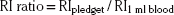Chapter 138 Management of Cerebrospinal Fluid Leaks
Cerebrospinal fluid (CSF) fistula is a serious and potentially fatal condition whose successful management requires a fundamental multidisciplinary approach. Until recently, the management of this condition was almost exclusively neurosurgical. Over the past decade, however, otolaryngologists skilled in functional endoscopic sinus surgery have contributed considerably to the surgical armamentarium, and the endoscopic approach is considered by some of those surgeons to have become the standard of care most specially in CSF leaks of the anterior and middle skull base.1 Although the endoscopic approach may be well-suited to the majority of cases encountered in otolaryngologic practice, it does not exhaust the techniques with which the neurosurgeon must be conversant in view of the full range of anatomic and pathophysiologic conditions confronted in neurosurgical practice. This chapter, therefore, addresses both the traditional neurosurgical approaches and the more recent endoscopic techniques. In addition, it reviews the use of glues, engineered tissues, and tissue substitutes in the management of CSF fistulas.
Historical Overview
The correlation of posttraumatic rhinorrhea with leakage of CSF was made in the 17th century by a Dutch surgeon, Bidloo the Elder.2,3 Cases in which apparently nontraumatic CSF rhinorrhea resulted from increased intracranial pressure were then reported by Miller in 18264 and by King in 1834.5 The full significance of CSF fistulas was not elucidated until 1884, however, when Chiari6 demonstrated a fistulous connection between a pneumatocele in the frontal lobes and the ethmoid sinuses of a patient who died of meningitis following rhinorrhea. The introduction of roentgenography enabled the diagnosis of a fistula to be made in vivo through the detection of intracranial air,7 ultimately leading to the development of pneumoencephalography as a diagnostic procedure8 and, less directly, to the refinement of surgical techniques for the repair of CSF fistulas.9,10
Despite a number of early attempts, successful repair was not consistently achieved until the mid-1930s. In 1937, Cairns11 published a series of cases demonstrating that CSF leaks could be repaired by the extradural application of fascia lata. The actual need for surgical intervention was regarded as unproven, however, and the indications remained controversial until the latter part of World War II.
By 1944, Dandy12 advocated surgical repair of any CSF leak within 2 weeks of onset to prevent meningitis. In British neurosurgery, Lewin’s review of the British combat experience and of a large series of basilar skull fractures13,14 became the basis for the adoption of aggressive operative management as the standard of care. Lewin demonstrated that the cessation of an active leak did not itself eliminate the risk of meningitis, because the possibility of intermittent communication with the contaminated extracranial space persisted unless repaired. By the mid-1950s, it became virtually axiomatic to operate on all CSF fistulas, except for some that had a well-understood cause and that closed spontaneously within several days of onset.
The recent enthusiasm for extradural endoscopic approaches to the skull base reflects both the advancement of endoscopic technology and the relative safety of extradural techniques. In fact, Cairns and Dandy both advocated the extradural approach because of its safety. As intradural surgery became safer in the years following World War II, the extradural approach was supplanted, at least among neurosurgeons.15 Evidence upon which to base a consensus regarding the more successful operative route has yet to be determined.
The introduction of minimally invasive approaches to CSF fistulas, whether microscopic or endoscopic, has simultaneously facilitated the extradural approach and blurred the distinction between truly extradural and intradural repair.1,16–18
For purposes of this discussion, endoscopic repair is considered extradural when it is intended to patch or plug a fistula from outside in, and is considered intradural when it is intended to facilitate the localization or repair of a fistula by placing a plug or graft intradurally.19,20
The primary danger of CSF leakage has been framed in terms of the potential for meningitis, and the primary indication for treatment has been driven by the rarity of spontaneous and permanent cessation of the leak. Why should some leaks stop and others not? Why should some recur? Why should 20% or more of repairs that are done come to failure? With these questions in mind, the principle promoted by Lewin13 (i.e., the idea that all cranial CSF fistulas, without exception, be repaired surgically unless they resolve spontaneously within 5 days or a week) has come under increasing scrutiny. The observations that have led to a reconsideration of the Lewin principle are as follows:
1. Some fistulas seem to heal spontaneously with time and stay closed, especially if external CSF drainage is used as an adjunctive maneuver.
2. A recurrence rate of 6% to 25% and a not inconsequential historical operative morbidity and mortality accompany the various forms of reparative surgery.
3. The incidence and severity of meningitis in otherwise uncomplicated CSF leaks may be diminished by treatment with antibiotics.3,21–29
Conventions and Definitions
Transcranial CSF leaks fall into two major categories: traumatic leaks and the so-called spontaneous or nontraumatic leaks. The traumatic group, in turn, is divided into two groups: acute or early leaks that present within 1 week of injury and delayed leaks that occur months or years later. The nontraumatic group is also divided into subsets, including leaks associated with intracranial mass lesions, congenital defects of the skull base, osteomyelitis, osteonecrosis (and other causes of bony erosion), and focal cerebral atrophy5; and leaks associated with an ill-defined group of acquired hernias, meningoceles, and meningeal diverticula perforating pneumatized bone in the anteromedial middle fossa.30 Nontraumatic fistulas are divided again into high-pressure and low-pressure categories.5,31 There is good reason to apply an analogous nosology to the traumatic group as well.25 Iatrogenic or postoperative leaks are usually included in the category of traumatic fistulas.
Spinal CSF leaks can be classified similarly. Most spinal CSF leaks are postoperative and therefore traumatic. A number of rare congenital anomalies can give rise to meningopleural or meningoperitoneal fistulas.32 The distinction between high-pressure and low-pressure fistulas is particularly important in the management of spinal leaks. In children with spinal dysraphism or other anomalies, the leak may be the first expression of hydrocephalus or shunt failure.33,34
Cause and Epidemiology
Traumatic Leaks
The most common cause of CSF leaks is head trauma, particularly basilar skull fracture.35 In Lewin’s series of 100 patients with head injury,13 7% had basal skull fractures, and 2% had CSF leaks. A CSF leak was detected in 2.8% of 1250 head injuries and in 11.5% of the basilar fractures studied by Brawley.22 In another study of 1077 skull fractures, including a particularly large proportion of high-speed road traffic accidents, 20.8% of 168 basilar skull fractures had an acute CSF leak.36 The association of incidence with high speed, although not rising to the level of a correlation, is certainly suggestive. The incidence in cases of penetrating missile injuries is comparable: in 1133 cases, 101 (8.9%) developed a CSF fistula. The proportion is somewhat higher with transventricular penetration.37 Traumatic CSF leaks typically begin within 48 hours, and it is estimated that 95% of them will be evident within 3 months of injury.38,39
In childhood, the incidence of traumatic CSF leaks is far lower at 1% or less of closed head injuries.40 This disparity may be caused by differences in fragility between the adult and the pediatric skull, as well as by the lack of development of the air sinuses in children. As a rule, the frontal sinuses become visible between the 4th and 12th year, and they are always detected by the 15th year. These sinuses are often asymmetric until age 20 years. The ethmoids are present at birth, enlarge by age 3 years, and are fully formed by age 16 or 17 years. The cavity of the sphenoid sinus is usually recognizable by age 4 years and is fully developed by puberty. In the pediatric age range, the interpretation of sinus x-ray studies is often difficult because of small size, variations in development, and normal calcification and clouding.35
Spontaneous Leaks
This term should be restricted to leaks explained neither by trauma nor by any other cause and because there have been few, if any, collected series of nontraumatic leaks rigorously studied, there are insufficient data to extrapolate quantitative estimates of incidence or cause.31 Tumors and increased intracranial pressure (ICP) are highly correlated with nontraumatic leaks. Anecdotal series suggest that pituitary tumors are the most common neoplastic cause of spontaneous CSF leaks. Increased ICP may be present or absent. Because of the structures eroded by sellar masses, such leaks generally present as rhinorrhea.33,41,42 Other presentations, including a serous otitis media, have also been reported.43–45 On the other hand, there is an intriguing report of pituitary hyperemia in the context of nontraumatic CSF leak masquerading as pituitary adenoma in three patients. After surgical repair of the leak, the magnetic resonance imaging (MRI) abnormalities, including an enlarged pituitary resembling pituitary tumor, reverted to normal.45
Postoperative Leaks
Although more radical approaches to cerebellopontine angle lesions, to tumors straddling the nasopharynx and the anterior and middle fossae, and to the skull base as a whole appear to have increased the prevalence of CSF leaks of all types, an accurate estimate of the incidence of incisional CSF leaks is difficult to provide. A recent study reports a 12% incidence (10 patients) in 85 posterior fossa procedures, but this figure may not pertain to other types of craniotomy.46
In large series CSF fistula occurs in 1.4% to 22% of operations for cerebellopontine angle tumor. The wide range may reflect the fact that it often proves necessary to report results encompassing many years and spanning important variations in technique in order to achieve significance.46–50 The incidence of leakage has been reduced by careful technique, including waxing and plugging mastoid air cells as they are opened and placing a graft of adipose tissue in the opened porus acusticus.51–54 The use of endoscopy to inspect the craniectomy site for unsealed air sinuses has also been advocated.55 Several recent series demonstrate that the incidence can be reduced below 11%, and that much lower rates are achievable, but that the incidence of leak seems relatively consistent irrespective of surgical approach (e.g., posterior fossa, transmastoid, or middle fossa).46,48–51,54 It is noteworthy that rhinorrhea, a classic false localizing sign in this setting, may be the presenting sign in up to 50% of leaks.46
In trans-sphenoidal approaches to the pituitary, leaks occur in 1.4% to 6.4%.56–58 In a small series reporting outcomes after the endoscopic transnasal trans-sphenoidal approach, the incidence was 14% (1/7).59
Endoscopic techniques were developed to improve visualization in the hope of reducing complications associated with blind instrumentation. It is not immediately obvious that the prevalence of CSF fistula has changed substantially, however. Estimates of incidence range from 0.002% to 2.9%.60,61 The latter figure was derived from a study explicitly designed to maximize the likelihood of diagnosing occult leaks using β-trace protein (prostaglandin D synthase) analysis and a 6-month follow-up.61
Pneumocephalus
Intracranial air, a pathognomonic sign of CSF fistula after trauma or spontaneous rhinorrhea (but not pathognomonic after surgery), is demonstrable in approximately 20% of patients with CSF leaks.62 Pneumocephalus is post-traumatic in 75% of these patients and is spontaneous, or otherwise unexplained, in 10%.63
Meningitis
Meningitis occurs in approximately 20% of acute post-traumatic leaks and in 57% of delayed leaks.35 The incidence of meningitis in nontraumatic CSF leaks has not been well documented. Anecdotally, copious, continuous leakage of the high-pressure type is less likely to be associated with meningitis than is intermittent leakage.31 The overall risk of meningitis associated with traumatic CSF leaks of all types is on the order of 25%.1,14,64–67 In neurosurgical postoperative leaks, the incidence of meningitis can be calculated to be on the order of 20%, but this figure is admittedly complicated by the problem of distinguishing aseptic from bacterial meningitis, and by the complexity introduced by factors such as steroid administration, chronic illness, and immunosuppression that might impair wound healing and predispose to both leaks and meningitis. The high incidence of delayed infection in military penetrating head injuries is demonstrably correlated to CSF fistulas, actively leaking or not.68,69
Defining and Localizing a Fistula
The management of CSF leaks involves three steps1: (1) confirming that the leaking fluid is really CSF,2 (2) delineating the site of the fistula, and (3) defining its mechanism.3
Evidence to Confirm the Presence of Cerebrospinal Fluid
Glucose
The presence of glucose in clear leaking fluid has been used historically to differentiate CSF from nasal secretions and other sources of serous or serosanguinous drainage. The concentration of glucose in CSF equals or exceeds 50% of the serum concentration except as follows1: during meningitis,2 after subarachnoid hemorrhage, or under other unusual circumstances.3 The glucose concentration in nasal secretions, in contrast, is 10 mg/dl or less.70
Quantitative measurements of glucose concentration are diagnostic. Qualitative spot tests, such as those provided by chemical testing strips (e.g., Clinistix, Dextrostix, Uristix, or Tes-Tape), are not definitive for two reasons.1 First, the glucose oxidase test on which they are based is too sensitive, turning positive at values less than 20 mg/100 ml of glucose.2 Second, normal nasopharyngeal secretions often elicit false-positive reactions even in the absence of glucose.71,72 Thus, although a negative glucose oxidase reaction effectively eliminates the possibility of CSF rhinorrhea, a positive result does not diagnose it unequivocally.
