Chapter 54 Management of acute pancreatitis and complications
Overview
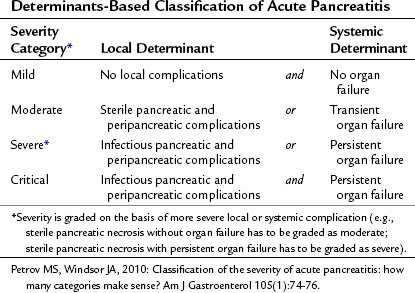
The severity of acute pancreatitis (AP) varies from mild uncomplicated disease to critical disease associated with both local and systemic complications (see Chapters 52 and 53). It is important to determine the severity of acute pancreatitis in the individual patient for triage, treatment, and prognosis. Since 1992, when the International Symposium on Acute Pancreatitis in Atlanta published its consensus, it has become customary to define the severity of acute pancreatitis as either mild or severe (Bradley, 1993). Acute pancreatitis is severe when it is associated with local or systemic complications. Although the Atlanta classification was a significant advance, it is now accepted that the dichotomous approach fails to capture clinically relevant categories of patients (Petrov & Windsor, 2010). For instance, it does not allow for discrimination among patients with transient and persistent organ failure, those with sterile and infectious local complications, and those with either local or systemic complications.
A large body of evidence now demonstrates that the two key determinants of severity in acute pancreatitis are organ failure—absent, transient, or persistent—and pancreatic complications—absent, noninfectious, or infectious. Determinants-based classification of the severity of acute pancreatitis composed of four categories (Table 54.1) appears to be more useful for the clinical assessment of severity in individual patients and for comparing groups of patients (Petrov & Windsor, 2010). The distinct advantages of this new classification is that it uses widely accepted and unambiguous terms, it can be applied in both early and late phases of acute pancreatitis, it can facilitate communication between treating physicians, and it promotes standardization for management of acute pancreatitis (Petrov et al, 2010).
Although many aspects of the management of acute pancreatitis remain controversial, significant overall progress has been made during the last few decades, evidenced by a reduction in morbidity and mortality rates (Lowenfels et al, 2009; Banks & Freeman, 2006). The improved outcomes have not been due to any treatments based on specific, critical pathophysiology. A wide range of drugs has been evaluated in randomized controlled trials (RCTs) and have proved ineffective in the treatment of acute pancreatitis. These include aprotinin, atropine, calcitonin, fresh frozen plasma, glucagon, gabexate, glucocorticoids, lexipafant, nonsteroidal antiinflammataory drugs (NSAIDs), and octreotide. The overall improvement in outcome for patients with acute pancreatitis has been due to a combination of factors that include improvements in intensive care medicine, imaging techniques, severity prediction, and selection of patients for endoscopic retrograde cholangiopancreatography (ERCP) and surgery.
General Management
Pain Management
Pain is the cardinal symptom of acute pancreatitis, and its relief is a clinical priority. The strategy in patients with acute pancreatitis, as for all acute pain, is the staged use of analgesics. Although some debate still surrounds step-up and step-down approaches, it is best practice to use NSAIDs for the management of mild pain (Pezzilli et al, 2007). The potential risk of bleeding as a result of the antiplatelet effect of NSAIDS has not been an issue in practice. For patients with moderate and severe pain, and for those for whom NSAIDs are inadequate, the second step is to use a weak opioid; the third step is to use a strong opioid.
Although experimental findings show that morphine can induce spasm of the sphincter of Oddi and exacerbate the severity of acute pancreatitis, it is worth noting that the avoidance of morphine as an analgesic has not been supported by clinical studies (Economou & Ward-McQuaid, 1971). A comprehensive review of the literature shows that all opiates increase the pressure in the sphincter of Oddi but that physiologic effect is of marginal clinical relevance (Thompson, 2001).
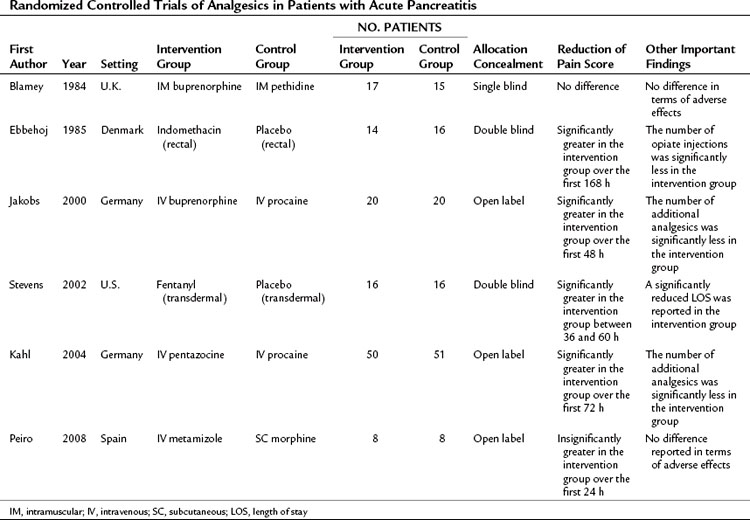
Different analgesics have been compared in patients with acute pancreatitis, and the six published RCTs are summarized in Table 54.2 (Blamey et al, 1984; Ebbehoj et al, 1985; Jakobs et al, 2000; Kahl et al, 2004; Peiro et al, 2008; Stevens et al, 2002). These trials had a different study design, evaluated different analgesics, and had small sample sizes; only three of the trials were double-blinded. From these studies it can be said that no credible clinical evidence supports avoiding the use of morphine in treating the pain associated with acute pancreatitis; however, the NSAID of choice is metamizole (2 g/8 h IV), and the opioid of choice is buprenorphine (0.3 g/4 h IV). There is no evidence to support the use of parenterally administered local anesthetics such as procaine in the management of pain associated with acute pancreatitis. However, the lack of high-quality evidence means that the choice of analgesic and the strategy for administration remain uncertain. No recent guidelines relating to the management of acute pancreatitis provide a specific recommendation regarding pain management (Loveday et al, 2010).
Fluid Resuscitation
Acute pancreatitis can be associated with substantial third-space fluid losses, and the resultant hypovolemia that impairs the microcirculation of the pancreas is a major determinant of pancreatic necrosis. The reflex splanchnic vasoconstriction in response to hypovolemia may compound pancreatic hypoperfusion and further predispose to ischemia. Fluid resuscitation is one of the most important aspects of the early management of acute pancreatitis (Gardner et al, 2008; Talukdar & Vege, 2009) and is the intervention most likely to improve outcome. No high-level evidence describes the optimal resuscitation fluid, required fluid rate, or best marker to guide resuscitation and indicate its adequacy (Banks & Freeman, 2006; Pandol et al, 2007). It is not even known whether colloids or crystalloids are more effective in improving pancreatic microcirculation and outcome. The initial goal of fluid resuscitation is to restore circulating blood volume (euvolemia), with the aim of normalizing heart rate, blood pressure, central venous pressure, and urine output, even though these do not reflect the adequacy of pancreatic and splanchnic perfusion (Flint & Windsor, 2003). In general, urine output should be restored at greater than 0.5 mL/h/kg body weight, and the central venous pressure should be restored to between 8 and 12 cm H2O. Swan-Ganz monitoring can be helpful in hemodynamically unstable patients, and some evidence shows that as an independent risk factor of pancreatitis severity and a marker of hydrations status, hematocrit might be useful with fluid resuscitation (Pitchumoni et al, 2005; Wu et al, 2009). Adequate fluid resuscitation is likely when the hematocrit is restored to between 30% and 35%. A number of other approaches to guide resuscitation offer promise but have not become established in clinical practice. An example is the use of intramucosal pH (pHi) derived by nasogastric tonometry (Juvonen et al, 2000a; van Haren et al, 2007), which is predictive of severity and reflects the adequacy of perfusion in the splanchnic circulation.
Antibiotics
Although the use of broad-spectrum antibiotics to treat the established infection in acute pancreatitis is a well-established practice, the use of prophylactic antibiotics has been controversial for decades. Three RCTs in the 1970s failed to demonstrate a beneficial effect of antibiotic prophylaxis, probably because of a small sample size, inappropriate selection of antibiotics—such as ampicillin, which does not sufficiently penetrate the pancreas—and inclusion of patients with mild pancreatitis (Table 54.3; Craig et al, 1975; Finch et al, 1976; Howes et al, 1975). Between 1993 and 2009, several randomized, controlled, open-label trials were published evaluating the efficacy of prophylactic antibiotic treatment in patients with severe acute pancreatitis (Delcenserie et al, 1996; Pederzoli et al, 1993; Rokke et al, 2007; Sainio et al, 1995; Schwarz et al, 1997; Spicak et al, 2003; Xue et al, 2009). The results of these trials were conflicting. Although some RCTs demonstrated a significant reduction of infectious complications and mortality with the use of prophylactic antibiotics, others failed to do so (see Table 54.3). Only three double-blind, placebo-controlled RCTs were published between 2004 and 2009, and all of them were unable to show a beneficial effect of antibiotic prophylaxis regarding infectious pancreatic complications, the need for surgery, and mortality (Dellinger et al, 2007; Garcia-Barrasa et al, 2009; Isenmann et al, 2004). This is in line with the findings of a meta-analysis that showed an inverse relationship between methodologic quality of the studies and impact of antibiotic prophylaxis on mortality (de Vries et al, 2007).
