CHAPTER 100 Malignant Neoplasms of the Oropharynx
About 10% to 12% of all malignancies of the upper aerodigestive tract occur in the oropharynx. Approximately 130,000 new cases were detected worldwide in 2002.1 Histopathologically, most malignancies found in the oropharynx (>90%) are squamous cell carcinomas (SCCs) with an incidence of 1 to 3/100,000 in the United States and Europe per year. Although the male population has a higher prevalence of oropharyngeal cancer, future counts will depend on tobacco and alcohol consumption behavior, which is the major risk factor for oropharyngeal carcinoma, followed by occupational and environmental pollutants and endogenous risks.
Anatomy of the Oropharynx
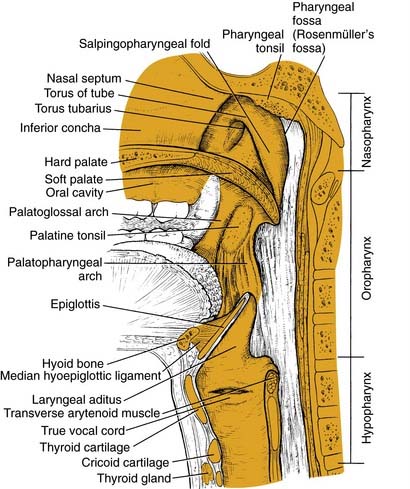
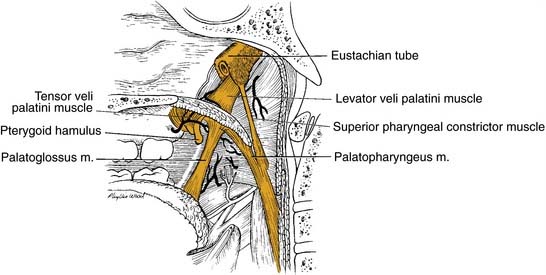
The structure of the oropharynx is complex and can be subdivided into the soft palate, tonsils, and base of tongue respecting different site-specific characteristics. The oropharyngeal borders are the soft palate superiorly and the hyoid bone and the vallecula inferiorly. The ventral border is the base of tongue, which ends at the circumvallate papilla. Laterally and dorsally the oropharynx contains the tonsillar region and the lateral and dorsal pharyngeal wall, respectively. In the following sections, the anatomic sites are described separately, accounting for their specific characteristics (Fig. 100-1).
Tonsillar Fossae
Base of Tongue
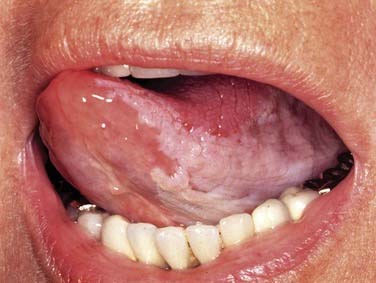
The base of tongue reaches from the circumvallate papilla to the vallecula and to the glossopalatine sulci laterally. The tongue base is rich in lymphatics and contains part of Waldeyer’s ring. The blood supply is covered by the lingual arteries. Innervation of the tongue musculature is provided by the hypoglossal nerve (Fig. 100-2).
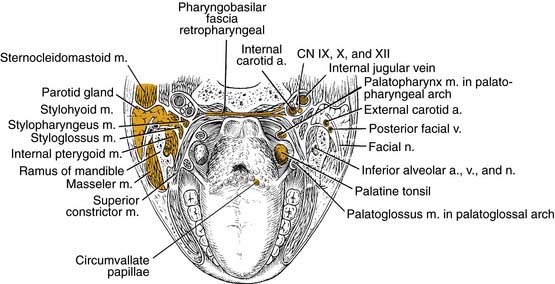
Oropharyngeal Wall
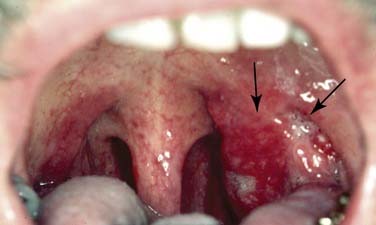
The posterior pharyngeal wall of the oropharynx reaches from the region of the soft palate to the epiglottis and borders the tonsillar fossae and the lateral aspect of the piriform sinuses laterally. The wall is composed of superior constrictor muscle and the buccopharyngeal and pharyngobasilar fascia covered by the pharyngeal mucosa (Fig. 100-3).
Lymphatics
The lymphatic pattern of the oropharynx is complex, and lymphatic spread from malignant lesions depends on size and location of the primary malignancy. Its understanding is of high importance for the outcome of surgical and/or radiotherapy treatment of oropharyngeal lesions. A number of previous studies have defined the routes of lymphatic spread in the head and neck.2–4 The clinical neck levels are defined by levels I to VI with subdivisions A and B for levels I, II, and V (Fig. 100-4).5
Oropharyngeal carcinoma is predisposed to drain to levels II, III, and IV with possible further spread to other regions in extensive disease.6 However, primary drainage is to the jugulodigastric nodes in the upper deep jugular chain (level II) and to the retropharyngeal and parapharyngeal nodes in the retropharyngeal and parapharyngeal space, respectively. Other metastatic spread may involve midcervical (level III) and lower cervical nodes (level IV). Skip metastases to other clinical levels are extremely rare. Shah described in a retrospective study of 1119 radical neck dissection specimens that tumors of the oropharynx characteristically metastasized to lymph node levels II to IV. There was no positive node involvement of level V when levels II to IV were negative.7
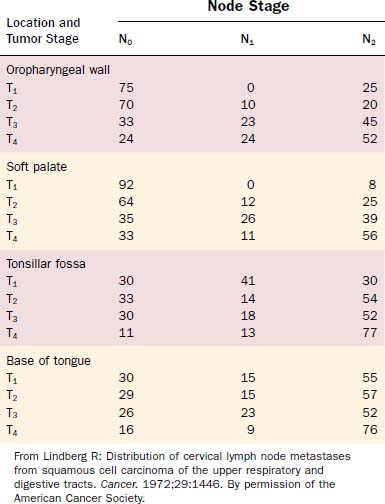
Attention should be spent on the location of the primary malignancy near the midline. Tumors of the base of tongue, the soft palate, and the posterior pharyngeal wall have a higher incidence of bilateral lymphadenopathy. These considerations should influence therapeutic planning to optimize therapy and minimize patient morbidity (Figs. 100-5 and 100-6, Table 100-1).
Pathology
SCC is the most common malignancy of the oropharynx, comprising more than 90% of all malignant tumors of that origin. Other malignant transformations are lymphomas, lymphoepithelial carcinoma, minor salivary gland tumors, malignant melanomas, and other rare malignancies. The World Health Organization Classification of Tumours outlines the presently known malignancies of the oropharynx (Table 100-2).8
Table 100-2 World Health Organization Classification of Malignant Tumors of the Oropharynx
Epithelial Precursor Lesions
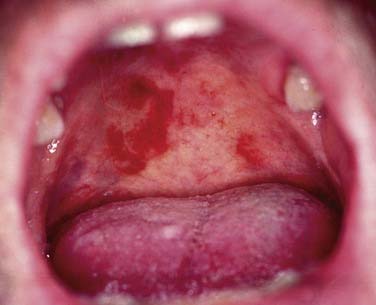
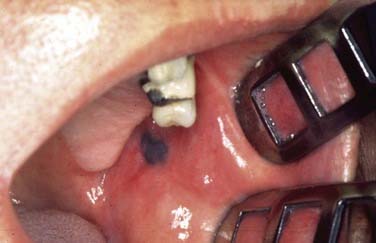
In general, precursor lesions of the oropharynx clinically present as white patches (leukoplakia) (Fig. 100-7) or red patches (erythroplakia). Some lesions appear as mixed variants with white and red components. Most white lesions do not show dysplastic cells and relate to hyperplasia. However, erythroplakia or mixed lesions frequently display dysplasia. Whereas leukoplakia rarely undergoes malignant transformation and may even regress after elimination of underlying etiologic factors, erythroplakia can often lead to malignancy.
Carcinoma in situ is described as malignant transformation without invasion, which is difficult to distinguish morphologically. WHO experts recommend that CIS be diagnosed when full-thickness architectural abnormality and severe cytologic atypia is found.9
Squamous Cell Carcinoma
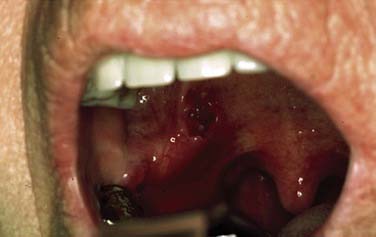
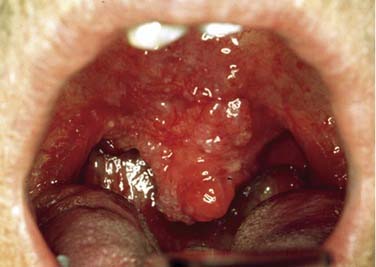
SCC is defined as an invasive epithelial neoplasm with varying degrees of squamous differentiation and a propensity to early and extensive lymph node metastases, occurring predominantly in alcohol- and tobacco-using adults in the fifth and sixth decades of life.10 The clinical appearance varies and presents as exophytic, flat, ulcerated, verrucoid, or papillary in growth (Fig. 100-8). Independent from the gross appearance, histopathologic patterns of invasion demonstrate multiple variations as well. SCC can vary from keratinizing to nonkeratinizing and well differentiated to poorly differentiated. Severe dysplasia and CIS are common elements found in association with invasive SCC. Invasive carcinoma eliminates the regular architecture and can include lympho-vascular space invasion, neurotropism, and infiltration of other tissue components such as muscle or cartilage, demonstrating aggressive behavior of the tumor.
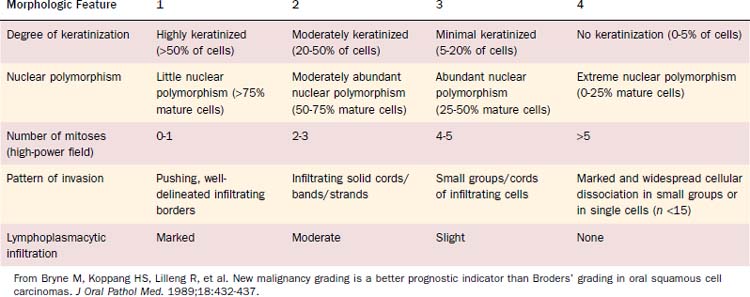
Malignancy grading can be a prognostic marker, although different grading systems have been proposed with diverse impact on prognosis. Broders suggested a four-tiered grading system for carcinoma of the lip in 1929.11 His grading system allows assessment of tumor differentiation with some predictability of prognosis because poorly differentiated tumors are more likely to recur.12,13 However, the grading system has also been shown to lack consistent prediction of prognosis using multivariate analyses.14,15 A subsequent multivariate analysis on 77 patients with oropharyngeal cancer unveiled only the invasive pattern of the tumor to be an independent predictor for patients’ survival.16 Anneroth and colleagues17 proposed that grading should include six morphologic features: degree of keratinization, nuclear polymorphism, number of mitoses, pattern of invasion, stage of invasion, and lymphoplasmacytic infiltration. Bryne and colleagues18 revised this grading method, applying Anneroth’s criteria to the most anaplastic fields in the areas of highest infiltration. This invasive cell grading was demonstrated to be a highly significant predictor for oral SCC patients’ outcome and survival.18–20 Table 100-3 summarizes the invasive cell grading system (ICG) by Bryne and colleagues.
