Chapter 80 Management of acute poisoning
Acute poisoning remains one of the commonest medical emergencies, accounting for 5–10% of hospital medical admissions. Although in the majority of cases the drug ingestion is intentional, the in-hospital mortality remains low (< 0.5%).1 There are specific antidotes available for a small number of poisons and drugs; however, in most intoxications, basic supportive care is the main requirement and recovery will follow. This chapter is a hands-on guide to the general management of acute poisoning and drug intoxication. Larger reference books should be sourced, or internet-based information services (e.g. Toxbase – http://www.spib.axl.co.uk) referred to, if specific detail is required.
GENERAL PRINCIPLES
CLINICAL EXAMINATION
A standard clinical examination should be carried out, looking particularly for needle marks or evidence of previous self-harm. The Glasgow Coma Scale, although designed for head-injured patients, is frequently used. However, descriptive documentation of the degree of impaired consciousness is much more valuable. When patients are unconscious and no history is available, the diagnosis depends upon excluding other common causes of coma (Table 80.1) and consideration of any circumstantial evidence. Specific attention should be paid to the temperature, pupil size, respiratory and heart rate, as these may help to restrict the list of potential toxins (Table 80.2).
Table 80.2 Clinical effects of the common poisons
| Convulsions | Tricyclics, isoniazid, lithium, amfetamines, theophylline, carbon monoxide, phenothiazines, cocaine |
| Skin | |
| Bullae | Barbiturates, tricyclics |
| Sweating | Salicylates, organophosphates, amfetamines, cocaine |
| Pupils | |
| Constricted | Opioids, organophosphates |
| Dilated | Hypoxia, hypothermia, tricyclics, phenothiazines, anticholinergics |
| Temperature | |
| Pyrexia | Anticholinergics, tricyclics, salicylates, amfetamines, cocaine |
| Hypothermia | Barbiturates, alcohol, opioids |
| Cardiac rhythm | |
| Bradycardia | Digoxin, β-blockers, organophosphates |
| Tachycardia | Salicylates, theophylline, anticholinergics |
| Arrhythmias | Digoxin, phenothiazines, tricyclics, anticholinergics |
INVESTIGATIONS
Important initial investigations include:
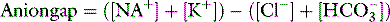 : It is normally 10–14. Ethanol, methanol, ethylene glycol, metformin, cyanide, isoniazid or salicylates are the most frequent causes a high anion gap metabolic acidosis in clinical toxicology.
: It is normally 10–14. Ethanol, methanol, ethylene glycol, metformin, cyanide, isoniazid or salicylates are the most frequent causes a high anion gap metabolic acidosis in clinical toxicology.GUT DECONTAMINATION
EMESIS
Ipecacuanha-induced emesis is no longer recommended for two reasons:2 first, it is ineffective at removing significant quantities of poisons from the stomach and, second, it limits the use of activated charcoal.
GASTRIC LAVAGE
Unless performed within 1 hour of drug ingestion, it is no longer recommended.3 The joint American and European toxicologists’ statement states that if performed after this time the amount of poison removed is insignificant, and lavage may only propel unabsorbed poison into the small intestine. However, this view has recently been questioned and is based on limited data.4 Prior intubation is essential when laryngeal competence is absent or doubtful, especially because, in the majority of overdoses, pulmonary aspiration is more lethal than the ingested drug.
ACTIVATED CHARCOAL
Activated charcoal (AC) remains the first-line treatment for most acute poisonings.5 Owing to its large surface area and porous structure it is highly effective at adsorbing many toxins with few exceptions. Exceptions include elemental metals, pesticides, strong acids and alkalis, and cyanide. It should be given to all patients who present within 1 hour of ingestion, although it is also acceptable to administer it after 1 hour if it follows an overdose of a substance that slows gastric emptying (e.g. opioids, tricyclic antidepressants). Because of international guidelines recommending administration of AC within 1 hour, it is vital to identify rapidly those who present after a potentially serious overdose so that it can be given swiftly.6
Repeated doses of AC can increase the elimination of some drugs by interrupting their entero-enteric and enterohepatic circulation. Indications for repeated dose AC are shown in Table 80.3.7 AC is given in 50 g doses for adults and 1 g/kg for children. It commonly causes vomiting; therefore consider giving an antiemetic prior to administration. Repeated doses are given at 4-hourly intervals.
