7 Management of Cardiac Arrest and Post–Cardiac Arrest Syndrome
• The ultimate outcome of patients with cardiac arrest is frequently poor but can be improved by immediate bystander cardiopulmonary resuscitation, early defibrillation, and postresuscitation treatment.
• The classic ABC (airway, breathing, circulation) prioritization has changed to CAB to reinforce and prioritize early initiation of high-quality chest compressions and deemphasize early invasive airway management.
• Early defibrillation in patients with ventricular fibrillation and pulseless ventricular tachycardia is aided by various strategies, including automatic external defibrillators and improved provider awareness.
• Interruptions in chest compressions during cardiopulmonary resuscitation should be limited to maximize resuscitation; such interruptions can contribute to an unfavorable outcome.
• Postresuscitation care should include consideration of both induced hypothermia and urgent coronary reperfusion therapy.
Epidemiology
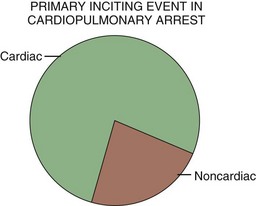
In the United States, sudden cardiac death accounts for approximately 200,000 to 500,000 deaths per year, with nearly half of these events occurring outside the hospital. Regarding the primary inciting event, cardiac causes of sudden cardiac death are most common (Fig. 7.1). Even though individuals with established cardiac disease have a greater than 50% incidence of sudden death, only a minority of cardiac arrest incidents occur in this population. It is estimated that half of all deaths from cardiovascular disease are sudden and unexpected and occur soon after the onset of symptoms. Patient age at the time of cardiac arrest has two distinct peaks—infants younger than 6 months and adults 45 to 75 years of age. Most sudden deaths occur outside the hospital and are often unwitnessed.1,2
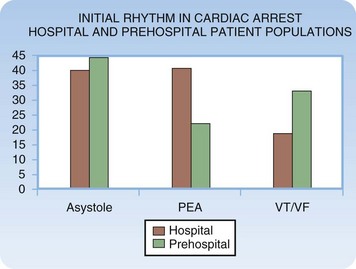
When resuscitation is successful, cardiac dysrhythmias remain a primary concern in the early phase of management of cardiac arrest. The four basic dysrhythmias encountered in cardiac arrest victims include pulseless ventricular tachycardia (VT), ventricular fibrillation (VF), asystole, and pulseless electrical activity (PEA). Pulseless VT and VF result in death unless treated aggressively and rapidly. Asystole, or effective absence of cardiac electrical activity, is the true cardiac arrhythmia (i.e., absence of any rhythm). PEA constitutes a diverse range of rhythms and related clinical scenarios. PEA is an electrical rhythm (i.e., the cardiac rhythm) with absence of discernible mechanical contraction of the heart and no detectable perfusion. The frequency of the dysrhythmias differs depending on the clinical setting (Fig. 7.2).
General Management Considerations
Resuscitation rates in patients with out-of-hospital and in-hospital cardiac arrest remain poor despite significant advances in the medical sciences. Contemporary research and recommendations have demonstrated that basic life support (BLS) interventions are very important therapies that have a positive impact on outcome.3 This same body of thought and investigation has suggested that advanced life support (ALS) treatments are of less value than originally thought.3 In addition, studies have demonstrated that early access to ALS treatment may be of less importance than previously believed.3,4 In the final phase of the Ontario Prehospital Advanced Life Support trial it was reported that in a community in which early CPR and early defibrillation are achieved, there is no survival benefit with the addition of prehospital ALS interventions.3 ALS is of value, but it is limited and less valuable than BLS measures in the early phase of most cardiac arrest events.
The 2010 guidelines of the American Heart Association (AHA) further deemphasizes advanced interventions. The 2010 AHA Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care list five goals in the approach to cardiac arrest resuscitation: rapid activation of the emergency response team, effective and high-quality chest compressions, early access to defibrillation, effective ALS, and coordinated postresuscitation care.5 Only one of these five goals addresses ALS interventions.
Cardiopulmonary Resuscitation
Numerous investigations have demonstrated that chest compressions are delayed, frequently interrupted, and of poor quality. Wik et al found that CPR was performed only 48% of the time when indicated and that when it was performed, the mean rate was just 64 compressions per minute, with an appropriate depth (at least 5 cm) attained in only 28% of cases.6 Wang et al. and others reported that CPR was frequently interrupted for prolonged periods, anywhere from 1.8 to 7 minutes, to perform endotracheal intubation.7,8
One of the key components of the 2010 AHA guidelines5 is the change from performing the ABCs (airway, breathing, circulation) to the CABs,9 thus demonstrating how important compressions are relative to airway management and oxygenation—particularly early in cardiac arrest. Chest compression–only CPR has been proposed as an alternative method of basic resuscitation that is superior to traditional CPR with chest compressions and ventilations. The basic component of compression-only CPR is the performance of continuous, uninterrupted chest compressions of high quality. Compression-only CPR suggests that early management of the airway is not a priority.
Compression-only CPR has been shown to have similar efficacy to conventional CPR in terms of neurologically intact survival at 1 year in victims of witnessed cardiac arrest.10 In patients with an initially shockable rhythm, Kellum et al. demonstrated an increased survival rate with neurologically intact status after receiving compression-only CPR.11 The SOS-KANTO (Survey of Survivors After out-of Hospital Cardiac Arrest in the Kanto Area of Japan) trial reviewed 4068 patients with witnessed out-of-hospital cardiac arrests, 1151 of whom received bystander CPR. The 439 subjects who received hands-only CPR showed similar neurologic outcome at 30 days as did those who received conventional CPR (6% versus 4% of survivors); no benefit was seen with the addition of mouth-to-mouth ventilation.12 In 2008 using the concept of minimally interrupted cardiac resuscitation, Bobrow et al. demonstrated that cycles of 200 continuous compressions, followed by electrical defibrillation with immediate resumption of compressions before endotracheal intubation, resulted in an increased survival rate (1.8% versus 5.4%) in all patients. In the witnessed VF subgroup, the survival rate increased from 4.7% to 17.6%.13
Electrical Therapy
The most recent analysis of the timing of initial defibrillation suggests that there is no benefit with delayed defibrillation, even in patients with prolonged downtimes. Simpson et al. performed a meta-analysis of the existing literature and noted that no benefit was found in delaying the first shock in patients with prolonged downtimes or unwitnessed arrest (or both).14 The cumulative data demonstrated no benefit in providing chest compression before defibrillation versus immediate defibrillation and also no harm in performing CPR before the initial defibrillation. The 2010 AHA guidelines5,9 also acknowledged that the literature does not support a chest compression–first approach, thus suggesting that clinicians should provide both therapies (chest compression and defibrillation) and base the time of the first defibrillation on analysis of the setting, personnel, and equipment parameters for individual cases.