The present WHO grading system has been recommended for histopathologic typing of oropharyngeal cancers by the American Joint Committee on Cancer (AJCC).21 This grading system is based on Broders’ system and includes three grades, which are described in Table 100-4.
Table 100-4 World Health Organization Grading System for Oral Squamous Cell Carcinoma
| Grade | Description |
|---|---|
| 1 | Well differentiated. Histologic and cytologic features closely resemble those of the squamous epithelial lining of the oral mucosa. There are varying proportions of basal and squamous cells with intercellular bridges; keratinization is a prominent feature; few mitotic figures are seen and atypical mitoses or multinucleated epithelial cells are extremely rare; nuclear and cellular polymorphism is minimal. |
| 2 | Moderately differentiated. This is a neoplasm with features intermediate between well differentiated and poorly differentiated. Compared with well-differentiated squamous cell carcinoma, these tumors have less keratinization and more nuclear and cellular pleomorphism; there are mitotic figures and some are abnormal in form; intercellular bridges are less conspicuous. |
| 3 | Poorly differentiated. Histologically and cytologically there is only a slight resemblance to the normal stratified squamous epithelium of the oral mucosa. Keratinization is rarely present, and intercellular bridges are extremely scarce; mitotic activity is frequent and atypical mitoses can readily be found; cellular and nuclear pleomorphism are obvious and multinucleated cells may be frequent. |
From Pindborg JJ, Reichert PA, Smith CJ, et al, eds. Histological Typing of Cancer and Precancer of the Oral Mucosa. 2nd ed. Berlin: Springer-Verlag; 1997:11. By permission of the publisher.
Lymphoepithelial Carcinoma
The lymphoepithelial carcinoma is defined as a poorly differentiated SCC or undifferentiated carcinoma, accompanied by a prominent reactive lymphoplasmacytic infiltration. The lymphoepithelial carcinoma is rare and diagnosed in 0.8% to 2% of all oral and oropharyngeal malignancies.22 More than 90% of all lymphoepithelial carcinoma in the oral and oropharyngeal region is found in the tonsils and the base of tongue. Some are found in the buccal mucosa and the palate.
The histopathologic pattern is described as invasive, comprising syncytial sheets and clusters of carcinoma cells with prominent nucleoli, ill-defined cell borders, and often intact surface. The tumor sites are accompanied by rich lymphoplasmacytic infiltrate. These tumors are radiosensitive and local control can be achieved in a high percentage of cases.23
Salivary Gland Tumors
Among malignant salivary gland tumors, 9% to 23% are found in the oral cavity and oropharynx.24–26 Nearly half of the salivary gland tumors in the oral cavity and oropharynx are malignant.24 However, most neoplasms of minor salivary glands arise in the oral cavity. The most common oropharyngeal sites are the (soft) palate, the tonsillar fossa, and base of tongue. Oropharyngeal salivary gland tumors are rare with only 1.1% to 3.3% of all minor salivary gland tumors.24,25,27 All malignant salivary gland tumors that can possibly arise in the oropharynx are listed in Table 100-2. Among those, adenoid cystic carcinoma and the mucoepidermoid carcinoma are most frequent.
The adenoid cystic carcinoma is relatively common in minor salivary glands. A large series describes 42.5% of this lesion to be found in minor glands and 20.5% of minor salivary gland adenoid cystic carcinoma in the oral/oropharyngeal space.28 They usually present as slow-growing masses, sometimes with ulceration of the palate. Symptoms like pain, signaling nerve invasion, suggest progressive disease. The histopathologic pattern is cylindromatous or cribriform, although some can show tubular areas or appear solid.29 Cribriform lesions have a more favorable prognosis than solid tumors. The spread of adenoidcystic lesions is commonly hematogenous, typically in the lung and bones, and lymph node involvement is rather rare. Therefore neck dissection is generally reserved for patients with palpable disease.30 In case of radiation therapy for these rather moderately radiosensitive tumors, neutron beam radiation is recommended.31 Prognosis is negatively influenced by minor salivary gland as origin, tumor size greater than 4 cm, osseous invasion, advanced stage, and local recurrence.32,33
Mucoepidermoid carcinoma is the most common salivary gland malignancy and accounts for 9.5% to 23% of all minor salivary gland tumors.25,34,35 Half of the often asymptomatic cases arise in the palate, whereas the base of tongue and other oropharyngeal locations are rare.36 They are commonly well differentiated and appear as a bluish swelling. Some show granulation or a papillary surface. Oropharyngeal tumors can cause symptoms of dysphagia. Histopathologically, a mixture of epidermal epithelium and mucous membrane-producing cells is found. Treatment usually includes wide surgical excision, and neck dissection appears to be beneficial in patients with suspected or clinically obvious metastases. Olsen and colleagues reported on a series of oropharyngeal mucoepidermoid carcinoma at Mayo Clinic. Based on a follow-up of at least 10 years, recurrence rate was significantly reduced after wide local excision, including bone if necessary compared with simple excision with closer margins. Of the patients in the series, 24% presented with positive lymph nodes.37 Patients treated with primary radiation or postoperative radiation did not profit in terms of local control or survival in this evaluation; however, numbers might be too small to draw final conclusions and radiotherapy might be beneficial in case of adverse effects in those patients.
Soft Tissue Tumors
Kaposi’s sarcoma is a locally aggressive growing neoplasm, which might present as cutaneous but also as mucosal lesion, showing multiple patches and nodules (Fig. 100-9). It can also affect lymph nodes and visceral organs. It rarely metastasizes and is a member of intermediate-type vascular tumors. It is associated with human herpes virus 8 (HHV 8) infections. Kaposi’s sarcoma occurs as an indolent variant in elderly men in the Mediterranean and Eastern Europe, as endemic disease in equatorial Africa, and in immunosuppressed patients after organ transplantation or in case of human immunodeficiency virus (HIV) infection.38
Patients might be treated with surgery, radiation, and chemotherapy, depending on the epidemiology of the disease. The incidence and course of the disease has improved with antiretroviral therapy in HIV-infected patients.38
Hematolymphoid Tumors
On the basis of the complex and widespread lymphatic tissue in the oropharynx, lymphoid malignancies often occur in this area. Non-Hodgkin’s lymphomas (NHLs) are found in the palatine tonsils, palate, base of tongue, and other oropharyngeal sites. Etiologic factors are yet unknown in most patients, although some lymphoma patients might suffer from an immunodeficiency. Clinical symptoms can be fullness of the throat, dysphagia, snoring, and pain. Systemic symptoms are rare.39 The lesions present as exophytic mass, submucosal swelling, and sometimes ulcerations (Figs. 100-10 and 100-11).
Most NHLs in the oropharynx are B-cell lymphomas, with diffuse large B-cell lymphoma being the predominant type. Histopathologically the stroma is densely infiltrated with lymphoma cells, which vary depending on the histologic type. Treatment is based on radiotherapy and/or chemotherapy depending on the histologic type and stage. Studies have demonstrated a benefit for adjuvant chemotherapy along with radiation compared with radiation treatment only.40,41 The 5-year survival rate for localized disease has been reported from 50% to more than 80%.41–45
Mucosal Malignant Melanoma
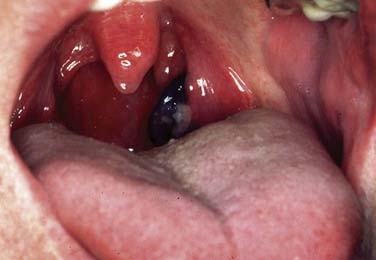
Although malignant melanoma (MM) most commonly manifests as skin lesions, it may also arise from melanocytes in the mucosa, but only 1.3% of all melanomas are mucosal melanomas (Figs. 100-12 and 100-13). Of those, 55.4% are mucosal MMs of the head and neck.46 They are characterized as malignant transformed melanocytes at the epithelial–connective tissue interface with migration into the epithelium and the connective tissue, respectively. Oropharyngeal mucosal melanoma is exceedingly rare, with more mucosal MM of the head and neck occurring in the oral cavity (50%).46,47
No known etiologic factors exist for mucosal MM of the head and neck. Clinically they can present as black, gray, or reddish, and they are rarely amelanotic. They typically consist of widespread, multiple, pigmented lesions with or without nodules. Ulcerations and bone infiltration are common and oral bleeding, dysphagia, and sensations of pain can be symptoms before diagnosis. Mucosal MM is usually diagnosed in an advanced stage with most tumors showing invasive character. However, 20% are in situ lesions.47 Aggressive surgical resection is still the primary treatment of choice, although many adjuvant modalities including different chemotherapy regimens and radiotherapy have been applied. Despite surgically achieved possible local control, 5-year survival of pharyngeal melanoma is only 13%.46,48,49 Developments for improved treatment options are limited because mucosal MM is a rare disease lacking clinical treatment trials. By early detection, melanoma patients have the best chance for cure and physician’s threshold for biopsy of a suspected lesion should be low.
Squamous Cell Carcinoma
Etiology
Predominant and synergistic acting risk factors for SCC of the oropharynx are tobacco and alcohol consumption.50 These factors account for about 75% of oral and oropharyngeal carcinoma in Western countries.50,51 For the highest consumption levels, relative risks from 70 to 100 have been reported compared with lowest consumption levels. A super-multiplicative effect in case of contact with tobacco and alcohol substances has been shown for oral and oropharyngeal cancers in case-control studies.52
Tobacco chewing is a common habit, particularly in India, parts of Southeast Asia, the Middle East, China, and Taiwan. Tobacco is often consumed in betel quids containing areca nut, which has been classified as human carcinogen by the IARC in 2003. Smokeless tobacco is a major risk factor for oropharyngeal cancer in these parts of the world.53 In India about 50% of oropharyngeal cancers in male and 90% in female are caused by chewing.54
Human papillomavirus (HPV) infection is another well-established cancer risk factor. Genotypes HPV 16 and 18, known causes for uterine cervix and skin cancer, are found in 50% of tonsillar and oropharyngeal SCC, and studies suggest that up to 40% of oropharyngeal cancer cases may be due to HPV infection.55 The incidence of HPV-associated oropharyngeal primaries is rising according to recent reports—up to 70% of all presenting cases in some series. The cohort of patients harboring this form of the disease is younger by approximately 10 years and has a distinctly favorable prognosis. Epidemiologic studies propose that having multiple sexual partners is significantly associated with HPV-related tumors.1
In addition to exogenic risk factors, multiple endogenic risks have been identified and associated with head and neck cancer.56 DNA repair, differences in mutagen sensitivity, and alteration of genes such as epidermal growth factor receptor (EGFR) have shown their impact on head and neck cancer and influenced modern therapy and prevention strategies.