Table 54.3 Randomized Controlled Trials of Intravenous Antibiotic Prophylaxis vs. No Antibiotics in Patients with Acute Pancreatitis
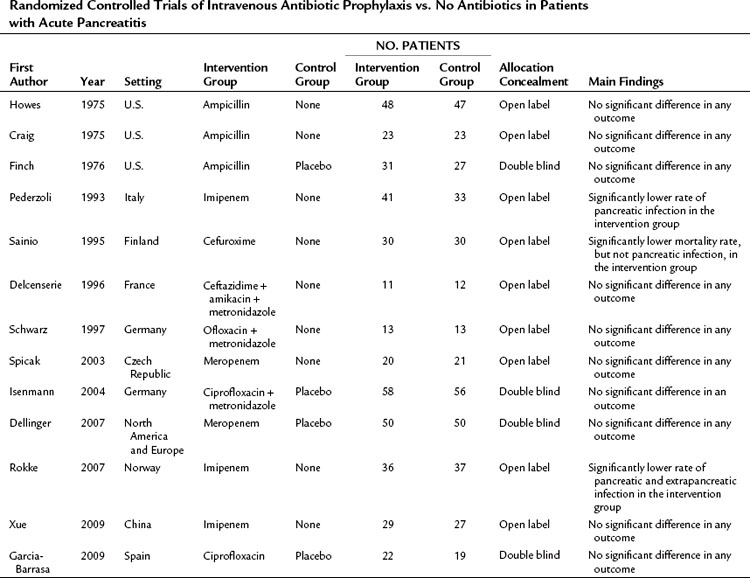
Several attempts have been made to statistically aggregate data on the use of prophylactic antibiotics in acute pancreatitis. Although only two new RCTs were published from 2006 through 2007, it is notable that seven of the 10 meta-analyses were published within this period (Petrov, 2008). Thirteen RCTs were included in these seven meta-analyses. Because of different inclusion criteria and various meta-analysis techniques used, concordance is lacking, and they provide contradictory recommendations regarding the role of prophylactic antibiotics in reducing the risk of pancreatic infectious complications. Overall, it appears that the most recent studies do not support the use of prophylactic antibiotics to reduce the frequency of pancreatic infectious complications, surgical intervention, and death in patients with acute pancreatitis. Therefore routine broad-spectrum prophylactic antibiotics in patients with severe acute pancreatitis cannot be recommended on the basis of current evidence.
Nutritional Management
Severe acute pancreatitis is associated with a cytokine-mediated systematic inflammatory response and a hypercatabolic state (Windsor & Hammodat, 2000; see Chapter 10). The adverse consequences of this include protein-calorie malnutrition, expansion of the extracellular fluid compartment, and immune suppression. A meta-analysis of RCTs showed that nutritional support, both enteral and parenteral, significantly reduced risk of mortality in patients with acute pancreatitis compared with no nutritional support (Petrov et al, 2008c). Nutritional support is thus an essential part of the management of patients with severe acute pancreatitis (Banks & Freeman, 2006; Pandol et al, 2007).
Type of Feeding
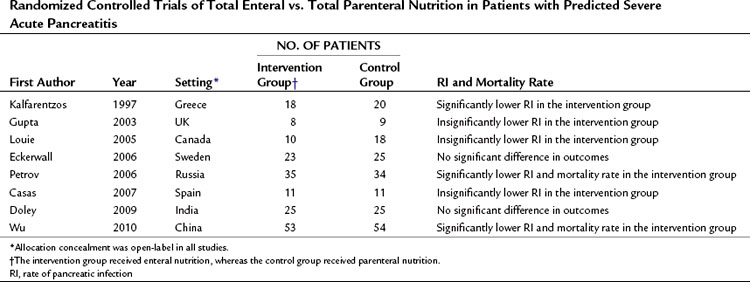
A number of RCTs compared total PN and total EN in the management of predicted severe AP (Table 54.4; Casas et al, 2007; Doley et al, 2009; Eckerwall et al, 2006; Gupta et al, 2003; Kalfarentzos et al, 1997; Louie et al, 2005; Petrov et al, 2006; Wu et al, 2010). A meta-analysis of high-quality RCTs has shown a significant twofold reduction in the risk of total and pancreatic infectious complications and a 2.5-fold reduction in the risk of death in patients receiving total EN (Petrov et al, 2008f). The basis for the clinical benefits of EN over PN remain unclear, although EN may prevent or attenuate mucosal barrier failure and bacterial translocation (Windsor & Hammodat, 2000). Intestinal permeability studies are a surrogate marker for clinically relevant events and provide conflicting results. Three clinical studies showed increased intestinal permeability to both micromolecules and macromolecules in patients with severe acute pancreatitis when compared with mild acute pancreatitis and healthy volunteers (Ammori et al, 1999; Juvonen et al, 2000b; Nagpal et al, 2006). But an early RCT from the United Kingdom (Powell et al, 2000) in which patients with predicted severe AP were randomized to receive either EN or no artificial nutritional support showed significantly increased intestinal permeability by day 4 in patients allocated to the EN group. Similarly, a recent RCT from Sweden comparing nasogastric EN and PN in patients with predicted severe acute pancreatitis demonstrated impaired gut permeability on day 3 in the EN group (Eckerwall et al, 2006). This difference might be explained by the inclusion of a considerable number of patients with mild AP in whom intestinal permeability is unlikely to change (Petrov et al, 2008f).
Route of Enteral Feeding
Nasogastric (NG) tube insertion may be more practical than nasojejunal (NJ), as the latter often requires endoscopy or radiology expertise, the transfer of patients within the hospital, and a delay in starting feeding. However, NJ EN has been preferred to NG EN because of a fear about pancreatic stimulation. Pancreatic response to feeding was studied in human volunteers, and it was shown that all forms of EN, with the exception of NJ feeding, stimulate pancreatic secretion (O’Keefe et al, 2003). By contrast, a study in patients with acute pancreatitis showed a significantly lower rate of secretion of trypsin, amylase, and lipase in comparison with healthy subjects (O’Keefe et al, 2005). Moreover, it was shown that the greater the severity of acute pancreatitis, the greater the reduction in pancreatic enzyme secretion as a response to duodenal feeding, probably reflecting greater injury of acinar cell mass. The possible clinical implication of these findings is that NG EN may not aggravate the course of acute pancreatitis, as was previously suggested.
Two RCTs have compared NG EN with NJ EN in patients with severe acute pancreatitis (Eatock et al, 2005; Kumar et al, 2006). These studies demonstrated the feasibility, safety, and tolerance of NG EN, but a meta-analysis did not demonstrate a statistically significant reduction in risk of death (Petrov et al, 2008b). Before NG EN can be considered the standard of care in the nutritional support of patients with AP, a well-designed and adequately powered RCT comparing mortality and morbidity of NG EN and NJ EN is required. A practical compromise is to start EN through an NG tube after the patient has been resuscitated and for the tube to be advanced when endoscopic or radiologic assistance is available.
Type of Enteral Feeding
The concept of “pancreatic rest” has also influenced decisions about the type and content of enteral feeding. The preferred formulations, elemental and semi-elemental formulas (Roberts, 2001; Petrov, 2007), did not require pancreatic enzymes for digestion and absorption. However, the major disadvantage of elemental and semi-elemental formulas is the cost, which is reportedly threefold to sevenfold higher than that of polymeric formulas. A recent meta-analysis of RCTs compared these two formula types in terms of feeding intolerance, infectious complications, and mortality and found that the use of polymeric over elemental and semi-elemental feedings did not result in reduced tolerance in patients with acute pancreatitis and appeared to reduce the risk of infectious complications and death (Petrov et al, 2009a). Thus the use of elemental and semi-elemental formulas confers no apparent advantage over relatively inexpensive polymeric formulas.