The other primary form of electrical therapy used in managing cardiac arrest is transcutaneous cardiac pacing. Very early use of transcutaneous ventricular pacing can be considered, although conclusive supporting evidence is lacking. Transcutaneous pacing is much less effective after the loss of spontaneous circulation or prolonged cardiac arrest.15,16 Transcutaneous pacing is a rapid, minimally invasive means of treating asystole and PEA bradyarrhythmias. Transcutaneous pacing electrodes are applied to the skin of the anterior and posterior chest walls, and pacing is initiated with a portable pulse generator. In an emergency situation, this type of pacing technique is easily and rapidly accomplished when compared with other methods of cardiac pacing.
Pharmacologic Therapy
Although numerous medications may be used in a resuscitation event, several “code drugs” are of potential importance, including epinephrine, vasopressin, atropine, amiodarone, lidocaine, magnesium, calcium, and sodium bicarbonate. Research into resuscitation after cardiac arrest has demonstrated that BLS interventions significantly contribute to favorable outcomes and that ALS treatments are less valuable than originally thought.3,17 A review of the issue has suggested that the use of cardioactive medications can increase the rate of successful resuscitation but does not alter the ultimate survival rate or have an impact on neurologic status at discharge among survivors.18
Atropine is a parasympatholytic drug that enhances both sinoatrial node automaticity and atrioventricular conduction via direct vagolytic action. In cardiac arrest, atropine can be considered in patients with both asystole and PEA, particularly those with bradydysrhythmic electrical activity. It is important to note that the AHA has removed atropine from all cardiac arrest algorithms.5,9 Removal of atropine is based not on any negative impact on patient outcome but on a significant lack of benefit.5,9 Atropine is still recommended in patients with compromising bradydysrhythmia with intact perfusion.5,9,19,20
Acute thrombosis with or without embolization, either coronary or pulmonary, can cause cardiac arrest. Many investigators have considered the early use of fibrinolytic agents in the management of cardiac arrest with known or presumed acute thrombosis. Anecdotal reports describe the cases of adult patients who have been successfully resuscitated following the administration of a fibrinolytic agent when the condition leading to the arrest was acute myocardial infarction (AMI) or acute pulmonary embolism.21 The 2010 AHA guidelines state that the evidence is insufficient to advocate the routine use of fibrinolytic agents during cardiac arrest but that its use should be considered on a case-by-case basis. Fibrinolytic agents are a level IIb recommendation in cardiac arrest secondary to pulmonary embolism.5,9
The Airway
The AHA has moved away from the airway-first strategy with a reordering of the resuscitation alphabet from the ABCs to the CABs, thus highlighting the relative importance of circulation over airway management.5,9 This change in strategy is based on the relative importance of circulation but also on the fact that airway interventions, particularly placement of an invasive airway, can interrupt continuous chest compressions and lessen the central nervous system (CNS), cardiac, and systemic perfusion produced by CPR. The airway should be managed invasively once appropriate chest compressions have been initiated and sustained and defibrillation has taken place. Management of the airway must not hinder appropriate chest compressions and other basic interventions. In cardiac arrest scenarios caused by a compromised airway or inadequate oxygenation and ventilation, or both, attention to invasive management of the airway is important.
