CHAPTER 240 Management of Acute Peripheral Nerve Injuries
It is important to have at least basic knowledge about the pertinent mechanisms of nerve degeneration and regeneration, with an emphasis on basic mechanisms relevant for trauma. The degree of nerve damage determines the potential for spontaneous recovery. The mechanism and type of injury, as well as objective clinical findings, play a major role in assessment. The better the combined information from the history and clinical examination, the easier it will be to assess the trauma and the level and depth of nerve injury. Electrophysiologic investigation, or nerve potential investigation (NPI) before and during surgery is a precious adjunct in the evaluation of potential for recovery. We emphasize the pivotal role of timing for satisfactory outcome. The advantage of urgent repair and the detrimental effect of neglected repair are clear. Unfortunately, timing and the chance for early exploration and repair are not always in the hands of the surgeon. This is most obvious in a subset of nerve injuries: those that are iatrogenic (see Chapter 246).
Pathophysiologic Aspects of Nerve Trauma
Nerve Architecture
Most peripheral nerves are mixed and contain motor, sensory, nociceptive, and autonomic fibers.1 The autonomic fibers are responsible for sudomotor control, vasoreactivity, and piloerection. The main constituent of a peripheral nerve is connective tissue, which can vary from 85% in the sciatic nerve at hip level to about 25% for the peroneal nerve at fibular level.2 The external sheath, the epineurium, is constituted from connective tissue with an amount of collagen and elastic fiber; it contains abundant blood vessels and protects the nerve against compression. The endoneurium provides connective tissue support for the nerve fibers. A certain degree of fiber undulation enables a physiologic amount of nerve stretch.3,4 Nerve elongation below 12% of its resting length can be compensated for without functional damage, but venous flow is already blocked when a nerve is stretched by 8%, and a stretch of 16% produces ischemia.5 Attached to the epineurium is another capillarized outer gliding layer, by some referred to as the mesoneurium or paraneurium, that is traversed by vascular channels supplying the nerve. This mesoneurium also allows the nerve to glide between tissue planes during physiologic movement.6–8 It is important to restore and retain gliding of injured nerve in order to prevent its tethering and entrapment.4,9 The epineurium includes the internal epineurium or interfascicular epineurium, which ensheathes the fascicles. This is less compact than the external epineurium. Directly around each fascicle is the perineurium, which is constituted by perineurial cells in between circular, oblique, and longitudinal strands of collagen fibers. The perineurium is responsible for maintaining the physiologic milieu of the conducting elements, and it acts as a diffusion or blood-nerve barrier. Breaching the perineurium interferes with conduction and provokes demyelination of the contained nerve fibers.10 The role of perineurial fibroblasts in trauma appears to be underestimated because they initiate and maintain the connective tissue reaction and as such contribute to neuroma development.11–13 Neuroma formation should be regarded as nondirected and a futile attempt at regeneration, where axons do not manage to reach the appropriate distal basal lamina and cannot reconnect to their natural target organ.
The vascular supply of peripheral nerves is abundant. At regular intervals, collateral vessels enter through the mesoneurium to connect to the nerve’s central artery.14 If a nerve is circumferentially mobilized, several of these “segmental” arteries are disconnected, and the central vessel maintains the blood supply.10 It is therefore possible to circumferentially mobilize a nerve over a relatively long stretch without putting the nerve at ischemic risk.10 Some of these extrinsic pedicles sustain vascularized nerve grafts, as in the case of the ulnar nerve.15,16 Nerves, however, differ extensively in their vascular supply; the tibial, for example, is more richly supplied than the common peroneal nerve at knee level.17
Functional Segregation
The fascicles within a nerve form a plexus. The fascicles cross-connect and do not run like copper cables aligned longitudinally from proximal to distal but rather exchange nerve fibers with other fascicles. This exchange and cross-connection of fascicles is more predominant proximally and decreases in a distal direction.2 Plexus formation fades toward the periphery. This fact was used to explain why interfascicular repair could not restore the original anatomy, and even less so the more proximal the lesion is. However, this exchange of fascicles acts as additional supply, and Sunderland demonstrated that the nerve fibers are topographically segregated according to function. There is functional segregation of bundles even proximally. The microneurographic findings of Schady and associates on median nerves supported that the intraneural fascicular plexus acts “as a safety mechanism allowing for nondermatomal overlap at proximal level.”18
Relevant Aspects of Degeneration
Any persistent disruption of the axonal transport results in a wallerian type of degeneration,19 and it is important to keep in mind that this also applies for compressive lesions. In terms of classification, disruption of axonal transport at least implies axonotmesis (Seddon)20 or a type 2 Sunderland lesion.21 The gravest form of nerve injury is a complete transection, a neurotmesis (Seddon) or Sunderland type 5 lesion. The “visible” wallerian degeneration as such is propagated through several nodes of Ranvier in a centripetal direction and all the way to the end organ in a centrifugal direction. Distal to the neuroma, the axons thus completely degenerate, leaving only “empty” endoneural sleeves with basal laminar structures. The nature of this process described by Waller in 1850 (on cranial nerves in frogs!), however, extends well beyond such a peripheral level and affects the cell body, Schwann cell envelope, and myelin sheath, as well as endoneurial cells, and ultimately the end organs.22 Neurapraxia implies only a focal conduction block. Conduction distal to the lesion is maintained. If there is no conduction distal to the injured segment the lesion is degenerative. The senior author (RB) and his colleagues found that the interval between injury and failure of distal neuromuscular conduction ranges from 48 to 160 hours.
Isolated injury of the perineurium ignites a cascade that can end in axon loss.23 The effect of a perineurial window is herniation and subsequent demyelination and degeneration of fibers. Blood vessels course through the perineurium, and their tight junctions constitute the blood-nerve barrier.
A nondegenerative lesion (conduction block, neurapraxia) is unlikely, when a nerve palsy is complete (which includes vasomotor and sudomotor paralysis), when there is neuropathic pain, and when there is a strong Hoffmann-Tinel sign24–27 at the level of the lesion that indicates the rupture of axons. Tinel made a clear distinction between this sign, evoked after traumatic neuropathy, and the hypersensitivity of the nerve trunk in nontraumatic compressive neuropathies (“neuralgia”). A strongly positive Hoffmann-Tinel sign indicates rupture of axons and can be found on the day of injury. Regeneration of axons can be confirmed and followed when a centrifugally moving Hoffmann-Tinel sign is persistently stronger than at the suture line. In failed repair, the Hoffmann-Tinel sign at the suture line remains stronger than at the growing point. Absent distal progression indicates failure of regeneration. After axonotmesis, the Hoffmann-Tinel sign advances faster than it does after nerve repair (about 2 mm per day).
Nonetheless, an advancing Hoffman-Tinel sign, because it only indicates the presence of fine fibers, does not ensure useful recovery. When tested for several months after injury, however, absence of the sign is an important negative finding. In a critical early report that addressed this issue, 50% of soldiers who had an advancing Tinel sign all the way to the hand never had useful median nerve recovery after elbow-level gunshot wound injuries to the nerve.28
Relevant Aspects of Regeneration
Axons begin to sprout from the growth cone.29 The axon tips follow the preceding bands of Büngner,30 which are processes of Schwann cells.31 A basal lamina serves as a guiding matrix and track. Axons grow with an approximate speed of 1 mm per day; in proximity to the cord, axons sprout at a faster pace of 2 to 3 mm per day. In addition, however, there is delay until the growth starts, delay at suture lines, distal delay due to diminished growth pace, and terminal delay, when axons are progressively myelinized and reach the distal areas of innervation.
The pivotal question is, therefore, when such a point of no return will be reached. As a rule of thumb, muscle that has been denervated for 18 months is thought to be irreversibly degenerated,32,33 but a precise answer cannot be given. This is why Brushart expressed that ideal reinnervation could be expected before 1 to 3 months of degeneration, functional reinnervation for up to 1 year, and no reinnervation after 3 years.34 These restrictions apply for motor nerves, however, because the reaction of muscle spindles and of cutaneous sensory organs is slower.
Timing of Operative Therapy
The Schwann cells, the skin, and other target organs are rich sources of neurotrophins that are essential for the development, maturation, and maintenance of the cell bodies. Transection of a peripheral nerve leads to central cell death by deprivation of neurotrophins (i.e., nerve growth factor). Progressive neuronal death, ischemia, and fibrous proliferation are important limiting factors for useful recovery.35 There is extensive evidence for progressive neuronal death to occur after a critical time window has passed. Sensory and motor neurons appear to differ in the onset of such cell loss. There is also evidence that early nerve repairs can stop this process of neuron loss.36 Early repair improves the reconstructed nerve’s regenerative capacity. The efficacy of axonal regeneration is significantly affected by the amount of cell loss already present at the time of repair.37–40 Central changes after peripheral nerve injury are more extreme in more proximal, more extensive, and more violent injuries and thus are an important factor in prognosis after repair.22,41
When a nerve lesion is deep enough to interrupt the axons and to separate them from the cell nucleus, wallerian degeneration19 (anterograde degeneration) is the consequence. This is not a consequence exclusively of complete transection but also of profound crush or severe traction, as happens in violent blunt injuries. When there is no regeneration of axons into the distal stump, changes will occur in the target organ that over time become irreversible:42,43 Motor end plates disappear, and the denervated muscle becomes fibrosed. Studies of central and peripheral conduction are of inestimable value in the analysis of injuries to the brachial plexus when these can be operated within 60 hours of the injury. This implies that injured nerves should be explored and repaired as soon as possible, unless there are realistic chances that the lesion bears enough potential for spontaneous regeneration to a functional level. In those cases, observation is justified. However, a favorable lesion might also progress to a less favorable one if the original cause is not addressed. Recovery is likely for the nerve accidently encircled by a suture or crushed under a plate if the cause is urgently removed. If that cause remains for days or weeks, a much more unfavorable lesion develops. One also cannot overemphasize the significance of the worsening pain and the deepening of nerve lesion caused by expanding hematoma or ischemia. The vulnerability of the lumbosacral plexus to expanding hematoma was discussed by Donaghy.44 Nerves crushed in a swollen ischemic limb or in a tense compartment progress from conduction block to much deeper and much less favorable degenerative lesions.
Some crucial clinical insights regarding timing have been known for a long time. In 1908, Sherren examined 50 cases of acute suture performed at the London Hospital.44a Pain sensibility recovered before touch sensibility; tactile localization continued to improve for more than 2 years. Recovery of power was rather slower. There was no recovery in only one patient, whose wound had become infected. Sherren recommended primary suture “because the prognosis after secondary suture is more unfavourable.”44b In 1924, Platt and Bristow reviewed the late results of nerve injuries treated in the First World War and noted the “extreme perfection attained after so-called primary suture,” acknowledging that the more severe gun shot wounds of nerves were treated by secondary suture.44c In 1937, Platt and Bristow Platt wrote, “in primary sutures performed under ideal conditions, complete recovery of motor power and recovery of protopathic sensibility at least, is to be expected … however, in more extensive wounds with widespread bruising and multiple tendon injuries, and in wounds in which infection had already secured a hold, partial or complete failure after primary suture is almost inevitable.”44d Nine years later, Zachary and Holmes compared 55 cases of primary sutures of nerves referred to the Peripheral Nerve Injury Centre at the Wingfield-Morris Hospital, Oxford, England, during the years 1940 to 1944, with 36 “early” secondary sutures. The results of secondary suture were distinctly better. The primary repair was resected and the nerve resutured in 16 patients. Histologic examination of the resected material revealed a number of causes for the failure of the first operation: poor matching of the proximal and distal stumps; coarse suture material lodged between the stumps; separation of the stumps; and dense scar between the stumps or within the distal stump. Zachary and Holmes concluded, “Formal nerve suture should be undertaken at the earliest moment when it is possible to recognise the extent of damage to the nerve, excise the injured segment, and bring together the mobilised nerve ends without the prospect of undue post operative tension.” These early contributions came at a time when antibiotics were unavailable and when grafting of nerves was rarely performed in Great Britain.
Glasby used the freeze-thawed muscle graft (FTMG) in a series of experiments that provided evidence about the factors governing the quality of regeneration after nerve repair.45 The findings were decisive. Regeneration was worsened by delay and also by association with arterial injury, fractures, or cavitation and hematoma.46,47
Improved results after urgent repair have been reported for the median and ulnar nerves,48–51 the radial nerve,52 the musculocutaneous nerve,53 the sciatic nerve,54 the common peroneal nerve,55,56 and the closed traction lesion of the brachial plexus.51,57 Kato and coworkers51 showed that recovery of function and relief of pain were decisively better in urgent repairs of the closed traction lesion of the brachial plexus. The spinal accessory nerve appears to be the one exception to the rule because it exhibits good recovery of function even after substantially delayed repairs. Uncomplicated open wounds and nerves can be left for 24 hours until an experienced surgeon can address them. When, however, a nerve injury is associated with damage to a major blood vessel, with an impending threat of peripheral ischemia, or with increasing pressure within a fascial compartment, delay is not permissible. The case is an emergency, as it is with cases of open fracture or fracture-dislocation.