Because the gastrointestinal tract is the largest immune organ, containing approximately 65% of the immune tissue in the body, it has been considered that the use of immune-enhanced enteral formulations might increase the beneficial effects of EN in acute pancreatitis (Bengmark, 1999; Schloerb, 2001). Several trials were performed in different clinical settings, which suggested that immunonutrition might have the potential to modify the inflammatory response. The results of RCTs that compared the use of immune-enhanced and standard enteral formulas were statistically aggregated in several meta-analyses (Beale et al, 1999; Heyland et al, 2001; Heys et al, 1999). The most recent and comprehensive systematic review of 2419 patients from 22 RCTs (Heyland et al, 2001) found that the effect of immune-enhancing EN may depend on the subset of the analyzed patients. In particular, no effect of immunonutrition on the risk of infectious complications or death was reported within the subgroup of critically ill patients. At the same time, administration of a formula high in arginine in a combined group of critically ill and elective surgery patients was associated with a statistically significant reduction in infectious complications and a trend toward lower mortality rates in comparison with other immune-enhancing diets. A recent meta-analysis of RCTs in patients with acute pancreatitis did not show any clinical beneficial effect of immunonutrition when compared with standard EN (Petrov et al, 2008a).
Tolerance of Enteral Feeding
Dieticians generally agree on the caloric target in patients with acute pancreatitis: 30 kcal/kg and 1.5 g/kg of protein daily based on ideal body weight (Andersson et al, 2009; Olah & Romics, 2008). To ensure tolerance of EN, it is usually commenced at a low rate of 25 to 30 mL/h and increased incrementally over a day or more, until the desired caloric intake is reached. Despite this approach, EN can be associated with feeding intolerance in some patients with acute pancreatitis. Most commonly, the intolerance manifests as abdominal distension, delayed gastric emptying, gastroesophageal reflux, and diarrhea, all of which may develop in the presence of sepsis or with a significant clinical deterioration. In general, tolerance is achieved when EN is provided without development of one of these complications (Bankhead et al, 2009; Mallampalli et al, 2000). Various strategies to improve tolerance to EN require clinical studies, which are also needed to investigate markers of gut motility, absorption, and blood flow that can be easily applied at the bedside.
Timing of Enteral Feeding
The best time to start EN in patients with acute pancreatitis has never been studied. The indirect evidence in regard to this is derived from trials of EN versus PN only. Some authors have demonstrated clinical benefits of early enteral nutrition, but others have demonstrated the favorable effects of delayed enteral feeding (Petrov et al, 2009b). Early EN should help maintain gut integrity (enterocyte population) and function (motility) and reduce bacterial translocation and ileus. It should also help to achieve caloric targets more quickly. But early nutrition is not without risk, particularly in hemodynamically unstable patients and in those requiring inotropic support. These patients appear to be at an increased risk of nonocclusive mesenteric ischemia, and it is best to commence EN after adequate resuscitation.
Therapeutic Endoscopic Retrograde Cholangiopancreatography
Endoscopic retrograde cholangiopancreatography (ERCP) with endoscopic sphincterotomy (ES) has been promoted as a proven intervention in patients with acute biliary pancreatitis since the early 1990s (see Chapters 18 and 27). This was based on the findings of two RCTs, from the United Kingdom and Hong Kong, of early ERCP (within 24 to 48 hours of admission) with or without ES versus conservative treatment (Neoptolemos et al, 1988; Fan et al, 1993). Both trials demonstrated that early ERCP was associated with a reduction in complications, but not in mortality, and only in patients with predicted severe acute pancreatitis.
Some evidence suggests that the duration of biliary obstruction, rather than the predicted severity of acute pancreatitis, is the most important determinant of outcome (Acosta et al, 1997, 2006). This is probably due to concomitant cholangitis secondary to the obstruction and probably best explains the usefulness of ERCP in the context of acute biliary pancreatitis (Petrov, 2009). The first multicenter RCT to examine the role of ERCP in acute pancreatitis was designed to include only patients without evidence of biliary obstruction (Folsch & Neoptolemos, 2002). This German study did not find any benefit of early ERCP (within 72 h after onset of symptoms) over conservative treatment. The most recent RCT, from Argentina, found that early ERCP in patients with biliary obstruction, defined by laboratory and radiologic criteria, and without evidence of acute cholangitis conferred no benefit (Oria et al, 2007).
Two important meta-analyses were published in 2008. The first found that compared with conservative treatment, early ERCP in patients with both predicted mild and predicted severe acute pancreatitis did not decrease the incidence of local pancreatic complications or mortality rate (Petrov et al, 2008d). The second meta-analysis was designed to negate the confounding effect of acute cholangitis and demonstrated no benefit of early ERCP over conservative treatment in terms of complications and mortality in patients with predicted mild and predicted severe acute pancreatitis (Petrov et al, 2008e). The conclusion to be drawn from these studies is that early ERCP is indicated in patients with acute pancreatitis if acute cholangitis is evident (see Chapter 43) but not for those with just cholestasis (Petrov, 2009). Although cholestasis can reflect a persisting main bile duct stone, it might also be due to edema of the ampulla secondary to stone passage to the duodenum and may thus be expected to improve over the first few days of admission. Persistent cholestasis without cholangitis may require an ERCP but not usually in the acute setting.
Cholecystectomy
Recurrent attacks of gallstone pancreatitis carry a morbidity rate of up to 40% (Banks & Freeman, 2006), and an early laparoscopic cholecystectomy is widely advocated within the same hospital admission (see Chapter 30). The optimal timing of cholecystectomy has been controversial. The U.K. guidelines for the management of acute pancreatitis recommend that all patients with biliary pancreatitis undergo definitive management of gallstones during the same hospital admission (index cholecystectomy; Working Group, 2005). However, some surgeons have concerns that early surgery may be associated with an increase in operative difficulty and hence morbidity and conversion rate; therefore they advocate cholecystectomy after a period of 4 to 6 weeks to allow resolution of the inflammatory process (interval cholecystectomy; Green, 2008; Larson et al, 2006). These two approaches have been compared in several RCTs, which demonstrated no difference in complication rates between index and interval cholecystectomy; however, index cholecystectomy resulted in a significantly shorter length of hospital stay (Siddiqui et al, 2008). It is worth mentioning that patients with acute biliary pancreatitis were excluded from these trials. An adequately powered RCT of index versus interval cholecystectomy is needed to draw a firm conclusion about the optimal timing of cholecystectomy in patients with acute biliary pancreatitis.
Management of Systemic Complications
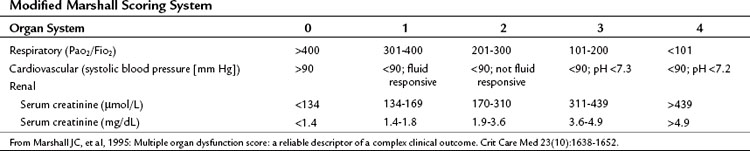
Most patients with acute pancreatitis have an initial sudden inflammatory respiratory syndrome response, others develop multiple organ dysfunction syndrome (MODS) in the first few days, and some develop MODS later in response to infectious complications (see Chapters 52 and 53). Patients with severe and critical acute pancreatitis are best managed in an intensive care environment to allow for optimal monitoring of fluid resuscitation and organ function and the early identification of life-threatening local or systemic complications. Systemic complications and organ failure are not an all-or-nothing phenomenon, rather a continuous spectrum exists between normal function of an organ system and its complete failure (Table 54.5; Marshall et al, 1995).
Some of the systemic complications of acute pancreatitis—coagulation abnormalities that may range from intravascular thrombosis to disseminated intravascular coagulation, metabolic disturbances (hyperlipidemia, hypocalcemia), liver failure, encephalopathy, and Purtscher retinopathy—are rare in clinical practice and beyond the scope of this chapter. In contrast, respiratory, cardiovascular, renal, and intestinal dysfunction are the most common systemic complications (Banks & Freeman, 2006; Pandol et al, 2007).
Respiratory failure is the most common systemic complication. The pathogenesis involves inflammatory cytokines released from the pancreas and the action of phospholipase A2 and other pancreatic catabolic enzymes on the lung. The clinical diagnosis of incipient respiratory failure is based on tachypnea and low oxygen saturation. A chest radiograph may confirm pleural effusions, pulmonary edema, and features of acute respiratory distress syndrome. Management is supportive, with oxygen supplementation and analgesia, and some patients will ultimately require endotracheal intubation and mechanical ventilation. Lung-protective ventilation strategies should be instituted, such as adequate positive end-expiratory pressures, permissive hypercapnia, and tidal volumes of 6 mL/kg ideal body weight (Nathens et al, 2004).
Patients with severe and critical acute pancreatitis can develop hypotension (arterial pressure <90 mm Hg) secondary to vasodilation and decreased systemic vascular resistance, and sometimes as a result of cardiotoxicity. Appropriate management consists of close hemodynamic monitoring, intravenous fluid resuscitation, and inotropic medications—dopamine at 2 to 10 µg/kg/min or dobutamine at 2 to 10 µg/kg/min—if indicated (Tonsi et al, 2009).
In the setting of acute pancreatitis, acute renal failure is usually secondary to decreased renal perfusion pressure with hypovolemia and is sometimes secondary to ischemic acute tubular necrosis. The clinical diagnosis is based on acute oliguria and an acute elevation in blood urea nitrogen and serum creatinine (>0.5 mg/dL or 44 mmol/L; Nathens et al, 2004; Tonsi et al, 2009). Treatment is supportive and focused on restoring renal perfusion and providing dialysis if required.
