Chapter 156 Management of Cervical Spondylotic Myelopathy
Cervical spondylotic myelopathy (CSM) is caused by a reduction of the sagittal diameter of the cervical spinal canal as a result of congenital and degenerative changes in the cervical spine.1–3 It is the most common type of spinal cord dysfunction in patients over the age of 55 years and it is the most common cause of acquired spastic paraparesis (quadriparesis) in adults.4,5 Risk factors for spondylosis include cigarette smoking, frequent lifting, and diving.6
Degenerative changes of cervical spondylosis occur in the five articulations that comprise the cervical motion segment: the intervertebral disc, the two facet joints, and the two false uncovertebral joints (of Luschka). The normal aging process of the spine results initially in disc desiccation and results in loss of disc height. This change is thought to bring the uncovertebral joints into contact, thereby disrupting the normal biomechanics of the facet joints. Osteophyte formation, ligamentum flavum hypertrophy, and facet and uncovertebral joint eburnation may then occur as a reaction to the abnormal biomechanics.6,7 These degenerative changes most commonly occur at C5–C6 and C6–C7.8 White and Panjabi classify these changes as “static” factors involved in the pathogenesis of cervical spondylotic myelopathy.9 Also included in this category are congenital spinal canal stenosis (less than 13 mm anteroposterior diameter) and disc herniation. White and Panjabi also describe “dynamic” factors, which are abnormal forces placed on the spinal column and cord during flexion and extension under normal physiologic loads. For example, repetitive traumatic compression of the spinal cord against an osteophyte during normal flexion and extension of the cervical spine is defined as a dynamic factor.4,10,11
In addition to the direct mechanical compression of neural elements, CSM may also be the result of spinal cord ischemia. Ischemia can result from compression of large arterial feeders to the spinal cord such as the anterior spinal artery, reduced flow through the penetrating arteries of the spinal cord or the pial plexuses, or impairment in venous outflow resulting in venous hypertension.4,12–14
The natural history of CSM appears to be one of progressive disability.15,16 It should be noted that there are some limitations in determining this natural history; studies are hampered by bias, heterogeneous patient populations, lack of long-term follow-up, and the use of qualitative rather than quantitative outcome measures.17 In fact, Clarke and Robinson asserted that once the disorder was recognized, neurologic function did not return to normal.3 In their series, 75% had episodic progression; 20% had slow, steady progression; and 5% had a rapid onset of symptoms followed by a prolonged period of stable disease. Nurick observed that patients with mild disability tended to have a better prognosis while patients over the age of 60 years tended to progress.18 Moreover, patients who have had symptoms longer than 2 years show no improvement despite treatment.4,19
Symptom duration may have a negative impact on natural history. Sadasivan et al. reported on 22 patients with symptomatic CSM for an average 6.3 years.20 All patients were reported to be Nurick II myelopathy at diagnosis. All progressed over the study period, one to grade III, 17 to grade IV, and 4 to grade V. Gait was involved in 100%, weakness in 45%, and determity in 72% of patients.
Pathologic Anatomy and Cervical Spondylotic Myelopathy
There are static, degenerative factors, including intervertebral disc bulging, dorsal vertebral body osteophytes, ossification of the posterior longitudinal ligament, uncovertebral and facet joint hypertrophy, ligamentum flavum, and facet joint capsule laxity and infolding. Collapse of the intervertebral disc space has the potential to lead to a degenerative cascade. As the disc-space height decreases, the uncovertebral joints approximate and are loaded abnormally, increasing the likelihood of degenerative spurring. Disc space collapse also causes a rostral–caudal translation, leading to similar degenerative spurring in the facet joints and laxity and buckling of the facet capsules and ligamentum flavum. All of these contribute to static narrowing of the spinal canal (Fig. 156-1).
Patient Presentation and Physical Examination
During the assessment of the patient with suspected CSM, a directed review of systems (ROS) is an important portion of the evaluation, since patients may dismiss or not think to volunteer mild symptoms. Questions are generally directed toward the upper extremity, lower extremity, and bladder function. This is consistent with the myelopathy grading system described by Benzel (modification of the myelopathy scale devised by the Japanese Orthopaedic Association).21 Upper extremity dysfunction can be ascertained by assessing for handwriting deterioration, increasing frequency of dropping objects, difficulty with fine motor skills such as fastening clasps or buttons, and the feeling of numbness and tingling in the hands. The term “myelopathy hand” had been used to describe nondermatomal paresthesias in the hands, a sense of clumsiness in the hands, and interosseous wasting.22,23 Myelopathy hand can be confused with peripheral nerve compression syndromes such as carpal tunnel, cubital tunnel, and thoracic outlet syndrome. Questioning regarding lower extremity function is directed toward balance and gait dysfunction, which is related in part to spasticity. Paresthesias and weakness in the lower extremities may also be a prominent complaint. Finally, inquiring about bladder function is important. Bladder dysfunction is not as common a finding as extremity problems but may be present in the form of incontinence or retention.24
The physical examination of the patient with suspected CSM includes a generalized neurologic examination, which can then focus more specifically based on the history. A thorough examination can differentiate CSM from those problems stemming rostral and caudal to the cervical spine and from motor neuron disease and peripheral neuropathy. The examination begins with observation of the patient’s posture, gait, and general appearance and fluidity of movements. Posture assessment may demonstrate sagittal and/or coronal imbalances. Gait examination may show a wide-based, spastic gait, and subtle changes may manifest only when the patient is asked to increase the pace of walking. Cranial nerve evaluation is included. Extremity function includes a motor and sensory examination from C4 to T1 and L2 to S1 bilaterally. Note that when assessing thoracic sensory levels, patients with cervical myelopathy can have variable sensory levels localizing to the thoracic spine.25 Deep tendon reflexes (DTRs) are typically hyperactive. This is not always the case because coexisting peripheral neuropathy such as diabetes can alter DTRs. Coexisting lumbar spinal stenosis can present with hyperactive upper extremity reflexes but normal or hypoactive lower extremity reflexes. Additional reflexes include Babinski’s reflex and assessment for clonus in the lower extremities, and Hoffman’s reflex, the inverted radial reflex, and finger escape sign in the upper extremities. Other reflexes that may help localize the site of compression are the pectoralis reflex, scapulohumeral reflex, and jaw jerk. Finally, cervical spine range of motion may be restricted. Associated findings may include a positive Spurling’s sign and Lhermitte’s sign.
Radiologic Evaluation
Plain radiographs are still an important part for the evaluation of the patient with CSM. The views obtained include anteroposterior (AP), lateral, flexion/extension, and obliques. AP radiographs allow for the evaluation of coronal alignment (scoliosis) and for the presence of anomalies such as cervical ribs or large C7 transverse processes (sometimes associated with fibrous bands). The lateral view provides the most information. It demonstrates the sagittal balance or the amount of lordosis/kyphosis. The lateral view also demonstrates the amount of disc space narrowing, vertebral body osteophytes, the AP diameter of the spinal canal, and sometimes the ossification of the posterior longitudinal ligament. The Pavlov ratio, defined as the ratio of the sagittal diameter of the spinal canal to the sagittal diameter of the vertebral body, is determined using lateral radiographs.26 A ratio of less than 0.8 has been associated with an increased risk for developing myelopathy. Lateral flexion/extension views evaluate for translation and angulation abnormalities, which can provide information regarding dynamic cord compression. Flexion/extension views, combined with a static lateral view, demonstrate the ability of the patient to achieve lordosis, either globally or focally, which can be important for deciding between surgical approaches. Lastly, oblique views provide information about foraminal narrowing, particularly due to uncovertebral joint spurring.