Reservoir Sign
It is widely held that true CSF leaks produce quantities of fluid sufficient for collection and quantitative analysis at some time in their course. The reservoir sign, the ability of a patient to produce CSF at will by positioning the head in a certain way, is generally taken to be quite specific for a fistula with pooling in the sphenoid sinus.30 Although Dandy12 believed that this sign would differentiate leakage through the frontal sinus from ethmoidal and sphenoidal leaks, it is not reliably localizing.
Other Confirmatory Evidence
The finding of unusually low opening pressure in the lumbar subarachnoid space is corroborative evidence for CSF leak. Unilateral or bilateral anosmia is associated with defects or leaks in the region of the cribriform plate and the fovea ethmoidalis. Olfaction may be preserved, however, in cases of spontaneous CSF rhinorrhea with congenital defects of the cribriform fossa.31,64 Optic nerve lesions point to the tuberculum sella, the sphenoid sinus, and the posterior ethmoids as the likely site of injury. Impaired vestibular function, facial nerve palsy, and cochlear damage accompany fractures in the temporal bone.
Imaging Techniques
Imaging techniques are used to detect intracranial air, fractures and defects in the skull base, mass lesions, and hydrocephalus, and to demonstrate flow through fistulas or skull defects.73 Plain films, multiplanar tomography, computed tomography (CT), and MRI have been used to delineate the anatomy and pathology of the skull base, sinuses, and calvaria. Radiographic data must be interpreted in the context of clinical findings. As always, positive data obtained from radiography are helpful, but negative data are often meaningless.
Plain Radiography and Computed Tomography
Plain films and CT are examined for evidence of fracture; air/fluid levels in the frontal, ethmoidal, and sphenoid sinuses; intracranial air; chronic increased intracranial pressure; erosion of bone by tumor or infection; congenital anomalies; and penetrating objects. Although multiplanar tomography provided exquisite detail of bony anatomy, it is of historical interest only, having been supplanted by high-resolution thin-slice CT with overlapping cuts.74 Contrast cisternography in conjunction with CT provides dynamic information about flow patterns of CSF.75 Similar information is also obtainable from MRI in addition to providing superb detail of soft tissue pathology at the skull base and in the nasopharynx.76
Tracers
The categorical proof of CSF fistula is the ability to retrieve extracranially a tracer substance injected into the CSF. The nonradioactive substances injected into the CSF historically have included methylene blue, phenolsulfonphthalein, indigo carmine, and fluorescein.77,78 Only indigo carmine and fluorescein remain in common use for intraoperative visualization: the others proved unacceptably toxic.5
In the presence of an active leak, cotton pledgets placed along the anterior roof of the nose, the posterior roof and the sphenoethmoid recess, and the middle meatus and below the posterior end of the inferior turbinate can be used to confirm that a leak exists. When differentially stained or contaminated by radioactive isotopes, they indicate the location of the leak.29 The following procedure has been recommended to localize a leak with fluorescein:
2. Ten milliliters of spinal fluid are withdrawn after measuring the opening pressure.
3. The fluid is mixed with 0.5 ml of 5% fluorescein.
4. The mixture is slowly reinjected intrathecally.
5. The patient assumes a recumbent position for about 30 minutes, the time depending on the size of the leak.
6. The pledgets are removed and examined under ultraviolet illumination or a direct inspection can be done with the use of the endoscope if the CSF leak is suspected in the anterior cranial fossa.
The findings are interpreted in Table 138-1. In addition, the middle ear is examined for evidence of staining, an indication of leakage.
| Location of Stain | Probable Site of Fistula |
|---|---|
| Anterior nasal | Cribriform plate or anterior ethmoidal roof |
| Posterior nasal or sphenoethmoidal | Posterior ethmoid or sphenoid sinus |
| Middle meatus | Frontal sinus |
| Below posterior end of inferior turbinate | Eustachian tube (middle fossa) |
| Behind tympanic membrane | Not accurately predicted |
Caveat: Fluorescein has been associated with severe adverse reactions in this application; see subsequent discussion.
The use of intrathecal fluorescein injection is quite controversial. Although otolaryngologists continue to recommend and utilize it routinely, neurosurgeons have become wary of its use because of reports of transverse myelitis and other serious adverse events such as seizures.79 Indigo carmine may be preferred by some neurosurgeons, not only because of its safety record but also because it is more visible than fluorescein to the unaided eye. This characteristic makes it useful to check for the presence of CSF fistulas intraoperatively, when ultraviolet illumination may not be readily available.30
Radioactive tracers such as 131I (RISA) were widely used for cisternography in the past but have most recently been replaced by 111In DTPA, an isobaric tracer that combines improved physical properties, fewer adverse reactions, better imaging quality, and shorter half-life (2.8 days). 169Yb DTPA and 99mTc albumin have also been approved for CSF imaging, but these tracers suffer from suboptimal imaging characteristics and half-lives (32 days and 6 hours).
Isotope cisternography is an effective method by which to demonstrate the existence of a CSF leak, but it becomes inaccurate for purposes of localization when “flooded” by a high-volume leak. The tracer saturates the pledgets and contaminates surrounding tissues with radioactivity so that differential localization becomes impossible.35,80,81
The importance of the timing of radioactive contamination is insufficiently appreciated. Active leaks can be documented by contamination of accurately placed pledgets within 0.5 to 2 hours. Slow or intermittent leaks can sometimes be detected by leaving the pledgets in longer or by replacing them over 6 to 48 hours. The danger lies in overinterpretation or misinterpretation of the data. There are two confounding mechanisms. First, the isotope can be absorbed into the bloodstream from the CSF and undergo secondary secretion into the nasopharynx.82 Second, there exists an alternate pathway for isotope secretion, first recognized in normal dogs and subsequently documented in normal human volunteers, involving active transport from the CSF and passage via the olfactory nerves, or passive lymphatic drainage leading to contamination of nasopharyngeal secretions.83
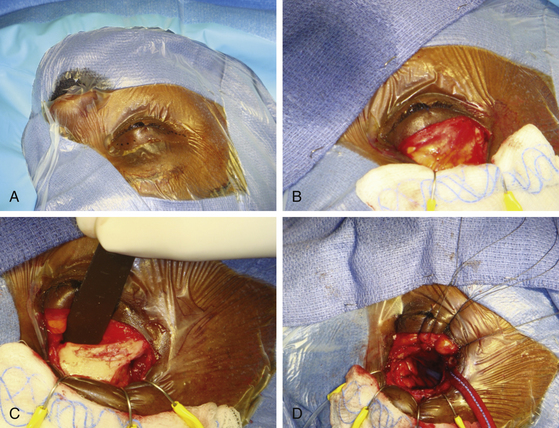
The accuracy of faintly positive tests can be improved slightly by calculating a radioactivity index (RI) ratio. This ratio compares the radioactivity in counts per minute of an exposed pledget with that of 1 ml of blood, as follows:
Two additional points deserve mention. First, it has been suggested that tracer injected into the cervical subarachnoid cistern can be forced through a slow, intermittent, or low-pressure leak by raising the CSF pressure with saline or artificial CSF delivered via a constant infusion pump.84 Second, scans carried out 24 and 48 hours after injection of isotopes can help define the mechanism of a leak by detecting defects in CSF absorption and circulation.
Cisternography
Early attempts at demonstrating CSF leaks by injecting air or iophendylate (Pantopaque) into the subarachnoid space failed to produce consistently satisfactory images. The combination of high-resolution CT and metrizamide, or of other form of cisternography with water-soluble contrast agents, yields excellent visualization of active leaks. Smaller fistulas have been demonstrated through stressing the barriers to CSF flow by having the patient cough or carry out a Valsalva maneuver.35 For maximal contrast enhancement, cisternal injections can be performed via C1–C2 punctures. Overlapping views in both the coronal and the axial planes are required.74 Direct coronal studies are preferable to reconstructed images. The risk of provoking seizures and aseptic or chemical meningitis following cisternography should be discussed with the patient.
A newer and safer technique utilizes MRI technologies to visualize CSF flow.76,85–88 A full discussion of the technique is outside the scope of this chapter, but heavily T2-weighted fast spin–echo studies with fat suppression and video reversal of the images are reported to yield sensitivity, specificity, and accuracy of 0.87, 0.57, and 0.78, respectively, in a study in which 65% of patients eventually underwent surgical exploration.86
MR remains less effective than CT in demonstrating bony anatomic detail. It is absolutely reasonable to require both for diagnostic purposes and in conjunction with the preoperative planning.89–94
Immunologic Methods
Immunologic methods differentiate between proteins in CSF and those in nasopharyngeal secretions.95,96 Irjala and colleagues97 have described the use of an immunofixation technique for the identification of microaliquots (100 μl) of CSF by demonstrating two electrophoretically characteristic bands of transferrin. The B1 fraction consists of normal transferrin and sometimes two variant fractions. The B2 fraction characteristic of CSF contains smaller amounts of neuraminic acid. This method is not subject to contamination from other body fluids (e.g., tears or nasal secretions). The immunofixation method could theoretically be used for localization of the leak by differential suction techniques in the nasopharynx, but large-scale clinical trials of this method have yet to be reported. Another surrogate marker for CSF is β-trace protein (prostaglandin D synthase), as reported by Arrer and colleagues.98 The β-trace protein test is reported to offer an overall accuracy of 95.7%, a specificity of near 100%, and a sensitivity of 91.2%.99
Anatomic Considerations: Sites of Leakage
Spontaneous
Nontraumatic leaks are usually confined to a single region where an anatomic defect is demonstrable, but exceptions have been noted.100 It is usually easier to demonstrate the defect than the leak. High-pressure leaks that act as safety valves for hydrocephalus occur where the skull is thinnest, usually the cribriform fossa and the sellar region. This is the case, for example, in Crouzon’s disease and osteopetrosis (Albers-Schönberg disease).