Clinical Presentation, Patterns of Spread
The most frequent location for oropharyngeal tumors is the tonsillar fossa, with the palatine tonsil and the anterior tonsillar pillar. They commonly present as foreign body, dysphagia, otalgia, or impeded jaw mobility caused by infiltration of the periosteum or bone of the mandible or the pterygoid muscles in extended cases. Physical examination can show exophytic or ulcerated lesions, dysplasia, and/or inflammation reaction. Extension into the base of tongue inferiorly and the soft palate superiorly is common, with 55% and 60% in a study by Perez and colleagues, respectively.57 Lymphatic drainage is directed primarily to level II nodes but can involve level III parapharyngeal and retropharyngeal nodes level I, IV, and V, depending on stage of presentation.58–60 Lindberg described nodal metastasis for cancer of the tonsil in 76% of cases compared with 45% in patients with cancer of the tonsillar pillar, with most common node involvement in level II for both locations. Contralateral nodal disease was found in 11% of patients with cancer of the tonsil and 5% with primary malignancy of the pillar.61 However, in these studies no mention is given of proximity to the midline, which is the most frequent reason a lateralized tumor epicenter in the oropharynx would present with “contralateral” neck metastasis. Differences might be based on higher-staged groups with tonsillar lesions, and likelihood of contralateral lymphatic spread is less than 5% for T1 and T2 cancers.
Cancer of the base of tongue is particularly difficult to detect and often becomes clinically evident in an advanced stage. This is due to relatively late clinical symptoms because the base of tongue is nearly without pain fibers. Moreover, the assessment of the base of tongue can be more difficult during physical examination due to prominent lingual tonsils or submucosal location, and deep basal areas might not completely present even using operative endoscopy. As in the palatine tonsil, occult carcinoma with clinically prominent metastatic disease, typically in neck level II, is often found in the base of tongue and is often the presenting feature of this disease. As with neck metastases primarily clerived from tonsils, some of these neck masses can be misdiagnosed as branchial cleft cysts or, on biopsy, as branchiogenic carcinomas.62 To rule out such findings, meticulous microendoscopic examination (see Fig. 100-12) and biopsy of suspected lesions are mandatory. Base of tongue cancer has a higher frequency of bilateral metastases (up to 20%), which is mainly due to proximity to the midline but enhanced by a rich lymphatic drainage of the base of tongue and tendency to late clinical presentation with upstaging. Ipsilateral node involvement can be detected in more than 70% of cases.63
Diagnostic Evaluation
Anamnesis
A thorough anamnesis is part of any comprehensive evaluation of head and neck cancer patients. Symptoms at first presentation of oropharyngeal cancer patients are commonly dysphagia and/or odynophagia, oral bleeding, otalgia, or changes in speech. In addition to the history of risk factors such as alcohol and tobacco consumption, xenobiotic exposure at working places and in the environment should be evaluated. Dietary and social habits are also worthwhile to evaluate. Knowing about possible risk factors for head and neck cancer in individual patients enables for future elimination and enlarges the chances for prevention of recurrent disease. Also, better results and improved treatment tolerance are obtained for patients who quit smoking.64 Other factors such as social aspects, family support, and general health status have great impacts on treatment and outcome and should therefore be consequently evaluated.
Imaging
Gray-scale and Doppler ultrasonography of the neck have proven to be valuable methods to evaluate lymphadenopathy of the neck. Using ultrasound for cervical assessment has advantages. It is a relatively inexpensive procedure with real-time imaging and can be frequently performed without radiation exposure. However, there is limitation to ultrasonography in patients with head and neck malignancies. Retropharyngeal nodes and deep structures are not accessible using ultrasound. Furthermore, ultrasound has deficits describing extracapsular spread and infiltration of soft tissue and bone. Moreover, the oropharyngeal primary usually cannot be detected using ultrasonography.65
Most oropharyngeal malignancies today are evaluated using computed tomography (CT) or magnetic resonance imaging (MRI). Sometimes the imaging technique preferred simply depends on its availability. Each has its advantages and disadvantages. Some oropharyngeal malignancies involve bony structures such as the maxilla, mandible, cervical spine, and skull base, and a CT scan might be helpful in interpretation of bone infiltration, although modern MRI precisely demonstrates infiltration of the periosseous membrane and allows for exact interpretation of bone involvement. In some cases panoramic x-ray views of the mandible can support identification of mandibular infiltration. Especially in deep invasive malignancies, MRI is helpful, although it can lead to false-negative results regarding superficial lesions. Although magnetic resonance scanning is generally the most recommended imaging method for the evaluation of the oropharynx and the neck, both CT and MRI have been used in such determinations.66 Positron emission tomography (PET)/CT plays an increasing role for the management of head and neck SCC. It can unveil unknown primary tumor sites and synchronous primary tumors, regional lymph node metastases, and distant metastases. It plays a lesser role for T-staging of a known primary based on decreased anatomic definition compared with MRI and contrast-enhanced CT.67 Its reliability may be limited by previous surgical or radiation therapy. Recent studies suggest that PET/CT is more accurate 2 to 3 months after completion of radiation therapy.68–70 Whereas a negative PET/CT is found to be highly reliable, positive results must be correlated with findings in physical examination and cross-sectional imaging modalities because nonspecific inflammation may cause false-positive results.71
In addition to evaluation of locoregional tumor spread, imaging systems are used to screen for distant metastatic spread in organs such as the lung, liver, skeletal system, and brain. On the basis of the primary malignancy and the accompanying likelihood of distant metastases, regular chest radiographs and ultrasonography of the abdomen might be sufficient staging methods. In many cases, CT scanning of the lung and CT and/or MRI for the abdomen are recommended. In recent years, FDG PET/CT showed growing importance for the detection of distant metastatic spread.71
Endoscopy, Biopsy, and Frozen Section
To further diagnose oropharyngeal lesions, a biopsy and histopathologic examination must be performed. Biopsies of oropharyngeal tumors might be taken under local anesthesia. Lymphadenopathy without perceptible primary lesion can be evaluated using fine-needle aspiration (FNA). Best diagnostic results are found for FNA using ultrasound of the neck.72
Some oropharyngeal lesions are not sufficiently accessible in the office setting and may require general anesthesia to biopsy an adequate-sized specimen. Occasionally small oropharyngeal malignancies can be difficult to detect in physical examination, as well as in imaging systems, and demand further diagnostics (e.g., using endoscopes with or without microscopic assistance) (Fig. 100-14). Microscopic evaluation is a sensitive procedure for the identification of unknown primary lesions, which can often be detected in the base of tongue or tonsillar fossa, respectively. The panendoscopy in general anesthesia is an important tool not only to detect and biopsy such lesions and to define tumor extension but also to rule out any secondary malignancy, which can be found in head and neck cancer patients and includes endoscopy of the bronchi and esophagus.
Frozen sections as confirmation of a suspected malignancy and as criteria for tumor-free margins have been critically evaluated in the past.73 The reliability of frozen sections depends on the experience and technique of the pathologist and requires close interdisciplinary communication. The accuracy of frozen sections is close to the final diagnosis (>90%).74 Considering the possibility of false-positive results, depending on the examiner’s expertise, frozen sections cannot be generally recommended as a basis for final treatment, however. The final treatment should be based on standard histopathology.
Staging
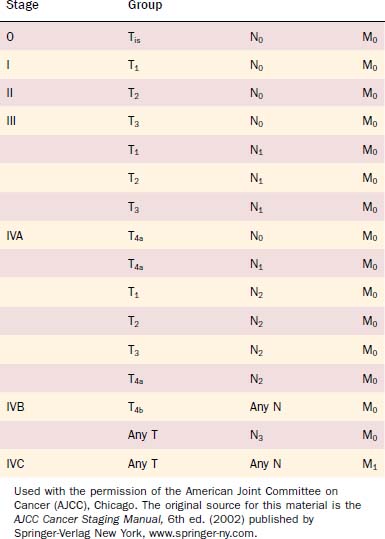
The Union Internationale Contre Cancer (UICC) and the American Joint Committee on Cancer (AJCC) in its Cancer Staging Manual (sixth edition) have defined the current staging system for oropharyngeal cancer.75,76 Both classifications correspond exactly. Tables 100-5 and 100-6 present an overview of the present oropharyngeal staging system.
Table 100-5 Definition of the Tumor Node Metastasis Staging System
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| Oropharynx | |
| T1 | Tumor ≤ 2 cm in greatest dimension |
| T2 | Tumor > 2 cm but ≤ 4 cm in greatest dimension |
| T3 | Tumor > 4 cm in greatest dimension |
| T4a | Tumor invades the larynx, deep or extrinsic muscle of tongue, medial pterygoid, hard palate, or mandible |
| T4b | Tumor invades lateral pterygoid muscle, pterygoid plates, lateral nasopharynx, or skull base or encases carotid artery |
| Regional Lymph Nodes (N) | |
| Oropharynx and Hypopharynx | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral lymph node ≤ 3 cm in greatest dimension |
| N2 | Metastasis in a single ipsilateral lymph node > 3 cm but ≤ 6 cm in greatest dimension, or in multiple ipsilateral lymph nodes, none > 6 cm in greatest dimension, or in bilateral or contralateral lymph nodes, none > 6 cm in greatest dimension |
| N2a | Metastasis in a single ipsilateral lymph node > 3 cm but ≤ 6 cm in greatest dimension |
| N2b | Metastasis in multiple ipsilateral lymph nodes, none > 6 cm in greatest dimension |
| N2c | Metastasis in bilateral or contralateral lymph nodes, none > 6 cm in greatest dimension |
| N3 | Metastasis in a lymph node > 6 cm in greatest dimension |
| Distant Metastasis (M) | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago. The original source for this material is the AJCC Cancer Staging Manual, 6th ed. (2002) published by Springer-Verlag New York, www.springer-ny.com.
Experts have debated and evaluated the clinical use of the staging system.77 The fifth edition of the tumor node metastasis (TNM) classification divided stage IV tumors into three subcategories (IVA, IVB, and IVC), which already caused relevant changes to the fourth edition.78 The sixth edition of the TNM classification introduced a subdivision of advanced tumors of group T4 into T4a (lower risk) and T4b (higher risk), which describes the risk of uncontrolled disease in patients with tumor extension beyond the site of origin. T4a disease describes tumors with reasonable opportunity for disease control on the basis of the resectability of structures involved such as larynx, deep/extrinsic muscles of the tongue, medial pterygoid, mandible, and hard palate. T4b describes tumors with certainty of poor outcome showing involvement of the lateral pterygoid muscle, pterygoid plates, lateral nasopharynx, skull base, or carotid artery. O’Sullivan and Shah79 suggested invasion of the prevertebral fascia to be classified as high risk and therefore included into subgroup T4b. T4a and T4b in combination with categories for N (regional lymph node disease) and M (distant metastasis) assist the classification of the advanced tumor stage IV into the subgroups with advanced lower-risk stage IVA (potentially curable), advanced higher-risk IVB (of dubious curability), and stage IVC with distant metastatic disease (undoubtedly incurable). Despite some suggested additions, the sixth edition of TNM classification offers valuable assistance in clinical management and research. Changes in the staging system over the past decades and improvements of staging tools such as modern imaging systems must be taken into consideration when former clinical studies about outcome and prognosis are evaluated.