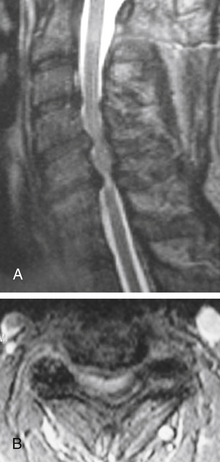
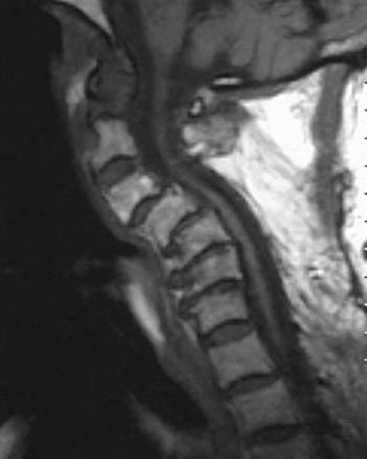
MRI is invaluable for evaluation of soft-tissue compressive structures as well as assessing the spinal cord itself. The intervertebral disc and ligamentous and capsular structures are all well visualized in the axial and sagittal planes. Perhaps the greatest advantage of MRI in CSM is its ability to directly visualize the spinal cord. Parenchymal changes such as myelomalacia signal changes and syrinx formation are readily identified (Fig. 156-2). MRI may also have a prognostic role. High-signal-intensity abnormalities on T2-weighted images and low-signal-intensity changes on T1-weighted MRIs are not uncommon findings in patients with CSM. A recent retrospective review correlating MRI findings to operative outcomes suggested that low-signal-intensity changes on the T1-weighted images preoperatively indicated a poor prognosis.27 A recent guideline article focusing on predictors of outcome found consensus that the presence of low signal of preoperative T1-weighted images, multisegment and focal high signal on T2-weighted images and the presence of cord atrophy are indicators or poor outcome.28,29 The addition of gadolinium is useful in cases of suspected infection or tumor and in patients with prior spine surgery.
Myelography combined with CT can serve several important roles. The myelogram can be evaluated with the cervical spine in flexed and extended postures, providing details regarding dynamic compression. The CT provides the most detailed information about bony pathology such as osteophytes and ossified posterior longitudinal ligament (OPLL), and in the case of significant OPLL, can influence the decision on surgical approach. It is better at differentiating bony from soft tissue than MRI, and it has been suggested that CT myelography and MRI should be considered complementary, not interchangeable.30
Somatosensory and Motor-Evoked Potential Monitoring
Somatosensory (SSEP) and motor-evoked potential recording (MEP and EMG have been used as tools of prognostic indication for CSM. Lyu et al. performed preoperative MEP and SEP in 39 patients with CSM who were to undergo surgical decompression.31,32 The authors judged improvement postoperatively by using pre- and post-operative JOA scores and 6-month neurologic recovery rates. The mean recovery rate was 51%, and this did not correlate with sex, arm or leg MEP or tibial SEP recordings. Neurological recovery rate, however, significantly correlated with normal SEP results. Morshita et al.32 performed pre- and post-operative median nerve SEPs in 14 patients with CSM with underwent cervical decompression. The authors demonstrated a statistically significant correlation between 1-week postoperative improvement in median nerve SEPs and 12-week postoperative JOA score for recovery rate. On the other hand, a lack of improvement in the median nerve SSEP in the early decompression period was associated with a poor neurological outcome. This data and others was judged to provide class II evidence supporting the use of median nerve SSEPs in predicting outcome following treatment of CSM.33
The use of EMG has not been shown to be convincingly predictive of recovery in CSM.28 It should be noted that EMG evidence of radiculopathy has been shown to be predictive of developing myelopathy in patients with cervical stenosis that is asymptomatic.17
Nonsurgical Treatment
Controversy exists regarding the natural history of patients with spondylotic myelopathy. This stems from the lack of randomized prospective studies on the issue and the reflection that the majority of studies that exist in the literature contain a heterogeneous population of patients. The natural history of cervical myelopathy is a slow, stepwise progressive deterioration. Lees and Turner reported episodic, stepwise deterioration in 75% of their patients.16 Patients with mild symptoms had the best prognosis with regard to lack of progression.
Conservative treatment consists of measures aimed at decreasing symptoms and increasing function. Options include cervical orthoses, physical therapy, medication, and epidural steroids. Medications include anti-inflammatories, analgesics (opioids), muscle relaxants, and steroids. Some or all of these conservative measures may lead to some improvement in 30% to 50% of patients, depending on the grading criteria utilized.34,35 This is especially true for those with only mild symptoms, that is, mild hand and arm symptoms but without impairment of daily tasks.
Careful conservative management may be considered for those with mild myelopathy, but caution should be taken with those with moderate to severe symptoms. No difference at 2 years was observed between those who received surgical decompression and those who did not in patients with only mild myelopathy.36 In contrast, those with severe myelopathy rarely improve with conservative measures. Fourteen of fifteen patients with severe disability remained so in one series.16
Ultimately, most patients with cervical spondylotic myelopathy undergo surgery. Those with moderate or severe symptoms should undergo early surgical decompression. Many surgical studies have demonstrated that shortening symptom duration prior to decompression results in improved outcome.37 Patients with only mild symptoms may be closely followed and decompression performed when there is deterioration or lack of improvement.
Operative Management
Ventral Approach
The ventral approach to the cervical spine to address spondylosis has been successfully used for nearly 50 years.38–40 This approach provides direct access for spinal cord and nerve root decompression secondary to herniated intervertebral discs, dorsal vertebral body osteophytes, ossified posterior longitudinal ligament, and uncovertebral joint hypertrophy. Another important benefit of the ventral approach is the ability to correct kyphotic deformity through discectomy or corpectomy.41 Although some advocate ventral discectomy without arthrodesis in certain clinical situations, decompression in the setting of CSM is accompanied by a stabilization procedure with the goal of arthrodesis (artificial disc placement would be an exception). Ventral decompression options include discectomy (either single or multiple levels), corpectomy (either single or multiple levels), or a combination of discectomy and corpectomy. Arthrodesis can be accomplished by using autograft or allograft bone, or a combination. A variety of metallic and carbon fiber cages have been used, typically packed with autograft or allograft bone, and all of these options can be performed with or without a ventral cervical plate (Fig. 156-3).
Discectomy versus Corpectomy
Another issue when considering discectomy versus corpectomy is the fusion rate. Certainly, the literature demonstrates successful fusion rates for single-level ventral discectomy as high as 96%.42 However, as the number of discectomies increases, so does the nonunion rate.43,44 A corpectomy procedure has the advantage of having fewer sites to fuse (one rostral and one caudal), compared with a multilevel discectomy procedure (rostral, caudal at all intervening sites). In one retrospective review, the corpectomy group had a significantly higher fusion rate when compared with a multilevel discectomy group. However, there were more graft-related complications in the corpectomy group, the clinical outcomes were not significantly different, and these procedures were done without instrumentation.45 Multiple-strut instrumented fusions have a theoretical mechanical and fusion potential advantage, particularly when utilizing intermediate points of fixation.41
Autograft versus Allograft
The use of autograft bone for ventral cervical spine arthrodesis has long been the standard with which to compare other techniques. Structural autograft is typically in the form of tricortical iliac crest or fibula. However, the morbidity associated with these harvest procedures has lead to the use of structural allograft alternatives. Iliac crest bone graft harvest has been associated with a high incidence of local pain, lasting up to a year in one third of patients. Other potential pitfalls include injury to the lateral femoral cutaneous nerve, fracture of the ilium, hematoma formation, and infection. Donor site complications have been reported to be as high as 20%.46
The literature provides mixed results when comparing autograft and allograft for ventral discectomy and fusion procedures. Some investigators report equivalent rates of fusion between autograft and allograft recipients while others demonstrate clearly superior results when using autograft.47–50 A common finding, whether using autograft or allograft, is that increasing the number of discectomies per patient also increases the pseudoarthrosis rate.