The middle fossa can be the site of CSF leaks that are direct in that they do not cross the inner ear. Such fistulas have been described mainly in conjunction with a pneumatized temporal fossa. Pulsatile CSF forces induce additional thinning of the bone and enlargement of the pits and small bony defects that are normally present. The leptomeninges and brain herniate, thinning the dura and leading to rupture of the arachnoid. The leak may be constant or intermittent depending on several factors, such as the underlying intracranial pressure, whether an arachnoid diverticulum is created, and whether brain tissue temporarily obliterates the leak. A similar sequence of events has been postulated to explain CSF leaks in the empty sella syndrome30 and in focal atrophy.31
Indirect fistulas through the temporal bone are the most elusive. In extralabyrinthine fistulas the defect is in the middle fossa in the region of the tegmen tympani. In intralabyrinthine fistulas, CSF escapes into the labyrinth through the subarachnoid space of the posterior fossa. In either case, the leak can present as otorrhea or, when the tympanic membrane is intact, as rhinorrhea.62 The possibility of temporal bone dysplasia should be investigated whenever a patient with severe hearing loss develops unexplained or recurrent meningitis.30,35,101 For example, in the Mondini malformation (i.e., unreactive ear with a shortened cochlear coil, dilated semicircular canal system, and widened inner ear vestibule), it is hypothesized that a widened, patent cochlear aqueduct allows CSF to pass from the subarachnoid space to the inner ear via a leak in the oval window. Other proposed routes include defects of the scala tympani; of the footplate of the stapes; or of the thin, bony plate separating the internal auditory canal and the inner ear vestibule and perforated by nerve fibers innervating the utricular and saccular maculas.102–104
Initial Management
In traumatic leaks, antibiotics have not been proven effective in changing the incidence of meningitis and are no longer recommended routinely.15–17,21,36,105–109 For postoperative leaks, however, prophylactic antibiotics are commonly, if not universally, employed. There is some theoretical justification for distinguishing between the two situations. Several principles are useful to keep in mind when prescribing prophylactic antibiotics108,110:
1. Patients should not be kept on antibiotics indefinitely in the hope that a leak will seal; a trial of conservative therapy is reasonable, but the end point should be decided a priori.
2. Wide-spectrum antibiotics are not desirable for prophylaxis; the most specific antibiotic capable of eliminating the potential pathogens should be used.
3. Patients of different ages and in different locations harbor different vulnerabilities because of changes in nasopharyngeal and environmental flora; thus, Haemophilus influenzae is a common cause of meningitis in children and in the elderly, whereas diplococcus is more common in healthy adults.
4. Patients can develop meningitis even while on prophylactic antibiotics; after the usual investigations are carried out, the antibiotics are changed to cover the appropriate organisms and sensitivities.
External Drainage of Cerebrospinal Fluid
External drainage of CSF has been used in various forms for many years. External ventricular drainage111,112 has been replaced in most centers by continuous lumbar drainage, first described in 1963.113 Since then, lumbar drainage has been found useful in controlling and sometimes curing CSF leak of every cause.23–25 McCoy114 provided theoretical justification for the initial management of CSF fistulas with CSF diversion by demonstrating that granulation can seal the fistulas, provided that the leakage has stopped. Lumbar drainage should be considered, therefore, whenever positioning alone does not eliminate, or at least significantly diminish, a leak within 24 hours.
Prevention of Infection with Indwelling Subarachnoid Catheters
The infection rate with indwelling catheters ranges in some series as high as 10% or more.115 The risk is lower with lumbar catheters.26 Infection can be reduced by prophylactic antibiotics (potentially, by two thirds115) and by externalization of the catheter through an extended subcutaneous tunnel.116
Prophylaxis with antibiotics is continued for 8 to 24 hours after the catheter is withdrawn. Daily samples of CSF are obtained; cultured; examined by Gram stain; and analyzed for cell count and differential, glucose, and protein. The presence of a catheter does not, of its own accord, lower the CSF glucose or evoke a major leukocytotic reaction; the cell count and glucose concentration remain quite stable in uninfected CSF over 4 to 9 days. Any persisting variation of 2 standard deviations or more from the cumulative average cell count and glucose concentration over several days is cause for concern and careful reexamination of the CSF for signs of opportunistic infection.117 So too would be the emergence of any clinical signs or symptoms of meningeal irritation.
External drainage has been maintained in large series for 10 days without infection. Longer durations have been reported in exceptional cases.25 By analogy with central venous access lines, it may be wise to change catheters if drainage is continued beyond 7 days.
How Long to Drain
The data on which these recommendations are made are empirically derived. Drainage should be continued for 3 to 5 days after stoppage of the leak to allow healing. If leakage recurs, operative repair is indicated. If the underlying problem is increased intracranial pressure or hydrocephalus, implantation of a drain acts purely as a temporizing maneuver: no “cure” is effected. Similarly, the patient whose leak is not controlled by external drainage should be considered for early operation. In Findler’s series of 50 patients,29 drainage of 350 to 420 ml daily was continued for an average of 10 days, with a leak recurrence rate of 14%. There was an additional 8% incidence of delayed leak at the site of lumbar puncture.
Pharmacologic Adjuvants to Drainage
Pharmacologic agents such as acetazolamide (Diamox) that retard the production of CSF may be helpful in reducing CSF pressure after the drain has been removed.118 They are temporizing agents only.
Complications
Calcaterra18 records one case of fatal postoperative suboccipital hemorrhage attributed to overdrainage of CSF in an elderly patient. Similar complications have followed spinal anesthesia.119–121 Overdrainage of CSF can also cause life-threatening pneumocephalus.64,122–124 The CSF pressure should be lowered, and may even be lowered substantially, but should not be reduced to less than 0 through negative pressure.
The acute reduction of CSF pressure can also precipitate headache, nausea, and vomiting. This reaction can be prevented, according to Findler and colleagues,29 by gradually lowering the pressure and increasing the drainage over several days. An accidental siphon effect can be avoided by relating the height of the drainage valve or the drainage bag to the level of the ventricular system rather than the bed. In this way, the pressure column remains constant as the bed is raised and lowered or as the patient is moved. Most commercial systems incorporate a micropore-filtered air port to prevent siphonage. Improvised systems are generally unable to include such a port, and positioning becomes critical.
There has been long-standing concern regarding the possibility of inducing meningitis by retrograde migration of bacteria into an open fistula under the influence of negative CSF pressure induced by an external drain.5 This seems to be an extremely rare complication, avoidable by maintaining a low but steady positive pressure in the CSF.
Dural-cutaneous fistulas can occur at the site of catheter insertion, particularly in the setting of a high-pressure leak. Most such fistulas stop spontaneously or seal with a single stitch. Low-pressure or normal-pressure leaks can also be sealed by an injection of 10 to 20 ml of autologous blood as an epidural blood patch. The technique has been shown by a number of studies to improve over natural history125–129 with a success rate of 93%.130,131
Epidural blood patching has resulted in symptomatic mass effect, hemorrhagic complications,132 and infection. Surgical repair of the dura may still be needed. External lumbar drainage is contraindicated in the context of increased intracranial pressure from a mass in the posterior fossa because of the danger of precipitating herniation through the foramen magnum.
Operative Management
Acute PostTraumatic Leaks
Some types of leak have a relatively high probability of sealing spontaneously. Most acute posttraumatic leaks stop within 10 days of injury: in Mincy’s classic series3 of 54 cases of rhinorrhea in frontal fossa injury, 35% had stopped within 24 hours, 68% within 48 hours, and 85% within 1 week. Similar numbers emerge even from series of complex craniofacial injuries.39 The use of lumbar drainage may further increase the rate of sealing.
There are three classic indications for surgical intervention: (1) a bout of meningitis, (2) pneumocephalus, or (3) an active leak (persistent or recurring). Lewin’s insistence on surgery for virtually all leaks was based on the threat of meningitis. There is no evidence that early surgical repair offers any significant improvement over natural history in patients with acute posttraumatic leaks that cease spontaneously within the first week after injury.
Most leaks or dural tears associated with midface fractures stop permanently when the facial fractures are reduced.39,133 Meningitis is relatively uncommon in dural tears associated with facial fractures despite the fact that the incidence of dural laceration in facial fractures (43%) is higher than in closed head injuries (7%) and that CSF leak occurs far more commonly (36%).133
Postoperative Leaks
In the series by Spaziante and colleagues57 of 140 trans-sphenoidal operations, four of six leaks stopped with lumbar drainage alone. Ciric58 estimates that 2% of transphenoidal cases require reoperation for CSF leak. The statistics in series of posterior fossa lesions is more variable,46,48–50 with reoperation required in 75% of one well-documented series.49
Indications for Surgical Intervention
Prompt surgical intervention may be indicated under the following circumstances:
1. Acute traumatic or postoperative leaks that recur or persist after 10 to 13 days of conservative management, including external drainage
2. Proven intermittent or delayed leaks
3. High-pressure leaks acting as a “safety valve” for hydrocephalus
4. Leaks associated with erosion, destruction, disruption, or severe comminution of the skull base or of the paranasal sinuses
5. Leaks associated with congenital dysplasias of the brain, skull base, orbit, or ear, particularly after a bout of meningitis
6. Leaks caused by high-energy missile wounds
7. Postoperative rhinorrhea and otorrhea that cannot be controlled by position and drainage, especially when the air sinuses have been violated as part of the operative route
8. High-volume leaks through the petrous bone and the sella are particularly recalcitrant to conservative management
Open or endoscopicWith tissue transfer, placement of fat plug or muscle pledget, injection of fibrin or other glues, applications of engineered tissue or tissue substitutes
Sinus repair and ablation as necessary
Repair of posterior sinus wall or cranialization of sinus and packing
Craniotomy
Anterior Fossa
1. Dural tears are virtually inevitable in the course of dissection.
2. Areas of cerebral tissue herniation into bony defects cannot be easily visualized.
The fistula is often betrayed by a palpable or visible dural defect or by a contusion, adhesion, or herniation of cerebral tissue. An obvious fistula is sealed by inserting a plug of abdominal fat and then covering the defect with a free or reflected flap of dura. The dura can be obtained from the adjacent bone or from the falx cerebri, depending on the location of the fistula. Alternatively, we prefer a free patch of pericranium, temporalis fascia, fascia lata, or lyophilized dura that can be sutured to the surrounding dura and, if needed, used to reinforce a plug of fat. Fat forms a more durable plug than does muscle: muscle fibroses and shrinks, whereas fat remains viable by recruiting a blood supply from adjacent tissues. Simple dural laceration can often be sutured primarily. A dural patch graft may be inserted when necessary. Dural grafts should be harvested from autologous tissue (e.g., temporalis fascia, pericranium, fascia lata, or transversalis fascia) or from commercially prepared cadaver tissue (e.g., dura, pericardium, or amniotic sac).