The primary limitation of very early repair is that there is a significant risk that even a very experienced surgeon will be unable to determine how much of the proximal and distal stump must be resected. Leaving in place any length of nerve that is irretrievably damaged is often the cause of failed repairs—such as those observed by Zachary and Holmes that had dense scar between the stumps. Because of this, when there is any component of blunt injury involved, a delay of a few weeks may be necessary.10 On the other hand, urgent operation enables detection of rupture of the perineurium, and studies of proximal and distal conduction ease the difficulties relating to the appropriate level of section.
The World War II experience with the urgent suture of nerves was dismal.58,59 The results of delayed repair, in special units, were surprisingly good, and they fell a little short of modern series.59A Urgent repair of nerves injured in current conflicts show promise.66A
Classifications and Grading
Seddon distinguished among three types of nerve injury in his 1943 classification20:
Sunderland’s classification system, which was introduced in 1951, is more elaborate and recognizes five degrees of severity.60 A neurapraxic lesion is the equivalent to a Sunderland type I lesion. In principle, lesions with axonolysis (as equivalent to Seddon’s axonotmesis) are further differentiated between those that have an intact endoneurium (Sunderland type II) and those that have not (Sunderland type III). A type IV lesion denotes complete disruption of the contents of the epineurium, both connective tissue elements and axons, but with the nerve still in continuity, and a type V lesion is a severed or pulled-apart nerve that is not in continuity.
The question remains of what way classifications can actually really depict the clinical picture and as such have relevance for clinical decision making. In theory, falling in the continuum between need to graft a lesion and leaving the lesion alone after decompression and external neurolysis is a Sunderland type III lesion, in which there is considerable internal fibrosis that effectively precludes that enough sprouting axons overcome this soft tissue barrier to sprout distally in an orderly fashion. Some patients with Sunderland type III lesions recover, and some do not. This is why nerve action potential (NAP) recording is valuable for type II, III, and IV lesions. In this Sunderland classification threshold situation, there is axonolysis, the endoneurium is destroyed and fibrotic,61 and the perineurium theoretically could still be intact—and yet such a lesion has a poor chance for recovery without grafts.
It helps clinical decision making with regard to surgery to simplify and distinguish a nondegenerative (focal conduction block) from a degenerative nerve62,63 lesion with axonal division and ongoing wallerian degeneration (axonal discontinuity in all its forms: axonotmesis and neurotmesis, or the Sunderland equivalent spectrum from types II to V).64
Appearance and forms of Traumatic Nerve Injury
Inflicted injuries are either open or closed. In open injuries, we differentiate tidy from untidy wounds. Closed injuries are predominantly due to traction. The rate of associated arterial injury is high in traction, in penetrating missile wounds, and of course in open injuries by knife. Etiology of acute peripheral nerve injury includes penetrating causes, crush, traction, and ischemia, with thermal and electrical lesions being rare. The conceivable mechanisms are by knife and gunshot, which usually create open wounds, and combinations of compression, contusion with stretch, and traction, which more often cause closed lesions. Lacerations are inflicted by glass, knife, fan, saw blade, auto parts, fractured bone (Fig. 240-1), and more.
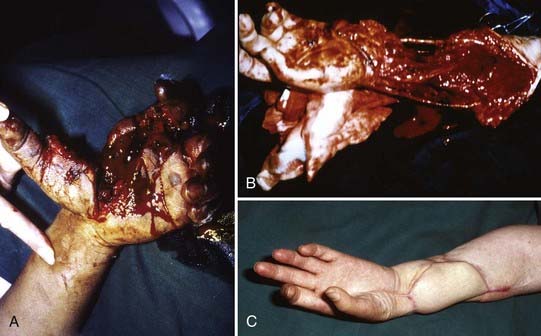
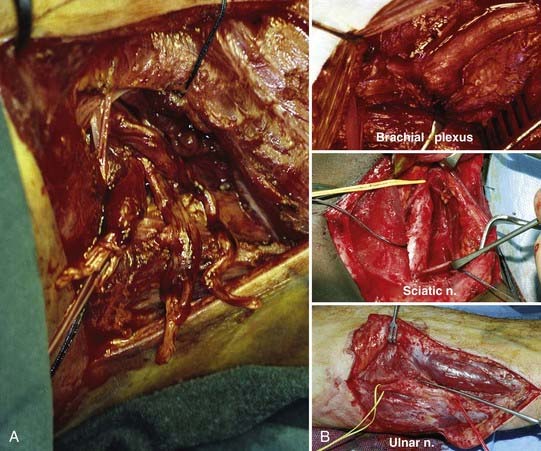
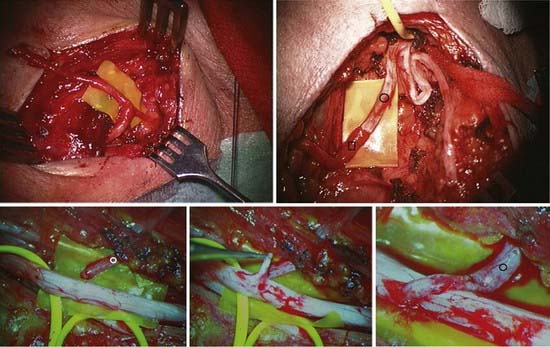
FIGURE 240-1 Blunt radial nerve trunk (RN) transection below triceps branch level2,3 due to humerus fracture. Patient was referred late, after wrist and hand extension never returned despite completely recovered triceps function. A, Proximal stump of radial nerve encircled, already removed plate held up against completely healed humerus; distal, badly disrupted stump encircled in upper yellow vessel loop tunneled out on ventral side of arm, where distal part of radial nerve was exposed through extra incision in order to follow nerve from distal to proximal. B, Close-up view showing main radial nerve trunk (RN) with functional triceps branches, and frayed, widespread distal stump. C, Radiographs displaying fresh humerus fracture, before (left) and after (right) plating.
The nerve injuries of war or civil conflict are associated with vascular injuries in at least one third of cases, and comminuted fractures of the long bones are frequent. There may be massive destruction of muscle and skin with associated visceral injury.65,66,66A
Iatrogenic nerve lesions are a special and frequent entity.67–69 The operated iatrogenic lesions at the University of Ulm/BKH Günzburg were 94% of the time inflicted during surgery.70,71 One fifth to one fourth of our operated nerve trauma cases are iatropathic in nature67–70 Unfortunately, delayed referral is predominant.
The exact time course and longitudinal expansion of neuromas in humans are still a somewhat nebulous matter, especially with regard to lesions in continuity. Successful replantations (Fig. 240-2) gave clinical clues that the process of “neuroma expansion” cannot take too long because otherwise there would never be recovery of nerve function in such a setting of primary repair where nerve ends are trimmed and cut back to viable-looking structures, only for several millimeters.72
A nerve can be surrounded by scar tissue, and the scar tissue can be continuous to within the nerve, or the scar tissue can be almost exclusively within the nerve, contained within the epineurium. Obviously, therefore, a nerve lesion in continuity can be a greater diagnostic challenge and sometimes at surgical exploration can even look rather normal from the outside: the epineurium may be intact, yet internally all the fascicles of the segment might be completely replaced by fibrous neuroma tissue. Fortunately, the firmness and segmental distention of a neuroma-in-continuity often leave no doubt about the unchangeable completeness of the lesion. Therefore, inspection and palpation give decisive clues at surgical exploration and are complemented by an intraoperative electrophysiologic examination to rule out ongoing substantial spontaneous recovery across the lesion or across part of the lesion.10
Delayed nerve repair worsens and sometimes precludes the chances for good functional outcome. However, one does not want to operate on lesions that might have recovered spontaneously. A distal focal lesion that does not substantially recover after 6 to 8 weeks will not fare better after 5 to 6 months, but the outcome after surgery will be worse. Intraoperative nerve action potentials (see Chapter 239) yield reliable information about focal lesions in continuity at any time after injury, if caused by fracture, gunshot, contusion, or stretch. To generate and conduct such a nonsummated compound nerve action potential across a lesion, several thousand functioning fibers greater than 6 µm are required (in contrast to neuromatous minifascicles, which are not sufficient to reconstitute function).73 In cases in which a nerve action potential could be recorded across a lesion, Kline and colleagues (1973) found a 93% rate of functional recovery in a very large series of patients in continuity. It is important to avoid delay, and the prognosis for the sciatic trunk or its divisions injured by fractures or dislocations of the lower limb is so poor that the clinician should have cogent reasons for not exploring the nerve.
Closed Traction Injury
If complicated by an arterial lesion, this group of injuries has an unfavorable prognosis.
The most common type of civilian nerve trauma most likely is a stretch-related injury in context with a motor vehicle crash.66,74,75 Three percent (2.8%) of a Canadian trauma population of 5777 patients treated between 1986 and 1996 sustained associated peripheral nerve injuries (162 of 5777 patients).74 Motor vehicle crashes were the predominant cause (46%). Eighty-three percent of the patients were male, and the mean age was 34.6 years. Seventy-five percent affected the upper extremities (121 of 162 injuries). The radial nerve was the most frequently injured nerve, with 36% (58 of 162 injuries), and the peroneal nerve was the most frequently injured lower limb nerve with 24% (39 of 162 injuries). Surgery was required in 54%. Brachial plexus injuries were identified in 1.2% of multitrauma patients presenting to the same major regional trauma facility (54 of 4538 patients seen at Sunnybrook Hospital, Toronto, Canada).75
Open Injury with Transection
In contrast, blunt injuries (![]() Web Fig. 240-1), commonly caused by open fractures or penetrating missile injuries, are better approached with a delayed end-to-end suture. There is extensive tissue damage with a high risk for sepsis. Retraction of stumps is usually performed. If possible, it is helpful to gently approximate nerve stumps to each other or to adjacent structures at the first operation. The nerve stumps are readapted after resection of the stump neuromas within a 2- to 3-week interval. This form of intentionally delayed repair due to unfavorable soft tissue condition at the time of injury is termed delayed end-to-end suture.
Web Fig. 240-1), commonly caused by open fractures or penetrating missile injuries, are better approached with a delayed end-to-end suture. There is extensive tissue damage with a high risk for sepsis. Retraction of stumps is usually performed. If possible, it is helpful to gently approximate nerve stumps to each other or to adjacent structures at the first operation. The nerve stumps are readapted after resection of the stump neuromas within a 2- to 3-week interval. This form of intentionally delayed repair due to unfavorable soft tissue condition at the time of injury is termed delayed end-to-end suture.
Unfortunately, the bulk of lacerating injuries (![]() Web Fig. 240-2) are blunt and also have ragged, contused, and compressed margins, necessitating more nerve resection and graft interposition.
Web Fig. 240-2) are blunt and also have ragged, contused, and compressed margins, necessitating more nerve resection and graft interposition.
Sharp transections can often be sutured end to end, and their prognosis is excellent.
Penetrating Missile Wounds
Penetrating missile wounds are blunt injuries and mainly result in contusion and stretch. Most are caused indirectly because the projectile trajectories do not strike the nerve in 85% of cases. However, many of the recited data in the literature have been carried over from older reports76 and thus describe injuries caused by older and completely different firearms.10,77–79 More current reports are related to the Serbo-Croatian wars and Middle Eastern combat activity.66,66a,80 For example, brachial plexus lesions associated with complete loss of function have been demonstrated to benefit from surgery, particularly in those associated with injuries to C5, C6, C7, and lateral and posterior cord.80,81 High-velocity missiles can have a direct impact but also can cause impact by the produced shock wave and by cavitation. A “near miss” creates a shock wave with instant high-pressure damage (explosion) on the way to the nerve, followed by low-pressure damage (implosion) on the way out.10,81–84 A serious problem is the open wound. The damage with a near or close miss can range from mere neurapraxia to a complete degenerative lesion. Lesions that show no spontaneous recovery are thus usually explored with delay and then often need graft repair. Of course, pain syndromes and associated other injuries are a frequent concern. The Red Cross wound classification system is helpful for describing this type of injury.66a,85
Iatrogenic Injury
A whole chapter of this edition is devoted to iatrogenic injury lesion complex (see Chapter 246), and there is a reason: in some centers, these lesions make up 20% to 25% of operated nerve injuries.67–70,86,87 Because of its importance for a nerve surgeon’s practice, some facts about this entity deserve reiteration. Despite the fact that cases of inadvertently injured nerves are not declining, and all mechanisms have been described, the appropriate treatment usually still is delayed. Treatment regimens should follow exactly those for traumatic nerve injuries of other origin. One element of delay certainly is that clinicians frequently embark on false hopes whenever a nerve injury has been induced through the hands of a physician. Whenever there is a wound over a nerve that is nonfunctional, chances come close to certainty that this nerve would benefit from exploration because it is probably cut.