Management of Local Complications
Type of Intervention
Another important consideration is the type of intervention. There are many different interventions, and the challenge is to select the intervention that is appropriate for the particular local complication, taking into account the anatomic location, infection status, complexity of any target lesions, the physiologic status and comorbidity of the individual patient, and the availability of expertise with the type of intervention. A review of current guidelines highlights the absence of level 1 evidence to guide decision making regarding the types of intervention (Loveday et al, 2008). Two broad philosophies are evolving in this regard. Many consider open surgical drainage with necrosectomy to be the gold standard in the management of infected pancreatic necrosis, and they reserve less invasive interventions for subsequent complications, such as percutaneous tube drainage of residual fluid complications. Such a step-down approach contrasts with the approach that advocates starting with less invasive interventions, such as percutaneous or endoscopic drainage, and only employing open surgical techniques later in the disease course in those who fail to respond (i.e., the step-up approach). These two approaches have been subjected to an RCT demonstrating that a minimally invasive step-up approach results in a reduced rate of the composite endpoint of major complications or death in patients with necrotizing pancreatitis and suspected or confirmed infected necrotic tissue (van Santvoort et al, 2010).
Classification of Interventions
In response to the need to standardize the description of invasive interventions to facilitate communication, comparison, and controlled trials (Windsor, 2007), progress has been made with the introduction of the VRP classification system, based on the ICD-10 approach (Loveday et al, 2011). It uses three dimensions, combining the method of visualization of the lesion, route taken to reach the lesion, and the purpose of the intervention (Table 54.6). Visualization includes open procedures, in which the operative site is exposed through the skin incision; endoscopic procedures, in which the operative site is visualized with an endoscope (gastroscope, laparoscope, or nephroscope); radiologic procedures that use computed tomography (CT), ultrasound, or fluoroscopy to visulalize lesions; and hybrid procedures that combine endoscopic and radiologic techniques. The routes taken by these interventions include the external route into the body, by mouth or gastrostomy tube, or the percutaneous route, through the skin; the internal routes used to reach a target lesion might pass through the gastrointestinal wall, peritoneum, or retroperitoneum.
| V1 Radiologic | Using only radiologic modalities (e.g., fluoroscopy, CT, US, MR) to visualize and assist entering the target lesion |
| V2 Endoscopic | Using any white-light endoscopic instrument (e.g., flexible or rigid endoscope, urologic endoscope) to visualize the target lesion |
| V3 Combined | Using an endoscopic technique as the primary mode of visualization, assisted by a real-time radiologic modality |
| V4 Open | Using any method in which skin and any other body layers are cut to expose the site of the procedure |
| Vx | Insufficient information |
| Vz | Other visualization technique |
| R1 Per os transpapillary | External orifice entry point, internal route traversing papilla to enter pancreatic duct |
| R2 Per os transmural | External orifice entry point, internal route traversing gastrointestinal wall |
| R3 Percutaneous retroperitoneal | Skin external entry point, internal route traversing retroperitoneum |
| R4 Percutaneous transperitoneal | Skin external entry point, internal route traversing peritoneum |
| R5 Percutaneous transmural | Skin external entry point, internal route traversing gastrointestinal wall |
| Rx | Insufficient information |
| Rz | Other route |
| P1 Drainage | Letting out fluid and/or solid necrotic matter, externally out of the body or internally into the gastrointestinal tract |
| P2 Lavage | Flushing away solid necrotic matter with fluid to facilitate external or internal drainage |
| P3 Fragmentation | Breaking down solid necrotic matter by instrumental or mechanical disruption to facilitate external or internal drainage |
| P4 Debridement | Taking or cutting out solid necrotic matter with either sharp or blunt dissection |
| P5 Excision | Cutting out all or part of the pancreas, including healthy tissue, with the intention to fully remove all necrotic matter |
| Px | Insufficient information |
| Pz | Other purpose |
Procedures should be classified using a single value from each component, and the highest applicable value should be selected.
CT, computed tomography; US, ultrasound; MR, magnetic resonance
Loveday BP, et al, 2011: A comprehensive classification of invasive procedures for treating the local complications of acute pancreatitis based on visualization, route, and purpose. Pancreatology 11:406-413.
Open Surgical Necrosectomy
Three general variations of open necrosectomy are currently practiced: 1) open necrosectomy with open or closed packing, 2) open necrosectomy with continuous closed postoperative lavage, and 3) programmed open necrosectomy. Although these are broadly similar in terms of the necrosectomy, they differ in terms of how they provide egress routes for infected fluid, debris, and tissue. The abdomen is entered though a midline or bilateral subcostal incision. The latter is preferred, as it minimizes contamination of the greater sac and allows easy access to the extremities of the gland. The pancreas is exposed by dividing the gastrocolic omentum or gastrohepatic omentum to access the pancreas through the lesser sac. The body and tail of the pancreas (Fig. 54.1) can also be exposed by elevating the transverse colon and gaining access to the lesser sac via the transverse mesocolon (Fig. 54.2). Inflammatory adhesions may exist between the pancreas and stomach or transverse mesocolon, and great care is required during exposure. It is generally useful to take down both the hepatic and splenic flexures, if possible, as this will facilitate exposure and reduce the risk of colonic fistula secondary to drain erosion. When the process involves the head of the pancreas, access might require medial mobilization of the duodenum.
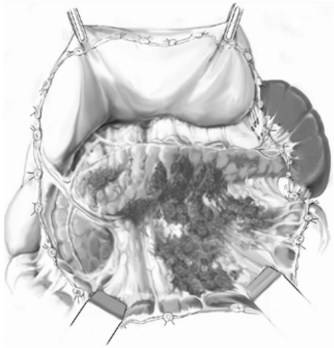
FIGURE 54.1 Access to the pancreas via the gastrocolic omentum.
(From Uhl W, et al, 2007: Necrosectomy. In Clavien P-A, et al (eds): Atlas of Upper Gastrointestinal and Hepato-Pancreato-Biliary Surgery. New York, Springer, pp 893-915.)
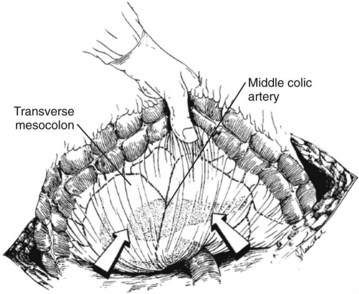
FIGURE 54.2 Access to the pancreas via the transverse mesocolon.
(From Mithofer K, et al, 1997: Interventional and surgical treatment of pancreatic abscess. World J Surg 21[2]:162-168.)
Open Necrosectomy with Closed Packing
The goal of necrosectomy with closed packing is to perform a single operation by performing a thorough debridement and removal of necrotic and infected tissue to minimize the need for reoperation or subsequent drainage (Pezzilli et al, 2007). Primary closure of the abdomen is the intention. Once the necrotic cavity is opened, fluid collections are drained, and the exposure is enlarged to allow debridement of all areas of necrosis, including those in the perirenal and paracolic spaces. Early advocates used gauze-stuffed Penrose drains placed via separate stab incisions, but many variations are in practice with regard to the type and number of drains. Between 2 and 12 drains are placed with the intention to fill the cavity and provide some compression. Additional silicone drains (Jackson-Pratt) are placed in the pancreatic bed and lesser sac for fluid drainage. The stuffed Penrose drains are removed one every other day, starting 5 to 7 days postoperatively, allowing a slow collapse of the cavity. The silicone drains are removed later.
Open Necrosectomy with Open Packing
The difference with this approach is that the abdomen is left open after debridement and packing of the pancreatic bed, lesser sac, and retroperitoneum (Gotzinger, 2008). Drains are placed in addition to the packing. Sometimes the abdomen is closed, and the packing is accessed via separate flank incisions (Nakasaki et al, 1999). Open packing techniques have been reported to have higher incidences of fistulae, bleeding, and incisional hernias as well as a slightly higher mortality rate (Heinrich et al, 2006). However, it should be noted that no prospective trials have compared open packing with any other technique.
Open Necrosectomy with Continuous Closed Postoperative Lavage
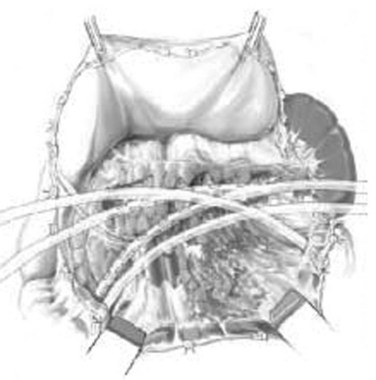
In this approach, debridement is followed by continuous peripancreatic lavage to remove infected necrotic debris, peripancreatic exudates, and extravasated pancreatic exocrine fluid (Fernandez-del Castillo et al, 1998). The large silicone drainage catheters are placed into the pancreatic bed to inflow and outflow isotonic irrigation fluid (Fig. 54.3). During closure, a closed peripancreatic compartment is made by resuturing the gastrocolic and duodenocolic ligaments. Postoperative continuous lavage is instituted at 1 to 10 L per day and should be continued until the effluent is clear and the patient shows improvement in clinical and laboratory parameters (Wig et al, 2004). No evidence is available to suggest the best irrigation fluid, the optimal number or caliber of drains, or the duration of irrigation.