Middle Fossa
Air can be insufflated through specially designed tubes that seal the nares and occlude the posterior pharynx during surgery. By flooding the field with saline, it is sometimes possible to identify, by the bubbles, a fistula that would not otherwise be evident.66 As a rule, it is simpler and more prudent to cover the entire anterior or middle fossa with a graft than to count on this technique.
Posterior Fossa
Postoperative CSF leaks can present as rhinorrhea or otorrhea, or as trickles through the suture line, with or without an incisional collection. Leakage through the suture line may be self-limiting and may not lead to other serious complications, but it should not be regarded as normal.
Postoperative leaks through the mastoid or through the temporal bone can be quite challenging. It is standard technique to wax or to otherwise seal any opened mastoid air cells intraoperatively. This task can be facilitated endoscopically.48–5066 Some surgeons also recommend plugging the porus acusticus with fat when it has been enlarged for tumor removal. Watertight closure of the dura is achieved using a dural graft, and other precautions are routinely deployed as well.47–53
In the event of a persistent leak, reexploration of the incision is indicated.47–53 Some surgeons prefer an extradural approach via mastoidectomy, particularly for recalcitrant cases. When the ear is nonfunctional, this approach, combined with an obliteration of the inner and middle ear, ensures the maximal likelihood of detection and obliteration of the site of leakage. The approach does not address the problem of altered CSF dynamics, however.
For the so-called “spontaneous” leaks, such as those associated with the Mondini malformation, the extradural approach is generally adopted. These are complex cases, and multiple layer closures may be required.134
Combined Craniotomy and Reduction of Facial Fractures
Most CSF leaks associated with fractures of the midface can be managed by reducing the facial fracture via a craniotomy. No definitive rules can be given for these injuries; treatment must be carefully individualized and often requires a team approach using professionals skilled in ear, nose, and throat surgery; dental surgery; ophthalmology; plastic surgery; and neurosurgery.39
Extracranial and Endoscopic Approaches
Indications
There are four situations for which the extracranial approach is particularly well adapted:
1. Discrete and definable normal pressure leaks through the cribriform plate or adjacent ethmoid labyrinth
2. Fractures that abut on an air sinus, particularly when the bony defect is limited to the cranial wall of that sinus
3. Postoperative leaks after trans-sphenoidal surgery
4. Leaks through the oval window, petrous bone, or other parts of the ear
Special Techniques
The key to the extradural approach is to identify and seal the leak or leaks with some combination of dural graft or substitute, bone or bone substitute, and packing. Although the insertion of bone or bone substitute to reconstitute the skull base and hold the dural graft in place is not universally practiced, it is strongly advocated by some.136 There is a view that reinforcement with bone is unnecessary because the air sinus below the leak should in any event be packed, a maneuver that serves both to reinforce the dural repair and to maintain the graft in position. The packing acts as a seal in its own right and serves to hold mucoperiosteal, periosteal, free fascia lata, or other grafts against the dura. A bone graft serves the same purpose, in theory, but it can be difficult to place and does not in and of itself act as a substantial barrier to CSF flow, unlike the ablated sinus packed with fat or muscle. Adipose tissue is generally preferred over muscle; anecdotally, it seems less likely to fibrose and is easier to harvest. Some centers have substituted cancellous iliac crest for fat. Others advocate attaching a graft with fibrin glue, packing the sinuses temporarily, and forgoing sinus ablation.136,137 Irrespective of the choice of packing material, the mucosa of the sinus must be stripped to avoid mucocele formation if it is to be ablated.
Transfrontal extradural procedures can be carried out either through a forehead incision or via a bicoronal incision. There is one important advantage to the bicoronal incision: should it be necessary to obtain a more generous view of the frontal fossa, a craniotomy can be carried out without difficulty. The anterior wall of the frontal sinus is removed with a Stryker saw or the Midas Rex with the C1 attachment, following a template obtained from a 72-inch sinus film. The posterior wall is fully exposed: the mucosa is resected, and enough bone is removed to display the dural defect. The dura can be patched or sutured primarily. Depending on the extent of damage, the fragments of the posterior wall can be replaced or totally removed, thereby cranializing the frontal sinus. In either case, the sinus is ablated with fat or bone, and the frontal wall is restored. A modification to the transfrontal approach is the subfrontal approach, which preserves the anterior wall of the frontal sinus. The subfrontal approach is performed via a bicoronal incision. A naso-orbitofrontal craniotomy is performed en bloc providing wide exposure of the entire posterior wall of the frontal sinus as well as access to the cribriform plate and planum spenoidale from below138 (Figures 138-1 and 138-2).
Endoscopic techniques have been adapted to each of these approaches. In the event that a leak can be pinpointed, and the necessary tissue manipulation achieved, endoscopic procedures have the advantage of reduced hospitalization and, quite often, better visualization. In most cases three-dimensional visualization is lost and the field of view is limited. Still, once the technique has been mastered, endoscopy offers remarkable versatility.140–142
Depending on the angle required, the width of the desired window, the site of the leak, and the surgeon’s preference, the sphenoid sinus and the sella can be approached trans-septally via a sublabial or a transnasal route, or transethmoidally via an external rhinotomy incision. The first approach is more familiar to neurosurgeons; but the transethmoidal approach is shorter, gives a wider exposure, permits more complete resection of the sphenoid septae, and overcomes some of the difficulties of endoscopic visualization of the lateral extensions of the sphenoid sinus, where spontaneous leaks are known to arise.137 This is an important consideration in reoperation for CSF leak after trans-sphenoidal surgery, for example. The leak can often be sealed by reconstructing the sellar floor and packing the ethmoid or sphenoid sinus. A flap of mucoperiosteum can be elevated with or without the underlying cartilage and folded over the dural defect, sometimes after the interposition of free fascia lata graft. The wider the view, the easier the procedure.139–144
The endoscopic endonasal approach is useful to seal CSF leaks in the parasellar area,139–142 mucoperiosteum from the inferior turbinate serves as a convenient source of tissue.139 Dural defects up to 10 mm × 10 mm have been successfully repaired, including complex congenital malformations of the skull base.144
If an open extracranial approach to the cribriform fossa and fovea ethmoidalis is preferred, these structures are best approached through a curved naso-orbital incision and a complete ethmoidectomy. A flap rotated from the middle turbinate or the septum is used to cover the cribriform plate and the ethmoid roof from below. The posterior ethmoidal artery is a landmark situated directly anterior to the optic nerve.
Evolving Techniques
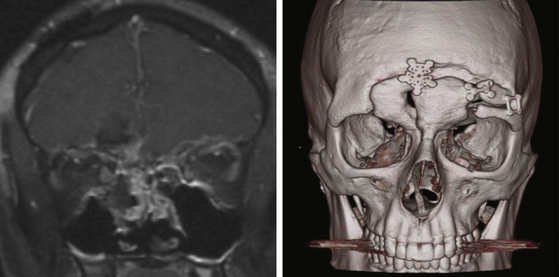
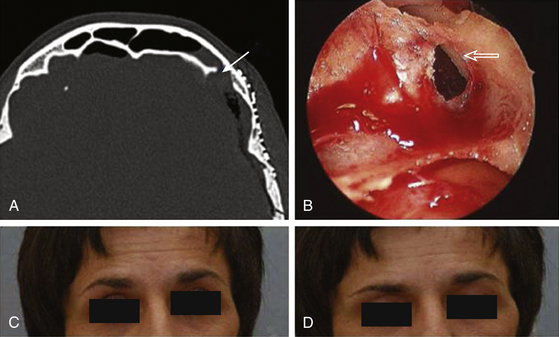
With the rise of endoscopic endonasal techniques the majority of cases of cerebrospinal fluid (CSF) leaks in the anterior and middle fossa can be treated through a transnasal endoscopic approach. Nevertheless, frontal sinus CSF leaks, especially far lateral, posterior and superiorly based leaks, remain difficult to access with transnasal endoscopic techniques. While CSF leaks in these hard to reach sites may be repaired with the more traditional bicoronal bifrontal craniotomy approach, the less invasive approach will be desirable. Novel techniques using transpalpebral approaches with minicraniotomy and endoscopic illumination have recently been described.145,146 The transpalpebral approach uses a limited eyelid incision, involves a minicraniotomy, preserves the frontal sinus function, and is associated with a rapid recovery. All patients selected for this approach undergo computed tomographic imaging studies with intraoperative image guidance protocol. The transpalpebral approach to the anterior cranial base starts with an upper eyelid crease incision through which the superior orbital rim is exposed (Figure 138-3). The arcus maginalis is sharply incised, and the supraorbital neurovascular bundle is released from its notch or foramen and protected. Subperiosteal elevation of the frontalis muscle and forehead skin exposes the frontal bone and supraorbital bar. A minifrontoorbital craniotomy is designed based on the location of the defect to provide the most direct approach for instrumentation. A 2 × 2 cm craniotomy limited to the anterior wall of the frontal allows introduction of an endoscope and multiple instruments. Through this access, defects along the posterior wall of the frontal sinus are addressed and the CSF leak site repaired in onlay and underlay grafts.
Transpalpebral Approach with Miniorbitofrontal Craniotomy for Repair of Anterior Cranial Base CSF Leaks
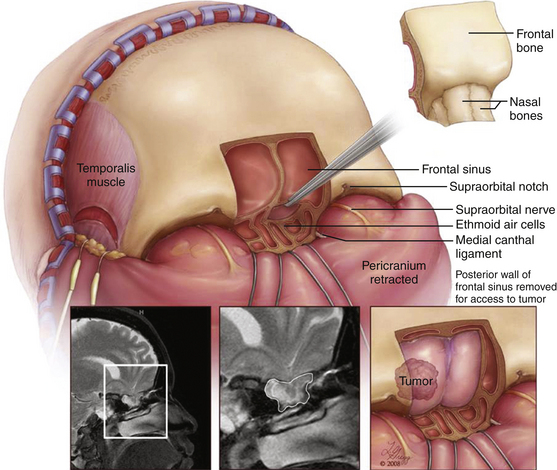
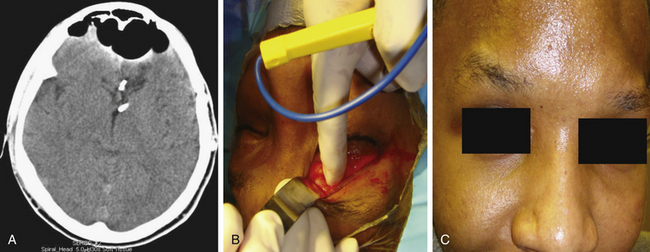
Case 1. A 40-year-old female underwent orbitotemporal craniotomy for clipping of an aneurysm. She developed postoperative CSF rhinorrhea and significant forehead swelling. On CT scan, the frontal sinus was opacified and a defect along the anterior cranial vault was identified as the source of leak (Figure 138-4). The location of the defect, involving the posterior superior-lateral portion of the frontal sinus, was determined to be beyond easy access with a transnasal endoscopic approach. To avoid reopening the orbitotemporal craniotomy and risk further disruption of the dural repair, a transblepharoplasty minicraniotomy approach was used to expose the cranial and dural defect (Figure 138-4). Excellent endoscopic visualization of the dural defect was achieved through a 15-mm × 15-mm craniotomy window. Direct access allowed bimanual instrumentation and repair of the defect using DuraGen (Integra Life Sciences, Plainsborough, NJ), fascia lata free graft, and fibrin glue. Operative time was approximately 1 hour. At the 1-year follow-up. there was no evidence of CSF leak and the eyelid had healed with excellent cosmetic results.