Inappropriate Nerve Tumor Removal
Most larger series of iatrogenic nerve injury include cases in which a benign peripheral nerve sheath tumor (PNST), usually a schwannoma, has been excised altogether with the parent nerve.10,68,70,88 Laterally placed schwannomas are thought to be ganglia, and the centrally placed ones are misinterpreted as malignant tumors. Fine-needle biopsy of benign schwannomas is a cause not only of erroneous diagnosis but also of painful nerve injury and functional deficit.88,89
Whenever it is evident that a benign schwannoma (or less likely, neurofibroma) has been resected together with a functional important parent nerve, reconstruction should be planned as soon as possible. In such cases, we apply the same principles as for any other acute nerve injury, which are outlined in this text, provided no malignancy was involved.10,69,88,90 It is essential to review the pathologic material in such cases to confirm the tumor type and grade and to provide further clues to the removal of normal nerve tissue. The same principles as for any other nerve reconstruction apply, and the approaches are nerve dependent. The lesion is approached from distal and proximal healthy planes and tissues to prevent further nerve damage. At times, it might actually be difficult to discern scar from tumor remnant. The nerve gap is usually bridged with autologous nerve. The distal and proximal nerve stumps must be resected back to healthy, tumor-free fascicular tissue. A frozen section helps to confirm a tumor and neuroma-free fascicular pattern. In contrast to the diagnostic dilemma that can be encountered with attempts to differentiate benign from malignant PNST, it should be no problem to confirm that the section does not contain residual tumor, or neuroma. In some situations, a split repair is most appropriate (e.g., if one portion of the nerve is still in continuity and functional, but a considerable other half has been excised, leading to functional loss). With regard to prognosis after reconstruction under those special circumstances, it is difficult to quote data because no comparable series are reported on this very confined set of patients. A potentially higher risk for recurrence most likely is only a very theoretical consideration because recurrence generally is not a problem with benign PNST.
Clinical Assessment
History, Symptoms, and Signs
The inexperienced surgeon usually is enlightened by the opportunity to observe what a thorough and nevertheless quick systematic examination by an expert can yield with regard to exact branch localization, level and extent of injury, and potential for recovery. To detect the level, thorough knowledge of branching pattern and supplied muscles and sensory area is crucial. A helpful guide is a systematic table and scheme that is routinely used to document the findings. It is valuable to develop an individual systematic sequence of muscles to examine for each nerve, which usually follows the innervated areas and thus branches from proximal to distal. The actual steps in testing the individual muscles is something that is best learned from an expert. By far, the best guide to examination is still O’Brian’s Aids to the Examination of the Peripheral Nervous System.91 There are some “trick movements” that can compensate for loss of nerve and muscle function by activating other muscle groups supplied by different and more functional nerves.10 Thus, sometimes a complete lesion is deemed partial and followed instead of being repaired. Examples of trick movements include the following:
The BMRC was developed by Highet for war wounds in 1943100 and was later used in poliomyelitis. Thus, in the BMRC system, grade 3 is only contraction against gravity, 4 mild or moderate pressure, and 5 normal. In contrast, the Louisiana State University Hospital (LSUH) system10 grades are as follows: 1—observable contraction but not enough to overcome gravity, 2—against gravity only, 3—against gravity and mild pressure, 4—against gravity and moderate pressure, and 5—close to normal.
Sensitivity and sympathetic function give precious clues to the completeness or extent of functional loss. Apart from weakness or paralysis of muscles, the early signs of nerve injury are alteration or loss of sensibility, vasomotor and sudomotor paralysis in the distribution of the affected nerve, and an abnormal sensitivity over the nerve at the point of injury. After severe injury of a nerve with a cutaneous sensory component, the skin in the distribution of the affected nerve is warm and dry starting within 48 hours of trauma. If possible, sensation to light touch and pinprick, vibration sense, position sense, and ability to localize stimuli should be tested and the affected area of skin recorded. Anhidrosis can easily be checked with loupes or an ophthalmoscope set on ±20 if in doubt. Warming of the skin, color change, and capillary pulsation in the fingertips indicate vasomotor paralysis. Ischemia affects the large fibers first, and thus discriminative sensibility and vibration sense are lost early.92 The palmar and plantar skin is scrutinized for changes in color and in sweating.
A painful nerve is injured or compressed, or both. The occurrence of pain after injury often means that the noxious process is continuing (![]() Web Fig. 240-3). Acute neuropathic pain is characterized by loss of sensation; by painful, spontaneous sensory symptoms throughout the nerve’s territory (dysesthesia); and by lancinating or shooting pain irradiating into the distribution of a main nerve. A constant crushing, bursting, or burning pain in the otherwise undamaged hand or foot indicates serious and continuing injury to major trunk nerves. Progression of sensory loss with a deep bursting or crushing pain within the muscles of the limb, often accompanied by allodynia, can indicate impending critical ischemia. A regular feature of injury caused by critical ischemia is neurostenalgia, which indicates continuation of the noxious process and sometimes also deepening of the lesion. Partial nerve injuries are at times excruciatingly painful. Causalgia is uncommon, and it responds well to the correct operation. Deafferentation pain is related to the death of neurons on the dorsal root ganglion (herpes zoster) or to lesions of the dorsal root of the spinal nerve.90a
Web Fig. 240-3). Acute neuropathic pain is characterized by loss of sensation; by painful, spontaneous sensory symptoms throughout the nerve’s territory (dysesthesia); and by lancinating or shooting pain irradiating into the distribution of a main nerve. A constant crushing, bursting, or burning pain in the otherwise undamaged hand or foot indicates serious and continuing injury to major trunk nerves. Progression of sensory loss with a deep bursting or crushing pain within the muscles of the limb, often accompanied by allodynia, can indicate impending critical ischemia. A regular feature of injury caused by critical ischemia is neurostenalgia, which indicates continuation of the noxious process and sometimes also deepening of the lesion. Partial nerve injuries are at times excruciatingly painful. Causalgia is uncommon, and it responds well to the correct operation. Deafferentation pain is related to the death of neurons on the dorsal root ganglion (herpes zoster) or to lesions of the dorsal root of the spinal nerve.90a
Electrophysiology
After nerve exposure, electrodiagnostic work is of inestimable value to assess whether a lesion in continuity has a chance for spontaneous recovery or will fare better with graft repair.10,93,94 Somatosensory evoked potentials (SSEPs) can help to check for root avulsion.95–97 NAPs for significant conduction require about 3000 to 4000 fibers of at least moderate size and some myelination across a lesion in continuity.10,73
Imaging
The role of imaging in nerve trauma is to rule out root avulsion,95,98,99 musculotendinous injury or tear (MRI, ultrasound), or bony injury (computed tomography [CT]), or to assess implanted metal (radiograph) in secondary nerve reconstructions. Ultrasound will gain increasing importance as an excellent means to assess whether a nerve has been torn apart or a neuroma-in-continuity has formed. Modern high-frequency ultrasound devices allow one to recognize fascicles within the nerve and, even more so, the lack of fascicles in case of internal scar (neuroma). However, fascicular continuity does not ensure, although it does favor, axonotmesis and future recovery of function.
Approaching Nerve Injury: A Treatment Plan Outline
Indications for Operation
The sooner the distal segment is reconnected to the cell body and to the proximal segment, the better the result will be. For the extreme case of replantation after traumatic amputation, O’Brian72 has indicated that primary suture of nerves provides the only chance of recovery.
Reasons not to proceed to repair of a transected nerve include the following:
Concerning nerves injured in the arm or the elbow, Seddon100 thought that recovery could be awaited if two conditions were met: (1) reasonable apposition of bony fragments, and (2) “complete certainty that there is no threat of ischemia of the forearm muscles.”
Recognition of Extent of Injury
Severance of a nerve with a cutaneous sensory component leads to well-defined loss of sensibility and to complete motor, sudomotor, and vasomotor paralysis in the distribution of the nerve. Conduction block is more likely to result in a patchy motor loss. Further, it is more likely to affect large axons more heavily than small ones. As such, vibration sense and sensibility to light touch are likely to be impaired, whereas pain sensibility may be unaffected. In the case of conduction block, axons are intact, and stimulation distal to the lesion will elicit a motor response. If, in contrast, the axons are damaged (degenerative lesion), stimulation distal to the lesion more than 6 days after the injury will not elicit a motor response.101 A diagnosis of neurapraxia cannot be made unless that time has passed after the injury.
Approach to Closed Injury and Lesions in Continuity
The closed traction injury is very destructive of nerves and of axial vessels. There is wide retraction of ruptured nerves and of vessels, and there is considerable longitudinal damage within the ruptured trunk. The outcome after nerve repair in this pattern of injury, when complicated by arterial lesion, is the worst of all groups. Failure of distal progression of the Hoffman-Tinel sign in a closed traction injury indicates rupture or other lesion impeding useful regeneration. After a degenerative lesion, recovery proceeds centrifugally, so that the most proximal muscle is reinnervated first. This also applies to sensibility. Recovery of sensibility may be preceded by recovery of sweating, and sometimes sweating may be restored without any later recovery of sensibility. In nondegenerative lesions, recovery does not usually follow such a centrifugal reinnervation pattern. Distal muscles can recover before proximal ones. Sensibility alteration is more patchy, and vasomotor and sudomotor paralysis is rare. Some of the most serious mistakes in the diagnosis and treatment of patients with injured nerves are made because the examiner fails to accurately assess the severity of the injury, that is, the distinction between a degenerative and a nondegenerative lesion. One almost infallible sign of profound injury is always present in the first 48 hours after extensive injury of a nerve with a cutaneous sensory component: because of the involvement of small as well as large fibers, the skin in the distribution of the affected nerve is warm and dry (anhidrosis and vasomotor paralysis). Ischemia first affects the large fibers, and discriminative sensibility and vibration sense are the first affected.92 It is important for the clinician to assess, as far as possible, the innervation of all muscles distal to the injury site on the day of injury and at subsequent examinations. Whether to leave alone a lesion in continuity after exposure or rather to resect and bridge the gap is a most difficult decision, and even more so when there is clinical evidence of “some recovery.” The decision certainly is favored, although not guaranteed, when intact fascicles can be shown traversing the lesion or by its indirect correlate, which is a compound nerve action potential across the lesion. For such a response to appear, several thousand axons need to have sprouted across the lesioned segment and conduct, and this process needs some time to occur. Stimulation above the lesion and recording from the nerve or individual fascicles below the nerve helps in most of these instances: a response of good amplitude may well indicate a good prognosis. A response in individual fascicles allows for separation of an intact part of a nerve from the damaged portion. The consistency and the diameter of the neuroma is also helpful. The harder and the larger the neuroma, the less likely is the chance for a good spontaneous recovery.
The stretch lesion in continuity is very difficult. The nerve, which is usually in the axilla or at the knee, is exposed after a severe closed traction injury and is found elongated by 30% or more. The epineurial blood vessels are torn, but individual bundles appear intact. The damaged segment may extend to as much as 25 cm. Resection and repair of such extensive lesions is not feasible. Useful recovery occurs naturally in as many as one third of these injuries.66a
Primary Repair: Urgent Surgery
Definition of Terms
The earlier the exploration is done, the easier the diagnosis is. Not only is the field free from scar tissue, but also the axons of the distal stump continue to conduct and the neuromuscular junctions continue to function for a few days. Stimulation of the distal stump produces a motor response, which permits the surgeon to identify bundles with a predominantly motor function. This is nowhere better shown than in the case of the brachial plexus (Fig. 240-3A); it is easy to diagnose rupture up to 3 days after injury but progressively more difficult after that time. With early operation, it is possible to match fascicular arrangements of the stumps; as time goes by, the matching becomes progressively more difficult. This is not all: with delay, there is progressive intraneural collagenization (Fig. 240-3B).
