestations of extracranial vertebral artery dissection include severe posterior cervical and occipital pain and stroke. More than 90% of patients with extracranial vertebral artery dissections present with ischemic symptoms of the brain stem, the thalamus, or the cerebral and/or cerebellar hemispheres.56 Lateral medullary infarctions commonly result in dysarthria, dysphagia, ataxia, vertigo, diplopia, and sensory disturbances (Wallenberg’s syndrome). TIAs occur less frequently than in extracranial internal carotid artery dissections.9
First-line treatment of extracranial vertebral artery dissections is anticoagulation with intravenous heparin followed by conversion to oral warfarin with a goal international normalized ratio of 2.0 to 3.0 for 3 to 6 months.3,31,32 Similar to extracranial carotid artery dissections, endovascular and surgical therapies are typically reserved for cases in which anticoagulation has failed due to persistent luminal irregularities with resulting thromboembolic events or hemodynamic insufficiency. Angioplasty and stent placement may effectively recanalize extracranial vertebral artery dissections.42,57 Dissection-induced extracranial vertebral artery occlusions may be treated with intravenous or intra-arterial pharmacological thrombolysis with or without stent deployment to achieve vessel recanalization.31,58,59 Vertebral artery sacrifice through endovascular obliteration or surgical ligation may be considered as tolerated in the setting of failed medical therapy. Extracranial-to-intracranial bypass procedures may be utilized prior to arterial sacrifice to improve symptomatic hemodynamic insufficiency related to dissection-induced arterial occlusion.60
Intracranial Arterial Dissection
The most common site of intracranial arterial dissections is the intradural vertebral artery (V4 segment). Although thromboembolic events may occur, the major risk of intracranial arterial dissections is subarachnoid hemorrhage due to the relatively thin arterial walls and weak surrounding milieu consisting of cerebrospinal fluid and brain parenchyma. Unruptured intracranial dissections typically follow a benign course, although the natural history of these lesions is poorly understood. Dissection appears to be a dynamic process with approximately 90% of stenoses resolving over time.61,62 However, patients remain at risk of subsequent thromboembolic events and subarachnoid hemorrhage while the intracranial arterial dissection heals. In a review of 457 patients presenting with intradural vertebral artery dissection, 79% presented with subarachnoid hemorrhage and recurrent hemorrhage occurred in 37% of patients, particularly within the first 24 hours of the initial bleed.63 Dissecting pseudoaneurysms of the vertebrobasilar arteries are estimated to account for 3% to 7% of all nontraumatic subarachnoid hemorrhage cases each year.2 Treatment of ruptured dissecting intracranial aneurysms should be performed emergently due to the high rate of recurrent hemorrhage estimated at 30% to 70%64,66 with mortality rates approaching 50%.65,66
Anticoagulation is not recommended for the treatment of intracranial arterial dissections due to the potential risk of subarachnoid hemorrhage. Despite this recommendation, Metso etal. treated 81 consecutive adult patients with intracranial dissections using intravenous heparin followed by conversion to oral warfarin for 3 months without any reported cases of intracranial hemorrhage.67 The mainstay treatment for intracranial dissections is surgical or endovascular therapy. At most institutions, surgical therapy is being replaced by neurointerventional techniques due to relatively high perioperative morbidity and mortality rates related mostly to lower cranial nerve deficits.63,68 Surgical options include ligation of the affected artery without or with extracranial-to-intracranial bypass and direct clipping or wrapping of associated intracranial dissecting pseudoaneurysms.63,68–73 Surgical procedures may be limited by the friable nature of the affected dissecting arteries and potential associated pseudoaneurysms.