Programmed Open Necrosectomy
The principle of this approach is to be more conservative with debridement, particularly if necrosum has not fully demarcated, with the intention of performing repeat procedures every 48 hours until debridement is no longer required. The pancreatic bed is drained or packed, and the abdomen is closed by suturing mesh or a zipper to the fascial edges of the wound. This allows multiple reentries and helps to prevent wound retraction, which aids in later delayed primary wound closure. In a proportion of patients, primary closure is not possible, and secondary intention healing is allowed to occur, accepting that this may require elective scar excision and repair of an incisional hernia. This procedure may be modified with the addition of intraabdominal vacuum sealing (negative pressure 50 to 75 mm Hg) to encourage granulation of the pancreatic bed, potentially reducing the number of operations and mortality (Olejnik et al, 2008).
Endoscopic Techniques
In 1996, Gagner described the first true endoscopic treatment of necrotizing pancreatitis, in which the pancreas was debrided using a laparoscopic approach. Over the last decade, a wide range of endoscopic approaches for pancreatic necrosectomy have been described, including infracolic laparoscopy, transgastric laparoscopy, hand-assisted laparoscopy, retroperitoneal laparoscopy, transgastric flexible endoscopy, flexible endoscopy via a percutaneous endoscopic gastrostomy, and retroperitoneal nephroscopy (Ammori, 2002; Baron & Morgan, 1999; Charnley et al, 2006; Cuschieri et al, 1998; Horvath et al, 2001; Parekh, 2006). This array of endoscopic techniques can be classified by the type of scope that is used: laparoscope, nephroscope, or flexible endoscope (Windsor, 2007). Although some endoscopic procedures do not utilize radiologic modalities, many are hybrid procedures that incorporate fluoroscopy or endoscopic ultrasound into the procedure.
Laparoscopic Techniques
Most laparoscopic techniques are minimally invasive versions of open surgical techniques, and they use an anterior or lateral approach. In Gagner’s original (1996) description of laparoscopic necrosectomy, two anterior routes—retrogastric retrocolic and transgastric—and one lateral route were described. In the retrogastric retrocolic route, a 30-degree laparoscope is introduced through the umbilical port following CO2 insufflation (15 mm Hg). Placement of additional ports depends on the location of the necrosis. Following necrosectomy, large sump drains are placed in the necrotic beds, and continuous lavage may be established to remove peritoneal contaminants. In the transgastric route, the stomach is opened anteriorly and posteriorly. Endoluminal ports are used, which maintain the tip of the port inside the stomach. Debridement is performed internally through the posterior stomach wall, in a technique akin to an open cystogastrostomy. It is also possible to use a transduodenal route for necrosis of the pancreatic head, although this can be more difficult. No drains are left in the stomach, but a drain may be placed over the incision in the anterior gastric wall. With the retroperitoneal route, the patient is placed in the left or right lateral position, and a small flank incision made. The three muscle layers of the abdominal wall are split, and a trocar is inserted. Using a 0-degree laparoscope and CO2 insufflation (10 to 15 mm Hg), a tract is made through to the pancreas. Once the necrotic areas have been identified, necrosectomy proceeds as when approached via a retrogastric retrocolic route.
These techniques have subsequently been modified. Of the lateral approaches, one of the most widely used laparoscopic techniques is videoscopic-assisted retroperitoneal debridement (VARD; Fig. 54.4), which was first described by Horvath and colleagues in 2001. The purpose of this procedure differs from those of open necrosectomy. Rather than performing complete removal of all infected and necrotic tissue, the purpose of VARD is to facilitate percutaneous drainage. In this technique, radiologic drainage of the lesion is first instituted. In the operating room, the patient is placed in a modified left lateral decubitus position, and a 4- to 5-cm incision is made in the left flank at the site of the drain. A finger is used to probe and confirm entry into the necrotic cavity. Fluid and loose necrotic debris are removed by suction, and two ports (10 to 12 mm) are inserted through the incision. The incision is sealed with wet sponges and towel clips to allow insufflation with CO2. Debridement of necrotic tissue is performed with hydrodissection and 10 mm forceps. Drains are placed for postoperative continuous lavage: a 10-Fr red rubber drain is brought through a separate anterolateral incision, and two 1.3-cm (0.5-inch) Penrose drains are placed in the original skin incision. An ostomy bag is then positioned over the flank incision and Penrose drains, and continuous lavage is performed through the red rubber drain at 200 mL/h for 5 days or until the effluent is clear.
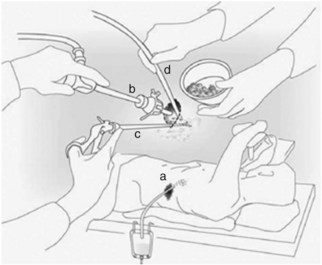
FIGURE 54.4 Videoscopic-assisted retroperitoneal debridement technique.
(From van Santvoort HC, et al, for the Dutch Acute Pancreatitis Study Group, 2007: Case-matched comparison of the retroperitoneal approach with laparotomy for necrotizing pancreatitis. World J Surg 31:1635-1642.)
Variations of the anterior laparoscopic approaches are well described. In addition to the retrogastric, recolic, and transgastric routes, a transmesocolic route may be used, in which the transverse colon is elevated to expose the pancreatic lesion in the lesser sac (Cuschieri et al, 1998). Laparoscopic ultrasound may be used to confirm the position of the lesion, and the transverse mesocolon is usually opened to the left of the middle colic vessels. Following necrosectomy, two or more drains are placed in the pancreatic bed for postoperative lavage of the lesser sac. Anterior approaches may also incorporate a hand-assist device; this is termed hand-assisted laparoscopic surgery (HALS; Parekh, 2006). Another recent publication described single-port laparoscopic necrosectomy (Bucher et al, 2008). Port placement (12 mm) is through postlaparotomy drain tracts, allowing introduction of both a 4-mm laparoscope and 5-mm instruments. Insufflation pressures are kept to 8 mm Hg, as higher pressures may promote bacterial translocation from the cavity. Debridement is performed with both hydrodissection and atraumatic grasping forceps, hemostasis is achieved with monopolar diathermy, and a sump drain is placed for continuous postoperative lavage.
Nephroscopic Techniques
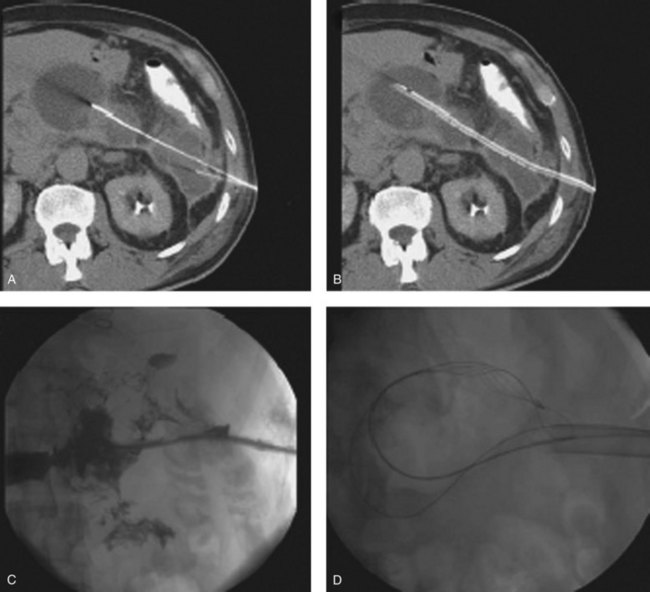
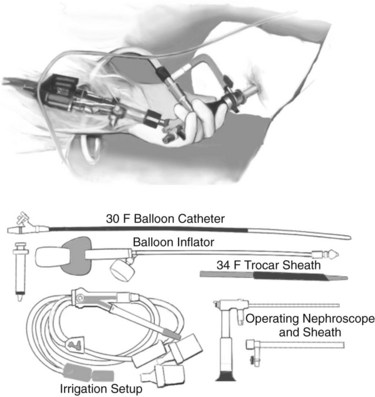
Nephroscopic techniques utilize warmed fluid to expand the necrotic cavity, irrigate turbid fluid, and maintain a clear visual field. Carter and colleagues (2000) were the first to describe the use of a nephroscope during necrosectomy, terming the procedure percutaneous necrosectomy. The principle underlying this procedure is the same as for an open procedure: debridement of devitalized tissue and establishment of a system for continuous postoperative lavage—with reduced physiologic stress on the patient. Percutaneous necrosectomy may only be used when the area of necrosis is accessible to percutaneous puncture, and it is contraindicated in the presence of bowel ischemia, perforated viscus, or significant preoperative hemorrhage. The first step is to insert a drainage catheter into the pancreatic lesion under CT guidance. The preferred path for drainage is between the lower pole of the spleen and the splenic flexure (Fig. 54.5), although in right-sided necrosis, a path through the gastrocolic omentum, anterior to the duodenum, may occasionally be used. The patient is then transferred to the operating room and positioned in the left lateral position, or for right-sided necrosis, in the right lateral position. A nephrolithotomy drape is used to collect irrigant fluid, and the drain tract is dilated to allow insertion of a 34-Fr Amplatz sheath. A nephroscope is inserted through the sheath into the cavity, and lavage is used to clear away debris and suppurative fluid (Fig. 54.6). Following necrosectomy, a 32-Fr soft drainage tube is left in the cavity. A smaller (8-Fr) catheter can be inserted through or alongside the larger drain to allow continuous postoperative lavage. This might be commenced at 250 mL/h with warmed dialysis fluid. Repeat procedures are often required after 2 to 10 days (Carter & Wysocki, 2008).