Management of Specific Dysrhythmias
Ventricular Fibrillation and Pulseless Ventricular Tachycardia
Clinical Presentation
Ventricular Fibrillation
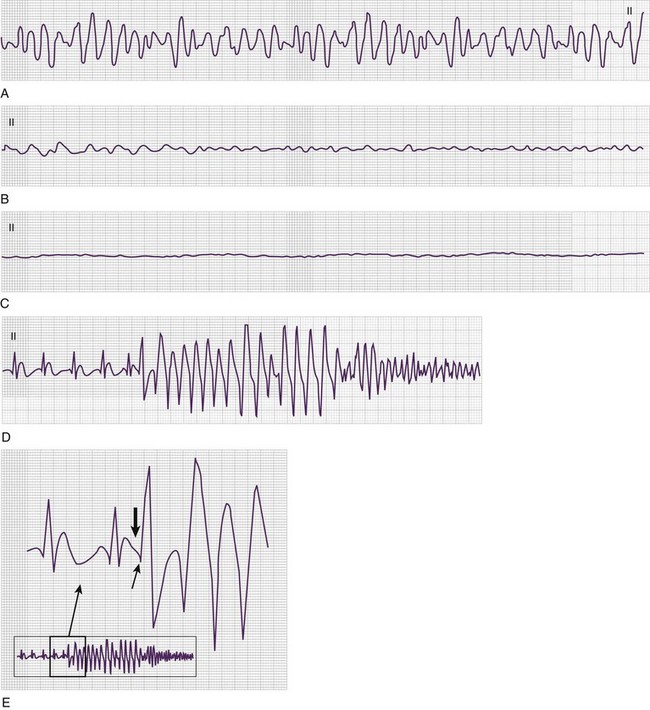
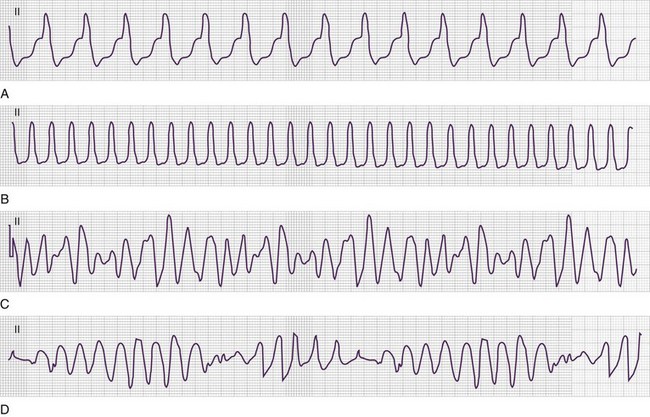
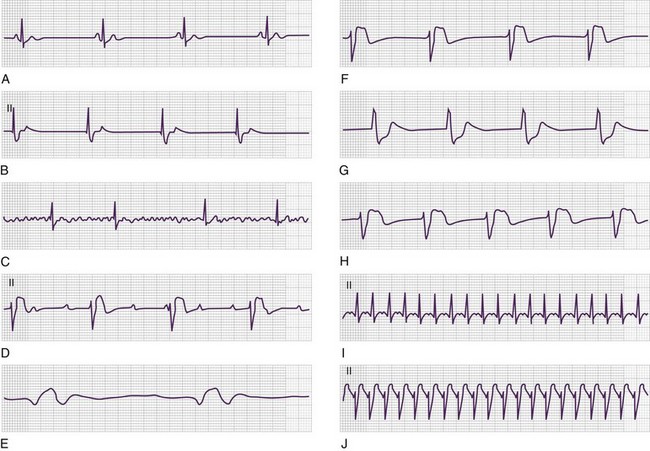
VF results in a lack of spontaneous perfusion except in the rare case of a witnessed, recent onset in which the patient is able to cough, thereby enabling perfusion to continue for a short period. VF is diagnosed electrocardiographically (Fig. 7.3) in pulseless and apneic patients by the presence of lower amplitude and chaotic activity. The rate of the deflections is usually between 200 and 500 depolarizations per minute. Morphologically, VF is divided into coarse (Fig. 7.3, A) and fine (Fig. 7.3, B and C). Coarse VF tends to occur early after cardiac arrest; is characterized by high-amplitude, or coarse, waveforms; and has a better prognosis than fine VF does. With continued cardiac arrest the amplitude dampens, with a less dramatic appearance of the dysrhythmia and fine VF (Fig. 7.3, B and C) ultimately being produced. The R-on-T phenomenon can result in VF as noted in Figure 7.3, D and E.
Ventricular Tachycardia
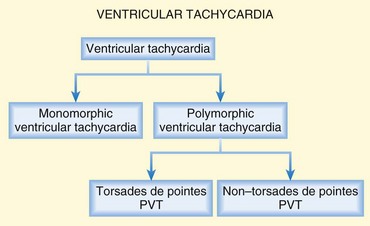
VT is defined as three or more ventricular beats in succession with a QRS complex duration of greater than 0.12 second and a ventricular rate greater than 100 or 120 beats/min (Fig. 7.4). Most instances of VT are characterized by very rapid rates; however, patients may have slower versions of VT, particularly if using amiodarone.
From an electrocardiographic perspective (Fig. 7.5; also see Fig. 7.4), the morphology of the VT is of importance. MVT (Fig. 7.5, A and B) is identified when each consecutive waveform has a single morphology; that is, the beat-to-beat variation in QRS complex morphology is negligible. The rate is usually between 140 and 180 beats/min and very regular. MVT is the most common form of VT and is seen in 65% to 75% of patients with VT in the out-of-hospital setting (Fig. 7.6).22,23 In patients with MVT, the cause of the dysrhythmia is usually myocardial scarring from a previous infarct.
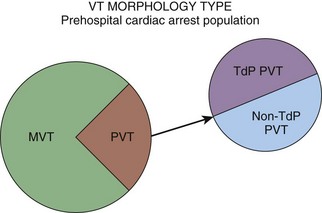
Fig. 7.6 Morphologic subtypes of ventricular tachycardia (VT) in prehospital cardiac arrest patients.
PVT (see Fig. 7.4) is characterized by a frequently changing QRS complex (see Fig. 7-5, C and D). The QRS complex tends to be greater than 0.12 second with beat-to-beat variations in morphology and width. Significant variability in the R-R interval and QRS complex axis is present. Its rate is usually more rapid than that of MVT, with a range of 150 to 300 beats/min, and PVT accounts for 25% to 30% of cases of VT in patients with out-of-hospital cardiac arrest (see Fig. 7.6).22,23
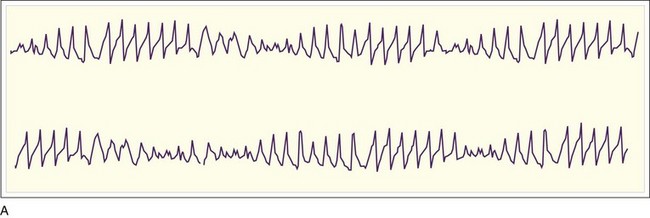
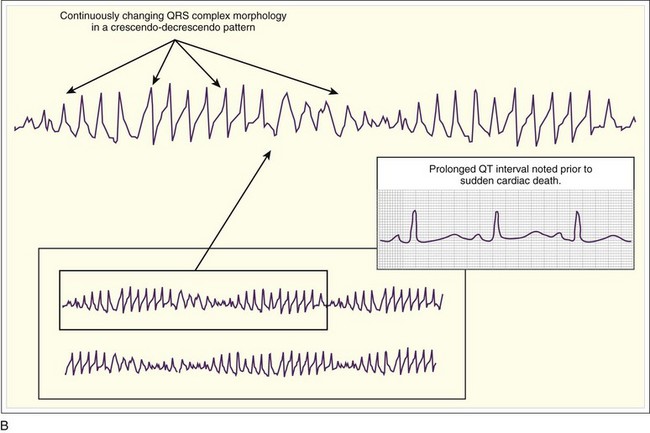
The PVT subtype TdP (see Fig. 7.4) demonstrates polymorphous QRS complexes that vary from beat to beat (Fig. 7.7, A and B; also see Fig. 7.5, D). The variation is often quite pronounced and easily observed. TdP has a highly characteristic electrocardiographic pattern; the literal translation of the French term torsades de pointes—”twisting of the points”—elegantly describes the appearance of the QRS complex as it varies in amplitude and appears to rotate about the isoelectric baseline in a semisinusoidal fashion. Also required for electrocardiographic (and clinical) diagnosis of TdP is demonstration of abnormal repolarization as manifested by prolongation of the QT interval on the electrocardiogram when the patient is in a supraventricular rhythm (either before or after cardiac arrest) (Fig. 7.7, B).