Oropharyngeal cancer is usually amenable to direct or endoscopic visualization and palpation, which in combination with imaging assessment enables a thorough clinical evaluation. Reasons for misjudgment remain. The examiner’s evaluation does not unveil microscopic and deep infiltration. Using MRI and CT scanning for further information results might be limited when examining superficial smaller lesions.62 Additionally, poor demarcation and accompanying inflammatory tissue reaction may affect interpretation of the images. Tumor size can be overestimated or underestimated by these factors and misjudged, and it can strongly influence therapeutic planning. Not only clinical but also postoperative staging might be affected (e.g., by shrinkage of the specimen and lack of clinical and surgical information). Moreover, large tumor extension might lead to difficulties about classification of the site of origin (e.g., whether a lesion derived from the oropharynx, hypopharynx, or supraglottic region). Because those tumor extensions commonly accompany high staging and comparably worse prognosis, there is less influence concerning management strategies but concerning scientific studies and statistical evaluation. Nevertheless, correspondence between the clinician and the pathologist is the key for best postoperative staging.
Therapeutic Management and Outcome
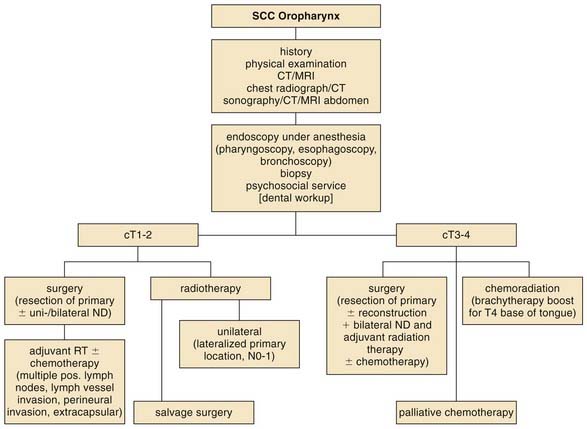
The main criterion for a successful treatment is locoregional control of the primary tumor. The choice of treatment modality depends on the location of the primary and the ability to control the primary and the regional node involvement. Figure 100-15 presents an informing algorithm for oropharyngeal cancer treatment. With improvements in locoregional control, distant metastases are a growing issue in the treatment of oropharyngeal cancers. To achieve this goal, surgery, radiotherapy, and chemotherapy can be administered alone or in combinations. In case of an early tumor stage, surgery and radiation therapy can be used for primary treatment. In later stages of oropharyngeal cancer, combinations of surgery, radiation therapy, and chemotherapy are recommended in most cases. The impact of additional therapies such as immunotherapy/biotherapy (e.g., epidermal growth factor receptor [EGFR] inhibitor) is currently investigated and their significance has yet to be evaluated. The decision-making process for treatment modality should include information from the latest review of the Cochrane Collaboration on the treatment on oral and oropharyngeal cancer. Only one study by Robertson (1998) compared surgery plus adjuvant radiotherapy and radiotherapy alone, fitting their criteria and therefore being included. The trial was prematurely stopped because of the unacceptably high number of deaths in the radiotherapy arm.80,81 However, in addition to outcome, post-therapeutic function associated with the patient’s quality of life must be considered in the decision about treatment.
With the use of modern free tissue transfer techniques, patients’ quality of life and functions like speech and swallowing have considerably improved after surgery with ablative defects.82 Patients should be thoroughly informed about possible treatment options to be included in the decision-making process. Complications and long-term effects possibly arising from surgical interventions or toxicity of chemoradiation therapy must be clearly assessed. As for all head and neck cancers, the clinician’s experience, the options given by the institution, and the patient’s individual reliability should be accounted for in order to achieve the optimal cancer treatment.
Soft Palate
Early disease of the soft palate (Fig. 100-16) shows favorable results for local control using surgery and using radiation therapy. Considering possible long-term side effects caused by radiation therapy and good reconstructive options with positive functional outcome, surgery (conventional or laser surgery) might be preferred. Some studies have been performed using radiation in combination with brachytherapy (Iridium-192 implant), without any proof of significant benefit.83–86
Advanced disease is commonly treated with surgery and adjuvant radiotherapy with or without chemotherapy. Radiation therapy did not demonstrate convincing results.87 However, Calais and colleagues88 showed more favorable results using radiation therapy with concomitant chemotherapy compared with radiation therapy alone as an alternative therapeutic modality for stage III and IV cancers.
The decision for performing a neck dissection is led by the propensity of soft palate cancers to metastasize even in early stages. Har-El and colleagues89 showed that more than 48% of patients with soft palate cancers present with clinical evidence of neck disease, and even in patients with no palpable or radiographic proof, more than 40% of their patients eventually had neck disease. As a consequence, patients with positive nodes should be treated with neck dissection (e.g., followed by radiation therapy). Also, patients with clinically negative neck should either receive elective neck dissection or radiation therapy. Some authors describe radiation therapy followed by neck dissection in clinically positive nodes as well.81 Yet higher complication rates have been found for neck dissection following radiation therapy in several studies, suggesting surgery as primary treatment.90 The closer the lesion is positioned toward the midline, the higher the likelihood for bilateral lymphatic spread and the necessity to perform bilateral neck dissection.91
Tonsillar Fossae
Early lesions of the tonsillar region can be treated with surgery or radiation. Both modalities offer good outcome and functional results. In most cases modern surgical techniques and instruments allow good transoral access to the tumor site without comorbidity worth mentioning. Procedures including mandibulotomy are rarely necessary and used only in some cases of extensive disease and reconstruction. Also, laser techniques add to the advancements of surgical precision and reduced morbidity.92,93 Local control rates up to 90% after surgery have been described.94,95 Comparing external radiation beam therapy (EBRT) with surgery, no differences in locoregional control or overall survival have been unveiled. However, complications based on high dosage for primary treatment and the effect on the patient’s quality of life must be considered and compared with those resulting from surgery.
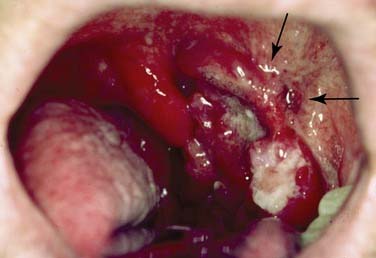
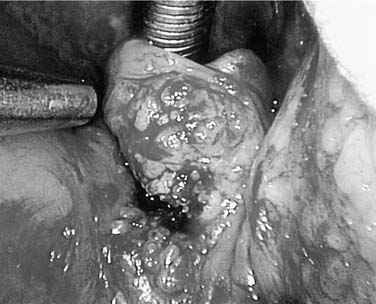
Advanced lesions (Fig. 100-17) are commonly treated with surgery and postoperative radiotherapy ± chemotherapy). One study was performed using single-modality (surgery vs. radiotherapy) treatments for stage III and IV cancers, with significantly better 5-year survival rates using surgery, even though more patients were stage IV in the radiated group.96 Foote and colleagues evaluated advanced cases treated with surgery ± adjuvant radiotherapy. After surgery with postoperative radiation, their overall survival rate was found to be 100% for stage III and 78% for stage IV cases, whereas patients who underwent solely surgery showed 56% and 43% survival, respectively.97 Another study from the Memorial Sloan-Kettering Cancer Center demonstrated favorable local control rates (T3 94%, T4 75%) and 64% disease-free survival. In case of positive or close surgical margins, 60 Gy or higher was administered and followed by a 93% local control rate.98
Spiro and Spiro compared radiation therapy and combined therapy with surgery followed by radiation retrospectively from 1969 until 1983. They found 3-year local control rates comparable with other investigators but for stage IV disease, although possibly biased against the combined treatment by preselection of high-staged cases.99 Recently, concomitant chemoradiation following surgery compared with postoperative radiation was investigated for head and neck cancers in Europe and the United States in two multicenter studies. For oropharyngeal tumors, the evidence for a benefit using concomitant chemoradiation therapy remains somewhat weak, although much stronger in patients with additional features such as extracapsular spread of lymph nodes.100,101 Parsons and colleagues presented a review of several studies comparing surgery ± radiation therapy with radiation ± neck dissection for tonsillar carcinoma. Despite comparable counts for 5-year survival and locoregional control, they criticize higher complication rates in the surgery group, although the data indicate that almost all fatal complications resulted in studies with radiation therapy performed before the surgery.102 Salvage surgery in the tonsillar region after primary radiation is accompanied by high mortality and low 5-year survival rates. One investigation by Gehanno and colleagues103 showed a 5-year survival of 24% and a mortality rate as high as 8% in 120 patients. Two investigations support the idea of brachytherapy salvage in this area with 5-year survival rate of 64% and a 2-year survival rate of 42%, respectively.104,105
In general, the clinically negative neck should be treated in case of an incidence of occult metastasis of 20% or higher.106 In patients with carcinoma of the tonsillar region, ipsilateral elective neck dissection is commonly performed even for small lesions and clinically negative necks. The latest considerations about neck dissections in clinically negative necks and the more selective surgery (e.g., sparing level IIb) have significantly reduced comorbidity and improved patients’ outcome.107 Postoperative radiation is often recommended in case of positive nodes, although prospective randomized trials exploring the role of radiation therapy after selective neck dissection are necessary.107 On the basis of the size and location of the primary metastasis and the ongoing higher likelihood of bilateral metastases, bilateral neck dissection or radiation treatment should be considered. This is true for T3 and T4 lesions, involvement of the tongue base, and neck findings greater than or equal to cN2. However, patients with T1-2 lesions and N0/N1 might be adequately treated with ipsilateral surgery/radiation.