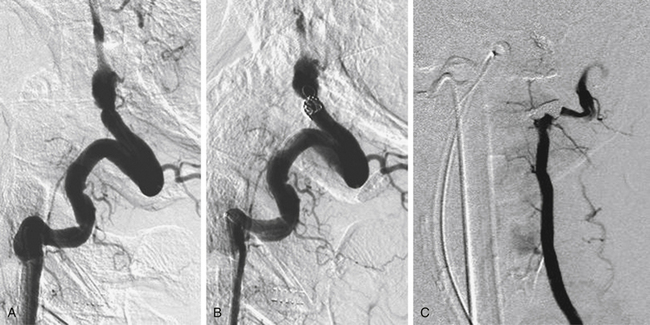
Endovascular techniques include parent artery occlusion and coil embolization of associated pseudoaneurysms without or with stent deployment. The need for prolonged antiplatelet therapy to prevent parent artery re-stenosis is a relative contraindication to stent placement in the setting of subarachnoid hemorrhage. Our neurovascular group attempts to use balloon assistance, if required, during coil embolization of ruptured dissecting pseudoaneurysms. Fig67-4 illustrates a ruptured dissecting intracranial vertebral artery pseudoaneurysm that was successfully treated with occlusion of the vertebral artery using coils. Several groups have successfully treated intracranial dissecting pseudoaneurysms via coil embolization alone.74–81 Rabinov etal. treated 26 patients diagnosed with vertebrobasilar dissecting pseudoaneurysms utilizing endovascular parent artery occlusion (n = 14) or pseudoaneurysm coiling (n = 12).72 Twenty patients (77%) presented with subarachnoid hemorrhage and 6 (23%) complained of headache or experienced stroke-like symptoms. The majority of patients (81%) had good clinical outcomes (modified Rankin Scale score ≤2). Three patients (11.5%) died in the periprocedural period. Stent-assisted coiling followed by stent-within-a-stent technique has been recently reported for treatment of ruptured vertebrobasilar dissecting artery aneurysms. Suh etal. successfully treated 11 patients with dissecting aneurysms affecting either the basilar artery, the dominant vertebral artery in the setting or poor collateralization, or the segment bearing the origin of the dominant posterior inferior cerebellar artery using this technique.82 Patients were not loaded with antiplatelet agents prior to the intervention, but were maintained on aspirin and clopidogrel for 3 months without any episodes of re-bleeding or stent re-stenosis. Only one patient died in the series due to complications from the initial subarachnoid hemorrhage. Stent deployment in combination with mechanical and/or chemical thrombolysis has been performed to successfully treat vertebrobasilar occlusion in patients presenting with acute stroke without significant hemorrhagic complications.58
Antiplatelet therapy vs. anticoagulation in cervical artery dissection: rationale and design of the Cervical Artery Dissection in Stroke Study (CADISS). Int J Stroke. 2007;2:292-296.
Beletsky V., Nadareishvili Z., Lynch J., et al. Cervical arterial dissection: time for a therapeutic trial? Stroke. 2003;34:2856-2860.
Donas K.P., Mayer D., Guber I., et al. Endovascular repair of extracranial carotid artery dissection: current status and level of evidence. J Vasc Interv Radiol. 2008;19:1693-1698.
Dziewas R., Konrad C., Drager B., et al. Cervical artery dissection—clinical features, risk factors, therapy and outcome in 126 patients. J Neurol. 2003;250:1179-1184.
Fisher C.M., Ojemann R.G., Roberson G.H. Spontaneous dissection of cervico-cerebral arteries. Can J Neurol Sci. 1978;5:9-19.
Kocaeli H., Chaalala C., Andaluz N., Zuccarello M. Spontaneous intradural vertebral artery dissection: a single-center experience and review of the literature. Skull Base. 2009;19:209-218.
Lyrer P., Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. 2003:CD000255.
Metso T.M., Metso A.J., Helenius J., et al. Prognosis and safety of anticoagulation in intracranial artery dissections in adults. Stroke. 2007;38:1837-1842.
Provenzale J.M., Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;193:1167-1174.
Rabinov J.D., Hellinger F.R., Morris P.P., et al. Endovascular management of vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol. 2003;24:1421-1428.
Schievink W.I. The treatment of spontaneous carotid and vertebral artery dissections. Curr Opin Cardiol. 2000;15:316-321.
Schievink W.I. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344:898-906.