Management of Pulseless Ventricular Tachycardia and Ventricular Fibrillation
Resuscitative management of these two malignant dysrhythmias is similar. The basic approach (Table 7.1) includes CPR, with an emphasis on high-quality chest compressions, and electrical defibrillation. Once defibrillation has occurred, CPR is resumed for an additional 2 minutes. If the dysrhythmia persists on electrocardiographic analysis, a vasopressor is administered, either vasopressin or epinephrine. Epinephrine should be used in 1-mg doses, preferably administered via an intravenous or intraosseous line. If given by endotracheal tube (ETT), a larger dose of two to three times the intravenous dose is recommended. Dosing through the ETT is not currently considered to be the most effective means of delivering the medication. Epinephrine should be readministered every 3 to 5 minutes during the resuscitation. In certain situations such as ingestion of a cardiotoxic drug, higher doses of epinephrine may be required, but this issue has not been explored. Vasopressin is administered via the intravenous or intraosseous routes at a dose of 40 international units (IU). In multiple studies of vasopressin alone or vasopressin versus epinephrine in prehospital and hospital-based populations, no significant difference has been found in survival to hospital discharge.24–26 A single dose of vasopressin may be used as either the first or second vasopressor administered, and epinephrine can be used in a primary or secondary role.
Table 7.1 Ventricular Fibrillation and Pulseless Ventricular Tachycardia
| TIME | TREATMENT PRIORITY | COMMENTS |
|---|---|---|
| Minute 0 | Chest compressions | Compressions should be performed at a rate of 100/min. Initial compressions may improve shock efficacy by increasing the amplitude of ventricular fibrillation. Pulse checks and rhythm analysis should be performed no more often than every 2 min. Defibrillation should take place as soon as a device is available. |
| Minute 0-1 | Defibrillation, followed by chest compressions IV access (if possible) |
Because of the increased first shock conversion rate with biphasic energy and the importance of hands-off time, high-energy shock is recommended. IV access should not interfere with chest compressions. |
| Minute 3 | Defibrillation, followed by immediate chest compressions Vasopressin, 40 IU Placement of an invasive airway (if possible) |
Vasopressin or epinephrine may be given interchangeably initially. Placement of an invasive airway should not interfere with chest compressions. If an invasive airway is not possible at this time, noninvasive management of the airway is appropriate. |
| Minute 5 | Defibrillation, followed by immediate chest compressions Amiodarone, 300 mg |
CPR should take place over 2-min periods with intervening attempts at defibrillation. Lidocaine is an acceptable alternative antiarrhythmic agent. |
| Minute 6 | Epinephrine, 1 mg | Medication is administered every 3-5 min for cardiac arrest; it should be realized that peak central levels may vary based on physiologic patient differences. If vasopressin is given first, epinephrine is administered in all subsequent doses. |
| Minute 7 | Defibrillation, followed by immediate chest compressions | CPR should not be interrupted for periods longer than 15 seconds at a time. |
| Minute 9 | Defibrillation, followed by immediate chest compressions Epinephrine, 1 mg |
Other medications should be considered in patients with an identified underlying cause. |
| Minute 10 | Amiodarone, 150 mg | Amiodarone can be repeated in 5 min at half the initial dose. |
CPR, Cardiopulmonary resuscitation; IV, intravenous.
After the administration of a vasopressor, CPR should be continued for 2 minutes, followed by a second defibrillation. If the VT or VF persists, amiodarone or lidocaine should be administered. Amiodarone is the preferred agent and should be considered in patients with pulseless VT or VF that is unresponsive to CPR, a vasopressor, and defibrillation. It is administered to a pulseless patient in a bolus dose of 300 mg intravenously, and it can be repeated with a second dose of 150 mg intravenously. Amiodarone has demonstrated a benefit over both placebo and lidocaine in this patient group. Even though rates of ROSC and survival to hospital admission were greater in amiodarone-treated patients, ultimate survival to hospital discharge was no different.27,28
PVT should be managed in similar fashion to pulseless MVT or VF. CPR, defibrillation, a vasopressor, and antidysrhythmic agents are appropriate therapeutic interventions. In two comparisons of MVT and PVT in prehospital patients with cardiorespiratory arrest, MVT occurred (60%) more often than PVT (15%) and TdP (15%).22,23 Clinical outcomes were similar in both rhythm groups with similar therapies. Patients with the subset of TdP also fared as well as the non-TdP PVT and MVT groups.22,23 If sustained, PVT is always unstable and requires immediate attention. Initial therapy is unsynchronized electrical cardioversion. Antiarrhythmic therapy is warranted, including the use of magnesium and amiodarone intravenously. Caution is advised because the rhythm frequently recurs.
Pulseless Electrical Activity
Definition
PEA is indicative of a very serious underlying medical event, such as profound hypovolemia, massive myocardial infarction, large pulmonary embolism, significant electrolyte disorder, or cardiotoxic overdose. PEA features the unique combination of no discernible cardiac mechanical activity (i.e., a pulseless state) with persistent cardiac electrical activity (i.e., the cardiac rhythm). Essentially, any dysrhythmia other than VT or VF may be seen (Figs. 7.8 and 7.9).
Clinical Presentation
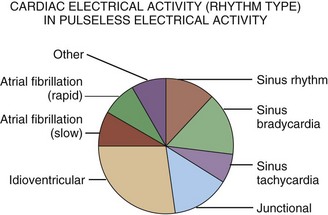
Causes of PEA are numerous (see Table 7.1), spanning all of acute care medicine, and include profound hypovolemia, cardiac tamponade, large anterior wall myocardial infarction, tension pneumothorax, massive pulmonary embolism, severe sepsis, anaphylactic reaction with shock, significant electrolyte abnormality (e.g., disorders of potassium), pronounced metabolic acidosis, substantial cardiotoxin ingestion, and hypothermia. These conditions have a final common pathophysiologic denominator of severe hypovolemia (absolute or relative), marked obstruction to flow, profound hypocontractility, or any combination of the three. The PEA rhythm manifestations are numerous. The most frequent dysrhythmias seen in PEA include idioventricular, junctional, and sinus bradycardia (see Figs. 7.8 and 7.9). Some causes of PEA are reversible if recognized early and treated correctly in the initial phases of resuscitation. Hemorrhagic shock, pulmonary embolism, pericardial effusion, and tension pneumothorax can all lead to PEA and are potentially reversible.