The comparison of autograft and allograft for ventral cervical corpectomy is less well studied compared with single-level discectomy. Corpectomy autograft options are tricortical iliac crest and fibula. The predominant corpectomy allograft is the fibular strut graft, although various allograft options exist. Presently, the literature supports the use of both auto-graft and allograft for cervical corpectomy.51–53
Instrumentation versus No Instrumentation
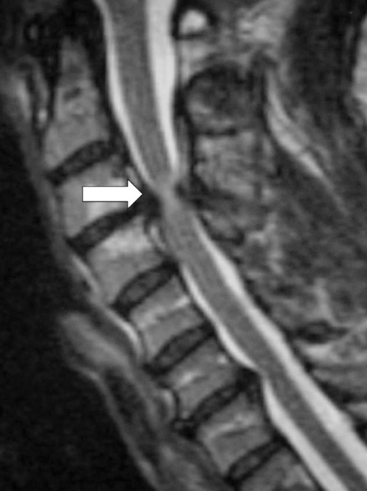
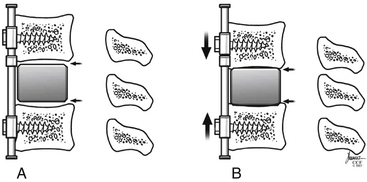
The addition of ventral plating to the cervical spine stemmed from the interest to provide immediate stability, increase arthrodesis rates, and prevent graft dislodgement. Biomechanical testing has demonstrated the ability of ventral cervical plating to enhance construct stability. This increased stability has translated into improved fusion rates clinically, in single as well as multilevel ventral discectomy procedures.54–56 However, the use of ventral plates for cervical corpectomies has not been as promising, especially for multilevel corpectomies. Graft displacement is reported to be the most common complication following cervical corpectomy (Fig. 156-4). One study failed to show any benefit by the application of a plate to patients with a single-level corpectomy, while others demonstrated satisfactory outcomes with plating in single- and multilevel corpectomies.53,57
The specifics of each plate may play a role in outcomes. For example, a static plate (one that does not allow settling) will offload the graft and will be a load-bearing device instead of a load-sharing device. Fusion at the rostral and caudal graft-host interface relies on axial loading. If the implant bears the entire load, this will tend to decrease graft loading and increase implant failure. Newer plate systems allow for some settling, sharing of the load between the implant and graft, and more normal loading characteristics during flexion and extension (Fig. 156-5). Published trials using these implants in corpectomy cases are not yet available. One fact that seems to be agreed upon is if decompression requires three or more contiguous corpectomies, supplemental dorsal stabilization should be seriously considered to help limit ventral graft-related complications.
Cervical Arthroplasty
Cervical arthroplasty has been applied to cervical degenerative disease. This has included myelopathy. There, however, is a concern of continued motion may result in microtrauma to the spinal cord and negatively affect outcome. Buchowski et al. reviewed the IDE trials of two commercially available artificial discs and their use for the treatment of myelopathy.58 The authors reported on 199 patients in which 106 underwent arthroplasty and 93 underwent arthrodesis. All demonstrated myelopathy. Valid outcome measures were utilized. The authors found that both groups demonstrated improvement following surgery. Improvement was similar between groups, with no worsening of myelopathy in the arthroplasty group. The study presents class II data that arthroplasty appears equivalent to arthrodesis for the treatment of single-level compression at the disc space. Other pathologies such as retrovertebral compression or OPLL were not assessed and no conclusions can be drawn.
Evidence
Fehlings and Arvin have provided an excellent editorial relative to the anterior approaches to the spine for CSM.28 Evidence has shown that there is no superiority of anterior cervical discectomy (ACD) and addition of fusion (ACDF) or instrumentation, or corpectomy with plating in the treatment of CSM.59 It appears that ACD and ACDF are equally effective in terms of long term outcome; however, ACDF results in more rapid reduction in neck pain and radiculopathy, and reduces the risk of kyphosis. For multi-level ventral decompression, the addition of fusion to ACD appears superior to ACD alone. The addition of instrumentation reduces the risk on nonunion and improves arm pain, but does not appear to improve long-term outcome versus ACD alone.
Ryken et al. have reviewed the evidence regarding fusion substrate and anterior cervical approaches.60 The use of autologous iliac crest bone, allograft, PEEK, and titanium appear equivalent with regard to fusion. BMP-2 is an option worth discussion for anterior surgery. There have been concerns regarding safety of this osteobiologic in anterior cervical fusions. This has been largely related to swelling after surgery.61 This complication has been reported to be much lower with the usage of lower doses.62 The FDA has released a warning regarding swelling and the use of BMP-2 in the anterior cervical spine and for this reason, the authors discourage its use in that the complications may outweigh the benefits.
Technique: Ventral Cervical Spine Approach
The incision should be placed at the approximate level of pathology by using surface anatomic landmarks. For a transverse incision, these include the hyoid bone (just rostral to the C3–4 level), the thyroid cartilage (at about C4–5), and the cricoid cartilage (approximately C5–6). The approach can be made on the left or right side. The recurrent laryngeal nerve takes a less predictable course on the right side, but there exists no evidence to suggest that this leads to an increased incidence of injury to this nerve following a right-sided exposure. When using a left-sided approach in the low cervical spine and cervicothoracic junction, the surgeon should keep in mind the proximity of the thoracic duct to the lateral confines of the exposure. Unless specific patient-related issues dictate the approach, surgeon comfort and familiarity, as well as surgeon’s hand dominance, should be used to determine the side of exposure.
Discectomy
Once the appropriate level is confirmed radiographically, electrocautery is used to elevate the medial aspect of the longus colli muscle to widen the exposure, visualize the uncovertebral joints, and provide a place under which a self-retaining retractor system can be positioned. Placing the blades of the retractors under the medial border of the longus colli muscle serves to protect the trachea and esophagus medially, the carotid sheath contents laterally, and the sympathetic chain bilaterally. Once the retractors are in place, several optional requests can be made of our anesthetic colleagues prior to proceeding with the decompression. Ensure that the temporal pulse can be palpated on the side of the carotid retraction (make sure it is palpable preoperatively). Additionally, ask the anesthesiologist to deflate and reinflate the endotracheal tube cuff, as there is some evidence this may decrease injury to the recurrent laryngeal nerve.63
Once decompression is complete, attention is directed to inserting the bone graft. Several autograft and allograft options exist and the choice of graft type will depend on the clinical situation and surgeon preference. The size of the graft will depend on the patient’s anatomy and will be measured once the decompression is complete. The height of the graft can be measured using calipers or intervertebral “lollipop” type spacers. The height of the graft is approximately 2 mm greater than the original disc height.64 The depth should be measured to avoid spinal cord compression and to allow 1 to 2 mm of countersinking beyond the anterior border of the vertebral body. If plate stabilization is to be used, it is applied at this point.