Base of Tongue
As with other locations of the oropharynx, early lesions might be treated with surgery and/or radiation therapy according to the literature. Published study results show that local control and survival do not significantly differ between the two modalities. Again, the functional outcome is an important factor to be considered in the decision-making process. Modern surgical applications such as transoral laser surgery have significantly improved surgical treatment and reduced morbidity.108 Latest investigations demonstrate highly effective surgical treatments with good functional outcome using transoral laser techniques. Grant and colleagues treated 59 patients with primary cancer of the base of tongue, among whom 11 had stage I and II disease. Their locoregional control for the entire patient collective was as high as 88%, and their recurrence-free survival was 84% with excellent postoperative function results measured.109 Steiner and colleagues reviewed 48 patients, 94% of whom were in stage III and IVA. The Kaplan-Meier 5-year recurrence rate was 20% and overall survival was 52% for T3 and T4 lesions. For T1 and T2 lesions, however, there was no local recurrence after 5 years and overall survival was 73%.110 New operative systems with even further chances of improvement like the transoral robotic surgery have been shown to be suitable and are under further evaluation.111 More extensive surgical procedures like transhyoid pharyngotomy or mandibulotomy are commonly reserved for patients with larger lesions. Radiation therapy is often performed as combination of external beam radiation therapy and brachytherapy with an interstitial 192-Iridium implant.63 Primary radiation therapy ± neck dissection without brachytherapy implant showed varying local control rates between 78% and 96% for T1 and 47% and 88% for T2 lesions.112–115 Others used external beam radiation in combination with brachytherapy implants and reported local control rates between 71% and 100% for T1 and T2 lesions.116–118 Houssett and colleagues compared surgery plus adjuvant radiation therapy, external beam radiation therapy plus 192-Iridium implant, and external beam radiation alone on stage T1 and T2 base of tongue carcinoma. They found comparable results for surgery plus adjuvant radiation and external beam radiation plus implant, although external beam radiation therapy alone showed an unacceptable failure rate twice as high as in the other two groups.113
Advanced disease of the base of tongue can be managed with two therapeutic strategies, one of which is resection with adjuvant radiation ± concomitant chemotherapy. Advances in oropharyngeal reconstruction in the past 2 decades can ensure good function and improved quality of life even when ending up with large defects of the tongue base and surrounding tissues.119 Zelefsky and colleagues performed a 7-year follow-up on 51 patients with carcinoma of the tongue base who received surgery and postoperative radiation therapy, most of those with T3 and T4 cancers. Their local control rate was as high as 94% and 75% in patients with T3 and T4 lesions, respectively. The disease-free survival for all patients was 64% after 7 years and the likelihood for distant metastases was 30%.117,120 Concurrent chemoradiation therapy has been shown to significantly improve local control and disease-free (59% vs. 41%) and overall survival (65% vs. 49%) compared with conventional radiation in a study by the European Organization for the Research and Treatment of Cancer (EORTC) reported by Bernier and colleagues in 2001. Acute toxicity (e.g., mucositis) was higher in the chemoradiation group, although chronic toxicity was comparable.101 In case of persistent or recurrent disease, surgical and radiotherapeutic modalities were evaluated. One study from 1980 performed by Pradhan and colleagues121 reported unsatisfying results after aggressive salvage surgery in most cases. However, on the basis of when the study was performed, this investigation contains many negatively affecting components that must be considered. Encouraging results were published by Grant and colleagues in 2006. They investigated patients with recurrent and residual disease showing local control rates of 75% and an overall survival of 54% after 2 years.122 Other groups have performed after-loading techniques with Iridium-192 in patients treated with radiation therapy ± surgery earlier. One group achieved local control in 59% and actuarial survival of 48%,123 and others achieved local control of 61% in base of tongue cancer patients.124 However, residual or recurrent disease of the tongue base remains a demanding therapeutic problem.
As for tonsillar carcinoma, tongue base lesions should be treated with elective, selective, modified radical, or radical neck dissection, depending on their clinical stage due to the high propensity of such cancers to lymphatic spread. Clinically negative necks should be treated less extensively to achieve reduced morbidity. Levels considered are II to V for base of tongue SCC. In case of primary chemoradiation therapy the neck is included in the planning, depending on the neck stage. In N2 to N3 disease some authors discuss planned neck dissection after surgery on the basis of the risk for residual occult disease.125 Cupino and colleagues favored neck dissection before definite primary chemoradiation in stage IV oropharyngeal cancer patients. Their small population of 25 patients had an actuarial locoregional control rate of 88% and an actuarial overall survival rate of 92% after 3 years.126 Independent from the modality used, the high incidence for bilateral occult or evident lymphatic disease commonly demands bilateral neck treatment.
Oropharyngeal Wall
Early lesions of the oropharyngeal wall can be treated with surgery and/or radiation therapy. Transoral conventional or laser surgery ± radiation therapy has shown to be a valid therapeutic option with low morbidity to the patients. In case of positive nodes surgery is commonly followed by radiation therapy ± chemotherapy. However, definitive radiation therapy is also sometimes used for small oropharyngeal lesions. An early analysis performed at M. D. Anderson Cancer Center showed survival rates of 71% (T1) and 73% (T2) using radiation therapy.127 In another early study from 1978, Marks and colleagues128 compared results for patients treated with radiation followed by surgery and those treated with definite radiation alone and found discouraging 17% survival for both groups and high complication rates for the group receiving surgery after radiation.
Most advanced lesions of the oropharyngeal wall are treated with surgical resection of the primary, bilateral neck dissection, and postoperative radiation therapy ± chemotherapy. Using modern reconstructive options, acceptable function often can be achieved. A retrospective analysis of posterior pharyngeal wall cancers by Spiro and colleagues129 showed 5-year survival rates ranging from 15% to 44% depending on tumor staging after surgical treatment with or without radiation therapy. However, in some cases resectability is not given and definitive chemoradiation therapy is applied. Some might use definitive radiation with addition of neck dissection. For posterior wall lesions the close proximity to the spinal cord is challenging for radiation planning and application.63
Transoral Approach
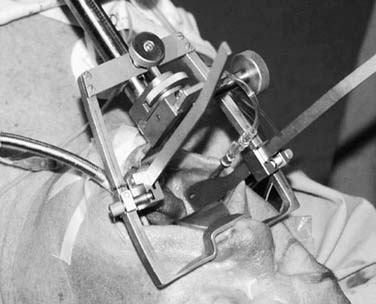
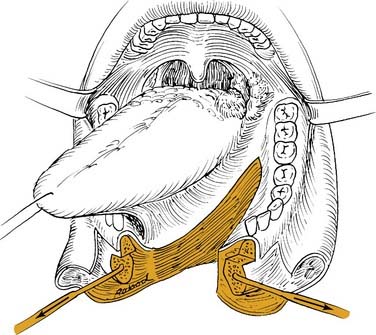
Most oropharyngeal lesions can be removed transorally. This is a minimally invasive approach, suitable for most small- and medium-sized lesions and even larger lesions in the upper part of the oropharynx. Necessary exposure can be achieved using the Stierlen (Fig. 100-18), McIver, Kastenbauer (Fig. 100-19), or Dingman mouth gag or endoscopes such as the Kleinsasser or Steiner for laser surgery of tongue base or deeply located lesions (Fig. 100-20).
The laser surgical approach either via microendoscopy or using a laser hand device is an excellent tool for oropharyngeal cancer resection with minimized morbidity to the patient.130 Other adequate options are electrocautery and cold knife or scissor. Using the CO2 laser, lesions of the soft palate, tonsil, pharyngeal wall, and tongue base can be resected (see Fig. 100-20). Depending on the individual skills of each surgeon, larger lesions involving more than one subsite can be resected with the laser. In case of involvement of the lateral pharyngeal wall/tonsillar fossa, depending on the depth of tumor growth, neck dissection should be addressed first. The medial portion of vessels such as the carotid can be easily protected with sponges before transoral laser resection. Laser surgery requirements such as adequate surgical training and local precautions in the entire operating room must be met. Today’s laser procedures enable minimal invasive access, reduced blood loss and morbidity, rapid secondary wound healing, and rehabilitation. Yet using electrocautery and scissors might be more suitable in selected cases.
Lateral and Transhyoid Pharyngotomy
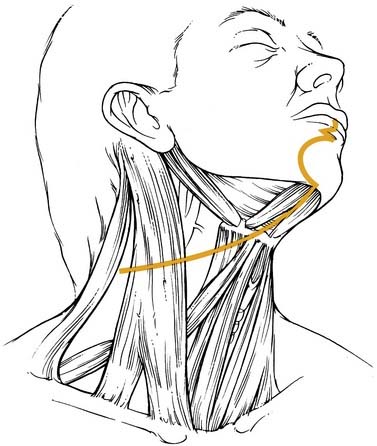
Some inferior lesions might not be accessible transorally or the patient’s neck mobility might not allow for reflexion and adequate vision of the lesion. In such cases lateral or transhyoid pharyngotomy can be performed. Those approaches can be combined and variations depend on the location and size of the tumor to be resected. The conventional lateral approach would leave the hypoglossal nerve cranially and the superior laryngeal bundle inferiorly (Fig. 100-21). Attention has to be spent on where to enter the pharynx in order not to cut into tumor tissue. In those cases, preliminary endoscopy can be recommended.
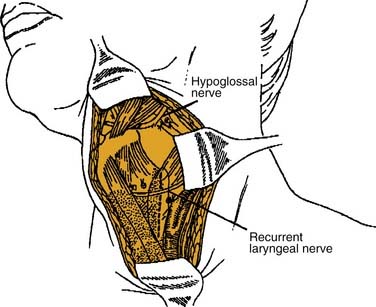
Figure 100-21. The epiglottis and tongue base are exposed through a lateral pharyngotomy approach.
(From Carrau RS. Lateral pharyngotomy. In: Myers EN, ed. Operative Otolaryngology: Head and Neck Surgery. Philadelphia: WB Saunders; 1997:242. Used with permission.)
Transhyoid pharyngotomy is achieved by freeing the hyoid from supraglottic musculature and entering the vallecula medially (Fig. 100-22). Again, tumor location has to be considered before entering the pharynx. The lesion is then visualized and thorough resection with adequate distance to the tumor can be performed. Separation of muscle structures like the stylohyoid or digastric muscle can further improve access to the surgical field. Using these approaches, there is no additional skin incision than that for the neck dissection and proper exposure in most cases in avoidance of lip and mandibular splitting. Yet the pharyngotomy can result in fistulas and closure of the pharynx has to be performed thoroughly inverting the mucosa using absorbable sutures.
Mandibular Swing Approach
The mandibular swing approach is commonly reserved for extensive lesions, which also often require reconstructive surgery. The incision of the skin is usually performed through the midline of the lip, followed by a zigzag-shaped line down to the mental fold, around the mentum, and continuing into the neck incision submentally (Fig. 100-23).
The soft tissue and gingiva, as well as the periosteum, are cut and the latter released from the bone only in the area of the mandibular split. Tissue must be handled carefully and later closure of the oral vestibule must be considered. A plate must be prepared to fit the contour of the mandible, and holes should be drilled and sized before mandibular splitting. The course of the dental roots must be considered before positioning the plate. The osteotomy is performed in the midline using an electric saw, although the cranial part of the mandible should be handled with an osteotomy in order of preservation of the roots of incisor teeth. The osteotomy can also be performed in a stair-step-like fashion. Then the floor-of-mouth soft tissues are divided and enough tissue must be left laterally toward the mandible for sufficient soft tissue closure with or without tissue transfer for reconstruction (Fig. 100-24). This approach allows the widest access to the oropharynx, although it is the most invasive. However, it might be necessary for extensive tumors and adequate exposure for resection and reconstruction. Other procedures for oropharyngeal access without mandibulotomy via the floor of mouth have been described, although indication seems limited and transoral approach might have been sufficient in the cases described.131
Follow-up
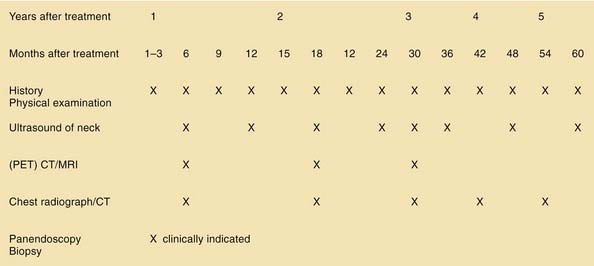
As with other head and neck tumors, the impact of follow-up on survival is controversial. However, it seems obvious that thorough follow-up enables early detection of possible recurrence and timely intervention with chances of further disease control. Figure 100-25 presents a suggested concept for the follow-up of oropharyngeal cancer patients. Follow-up schedules should always include the treating physician’s judgment about each individual patient’s compliance.