Schievink W.I., Piepgras D.G., McCaffrey T.V., Mokri B. Surgical treatment of extracranial internal carotid artery dissecting aneurysms. Neurosurgery. 1994;35:809-815. discussion 815-806
Thanvi B., Munshi S.K., Dawson S.L., Robinson T.G. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81:383-388.
Yamaura A. Nontraumatic intracranial arterial dissection: natural history, diagnosis, and treatment. Contemp Neurosurg. 1994;16:1-6.
1. Fisher C.M., Ojemann R.G., Roberson G.H. Spontaneous dissection of cervico-cerebral arteries. Can J Neurol Sci. 1978;5:9-19.
2. Sasaki O., Ogawa H., Koike T., et al. A clinicopathological study of dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1991;75:874-882.
3. Schievink W.I. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344:898-906.
4. Giroud M., Fayolle H., Andre N., et al. Incidence of internal carotid artery dissection in the community of Dijon. J Neurol Neurosurg Psychiatry. 1994;57:1443.
5. Schievink W.I., Mokri B., Whisnant J.P. Internal carotid artery dissection in a community. Rochester, Minnesota, 1987-1992. Stroke. 1993;24:1678-1680.
6. Bassetti C., Carruzzo A., Sturzenegger M., Tuncdogan E. Recurrence of cervical artery dissection. A prospective study of 81 patients. Stroke. 1996;27:1804-1807.
7. Schievink W.I., Mokri B., O’Fallon W.M. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330:393-397.
8. Yamaura A. Nontraumatic intracranial arterial dissection: Natural history, diagnosis, and treatment. Contemp Neurosurg. 1994;16:1-6.
9. Thanvi B., Munshi S.K., Dawson S.L., Robinson T.G. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81:383-388.
10. Schievink W.I., Mokri B., Piepgras D.G., Kuiper J.D. Recurrent spontaneous arterial dissections: risk in familial versus nonfamilial disease. Stroke. 1996;27:622-624.
11. Rouviere S., Michelini R., Sarda P., Pages M. Spontaneous carotid artery dissection in two siblings with osteogenesis imperfecta. Cerebrovasc Dis. 2004;17:270-272.
12. Schievink W.I., Bjornsson J., Piepgras D.G. Coexistence of fibromuscular dysplasia and cystic medial necrosis in a patient with Marfan’s syndrome and bilateral carotid artery dissections. Stroke. 1994;25:2492-2496.
13. Schievink W.I., Michels V.V., Piepgras D.G. Neurovascular manifestations of heritable connective tissue disorders. A review. Stroke. 1994;25:889-903.
14. Pezzini A., Del Zotto E., Archetti S., et al. Plasma homocysteine concentration, C677T MTHFR genotype, and 844ins68bp CBS genotype in young adults with spontaneous cervical artery dissection and atherothrombotic stroke. Stroke. 2002;33:664-669.
15. Guillon B., Berthet K., Benslamia L., et al. Infection and the risk of spontaneous cervical artery dissection: a case-control study. Stroke. 2003;34:e79-e81.
16. Houser O.W., Mokri B., Sundt T.M.Jr., et al. Spontaneous cervical cephalic arterial dissection and its residuum: angiographic spectrum. AJNR Am J Neuroradiol. 1984;5:27-34.
17. Johnston D.C., Chapman K.M., Goldstein L.B. Low rate of complications of cerebral angiography in routine clinical practice. Neurology. 2001;57:2012-2014.
18. Alecu C., Fortrat J.O., Ducrocq X., et al. Duplex scanning diagnosis of internal carotid artery dissections. A case control study. Cerebrovasc Dis. 2007;23:441-447.
19. Anderson C.M., Saloner D., Lee R.E., et al. Assessment of carotid artery stenosis by MR angiography: comparison with x-ray angiography and color-coded Doppler ultrasound. AJNR Am J Neuroradiol. 1992;13:989-1003. discussion 1005-1008
20. Steinke W., Rautenberg W., Schwartz A., Hennerici M. Noninvasive monitoring of internal carotid artery dissection. Stroke. 1994;25:998-1005.