The electrocardiographic rhythm may be a useful guide to the etiology and a key to successful resuscitation.29 Rapid, narrow–QRS complex tachycardic manifestations are associated with a somewhat better opportunity for survival. Profound hypovolemia is the most frequently encountered event in patients with PEA. In an attempt to maintain cardiac output, the heart will increase its rate. This increased rate will usually be in the form of sinus tachycardia. In patients with atrial fibrillation, a rapid ventricular response will be present and create a manifestation that is probably a pseudo-PEA cardiac arrest.
Only 2% to 3% of patients with PEA as the initial or primary dysrhythmia in cardiac arrest survive to hospital discharge neurologically intact. Electrocardiographic variables may be predictive of successful resuscitation.29,30 Rapid rhythm rates are much more frequently associated with ROSC than sinus bradycardia is. The width of the QRS complex is also potentially predictive of resuscitative outcome. Progressively wider QRS complexes are found in patients with a lower likelihood of restoration of spontaneous perfusion. In this risk prognostication application, an idioventricular rhythm is associated with a lower chance of resuscitation than is sinus bradycardia with a normal QRS complex duration. Other electrocardiographic findings encountered in PEA patients successfully resuscitated include the development of P waves and shortened QT intervals.
Management
Management of a patient in PEA arrest should focus on standard resuscitation treatments and rapid identification and correction of reversible causes, but it is otherwise similar to the treatment of pulseless VT and VF (Table 7.2).
Table 7.2 Etiologies, Pathophysiologic Events, and Specific Therapies for Cardiac Arrest with Pulseless Electrical Activity
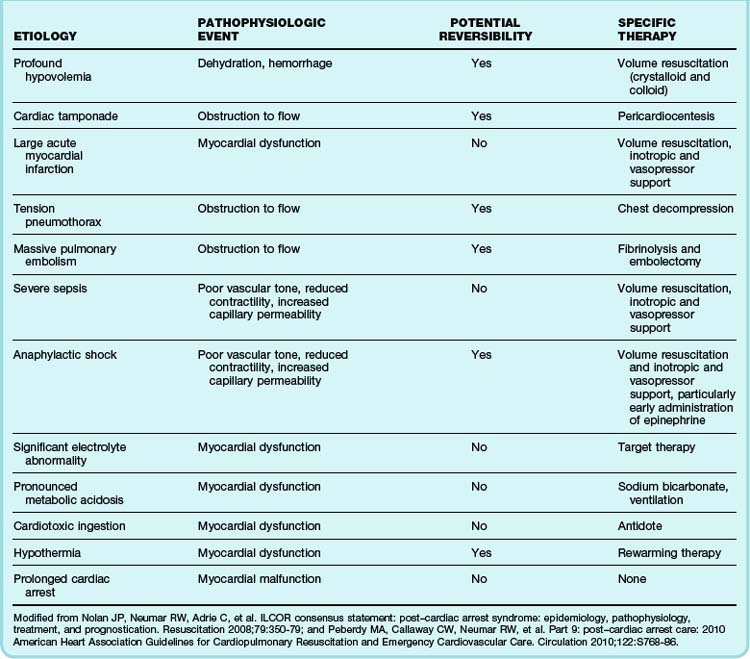
In the 2010 AHA guidelines, atropine was removed from the PEA and asystole algorithm. Atropine was removed not because of any harm induced but because of lack of efficacy.5,9 The clinician at the bedside should use clinical judgment to determine whether atropine should be administered in this rhythm scenario. Epinephrine is recommended at a dose of 1 mg intravenously, repeated every 3 to 5 minutes during resuscitation. Vasopressin, 40 IU intravenously, can also be used once in the resuscitation event. Aggressive volume replacement with either crystalloid or colloid is recommended. Attention to the various causes and focused treatment of PEA is encouraged early in the resuscitation (see Table 7.1).
Asystole
Clinical Presentation
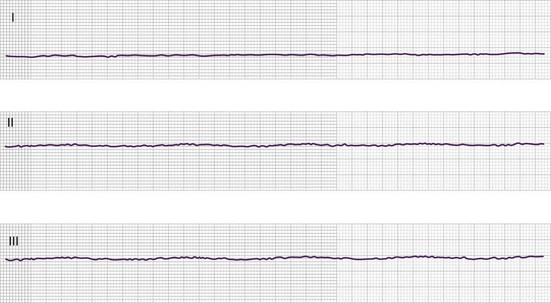
In asystole, the electrocardiogram demonstrates a flat line or nearly flat line (Fig. 7.10). Minimal undulations of the waveform resulting from electrocardiographic baseline drift can be seen. Several pitfalls must be avoided in the apparent asystolic manifestation, including monitor malfunction, disconnection of electrocardiographic leads, and fine VF with minimal amplitude in the imaging lead. The last potential error can be detected by confirming asystole with at least two leads oriented in perpendicular fashion (see Fig. 7.10).