Special Treatment Considerations
Data on oropharyngeal cancer treatment remain controversial. The Scottish Intercollegiate Guidelines Network (SIGN) found that randomized controlled trials comparing surgery versus radiotherapy ± chemotherapy for the treatment of primary lesions were unavailable for both early and advanced lesions. This is also true for the treatment of the neck.132 Although more studies were included in their analysis, the Cochrane Collaboration also criticized the lack of valid evidence for one modality or the other.77 The assessment of existing literature must include the time frame of the investigation because many surgical and radiotherapeutic, as well as chemotherapeutic applications, have been tremendously modified in the past.
Quality of life has been hardly addressed in the Cochrane literature despite being one of the most important factors for decision making concerning therapy.77 Selective studies offer quality-of-life assessments, yet scaling systems differ significantly and results are not comparable. Moreover, upcoming investigations should include adverse events and morbidity caused by the applied therapy. It is recommended to clearly separate studies on therapy and outcome on the basis of the exact tumor location respecting the complexity of the different head and neck subsites. Finally, treatment regimen might also be influenced by socioeconomic factors; thus elements such as length of hospital stay and costs should, ideally, be integrated in future studies.
Prognostic Factors
TNM status, general performance, and gender are known influential factors for prognosis in oropharyngeal cancer patients.91,133 Aebersold and colleagues134 found intratumoral microvessel distribution to effect local control and overall survival using definitive radiation therapy with unfavorable high microvessel density. In case of proliferating cell nuclear antigen (PCNA) and vascular endothelial growth factor (VEGF) receptor, no predictive effect was investigated in patients with oropharyngeal cancer independent from the treatment modality used. A high Ki-67 labeling index was found to be linked to a reduced time interval until relapse of disease after surgery and adjuvant radiation.134–136 Several studies have analyzed the negative impact of anemia on radiation therapy.133,137 Also, hypoxia measured in lymph node metastases from head and neck cancers reduced the response rate for radiation and chemoradiation therapy.138–140 Many other molecular markers such as EGFR and DNA-repair enzymes are currently under investigation. Regardless of these tested markers, one of the most important predictive factors seems to be the exposure to exogenous risk factors such as tobacco and alcohol, before and following treatment. Patient compliance must also be considered for future outcome. Different reviews have been performed to explore the effect of delay in commencing postoperative radiotherapy on patient’s outcome. Although it remains vague whether outcome is significantly impaired by a delayed postoperative radiotherapy, there is some evidence supporting time intervals between surgery and radiotherapy not to exceed 6 to 8 weeks.141,142 However, such prognostic factors are important for the evaluation of the ideal treatment modality and must be taken into account in the judgment of outcome in existing clinical studies.
Quality of Life
Quality of life is the major key for judgment of therapeutic success besides evaluation of locoregional control and survival. As mentioned earlier, multiple variants for life quality assessment exist and comparability is low. However, the importance of the issue has tremendously grown for head and neck cancer patients in the past and studies including objective criteria are necessary in order to compare the impact of different treatment modalities on patients’ quality of life. In acknowledgement of the problem, the International Classification of Functioning, Disability and Health (ICF) supported by the World Health Organization in 2007 initiated an international consensus meeting on head and neck cancer and developed a core set for this patient group. In future studies, this core set will enable a standardized quality of life assessment for head and neck cancer patients.143
Barnes L, Eveson JW, Reichart P, et al, editors. WHO Classification of Tumours, Pathology and Genetics Head and Neck Tumours. Lyon, France: WHO Blue Books, 2005.
Bernier J, Domenge C, Ozsahin M, et al. Postoperative radiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945-1952.
Browman GP, Wong G, Hodson I, et al. Influence of cigarelte smoking on the efficacy of radiation therepy in head and neck cancer. N Engl J Med. 1993;328:159-163.
Bryne M, Koppang HS, Lilleng R, et al. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol. 1992;166:375-381.
Cupino A, Axelrod R, Anne PR, et al. Neck dissection. Otolaryngol Head Neck Surg. 2007;137:416-421.
Ferlito A, Rinaldo A, Silver CE, et al. Effective and therapeutic selective neck dissection. Oral Oncol. 2006;42:14-25.
Grant DG, Salassa JR, Hinni ML, et al. Carcinoma of the tongue base treated by transoral laser microsurgery, part one: untreated tumors, a prospective analysis of oncologic and functional outcomes. Laryngoscope. 2006;116:2150-2155.
Greene FL, Page DL, Fleming ID, et al, editors. AJCC Cancer Staging Manual, 6th ed, New York: Springer Verlag, 2002.
Harrison LB, Sessions RB, Hong WK, editors. Head and Neck Cancer, 2nd ed, Philadelphia: Lippincott Williams and Wilkins, 2004.
International Classification of Functioning, Disability and Health (ICF). Core set on head and neck cancer. WHO http://www.who.int/classifications/icf/en/, 2007.
Marshak G, Popovtzer A. Is there any significant reduction of patients’ outcome following delay in commencing postoperative radiotherapy? Curr Opin Otolaryngol Head Neck Surg. 2006;14:82-84.
Oliver RJ, Clarkson JE, Conway DI, et al. Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment. Cochrane Database Syst Rev. CD006205, 2007.
O’Sullivan B, Shah J. New TNM staging criteria for head and neck tumors. Semin Surg Oncol. 2003;21:30-42.
Pellitteri PK, Ferlito A, Rinaldo A, et al. Planned neck dissection following chemoradiotherapy for advanced head and neck cancer: is it necessary for all? Head Neck. 2006;28:166-175.
Pindborg JJ, Reichert PA, Smith CJ, et al, editors. Histological Typing of Cancer and Precancer of the Oral Mucosa, ed 2, Berlin: Springer Verlag, 1997.
Quon A, Fischbein NJ, McDougall IR, et al. Clinical role of 18F-FDG PET/CT in the management of squamous cell carcinoma of the head and neck and thyroid carcinoma. J Nucl Med. 2007;48(Suppl 1):58S-67S.
Rosertson AG, Soutar DS, Paul J, et al. Early closure of a randomized trial: surgery and postoperative radiotherapy versus radiotherapy in the management of intra-oral tumours. Clin Oncol. (R Coll Radiol.). 1998;10:155-160.
Scottish Intercollegiate Guidelines Network (SIGN). Diagnosis and management of head and neck cancer. http://www.sign.ac.uk/, 2006.
Sobin LH, Wittekind C, editors. TNM Classification of Malignant Tumours, 6th ed, New York: Wiley-Liss, 2002.
Steiner W, Ambrosch P, editors. Endoscopic Laser Surgery of the Upper Aerodigestive Tract. New York: Thieme Stuttgart, 2000.
Steiner W, Fierek O, Ambrosch P, et al. Transoral laser microsurgery for squamous cell carcinoma of the base of the tongue. Arch Otolaryngol Head Neck Surg. 2003;129:36-43.
Wei WI, Ferlito A, Rinaldo A, et al. Management of the N0 neck—reference or preference. Oral Oncol. 2006;42:115-122.
Werner JA, Dunne AA, Myers JN. Functional anatomy of the lymphatic drainage system of the upper aerodigestive tract and its role in metastasis of squamous cell carcinoma. Head Neck. 2003;25:322-332.
Wippold FJ2nd. Head and neck imaging: the role of CT and MRI. J Magn Reson Imaging. 2007;25:453-465.
Znaor A, Brennan P, Gajalakshmi V, et al. Independent and combined effects of tobacco smoking, chewing and alcohol drinking on the risk of oral, pharyngeal and esophageal cancers in Indian men. Int J Cancer. 2003;105:681-686.
1. D’Souza G, Kreimer AR, Viscidi R, et al. Case control study of human papilloma virus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944-1956.
2. Werner JA, Dünne AA, Myers JN. Functional anatomy of the lymphatic drainage system of the upper aerodigestive tract and its role of metastasis of squamous cell carcinoma. Head Neck. 2003;25:322-332.
3. Fisch UP, Sigel ME. Cervical lymphatic system as visualized by lymphography. Ann Otol Rhinol Laryngol. 1964;73:870-882.
4. Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446-1449.
5. Robbins KT, Clayman G, Levine PA, et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology. Arch Otolaryngol Head Neck Surg. 2002;128:751-758.
6. Ferlito A, Rinaldo A, Silver CE, et al. Effective and therapeutic selective neck dissection. Oral Oncol. 2006;42:14-25.
7. Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160:405-409.
8. Barnes L, Eveson JW, Reichart P, et al, editors. WHO Classification of Tumours, Pathology and Genetics Head and Neck Tumours. Lyon, France: WHO Blue Books, 2005.
9. Gale N, Pilch BZ, Sidransky D, et al. Epithelial precursor lesions. In: Barnes L, Eveson JW, Reichart P, et al, editors. WHO Classification of Tumours, Pathology and Genetics Head and Neck Tumours. Lyon, France: WHO Blue Books, 2005.
10. Johnson N, Franceschi S, Ferlay J, et al. Squamous cell carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. WHO Classification of Tumours, Pathology and Genetics Head and Neck Tumours. Lyon, France: WHO Blue Books, 2005.
11. Broders AC. Squamous-cell epithelioma of the lip. JAMA. 1929;74:656-664.
12. Noguchi M, Kido Y, Kubota H, et al. Prognostic factors and relative risk for survival in N1-3 oral squamous cell carcinoma: a multivariate analysis using Cox’s hazard model. Br J Oral Maxillofac Surg. 1999;37:433-437.
13. Olsen KD, Caruso M, Foote RL, et al. Primary head and neck cancer. Histopathologic predictors of recurrence after neck dissection in patients with lymph node involvement. Arch Otolaryngol Head Neck Surg. 1994;120:1370-1374.
14. Valle-Zapico A, Fernandez FF, Suarez AR, et al. Prognostic value of histopathologic parameters and DNA flow cytometry in squamous cell carcinoma of the pyriform sinus. Laryngoscope. 1998;108:269-272.
15. Janot F, Klijanienko J, Russo A, et al. Prognostic value of clinicopathological parameters in head and neck squamous cell carcinoma: a prospective analysis. Br J Cancer. 1996;73:531-538.
16. Crissman JD, Liu WY, Gluckman JL, et al. Prognostic value of histopathologic parameters in squamous cell carcinoma of the oropharynx. Cancer. 1984;54:2995-3001.
17. Anneroth G, Batsakis J, Luna M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand J Dent Res. 1987;95:229-249.
18. Bryne M, Kopan HS, Lilleng R, et al. New malignancy grading is a better prognostic indicator than Broders’ grading in oral squamous cell carcinomas. J Oral Pathol Med. 1989;18:432-437.
19. Bryne M. Prognostic value of various molecular and cellular features in oral squamous cell carcinomas: a review. J Oral Pathol Med. 1991;20:413-420.
20. Bryne M, Koppang HS, Lilleng R, et al. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol. 1992;166:375-381.
21. Pindborg JJ, Reichert PA, Smith CJ, van der Wal I. Histological Typing of Cancer and Precancer of the Oral Mucosa, ed 2. Berlin: Springer Verlag; 1997.