Flexible Endoscopic Techniques
The use of flexible endoscopy to treat patients with PN has a long history. In 1991, Prinz and Olen recognized that some areas of the abdomen were difficult to access during open necrosectomy as a result of the inflammatory process, therefore a choledochoscope was passed through the open abdomen to debride necrosis located in the lower retroperitoneum following open necrosectomy (Prinz & Olen, 1991). Since that time, the use of flexible endoscopes has developed considerably, with many different techniques currently available, as discussed below. In these procedures, the endoscope is either inserted via an external orifice, usually the mouth, or a skin incision is made.
Peroral flexible endoscopic techniques follow an internal route through either the gastric/duodenal wall or duodenal papilla, and some authors consider this to be a form of natural orifice transluminal endoscopic surgery (Friedland et al, 2009). Initial descriptions of flexible endoscopic treatment of PN used lavage and drainage without instrumental debridement (Baron et al, 1996). A more aggressive approach was subsequently introduced, which demonstrated that necrotic tissue may be debrided with baskets, snares (Fig. 54.7), forceps, and suction (Seewald et al, 2005). A recent long-term multicenter study of transluminal endoscopic necrosectomy described 93 patients who underwent a mean of six interventions, starting at a mean of 43 days after the onset of severe acute pancreatitis (Seifert et al, 2009). Initial clinical success was obtained in 80% of patients but with a complication rate of 26% over 43 months of follow-up; 16% developed recurrent pancreatitis, 10% required further endoscopic procedures, 4% required surgical treatment, and the mortality rate was 7.5% at 30 days. Surgical necrosectomy was required in 12% of patients.
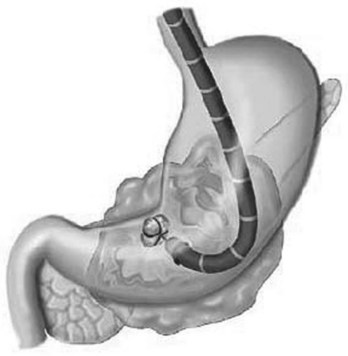
FIGURE 54.7 Pancreatic necrosis debridement with the use of flexible endoscope.
(From Seewald S, et al, 2005: Aggressive endoscopic therapy for pancreatic necrosis and pancreatic abscess: a new safe and effective treatment algorithm [videos]. Gastrointest Endosc 62[1]:92-100.)
The injection of contrast with fluoroscopy can be used to determine the extent of the cavity. The gastric perforation is dilated with balloons up to 20 mm. For lavage and drainage, a 7-Fr nasocystic (lavage) and a 10-Fr pigtail drain (drainage) are placed in the cavity (Fig. 54.8). Continuous lavage with 1500 mL/day saline is established. Necrosectomy may be performed with endoscopic instruments, such as a Dormia basket or polypectomy snare, and introduction of a forward-viewing endoscope into the necrotic cavity can be used for better visualization during the necrosectomy. Multiple necrosectomy procedures are usually required to clear the cavity of necrotic tissue. A similar procedure may be performed with external entry into the body via a percutaneous endoscopic gastrostomy (PEG) tube (Baron & Morgan, 1999).
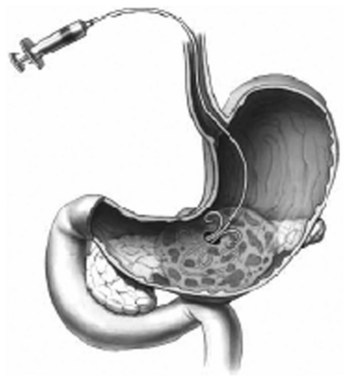
FIGURE 54.8 Transmural drainage of organized pancreatic necrosis.
(From Baron TH, et al, 2002: Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc 56[1]:7-17.)
Several techniques using flexible endoscopy through skin incisions have been described. The first was by Prinz and Olen (1991) after open necrosectomy, as detailed above. Following percutaneous necrosectomy with a nephroscope, subsequent debridement may be undertaken using a flexible endoscope, a so-called sinus-tract endoscopy (Carter et al, 2000). The drain tract is dilated to 45 Fr, and a flexible endoscope is inserted into the cavity for necrosectomy. A similar technique has been described following open necrosectomy via a translumbar incision, in which a flexible endoscope is inserted into the cavity for debridement of ongoing necrosis (Jakobs et al, 2000). These endoscopic debridements were initiated 10 days after the open necrosectomy and were carried out every 3 days for 4 weeks (approximately 8 to 10 sessions).
Radiologic Techniques
Interventional radiology techniques are assuming greater importance, particularly for initial sepsis control to allow definitive necrosectomy to be performed more electively (Jakobs et al, 2000). Ultrasound, fluoroscopy, or CT is used to guide the interventional radiologist into the pancreatic lesion. These radiologic modalities are also used to define the extent and composition of the lesion, visualize the instruments used, and determine the efficacy of the treatment procedure. The purpose of radiologic techniques may be to achieve either drainage, with or without lavage, or debridement.
Radiologic Drainage Techniques
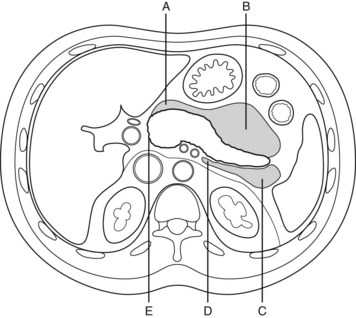
Drainage techniques are most effective if target lesions have a significant fluid component. They can be located in many sites but are most often in the lesser sac, anterior and left pararenal spaces, and other parts of the retroperitoneum (Fig. 54.9; Jakobs et al, 2000). Image-guided needle puncture of the lesion may involve a retroperitoneal or transperitoneal route and may be transmural (transgastric or transduodenal), but a retroperitoneal approach is preferred to reduce the risk of contamination and possible peritonitis (Jakobs et al, 2000). A guidewire is passed down the needle, and the tract is dilated to accommodate a catheter. Because catheters readily block with necrosum and debris, it is advisable to maximize catheter diameter to improve patency. Typically, catheters should have multiple sideholes and a minimum diameter of 12 Fr (Jakobs et al, 2000). Drain tracts may be serially dilated to accommodate larger catheters (20 to 24 Fr). Often multiple catheters are required, especially for large or complex lesions. To increase the efficacy of the drainage procedure, remove toxic factors, and help maintain catheter patency, lavage should be considered (Eatock et al, 2005; Jakobs et al, 2000). Theoretical concerns have been raised that lavage may spread infection, either from infected fluid spilling over into previously sterile cavities or from the increased intracavity pressure, resulting in translocation of bacteria into surrounding tissues. However, this has not been demonstrated as a major problem clinically, most likely because the pancreatic lesion is walled off in a fibrous capsule 4 to 6 weeks after the onset of acute pancreatitis.
The efficacy of radiologic drainage procedures is reduced with more solid lesions. In patients with PN treated with percutaneous catheter drainage, approximately half will be successful and will not require surgical intervention (Jakobs et al, 2000). Indications for surgical intervention in patients who have undergone percutaneous catheter drainage include persistent systemic or local manifestations of infected PN, physiologic deterioration despite the placement of a patent drain, persistent abdominal pain, and intolerance of oral intake after the systemic inflammatory response syndrome has resolved (Jakobs et al, 2000).