Case 2. A 20-year-old male sustain severe skull-base injuries from a gunshot wound and underwent a traditional open approach at another hospital to manage his acute injuries, including the use of pericranial flaps to repair a CSF leak. The patient, however, developed chronic CSF leak and marked pneumochephalus that persisted in spite of repeated open craniotomies and attempted endoscopic repair of the CSF leak and was transferred to our service for definitive procedure and CSF leak repair. The patient was clinically deteriorating from his pneumocephalus. We performed a transpalpebral approach for CSF leak repair. The site of the leak was localized to the lateral and posterior was of the frontal sinus (Figure 138-5 and Video). The CSF leak and pneumocephalus was successfully repaired after the first surgery through the transpalpebral approach.
The Transconjunctival Transorbital Approach for Anterior Cranial Base CSF Leaks
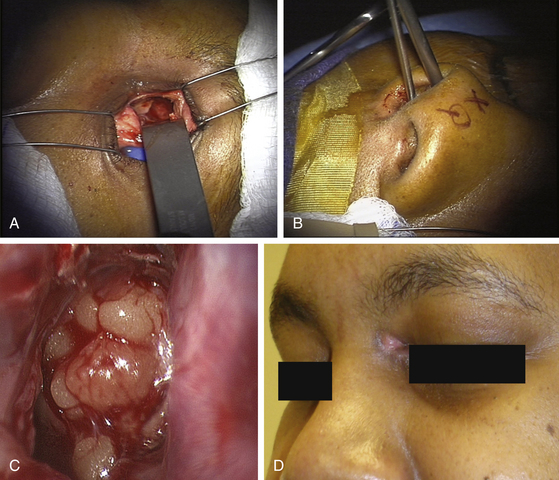
Case 3. A 20-year-old male presented with a chronic CSF leak that had failed several attempts at repair via the transnasal endoscopic approach at another institution. The defect was noted to be in the area of the planum sphenoidale based on a high-resolution CT scan with thin cuts. A precaroncular transconjunctival incision was used to expose the superior medial orbital wall. The anterior and posterior ethmoid arteries were identified and ligated. A 2-cm × 1.5-cm superior medial orbitotomy was performed under stereotactic image guidance. Under microscopic magnification, the site of CSF leak was localized and repaired with a fat-fascia graft placed via the orbital widow. This was subsequently reinforced via the endonasal endoscopic approach with a fat graft and fibrin glue. The CSF leak revolved after this procedure (Figure 138-6).
Glues, Tissue Substitutes, Engineered Biomaterials, and Other Technical Considerations
Parasellar leaks have been treated by injecting fibrin glue transmucosally under CT guidance.143 The use of tissue glue to reduce the need for sinus ablation has been mentioned. From a historical perspective, the management of CSF leaks has drawn heavily on the innovative use of glues, tissue substitutes, polymers, and engineered biomaterials to overcome the challenges of obtaining a permanent seal with reduced morbidity in relatively inaccessible areas.
There was a round of enthusiasm for methyl methacrylate; for a time, it was hailed as the panacea for CSF leaks.62,147 Long-term outcomes, however, were disappointing. Methacrylate was found to shrink; the leaks recurred. If infection set in, the plug became a septic nidus. The so-called “super-glues,” or cyanoacrylates, which are chemically related to methacrylate, also proved problematic, although they were more successful when used to adhere packing or supporting tissue in place rather than to create an adhesive layer between dura and patch.148–152 More importantly, it has become evident that the concept behind the use of methacrylate was faulty: it is the dura, not the bone, that requires repair. Should the underlying problem be hydrocephalus or increased intracranial pressure, the leak will not permanently stop until the intracranial pressures are adequately controlled.
Tissue Adhesives and Sealants
It was hoped that tissue adhesives might overcome some of the problems associated with obtaining a durable seal, especially in relatively inaccessible areas, and act as tissue sealants: they did not. The first generation of acrylic-based compounds proved particularly disappointing. In addition to carrying a risk of meningitis and of neural toxicity, particularly to the optic apparatus, they formed a barrier between layers of tissue, inhibiting granulation and preventing fibroblastic proliferation from fusing one layer to the next. With time, tissue adhesives became porous and resulted in recurring leaks.149–152 More recently, the use of autogenous or prepackaged fibrin clot adhesives has prompted a reconsideration of the role of tissue adhesives. Initial tests of Bioglue have been promising.153 Fibrin-based adhesives may be worth investigating in conjunction with relatively porous engineered tissue substitutes such as acellular cadaveric dermal matrix (ACDM), which is processed from human cadaver skin (AlloDerm, Life Cell Corp., Branchburg, NJ).154 An excellent review of tissue adhesives is offered by Preul and colleagues.155
Use of Cerebrospinal Fluid Shunts
High-pressure leaks cannot be sealed without reducing the intracranial pressure. The primary pathology must be treated first, either by resection of the space-occupying lesion or by reduction of CSF volume and flow in hydrocephalus. Nonetheless, several types of recalcitrant fistulas have been successfully treated with lumboperitoneal shunts.156,157
CSF shunting can be attempted in normal-pressure leaks when other means of repair have failed, or when, after exhaustive investigation, the site of the leak cannot be delineated. Lumboperitoneal shunting has been advocated as the only treatment needed for small leaks that cannot be visualized.84,158 This empirical approach assumes that the resistance to flow through the shunt will be less than the resistance to passage through the fistula. With shunt malfunction, the leak may recur. Moreover, tension pneumocephalus can occur when air is aspirated intracranially through an open fistula under negative pressure.62,122,123 The treatment for this complication is ligation of the shunt, initially, and replacement of the valve with a higher pressure unit once the mass effect has been treated and the air resorbed.
Treatment of Spinal Cerebrospinal Fluid Leaks
Spontaneous spinal CSF leaks rarely occur outside the setting of spinal dysraphism or unusual spinal anomalies. Some rare leaks have been attributed to bone spurs at the skull base or cervical spine.159 There is a rare and relatively young population subject to recurrent leaks at multiple sites without explanation,160 or with unusual abnormalities such as “nude nerve roots” in which the nerve root sleeve is absent at multiple levels.161 Another category, documented in three patients, is characterized by new onset of daily headaches and C1–C2 retrospinal fluid collections. The collections did not correlate with the sites of leakage in the lower cervical spine.162 Spontaneous leaks of this nature have responded to the percutaneous injection of fibrin sealants after epidural blood patches failed.163 Leaking meningeal diverticula and ill-defined connective tissue syndromes have also been implicated in spontaneous leaks.32,34,164,165 These leaks tend to occur at the cervicothoracic junction or the thoracic spine. CT myelography has been reported as the study of choice.32
Intraoperative observations suggest that the dura may both thicken and thin after multiple steroid injections. The dura is sometimes gossamer-thin and almost porous. The CSF that leaks from this location more accurately seeps than leaks. Multiple sites of leakage and recurrences are recognized when the dura is thin, even without a history of epidural steroid injections.160 Epidural fat grafts seem to contain this seepage in many, but not all, cases.
The prevalence of postoperative leaks is becoming well documented.166,167 Age, complexity of surgery, and especially reoperation are risk factors. In reoperation for herniated thoracic discs, for example, the incidence is approximately 7% (1/15).167
In meningomyeloceles and other dysraphic states, the repair of the leak becomes part of the repair of the anomaly. Increased intracranial pressure must be controlled before the leak stops. Except in open injuries, transcutaneous leaks, and obvious anomalies, the site of the leak may be quite difficult to determine.130 Isotope cisternography and CT myelography are usually accurate in active leaks.168 Most uncomplicated traumatic leaks seal within several days, so long as there is no ball-valve dural defect to resist healing. Certain maneuvers may be helpful: incisional leaks should be initially repaired by resuturing the wound and applying an abdominal binder over a pressure pad to increase resistance to CSF flow. External CSF drainage from the cervical subarachnoid space via C1–C2 is another alternative. The Touhy needle and drainage catheter must be inserted under fluoroscopy. Although the cervical subarachnoid catheters are more likely to kink, no other major difficulties have been encountered. If the leak persists over 10 days and if the intracranial pressure is normal, reexploration of the wound should be weighed.
A number of other techniques have been reported in the management of spinal leaks. Three are mentioned as additions to the surgeon’s armamentarium, although they cannot be recommended as a routine: (1) an infusion of 100 ml of 20% mannitol every 4 hours for 7 days and positioning in a head-down attitude for 1 week,169 (2) insertion of a fat plug through a limited midline durotomy for small rents of the anterior and lateral thecal walls,170 and (3) the use of tissue adhesives to seal the dura.171 It is particularly important not to confuse an infected serous exudate with a delayed spinal CSF leak. It is necessary to re-explore a recalcitrant postoperative leak to determine which tissue layers need to be repaired for the leak to be contained.
Appelbaum E. Meningitis following trauma to the head and face. JAMA. 1968;173:116-120.
Bachmann G., Nekic M., Michel O. Clinical experience with beta-trace protein as a marker for cerebrospinal fluid. Ann Otol Rhinol Laryngol. 2000;109(12):1099-1102.
Bell R.B., Dierks E.J., Homer L., Potter B.E. Management of cerebrospinal fluid leak associated with craniomaxillofacial trauma. J Oral Maxillofacial Surg. 2004;62(6):676-684.
Carrion E., Hertzog J.H., Medlock M.D., et al. Use of acetazolamide to decrease cerebrospinal fluid production in chronically ventilated patients with ventriculopleural shunts. Arch Dis Child. 2001;84:68-71.
Choi D., Spann B. Traumatic cerebrospinal fluid leakage: risk factors and the use of prophylactic antibiotics. Br J Neurosurg. 1996;10:571-575.
Chu E.A., Quiñones-Hinojosa, Boahene K.D.O. Transblepharoplasty orbitofrontal craniotomy for repair of lateral and posterior frontal sinus cerebrospinal fluid leak. Otol. Head and Neck Surgery. 2010.
Crawford J.S. Experiences with epidural blood patch. Anaesthesia. 1980;35:513-515.
Dandy W.E. Pneumocephalus (intracranial pneumatocele or aerocele). Arch Surg. 1926;12:949-982.
Dandy W.E. Treatment of rhinorrhea and otorrhea. Arch Surg. 1944;49:75-85.
Hilary A.B. Prophylactic antibiotics for post-traumatic cerebrospinal fluid fistula: a meta-analysis. Arch Otolaryngol Head Neck Surg. 1997;123:749-752.
Jho H.D., Carrau R.L., Ko Y., Daly M.A. Endoscopic pituitary surgery: an early experience. Surg Neurol. 1997;47:213-223.
Khrais T.H., Falcioni M., Taibah A., et al. Cerebrospinal fluid leak prevention after translabyrinthine removal of vestibular schwannoma. Laryngoscope. 2004;114(6):1015-1020.