22. Tsang WYW, Chan JKC, Westra W. Lymphoepithelial carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. WHO Classification of Tumours, Pathology and Genetics Head and Neck Tumours. Lyon, France: WHO Blue Books, 2005.
23. Tsang RW, Gospodarowicz MK, Pintilie M, et al. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol. 2003;21:4157-4164.
24. Eveson JW, Cawson RA. Tumors of the minor (oropharyngeal) salivary glands: a demographic study of 336 cases. J Oral Pathol. 1985;14:500-509.
25. Ellis GL, Auclair PL, Gnepp DR, editors. Surgical Pathology of the Salivary Glands. Philadelphia:: WB Saunders, 1991.
26. Seifert G, Miehlke A, Haubrich J, editors. Diseases of the Salivary Glands: Pathology-Diagnosis-Treatment-Facial Nerve Surgery. New York: Thieme Verlag Stuttgart, 1986.
27. Spiro RH, Koss LG, Hajdu SI, et al. Tumors of minor salivary origin. A clinicopathologic study of 492 cases. Cancer. 1973;31:117-129.
28. Gnepp DR, editor. Diagnostic Surgical Pathology of the Head and Neck. Philadelphia: WB Saunders, 2001.
29. Waldron CA, el Mofty SK, Gnepp DR. Tumors of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol. 1988;66:323-333.
30. Stell PM. Adenoid cystic carcinoma. Clin Otolaryngol Allied Sci. 1986;11:267-291.
31. Douglas JG, Lee S, Laramore GE, et al. Neutron radiotherapy for the treatment of locally advanced major salivary gland tumors. Head Neck. 1999;21:255-263.
32. Spiro RH, Huvos AG, Strong EW. Adenoid cystic carcinoma: factors influenceing survival. Am J Surg. 1979;138:579-583.
33. Eibling DE, Johnson JT, McCoy JPJr, et al. Flow cytometric evaluation of adenoid cystic carcinoma: correlation with histologic subtype and survival. Am J Surg. 1991;162:367-372.
34. Eveson JW, Cawson RA. Tumors of the minor (oropharyngeal) salivary glands: a demographic study of 336 cases. J Oral Pathol. 1985;14:500-509.
35. Waldron CA, el Mofty SK, Gnepp DR. Tumors of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol. 1988;66:323-333.
36. Neville BW, Damm DD, Weir JC, et al. Labial salivary gland tumors. Cancer. 1988;61:2113-2116.
37. Olsen KD, Devine KD, Weiland LH. Mucoepidermoid carcinoma of the oral cavity. Otolaryngol Head Neck Surg. 1981;89:783-791.
38. van der Waal I, Lamovec J, Knuutila S. Kaposi sarcoma. In: Barnes L, Eveson JW, Reichart P, et al, editors. WHO Classification of Tumours, Pathology and Genetics Head and Neck Tumours. Lyon, France: WHO Blue Books, 2005.
39. Berkowitz RG, Mahadevan M. Unilateral tonsillar enlargement and tonsillar lymphoma in children. Ann Otol Rhinol Laryngol. 1999;108:876-879.
40. Fujitani T, Takahara T, Hattori H, et al. Radiochemotherapy for non-Hodgkin’s lymphoma in palatine tonsil. Cancer. 1984;54:1288-1292.
41. Harabuchi Y, Tsubota H, Ohguro S, et al. Prognostic factors and treatment outcome in non-Hodgkin’s lymphoma of Waldeyer’s ring. Acta Oncol. 1997;36:413-420.
42. Fujitani T, Takahara T, Hattori H, et al. Radiochemotherapy for non-Hodgkin’s lymphoma in palatine tonsil. Cancer. 1984;54:1288-1292.
43. Barton JH, Osborne BM, Butler JJ, et al. Non-Hodgkin’s lymphoma of the tonsil. A clinicopathologic study of 65 cases. Cancer. 1984;53:86-95.
44. Makepeace AR, Fermont DC, Bennett MH. Non-Hodgkin’s lymphoma of the tonsil. Experience of treatment over a 27-year period. J Laryngol Otol. 1987;101:1151-1158.
45. Sunaba K, Shibuya H, Okada N, et al. Radiotherapy for primary localized (stage I and II) non-Hodgkin’s lymphoma of the oral cavity. Int J Radiat Oncol Biol Phys. 2000;47:179-183.
46. Patrick RJ, Fenske NA, Messina JL. Primary mucosal melanoma. J Am Acad Dermatol. 2007;56:828-834.
47. Speight PM. Mucosal malignant melanoma. In: Barnes L, Eveson JW, Reichart P, et al, editors. WHO Classification of Tumours, Pathology and Genetics Head and Neck Tumours. Lyon, France: WHO Blue Books, 2005.
48. Shah JP, Huvos AG, Strong EW. Mucosal melanomas of the head and neck. Am J Surg. 1977;134:531-535.
49. Snow GB, van der Esch EP, van Slooten EA. Mucosal melanomas of the head and neck. Head Neck Surg. 1978;1:24-30.
50. Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282-3287.
51. Negri E, La Vecchia C, Franceschi S, et al. Attributable risk for oral cancer in northern Italy. Cancer Epidemiol Biomarkers Prev. 1993;2:189-193.
52. Franceschi S, Talamini R, Barra S, et al. Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx, and esophagus in northern Italy. Cancer Res. 1990;50:6502-6507.
53. Znaor A, Brennan P, Gajalakshmi V, et al. Independent and combined effects of tobacco smoking, chewing and alcohol drinking on the risk of oral, pharyngeal, and esophageal cancers in Indian men. Int J Cancer. 2003;105:681-686.
54. Balaram P, Sridhar H, Rajkumar T, et al. Oral cancer in southern India: the influence of smoking, drinking, paan-chewing and oral hygiene. Int J Cancer. 2002;98:440-445.
55. Herrero R, Castellsaqué X, Pawlita M, et al. Human papillomavirus and oral: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772-1783.
56. Harreus UA, Kleinsasser NH, Zieger S, et al. Sensitivity to DNA-damage induction and chromosomal alterations in mucosa cells from patients with or without cancer of the oropharynx detected by a combination of Comet assay and fluorescence in situ hybridization. Mutat Res. 2004;563:131-138.
57. Perez CA, Purdy JA, Breaux SR, et al. Carcinoma of the tonsillar fossa: a nonrandomized comparison of preoperative radiation and surgery of irradiation alone: long-term results. Cancer. 1982;50:2314-2322.
58. Werner JA, Dünne AA, Myers JN. Functional anatomy of the lymphatic drainage system of the upper aerodigestive tract and its role in metastasis of squamous cell carcinoma. Head Neck. 2003;25:322-332.
59. Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446-1449.
60. Belz GT, Heath TJ. Lymphatic drainage from the tonsil of the soft palate in pigs. J Anat. 1995;187(Pt 2):491-495.
61. Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446-1449.
62. Soh KB. Branchiogenic carcinomas: do they exist? J R Coll Surg Edinb. 1998;43:1-5.
63. Hu KS, Harrison LB, Culliney B, et al. Cancer of oropharynx. In Harrison LB, Sessions RB, Hong WK, editors: Head and Neck Cancer, 2nd ed, Philadelphia: Lippincott Williams and Wilkins, 2004.
64. Browman GP, Wong G, Hodson I, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993;328:159-163.
65. Chan JM, Shin LK, Jeffrey RB. Ultrasonography of abnormal neck lymph nodes. Ultrasound Q. 2007;23:47-54.
66. Wippold FJ. Head and neck imaging: the role of CT and MRI. J Magn Reson Imaging. 2007;25:453-465.
67. Quon A, Fischbein NJ, McDougall IR, et al. Clinical role of 18F-FDG PET/CT in the management of squamous cell carcinoma of the head and neck and thyroid carcinoma. J Nucl Med. 2007;48(Suppl 1):58S-67S.
68. Andrade RS, Heron DE, Degirmenci B, et al. Posttreatment assessment of response using FDG-PET for patients treated with definitive radiation therapy for head and neck cancers. Int J Radiat Oncol Biol Phys. 2006;65:1315-1322.
69. Porceddu SV, Jarmolowski E, Hicks RJ, et al. Utility of positron emission tomography for the detection of disease in residual neck nodes after (chemo)radiotherapy in head and neck cancer. Head Neck. 2005;27:175-181.
70. Lonneux M, Lawson G, Ide C, et al. Positron emission tomography with fluorodeoxyglucose for suspected head and neck tumor recurrence in the symptomatic patient. Laryngoscope. 2000;110:1493-1497.
71. Quon A, Fischbein NJ, McDougall IR, et al. Clinical of 18F-FDG PET/CT in the management of squamous cell carcinoma of the head and neck and thyroid carcinoma. J Nucl Med. 2007;48(Suppl 1):58S-67S.
72. Righi PD, Kopecky KK, Caldemeyer KS, et al. Comparison of ultrasound-fine needle aspiration and computed tomography in patients undergoing elective neck dissection. Head Neck. 1997;19:604-610.
73. Black C, Marotti J, Zarovnaya E, et al. Critical evaluation of frozen section margins in head and neck cancer resections. Cancer. 2006;107:2792-2800.
74. Zarbo RJ, Hoffman GG, Howanitz PJ. Interinstitutional comparison of frozen-section consultation. A College of American Pathologists Q-Probe study of 79,647 consultants in 297 North American institutions. Arch Pathol Lab Med. 1991;115:1187-1194.
75. Sobin LH, Wittekind C, editors. TNM Classification of Malignant Tumours, 6th ed, New York: Wiley-Liss, 2002.
76. Greene FL, Page DL, Fleming ID, et al, editors. AJCC Cancer Staging Manual, 6th ed, New York: Springer Verlag, 2002.
77. Iro H, Waldfahrer F. Evaluation of the newly updated TNM classification of head and neck carcinoma with data from 3247 patients. Cancer. 1998;83:2201-2207.
78. Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803-1804.
79. O’Sullivan B, Shah J. New TNM staging criteria for head and neck tumors. Semin Surg Oncol. 2003;21:30-42.
80. Clarkson JE, Conway DI, Oliver RJ, et al. Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment. Cochrane Database Syst Rev. 17:CD006205, 2007.
81. Robertson AG, Soutar DS, Paul J, et al. Early closure of a randomized trial: surgery and postoperative radiotherapy versus radiotherapy in the management of intra-oral tumors. Clin Oncol. (R Coll Radiol.). 1998;10:155-160.
82. Smith RB, Sniezek JC, Weed DT, et al. Utilization of free tissue transfer in head and neck surgery. Otolaryngol Head Neck Surg. 2007;137:182-191.
83. Mazeron JJ, Marinello G, Crook J, et al. Definitive radiation treatment for early stage carcinoma of the soft palate and uvula: the indications for iridium 192 implantation. Int J Radiat Oncol Biol Phys. 1987;13:1829-1837.
84. Esche BA, Haie CM, Gerbaulet AP, et al. Interstitial and external radiotherapy in carcinoma of the soft palate and uvula. Int J Radiat Oncol Biol Phys. 1988;15:619-625.