Corpectomy
The vertebral body can then be removed in several ways. If fibular allograft is to be used as a strut graft, various-sized rongeurs can be used to remove, save, and morselize the vertebral body bone to pack inside of the canal of the strut graft. The other option is to use a high-speed burr to remove the vertebral body. The width of the corpectomy varies depending on individual patient anatomy and can range from 15 to over 20 mm. A corpectomy width of 16 to 18 mm is typically sufficient for decompression. Careful assessment of preoperative imaging (CT, MRI) can help determine appropriate decompression width. Another important factor to consider when evaluating the imaging studies preoperatively is the location of the vertebral arteries on the axial CT or MRI at the level(s) of planned decompression. Anomalous vertebral artery anatomy can place the artery at risk during a corpectomy because the artery can course closer to the midline than would otherwise be expected.65 Even without aberrant vertebral artery anatomy, excessively wide decompression places the vertebral arteries at risk. This is less of a concern with discectomy due to the uncovertebral joints.
There are several sources of bone graft for corpectomy defects and several ways to secure them in place. Graft options include structural iliac crest and fibular autograft, fibular allograft, allograft bone dowels, and metallic cages. Again, the clinical situation and surgeon preferences and experience help make this determination. As with discectomy, the decompression defect needs to be measured and the appropriate size graft fashioned. Distraction across the defect can be performed internally by means of an intervertebral distractor or externally through traction using Gardner-Wells tongs or a harness device. The rostral and caudal vertebral body end plates can be gently hollowed out using a high-speed burr to serve as docking sites for the strut graft. The ends of the strut can be rounded in a similar manner to provide optimal graft-host bony contact. There are a variety of creative options to help secure the strut between the vertebral bodies, and one such option is that proposed by Whitecloud and LaRocca.66 Instrumentation can then be applied if appropriate.
Complications Associated with Ventral Approach to the Cervical Spine
Neurologic Injury
Injury to the spinal cord or nerve roots is an uncommon occurrence with the ventral approach to the cervical spine. Neurologic complications reported by the Cervical Spine Research Society have an incidence of just over 1%, with dorsal approaches having a greater incidence than ventral approaches.67 When discussing spinal cord injury specifically, the incidence of injury is much lower. Identifiable causes include spinal cord compression, blunt trauma, and ischemia.
Injury of the recurrent laryngeal nerve (RLN) is a known complication of ventral cervical spine surgery. The reported incidence ranges from 1% to 11%.68 Overzealous retraction and endotracheal tube cuff pressure seem to be several intraoperative factors that can be controlled.63 Approaching from the right has also been suggested as a factor that increases the risk for injury to the RLN. This is due to the less predictable and often oblique course the nerve takes through the operative field. Although the anatomy of the nerve differs from right to left, there is no evidence that the side of exposure increases or decreases the incidence of injury. On the other hand, previous ventral spine surgery or a procedure such as thyroid surgery may influence the surgeon’s approach. If the right side is the preferred side, but the patient has had previous left-sided surgery, a left-sided approach should be considered. If this is not possible, then preoperative otolaryngology assessment of vocal cord function should be sought. If the previous surgery damaged the left RLN, proceeding with a contralateral approach risks complete vocal cord paralysis.
Vascular Injury
The major vascular structures with potential injury during a ventral cervical spine exposure are the carotid and vertebral artery. The carotid artery should be easily palpable during the procedure, and attention to detail on the exposure prevents injury. Using blunt self-retaining retractors and occasional release of the retractors help decrease carotid artery trauma. Additionally, the surgeon can mark the location of the ipsilateral temporal pulse, and then ask the anesthesiologist to make sure the pulse is palpable once any retractors are in place. The vertebral artery is at the highest risk for injury during corpectomy. Unlike at the level of the disc, where the uncovertebral joints provide some protection to the vertebral artery by limiting lateral dissection, the same protection is not present at the level of the vertebral body. Accordingly, preoperative imaging in the form of the axial images of a CT scan or MRI should be evaluated to determine a safe width for the corpectomy.65 These studies also demonstrate anomalous vertebral arteries. If a vertebral artery injury occurs, direct pressure with thrombin-soaked Gelfoam to control the hemorrhage is the first step. A variety of options have been proposed in the literature, including packing and observation, delayed endovascular procedures, artery ligation, and direct repair.69,70
Visceral Injury
Perforation of the esophagus can happen during the intraoperative period from sharp or excessive retraction and motorized instruments. Perforation can also occur in the postoperative period due to the esophagus wearing against prominent hardware or bone or from hardware failure.71 The reported incidence ranges from 0.2% to 0.9%.68 Early recognition and aggressive treatment, including irrigation and debridement, repair, broad-spectrum antibiotics, and total parenteral nutrition, should be sought to help prevent the devastating effects of mediastinitis and sepsis.
Pseudoarthrosis
Pseudoarthrosis or nonunion is the failure of a bony union to take place. This complication is often related to poor technique and planning. The bone graft-host site preparation is crucial. Maximal surface area contact between the host vertebral end plates and bone graft improves fusion chances. Autograft bone is likely to yield improved fusion rates, and increasing the number of fusion sites decreases fusion rates.43,44 Patient factors associated with lower fusion rates include smoking, diabetes, and any other immunocompromised states. The use of a ventral cervical plate has been shown to increase the fusion rates in smokers, improve fusion rates with allograft bone, and increase the fusion rate in multilevel discectomy procedures.54–56 Note that a pseudoarthrosis does not necessarily mean a poor outcome. Revision of a pseudoarthrosis is indicated if there are neurologic signs or symptoms or if the nonunion has led to deformity and mechanical axial pain that is resistant to nonoperative measures.
Implant Failure
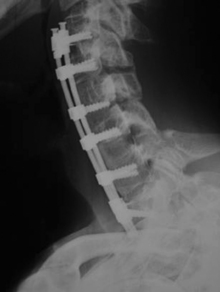
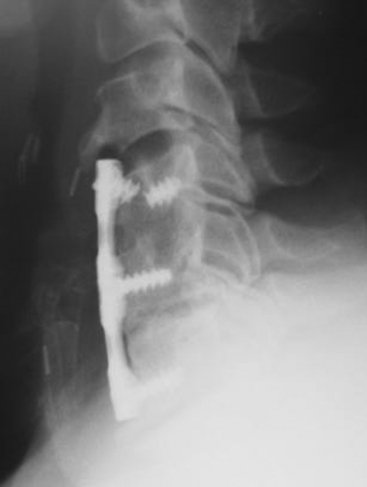
Failure of the instrumentation can occur in several ways. The implant can loosen, causing the screws to back out, for example. The other failure mode is fracture of the implant. Either the plate can break (typically where the cross-sectional area of the plate is the least) or the screw(s) can break (Fig. 156-6). The reason for implant failure should be sought and is typically associated with a pseudoarthrosis. Loose and fractured hardware can be observed closely, but progressive implant migration has the potential for serious complications; therefore, serious consideration should be given to its removal.
Construct Failure
Construct failure with the possibility of deformity may also be seen (see Fig. 156-4). The incidence of such depends on the surgical technique (i.e., discectomy vs. corpectomy) and the number of levels operated on. This complication is usually suspected by increasing cervical pain at a point at which fusion would be expected and/or by new neurologic deficit. Surgical revision may be required.
Operative Management
Dorsal Approach
As described above, most patients with cervical myelopathy eventually undergo surgical decompression. Options include ventral approaches, such as discectomy and corpectomy, posterior approaches, including laminectomy and laminoplasty, and combined approaches. Analysis of the sites of compression, number of levels involved, and spinal alignment generally dictate the most appropriate surgical approach. In general, dorsal approaches are reserved for patients with multilevel involvement, predominantly dorsal or circumferential compression, and a straight or lordotic cervical alignment.