Magliulo G., Sepe C., Varacalli S., Fusconi M. Cerebrospinal fluid leak management following cerebellopontine angle surgery. J Otolaryngol. 1998;27(5):258-262.
Mahaley M.S., Odom G.L. Complications following intrathecal injections of fluorescein. J Neurosurg. 1966;25:298.
Preul M.C., Bichard W.D., Spetzler R.F. Toward optimal tissue sealants for neurosurgery: use of a novel hydrogel sealant in a canine durotomy repair model. Neurosurgery. 2003;53(5):1189-1198. discussion 1198:1199
Raza S.M., Conway J.E., Li K.W., et al. A modified frontal-nasal-orbital approach to midline lesions of the anterior cranial fossa and skull base: technical note with case illustrations. Neurosurg Rev. 2010;33:63-70.
Schievink W.I., Morreale V.M., Atkinson J.L., et al. Surgical treatment of spontaneous spinal cerebrospinal fluid leaks. J Neurosurg. 1998;88:2430-2436.
Schlosser R.J., Bolger W.E. Nasal cerebrospinal fluid leaks: critical review and surgical considerations. Laryngoscope. 2004;113(2):255-265.
Scievink W.I., Meyer F.B., Atkinson J.J., Mokri B. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. J Neurosurg. 1996;84:598-605.
Selesnick S.H., Liu J.C., Jen A., Newman J. The incidence of cerebrospinal fluid leak after vestibular schwannoma surgery. Otol Neurotol. 2004;25(3):387-393.
Sencakova D., Mokri B., McClelland R.L. The efficacy of epidural blood patch in spontaneous CSF leaks. Neurology. 2001;57(10):1921-1923.
Spaziante R., de Divitiis E., Cappabianca P. Reconstruction of the pituitary fossa in transsphenoidal surgery: an experience of 140 cases. Neurosurgery. 1985;17:453-458.
Spetzler R.F., Wilson C.B. Management of recurrent CSF rhinorrhea of the middle and posterior fossa. J Neurosurg. 1978;49:393-397.
Yessenow R.S., McCabe B.F. The osteo-mucoperiosteal flap in repair of cerebrospinal fluid rhinorrhea: 20-year experience. Otolaryngol Head Neck Surg. 1989;101:555-558.
Zapalac J.S., Marple B.F., Schwade N.D. Skull base cerebrospinal fluid fistulas: a comprehensive diagnostic algorithm. Otolaryngol Head Neck Surg. 2002;126:669-676.
1. Schlosser R.J., Bolger W.E. Nasal cerebrospinal fluid leaks: critical review and surgical considerations. Laryngoscope. 2004;113(2):255-265.
2. Lewin W. Cerebrospinal fluid rhinorrhea in nonmissile head injuries. Clin Neurosurg. 1966;12:237-252.
3. Mincy J.E. Post-traumatic cerebrospinal fluid fistula of the frontal fossa. J Trauma. 1966;6:618-622.
4. Miller C. Case of hydrocephalus chronicus with some unusual symptoms and appearances on dissection. Trans Med Chir Soc Edinb. 1826;2:243-248.
5. Ommaya A.K. Spinal fluid fistulae. Clin Neurosurg. 1975;23:363-392.
6. Chiari H. Ueber einem Fall von Luftansammlung in den Ventrikeln des menchichen Gehirns. Z Heilkd. 1884;5:383-390.
7. Luckett W.H. Air in the ventricles of the brain, following a fracture of the skull: report of a case. Surg Gynecol Obstet. 1913;17:237-240.
8. Wilkins R.H. Neurosurgical Classics. New York and London: Johnson Reprint Corporation; 1965.
9. Grant F.C. Intracranial aerocele following fracture of the skull: report of a case with review of the literature. Surg Gynecol Obstet. 1923;36:251-255.
10. Dandy W.E. Pneumocephalus (intracranial pneumatocele or aerocele). Arch Surg. 1926;12:949-982.
11. Cairns H. Injuries of the frontal and ethmoidal sinuses with special reference to cerebrospinal fluid rhinorrhea and aeroceles. J Laryngol Otol. 1937;52:589-623.
12. Dandy W.E. Treatment of rhinorrhea and otorrhea. Arch Surg. 1944;49:75-85.
13. Lewin W. Cerebrospinal fluid rhinorrhea in closed head injuries. Br J Surg. 1954;42:1-18.
14. Lewin W. Cerebrospinal fluid rhinorrhea in nonmissile head injuries. Clin Neurosurg. 1966;12:237-252.
15. Eden K. Traumatic cerebrospinal rhinorrhoea: repair of a fistula by a transfrontal intradural operation. Br J Surg. 1941;29:299-303.
16. Dohlman G. Spontaneous cerebrospinal rhinorrhoea: case operated by rhinologic methods. Acta Otolaryngol (Stockh). 1948;67(Suppl):20-23.
17. McCabe N.F. The osteo-mucoperiosteal flap in repair of cerebrospinal fluid rhinorrhea. Laryngoscope. 1976;86:537-539.
18. Calcaterra T.C. Extracranial surgical repair of cerebrospinal rhinorrhea. Ann Otol. 1980;89:108-116.
19. Wormald P.J., McDonogh M. “Bath-plug” technique for the endoscopic management of cerebrospinal fluid leaks. J Laryngol Otol. 1997;111:1042-1046.
20. Sethi D.S., Chan C., Pillay P.K. Endoscopic management of cerebrospinal fluid fistulae and traumatic cephalocoele. Ann Acad Med Singapore. 1996;25:724-727.
21. Appelbaum E. Meningitis following trauma to the head and face. JAMA. 1968;173:116-120.
22. Brawley B., Kelly W. Treatment of skull fractures with and without cerebrospinal fluid fistula. J Neurosurg. 1967;26:57-61.
23. Einhorn A., Mizrahia E.M. Basilar skull fractures in children: incidence of CNS infection and the use of antibiotics. Am J Dis Child. 1978;132:1121-1124.
24. Krayenbuhl H.A. Questions and answers. Clin Neurosurg. 1967;14:23-24.
25. Leech P.J., Patterson R. Conservative and operative management for cerebrospinal leakage after closed head injury. Lancet. 1973;1:1013-1016.
26. Vourc’h G. Continuous cerebrospinal fluid drainage by indwelling spinal catheter. Br J Anaesth. 1963;35:118-120.
27. Aitken R.R., Drake C.G. Continuous spinal drainage in the treatment of postoperative cerebrospinal-fluid fistulae. J Neurosurg. 1964;21:275-277.
28. McCallum J., Maroon J.C., Janetta P.J. Treatment of postoperative cerebrospinal fluid fistulas by subarachnoid drainage. J Neurosurg. 1975;42:434-437.
29. Findler G., Sahar A., Beller A.J. Continuous lumbar drainage of cerebrospinal fluid in neurosurgical patients. Surg Neurol. 1977;8:455-457.
30. Kaufman B., Nulsen F.E., Weiss M.H., et al. Acquired spontaneous nontraumatic normal-pressure cerebrospinal fluid fistulas originating from the middle fossa. Radiology. 1977;122:379-387.
31. Ommaya A.K., Di Chiro G., Baldwain M., Pennybacker J.B. Non-traumatic cerebrospinal fluid rhinorrhoea. J Neurol Neurosurg Psychiatry. 1968;31:214-225.
32. Scievink W.I., Meyer F.B., Atkinson J.J., Mokri B. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. J Neurosurg. 1996;84:598-605.
33. Droste D.W., Krauss J.K. Oscillations of cerebrospinal fluid in nonhydrocephalic persons. Neurol Res. 1997;19:135-138.
34. Schievink W.I., Morreale V.M., Atkinson J.L., et al. Surgical treatment of spontaneous spinal cerebrospinal fluid leaks. J Neurosurg. 1998;88:2430-2436.
35. Park J.I., Strelzow V.V., Friedman W.H. Current management of cerebrospinal fluid rhinorrhea. Laryngoscope. 1983;93:1294-1300.
36. Dagi T.F., Meyer F.B., Poletti C.A. The incidence and prevention of meningitis after basilar skull fracture. Am J Emerg Med. 1983;3:295-298.
37. Meirowsky A.M., Caveness W.F., Dillon J.D., et al. Cerebrospinal fluid fistulas complicating missile wounds of the brain. J Neurosurg. 1981;54:44-48.
38. Zlab M.K., Moore G.F., Daly D.T., et al. Cerebrospinal fluid rhinorrhea: a review of the literature. Ear Nose Throat J. 1992;71:314-317.
39. Bell R.B., Dierks E.J., Homer L., Potter B.E. Management of cerebrospinal fluid leak associated with craniomaxillofacial trauma. J Oral Maxillofacial Surg. 2004;62(6):676-684.
40. Shulman K. Later complications of head injuries in children. Clin Neurosurg. 1971;19:371-380.
41. Nutkiewicz A., DeFeo D.R., Kohut R.I., Fierstien S. Cerebrospinal fluid rhinorrhea as a presentation of pituitary adenoma. Neurosurgery. 1980;6:195-197.
42. Haran R.P., Chandy M.J. Symptomatic pneumocephalus after transsphenoidal surgery. Surg Neurol. 1997;48:575-578.
43. Landy L.B., Graham M.D., Kartush J.M., LaRouere M.J. Temporal bone encephalocele and cerebrospinal fluid leaks. Am J Otol. 1996;17:461-469.
44. Piziak V.K., Gilliland P.F., Boyd G., et al. Pituitary tumor initially seen as serous otitis media. JAMA. 1984;251:3131-3132.
45. Mokri B., Atkinson J.L. False pituitary tumor in CSF leaks. Neurology. 2000;55(4):573-575.
46. Magliulo G., Sepe C., Varacalli S., Fusconi M. Cerebrospinal fluid leak management following cerebellopontine angle surgery. J Otolaryngol. 1998;27(5):258-262.
47. Horowitz N.H., Rizzoli H.V. Postoperative Complications of Intracranial Surgery. Baltimore: Williams & Wilkins; 1982.
48. Becker S.S., Jackler R.K., Pitts L.H. Cerebrospinal fluid leak after acoustic neuroma surgery: a comparison of the translabyrinthine, middle fossa, and retrosigmoid approaches. Otol Neurotol. 2003;24(1):107-112.
49. Sanna M., Taibah A., Russo A., et al. Perioperative complications in acoustic neuroma (vestibular schwannoma) surgery. Otol Neurotol. 2004;25(3):379-386.
50. Selesnick S.H., Liu J.C., Jen A., Newman J. The incidence of cerebrospinal fluid leak after vestibular schwannoma surgery. Otol Neurotol. 2004;25(3):387-393.
51. Shen T., Friedman R.A., Brackmann D.E., et al. The evolution of surgical approaches for posterior fossa meningiomas. Otol Neurotol. 2004;25(3):394-397.