Radiologic Debridement Techniques
In some specialized centers, radiologic techniques have been used to debride PN. These procedures are similar to percutaneous catheter drainage, as described above, but they also include removal of necrotic material with snares, baskets, or even by applying suction to a catheter during its removal (Economou & Ward-McQuaid, 1971; Jakobs et al, 2000; Thompson, 2001). Necrotic tissue may be fragmented with wires before attempting its extraction (Blamey et al, 1984; Jakobs et al, 2000), and lavage is recommended to flush out loosened necrotic debris.
Acosta JM, et al. Effect of duration of ampullary gallstone obstruction on severity of lesions of acute pancreatitis. J Am Coll Surg. 1997;184(5):499-505.
Acosta JM, et al. Early ductal decompression versus conservative management for gallstone pancreatitis with ampullary obstruction: a prospective randomized clinical trial. Ann Surg. 2006;243(1):33-40.
Ammori BJ. Laparoscopic transgastric pancreatic necrosectomy for infected pancreatic necrosis. Surg Endosc. 2002;16(9):1362.
Ammori BJ, et al. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg. 1999;3(3):252-262.
Andersson R, et al. Treatment of acute pancreatitis: focus on medical care. Drugs. 2009;69(5):505-514.
Bankhead R, et al. Enteral nutrition practice recommendations. JPEN. 2009;33(2):122-167.
Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379-2400.
Baron TH, Morgan DE. Endoscopic transgastric irrigation tube placement via PEG for debridement of organized pancreatic necrosis. Gastrointest Endosc. 1999;50(4):574-577.
Baron TH, et al. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology. 1996;111(3):755-764.
Beale RJ, et al. Immunonutrition in the critically ill: a systematic review of clinical outcome. Crit Care Med. 1999;27(12):2799-2805.
Bengmark S. Gut microenvironment and immune function. Curr Opin Clin Nutr Metab Care. 1999;2(1):83-85.
Besselink MG, et al. Minimally invasive “step-up approach” versus maximal necrosectomy in patients with acute necrotising pancreatitis (PANTER trial): design and rationale of a randomised controlled multicenter trial [ISRCTN13975868]. BMC Surg. 2006;6:6.
Blamey SL, et al. Analgesia in acute pancreatitis: comparison of buprenorphine and pethidine. Br Med J (Clin Res Ed). 1984;288(6429):1494-1495.
Bradley EL3rd. A clinically based classification system for acute pancreatitis: summary of the International Symposium on Acute Pancreatitis, Atlanta, September 11 through 13, 1992. Arch Surg. 1993;128(5):586-590.
Bucher P, et al. Minimally invasive necrosectomy for infected necrotizing pancreatitis. Pancreas. 2008;36(2):113-119.
Carter CR, et al. Percutaneous necrosectomy and sinus tract endoscopy in the management of infected pancreatic necrosis: an initial experience. Ann Surg. 2000;232(2):175-180.
Casas M, et al. Total enteral nutrition vs. total parenteral nutrition in patients with severe acute pancreatitis. Rev Esp Enferm Dig. 2007;99(5):264-269.
Charnley RM, et al. Endoscopic necrosectomy as primary therapy in the management of infected pancreatic necrosis. Endoscopy. 2006;38(9):925-928.
Craig RM, et al. The use of ampicillin in acute pancreatitis [letter]. Ann Int Med. 1975;83(6):831-832.
Cuschieri SA, et al. Laparoscopic infracolic approach for complications of acute pancreatitis. Semin Laparosc Surg. 1998;5(3):189-194.
de Vries AC, et al. Randomized controlled trials of antibiotic prophylaxis in severe acute pancreatitis: relationship between methodological quality and outcome. Pancreatology. 2007;7(5-6):531-538.
Delcenserie R, et al. Prophylactic antibiotics in treatment of severe acute alcoholic pancreatitis. Pancreas. 1996;13(2):198-201.
Dellinger EP, et al. Early antibiotic treatment for severe acute necrotizing pancreatitis: a randomized, double-blind, placebo-controlled study. Ann Surg. 2007;245(5):674-683.
Doley RP, et al. Enteral nutrition in severe acute pancreatitis. JOP. 2009;10(2):157-162.
Eatock FC, et al. A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis. Am J Gastroenterol. 2005;100(2):432-439.
Ebbehoj N, et al. Indomethacin treatment of acute pancreatitis: a controlled double-blind trial. Scand J Gastroenterol. 1985;20(7):798-800.
Eckerwall GE, et al. Early nasogastric feeding in predicted severe acute pancreatitis: a clinical, randomized study. Ann Surg. 2006;244(6):959-965. discussion 965-957
Economou G, Ward-McQuaid JN. A cross-over comparison of the effect of morphine, pethidine, pentazocine, and phenazocine on biliary pressure. Gut. 1971;12(3):218-221.
Fan ST, et al. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med. 1993;328(4):228-232.
Fernandez-del Castillo C, et al. Debridement and closed packing for the treatment of necrotizing pancreatitis. Ann Surg. 1998;228(5):676-684.
Finch WT, et al. A prospective study to determine the efficacy of antibiotics in acute pancreatitis. Ann Surg. 1976;183(6):667-671.
Flint RS, Windsor JA. The role of the intestine in the pathophysiology and management of severe acute pancreatitis. HPB. 2003;5(2):69-85.
Folsch UR, Neoptolemos J. Reason for performing endoscopic retrograde cholangiopancreatography (ERCP) and ES in a patient with severe gallstone pancreatitis even in the absence of main bile duct stones. Pancreas. 2002;24(4):412-414. author reply 414-417
Friedland S, et al. Endoscopic necrosectomy of organized pancreatic necrosis: a currently practiced NOTES procedure. J Hepatobiliary Pancreat Surg. 2009;16(3):266-269.
Gagner M. Laparoscopic treatment of acute necrotizing pancreatitis. Semin Laparosc Surg. 1996;3(1):21-28.
Garcia-Barrasa A, et al. A double-blind, placebo-controlled trial of ciprofloxacin prophylaxis in patients with acute necrotizing pancreatitis. J Gastrointest Surg. 2009;13(4):768-774.
Gardner TB, et al. Fluid resuscitation in acute pancreatitis. Clin Gastroenterol Hepatol. 2008;6(10):1070-1076.
Götzinger P, et al. Surgical treatment for severe acute pancreatitis: extent and surgical control of necrosis determine outcome. World J Surg. 2002;26(4):474-478.
Green JM. When is faster better? Operative timing in acute care surgery. Curr Opin Crit Care. 2008;14(4):423-427.
Gupta R, et al. A randomised clinical trial to assess the effect of total enteral and total parenteral nutritional support on metabolic, inflammatory and oxidative markers in patients with predicted severe acute pancreatitis (APACHE II ≥6). Pancreatology. 2003;3(5):406-413.
Heinrich S, et al. Evidence-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg. 2006;243(2):154-168.
Heyland DK, et al. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA. 2001;286(8):944-953.
Heys SD, et al. Enteral nutritional supplementation with key nutrients in patients with critical illness and cancer: a meta-analysis of randomized controlled clinical trials. Ann Surg. 1999;229(4):467-477.
Horvath KD, et al. A technique for laparoscopic-assisted percutaneous drainage of infected pancreatic necrosis and pancreatic abscess. Surg Endosc. 2001;15(10):1221-1225.
Howes R, et al. Evaluation of prophylactic antibiotics in acute pancreatitis. J Surg Res. 1975;18(2):197-200.
Isenmann R, et al. Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double-blind trial. Gastroenterology. 2004;126(4):997-1004.
Jakobs R, et al. Buprenorphine or procaine for pain relief in acute pancreatitis: a prospective randomized study. Scand J Gastroenterol. 2000;35(12):1319-1323.
Juvonen PO, et al. Gastric tonometry in assessing splanchnic tissue perfusion in acute pancreatitis. Scand J Gastroenterol. 2000;35(3):318-321.
Juvonen PO, et al. Gut permeability in patients with acute pancreatitis. Scand J Gastroenterol. 2000;35(12):1314-1318.
Kahl S, et al. Procaine hydrochloride fails to relieve pain in patients with acute pancreatitis. Digestion. 2004;69(1):5-9.
Kalfarentzos F, et al. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. Br J Surg. 1997;84(12):1665-1669.
Kumar A, et al. Early enteral nutrition in severe acute pancreatitis: a prospective randomized controlled trial comparing nasojejunal and nasogastric routes. J Clin Gastroenterol. 2006;40(5):431-434.
Larson SD, et al. Management of gallstone pancreatitis. Adv Surg. 2006;40:265-284.
Louie BE, et al. 2004 MacLean-Mueller prize—enteral or parenteral nutrition for severe pancreatitis: a randomized controlled trial and health technology assessment. Can J Surg. 2005;48(4):298-306.
Loveday BP, et al. Minimally invasive management of pancreatic abscess, pseudocyst, and necrosis: a systematic review of current guidelines. World J Surg. 2008;32(11):2383-2394.
Loveday BP, et al. High quantity and variable quality of guidelines for acute pancreatitis: a systematic review. Am J Gastroenterol. 2010;105:1466-1476.