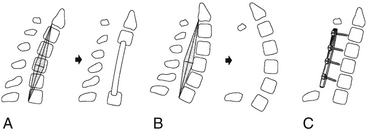
The aforementioned indications are relative and therefore not absolute. The location of spinal cord compression is intuitive and may be gleaned from review of neuroimaging. If multiple consecutive levels are involved in the stenosis (three or more), some would consider laminectomy over ventral multilevel corpectomy. This indication appears to be more surgeon dependent than borne out of the scientific literature. The most important issue regarding the choice of laminectomy, however, is preoperative spinal alignment. Laminectomy may be considered in cases of cervical lordosis and possibly straightening (see Fig. 156-7) of the spine, but avoided in kyphosis. Benzel has described a useful technique in determining effective cervical alignment.72 On a sagittal midline MRI of the cervical spine, a line is drawn from the dorsocaudal aspect of C2 to the dorsocaudal aspect of the C7 vertebral body. A perpendicular line is then drawn in the middle of the prior line. The width of this perpendicular line is determined by the surgeon’s experience and preferences. The lines are then connected, which creates an area that resembles a kite. If all cervical vertebral bodies lie in front or ventral to the kite, the spine is lordotic, and laminectomy is appropriate (Fig. 156-7). If the kite lies within the vertebral bodies, the spine is kyphotic, and laminectomy alone is not indicated. There are situations in which the dorsal vertebral bodies only line partly within the kite. In this circumstance, the spine is straight, and a ventral or dorsal approach may then be utilized.
Technique: Dorsal Cervical Spine Approach
Laminectomy
Following exposure and hemostasis, the levels to be decompressed should be fully identified. Many techniques exist to perform the laminectomy. A high-speed drill is used to create a trough on either side of midline at the facet-lamina junction. The trough should be carried down to the thin inner cortical margin of the lamina. A small rongeur is then used to complete the troughs to the level of the dura bilaterally. The laminae may then be removed en bloc. This technique avoids the need to place an instrument between the laminae and dura. Any remaining ligamentum flavum may then be removed sharply. Lateral decompression (i.e., nerve root) may then be performed, if necessary. Small no. 2 and no. 1 rongeurs may be used to perform the lateral decompression with care not to remove greater than 50% of the facet for fear of instability.73
Laminoplasty
The indications for laminoplasty versus laminectomy are surgeon dependent. Theoretically, laminoplasty should provide some aspect of stability and may then be useful in cases where current or future stability is in question. Furthermore, there is a theoretical reduced chance of epidural scar formation following laminoplasty compared with laminectomy.74 For example, it is useful in children with spinal cord tumors in which a multilevel laminectomy would be required.
Results
Laminectomy
Laminectomy appears to result in the restoration of neurologic function in some patients or at least halt the progression of the disease following surgery. Overall 68% to 85% of patients can be expected to have a good or excellent outcome.75–78
Epstein et al. reported on 1355 patients treated for cervical myelopathy.79 In those patients who were treated with a posterior approach, either extensive or limited laminectomies, 60% to 85% were improved. Age and gender had no effect on the results, although the investigators noted that no patient older than 70 years had an excellent result.
Of 84 patients reported by Collias and Roberts, improvement was seen in 70% of patients, stable neurologic status was seen in 29%, while 2% were worse following posterior decompression for cervical myelopathy.80 The patients who were worse following surgery had errors in their preoperative diagnosis. One patient had amyotrophic lateral sclerosis while the other had an astrocytoma of the spinal cord.
Laminectomy appears as effective as ventral procedures for the treatment of cervical spondylotic myelopathy.81 Some researchers have reported slightly superior results with ventral procedures.77 Although it is difficult to directly compare the two procedures based on reports in the literature, proper patient selection is probably the most important predictor of outcome following a dorsal procedure compared with ventral strategies.
Laminoplasty
Studies have demonstrated that laminoplasty can increase and maintain spinal canal diameter. In one report, the area of the spinal cord increased 7.4%, while the dural sac area increased 33.8%.82 Furthermore, clinical improvement has been demonstrated. Lee et al. performed laminoplasty on 25 patients with myelopathy.83 Eighteen months following surgery, 84% of patients demonstrated improvement in gait, 87% improved with regard to upper extremity function (numbness and tingling), while 77% improved in bowel and bladder function. Miyazaki et al. reported a 75.5% neurologic improvement following laminoplasty.84
Ultimately laminoplasty leads to fusion of the dorsal elements (i.e., those levels included in the laminoplasty). This results in a loss of cervical range of motion by 30% to 70%.65 This may alleviate axial neck pain following surgery.
Evidence
There is no class I evidence supporting one dorsal approach over the other. With adequate decompression all dorsal approaches have resulted in improved short-term outcomes. Laminectomy appears to have equal efficiency to laminoplasty and instrumented fusion at least in the short-term.85–87 Laminoplasty and laminectomy have the benefit of maintaining motion, but may be at the expense of postoperative kyphosis, especially laminectomy.
Complications Associated with Dorsal Approach to Cervical Spine
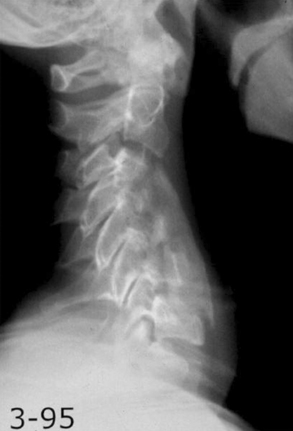
The most dreaded complication following a dorsal approach is kyphosis (Fig. 156-8). The incidence may be as high as 21% following laminectomy.88 Careful patient selection and meticulous surgical technique should lessen this complication. Theoretically, laminoplasty should have a lower incidence of postoperative kyphosis.
Nerve root injury may also be encountered following a dorsal procedure. It was reported to occur in 13% of cases in one study. It involved C5 and C6 most often with mainly motor symptoms. The researchers reported a mean recovery time of 5.4 months.89
Infection and wound breakdown may be higher with dorsal procedures compared with ventral ones (personal experience). Patients should be optimally mobilized and have adequate nutrition to attempt to lessen these complications. The incidence may be greater following laminoplasty because of an elevated block of laminae.90
Other potential complications include epidural hematoma. The incidence may be less following laminoplasty because of the retained block of laminae.90 CSF leak or vertebral injury may be encountered. Careful dissection and bone removal should lessen these complications.
Combined Approach (Ventral and Dorsal)
Once the decision for surgical intervention is made, three options are possible: ventral, dorsal,91–95 and combined ventral and dorsal.91,96–100 While all three options have proponents and detractors, the goals of surgery remain the same: decompress the neural elements and stabilize the spine if necessary. The advantages of the dorsal approach include familiarity, ease of decompression of multiple levels, and the ability to extend the decompression and/or fixation and fusion rostrally to the occiput or caudally to the thoracic spine. The major disadvantage of dorsal decompression is the requisite disruption of the posterior tension band resulting in a high rate of postlaminectomy kyphosis.72,88 This complication has led a number of researchers to caution against laminectomy or laminoplasty in patients with a pre-existing kyphosis, or even the relative kyphosis seen in patients with a “straightened” cervical spine72,99 (see Fig. 156-7). In some cases, a mild kyphosis can be corrected with a combination of dorsal decompression and posterior fixation, but this requires the presence of a flexible deformity.91 However, ventral compression of the neural elements cannot be directly addressed with a purely dorsal approach.