From 1956 on, primary or relatively acute suture of severed nerves was practiced at St. Mary’s Hospital whenever it was possible. There, in 1965, Michael Lawrence achieved an outstanding result from primary repair of all flexor tendons, radial and ulnar arteries, and median and ulnar nerves sectioned at the wrist. The policy was extended to revascularization of damaged limbs and to replantation of amputated hands and severe injuries of the brachial plexus.104
Having said that, most of the traumatic adult brachial plexus lesions unfortunately come to the attention of the nerve surgeon only with considerable delay, a fact that profoundly influences and worsens functional outcome.103
Contraindications to Early Repair
Of course, there is no need to explore the nerve if the clinician expects spontaneous recovery. Seddon had this to say about nerve lesions complicating closed fractures of the shaft and the lower end of the humerus: “however, on balance, it would seem proper to await spontaneous recovery of the nerve, provided the two conditions are satisfied. The first is reasonable apposition of the bony fragments, and the other complete certainty that there is no threat of ischaemia of the forearm muscles.”100 If however, the clinician elects to convert a closed fracture to an open one by internal fixation, it is wisest to expose nerves that are not working. The fracture surgeon who does not do this is asking for trouble.
Secondary Repair: Elective yet Early Enough
Definition of Terms
As already mentioned, the decision about whether to operate should be made as soon as possible, and surgery should take place before 3 months after trauma if there is no indication or chance for primary repair (e.g., late referral). A decision to observe is made in the setting of a nerve lesion that is in continuity in which useful spontaneous functional recovery is expected. Other reasons may include providing time for wound healing and recovery after primary trauma surgery or because a wound infection needed to be overcome. The clinician should focus on making the diagnosis while there are still signs of peripheral degeneration, which is the best time for intervention.104
Aspects of Diagnosis
Web Figure 240-3 ![]() demonstrates a bone fragment that we dissected out of a patient’s retroclavicular and supraclavicular brachial plexus during secondary reconstruction. The patient had complained of severe, electrifying pain shooting down into the hand at times and also locally, without having had signs of root avulsion (classic sign of impalement and also of the type of pain that occurs when a suture is inadvertently placed in nerve). It is desirable to have detailed reports about the early and preceding procedures and encountered problems. It is also necessary to inquire about other surgery and to coordinate this with the other surgical teams, especially in secondary nerve repair of complex trauma cases.
demonstrates a bone fragment that we dissected out of a patient’s retroclavicular and supraclavicular brachial plexus during secondary reconstruction. The patient had complained of severe, electrifying pain shooting down into the hand at times and also locally, without having had signs of root avulsion (classic sign of impalement and also of the type of pain that occurs when a suture is inadvertently placed in nerve). It is desirable to have detailed reports about the early and preceding procedures and encountered problems. It is also necessary to inquire about other surgery and to coordinate this with the other surgical teams, especially in secondary nerve repair of complex trauma cases.
In making a decision about supplementary examinations, the diagnosis and current status of recovery should be confirmed by NPI. If root avulsion has to be ruled out, postmyelographic thin-slice CT is necessary, unless a devoted radiologist can perform sequential MRI root levels at the spinal root entry and exit zones.95 If there is a bony element and fractures have been addressed at prior surgery, current radiographs and CT scans are reviewed to assess whether bone needs to be redressed or whether plates, screws, or wires can be removed during nerve exploration. One certainly does not like the idea of having the first-year trauma resident remove the “minimally invasive” metal after one has done a nerve reconstruction coursing over a plate (e.g., radial nerve at humerus). Sometimes, bony exostoses and overzealous callus formation need to be drilled or rongeured off, and we have encountered median, ulnar, radial, and suprascapular nerve and brachial plexus elements that had to be freed and drilled out of bone.
Aspects of Microsurgical Nerve Repair
The technical aspects of nerve repair are detailed in Chapter 244; certain aspects are emphasized here.
Microsurgical Principles Applied to Nerve
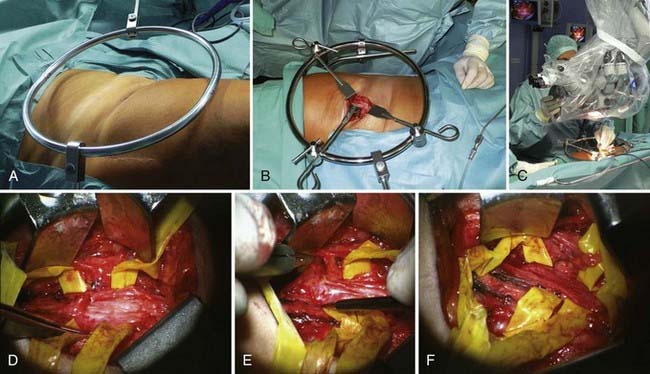
Nerve repair can be dismantled in four steps: exposure of pathology, decision making, reconstruction, and closure. The initial steps of the exploration follow the anatomic planes. Depending on the nerve and exposure, we might change the standing position several times if different viewing angles are of help, which is easier without the microscope in place (e.g., during the different early dissection steps of brachial plexus surgery). Changing the limb position during the case at times helps in some dissections of proximal and distal nerve segments (e.g., radial nerve lesion at humerus with posterior and anterior exposures); this needs to be accounted for when draping; movable arm boards facilitate this. Meticulous hemostasis by use of bipolar coagulation with a foot pedal is essential if one is to avoid a bloody field. Dissection in anatomic planes helps greatly in this regard because it will create less bleeding compared with “plowing through” from top to bottom. It is important not only to place grafts without tension but also to determine whether reasonable limb movement is possible after the grafts are placed and coapted. Millesi pointed out the great importance of preserving a nerve’s gliding planes,9 respecting and treasuring the synovium and fat planes. If grafts cannot be cut to length within the field, or will be pulled through tunnels (e.g., from infraclavicular to supraclavicular), a good solution is to use vessel loop as a template and to cut it to the desired length in situ, adding some extra length (theoretically 10% for graft shrinking due to retractive forces, plus some extra length for trimming of graft ends and for tensionless repair). In complex reconstructions with several gaps to bridge, it is essential to calculate beforehand how many grafts of what length need to be used. Background material to improve the visibility of the suture and proper hand rest are of great help. The choice of suture and needle size is an individual one (e.g., TK prefers 10-0 suture on a BV 6 needle). The first stitches determine whether the remainder of the grafting procedure will go smoothly or will be awkward. In other words, perfect alignment and strategic positioning of the initial grafts should be planned in a way to make the last graft placements and stitches optimal. Three knots suffice for every stitch. For the actual microsuture technique, there are such a variety of preferences that we find it hard to give a recommendation other than to exercise care. For example, one maneuver to regrab the needle is by resting the tip of it on the background material in order to pivot it until it has reached a good position to take it up again (TK). Microsuturing is a process that needs to be developed in order to find out which instruments, holds, and moves work best for the individual. Observation of different slick microsurgeons will, however, reveal some common traits: slow, controlled, unhasty, effective movements combined with very precise placements of grafts and stitches. Because effective microsurgeons manage to eliminate everything unnecessary, they can be fast with slow movements.
In many circumstances, fibrin glue has replaced suture or is used as an adjunct. For instance, RB now only sutures in direct repair and in transfers. One needs to be aware that glue only secures the position and does not provide the same tensile strength as suture. Further, glue does not permit the surgeon to “fish-mouth” the ends of the grafts to match as much as “lead out” and “lead in” fascicular structure as possible.10
Exploring Traumatized Nerve
General Principles and Use of Landmarks
It is paramount to have a thorough understanding of the normal anatomy, if one approaches distorted and scarred anatomy. As always in peripheral nerve surgery, good use should be made of anatomic landmarks. It is important to approach the lesion from virginal tissue and planes that are not scarred. This is why the lesion is approached from proximally and distally in a way that the nerve’s pristine ends are dissected and followed toward the lesion. This prevents inadvertent laceration of the nerve segments that are buried in scar. When the two “good sides” are dissected out, a much better understanding of the nerve’s current course can be developed. Frequently, the nerve will be completely displaced from its natural location. For lesions in continuity, it is especially important to not cut inadvertently into nerve during scar dissection. Often, nerve needs to be sculpted out of scar, such as by use of a 15-blade knife and scissors. Vascular loops or Penrose drains for larger nerves can help to lift the already developed nerve parts up in order to ease circumferential dissection. This also explains why these approaches usually necessitate larger incisions. Working in planes facilitates overview and prevents digging deep holes that necessitate aggravated retraction. When the nerve ends are completely dissected out, one has to resect back to normal fascicular tissue on both nerve ends by use of a razor blade or a fresh 15-blade knife. The sequentially cut nerve slices will show a gradual change from scar, to scar with some fascicles, and then to completely normal fascicular tissue, with a glossy, moist cross-sectional area and pouting fascicles. If in doubt, a frozen section can help to rule out scar tissue in the section.105 This is usually not necessary in the periphery but sometimes is more difficult at a brachial plexus spinal nerve level when the section comes close to, or is within, the unroofed foramen.
Nerve Repair Techniques
Neurolysis
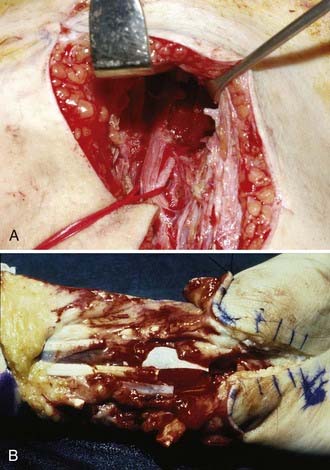
In external neurolysis (or neuroplasty), the nerve is set free from scar, organized hematoma, or bony fragments and callus. Usually, the nerve is released out of such an encasement in a circumferential manner. The epineurium is minimally breached with external neurolysis. This is a partial step to prepare the nerve for recordings and reconstruction if needed. It is done macroscopically and microscopically, depending on the findings. A step further, which at times is necessary, is internal neurolysis (or neuroplasty) (Fig. 240-4), which implies opening of the epineurium, or sometimes resection, in order to lyse certain fascicles or fascicle groups out of a scarred bed within the nerve. The dissection is within the interfascicular epineurium. As such, it is important to avoid damage not only to the fascicle but also to the ensheathing structure of functional fascicles, which is the perineurium. Damage to the perineurium leads to functional loss of the contained fascicle, or fascicular bundle (depending on the nerve, it can be monofascicular, oligofascicular, or polyfascicular). This step needs to be done with proper illumination and magnification. Internal neurolysis is used in the preparation of nerve ends for grafting, in the dissection of a neuroma-in-continuity (in which the neuromatous tissue is dissected away from intact fascicles), and in benign nerve sheath tumors (schwannomas and neurofibromas).
Intraoperative Decision Making: Is Grafting Necessary?
Nerve Suture and Grafting
If tensionless end-to-end suture is possible, the fascicles and the fascicular groups of the two stumps should be matched as precisely as possible. The longitudinal course of arteries in the epineurium greatly helps with correct alignment of the two stumps, if it is a fresh and smooth injury without a contusion element. The coaptation is preceded by preparation of the nerve stumps. Usually, despite sharp cut ends, both of the stumps need to be cut back for 1 mm or less to healthy-appearing fascicular structure, the hallmark of which is a glossy, moist-looking surface with pouting fascicles. The coaptation is started with two opposing lateral sutures, completed by three or four more sutures. If after nerve stump preparation and cutting back, a tensionless repair is not feasible, the defect is bridged with interposed autologous grafts.106 The grafts are sutured to the epineurium or individual fascicle (groups) of the recipient depending on the nerve caliber, type, and location. The nerve ends are prepared so that the fascicles protrude and are laid in the prepared bed orthodromically as a rule. Fixation is by glue or suture. The sutures unite the fascicles of the stumps with the grafts. Because the individual strands of the grafts are often the same size as the individual bundles, the suture unites epineurium of graft to perineurium of bundle. No discernible difference in outcome could be demonstrated between epineural and fascicular (or perineural) sutures.107 Orgell108 described a modified fascicular suture termed group fascicular suture and concluded that epineural suture was the “technique of choice for most acute nerve lacerations.” He pointed out that it was easier and faster and entailed less manipulation of the internal structure of the nerve than did fascicular suture. Kline and Hudson indicated a restriction of fascicular suture to “some oligofascicular nerves.”10 This is in contrast to Millesi, who prefers interfascicular suture and proposed that the epineurium was the main source of the infiltration of the suture line by fibroblasts.109
We prefer to combine bundle (fascicular) suture with epineurial suture on an individual basis, guided by the nerve stump structure. Dissection within the nerve is avoided because this surely leads to fibrosis. In the early days after division of a nerve, bundles are mobile within the epineurium, and epineurial suture increases the risk for malalignment.50 That is why the needle is passed through the condensed inner epineurium and the perineurium to secure accurate coaptation of larger bundles. The repair is completed by epineurial suture. In delayed repair, fibrosis within the epineurium stabilizes the bundles so that they cannot rotate within the epineurium. In these, epineurial suture may be adequate. The atrophy of the distal stump and the extent of fibrosis in both proximal and distal stumps increases the difficulties in neglected cases. In both primary and secondary suture, the areolar adventitial tissue is pushed back from each stump to expose the true epineurium.