Table 80.3 Drug intoxications where multiple-dose activated charcoal may be beneficial
WHOLE BOWEL IRRIGATION
This is a newer method of gastric decontamination that is indicated for a limited number of poisons.8 Whole bowel irrigation involves administration of non-absorbable polyethylene glycol solution to cause a liquid stool and reduce drug absorption by physically forcing contents rapidly through the gastrointestinal tract. Polyethylene glycol preparations are still occasionally used in surgical units for ‘bowel preparation’ prior to surgery. It may have arole in treating large ingestions of drugs that are not absorbed by AC. Indications include large ingestions of iron or lithium, ingestion of drug-filled packets/condoms (‘body packers’), and large ingestions of sustained-release or enteric-coated drugs (e.g. theophylline or calcium channel blockers). At present, efficacy is based on case reports alone.
ENHANCING DRUG ELIMINATION
URINARY ALKALINISATION
Intravenous sodium bicarbonate (approximately 1.26%) is infused to maintain a neutral balance and attempt to achieve a urine pH of approximately ≥ 7.5. The term ‘urine alkalinisation’ emphasises that urine pH manipulation is the prime objective of treatment; the terms ‘forced alkaline diuresis’ and ‘alkaline diuresis’ should be discontinued. According to international position statements it should be considered the treatment of choice for moderate to severe salicylate poisoning.9 Care must be taken to ensure the potassium does not fall rapidly.
SPECIFIC THERAPY OF SOME COMMON OR DIFFICULT OVERDOSES
AMPHETAMINES (INCLUDING ‘ECSTASY’, MDMA)
CLINICAL FEATURES
Symptoms of mild overdose include sweating, dry mouth and anxiety. Although the majority of ecstasy patients are dehydrated, a proportion have hyponatraemia from drinking water to excess. More severe features include hypertonia, hyperreflexia, hallucinations and hypertension. Supraventricular dysrhythmias may follow with coma, convulsions and the risk of haemorrhagic stroke. A hyperthermic syndrome may develop with hyperpyrexia leading to rhabdomyolysis, metabolic acidosis, acute renal failure, disseminated intravascular coagulation (DIC) and multiple organ failure.10
TREATMENT
AC should be considered up to 1 hour post ingestion. Benzodiazepines are useful for agitated or psychotic patients and may have a central effect in reducing tachycardia, hypertension and hyperpyrexia. If benzodiazepines fail to control hypertension, other classes of antihypertensives should be started, such as α-blockers, labetalol or direct vasodilators. Hypertonic saline may need to be considered in severe hyponatraemia. Hyperthermia should betreated in the standard manner including administration of cold fluids. Dantrolene has been used in the treatment of ecstasy-related hyperpyrexia; however, it has been suggested that it treats the effects and not the cause, and that it may be better to direct treatment at the central mechanisms of thermoregulation.11 Beyond this, there have been a few reports of the use of clozapine or olanzapine as a treatment for hyperpyrexia.
BENZODIAZEPINES
TREATMENT
AC can be given if patients present within 1 hour of ingestion, yet supportive treatment is usually all that is required. Flumazenil is a specific antagonist, but its brief duration of action limits its use to diagnostic purposes. Moreover, as flumazenil may cause other symptoms in patients who have ingested a cocktail of drugs (e.g. precipitation of fits in patients co-ingesting tricyclic antidepressants), its administration risks increasing morbidity and mortality.12
β-BLOCKERS
CLINICAL FEATURES
There is a wide variation in the individual response to β-blocker overdose. Those with cardiac disease are more at risk of complications. Hypotension and bradycardia predominate and are often refractory to standard resuscitation measures. The degree of heart block can range from a prolonged PR interval through to complete heart block and asystole. Cardiogenic shock and pulmonary oedema are not uncommon.13
TREATMENT
AC should be considered up to 1 hour post ingestion and multiple-dose charcoal in patients who have ingested sustained-release preparations. The role of atropine is not clear, although it is commonly used in patients who have bradycardia and hypotension. If symptomatic treatment fails, then other options such as glucagon (up to 10 mg i.v.), an adrenaline infusion or cardiac pacing are indicated. Although part of medical folklore, the evidence for glucagon is limited to animal studies.14 It often comes as a dried powder with a phenol diluent, so if used, the glucagons powder should be dissolved in 5% dextrose, since the phenol diluent in large amounts is a myocardial depressant.