The ventral approach allows the surgeon to directly decompress the spinal cord in cases of ventral pathology via discectomy, multiple level discectomies, or corpectomy. Moreover, it affords the surgeon the ability to perform a ventral release prior to any attempts to correct a cervical deformity. Although this strategy has been shown to be highly effective for patients with short segment stenosis, multisegment constructs have been plagued by historically high rates of pseudoarthrosis, bone graft subsidence, and graft dislocation.95,99 These complications are the result of the often suboptimal bony fixation sites and the reliance on screw/plate fixation as the only method of bony fixation allowed by the ventral approach.72 Some of these factors may be mitigated by the use of multiple points of fixation and the use of dynamic implants, but their true effectiveness has yet to be proven.72 A purely ventral approach, moreover, does not allow access for dorsal decompression of the spinal cord.
The use of a combined dorsal and ventral strategy affords all of the previously mentioned advantages of each and limits the disadvantages of each approach alone. Both dorsal and ventral compression can be addressed directly, resulting in a 360-degree decompression of the spinal cord. Optimal correction of sagittal plane deformities may be achieved by taking advantage of the mechanical advantage of dorsal fixation and osteotomies in conjunction with anterior releases and reconstruction of the ventral load-bearing column via interbody fusion techniques afforded by the ventral approach.72,97,100 The addition of dorsal fixation to a ventral construct may also serve to reduce the rates of pseudoarthrosis, especially in multisegment constructs. The dorsal fixation loads the ventral interbody graft, creating a compressive moment as well as increasing translational and torsional resistance, thereby increasing fusion rates via Wolff’s law.72 This may be especially useful in patients with poor bone quality, prior failed surgeries, or medical comorbidities that could compromise healing. However, a combined anterior-posterior fusion strategy may obviate the need for external orthoses in many cases.97,101 Some researchers recommend dorsal fixation when a fibular allograft is employed ventrally because it may take up to 2 years for a long strut to incorporate.102
The major disadvantage of a combined approach is the increase in morbidity and perceived increased risk for complications when compared with either dorsal or ventral strategies alone. However, acceptably low rates of morbidity and complications of 5% to 11% have recently been reported in the literature.97,100
While the majority of patients with cervical myelopathy can be treated by either a ventral or dorsal approach alone, a subset of patients may only be optimally treated by a circumferential approach. In general, a combined anterior-posterior strategy is indicated for any patient with multilevel spondylosis requiring extensive decompression, multilevel spondylosis with congenital cervical stenosis, multilevel spondylosis with kyphosis, postlaminectomy kyphosis, swan-neck deformity, or post-traumatic kyphosis.6 Moreover, a circumferential strategy should be considered in patients with poor bone quality, patients with comorbidities that may inhibit bony fusion or wound healing, and any patient that has failed a prior attempt at deformity correction (Fig. 156-9). While no specific contraindications to the combined approach exist, it should be used with discretion due to the relative increase in morbidity and surgical risk when compared with either a dorsal or ventral strategy in isolation.6
Conclusion
CSM is a common spinal disorder caused by a number of congenital and degenerative etiologies. Unfortunately, the natural history of CSM is one of progressive neurologic decline. Although some mild cases may be treated conservatively, most are treated surgically. The primary goal of surgical intervention in CSM is decompression of the neural elements to prevent disease progression. Secondarily, stabilization and fusion may often be indicated to prevent or correct deformity and to prevent further repetitive neural trauma. This goal can be accomplished through a variety of ventral, dorsal, and combined (ventral and dorsal) approaches as described above. The ideal approach for any given patient depends on a number of factors, including extent of disease (single level vs. multilevel), the site of spinal cord compression (dorsal, ventral, or both), the extent of cervical kyphosis, the general medical condition of the patient, and the surgeon’s experience and comfort with the approach. We generally prefer the ventral approach for patients with single level or limited disease, with mild kyphosis, or with purely ventral pathology. The dorsal approach is advocated for patients with multilevel or dorsal pathology. A combined approach is utilized for patients who require more extensive deformity correction than is possible via a ventral approach alone or for patients who have failed prior surgery. Regardless of the surgical strategy employed, we favor early intervention in most cases of CSM, given the apparent association between duration of symptoms and neurologic outcome.
Clarke E., Robinson P.K. Cervical myelopathy: a complication of cervical spondylosis. Brain. 1956;79:483-510.
Emery S.E., Bolesta M.J., Banks M.A., et al. Robinson anterior cervical fusion: comparison of the standard and modified techniques. Spine. 1994;19:660-663.
Montgomery D.M., Brower R.S. Cervical spondylotic myelopathy: clinical syndrome and natural history. Orthop Clin North Am. 1992;23:487-493.
White A.A., Panjabi M.M. Biomechanical considerations in the surgical management of cervical spondylotic myelopathy. Spine. 1988;13:856-860.
Zdeblick T.A., Hughes S.S., Riew K.D., et al. Failed anterior cervical discectomy and arthrodesis: analysis and treatment of thirty-five patients. J Bone Joint Surg Am. 1997;79:523-532.
1. Montgomery D.M., Brower R.S. Cervical spondylotic myelopathy: clinical syndrome and natural history. Orthop Clin North Am. 1992;23:487-493.
2. Heller J.G. The syndromes of degenerative cervical disease. Orthop Clin North Am. 1992;23:381-394.
3. Clarke E., Robinson P.K. Cervical myelopathy: a complication of cervical spondylosis. Brain. 1956;79:483-510.
4. Rao R. Neck pain, cervical radiculopathy, and cervical myelopathy: pathophysiology, natural history and clinical evaluation. Instr Course Lect. 2003;52:479-488.
5. Yonenobu K. Cervical radiculopathy and myelopathy: when and what can surgery contribute to treatment? Eur Spine J. 2000;9:1-7.
6. Truumees E., Herkowiz H.N. Cervical spondylotic myelopathy and radiculopathy. Instr Course Lect. 2000;49:339-360.
7. Adams C.B., Logue V. Studies in cervical spondylitic myelopathy. Brain. 1971;94:557-594.
8. Brown M.D. The pathophysiology of disc disease. Orthop Clin North Am. 1971;2:359-370.
9. White A.A., Panjabi M.M. Biomechanical considerations in the surgical management of cervical spondylotic myelopathy. Spine. 1988;13:856-860.
10. McCormick W.E., Steinmetz M.C., Benzel E.C. Cervical spondylotic myelopathy: make the difficult diagnosis then refer for surgery. Cleve Clin J Med. 2003;70(10):899-904.
11. Bernhardt M., Hynes R.A., Blume H.W., White A.A. Current concepts review: cervical spondylotic myelopathy. J Bone Joint Surg Am. 1993;75:119-128.
12. Shimomura Y., Hukuda S., Mizuno S. Experimental study of ischemic damage to the cervical spinal cord. J Neurosurg. 1954;28:565-581.
13. Ferguson R.J.L., Caplan L.R. Cervical spondylotic myelopathy. Neurol Clin. 1985;3:373-382.
14. Verbiest H. The management of cervical spondylosis. Clin Neurosurg. 1973;20:262-294.
15. Spillane J.D., Lloyde G.H.T. The diagnosis of lesions of the spinal cord in association with “osteoarthritic” disease of the cervical spine. Brain. 1952;75:177-186.
16. Lees F., Turner J.W.A. Natural history and prognosis of cervical spondylosis. BMJ. 1963;2:1607-1610.
17. Matz P.G., Anderson P.A., Holly L.T., et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:104-111.
18. Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:101-108.
19. Phillips D.G. Surgical treatment of myelopathy with cervical spondylosis. J Neurol Neurosurg Psychiatry. 1973;36:879-884.