Tensionless suture repair, irrespective of interfascicular or epineural graft coaptation, is the crucial component for successful microsurgical nerve suture. Trumble advised narrowing the gap by careful mobilization and by joint positioning.101a There are reported series of sutures of digital nerves with some tension.102,110–112
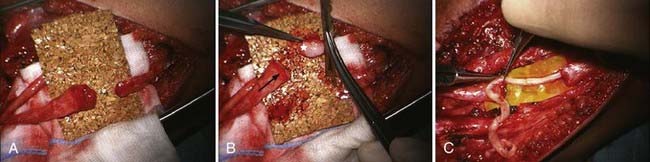
The principle steps of autologous interfascicular nerve grafting are removal of nerve stump neuromas of the recipient (Fig. 240-5) and cutting back to normal fascicular structure on both nerve ends; these are dependent on the findings and individual technical preferences for dissection of fascicle groups that match the individual graft pieces, followed by sequential insertion and coaptation of the grafts.
The extent of resection of a damaged stump in closed traction rupture is remarkably small if done primarily and is sufficient once an orderly and recognizable architecture of bundles is displayed. To dissect every fascicle out, as once was proposed, is not advised because this will create unnecessary additional damage to the recipient nerve and potentially stimulate fibrosis. Creating “fascicular fingers” in the form of a grouped fascicular repair sometimes enables a better match and alignment. A freshly cut or “raw” muscle is not a good bed for grafts, but fat flaps and synovium are. The technique to be applied at times even differs between proximal and distal coaptation side and is adjusted to the need for maximal, tensionless alignment. The objective, which should not be forgotten, simply is to have as many guiding rails for sprouting axons at disposal as possible. At the same time, these rails need to be reachable by external vascular blood supply from the wound bed; a perfect match is not feasible, but too generous use of interfascicular dissection risks increased fibrous reaction and scarring.113 Many sprouting axons will be misdirected and get lost on their way.114 The more rails that are available, the better the chances will be for axons to reach the target organ and thus for good functional recovery.
Interposition of an autologous nerve graft is achieved by one or multiple strands of a cutaneous nerve. The senior author (RB) uses nerves from the damaged upper limb whenever possible. If suture is not substituted by glue, the surgeon should attempt to minimize the number of sutures needed. When the graft is splayed out perfectly and not contorted along its long axis (in other words, well aligned), the adhesive forces cause the donor to stick to the recipient. In such cases, only one or two securing sutures are usually needed on each end.115,116 When using glue, a clump should be avoided because it can interfere with revascularization. Young and Medawar introduced the concept of fibrin suture in 1940 in rabbits, after a sutureless fibrin glue technique for securing union between divided intracanalicular facial nerve ends had been described as early as 1932 by Ballance and Duel.117 Narakas had popularized and refined its application for nerve repair in the 1980s.115
Harvesting Graft
The sural nerve is used frequently118,119; other cutaneous nerves that are in the operative field can be harvested provided that the produced donor site deficit is acceptable (e.g., medial cutaneous nerve of the forearm). The lateral cutaneous nerve of the forearm (LCNF) is found just lateral to the biceps tendon, and it can be displayed, in the lower part of the arm, between the biceps and brachialis muscles. About 15 cm of graft is available. The medial cutaneous nerve of the forearm (MCNF) is taken from the arm through a curvilinear incision. About 30 cm of graft is available, and this is the nerve of choice because the donor defect is so trivial. The terminal part of the posterior interosseous nerve has been used.120 The superficial radial nerve and lateral cutaneous nerve of the forearm should only be used when the parent nerve is irreparably damaged or when C5 and C6 are ruptured or avulsed. The sural nerve offers up to 45 cm of graft, but it should not be used in the ipsilateral tibial nerve in the leg because this seriously adds to the loss of sensation in the foot and ankle; it is better to use the MCNF. It is important to always avoid the formation of a painful neuroma, and donor nerves should be cut cleanly deep to the deep fascia of the limb.
If the cutaneous nerve caliber matches that of the nerve to be reconstructed (often with monofascicular or oligofascicular nerves), one interposed graft can be bridged with epineural sutures, after the mesoneurium and soft tissue have been cleaned from the recipient nerve ends. Various methods for harvest of the sural nerve have been proposed: single long longitudinal incision, multiple stair-step incisions, and more recently, endoscopic methods.118,121–124 The major drawback of minimal access methods is that usable branches may be sectioned and not removed as graft material. With a longitudinal incision, one can harvest under direct vision and in the prone and supine position. Certainly, it is also possible to use several transverse incisions to prevent a large open wound. The latter technique potentially exposes the nerve to the risk for traction injury and of damage to communicating branches from the common peroneal nerve. When maximal lengths need to be harvested (sural nerve), we like to lift the skin at the proximal and distal incisional ends and gently grasp the proximal nerve, cut distal to it (nontraumatized end), and keep the proximal end grasped to be able to coagulate its free fascicles in an attempt to “seal” the end. The proximal end of the cut nerve needs to be buried deep to fascia to prevent neuroma pain. We routinely make use of a longer-lasting local anaesthetic directly at the nerve end and along the skin incision. In using a long incision, it is essential to maintain meticulous hemostasis and to close layer by layer (the proximal part for example will be underneath gastrocnemius fascia). A bandage from foot to below knee will help to prevent subcutaneous hemorrhage. At the end of the operation, the lower leg is slightly elevated to ease venous drainage and prevent swelling. After its excision, a donor site defect in the form of a hypoesthetic area of variable size at the lateral aspect of the lower leg and foot will remain, mainly around the area posterior and anterior to external malleolus and at the proximal lateral aspect of the forefoot. With time, the area will diminish slightly in size owing to collateral branch reinnervation. The patient needs to be aware of the foreseeable donor site deficits.
Tunneling of Grafts
Nerve grafts can easily be tunneled between two skin incisions if longer stretches need to be covered and it is not desired to enlarge the skin incision (e.g., a formerly open wound that had been superficially compromised before; ![]() Web Fig. 240-4). In such a case, the grafts can be bundled, sewn together to a vessel loop, and carefully pulled through the tunnel. For shorter distances, it is sufficient to bluntly create a tunnel and pull a vessel loop with the grafts attached by suture. The sutured graft tips need to be resected once they are in place.
Web Fig. 240-4). In such a case, the grafts can be bundled, sewn together to a vessel loop, and carefully pulled through the tunnel. For shorter distances, it is sufficient to bluntly create a tunnel and pull a vessel loop with the grafts attached by suture. The sutured graft tips need to be resected once they are in place.
Nerve Transfers
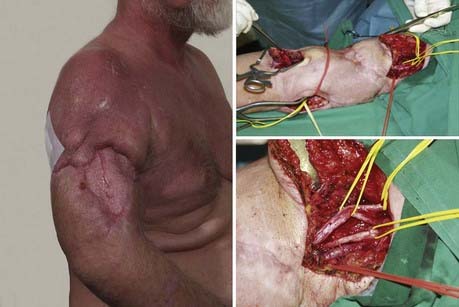
In principle, an uninjured nerve is used as an axon donor and is therefore transected, usually distally, and transferred to the distal stump of an injured nerve to reinnervate the recipient nerve’s target organ (Fig. 240-6). The first transfers were described decades ago125–127: Harris and Low suggested the principle in 1903; Tuttle128 recommended the use of the accessory nerve and portion of the upper cervical plexus in 1913, Vulpius and Stoffel129 the pectoral nerve in 1913, and Foerster130 the long thoracic nerve in 1929; Yeoman and Seddon131 (1961) and Fantis and Slezak132 (1967) suggested the intercostal nerves. Yeoman proposed reinnervation of the biceps muscle by intercostal nerves, and Seddon reported these cases in 1963.100 Nagano and colleagues developed this method for the treatment of avulsion injuries of the brachial plexus.133 The principle has been extended to the reinnervation of free muscles,134 and other applications have been described.135,136 Oberlin and coworkers137 pioneered and successfully employed one or two fascicles of the ulnar nerve to reinnervate the motor branch of the biceps in brachial plexus palsies, with outstanding restoration of muscle strength, a concept further applied by Mackinnon and colleagues,138 who also reported earlier with Brandt and associates139 on use of medial pectoral branch to reinnervate the musculocutaneous nerve. Gu and associates140 described the use of the contralateral seventh cranial nerve to reinnervate the limb after complete lesion of the brachial plexus. According to Chen and Gu,141 the contralateral seventh nerve is a more effective axon donor than the phrenic nerve, and a vascularized interposed ulnar nerve graft was superior to a nonvascularized graft to the recipient.
In cases of high common peroneal nerve palsy, the nerve bundle to the lateral head of the gastrocnemius and the soleus has been transferred to the anterior tibial branch of the common peroneal nerve by Gousheh and Babaei.142
The senior author (RB) has performed more than 2500 nerve transfers in 1500 patients since 1979, and most were for patients with severe injuries to the brachial plexus. Some of these transfers should be emphasized because they are particularly reliable and important. First comes reinnervation of the paralyzed serratus anterior by transfer of the deep division of the intercostal nerves of T3 and T4 to the nerve to serratus anterior. This operation has been successful in about 90% of cases. Next comes Oberlin’s operation, transfer of a fascicle or fascicular bundle from the ulnar nerve to the musculocutaneous nerve branch to biceps, which was successful in more than 80% of cases. The spinal accessory nerve is a powerful motor donor. Functional elevation and lateral rotation at the shoulder was achieved in more than 70% of 250 cases in which the lesion was confined to the fifth and sixth spinal nerves. In cases of complete preganglionic injury, the spinal accessory nerve is transferred to the ventral root of one of the avulsed spinal nerves. Some useful function followed in more than 50% of these operations. Accessory nerve to biceps branch of musculocutaneous is a reliable operation but requires an intervening graft. The experience at Stanmore with intercostal transfer does not match that of Japanese groups; this experience was shared by Narakas and Allieu.57 Functional flexion at the elbow was regained in just fewer than 40% of 300 cases, but some of these patients experienced a welcome improvement in pain. Success has followed in a smaller number of operations where intercostal nerves were transferred to such nerves as the thoracodorsal, medial pectoral, and axillary (circumflex) nerves to the medial head of triceps and to extensor carpi radialis brevis. More recent experience with transfer of intact triceps branches to axillary nerve for deltoid paralysis has also been favorable.
We have little experience with nerve transfers within the distal upper limb. Exceptions are cases of transfer of the dorsal branch of the ulnar cutaneous nerve onto the median nerve for restoration of sensation. Battiston and Lanzetta143 reported seven cases of unfavorably high ulnar nerve palsies treated by transfer of the palmar cutaneous branch of the median and of the anterior interosseus nerve before it entered the quadratus into the ulnar nerve at the wrist. Good results were achieved in six cases. Ozkan and coworkers144 related impressive results from transfer of a digital nerve in irreparable median or ulnar nerve lesions. Eighteen of 20 patients experienced improvement after transferring nerves from inner to outer digits; sensory retraining was used in these patients.
Alternative Methods of Grafting
Direct Muscular Neurotization
Rarely there is a clear lesion in discontinuity, yet there is no distal nerve stump available (e.g., axillary and deltoid, accessory and trapezius). Cases like this can sometimes be worth a direct muscular neurotization because there is nothing to lose apart from an interposed autologous cutaneous nerve graft.145–148 The importance in spreading out the fascicles in a fan-like manner and burying them in intermysial folds before fixing them in place (e.g., fibrin glue) has been pointed out.149
Conduits
So far, conduits have only been useful for defects up to 3 cm and for cutaneous nerves (Fig. 240-7).150–155 Research is aimed at tissue-engineered, bioartificial156 tubes or other autograft surrogates that supply a matrix in concert with neurotrophic factors and cultured cells.157–159 Schlosshauer and associates gave a detailed review on the current status of synthetic nerve guide implants.157 Autologous Schwann cells have already been cultured from nerve stump and neuroma areas and implanted into predegenerated sural grafts, tendons, and freeze-thawed muscle grafts.