52. Montgomery W.W. Surgery for acoustic neurinoma. Ann Otolaryngol. 1973;82:428-444.
53. Ojemann R.G. Microsurgical suboccipital approach to cerebellopontine angle tumors. Clin Neurosurg. 1978;25:461-479.
54. Khrais T.H., Falcioni M., Taibah A., et al. Cerebrospinal fluid leak prevention after translabyrinthine removal of vestibular schwannoma. Laryngoscope. 2004;114(6):1015-1020.
55. Valtonen H.J., Poe D.S., Heilman C.B., Tarlov E.C. Endoscopically assisted prevention of cerebrospinal fluid leak in suboccipital acoustic neuroma surgery. Am J Otol. 1997;18:381-385.
56. Horowitz N.H., Rizzoli H.V. Postoperative Complications of Intracranial Surgery. Baltimore: Williams & Wilkins; 1982.
57. Spaziante R., de Divitiis E., Cappabianca P. Reconstruction of the pituitary fossa in transsphenoidal surgery: an experience of 140 cases. Neurosurgery. 1985;17:453-458.
58. Ciric I. Comment on Spaziante. Neurosurgery. 1985;17:458. et al
59. Aust M.R., McCaffrey T.V., Atkinson J. Transnasal endoscopic approach to the sella turcica. Am J Rhinol. 1998;12(4):283-287.
60. Castillo L., Verschuur H.P., Poissonnet G., et al. Complications of endoscopically guided sinus surgery. Rhinology. 1996;34(4):215-218.
61. Bachmann G., Djenabi U., Jungehulsing M., et al. Incidence of occult cerebrospinal fluid fistula during paranasal sinus surgery. Arch Otolaryngol Head Neck Surg. 2002;128(1):11299-11302.
62. Bakay L., Glasauer F.E. Head Injury. Boston: Little, Brown; 1980.
63. Markham J.W. The clinical features of pneumocephalus based upon a survey of 284 cases with report of 11 additional cases. Acta Neurochir. 1967;16:1-78.
64. Hubbard J.L., Thomas J.M., Pearson B.W., Laws E.R. Spontaneous cerebrospinal fluid rhinorrhea: evolving concepts in diagnosis and surgical management based on the Mayo Clinic experience from 1970 through 1981. Neurosurgery. 1985;16:314-321.
65. Flanagan J.C., McLachlan D.L., Shannon G.M. Orbital roof fractures: neurologic and neurosurgical considerations. Ophthalmology. 1980;87:325-329.
66. Ray B.S., Bergland R.M. Cerebrospinal fluid fistula: clinical aspects, techniques of localization, and methods of closure. J Neurosurg. 1969;30:399-405.
67. Jamieson K.G., Yelland J.D.N. Surgical repair of the anterior fossa because of rhinorrhea, aerocele, or meningitis. J Neurosurg. 1973;39:328-331.
68. Management of cerebrospinal fluid leaks. J Trauma. 2001;51(suppl 2):S29-S33.
69. Bernal-Sprekelsen M., Bleda-Vazquez C., Carrau R.L. Ascending meningitis secondary to traumatic cerebrospinal fluid leaks. Am J Rhinol. 2000;14:257-259.
70. Kosoy J., Trieff N., Winkelmann P., et al. Glucose in nasal secretions. Arch Otolaryngol. 1975;95:225-229.
71. Healy C.E. Significance of a positive reaction for glucose in rhinorrhea. Clin Pediatr. 1969;8:239.
72. Kirsch A.P. Diagnosis of cerebrospinal fluid rhinorrhea: lack of specificity of the glucose oxidase Tes-Tape. J Pediatr. 1967;71:718.
73. Ghoshhajra K. Radiologic techniques for identification and localization of cerebrospinal fluid fistulae. Semin Neurol. 1982;2:115-125.
74. Levy J.M., Christensen F.K., Nykamp P.W. Detection of a cerebrospinal fluid fistula by computed tomography. AJR Am J Roentgenol. 1978;131:344-345.
75. Ahmadi J., Weiss M.H., Segali H.D., et al. Evaluation of cerebrospinal fluid rhinorrhea by metrizamide computed tomographic cisternography. Neurosurgery. 1985;16:54-60.
76. Matsumura A., Anno I., Kimura H., et al. Diagnosis of spontaneous intracranial hypotension by using magnetic resonance myelography: case report. J Neurosurg. 2000;92(5):873-876.
77. Strauss H. Fluorescein als indikator fuer die nierenfunktion. Berliner Klin Wchschr. 1913;50:2226-2227.
78. Fox N. Cure in a case of cerebrospinal rhinorrhea. Arch Otolaryngol. 1933;17:85-86.
79. Mahaley M.S., Odom G.L. Complications following intrathecal injections of fluorescein. J Neurosurg. 1966;25:298.
80. Staab E.V., Shirkhoda A. Cerebrospinal fluid scanning. Clin Nucl Med. 1981;6:103-109.
81. Coletti P.M., Siegel M.E. Posttraumatic lumbar cerebrospinal fluid leak: detection by retrograde In-111-DTPA myeloscintography. Clin Nucl Med. 1981;6:403-404.
82. Hasegawa M., Watanabe I., Hiratsuka H., et al. Transfer of radioisotope from CSF to nasal secretion. Acta Otolaryngol (Stockh). 1983;95:359-364.
83. Di Chiro G., Stein S.C., Harrington T. Spontaneous cerebrospinal fluid rhinorrhea in normal dogs: radioisotope studies of an alternate pathway of CSF drainage. J Neuropathol Exp Neurol. 1972;31:447-453.
84. Spetzler R.F., Wilson C.B. Management of recurrent CSF rhinorrhea of the middle and posterior fossa. J Neurosurg. 1978;49:393-397.
85. El Jamel M.S., Pidgeon C.N., Toland J., et al. MRI cisternography and the localization of CSF fistulae. Br J Neurosurg. 1994;8:433-437.
86. El Gammal T., Sobol W., Wadlington V.R., et al. Cerebrospinal fluid fistula: detection with MR cisternography. Am J Neuroradiol. 1998;19(4):627-631.
87. Moayeri N.N., Henson J.W., Schaefer P.W., Zervas N.T. Spinal dural enhancement on magnetic resonance imaging associated with spontaneous intracranial hypotension: report of three cases and review of the literature. J Neurosurg. 1998;88(5):912-918.
88. Shetty P.G., Schroff M.M., Sahani D.V., Kirtane M.V. Evaluation of high-resolution CT and MR cisternography in the diagnosis of cerebrospinal fluid fistula. Am J Neuroradiol. 1998;19:633-639.
89. Lloyd M.N.H., Kimber P.M., Burrows E.H. Post-traumatic cerebrospinal fluid rhinorrhea: modern high-definition computed tomography is all that is required for the effective demonstration of the site of leakage. Clin Radiol. 1994;49:100-103.
90. Zapalac J.S., Marple B.F., Schwade N.D. Skull base cerebrospinal fluid fistulas: a comprehensive diagnostic algorithm. Otolaryngol Head Neck Surg. 2002;126:669-676.
91. Bernal-Sprekelsen M., Bleda-Vazquez C., Carrau R.L. Ascending meningitis secondary to traumatic cerebrospinal fluid leaks. Am J Rhinol. 2000;14:257-259.
92. Marshall A.H., Jones N.S., Robertson I.J.A. An algorithm for the management of CSF rhinorrhea illustrated by 36 cases. Rhinology. 1993;37:182-185.
93. Bateman N., Jones N.S. Rhinorrhoea feigning cerebrospinal fluid leak: nine illustrative cases. J Laryngol Otol. 2000;114:462-464.
94. Meco C., Oberascher G. Comprehensive algorithm for skull base dural lesion and cerebrospinal fluid fistula diagnosis. Laryngoscope. 2004;114(6):991-999.
95. Ricchetti A., Burkhard P.R., Rodrigo N., et al. Skull base cerebrospinal fluid fistula: a novel detection method based on 2-dimensional electrophoresis. Head Neck. 2004;26(5):464-469.
96. Oberascher G., Arrer E. First clinical experience with (beta)2-transferrin in cerebrospinal fluid oto-and rhinoliquorrhea. HNO. 1986;34:151-155.
97. Irjala K., Suonpaa J., Laurent B. Identification of CSF leakage by immunofixation. Arch Otolaryngol. 1979;105:447-448.
98. Arrer E., Meco C., Oberascher G., et al. (Beta)-trace protein as a marker for cerebrospinal fluid rhinorrhea. Clin Chemistry. 2002;48:939-941.
99. Bachmann G., Nekic M., Michel O. Clinical experience with beta-trace protein as a marker for cerebrospinal fluid. Ann Otol Rhinol Laryngol. 2000;109(12 Pt 1):1099-1102.
100. Schlosser R.J., Bolger W.E. Management of multiple spontaneous nasal meningoencephaloceles. Laryngoscope. 2002;112:980-985.
101. Parisier S.C., Briken E.A. Recurrent meningitis secondary to idiopathic oval window CSF leak. Laryngoscope. 1976;86:1503-1515.
102. Nenzelius C. On spontaneous cerebrospinal otorrhea due to congenital malformations. Acta Otolaryngol (Stockh). 1951;39:314-328.
103. Bottema T. Spontaneous cerebrospinal fluid otorrhea. Arch Otolaryngol. 1975;101:693-694.
104. Rice W.J., Waggoner L.G. Congenital cerebrospinal fluid otorrhea via defect in the stapes footplate. Laryngoscope. 1967;77:341-349.
105. Ignelzi R.J., VanderArk G.D. Analysis of the treatment of basilar skull fractures with and without antibiotics. J Neurosurg. 1975;43:75-78.
106. Eljamel M.S., Foy P.M. Acute traumatic CSF fistulae: the risk of intracranial infection. Br J Neurosurg. 1990;4:381-385.
107. Choi D., Spann B. Traumatic cerebrospinal fluid leakage: risk factors and the use of prophylactic antibiotics. Br J Neurosurg. 1996;10:571-575.
108. Hilary A.B. Prophylactic antibiotics for post-traumatic cerebrospinal fluid fistula: a meta-analysis. Arch Otolaryngol Head Neck Surg. 1997;123:749-752.
109. Bernal-Sprekelsen M., Bleda-Vazquez C., Carrau R.L. Ascending meningitis secondary to traumatic cerebrospinal fluid leaks. Am J Rhinol. 2000;14:257-259.
110. Dagi T.F., Ojemann R.G., Zervas N.T. Incidence and prevention of infection after neurosurgical operations. In: Thompson R.A., Green J.R. Infectious Diseases of the Central Nervous System. Jamaica, NY: Spectrum Publications; 1984:155-173.
111. Ingraham F.D., Campbell J.B. An apparatus for closed drainage of the ventricular system. Ann Surg. 1941;114:1096-1098.
112. White R.J., Dakters J.G., Yashon D., et al. Temporary control of cerebrospinal fluid volume and pressure by means of an externalized valve-drainage system. J Neurosurg. 1969;30:264-269.
113. Vourc’h G. Continuous cerebrospinal fluid drainage by indwelling spinal catheter. Br J Anaesth. 1963;35:118-120.
114. McCoy G. Cerebrospinal rhinorrhea: a comprehensive review and a definition of the responsibility of the rhinologist in the diagnosis and treatment. Laryngoscope. 1963;73:1125-1157.