20. Sadasivan K.K., Reddy R.P., Albright J.A. The natural history of cervical spondylotic myelopathy. Yale J Biol Med. 1993;66:235-242.
21. Benzel E.C., Lancon J., Kesterson L., et al. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord. 1991;4(3):286-295.
22. Ono K., Ebara S., Fuji T., et al. Myelopathy hand: new clinical signs of cervical cord damage. J Bone Joint Surg Br. 1987;69:215-219.
23. Voskuhl R.R., Hinton R.C. Sensory impairment in the hands secondary to spondylotic compression of the cervical spinal cord. Arch Neurol. 1990;47(3):309-311.
24. Sakakibara R., Hattori T., Tojo M., et al. The location of the paths subserving micturation: studies in patients with cervical myelopathy. J Auton Nerv Syst. 1995;55(3):165-168.
25. Adams K.K., Jackson C.E., Rauch R.A., et al. Cervical myelopathy with false localizing sensory levels. Arch Neurol. 1996;53(11):1155-1158.
26. Pavlov H., Torg J.S., Robie B., Jahre C. Cervical spinal stenosis: determination with vertebral body ratio method. Radiology. 1987;164:771-775.
27. Morio Y., Teshima R., Nagashima H., et al. Correlation between operative outcomes of cervical compression myelopathy and MRI of the spinal cord. Spine. 2001;26(11):1238-1245.
28. Fehlings M.G., Arvin B. Surgical management of cervical degenerative disease: the evidence related to indications, impact and outcome. J Neurosurg Spine. 2009;11:97-100.
29. Mummaneni P.V., Kaiser M.G., Matz P.G., et al. Preoperative patient selection with magnetic resonance imaging, computed tomography, and electroencephalography: does the test predict outcome after cervical surgery? J Neurosurg Spine. 2009;11:119-129.
30. Shafaie F.F., Wippold F.J., Gado M., et al. Comparison of computed tomography myelography and magnetic resonance imaging in the evaluation of cervical spondylotic myelopathy and radiculopathy. Spine. 1999;24:1781-1785.
31. Lyu R.K., Tang L.M., Chen C.J., et al. The use of evoked potentials for clinical correlation and surgical outcome in cervical spondylotic myelopathy with intramedullary high signal intensity on MRI. J Neurol Neurosurg Psychiatry. 2004;75:256-261.
32. Morshita Y., Hida S., Naito M., Matsushima U. Evaluation of cervical spondylotic myelopathy using somatosensory-evoked potentials. Int Orthop. 2005;29:343-346.
33. Holly L.T., Matz P.G., Anderson P.A., et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:112-118.
34. LaRocca H. Cervical spondylotic myelopathy: natural history. Spine. 1988;13:854-855.
35. Way Y.L., Tsau J.C., Huang M.H. The prognosis of patients with cervical spondylotic myelopathy. Kaohsiung J Med Sci. 1997;13:425-431.
36. Fouyas I.P., Statham P.F., Sandercock P.A. Cochrane review on the role of surgery in cervical spondylotic radiculomyelopathy. Spine. 2002;27:736-747.
37. Yamazaki T., Yanaka K., Sato H., et al. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52:122-126.
38. Robinson R.A., Smith G.W. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. Bull Johns Hopkins Hosp. 1955;96:223-224.
39. Smith P.N., Knaub M.A., Kang J.D. Anterior cervical approaches for cervical radiculopathy and myelopathy. Instr Course Lect. 2003;52:455-463.
40. Cloward R.B. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15:602-617.
41. Steinmetz M.P., Kager C., Benzel E.C. Anterior correction of postsurgical cervical kyphosis. J Neurosurg (Spine 2). 2002;97:277-280.
42. Emery S.E., Bolesta M.J., Banks M.A., et al. Robinson anterior cervical fusion: comparison of the standard and modified techniques. Spine. 1994;19:660-663.
43. Emery S.A., Fisher J.R., Bohlman H.H. Three-level anterior cervical discectomy and fusion: radiographic and clinical results. Spine. 1997;22:2622-2625.
44. Swank M.L., Lowery G.L., Bhat A.L., et al. Anterior cervical allograft arthrodesis and instrumentation: multilevel interbody grafting or strut graft reconstruction. Eur Spine J. 1997;6:138-143.
45. Hilibrand A., Fye M., Emery S., et al. Improved arthrodesis with strut grafting after multilevel anterior cervical decompression. Spine. 2002;27:146-151.
46. Whitecloud T.S.III. Complications of anterior cervical fusion. Instr Course Lect. 1978;27:223-227.
47. Young W.F., Rosenwasser R.H. An early comparative analysis of the use of fibular allograft versus autologous iliac crest graft for interbody fusion after anterior cervical discectomy. Spine. 1993;18:1123-1124.
48. Shapiro S. Banked fibula and the locking anterior cervical plate in anterior cervical fusions following cervical discectomy. J Neurosurg. 1996;84:161-165.
49. Bishop R.C., Moore K.A., Hadley M.N. Anterior cervical interbody fusion using autogeneic and allogeneic bone graft substrate: a prospective comparative analysis. J Neurosurg. 1996;85:206-210.
50. An H.S., Simpson J.M., Glover J.M., et al. Comparison between allograft plus demineralized bone matrix versus autograft in anterior cervical fusion: a prospective multicenter study. Spine. 1995;20:2211-2216.
51. Bernard T.N.Jr., Whitecloud T.S.III. Cervical spondylotic myelopathy and myeloradiculopathy: anterior decompression and stabilization with autogenous fibula strut graft. Clin Orthop. 1987;221:149-160.
52. Eleraky M.A., Llanos C., Sonntag V.K. Cervical corpectomy: report of 185 cases and review of the literature. J Neurosurg. 1999;90(suppl 1):35-41.
53. Mayr M.T., Subach B.R., Comey C.H., et al. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg. 2002;96:10-16.
54. Wang J.C., McDonough P.W., Endow K., et al. The effect of cervical plating on single-level anterior cervical discectomy and fusion. J Spinal Disord. 1999;12:467-471.
55. Wang J.C., McDonough P.W., Kanim L.E., et al. Increased fusion rates with cervical plating for three-level anterior cervical discectomy and fusion. Spine. 2001;26:643-647.
56. Kaiser M.G., Haid R.W., Subach B.R., et al. Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery. 2002;50(2):229-236.
57. Epstein N.E. Reoperation rates for acute graft extrusion and pseudoarthrosis after one-level anterior corpectomy and fusion with and without plate instrumentation: etiology and corrective management. Surg Neurol. 2001;56:73-81.
58. Buchowski J.M., Anderson P.A., Sekhon L., Riew K.D. Cervical disc arthroplasty compared with arthrodesis for the treatment of myelopathy. J Bone Joint Surg Am. 2009;91:223-232.
59. Matz P.G., Holly L.T., Mummaneni P.V., et al. Anterior cervical surgery for the treatment of cervical degenerative myelopathy. J Neurosurg Spine. 2009;11:170-173.
60. Ryken T.C., Heary R.F., Matz P.G., et al. Techniques for cervical interbody grafting. J Neurosurg Spine. 2009;11:203-220.
61. Smucker J.S., Rhee J.M., Singh K., et al. Increased swelling complications associated with off-label usage of rhBMP in the anterior cervical spine. Spine. 2006;31(24):2813-2819.
62. Tumialan L.M., Pan J., Rodts G.E., Mummaneni P.V. The safety and efficacy of anterior cervical discectomy and fusion with polyetheretherketone spacer and recombinant human bone morphogenic protein-2: a review of 200 patients. J Neurosurg Spine. 2008;8(6):529-535.