Management
Resuscitation should follow a similar algorithm as that for PEA (Table 7.3), with the notable exception of consideration of cardiac pacing. Even though several trials have not demonstrated a benefit of transcutaneous pacing in patients with asystolic arrest,15,16 it is not unreasonable to consider a short course of cardiac pacing if performed very early in the resuscitation. A comment should be made on the choice of vasopressor. One large study demonstrated a short-term survival benefit of vasopressin with respect to epinephrine in these patients, and this benefit remained apparent at hospital discharge.26
| TIME | TREATMENT PRIORITY | COMMENTS |
|---|---|---|
| Minute 0 | Chest compressions | Compressions should be performed at a rate of 100/min. Pulse checks and rhythm analysis should be performed no more frequently than every 2 min. With a narrow-complex rhythm, assess for occult blood flow (i.e., pseudo-PEA). |
| Minute 1 | Chest compressions IV or IO access Vasopressor |
Perform interventions but do not interrupt chest compressions for more than 15 sec after each 2-min cycle. Early IV or IO access for drug administration is now preferred. Vasopressin or epinephrine may be given interchangeably initially. |
| Minute 2 | Chest compressions Placement of an invasive airway |
Placement of an invasive airway is not a priority unless bag-mask ventilation is inadequate. An invasive airway can be placed, but its placement should not interrupt chest compressions. Atropine, 1 mg intravenously, can be given at this time if so desired, though its impact is minimal. |
| Minute 4 | Chest compressions Vasopressor |
Chest compressions should be interrupted only to change compressors (no longer than 15 sec). If epinephrine was given initially, vasopressin can now be administered; epinephrine is then administered in all subsequent doses. |
| Minute 5 | Chest compressions Supplemental medications |
Underlying causes may necessitate the administration of adjunctive medications. |
| Minute 7 | Chest compressions Vasopressor |
Medication should be administered every 3-5 min for cardiac arrest; it should be realized that peak central levels may vary based on physiologic patient differences. Atropine, 1 mg intravenously, can be given at this time if so desired. |
| Minute 10 | Chest compressions Vasopressor |
At 10 min the outcome-related implications should be weighed because survival to discharge decreases after this point without ROSC. |
IO, Intraosseous; IV, intravenous; PEA, pulseless electrical activity; ROSC, return of spontaneous circulation.
Special Arrest Populations and Scenarios
Traumatic Injury
Multiple traumatic causes can lead to cardiac arrest. The prognosis of patients with out-of-hospital cardiac arrest as a result of trauma is poor, particularly in the setting of blunt injury.31
In cases of traumatic arrest in which a clear etiology is not readily apparent, aggressive resuscitation is indicated, although the prognosis is bleak. General trauma management should be performed while specific therapy is aimed at the medical portion of the cardiorespiratory arrest. Standard ALS interventions aimed at management of the dysrhythmia should be pursued with the realization that the likelihood of successful resuscitation is minimal. Pulseless VT and VF should be defibrillated immediately on recognition. In most traumatic situations, the underlying etiology must be corrected for return of circulation to occur.5,9 Medical therapies are unlikely to correct traumatized tissue and restore spontaneous circulation.
If definitive surgical intervention is available, resuscitative thoracotomy may be a reasonable intervention for a subset of trauma patients. This subset includes any traumatic arrest patient with the following features: witnessed arrest in the emergency department, arrest time less than 5 minutes with penetrating cardiac injury, arrest time less than 15 minutes with penetrating thoracic injury, or any exsanguinating abdominal vascular injury in which secondary signs of life are present (e.g., pupillary reflexes, spontaneous movement, organized electrocardiographic activity).5,9,32 Thoracotomy allows internal cardiac massage and defibrillation, relief of pericardial tamponade, direct control of cardiac or thoracic hemorrhage, and cross-clamping of the aorta. Many of these interventions require a high degree of technical skill and should be attempted only by experienced providers; however, outcomes are predictably poor.
Status Asthmaticus
External compression of the thorax during the expiratory phase to maximize exhalation has been proposed,33 but its use remains controversial and it will probably be difficult to coordinate during compressions. Needle or tube thoracostomy decompression should be performed if pneumothorax is detected or suspected. Bilateral decompression for refractory arrest is warranted given the potential for masking the lateralizing signs of pneumothorax.
Pregnancy
Cesarean delivery should be accomplished as soon as cardiac arrest is identified in a pregnant patient. The highest survival rates for infants older than 24 to 25 weeks’ gestational age occurs in deliveries performed within 5 minutes of arrest of the mother. This intervention is most appropriately performed by a multidisciplinary team skilled in emergency obstetric surgery.34
Pregnant patients should be placed in the left lateral decubitus position. Displacement of the gravid uterus should be performed by elevating the right hip and lumbar region 15 to 30 degrees from the supine position. Manual displacement of the uterus by lifting it with two hands and directing it toward the upper left part of the patient’s abdomen can be performed before blanket roll placement and afterward to optimize venous return.34
Postresuscitation Phase
Post–Cardiac Arrest Syndrome
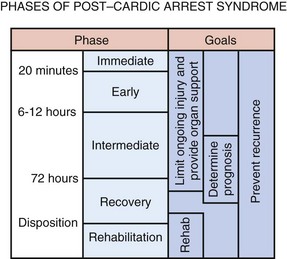
Management of resuscitated cardiac arrest patients has undergone significant change in the past decade. The concept of post–cardiac arrest syndrome has been developed.35,36 This syndrome, thought to occur in the initial 72 hours after ROSC, is a unique pathophysiologic entity found in patients who have been resuscitated from cardiorespiratory arrest. The primary considerations include CNS injury, myocardial dysfunction, systemic hypoperfusion with reperfusion-related damage, and the precipitating event. Brain injury from ischemia is responsible for many patient deaths, including approximately 70% of patients resuscitated after out-of-hospital arrest and 25% of individuals resuscitated after in-hospital arrest. The basic reasons for CNS injury include a limited tolerance for brain ischemia coupled with a unique response to reperfusion. Brain injury is an ongoing phenomenon, even after successful resuscitation, that usually results from a number of different pathophysiologic events. Injury can continue for 6 to 72 hours after the return of spontaneous perfusion despite adequate systemic blood pressure.
Postresuscitation Management
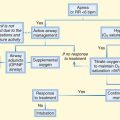
Management considerations (Figs. 7.11 and 7.12) must address the following issues: CNS injury, myocardial dysfunction, systemic hypoperfusion, multiorgan failure, and the precipitating cause of the cardiac arrest. Multidisciplinary critical care support along with appropriate respiratory and hemodynamic support is vital. Two specific treatment considerations, therapeutic (induced) hypothermia and emergency coronary reperfusion, must be emphasized in these resuscitated patients.
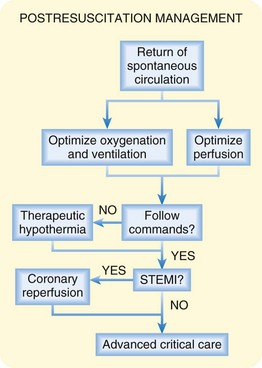
Fig. 7.12 Resuscitative management of patients with post–cardiac arrest syndrome.