CALCIUM CHANNEL BLOCKERS
TREATMENT
AC should be considered up to 1 hour post ingestion and multiple-dose charcoal in patients who have ingested sustained-release preparations. Treatment remains supportive; however i.v. calcium chloride is often given in patients who remain hypotensive despite fluid administration.15 Atropine is often used for bradycardia, and occasionally cardiac pacing may be necessary. In persistent low cardiac output states, phosphodiesterase inhibitors, levosimendan or high-dose insulin may need to be considered.16
CARBON MONOXIDE (CO)
TREATMENT
After basic resuscitative measures, high-flow oxygen (up to 100% if possible) should be administered and continued until the COHb level is less than 5%. This sometimes takes up to 24 hours. Hyperbaric oxygen (HBO), although often used, remains controversial. There have been eight randomised controlled trials of HBO versus normobaric oxygen. Unfortunately, many of these are of poor quality and it is not possible to show any statistical benefit of HBO in reducing neurological sequelae at 1 month.18 Considering the frequently encountered logistical difficulties in transferring an unconscious patient to an HBO centre, it cannot be recommended based on the data currently available.
CHLOROQUINE
TREATMENT
AC should be considered up to 1 hour post ingestion. Vasopressors may be required until hypotension is reversed, together with diazepam for agitated patients. Hypokalaemia is common and may be protective in the early stage. It is self-correcting and consequently aggressive potassium replacement is not recommended.19
COCAINE
CLINICAL FEATURES
Features of severe intoxication include hyperreflexia, drowsiness and convulsions.20 Severe hypertension may cause subarachnoid or intracerebral haemorrhage, and coronary artery spasm may result in myocardial infarction or ventricular arrhythmias; fatalities generally occur early. Hyperthermia associated with rhabdomyolysis, acute renal failure and DIC may also occur.
CYANIDE
TREATMENT
Inhaled amyl nitrate and 100% oxygen may well have been given at the scene, since it is present in ‘cyanide antidote kits’. Following this there are a number of options:
DIGOXIN
TREATMENT
AC should be considered up to 1 hour post ingestion and multiple-dose charcoal may be effective by interrupting enterohepatic recirculation of the drug. A digoxin serum level may be helpful although it does not equate to the total body burden. Hyper/hypokalaemia and hypomagnesaemia should be corrected. Cardiac pacing may be necessary to control symptomatic bradyarrhythmias and amiodarone may be useful for tachyarrhythmias. DC shock should be avoided if possible, but when essential must be initiated at low energy levels. Digoxin-specific antibodies are indicated in severe hyperkalaemia resistant to basic treatment, bradycardia resistant to atropine, or ventricular arrhythmias; repeated dosing is sometimes required in chronic toxicity as tissue redistribution of digoxin from deeper stores occurs.21
GAMMA-HYDROXYBUTYRIC ACID (GHB)
CLINICAL FEATURES
In moderate to high ingestion (> 50 mg/kg), coma, convulsions, bradycardia, hypotension and severe respiratory depression can be seen, and other CNS depressant drugs potentiate the effects. Most patients who present to hospital merely require supportive care, and even those who present in coma are often awake within 2–4 h; very few require intensive care.22
METHANOL AND ETHYLENE GLYCOL
CLINICAL FEATURES
Methanol and ethylene glycol are relatively non-toxic, but their ingestion is a medical emergency, because of their metabolism (following a latent period of 12–18 hours) to formic acid and glycolic acid respectively. These toxic metabolites account for the metabolic acidosis, ocular toxicity, renal failure and mortality that are occasionally seen. Mild features include dizziness, drowsiness and abdominal pain. When treatment is delayed, metabolicacidosis develops with drowsiness, coma and convulsions. The osmolal and the anion gap are increased.