21. Provenzale J.M., Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;193:1167-1174.
22. Fleischmann D. Present and future trends in multiple detector-row CT applications: CT angiography. Eur Radiol. 2002;12(suppl 2):S11-S15.
23. Elijovich L., Kazmi K., Gauvrit J.Y., Law M. The emerging role of multidetector row CT angiography in the diagnosis of cervical arterial dissection: preliminary study. Neuroradiology. 2006;48:606-612.
24. Magarelli N., Scarabino T., Simeone A.L., et al. Carotid stenosis: a comparison between MR and spiral CT angiography. Neuroradiology. 1998;40:367-373.
25. Brenner D.J., Hall E.J. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277-2284.
26. Raelson C.A., Kanal K.M., Vavilala M.S., et al. Radiation dose and excess risk of cancer in children undergoing neuroangiography. AJR Am J Roentgenol. 2009;193:1621-1628.
27. Anson J., Crowell R.M. Cervicocranial arterial dissection. Neurosurgery. 1991;29:89-96.
28. O’Connell B.K., Towfighi J., Brennan R.W., et al. Dissecting aneurysms of head and neck. Neurology. 1985;35:993-997.
29. Mokri B., Silbert P.L., Schievink W.I., Piepgras D.G. Cranial nerve palsy in spontaneous dissection of the extracranial internal carotid artery. Neurology. 1996;46:356-359.
30. Sila C.A., Furlan A.J., Little J.R. Pulsatile tinnitus. Stroke. 1987;18:252-256.
31. Dziewas R., Konrad C., Drager B., et al. Cervical artery dissection—clinical features, risk factors, therapy and outcome in 126 patients. J Neurol. 2003;250:1179-1184.
32. Schievink W.I. The treatment of spontaneous carotid and vertebral artery dissections. Curr Opin Cardiol. 2000;15:316-321.
33. Hart R.G., Easton J.D. Dissections. Stroke. 1985;16:925-927.
34. Mokri B., Piepgras D.G., Houser O.W. Traumatic dissections of the extracranial internal carotid artery. J Neurosurg. 1988;68:189-197.
35. Sturzenegger M. Spontaneous internal carotid artery dissection: early diagnosis and management in 44 patients. J Neurol. 1995;242:231-238.
36. Lyrer P., Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. 2003:CD000255.
37. Beletsky V., Nadareishvili Z., Lynch J., et al. Cervical arterial dissection: time for a therapeutic trial? Stroke. 2003;34:2856-2860.
38. Antiplatelet therapy vs. anticoagulation in cervical artery dissection: rationale and design of the Cervical Artery Dissection in Stroke Study (CADISS). Int J Stroke. 2007;2:292-296.
39. Albuquerque F.C., Han P.P., Spetzler R.F., et al. Carotid dissection: technical factors affecting endovascular therapy. Can J Neurol Sci. 2002;29:54-60.
40. Biondi A., Katz J.M., Vallabh J., et al. Progressive symptomatic carotid dissection treated with multiple stents. Stroke. 2005;36:e80-e82.
41. DuBose J., Recinos G., Teixeira P.G., et al. Endovascular stenting for the treatment of traumatic internal carotid injuries: expanding experience. J Trauma. 2008;65:1561-1566.
42. Joo J.Y., Ahn J.Y., Chung Y.S., et al. Treatment of intra- and extracranial arterial dissections using stents and embolization. Cardiovasc Intervent Radiol. 2005;28:595-602.
43. Kadkhodayan Y., Jeck D.T., Moran C.J., et al. Angioplasty and stenting in carotid dissection with or without associated pseudoaneurysm. AJNR Am J Neuroradiol. 2005;26:2328-2335.
44. Malek A.M., Higashida R.T., Phatouros C.C., et al. Endovascular management of extracranial carotid artery dissection achieved using stent angioplasty. AJNR Am J Neuroradiol. 2000;21:1280-1292.