63. Apfelbaum R.I., Kriskovich M.D., Haller J.R. On the incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine. 2000;25(22):2906-2912.
64. An H.S., Evanish C.J., Nowicki B.H., et al. Ideal thickness of Smith Robinson graft for anterior cervical fusion: a cadaveric study with computed tomographic correlation. Spine. 1993;18:2043-2047.
65. Curylo L.J., Mason H.C., Bohlman H.H., et al. Tortuous course of the vertebral artery and anterior cervical decompression: a cadaveric and clinical case study. Spine. 2000;25(22):2860-2864.
66. Whitecloud T.S.III, LaRocca H. Fibular strut graft in reconstructive surgery of the cervical spine. Spine. 1976;1:33.
67. Graham J.J. Complications of cervical spine surgery: a five-year report on a survey of the membership of the Cervical Spine Research Society by the Morbidity and Mortality Committee. Spine. 1989;14:1046-1050.
68. Patel C.K., Fischgrund J.S. Complications of anterior cervical spine surgery. Instr Course Lect. 2003;52:465-469.
69. Pfeifer B.A., Freidberg S.R., Jewell E.R. Repair of injured vertebral artery in anterior cervical procedures. Spine. 1994;19:1471-1474.
70. Golfinos J.G., Dickman C.A., Zabramski J.M., et al. Repair of vertebral artery injury during anterior cervical decompression. Spine. 1994;19:2552-2556.
71. Newhouse K.E., Lindsey R.W., Clark C.R., et al. Esophageal perforation following anterior cervical spine surgery. Spine. 1989;14:1051-1053.
72. Benzel E.C. Biomechanics of Spine Stabilization. Rolling Meadows, IL: American Association of Neurological Surgeons; 2001.
73. Zdeblick T.A., Abitbol J.J., Kunz D.N., et al. Cervical stability after sequential capsule resection. Spine. 1993;18(14):2005-2008.
74. Kawaguchi Y., Kanamori M., Ishihara H., et al. Minimum 10-year follow-up after en bloc cervical laminoplasty. Clin Orthop Rel Res. 2003;411:129-139.
75. Fujiwara K., Yonenobu K., Ebara S., et al. The prognosis of surgery for cervical compression myelopathy: an analysis of the factors involved. J Bone Joint Surg Br. 1989;71:393-398.
76. Epstein N.E., Epstein J.A. Operative management of cervical spondylotic myelopathy: techniques and results of laminectomy. In: Clark C.R., Ducker T.B., Dvorak J., et al. The Cervical Spine. 3rd ed. Philadelphia: Lippincott-Raven; 1998:839-848.
77. Herkowitz N.H. A comparison of anterior cervical fusion, cervical laminectomy, and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine. 1988;13:774-780.
78. Malone D.G., Benzel E.C. Laminotomy and laminectomy for spinal stenosis causing radiculopathy or myelopathy. In: Clark C.R., Ducker T.B., Dvorak J., et al. The Cervical Spine. 3rd ed. Philadelphia: Lippincott-Raven; 1998:817-823.
79. Epstein J.A., Janin Y., Carras R., Lavine L.S. A comparative study of the treatment of cervical spondylotic myeloradiculopathy: experience with 50 cases treated by means of extensive laminectomy, foraminotomy, and excision of osteophytes during the past 10 years. Acta Neurochir (Wien). 1982;61:89.
80. Collias J.C., Roberts M.P. Posterior surgical approaches for cervical disc herniation and spondylotic myelopathy. In: Schmidek H.H., editor. Operative Neurosurgical Techniques: Indications, Methods, and Results. 4th ed. Philadelphia: Harcourt; 2000:2016-2028.
81. Gorter K. The influence of laminectomy on the course of cervical myelopathy. Acta Neurochir (Wien). 1976;33:265.
82. Aita I., Hayashi K., Wadano T., Yabuki T. Posterior movement and enlargement of the spinal cord after cervical laminoplasty. J Bone Joint Surg Br. 1998;80(1):33-37.
83. Lee T.T., Manzano G.R., Green B.A. Modified open-door cervical expansile laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86:64-68.
84. Miyazaki K., Hirohuji E., Ono S., et al. Extensive simultaneous multi-segmental laminectomy and posterior decompression with posterolateral fusion. J Jpn Spine Res Soc. 1994;5:167.
85. Matz P.G., Anderson P.A., Groff M.W., et al. Cervical laminoplasty for the treatment of cervical degenerative myelopathy. J Neurosurg Spine. 2009;11:157-169.
86. Mummaneni P.V., Kaiser M.G., Matz P.G., et al. Cervical surgical techniques for the treatment of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:130-141.
87. Ryken T.C., Heary R.F., Matz P.G., et al. Cervical laminectomy for the treatment of cervical degenerative myelopathy. J Neurosurg Spine. 2009;11:142-149.
88. Kaptain G.J., Simmons N., Replogle R.E., et al. Incidence and outcome of kyphotic deformity following laminectomy for cervical spondylotic myelopathy. J Neurosurg (Spine 2). 2000;93:199-204.
89. Dai L., Ni B., Yuan W., Jia L. Radiculopathy after laminectomy for cervical compression myelopathy. J Bone Joint Surg Br. 1998;80:846-849.
90. Yonenobu K., Yamamoto T., Ono K. Laminoplasty for myelopathy: indications, results, outcome, and complications. In: Clark C.R., Ducker T.B., Dvorak J., et al. The Cervical Spine. 3rd ed. Philadelphia: Lippincott-Raven; 1998:849-864.
91. Abumi K., Shono Y., Taneichi H. Correction of cervical kyphosis using pedicle screw fixation systems. Spine. 1999;24:2389-2396.
92. Butler J.C., Whitecloud T.S.III. Postlaminectomy kyphosis: causes and surgical management. Clin Orthop North Am. 1992;23:505-511.
93. Cattrell H.S., Clark G.J.Jr. Cervical kyphosis and instability following multiple laminectomies in children. J Bone Joint Surg Am. 1967;49:713-720.
94. Herman J.M., Sonntag V.K. Cervical corpectomy and plate fixation for post-laminectomy kyphosis. J Neurosurg. 1994;80:963-970.
95. Zdeblick T.A., Hughes S.S., Riew K.D., et al. Failed anterior cervical discectomy and arthrodesis: analysis and treatment of thirty-five patients. J Bone Joint Surg Am. 1997;79:523-532.
96. Heller J.G., Silcox D.H.III, Sutterlin C.E.III. Complications of posterior cervical plating. Spine. 1995;20:2442-2448.
97. McAfee P.C., Bohlman H.H., Ducker T.B. One stage anterior cervical decompression and posterior stabilization: a study of one hundred patients with a minimum of two years of follow-up. J Bone Joint Surg Am. 1995;77:1791-1800.
98. Savini R., Parisini P., Cervellati S. The surgical treatment of late instability of flexion-rotation injuries in the lower cervical spine. Spine. 1987;12:178-182.
99. Albert T.J., Vacarro A. Postlaminectomy kyphosis. Spine. 1998;23:2738-2745.
100. Schultz K.D., McLaughlin M.R., Haid R.W., et al. Single-stage anterior-posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg (Spine 2). 2000;93:214-221.
101. Rushton S.A., Albert T.J. Cervical degenerative disease: rationale for selecting the appropriate fusion technique (anterior, posterior and 360 degree). Orthop Clin North Am. 1998;29:755-777.
102. Bohlman H.H., Emery S.E. The pathophysiology of cervical spondylosis and myelopathy. Spine. 1988;13:843-846.