85. Mazeron JJ, Crook J, Martin M, et al. Iridium 192 implantation of squamous cell carcinomas of the oropharynx. Am J Otolaryngol. 1989;10:317-321.
86. Pernot M, Malissard L, Taghian A, et al. Velotonsillar squamous cell carcinoma: 277 cases treated by combined external irradiation and brachytherapy—results according to extension, localization, and dose rate. Int J Radiat Oncol Biol Phys. 1992;23:715-723.
87. Amdur RJ, Mendenhall WM, Parsons JT, et al. Carcinoma of the soft palate treated with irradiation: analysis of results and complications. Radiother Oncol. 1987;9:185-194.
88. Calais G, Alfonsi M, Bardet E, et al. Stage III and IV cancers of the oropharynx: results of a randomized study of Gortec comparing radiotherapy alone with concomitant chemotherapy. Bull Cancer. 2000;87(Spec No):48-53.
89. Har-El G, Shaha A, Chaudry R, et al. Carcinoma of the uvula and midline soft palate: indication for neck treatment. Head Neck. 1992;14:99-101.
90. Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94:2967-2980.
91. Werner JA, Dunne AA, Myers JN. Functional anatomy of the lymphatic drainage system of the upper aerodigestive tract and its role in metastasis of squamous cell carcinoma. Head Neck. 2003;25:322-332.
92. Eckel HE, Volling P, Pototschnig C, et al. Occult neck disease. Laryngoscope. 1995;105:53-60.
93. Eckel HE, Volling P, Ebeling O, et al. Transoral laser surgery for oral carcinoma. Adv Otorhinolaryngol. 1995;49:185-190.
94. Foote RL, Schild SE, Thompson WM, et al. Tonsil cancer. Patterns of failure after surgery alone and surgery combined with postoperative radiation therapy. Cancer. 1994;73:2638-2647.
95. Perez CA, Patel MM, Chaos KS, et al. Carcinoma of the patellar fossa: prognostic factors and long-term therapy outcome. Int J Radiat Oncol Biol Phys. 1998;42:1077-1084.
96. Hicks WLJr, Kuriakose MA, Loree TR, et al. Surgery versus radiation therapy as single-modality treatment of tonsillar fossa carcinoma: the Roswell Park Cancer Institute experience (1971-1991). Laryngoscope. 1998;108:1014-1019.
97. Foote RL, Hilgenfeld RU, Kunselman SJ, et al. Radiation therapy for squamous cell carcinoma of the tonsil. Mayo Clin Proc. 1994;69:525-531.
98. Zelefsky MJ, Harrison LB, Armstrong JG. Long-term treatment results of postoperative radiation therapy for advanced stage oropharyngeal carcinoma. Cancer. 1992;70:2388-2395.
99. Spiro JD, Spiro RH. Carcinoma of the tonsillar fossa. An update. Arch Otolaryngol Head Neck Surg. 1989;115:1186-1189.
100. Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937-1944.
101. Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945-1952.
102. Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94:2967-2980.
103. Gehanno P, Depondt J, Guedon C, et al. Primary and salvage surgery for cancer of the tonsillar region: a retrospective study of 120 patients. Head Neck. 1993;15:185-189.
104. Peiffert D, Pernot M, Malissard L, et al. Salvage irradiation by brachytherapy of velotonsillar squamous cell carcinoma in a previously irradiated field: results in 73 cases. Int J Radiat Oncol Biol Phys. 1994;29:681-686.
105. Puthawala AA, Syed AM, Gates TC. Iridium-192 implants in the treatment of tonsillar region malignancies. Arch Otolaryngol. 1985;111:812-815.
106. Wei WI, Ferlito A, Rinaldo A, et al. Management of the N0 neck—reference or preference. Oral Oncol. 2006;42:115-122.
107. Ferlito A, Devaney KO, Rinaldo A, et al. Neuroendocrine neoplasms of the larynx: advances in identification, understanding, and management. Oral Oncol. 2006;42:14-25.
108. Steiner W, Ambrosch P, editors. Endoscopic Laser Surgery of the Upper Aerodigestive Tract. New York: Thieme Stuttgart, 2000.
109. Grant DG, Salassa JR, Hinni ML, et al. Carcinoma of the tongue base treated by transoral laser microsurgery, part one: untreated tumors, a prospective analysis of oncologic and functional outcomes. Laryngoscope. 2006;116:2150-2155.
110. Steiner W, Fierek O, Ambrosch P, et al. Transoral laser microsurgery for squamous cell carcinoma of the base of tongue. Arch Otolaryngol Head Neck Surg. 2003;129:36-43.
111. O’Malley BWJr, Weinstein GS, Snyder W, et al. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope. 2006;116:1465-1472.
112. Mashberg A, Meyers H. Anatomical site and size of 222 early asymptomatic oral squamous cell carcinomas: a continuing prospective study of oral cancer. II. Cancer. 1976;37:2149-2157.
113. Housset M, Baillet F, Dessard-Diana B, et al. A retrospective study of three treatment techniques for T1-T2 base of tongue lesions: surgery plus postoperative radiation, external radiation plus interstitial implantation and external radiation alone. Int J Radiat Oncol Biol Phys. 1987;13:511-516.
114. Spanos WJJr, Shukovsky LJ, Fletcher GH. Time, dose, and tumor volume relationships in irradiation of squamous cell carcinomas of the base of the tongue. Cancer. 1976;37:2591-2599.
115. Foote RL, Parsons JT, Mendenhall WM, et al. Reponse to Goffinet, Harrison, Puthawala, and Syed. Int J.Radiat Oncol Biol Phys. 1991;21:868-869.
116. Goffinet DR, Fee WEJr, Wells J, et al. 1921r pharyngoepiglottic fold interstitial implants. The key to successful treatment of base tongue carcinoma by radiation therapy. Cancer. 1985;55:941-948.
117. Harrison LB, Zelefsky MJ, Sessions RB, et al. Base-of-tongue cancer treated with external beam irradiation plus brachytherapy: oncologic and functional outcome. Radiology. 1992;184:267-270.
118. Puthawala AA, Syed AM, Eads DL, et al. Limited external beam and interstitial 192iridium irradiation in the treatment of carcinoma of the base of the tongue: a ten year experience. Int J Radiat Oncol Biol Phys. 1988;14:839-848.
119. Sabri A. Oropharyngeal reconstruction: current state of the art. Curr Opin Otolaryngol Head Neck Surg. 2003;11:251-254.
120. Zelefsky MJ, Harrison LB, Armstrong JG. Long-term treatment results of postoperative therapy for advanced stage oropharyngeal carcinoma. Cancer. 1992;70:2388-2395.
121. Pradhan SA, Rajpal RM, Kothary PM. Surgical management of postradiation residual/recurrent cancer of the base of the tongue. J Surg Oncol. 1980;14:201-206.
122. Grant DG, Salassa JR, Hinni ML, et al. Carcinoma of the tongue base treated by transoral laser microsurgery, part two: persistent, recurrent and second primary tumors. Laryngoscope. 2006;116:2156-2161.
123. Langlois D, Hoffstetter S, Malissard L, et al. Salvage irradiation of oropharynx and mobile tongue about 192 iridium brachytherapy in Centre Alexis Vautrin. Int J Radiat Oncol Biol Phys. 1988;14:849-853.
124. Mazeron JJ, Marinello G, Crook J, et al. Definitive radiation treatment for early stage carcinoma of the soft palate and uvula: the indications for iridium 192 implantation. Int J Radiat Oncol Biol Phys. 1987;13:957-962.
125. Pellitteri PK, Ferlito A, Rinaldo A, et al. Planned neck dissection following chemoradiotherapy for advanced head and neck cancer: is it necessary for all? Head Neck. 2006;28:166-175.
126. Cupino A, Axelrod R, Anne PR, et al. Neck dissection followed by chemoradiotherapy for stage IV (N+) oropharynx cancer. Otolaryngol Head Neck Surg. 2007;137:416-421.
127. Meoz-Mendez RT, Fletcher GH, Guillamondegui OM, et al. Analysis of the results of irradiation in the treatment of squamous cell carcinomas of the pharyngeal walls. Int J Radiat Oncol Biol Phys. 1978;4:579-585.
128. Marks JE, Freeman RB, Lee F, et al. Pharyngeal wall cancer: an analysis of treatment. Int J Radiat Oncol Biol Phys. 1978;4:587-593.
129. Spiro RH, Kelly J, Vega AL, et al. Squamous carcinoma of the posterior pharyngeal wall. Am J Surg. 1990;160:420-423.
130. Steiner W, Fierek O, Ambrosch P, et al. Transoral laser microsurgery for squamous cell carcinoma of the base of the tongue. Arch Otolaryngol Head Neck Surg. 2003;129:36-43.
131. Basterra J, Bagán JV, Alba JR, et al. Oropharyngectomy without mandibulotomy in advanced stage (T3-T4) oropharyngeal cancer. Acta Otolaryngol. 2007;127:874-879.
132. Scottish Intercollegiate Guidelines Network (SIGN). Diagnosis and management of head and neck cancer. http://www.sign.ac.uk/. accessed Nov. 2007
133. Johansen LV, Grau C, Overgaard J. Squamous cell carcinoma of the oropharynx—an analysis of treatment results in 289 consecutive patients. Acta Oncol. 2000;39:985-994.
134. Aebersold DM, Beer KT, Laissue J, et al. Intratumoral microvessel density predicts local treatment failure of radically irradiated squamous cell cancer of the oropharynx. Int J Radiat Oncol Biol Phys. 2000;48:17-25.
135. Sittel C, Eckel HE, Damm M, et al. Ki-67 (MIB1), p53, and Lewis-X (LeuM1) as prognostic factors of recurrence in T1 and T2 laryngeal carcinoma. Laryngoscope. 2000;110:1012-1017.
136. Jaskulski D, deRiel JK, Mercer WE, et al. Inhibition of cellular proliferation by antisense oligodeoxynucleotides to PCNA cyclin. Science. 1988;240:1544-1546.
137. Overgaard J, Hansen HS, Overgaard M, et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiother Oncol. 1998;46:135-146.
138. Nordsmark M, Overgaard J. A confirmatory prognostic study on oxygenation status and loco-regional control in advanced head and neck squamous cell carcinoma treated by radiation therapy. Radiother Oncol. 2000;57:39-43.
139. Vanselow B, Eble MJ, Rudat V, et al. Oxygenation of advanced head and neck cancer: prognostic marker for the response to primary radiochemotherapy. Otolaryngol Head Neck Surg. 2000;122:856-862.
140. Brizel DM, Sibley GS, Prosnitz LR, et al. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285-289.
141. Marshak G, Popovtzer A. Is there any significant reduction of patients’ outcome following delay in commencing postoperative radiotherapy. Curr Opin Otolaryngol Head Neck Surg. 2006;14:82-84.
142. Fietkau R. [Effects of the time interval between surgery and radiotherapy on the treatment results]. Strahlenther Onkol. 2000;176:452-457.
143. ICF—International Classification of Functioning, Disability and Health. Core set on head and neck cancer. WHO http://www.who.int/classifications/icf/en accessed Nov. 2007