TREATMENT
AC does not adsorb either methanol or ethylene glycol. The metabolic acidosis should be treated with sodium bicarbonate and the serum electrolytes measured. Ethanol prevents formation of the toxic metabolites and previously has been the most established treatment. However, ethanol dosing is complex, requires repeated monitoring and has adverse effects. The recent introduction of fomepizole (4-methypyrazole), although expensive, has significantly simplified the treatment of ethylene glycol and methanol poisoning, and is now strongly recommended as first-line therapy in some national guidelines.23,24 It has been shown to be safe and effective, with minimal adverse effects,25 and potentially prevents the need for haemodialysis in patients presenting with visual disturbances or severe acidosis.26 If the ethylene glycol blood concentration is > 20 mg/dl or if there is a good history of ethylene glycol/methanol ingestion with an osmolal gap > 10, then patients should receive a loading dose of 15 mg/kg, followed by 10 mg/kg every 12 hours for 48 hours.
Folinic acid should also be given since it enhances the metabolism of formic acid.
NON-STEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDs)
CLINICAL FEATURES
NSAIDs are commonly ingested in overdose. Fortunately, however, serious problems, other than a bad attack of gastritis, are rare. Exceptions to this rule include mefenamic acid or large ingestions of ibuprofen where self-limiting convulsions and renal failure have been reported.27 Patients who do not develop symptoms within 4 hours are unlikely to experience delayed toxicity.
PARACETAMOL
In normal adults, doses > 10 g may exceed the ability of hepatic glutathione to conjugate the toxic metabolite. Plasma concentrations > 200 mg/l at 4 hours or ≥ 50 mg/l at 12 hours (Figure 80.1) are usually associated with hepatic damage. Treatment should begin at lower levels for those considered to be high risk (see Table 80.4). While i.v. acetylcysteine administered more than 16 hours after ingestion may not prevent severe liver damage, it should still be given since outcome from paracetamol-induced fulminant hepatic failure is improved.28 Although severe hepatic injury has a 10% mortality, the majority of patients recover within 1–2 weeks.
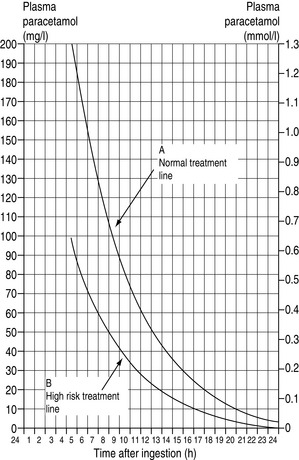
Figure 80.1 Treatment lines following paracetamol overdose in relation to time after ingestion and plasma levels.
(Figure from Paracetamol Information Centre, London and the Welsh National Poisons Unit, Cardiff.)
Table 80.4 Those at high risk of liver damage following paracetamol overdose
TREATMENT
PARAQUAT
In adult humans the lethal dose is 3–6 g (i.e. 15–30 ml of 20% w/v liquid concentrate). The mortality rate in patients ingesting the liquid concentrate is about 45%.30 The lung is the primary target organ, with the injury being enhanced by oxygen. Peak concentrations are achieved between 0.5 and 2.0 hours.
SALICYLATES (ASPIRIN)
TREATMENT
Multiple-dose AC may be effective but is not established. Vitamin K and glucose are used to correct hypoprothrombinaemia and hypoglycaemia. Urinary alkalinisation (see above) will decrease the amount of non-ionised drug available to enter tissues, but is hazardous and should only be used for the most severely ill patients. Extracorporeal techniques are very effective in removing salicylates and correcting acid–base disturbance. Although indications for their use are yet to be defined, they should be considered for severe cases.31
SELECTIVE SEROTONIN REUPTAKE INHIBITORS (SSRIs) or 5-HT Drugs
Drugs include citalopram, fluoxetine, fluvoxamine, paroxetine and sertraline. These drugs have increasinglyreplaced the tricyclic antidepressants and generally appear to be much safer in overdose.
THEOPHYLLINE
CLINICAL FEATURES
Acute theophylline poisoning is potentially very serious and severe poisoning carries a high mortality. Toxic effects such as agitation, tremor, nausea, vomiting and sinus tachycardia become evident at < 30 mg/l (167 μmol/l). Concentrations > 60 mg/l (333 μmol/l) in acute poisoning or > 40 mg/l (222 μmol/l) in chronic usage frequently result in seizures, malignant ventricular arrhythmias, severe hypotension and death.34 A key feature is hypokalaemia, which predisposes to arrhythmias and rhabdomyolysis. Measuring plasma theophylline levels confirms the ingestion and may help in deciding elimination methods; in the majority of poisoned patients they do not aid management. Sustained-release preparations may result in delayed onset and prolonged toxicity.