45. Donas K.P., Mayer D., Guber I., et al. Endovascular repair of extracranial carotid artery dissection: current status and level of evidence. J Vasc Interv Radiol. 2008;19:1693-1698.
46. Yi A.C., Palmer E., Luh G.Y., et al. Endovascular treatment of carotid and vertebral pseudoaneurysms with covered stents. AJNR Am J Neuroradiol. 2008;29:983-987.
47. Arnold M., Nedeltchev K., Sturzenegger M., et al. Thrombolysis in patients with acute stroke caused by cervical artery dissection: analysis of 9 patients and review of the literature. Arch Neurol. 2002;59:549-553.
48. Georgiadis D., Baumgartner R.W. Thrombolysis in cervical artery dissection. Front Neurol Neurosci. 2005;20:140-146.
49. Zaidat O.O., Fernandes Filho J.A., Singh G., Suarez J.I. Thrombolytic therapy for acute extra-cranial artery dissection: report of two cases. Arq Neuropsiquiatr. 2001;59:936-938.
50. Abboud H., Houdart E., Meseguer E., Amarenco P. Stent assisted endovascular thrombolysis of internal carotid artery dissection. J Neurol Neurosurg Psychiatry. 2005;76:292-293.
51. Cohen J.E., Gomori J.M., Grigoriadis S., et al. Intra-arterial thrombolysis and stent placement for traumatic carotid dissection with subsequent stroke: a combined, simultaneous endovascular approach. J Neurol Sci. 2008;269:172-175.
52. Mourand I., Brunel H., Vendrell J.F., et al. Endovascular stent-assisted thrombolysis in acute occlusive carotid artery dissection. Neuroradiology. 2009.
53. Schievink W.I., Piepgras D.G., McCaffrey T.V., Mokri B. Surgical treatment of extracranial internal carotid artery dissecting aneurysms. Neurosurgery. 1994;35:809-815. discussion 815-816
54. Plaza I., Diez-Tejedor E., Lara M., Barreiro P. [Spontaneous dissection of the vertebral artery.]. Rev Neurol. 1996;24:163-171.
55. Vickers A., Zollman C. ABC of complementary medicine. The manipulative therapies: osteopathy and chiropractic. Bmj. 1999;319:1176-1179.
56. Caplan L.R., Zarins C.K., Hemmati M. Spontaneous dissection of the extracranial vertebral arteries. Stroke. 1985;16:1030-1038.
57. Komotar R.J., Mocco J., Samuelson R.M., et al. Rapidly successive, symptomatic, bilateral, spontaneous vertebral artery dissections: treatment with stent reconstruction. Surg Neurol. 2009;72:300-305.
58. Cerrato P., Berardino M., Bottacchi E., et al. Vertebral artery dissection complicated by basilar artery occlusion successfully treated with intra-arterial thrombolysis: three case reports. Neurol Sci. 2008;29:51-55.
59. Price R.F., Sellar R., Leung C., O’Sullivan M.J. Traumatic vertebral arterial dissection and vertebrobasilar arterial thrombosis successfully treated with endovascular thrombolysis and stenting. AJNR Am J Neuroradiol. 1998;19:1677-1680.
60. da Costa L.B. Tymianski M. Symptomatic non-atherosclerotic bilateral extracranial vertebral artery occlusion treated with extracranial to intracranial bypass: case report. Arq Neuropsiquiatr. 2006;64:664-667.
61. de Bray J.M., Penisson-Besnier I., Dubas F., Emile J. Extracranial and intracranial vertebrobasilar dissections: diagnosis and prognosis. J Neurol Neurosurg Psychiatry. 1997;63:46-51.
62. Yoshimoto Y., Wakai S. Unruptured intracranial vertebral artery dissection. Clinical course and serial radiographic imagings. Stroke. 1997;28:370-374.
63. Kocaeli H., Chaalala C., Andaluz N., Zuccarello M. Spontaneous intradural vertebral artery dissection: a single-center experience and review of the literature. Skull Base. 2009;19:209-218.