Loveday BP, et al. A comprehensive classification of invasive procedures for treating the local complications of acute pancreatitis based on visualization, route, and purpose. Pancreatology. 2011;11:406-413.
Lowenfels AB, et al. The changing character of acute pancreatitis: epidemiology, etiology, and prognosis. Curr Gastroenterol Rep. 2009;11(2):97-103.
Mallampalli A, et al. Defining tolerance to enteral feeding in the intensive care unit. Clin Nutr (Edinburgh). 2000;19(4):213-215.
Marshall JC, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638-1652.
Nagpal K, et al. Evaluation of intestinal mucosal permeability function in patients with acute pancreatitis. Am J Surg. 2006;192(1):24-28.
Nakasaki H, et al. A surgical treatment of infected pancreatic necrosis: retroperitoneal laparotomy. Dig Surg. 1999;16(6):506-511.
Nathens AB, et al. Management of the critically ill patient with severe acute pancreatitis. Crit Care Med. 2004;32(12):2524-2536.
Neoptolemos JP, et al. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988;2(8618):979-983.
O’Keefe SJ, et al. Physiological effects of enteral and parenteral feeding on pancreaticobiliary secretion in humans. Am J Physiol Gastrointest Liver Physiol. 2003;284(1):G27-G36.
O’Keefe SJ, et al. Trypsin secretion and turnover in patients with acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2005;289(2):G181-G187.
Olah A, Romics LJr. Early enteral nutrition in acute pancreatitis: benefits and limitations. Langenbecks Arch Surg. 2008;393(3):261-269.
Olejnik J, et al. Acute necrotizing pancreatitis: intra-abdominal vacuum sealing after necrosectomy. Hepatogastroenterology. 2008;55(82-83):315-318.
Oria A, et al. Early endoscopic intervention versus early conservative management in patients with acute gallstone pancreatitis and biliopancreatic obstruction: a randomized clinical trial. Ann Surg. 2007;245(1):10-17.
Pandol SJ, et al. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132(3):1127-1151.
Parekh D. Laparoscopic-assisted pancreatic necrosectomy: a new surgical option for treatment of severe necrotizing pancreatitis. Arch Surg. 2006;141(9):895-902.
Pederzoli P, et al. A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem. Surg Gynecol Obstet. 1993;176(5):480-483.
Peiro AM, et al. Efficacy and tolerance of metamizole versus morphine for acute pancreatitis pain. Pancreatology. 2008;8(1):25-29.
Petrov MS. Enteral nutrition: goody or good-for-nothing in acute pancreatitis? Am J Gastroenterol. 2007;102(8):1828-1829. author reply 1829-1830
Petrov MS. Meta-analyses on the prophylactic use of antibiotics in acute pancreatitis: many are called but few are chosen. Am J Gastroenterol. 2008;103(7):1837-1838. author reply 1838-1839
Petrov MS. Early use of ERCP in acute biliary pancreatitis with(out) jaundice: an unjaundiced view. JOP. 2009;10(1):1-7.
Petrov MS, et al. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg. 2006;23(5-6):336-344.
Petrov MS, et al. Advanced enteral therapy in acute pancreatitis: is there a room for immunonutrition? A meta-analysis. Int J Surg (Lond). 2008;6(2):119-124.
Petrov MS, et al. Nasogastric tube feeding in predicted severe acute pancreatitis: a systematic review of the literature to determine safety and tolerance. JOP. 2008;9(4):440-448.
Petrov MS, et al. Systematic review: nutritional support in acute pancreatitis. Aliment Pharmacol Ther. 2008;28(6):704-712.
Petrov MS, et al. Does endoscopic retrograde cholangiopancreatography reduce the risk of local pancreatic complications in acute pancreatitis? A systematic review and meta-analysis. Surg Endosc. 2008;22(11):2338-2343.
Petrov MS, et al. Early endoscopic retrograde cholangiopancreatography versus conservative management in acute biliary pancreatitis without cholangitis: a meta-analysis of randomized trials. Ann Surg. 2008;247(2):250-257.
Petrov MS, et al. Enteral nutrition and the risk of mortality and infectious complications in patients with severe acute pancreatitis: a meta-analysis of randomized trials. Arch Surg. 2008;143(11):1111-1117.
Petrov MS, et al. Systematic review and meta-analysis of enteral nutrition formulations in acute pancreatitis. Br J Surg. 2009;96(11):1243-1252.
Petrov MS, et al. A systematic review on the timing of artificial nutrition in acute pancreatitis. Br J Nutr. 2009;101(6):787-793.
Petrov MS, et al. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813-820.
Pezzilli R, et al. A prospective multicentre survey on the treatment of acute pancreatitis in Italy. Dig Liver Dis. 2007;39(9):838-846.
Pitchumoni CS, et al. Factors influencing mortality in acute pancreatitis: can we alter them? J Clin Gastroenterol. 2005;39(9):798-814.
Powell JJ, et al. Randomized controlled trial of the effect of early enteral nutrition on markers of the inflammatory response in predicted severe acute pancreatitis. Br J Surg. 2000;87(10):1375-1381.
Prinz RA, Olen R. Endoscopic evaluation of infected pancreatic necrosis. Surg Laparosc Endosc. 1991;1(3):195-197.
Roberts PR. Nutritional support in acute pancreatitis: an update on management issues. Semin Resp Critical Care Med. 2001;22(1):29-34.
Rokke O, et al. Early treatment of severe pancreatitis with imipenem: a prospective randomized clinical trial. Scand J Gastroenterol. 2007;42(6):771-776.
Sainio V, et al. Early antibiotic treatment in acute necrotising pancreatitis. Lancet. 1995;346(8976):663-667.
Schloerb PR. Immune-enhancing diets: products, components, and their rationales. JPEN. 2001;25(2 Suppl):S3-S7.
Schwarz M, et al. Antibiotic use in necrotizing pancreatitis: results of a controlled study [in German]. Dtsch Med Wochenschr. 1997;122(12):356-361.
Seewald S, et al. Aggressive endoscopic therapy for pancreatic necrosis and pancreatic abscess: a new safe and effective treatment algorithm [videos]. Gastrointest Endosc. 2005;62(1):92-100.
Seifert H, et al. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD Study). Gut. 2009;58(9):1260-1266.
Siddiqui T, et al. Early versus delayed laparoscopic cholecystectomy for acute cholecystitis: a meta-analysis of randomized clinical trials. Am J Surg. 2008;195(1):40-47.
Spicak J, et al. Antibiotic prophylaxis of infectious complications of acute pancreatitis: the results of randomised study by meropenem. Ceska Slovenska Gastroenterol Hepatol. 2003;57:6.
Stevens M, et al. Transdermal fentanyl for the management of acute pancreatitis pain. Appl Nurs Res. 2002;15(2):102-110.
Talukdar R, Vege SS. Recent developments in acute pancreatitis. Clin Gastroenterol Hepatol. 2009;7(11 Suppl):S3-S9.
Thompson DR. Narcotic analgesic effects on the sphincter of Oddi: a review of the data and therapeutic implications in treating pancreatitis. Am J Gastroenterol. 2001;96(4):1266-1272.
Tonsi AF, et al. Acute pancreatitis at the beginning of the 21st century: the state of the art. World J Gastroenterol. 2009;15(24):2945-2959.
van Haren FM, et al. Gastrointestinal perfusion in septic shock. Anesth Intensive Care. 2007;35(5):679-694.
van Santvoort HC, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362(16):1491-1502.
Wig JD, et al. Closed lesser sac lavage in the management of pancreatic necrosis. J Gastroenterol Hepatol. 2004;19(9):1010-1015.
Windsor JA. Minimally invasive pancreatic necrosectomy. Br J Surg. 2007;94(2):132-133.
Windsor JA, Hammodat H. Metabolic management of severe acute pancreatitis. World J Surg. 2000;24(6):664-672.
Working Party of the British Society of Gastroenterology, Association of Surgeons of Great Britain and Ireland, Pancreatic Society of Great Britain and Ireland, Association of Upper GI Surgeons of Great Britain and Ireland. UK guidelines for the management of acute pancreatitis. Gut. 2005;54(Suppl 3):iii1-9.
Wu BU, et al. Early hemoconcentration predicts increased mortality only among transferred patients with acute pancreatitis. Pancreatology. 2009;9(5):639-643.
Wu XM, et al. Total enteral nutrition in prevention of pancreatic necrotic infection in severe acute pancreatitis. Pancreas. 2010;39(2):248-251.
Wysocki AP, McKay CJ, Carter CR. Infected pancreatic necrosis: minimizing the cut. ANZ J Surg. 2010;80:58-70.
Xue P, et al. Effect of antibiotic prophylaxis on acute necrotizing pancreatitis: results of a randomized controlled trial. J Gastroenterol Hepatol. 2009;24(5):736-742.