Interposed Freeze-Thawed Muscle
Interposed freeze-thawed muscle47 was a promising technique because muscle as a donor is more readily available as an autologous repair material than nerve and results in less donor site morbidity.160 The idea, which led to experimentation with this type of autologous nerve graft substitute, is that muscle also has a longitudinal equivalent to the most important guiding structure for sprouting axons: a basal laminar layer.113 It could be reproduced in several series and experiments that axons are indeed able to sprout across a defect that was bridged with interposed freeze-thawed muscle. The freeze-thawing is necessary to destroy most of the viable biostructure, leaving only the basal laminar scaffold alive. Pereira and associates161 reported promising results for sensory nerve repair in 89% of 38 patients with nerve damage and painful neuromas due to leprosy. Other groups’ functional results were far from good; one of the main problems appears to be that axons do not sprout target oriented but rather diffusely all over the muscle piece, preventing a substantial number of axons from reaching the target organ.157 The method has proven useful in some cases of painful neuromas affecting cutaneous nerves.10a
Allograft
Mackinnon revitalized the use of allografts in a case of extensive loss of the tibial nerve in a 12-year-old boy.162 However, even with potential functional benefit, necessary temporary immunosuppression and the as yet unknown risk for viral infection (e.g., Epstein-Barr virus) still pose a substantial problem.163 Brenner and colleagues gave a good account of the array of potential complications in their discussion of complications due to hand transplantations in 2002.164
The vascularized nerve graft was a disappointment,165 and its use is mainly confined to contralateral C7 transfer with vascularized ulnar nerve interposition.134 Survival of such a long graft depends on its revascularization. Seddon in 1963100 concluded, “there is a critical diameter for survival of graft taken from normal nerve: the radial nerve falls within the limit, the common peroneal nerve beyond it.” Pedicled grafting had already been introduced by Strange in 1947 to enhance the viability of such trunk nerves as the ulnar.166 This led to the development of the free vascularized ulnar nerve, supplied by the ulnar vessels by Bonney and colleagues in the 1970s and reported by them in 1984.167 The first free vascularized nerve graft was described by Taylor and Ham in 1976.15 However, the theoretical advantage could not be replicated clinically, and its use has been limited to cases where the gap to bridge is long (e.g., contralateral C7 transfers) and where the wound bed is particularly scarred.
Central Repair
Central repair, or reimplantation of avulsed spinal nerves, is a fascinating concept.168 The first case of reimplantation was performed in 1977 and reported in 1979169; Carlstedt translated his extensive experimental work from primate models to clinical application and has demonstrated some functional benefit in humans.170–172 Since 1995, 45 operations have been performed in cases of complete lesion with 4 to 5 avulsions. Surgical procedures are contraindicated in patients with rupture of the subclavian or vertebral arteries and also in patients showing signs of affliction of the spinal cord. Transforaminal endoscopic examination of the cord is helpful. Regeneration into proximal muscle, notably pectoralis major, has been confirmed in all patients. Useful functions, including adduction, medial rotation, forward flexion at the shoulder, and flexion and extension at the elbow, have been regained in 21 patients. Some patients have done better than this.172a Fournier reckoned that 60% of the motor neurons of the anterior horn were dead by 6 weeks after avulsion and that reimplantation increased the percentage of surviving motor neurons to 80% at 6 weeks after injury.175,176
Outcome
Other important factors177–179 are the type and level of nerve affected, associated injuries, extent of lesion, age of patient, graft length, type of necessary repair (neurolysis versus graft), and applied technique.
Overall, proper repair can improve functionality in 50% to 70% of cases.
The functionality and usefulness of recovery depend mainly on the number of axons reaching the correct target organ and on progressive myelination of those axons.10 Therefore, the number of potential sprouting channels provided by grafts or more direct repair is important, as is the length of the gap after resection and the extent of fibroblastic infiltration of the interposed grafts or sutured nerves. In addition to delay between injury and repair, this regenerative process is influenced by the quality of repair, the degree of damage to the nerve not only during injury but also during repair, and the speed of axonal growth and regeneration.
Every week that passes in delay of repair means progressive atrophy of target tissues as well as cell body degeneration, progressive cell number loss, and central nervous system changes. Omer180 once confirmed the early findings of Woodhall and Beebe177: “delay in suture induces a loss of, on average, about 1% of maximal performance for every six days of delay.” An exponential decay of best obtainable regeneration over time most likely reflects this process more precisely. The prognostic effect of level of lesion can be appreciated in those nerves with a long course: the radial, median, and ulnar nerves. A technically sound ulnar repair at wrist level can bring the function of the hand intrinsics back. This is rather exceptional, however, even for primary sutures of the ulnar nerve at the elbow, let alone at the axilla. Repair of the posterior interosseus nerve can restore the function of fingers and thumb; however, this functional gain is rather unusual after repair of the radial nerve at the spiral groove level. In contrast, urgent repairs of C5, C6, and C7, as well as the upper and middle trunk, can achieve results comparable to those seen after repair of combined injuries to more terminal branches of the brachial plexus. Reconstruction of the peroneal nerve will yield worse results than that of the tibial nerve. Ruptures of the axillary nerve are often associated with rotator cuff tears in older patients, which have a bad effect on the recovery of function.
, Birch R. Iatrogenous injuries. Surgical Disorders of the Nerves. 2nd ed. London: Churchill Livingstone. 2010.
Hall S. The response to injury in the peripheral nervous system. J Bone Joint Surg Br. 2005;87:1309-1319.
Kato N, Htut M, Taggart T, et al. The effects of operative delay on the relief of neuropathic pain after injury to the brachial plexus: a review of 148 cases. J Bone Joint Surg Br. 2006;88:756-759.
Kline D, Hudson A. Kline & Hudson’s Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors. Philadelphia: Saunders; 2008.
Kretschmer T, Heinen C, Antoniadis G, et al. Iatrogenic nerve injuries. Neurosurg Clin N Am. 2009:73-90, vii.
Kretschmer T, Antoniadis G, Braun V, et al. Evaluation of iatrogenic lesions in 722 surgically treated cases of peripheral nerve trauma. J Neurosurg. 2001;94:905-912.
Lawson GM. The peripheral sensory nervous system: dorsal root ganglion neurones. In: Dyck P, Thomas PK, editors. Peripheral Neuropathy. Philadelphia: Elsevier; 2005:163-202.
Mackinnon S, Dellon A. Ischemia of nerve: loss of vibration sensibility. In: Mackinnon S, Dellon A, editors. Surgery of the Peripheral Nerve. New York, Stuttgart: Georg Thieme Verlag; 1988:57.
Nagano A, Tsuyama N, et al. Direct nerve crossing with the intercostal nerve to treat avulsion injuries of the brachial plexus. J Hand Surg Am. 1989;14(6):980-985.
Narakas AO. Thoughts on neurotisation of nerve transfers in irreparable lesions. In: Tertzis J, editor. Microreconstruction of Nerve Injuries. Philadelphia: Saunders; 1987:447-454.
O’Brian M. Aids to the Examination of the Peripheral Nervous System. Philadelphia: Saunders; 2000.
Schady W, Ochoa JL, Torebjörk HE, et al. Peripheral projections of fascicles in the human median nerve. Brain. 1983;106:745-760.
Seddon H. Three types of nerve injury. Brain. 1943;66:237-288.
1 Lawson GM. The peripheral sensory nervous system: dorsal root ganglion neurones. In: Dyck P, Thomas PK, editors. Peripheral Neuropathy. 4th ed. Philadelphia: Elsevier; 2005:163-202.
2 Sunderland S. Peripheral nerve trunks. In Nerve and Nerve Injuries, 2nd ed, Edinburgh: Churchill Livingsone; 1978:31-60.
3 Haninec P. Undulating course of nerve fibres and bands of Fontana in peripheral nerves of the rat. Anat Embryol (Berl). 1986;174:407-411.
4 Tillett RL, Afoke A, Hall SM, et al. Investigating mechanical behaviour at a core-sheath interface in peripheral nerve. J Peripher Nerv Syst. 2004;9:255-262.
5 Lundborg G, Rydevik B. Effects of stretching the tibial nerve of the rabbit. A preliminary study of the intraneural circulation and the barrier function of the perineurium. J Bone Joint Surg Br. 1973;55:390-401.
6 Wilgis EF, Murphy R. The significance of longitudinal excursion in peripheral nerves. Hand Clin. 1986;2:761-766.
7 Lundborg G. Vascular systems. In: Nerve Injury and Repair. London: Churchill Livingstone; 1988:42-46.
8 Lundborg G. The nerve trunk. In: Nerve Injury and Repair. London: Churchill Livingstone; 1988:32-63.
9 Millesi H, Zoch G, Rath T. The gliding apparatus of peripheral nerve and its clinical significance. Ann Chir Main Memb Super. 1990;9:87-97.
10 Kline D, Hudson A, Spinner R, et al. Kline & Hudson’s Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors, 2nd ed. Philadelphia: Saunders; 2008.
10a Birch R. Operating on the nerves. Surgical Disorders of the Nerves. 2nd ed. London: Springer. 2010.
11 Dreesmann L, Ahlers M, Schlosshauer B. The pro-angiogenic characteristics of a cross-linked gelatin matrix. Biomaterials. 2007;28:5536-5543.
12 Dreesman L. Zelluläre Mechanismen beim Neuro Tissue Engineering [Cellular Mechanisms at Neuro Tissue Engineering]. Stuttgart Hohenheim: Faculty of the Natural Sciences, Institute for Zoology/Biosciences, Universiiät Hohenheim; 2007.
13 Lietz M, Dreesmann L, Hoss M, et al. Neuro tissue engineering of glial nerve guides and the impact of different cell types. Biomaterials. 2006;27:1425-1436.
14 McManis P, Low P, Lagerlund T. Nerve blood flow and microenvironment. In: Dyke P, Thomas P, editors. Peripheral Neuropathy. Philadelphia: Saunders; 2005:671.
15 Taylor GI, Ham FJ. The free vascularized nerve graft. A further experimental and clinical application of microvascular techniques. Plast Reconstr Surg. 1976;57:413-426.
16 Hong MK, Hong MK, Taylor GI. Angiosome territories of the nerves of the upper limbs. Plast Reconstr Surg. 2006;118:148-160.
17 Kadiyala RK, Ramirez A, Taylor AE, et al. The blood supply of the common peroneal nerve in the popliteal fossa. J Bone Joint Surg Br. 2005;87:337-342.
18 Schady W, Ochoa JL, Torebjork HE, Chen LS. Peripheral projections of fascicles in the human median nerve. Brain. 1983;106:745-760.
19 Waller A. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of the Primitive fibres. XX. Philos Trans R Soc Lond. 1850;140;:423-429.
20 Seddon H. Three Types of Nerve Injury. Brain. 1943;66:237-288.
21 Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain Res. 1951;74:491-516.
22 Stoll G, Jander S, Myers RR. Degeneration and regeneration of the peripheral nervous system: from Augustus Waller’s observations to neuroinflammation. J Peripher Nerv Syst. 2002;7:13-27.
23 Jabaley M. Internal topography of peripheral nerves as related to repair. In: Geleberman R, editor. Operative Nerve Repair and Reconstruction. Philadelphia: JB Lippincott; 1991:231-240.
24 Hoffmann P. Weiteres über das Verhalten frisch regenerierter Nerven und über die Methode, den Erfolg einer Nervennaht frühzeitig zu beurteilen. Medizinische Klinik. 1915;11:856-858.
25 Hoffmann P. Über eine Methode, den Erfolg einer Nervennaht zu beurteilen. Medizinische Klinik. 1915;11:359-360.
26 Tinel J. Nerve Wounds. Authorised translation Rothwll F. Revised and edited by Joll CA. London: Ballière Tindal and Cox; 1917.
27 Buck-Gramcko D, Lubahn JD. The Hoffmann-Tinel sign. 1915. J Hand Surg Br. 1993;18:800-805.
28 Henderson WR. Clinical assessment of peripheral nerve injuries; Tinel’s test. Lancet. 1948;2:801-805.
29 Abercombie M, Jonson M. The outwandering of cells in tissue cultures of nerves undergoing Wallerian degeneration. J Exp Biol. 1942;19:266-283.
30 Büngner OV. Über die Degenerations- und Regenerationsvorgänge am Nerven nach Verletzungen. Beitr Pathol Anat. 1891;10:321-393.
31 Levi-Montalcini R, Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951;116:321-361.
32 Richter HP. Reinnervation of skeletal muscle. A survey. Zentralbl Neurochir. 1991;52:109-117.
33 Richter HP. Impairment of motor recovery after late nerve suture: experimental study in the rabbit. Part 1: functional and electromyographic findings. Neurosurgery. 1982;10:70-74.
34 Brushart T. Nerve repair and grafting: degenerative changes in muscle. In: Green D, Hotchkiss R, Pederson W, editors. Green’s Operative Hand Surgery. 4th ed. New York: Churchill Livingstone; 1999:1384-1385.