64. Aoki N., Sakai T. Rebleeding from intracranial dissecting aneurysm in the vertebral artery. Stroke. 1990;21:1628-1631.
65. Dohi K., Kubota M., Hamada H., et al. [Compression of medulla oblongata by the dissecting aneurysm of the vertebral artery 7 years after its rupture: case report.]. No Shinkei Geka. 1994;22:1067-1070.
66. Mizutani T., Aruga T., Kirino T., et al. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995;36:905-911. discussion 912-913
67. Metso T.M., Metso A.J., Helenius J., et al. Prognosis and safety of anticoagulation in intracranial artery dissections in adults. Stroke. 2007;38:1837-1842.
68. Sugiu K., Tokunaga K., Watanabe K., et al. Emergent endovascular treatment of ruptured vertebral artery dissecting aneurysms. Neuroradiology. 2005;47:158-164.
69. Ausman J.I., Diaz F.G., Mullan S., et al. Posterior inferior to posterior inferior cerebellar artery anastomosis combined with trapping for vertebral artery aneurysm. Case report. J Neurosurg. 1990;73:462-465.
70. Nussbaum E.S., Madison M.T., Myers M.E., et al. Dissecting aneurysms of the posterior inferior cerebellar artery: retrospective evaluation of management and extended follow-up review in 6 patients. JNeurosurg. 2008;109:23-27.
71. Oka F., Shimizu H., Matsumoto Y., et al. Ischemic stroke due to dissection of intracranial internal carotid artery: implications for early surgical treatment. Surg Neurol. 2008;69:578-584. discussion 584-575
72. Rabinov J.D., Hellinger F.R., Morris P.P., et al. Endovascular management of vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol. 2003;24:1421-1428.
73. Yonekawa Y., Zumofen D., Imhof H.G., et al. Hemorrhagic cerebral dissecting aneurysms: surgical treatments and results. Acta Neurochir Suppl. 2008;103:61-69.
74. Ahn J.Y., Han I.B., Kim T.G., et al. Endovascular treatment of intracranial vertebral artery dissections with stent placement or stent-assisted coiling. AJNR Am J Neuroradiol. 2006;27:1514-1520.
75. Anxionnat R., de Melo Neto J.F., Bracard S., et al. Treatment of hemorrhagic intracranial dissections. Neurosurgery. 2003;53:289-300. discussion 300-281
76. Cellerini M., Mangiafico S., Ammannati F., et al. Ruptured, dissecting posterior inferior cerebellar artery aneurysms: endovascular treatment without parent vessel occlusion. Neuroradiology. 2008;50:315-320.
77. Isokangas J.M., Siniluoto T., Tikkakoski T., Kumpulainen T. Endovascular treatment of peripheral aneurysms of the posterior inferior cerebellar artery. AJNR Am J Neuroradiol. 2008;29:1783-1788.
78. Kurata A., Ohmomo T., Miyasaka Y., et al. Coil embolization for the treatment of ruptured dissecting vertebral aneurysms. AJNR Am J Neuroradiol. 2001;22:11-18.
79. Manabe H., Hatayama T., Hasegawa S., et al. Coil embolisation for ruptured vertebral artery dissection distal to the origin of the posterior inferior cerebellar artery. Neuroradiology. 2000;42:384-387.
80. Peluso J.P., van Rooij W.J., Sluzewski M., et al. Endovascular treatment of symptomatic intradural vertebral dissecting aneurysms. AJNR Am J Neuroradiol. 2008;29:102-106.
81. Zhao W.Y., Krings T., Alvarez H., et al. Management of spontaneous haemorrhagic intracranial vertebrobasilar dissection: review of 21 consecutive cases. Acta Neurochir (Wien). 2007;149:585-596. discussion 596
82. Suh S.H., Kim B.M., Park S.I., et al. Stent-assisted coil embolization followed by a stent-within-a-stent technique for ruptured dissecting aneurysms of the intracranial vertebrobasilar artery. Clinical article. J Neurosurg. 2009;111:48-52.