115. Wyler A.R., Kelly W.A. Use of antibiotics with external ventriculostomies. J Neurosurg. 1972;37:185-187.
116. Friedman W.A., Vries J.K. Percutanous tunnel ventriculostomy: summary of 100 procedures. J Neurosurg. 1980;53:662-665.
117. Dagi TF, Ondra SL: The role of artificial intelligence systems in neurosurgical intensive care. International Congress on Trends in Neurosurgery, Diagnostic and Surgical Perspectives, Vienna, Austria, May 14-17, 1986.
118. Carrion E., Hertzog J.H., Medlock M.D., et al. Use of acetazolamide to decrease cerebrospinal fluid production in chronically ventilated patients with ventriculopleural shunts. Arch Dis Child. 2001;84:68-71.
119. Brownridge P. Spinal anesthesia revisited: an evaluation of subarachnoid block in obstetrics. Anaesth Intensive Care. 1984;12:334-342.
120. Rudehill A., Gordon E., Rahn T. Subdural haematoma: a rare but life-threatening complication after spinal anaesthesia. Acta Anaesthesiol Scand. 1983;27:376-377.
121. Benzon H.T. Intracerebral hemorrhage after dural puncture and epidural blood patch: nonpostural and noncontinuous headache. Anesthesiology. 1984;60:258-259.
122. Ikeda K., Nakano M., Tani E. Tension pneumocephalus complicating ventriculoperitoneal shunt for cerebrospinal fluid rhinorrhea: case report. J Neurol Neurosurg Psychiatry. 1978;41:319-322.
123. Little J.R., McCarty C.S. Tension pneumocephalus after insertion of ventriculoperitoneal shunt for aqueductal stenosis. J Neurosurg. 1976;44:383-385.
124. Jooma R., Grant D.N. Cerebrospinal fluid rhinorrhea and intraventricular pneumocephalus due to intermittent shunt obstruction. Surg Neurol. 20, 1983. 231-124
125. Katz J. Treatment of a subarachnoid-cutaneous fistula with an epidural blood patch. Anesthesiology. 1984;60:603-604.
126. Digiovanni A.J., Galbert M.W., Wahle W.M. Epidural injection of autologous blood for post-lumbar puncture headache. I: additional clinical experiences and laboratory investigation. Anesth Analg. 1972;51:226-228.
127. Crawford J.S. Experiences with epidural blood patch. Anaesthesia. 1980;35:513-515.
128. Casement B.A., Danielson D.R. The epidural blood patch: are more than two ever necessary? Anesth Analg. 1984;63:1033-1035.
129. Rosenberg P.H., Heavner J.E. In vitro study of the effect of epidural blood patch on leakage through a dural puncture. Anesth Analg. 1985;64:501-504.
130. Harrington H., Tyler H.R., Welch K. Surgical treatment of post-lumbar puncture dural CSF leak causing chronic headache. J Neurosurg. 1982;57:703-707.
131. Sencakova D., Mokri B., McClelland R.L. The efficacy of epidural blood patch in spontaneous CSF leaks. Neurology. 2001;57(10):1921-1923.
132. Reynolds A.F., Hameroff S.R., Blitt C.D., et al. Spinal subdural epiarachnoid hematoma: a complication of a novel epidural blood patch technique. Anesth Analg. 1980;59:702-703.
133. O’Brien M.D., Reade P.C. The management of dural tear resulting from mid-facial fracture. Head Neck Surg. 1984;6:810-818.
134. da Cruz M.J., Ahmed S.M., Moffat D.A. An alternative method for dealing with cerebrospinal fluid fistulae in inner ear deformities. Am J Otol. 1998;19:288-291.
135. Kelley T.F., Stankiewicz J.A., Chow J.M., et al. Endoscopic closure of postsurgical anterior cranial fossa cerebrospinal fluid leaks. Neurosurgery. 1996;39:743-746.
136. Bolger W.E., McLaughlin K. Cranial bone grafts in cerebrospinal fluid leak and encephalocele repair: a preliminary report. Am J Rhinol. 2003;17(3):153-158.
137. Mortuaire G., Louis E., Pellerin P. Sphenoidal cerebrospinal fluid rhinorrhea: an original surgical approach. J Craniofac Surg. 2004;15(3):458-463.
138. Raza S.M., Conway J.E., Li K.W., et al. A modified frontal-nasal-orbital approach to midline lesions of the anterior cranial fossa and skull base: technical note with case illustrations. Neurosurg Rev. 2010;33:63-70.
139. Yessenow R.S., McCabe B.F. The osteo-mucoperiosteal flap in repair of cerebrospinal fluid rhinorrhea: 20-year experience. Otolaryngol Head Neck Surg. 1989;101:555-558.
140. Hughes R.G.M., Jones N.S., Robertson I.J.A. The endoscopic treatment of cerebrospinal fluid rhinorrhea: the Nottingham experience. J Laryngol Otol. 1997;111:125-128.
141. Jho H.D., Carrau R.L., Ko Y., Daly M.A. Endoscopic pituitary surgery: an early experience. Surg Neurol. 1997;47:213-223.
142. Gjuric M., Goede U., Keimer H., Wigand M.E. Endonasal endoscopic closure of cerebrospinal fluid fistulas at the anterior cranial base. Ann Otol Rhinol Laryngol. 1996;105:620-623.
143. Fraioli B., Pastore F.S., Floris R., et al. Computed tomography-guided transsphenoidal closure of postsurgical cerebrospinal fluid fistula: a transmucosal needle technique. Surg Neurol. 1997;48:409-413.
144. Van Den Abbeele T., Elmaleh M., Herman P., et al. Transnasal endoscopic repair of congenital defects of the skull base in children. Arch Otolaryngol Head Neck Surg. 1999;125(5):580-584.
145. Chu E.A., Quiñones-Hinojosa, Boahene K.D.O. Transblepharoplasty orbitofrontal craniotomy for repair of lateral and posterior frontal sinus cerebrospinal fluid leak. Otol Head Neck Surg. 2010;142(6):906-908.
146. Boahene K., Chu E., Lim A., Quiñones-Hinojosa A. Transpalpebral orbitofrontal craniotomy: a minimally invasive approach to anterior cranial vault lesions. Skull Base. 2010;20:237-244.
147. Jakoby R.K. The use of a methylmethacrylate seal in spinal fluid otorrhea and rhinorrhea. J Neurosurg. 1961;18:614-615.
148. Papini R.P.G., Niranjan N.S. Superglue sealant for persistent leakage of cerebrospinal fluid. Plast Reconstr Surg. 1993;91:371-372.
149. Lehman R.A.W., Hayes G.J., Martins A.N. The use of adhesive and lyophilized dura in the treatment of cerebrospinal rhinorrhea. J Neurosurg. 1967;26:92-95.
150. VanderArk G.D., Pitkethly D.T., Ducker T.B., et al. Repair of cerebrospinal fluid fistulas using a tissue adhesive. J Neurosurg. 1970;33:151-155.
151. Maxwell J.A., Goldware S.I. Use of tissue adhesive in the surgical treatment of cerebrospinal fluid leaks: experience with isobutyl-2 cyanoacrylate in 12 cases. J Neurosurg. 1973;39:332-336.
152. Mickey B.E., Samson D. Neurosurgical applications of the cyanoacrylate adhesives. Clin Neurosurg. 1982;29:429-444.
153. Kumar A., Maartens N.F., Kaye A.H. Reconstruction of the sellar floor using Bioglue following transsphenoidal procedures. J Clin Neurosci. 2003;10(1):92-95.
154. Agag R.L., Granick M.S., Omidi M., et al. Neurosurgical reconstruction with acellular cadaveric dermal matrix. Ann Plastic Surg. 2004;52(6):571-577.
155. Preul M.C., Bichard W.D., Spetzler R.F. Toward optimal tissue sealants for neurosurgery: use of a novel hydrogel sealant in a canine durotomy repair model. Neurosurgery. 2003;53(5):1189-1198. discussion 1198-1199
156. Greenblatt S.H., Wilson D.H. Persistent cerebrospinal fluid rhinorrhea treated by lumboperitoneal shunt: technical note. J Neurosurg. 1973;38:524-526.
157. Bret P., Hor F., Huppert J., et al. Treatment of cerebrospinal fluid rhinorrhea by percutaneous lumboperitoneal shunting: review of 15 cases. Neurosurgery. 1985;16:44-47.
158. Spetzler R.F.. Commentary on Bret P, et al, Neurosurgery, 1985;16:47
159. Vishteh A.G., Scievink W.I., Baskin J.J., Sonntag V.K. Cervical bone spur presenting with spontaneous intracranial hypotension: case report. J Neurosurg. 1998;89:483-484.
160. Schievink W.I., Maya M.M., Riedinger M. Recurrent spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension: a prospective study. J Neurosurg. 2003;99(5):840-842.
161. Schievink W.I., Jacques L. Recurrent spontaneous spinal cerebrospinal fluid leak associated with “nude nerve root” syndrome: case report. Neurosurgery. 2003;53(5):1216-1218. discussion 1218-1219
162. Schievink W.I., Maya M.M., Tourje J. False localizing sign of C1-C2 cerebrospinal fluid leak in spontaneous intracranial hypotension. J Neurosurg. 2004;100(4):639-644. [Erratum appears in J Neurosurg. 2004;100(6):1135.]
163. Schievink W.I., Maya M.M., Moser F.M. Treatment of spontaneous intracranial hypotension with percutaneous placement of a fibrin sealant: report of four cases. J Neurosurg. 2004;100(6):1098-1100.
164. Schrijver I., Schievink W.I., Godfrey M., et al. Spontaneous spinal cerebrospinal fluid leaks and minor skeletal features of Marfan syndrome: a microfibrillopathy. J Neurosurg. 2002;96(3):483-489.
165. Schievink W.I., Gordon O.K., Tourje J. Connective tissue disorders with spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension: a prospective study. Neurosurgery. 2004;54(1):65-70. discussion 70-71
166. Dickman C.A., Rosenthal D., Regan J.J. Reoperation for herniated thoracic discs. J Neurosurg. 1999;91(suppl 2):157-162.
167. Regan J.J., Aronoff R.J., Ohnmeiss D.D., Sengupta D.K. Laparoscopic approach to L4-L5 for interbody fusion using BAK cages: experience in the first 58 cases. Spine. 1999;24(20):2171-2174.
168. Gass H., Goldstein A.S., Ruskin R., et al. Chronic postmyelogram headache: isotopic demonstration of dural leak and surgical cure. Arch Neurol. 1971;25:108-170.
169. Rosenthal J.D., Hahn J.F., Martinez G.J. A technique for closure of leak of spinal fluid. Surg Gynecol Obstet. 1975;140:948-950.
170. Mayfield F.H., Kurokawa K. Watertight closure of the spinal dura mater: technical note. J Neurosurg. 1975;43:639-640.
171. Papadakis N., Mark V.H. Repair of spinal cerebrospinal fluid fistula with the use of a tissue adhesive: technical note. Neurosurgery. 1980;6:63-65.