(Modified from Nolan JP, Neumar RW, Adrie C, et al. ILCOR consensus statement: post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. Resuscitation 2008;79:350-79; and Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post–cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science. Circulation 2010;122:S768-86.)
Therapeutic hypothermia (TH), or induction of lower body temperatures in unresponsive patients resuscitated from cardiac arrest, is strongly encouraged in certain individuals. The AHA notes that “…therapeutic hypothermia in the resuscitated cardiac arrest patient can markedly improve outcome…both in terms of rates of survival and neurologic status.”35,36 TH is currently recommended for victims resuscitated after out-of-hospital cardiac arrest with initial rhythms of either pulseless VT or VF who remain unconscious. TH should also be considered in patients resuscitated from cardiac arrest associated with other initial rhythms (i.e., PEA and asystole) and in survivors of in-hospital cardiac arrest.35,36 The actual beneficial mechanisms for TH are not fully known, but the basic accepted mechanism involves suppression of the chemical cascade associated with the total-body ischemia and reperfusion injury. These mechanisms probably involve a number of different protective processes, including a reduction in the metabolic rate, oxygen use, and CNS electrical activity. TH probably interrupts the injury process at many points in the pathophysiologic response to cardiac arrest and subsequent reperfusion. Regardless of the modes of action of this intervention, TH has been demonstrated to provide CNS protection, reduce the incidence of multiorgan failure syndrome, increase survival, and improve functional abilities. The outcome after cardiac arrest with our present abilities remains suboptimal. Use of TH can increase the rate of functional survival, but it is not a miracle cure.
• Patients should be cooled as soon as possible after ROSC, ideally within 4 to 6 hours. Cooling should last for approximately 18 hours once the target temperature has been reached.
• The goal temperature is 32° C to 34° C.
• The patient is probably mechanically ventilated via an ETT.
• Pain medication and sedative agents are probably required for patient comfort and safety. Chemical paralytic agents are used with caution because seizures cannot be detected as easily.
• Shivering should be avoided if at all possible because of the related heat production, and appropriate treatment should be provided if it develops.
• Biometric surveillance should include an electrocar-diogram, blood pressure, core temperature, oxygen saturation, and end-tidal CO2 monitoring. Continuous electrocardiographic monitoring can also be considered. Laboratory studies should include electrolytes and serum glucose.
• The most appropriate cooling method is debatable. Two basic approaches have been advocated, but thus far neither therapy has proved to be superior. Rapid infusion of 2 L of chilled normal saline (4° C) represents the least challenging approach. The chilled saline is used in conjunction with cooling blankets and ice packs applied to the neck, armpits, groin, and other areas. The other technique, which is both more invasive and more expensive, involves the use of an intravascular cooling machine. This technique, once the intravascular catheter has been placed, is easier to use in terms of reaching the target temperature and maintaining this temperature.
• If instability develops, all cooling must be halted immediately and appropriate therapy initiated to address the current clinical situation.
The primary indications for such an intervention included ST-segment elevation myocardial infarction (STEMI) and new-onset left bundle branch block. Patients who demonstrate one of these findings on electrocardiography, either before or after arrest, are candidates for emergency coronary reperfusion. The majority of cases of primary cardiac arrest are associated with acute coronary syndromes, and it would seem reasonable to assume that emergency coronary reperfusion in patients with ROSC would be associated with improved outcomes. The AHA issued a policy statement in 201037 supporting the use of immediate catheterization in survivors of cardiac arrest who demonstrate electrocardiographic evidence of STEMI: “Patients resuscitated from [out-of-hospital cardiac arrest] with STEMI should undergo immediate angiography and receive PCI as needed.”
These two classic findings represent the primary indications for emergency coronary reperfusion. More recent investigation of this therapy has suggested that all patients suspected of having primary cardiac arrest can be considered for emergency coronary reperfusion. It is well known that the electrocardiogram is far from perfect in demonstrating evidence of AMI. Use of electrocardiography to determine which patients will benefit from emergency coronary reperfusion might potentially lead to some individuals missing out on such a benefit. The absence of ST-segment elevation after ROSC does not reliably rule out the presence of acute coronary artery occlusion.37,38 There has been increasing support in the literature39,40 for early coronary angiography in even these patients without ST-segment elevation.
The combination of TH and emergency coronary reper-fusion offers very promising results. This combination of postresuscitative therapy has demonstrated survival rates approaching 50% with intact or nearly intact functional status, and both PCI and TH can be safely performed simultaneously.41
Medical centers that are well equipped, aggressive, and experienced in the use of post-arrest interventions are more likely to demonstrate the best outcomes. It has been suggested that outcomes in victims of cardiac arrest are better at facilities that care for at least 50 such cases per year.37,42 Should postresuscitation care be regionalized? The Ontario Prehospital Advanced Life Support database demonstrates that transport time is not associated with outcome after cardiac arrest, thus suggesting that regionalization of this care might be appropriate.43
1 Neumar RW, Ward KR. Adult resuscitation. Marx JA, Hockberger RS, Walls RM, et al. Rosen’s emergency medicine: concepts and clinical practice, 6th ed, Philadelphia: Mosby, 2006.
2 Myerburg RJ, Castellanos A. Cardiac arrest and sudden cardiac death. Libby P, Bonow RO, Mann DL, et al. Libby: Braunwald’s heart disease: a textbook of cardiovascular medicine, 8th ed, Philadelphia: Saunders, 2007.
3 Stiell IG, Wells GA, Field B, et al. Advanced cardiac life support in out-of hospital cardiac arrest. N Engl J Med. 2004;351:647–656.
4 Larsen MP, Eisenberg MS, Cummins RO, et al. Predicting survival from out-of-hospital cardiac arrest: a graphic model. Ann Emerg Med. 1993;22:1652–1658.
5 Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S640–S656.
6 Wik L, Kramer-Johansen J, Myklebust H, et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005;293:299–304.
7 Wang HE, Simeone SJ, Weaver MD, et al. Interruptions in cardiopulmonary resuscitation from paramedic endotracheal intubation. Ann Emerg Med. 2009;54:645–652.
8 Bobrow BJ, Spaite DW. Do not pardon the interruption. Ann Emerg Med. 2009;54:653–655.
9 Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(suppl 3):S729–S767.