35 Xu QG, Zochodne DW. Ischemia and failed regeneration in chronic experimental neuromas. Brain Res. 2002;946:24-30.
36 Ma J, Novikov LN, Kellerth JO, Wiberg M. Early nerve repair after injury to the postganglionic plexus: an experimental study of sensory and motor neuronal survival in adult rats. Scand J Plast Reconstr Surg Hand Surg. 2003;37:1-9.
37 Terenghi G, Calder JS, Birch R, Hall SM. A morphological study of Schwann cells and axonal regeneration in chronically transected human peripheral nerves. J Hand Surg Br. 1998;23:583-587.
38 West CA, Davies KA, Hart AM, et al. Volumetric magnetic resonance imaging of dorsal root ganglia for the objective quantitative assessment of neuron death after peripheral nerve injury. Exp Neurol. 2007;203:22-33.
39 McKay Hart A, Brannstrom T, Wiberg M, Terenghi G. Primary sensory neurons and satellite cells after peripheral axotomy in the adult rat: timecourse of cell death and elimination. Exp Brain Res. 2002;142:308-318.
40 Ma J, Novikov LN, Wiberg M, Kellerth JO. Delayed loss of spinal motoneurons after peripheral nerve injury in adult rats: a quantitative morphological study. Exp Brain Res. 2001;139:216-223.
41 Hall S. The response to injury in the peripheral nervous system. J Bone Joint Surg Br. 2005;87:1309-1319.
42 Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat. 1999;194:1-14.
43 Windebank A, Mcdonald E. Neurotrophic factors in the peripheral nervous system. In: Dyck P, Thomas P, editors. Peripheral Neuropathy. 4th ed. Philadelphia: Saunders; 2005:377-386.
44 Donaghy M. Lumbosacral plexus lesions. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. 4 ed. Oxford: Elsevier; 2005:1375-1390.
45 Glasby MA. Nitrogen freezing of muscle grafts. J Bone Joint Surg Br. 1993;75:507.
46 Fullarton AC, Glasby MA, Lawson GM. Immediate and delayed nerve repair using freeze-thawed muscle allografts. Associated long-bone fracture. J Hand Surg Br. 1998;23:360-364.
47 Glasby MA, Fullerton AC, Lawson GM. Immediate and delayed nerve repair using freeze-thawed muscle autografts in complex nerve injuries. Associated arterial injury. J Hand Surg Br. 1998;23:354-359.
48 Leclercq DC, Carlier AJ, Khuc T, et al. Improvement in the results in sixty-four ulnar nerve sections associated with arterial repair. J Hand Surg Am. 1985;10:997-999.
49 Merle M, Amend P, Cour C, et al. Microsurgical repair of peripheral nerve lesions: a study of 150 injuries of the median and ulnar nerves. Peripher Nerve Repair Regen. 1986;2:17-26.
50 Birch R, Raji AR. Repair of median and ulnar nerves. Primary suture is best. J Bone Joint Surg Br. 1991;73:154-157.
51 Kato N, Htut M, Taggart M, et al. The effects of operative delay on the relief of neuropathic pain after injury to the brachial plexus: a review of 148 cases. J Bone Joint Surg Br. 2006;88:756-759.
52 Shergill G, Bonney G, Munshi P, Birch R. The radial and posterior interosseous nerves. Results of 260 repairs. J Bone Joint Surg Br. 2001;83:646-649.
53 Osborne AW, Birch RM, Munshi P, Bonney G. The musculocutaneous nerve. J Bone Joint Surg Br. 2000;82:1140-1142.
54 Sunderland S, McArthur RA, Nam DA. Repair of a transected sciatic nerve. A study of nerve regeneration and functional recovery: report of a case. J Bone Joint Surg Am. 1993;75:911-914.
55 Wilkinson MC, Birch R. Repair of the common peroneal nerve. J Bone Joint Surg Br. 1995;77:501-503.
56 Sedel L, Nizard RS. Nerve grafting for traction injuries of the common peroneal nerve. A report of 17 cases. J Bone Joint Surg Br. 1993;75:772-774.
57 Birch R. Regeneration. Surgical Disorders of the Nerves. 2nd ed. London: Springer. 2010.
58 Woodhall B, Nulsen FE, White J, Davis L. Neurosurgical implications. In: Woodhall B, editor. Peripheral Nerve Regeneration. Washington, D.C.: Veterans Administration, US Govt. Printing Office; 1957:569-638.
59 Lyons WR, Woodhall B. Atlas of Peripheral Nerve Injuries. Philadelphia: Saunders; 1949.
59a Zachary RB. Results of nerve sutures. In: Seddon HJ, editor. Peripheral Nerve Injuries, 282. Medical Research Council; 1954:354-387. Special Report Series
60 Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491-516.
61 Millesi H, Rath T, Reihsner R, Zoch G. Microsurgical neurolysis: its anatomical and physiological basis and classification. Microsurgery. 1993;14:430-439.
62 Birch R. Reactions to injury. Surgical Disorders of the Nerves. 2nd ed. London: Churchill Livingstone. 2010.
63 Thomas PK, Berthold CH, Ochoa J. Microscopic anatomy of the peripheral nervous system. In: Dyck PJ, Thomas PK, Griffin JW, et al, editors. Peripheral Neuropathy. 3rd ed. Philadelphia: Saunders; 1993:38.
64 Thomas PK, Holdorff B. Neuropathy due to physical agents. In: Dyck PJ, Thomas PK, Griffin JW, et al, editors. Peripheral Neuropathy. 3rd ed. Philadelphia: Saunders; 1993:990-1014.
65 Stanec S, Tonkovic I, Stanec Z, et al. Treatment of upper limb nerve war injuries associated with vascular trauma. Injury. 1997;28:463-468.
66 Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008;119:1951-1965.
66a Birch R. Compound nerve injuries. Surgical Disorders of the Nerves. 2nd ed. London: Churchill Livingstone. 2010.
67 Kretschmer T, Antoniadis G, Braun V, et al. Evaluation of iatrogenic lesions in 722 surgically treated cases of peripheral nerve trauma. J Neurosurg. 2001;94:905-912.
68 Khan R, Birch R. Iatropathic injuries of peripheral nerves. J Bone Joint Surg Br. 2001;83:1145-1148.
69 Birch R. Iatrogenous injuries. Surgical Disorders of the Nerves. 2nd ed. London: Churchill Livingstone. 2010.
70 Kretschmer T, Antoniadis G, Borm W, Richter HP. [Iatrogenic nerve injuries. Part 1: Frequency distribution, new aspects, and timing of microsurgical treatment]. Chirurg. 2004;75:1104-1112.
71 Kretschmer T, Heinen C, Antoniadis G, et al. Iatrogenic nerve injuries. Neurosurg Clin N Am. 2009;20:73-90.
72 O’Brian B. Microsurgery in the treatment of injuries. In: McKibbin B, editor. Recent Advances in Orthopaedics. Edinburgh: Churchill Livingstone; 1975:235-279.
73 Kline DG, Happel LT. Penfield Lecture. A quarter century’s experience with intraoperative nerve action potential recording. Can J Neurol Sci. 1993;20:3-10.
74 Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116-122.
75 Midha R. Epidemiology of brachial plexus injuries in a multitrauma population. Neurosurgery. 1997;40:1182-1188.
76 Puckett W. Damage to peripheral nerves due to high velocity missiles without direct hit. Neurosurg Rev. 1946;3:294-299.
77 Persad IJ, Reddy RS, Saunders MA, Patel J. Gunshot injuries to the extremities: experience of a U.K. trauma centre. Injury. 2005;36:407-411.
78 Samardzic MM, Rasulic LG, Grujicic DM. Gunshot injuries to the brachial plexus. J Trauma. 1997;43:645-649.
79 Hadley GP, Mars M. Gunshot injuries in infants and children in KwaZulu-Natal: an emerging epidemic? S Afr Med J. 1998;88:444-447.
80 Stewart MP, Birch R. Penetrating missile injuries of the brachial plexus. J Bone Joint Surg Br. 2001;83:517-524.
81 Kline DG. Civilian gunshot wounds to the brachial plexus. J Neurosurg. 1989;70:166-174.
82 Secer HI, Daneyemez M, Gonul E, Izci Y. Surgical repair of ulnar nerve lesions caused by gunshot and shrapnel: results in 407 lesions. J Neurosurg. 2007;107:776-783.
83 Secer HI, Daneyemez M, Tehli O, et al. The clinical, electrophysiologic, and surgical characteristics of peripheral nerve injuries caused by gunshot wounds in adults: a 40-year experience. Surg Neurol. 2008;69:143-152.
84 Kline DG, Kim D, Midha R, et al. Management and results of sciatic nerve injuries: a 24-year experience. J Neurosurg. 1998;89:13-23.
85 Coupland RM. War Wounds of Limbs. Oxford: Butterworth-Heineman; 1993.
86 Bonney GLW. Iatropathic lesions of peripheral nerves. Curr Orthop. 1997;11:255-266.
87 Wilbourn AJ. Iatrogenic nerve injuries. Neurol Clin. 1998;16:55-82.
88 Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246-255.
89 Knight DM, Birch R, Pringle J. Benign solitary schwannomas: a review of 234 cases. J Bone Joint Surg Br. 2007;89:382-387.
90 Kretschmer T, Antoniadis G, Heinen C, et al. Nerve sheath tumor surgery: case-guided discussion of ambiguous findings, appropriateness of removal, repeated surgery, and nerve repairs. Neurosurg Focus. 2007;22:E19.
90a Birch R. Pain. Surgical Disorders of the Nerves. 2nd ed. London: Churchill Livingstone. 2010.
91 O’Brian M. Aids to the Examination of the Peripheral Nervous System, 4 ed. Philadelphia: Saunders; 2000.
92 Mackinnon S, Dellon A. Ischemia of nerve: loss of vibration sensibility. In: Mackinnon S, Dellon A, editors. Surgery of the Peripheral Nerve. New York: Georg Thieme Verlag; 1988:57.
93 Smith S. Electrodiagnosis. In: Birch R, Bonney G, Parry CW, editors. Surgical Disorders of the Peripheral Nerves. London: Churchill Livingstone; 1998:467-490.
94 Bonney G, Gilliatt R. Sensory nerve conduction after traction lesion of the brachial plexus. Proc R Coll Med. 1958;51:365-367.
95 Carvalho GA, Nikkhah G, Matthies C, et al. Diagnosis of root avulsions in traumatic brachial plexus injuries: value of computerized tomography myelography and magnetic resonance imaging. J Neurosurg. 1997;86:69-76.
96 Oberle J, Antoniadis G, Rath SA, et al. Radiological investigations and intra-operative evoked potentials for the diagnosis of nerve root avulsion: evaluation of both modalities by intradural root inspection. Acta Neurochir (Wien). 1998;140:527-531.
97 Kline DG, Hudson AR. Discussion of: Carvalho et al, JNS 86:69-76. J Neurosurg. 1997;87:483-484.
98 Filler AG, Kliot M, Howe FA, et al. Application of magnetic resonance neurography in the evaluation of patients with peripheral nerve pathology. J Neurosurg. 1996;85:299-309.
99 Filler AG, Maravilla KR, Tsuruda JS. MR neurography and muscle MR imaging for image diagnosis of disorders affecting the peripheral nerves and musculature. Neurol Clin. 2004;22:643-682. vi-vii
100 Seddon HJ. Nerve grafting. J Bone Joint Surg Br. 1963;45:447-461.
101 Landau WM. The duration of neuromuscular function after nerve section in man. J Neurosurg. 1953;10:64-68.
101a Trumble TE. Overcoming defects in peripheral nerves. In: Gelberman RH, editor. Operative Nerve Repair and Reconstruction. Philadelphia: JB Lippincott; 1991:507-524.
102 Giddins GE, Wade PJ, Amis AA. Primary nerve repair: strength of repair with different gauges of nylon suture material. J Hand Surg Br. 1989;14:301-302.
103 Kandenwein JA, Kretschmer T, Engelhardt M, et al. Surgical interventions for traumatic lesions of the brachial plexus: a retrospective study of 134 cases. J Neurosurg. 2005;103:614-621.
104 Birch R. Clinical aspects of nerve injuries. In Surgical Disorders of the Nerves, 2nd ed, London: Churchill Livingstone; 2010:7-14.
105 Gschmeissner SE, Pereira JH, Cowley SA. The rapid assessment of nerve stumps. J Bone Joint Surg Br. 1991;73:688-689.
106 Clark WL, Trumble TE, Swiontkowski MF, Tencer AF. Nerve tension and blood flow in a rat model of immediate and delayed repairs. J Hand Surg Am. 1992;17:677-687.