TRICYCLIC ANTIDEPRESSANTS (TCAs)
CLINICAL FEATURES
These drugs remain the leading cause of death from overdose in patients arriving at the emergency department alive and account for up to one half of all overdose-related adult intensive care admissions.35 Features include anticholinergic effects such as warm dry skin, tachycardia, blurred vision, dilated pupils and urinary retention. Severe features include respiratory depression, reduced conscious level, cardiac arrhythmias, fits and hypotension. Arrhythmias may be predicted by a QRS duration > 100 ms on the ECG; a QRS duration of > 160 ms increases risk of seizures.36 All forms of rhythm and conduction disturbance have been described, and are not necessarily predicted by the ECG.37 Amoxapine typically causes features of severe poisoning in the absence of QRS widening. Cardiac toxicity is due mainly to quinidine-like actions, slowing phase 0 depolarisation of the action potential. Other mechanisms include impaired automaticity, cholinergic blockade and inhibition of neuronal catecholamine uptake. Toxicity is worsened by acidaemia, hypotension and hyperthermia.
TREATMENT
After supportive care as outlined above, including multiple-dose AC, continuous cardiac monitoring is essential. Increasing arterial pH to ≥ 7.45 significantly reduces the available free drug and this may be the best way to avoid TCA toxicity. Mild hyperventilation and 8.4% sodium bicarbonate in 50 mmol aliquots achieves this strategy and may improve outcome.38 Bicarbonate should probably be given in all cases of QRS prolongation (even in the absence of metabolic acidosis), malignant arrhythmias, hypotension or metabolic acidosis. If arrhythmias occur, avoid Class 1a agents; lidocaine may be best. Benzodiazepines are the drug of choice for sedation, treatment of seizures and may prevent emergence delirium.
1 Gunnell D, Ho D, Murray V. Medical management of deliberate drug overdose: a neglected area for suicide prevention? Emerg Med J. 2004;21:35-38.
2 American Academy of Clinical Toxicology; European Association of Poison Control Centres and Clinical Toxicologists. Position statement: ipecac syrup. Clin Toxicol. 1997;35:699-709.
3 American Academy of Clinical Toxicology; European Association of Poison Control Centres and Clinical Toxicologists. Position statement: gastric lavage. Clin Toxicol. 1997;35:711-719.
4 Eddleston M, Juszczak E, Buckley N. Does gastric lavage really push poison beyond the pylorus? A systematic review of the evidence. Ann Emerg Med. 2003;42:359-364.
5 Chyka PA, Seger D, Krenzelok EP, et al. American Academy of Clinical Toxicology; European Associationof Poisons Centres and Clinical Toxicologists. Position paper: single-dose activated charcoal. Clin Toxicol. 2005;43:61-87.
6 Karim A, Ivatts S, Dargan P, Jones A. How feasible is it to conform to the European guidelines on administration of activated charcoal within one hour of an overdose? Emerg Med J. 2001;18:390-392.
7 American Academy of Clinical Toxicology; European Association of Poison Control Centres and Clinical Toxicologists. Position statement and practice guidelines on the use of multi-dose activated charcoal in the treatment of acute poisoning. Clin Toxicol. 1999;37:731-751.
8 American Academy of Clinical Toxicology; European Association of Poison Control Centres and Clinical Toxicologists. Position statement: whole bowel irrigation. Clin Toxicol. 1997;35:753-762.
9 Proudfoot AT, Krenzelok EP, Vale JA. Position paper on urine alkalinisation. J Toxicol Clin Toxicol. 2004;42:1-26.
10 Hall AP, Henry JA. Acute toxic effects of ‘Ecstasy’ (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth. 2006;96:678-685.
11 Padkin A. Treating MDMA (‘Ecstasy’) toxicity. Anaesthesia. 1994;49:259.
12 Seger DL. Flumazenil – treatment or toxin? J Toxicol Clin Toxicol. 2004;42:209-216.
13 Shepherd G. Treatment of poisoning caused by beta-adrenergic and calcium-channel blockers. Am J Health Syst Pharm. 2006;63:1828-1835.