10 Iwami T, Kawamura T, Hiraide A, et al. Effectiveness of bystander-initiated cardiac-only resuscitation for patients with out-of-hospital cardiac arrest. Circulation. 2007;116:2900–2907.
11 Kellum MJ, Kennedy KW, Barney R, et al. Cardiocerebral resuscitation improves neurologically intact survival of patients with out-of-hospital cardiac arrest. Ann Emerg Med. 2008;52:244–252.
12 Ewy GA. Continuous-chest-compression cardiopulmonary resuscitation for cardiac arrest. Circulation. 2007;116:2894–2896.
13 Bobrow BJ, Clark LL, Ewy GA, et al. Minimally interrupted cardiac resuscitation by emergency medical services for out-of-hospital cardiac arrest. JAMA. 2008;299:1158–1165.
14 Simpson PM, Goodger MS, Bendall JC. Delayed versus immediate defibrillation for out-of-hospital cardiac arrest due to ventricular fibrillation: a systematic review and meta-analysis of randomized controlled trials. Resuscitation. 2010;81:925–931.
15 Hedges JR, Syverud SA, Dalsey WC, et al. Prehospital trial of emergency transcutaneous cardiac pacing. Circulation. 1987;76:1337–1343.
16 Cummins RO, Graves JR, Larsen MP, et al. Out-of-hospital transcutaneous pacing by emergency medical technicians in patients with asystolic cardiac arrest. N Engl J Med. 1993;328:1377–1382.
17 Valenzuela TD, Kern KB, Clark LL, et al. Interruptions of chest compressions during emergency medical systems resuscitation. Circulation. 2005;112:1259–1265.
18 Williamson K, Breed M, Brady WJ. The use of cardioactive medications in cardiac arrest. Emerg Med Clin North Am. 2012;30:65–75.
19 Brady WJ, Swart G, DeBehnke DJ, et al. The efficacy of atropine in the treatment of hemodynamically unstable bradycardia and atrioventricular block: prehospital and emergency department considerations. Resuscitation. 1999;41:47–55.
20 Swart G, Brady WJ, DeBehnke DJ, et al. Acute myocardial infarction complicated by hemodynamically unstable bradyarrhythmia: prehospital and emergency department treatment with atropine. Am J Emerg Med. 1999;17:647–652.
21 Bottiger BW, Arntz HR, Chamberlain DA, et al. Thrombolysis during resuscitation for out-of-hospital cardiac arrest. N Engl J Med. 2008;359:2651–2662.
22 Brady W, Meldon S, DeBehnke D. Comparison of prehospital monomorphic and polymorphic ventricular tachycardia: prevalence, response to therapy, and outcome. Ann Emerg Med. 1995;25:64–70.
23 Brady WJ, DeBehnke DJ, Laundrie D. Prevalence, therapeutic response, and outcome of ventricular tachycardia in the out-of-hospital setting: a comparison of monomorphic ventricular tachycardia, polymorphic ventricular tachycardia, and torsade de pointes. Acad Emerg Med. 1999;6:609–617.
24 Stiell IG, Hebert PC, Wells GA, et al. Vasopressin versus epinephrine for in-hospital cardiac arrest: a randomized controlled trial. Lancet. 2001;358:105–109.
25 Wenzel V, Krismer AC, Arntz HR, et al. A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. N Engl J Med. 2004;350:105–113.
26 Guyette FX, Guimond GE, Hostler D, et al. Vasopressin administered with epinephrine is associated with a return of a pulse in out-of-hospital cardiac arrest. Resuscitation. 2004;63:277–282.
27 Kudenchuck PJ, Cobb LA, Copass MK, et al. Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. N Engl J Med. 1999;341:871–878.
28 Dorian P, Cass D, Schwartz B, et al. Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation. N Engl J Med. 2002;346:884–890.
29 Mehta C, Brady WJ. Pulseless electrical activity in cardiac arrest: electrocardiographic presentations and management considerations based on the electrocardiogram. Am J Emerg Med. 2012;30:236–239.
30 Aufderheide TP, Thakur RK, Steuven HA, et al. Electrocardiographic characteristics in EMD. Resuscitation. 1989;17:183–193.
31 Rosemugy AS, Norris PA, Olson SM, et al. Prehospital traumatic cardiac arrest: the cost of futility. J Trauma. 1993;35:468–473.
32 ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112(24 suppl):IV1–203.
33 Van der Touw T, Mudiliar Y, Nayyar V. Cardiorespiratory effects of manually compressing the rib cage during tidal expiration in mechanically ventilated patients recovering from acute severe asthma. Crit Care Med. 1998;26:1361–1367.
34 Whitty JE. Maternal cardiac arrest in pregnancy. Obstet Gynecol. 2002;45:377–392.
35 Nolan JP, Neumar RW, Adrie C, et al. ILCOR consensus statement: post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. Resuscitation. 2008;79:350–379.
36 Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post–cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S768–S786.
37 Nichol G, Aufderheide TP, Eigel B, et al. Regional systems of care for out-of-hospital cardiac arrest: a policy statement from the American Heart Association. Circulation. 2010;121:709–729.
38 Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336:1629–1633.
39 Ewy GA, Kern KB. Recent advances in cardiopulmonary resuscitation: cardiocerebral resuscitation. J Am Coll Cardiol. 2009;53:149–157.
40 Reynolds JC, Callaway CW, El Khoudary SR, et al. Coronary angiography predicts improved outcome following cardiac arrest: propensity-adjusted analysis. J Int Care Med. 2009;24:179–186.
41 Batista LM, Lima FO, Januzzi JL, et al. Feasibility and safety of combined percutaneous coronary intervention and therapeutic hypothermia following cardiac arrest. Resuscitation. 2010;81:398–403.
42 Carr BG, Kahn JM, Merchange RM, et al. Inter-hospital variability in post-cardiac arrest mortality. Resuscitation. 2009;80:30–34.
43 Spaite DW, Stiell IG, Bobrow BJ, et al. Effect of transport interval on out-of-hospital cardiac arrest survival in the OPALS study: implications for triaging patients to specialized cardiac arrest centers. Ann Emerg Med. 2009;54:248–255.