107 Urbaniak JR. Fascicular nerve suture. Clin Orthop Relat Res. 1982;163:57-64.
108 Orgell M. Epineurial versus perineurial repair of peripheral nerves. In: Tertzis J, editor. Microreconstruction of Nerve Injuries. London: Saunders; 1987:97-100.
109 Millesi H. Interfascicular nerve grafting. Orthop Clin North Am. 1981;12:287-301.
110 Skoulis TG, Lovice D, von Fricken K, Terzis JK. Nerve expansion. The optimal answer for the short nerve gap. Behavioral analysis. Clin Orthop Relat Res. 1995;314:84-94.
111 Hentz VR, Rosen JM, Xiao SJ, et al. The nerve gap dilemma: a comparison of nerves repaired end to end under tension with nerve grafts in a primate model. J Hand Surg Am. 1993;18:417-425.
112 Hudson AR, Hunter D. Polyglycolic acid suture in peripheral nerve II: sutured sciatic nerve. Can J Neurol Sci. 1976;3:69-72.
113 Ide C. Nerve regeneration and Schwann cell basal lamina: observations of the long-term regeneration. Arch Histol Jpn. 1983;46:243-257.
114 Becker S, Penkert G. In vivo visualization of the neuromuscular junction before and after autologous nerve transfer in the rat. J Reconstr Microsurg. 2000;16:111-120.
115 Narakas A. The use of fibrin glue in repair of peripheral nerves. Orthop Clin North Am. 1988;19:187-199.
116 Maragh H, Meyer BS, Davenport D, et al. Morphofunctional evaluation of fibrin glue versus microsuture nerve repairs. J Reconstr Microsurg. 1990;6:331-337.
117 Ballance C, Duel A. The operative treatment of facial palsy. Arch Otolaryngol. 1932;15:1-70.
118 Ortiguela ME, Wood MB, Cahill DR. Anatomy of the sural nerve complex. J Hand Surg Am. 1987;12:1119-1123.
119 Mahakkanukrauh P, Chomsung R. Anatomical variations of the sural nerve. Clin Anat. 2002;15:263-266.
120 Reissis N, Stirrat A, Manek S, Dunkerton M. The terminal branch of posterior interosseous nerve: a useful donor for digital nerve grafting. J Hand Surg Br. 1992;17:638-640.
121 Coert JH, Dellon AL. Clinical implications of the surgical anatomy of the sural nerve. Plast Reconstr Surg. 1994;94:850-855.
122 Park SB, Cheshier S, Michaels D, et al. Endoscopic harvesting of the sural nerve graft: technical note. Neurosurgery. 2006;58(suppl):ONS-E180.
123 Chang DW. Minimal incision technique for sural nerve graft harvest: experience with 61 patients. J Reconstr Microsurg. 2002;18:671-676.
124 Capek L, Clarke HM, Zuker RM. Endoscopic sural nerve harvest in the pediatric patient. Plast Reconstr Surg. 1996;98:884-888.
125 Midha R. Nerve transfers for severe brachial plexus injuries: a review. Neurosurg Focus. 2004;16:E5.
126 Narakas AO. Thoughts on neurotisation of nerve transfers in irreparable lesions. In: Tertzis J, editor. Microreconstruction of Nerve Injuries. Philadelphia: Saunders; 1987:447-454.
127 Narakas AO, Hentz VR. Neurotization in brachial plexus injuries. Indication and results. Clin Orthop Relat Res. 1988;237:43-56.
128 Tuttle H. Exposure of the brachial plexus with nerve transplantation. JAMA. 1913;61:15-17.
129 Vulpius O, Stoffel A. Orhopaedische Operationshehre. Stuttgart: Ferdinand Euke; 1913.
130 Foerster O. Die therapie der Schussverletzungen der Peripheren Nerve. In: Handbuch der Neurologie. Berlin: Julius Springer; 1929:1677-1691.
131 Yeoman PM, Seddon HJ. Brachial plexus injuries: treatment of the flail arm. J Bone Joint Surg Br. 1961;43:4933.
132 Fantis A, Slezak Z. On the surgical management of brachial plexus injuries. Acta Chir Orthop Traumatol Cech. 1967;34:301-309.
133 Nagano A, Tsuyama N, Ochiai N, et al. Direct nerve crossing with the intercostal nerve to treat avulsion injuries of the brachial plexus. J Hand Surg Am. 1989;14:980-985.
134 Doi K, Sakai K, Kuwata N, et al. Double free-muscle transfer to restore prehension following complete brachial plexus avulsion. J Hand Surg Am. 1995;20:408-414.
135 Leechavengvongs S, Witoonchart K, Uerpairojkit C, et al. Combined nerve transfers for C5 and C6 brachial plexus avulsion injury. J Hand Surg Am. 2006;31:183-189.
136 Leechavengvongs S, Witoonchart K, Uerpairojkit C, Thuvasethakul P. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, part II: a report of 7 cases. J Hand Surg Am. 2003;28:633-638.
137 Oberlin C, Beal D, Leechavengvongs S, et al. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: anatomical study and report of four cases. J Hand Surg Am. 1994;19:232-237.
138 Mackinnon SE, Novak CB, Myckatyn TM, Tung TH. Results of reinnervation of the biceps and brachialis muscles with a double fascicular transfer for elbow flexion. J Hand Surg Am. 2005;30:978-985.
139 Brandt KE, Mackinnon SE. A technique for maximizing biceps recovery in brachial plexus reconstruction. J Hand Surg Am. 1993;18:726-733.
140 Gu YD, Zhang GM, Chen DS, et al. Seventh cervical nerve root transfer from the contralateral healthy side for treatment of brachial plexus root avulsion. J Hand Surg Br. 1992;17:518-521.
141 Chen L, Gu YD. An experimental study of contralateral C7 root transfer with vascularized nerve grafting to treat brachial plexus root avulsion. J Hand Surg Br. 1994;19:60-66.
142 Gousheh J, Babaei A. A new surgical technique for the treatment of high common peroneal nerve palsy. Plast Reconstr Surg. 2002;109:994-998.
143 Battiston B, Lanzetta M. Reconstruction of high ulnar nerve lesions by distal double median to ulnar nerve transfer. J Hand Surg Am. 1999;24:1185-1191.
144 Ozkan T, Ozer K, Gulgonen A. Restoration of sensibility in irreparable ulnar and median nerve lesions with use of sensory nerve transfer: long-term follow-up of 20 cases. J Hand Surg Am. 2001;26:44-51.
145 Askar I, Sabuncuoglu BT. Superficial or deep implantation of motor nerve after denervation: an experimental study—superficial or deep implantation of motor nerve. Microsurgery. 2002;22:242-248.
146 Holle J. [Muscular neurotization in the field of reconstructive surgery (author’s transl)]. Wien Klin Wochenschr Suppl. 1976;48:3-21.
147 Brunelli GA. Direct neurotization of muscles by presynaptic motoneurons. J Reconstr Microsurg. 2001;17:631-636.
148 Mackinnon SE, McLean JA, Hunter GA. Direct muscle neurotization recovers gastrocnemius muscle function. J Reconstr Microsurg. 1993;9:77-80.
149 Becker M, Lassner F, Fansa H, et al. Refinements in nerve to muscle neurotization. Muscle Nerve. 2002;26:362-366.
150 Lundborg G, Hansson HA. Regeneration of peripheral nerve through a preformed tissue space. Preliminary observations on the reorganization of regenerating nerve fibres and perineurium. Brain Res. 1979;178:573-576.
151 Lundborg G, Rosen B, Dahlin L, et al. Tubular repair of the median or ulnar nerve in the human forearm: a 5-year follow-up. J Hand Surg Br. 2004;29:100-107.
152 Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004;26:151-160.
153 Dahlin LB, Lundborg G. Use of tubes in peripheral nerve repair. Neurosurg Clin N Am. 2001;12:341-352.
154 Mackinnon SE, Dellon AL. A study of nerve regeneration across synthetic (Maxon) and biologic (collagen) nerve conduits for nerve gaps up to 5 cm in the primate. J Reconstr Microsurg. 1990;6:117-121.
155 Archibald SJ, Krarup C, Shefner J, et al. A collagen-based nerve guide conduit for peripheral nerve repair: an electrophysiological study of nerve regeneration in rodents and nonhuman primates. J Comp Neurol. 1991;306:685-696.
156 Lundborg G. Alternatives to autologous nerve grafts. Handchir Mikrochir Plast Chir. 2004;36:1-7.
157 Schlosshauer B, Dreesmann L, Schaller HE, Sinis N. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery. 2006;59:740-747.
158 Dahlin L, Brandt J, Nilsson A, et al. Schwann cells, acutely dissociated from a predegenerated nerve trunk, can be applied into a matrix used to bridge nerve defects in rats. Acta Neurochir Suppl. 2007;100:57-59.
159 Nishiura Y, Brandt J, Nilsson A, et al. Addition of cultured Schwann cells to tendon autografts and freeze-thawed muscle grafts improves peripheral nerve regeneration. Tissue Eng. 2004;10:157-164.
160 Chen LE, Seaber AV, Urbaniak JR, Murrell GA. Denatured muscle as a nerve conduit: a functional, morphologic, and electrophysiologic evaluation. J Reconstr Microsurg. 1994;10:137-144.
161 Pereira JH, Palande DD, Narayanakumar TS, et al. Nerve repair by denatured muscle autografts promotes sustained sensory recovery in leprosy. J Bone Joint Surg Br. 2008;90:220-224.
162 Mackinnon SE. Nerve allotransplantation following severe tibial nerve injury. Case report. J Neurosurg. 1996;84;:671-676.
163 Larsen M, Habermann TM, Bishop AT, et al. Epstein-Barr virus infection as a complication of transplantation of a nerve allograft from a living related donor. Case report. J Neurosurg. 2007;106;:924-928.
164 Brenner MJ, Tung TH, Jensen JN, Mackinnon SE. The spectrum of complications of immunosuppression: is the time right for hand transplantation? J Bone Joint Surg Am. 2002;84:1861-1870.
165 Birch R, Dunkerton M, Bonney G, Jamieson AM. Experience with the free vascularized ulnar nerve graft in repair of supraclavicular lesions of the brachial plexus. Clin Orthop Relat Res. 1988;237:96-104.
166 Strange FG. An operation for pedicle nerve grafting. Br J Surg. 1947;34:423-425.
167 Bonney G, Birch R, Jamieson AM, Eames RA. Experience with vascularized nerve grafts. Clin Plast Surg. 1984;11:137-142.
168 Birch R, Bonney G, Parry CW. Reimplantation of avulsed spinal nerves. In: Surgical Disorder of the Peripheral Nerves. London: Churchill Livingstone; 1998:201-207.
169 Bonney G, Jamieson AM. Reimplantation of C7 and C8. Communication—Au syposium sur le plexus brachial. Int Microsurg. 1979;1:103-106.
170 Carlstedt T, Grane P, Hallin RG, Noren G. Return of function after spinal cord implantation of avulsed spinal nerve roots. Lancet. 1995;346:1323-1325.
171 Carlstedt T, Anand P, Hallin R, et al. Spinal nerve root repair and reimplantation of avulsed ventral roots into the spinal cord after brachial plexus injury. J Neurosurg. 2000;93(suppl):237-247.
172 Carlstedt T. Central Nerve Plexus Injury. London: Imperial College Press; 2007.
172a Carlstedt T, Hultgren T, Nyman T, et al. Cortical activity and hand function restoration in a patient after spinal cord surgery. Nat Rev Neurol. 2009;5:571-574.
173 Fournier HD, Mercier P, Menei P. Lateral interscalenic multilevel oblique corpectomies to repair ventral root avulsions after brachial plexus injury in humans: anatomical study and first clinical experience. J Neurosurg. 2001;95(suppl):202-207.
174 Cloward RB. Cervical cordotomy by the anterior approach. J Neurosurg. 1964;21:19-25.
175 Fournier HD, Mercier P, Menei P. Repair of avulsed ventral nerve roots by direct ventral intraspinal implantation after brachial plexus injury. Hand Clin. 2005;21:109-118.
176 Fournier HD, Mercier P, Menei P. [Spinal repair of ventral root avulsions after brachial plexus injuries: Towards new surgical strategies?]. Neurochirurgie. 2006;52:357-366.
177 VA Medical Monograph. Woodhall B, Beebe G, editors. Peripheral Nerve Regeneration: A Follow-Up Study of 3656 WWII Injuries. Washington DC: US Government Printing Office, 1956.
178 Seddon H. Peripheral Nerve Injuries. London: Her Majesty’s Stationary Office; 1954.
179 Lundborg G. A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg Am. 2000;25:391-414.
180 Omer GEJr. Tendon transfers in combined nerve lesions. Orthop Clin North Am. 1974;5:377-387.