14 Bailey B. Glucagon in beta-blocker and calcium channel blocker overdoses: a systematic review. J Toxicol Clin Toxicol. 2003;41:595-602.
15 DeWitt CR, Waksman JC. Pharmacology, pathophysiology and management of calcium channel blocker and beta-blocker toxicity. Toxicol Rev. 2004;23:223-238.
16 Megarbane B, Karyo S, Baud FJ. The role of insulin and glucose (hyperinsulinaemia/euglycaemia) therapy in acute calcium channel antagonist and beta-blocker poisoning. Toxicol Rev. 2004;23:215-222.
17 MacNab A, Anderson E, Susak K. Ingestion of cannabis: a cause of coma in children. Pediatr Emerg Care. 1989;5:238-239.
18 Buckley NA, Isbister GK, Stokes B, et al. Hyperbaric oxygen for carbon monoxide poisoning: a systematic review and critical analysis of the evidence. Toxicol Rev. 2005;24:75-92.
19 Jaeger A, Sauder P, Kopferschmitt J, et al. Clinical features and management of poisoning due to antimalarial drugs. Med Toxicol Adverse Drug Exp. 1987;2:242-273.
20 Paul S, York D. Cocaine abuse: an expanding healthcare problem for the 1990s. Am J Crit Care. 1992;1:109-113.
21 Bateman DN. Digoxin-specific antibody fragments: how much and when? Toxicol Rev. 2004;23:135-143.
22 Liechti ME, Kunz I, Greminger P, et al. Clinical features of gamma-hydroxybutyrate and gamma-butyrolactone toxicity and concomitant drug and alcohol. Drug Alcohol Depend. 2006;81:323-326.
23 Barceloux DG, Krenzelok EP, Olson K, et al. American Academy of Clinical Toxicology practice guidelines on the methanol poisoning. J Toxicol Clin Toxicol. 1999;37:537-560.
24 Barceloux DG, Bond GR, Krenzelok EP, et al. American Academy of Clinical Toxicology practice guidelines on the methanol poisoning. J Toxicol Clin Toxicol. 2002;40:415-446.
25 Brent J, McMartin K, Phillips S, et al. Fomepizole for the treatment of methanol poisoning. N Engl J Med. 2001;344:424-429.
26 Megarbane B, Borron SW, Trout H, et al. Treatment of acute methanol poisoning with fomepizole. Intensive Care Med. 2001;27:1370-1378.
27 Smolinske SC, Hall AH, Vandenberg SA. Toxic effects of nonsteroidal anti-inflammatory drugs in overdose. Drug Safety. 1990;5:252-274.
28 Keays R, Harrison PM, Wendon JA, et al. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled study. N Engl J Med. 1991;303:1026-1029.
29 Spiller HA, Winter ML, Klein-Schwartz W, et al. Efficacy of activated charcoal administered more than four hours after acetaminophen overdose. J Emerg Med. 2006;30:1-5.
30 Hwang KY, Lee EY, Hong SY. Paraquat intoxication in Korea. Arch Environ Health. 2002;57:162-166.
31 Dargan PI, Wallace CI, Jones AL. An evidenced based flowchart to guide the management of acute salicylate (aspirin) overdose. Emerg Med J. 2002;19:206-209.
32 Isbister GK, Bowe SJ, Dawson A, et al. Relative toxicity of selective serotonin reuptake inhibitors in overdose. J Toxicol Clin Toxicol. 2004;42:277-285.
33 McDaniel WW. Serotonin syndrome: early management with cyproheptadine. Ann Phamacother. 2001;35:870-873.
34 Shannon M. Predictors of major toxicity after theophylline overdose. Ann Intern Med. 1993;119:1161-1167.
35 Newton EH, Shih RD, Hoffman RS. Cyclic antidepressant overdose: a review of current management strategies. Am J Emerg Med. 1994;12:376-379.
36 Boehert MT, Lovejoy FH. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Eng J Med. 1985;313:474-479.
37 Harrigan RA, Brady WJ. ECG abnormalities in tricyclic antidepressant ingestion. Am J Emerg Med. 1999;17:387-393.
38 Hoffman JR, Votey SR, Bayer M, et al. Effect of hypertonic sodium bicarbonate in the treatment of moderate-to-severe cyclic antidepressant overdose. Am J Emerg Med. 1993;11:336-341.







