Male hypogonadism
Male hypogonadism is the clinical and/or laboratory syndrome that results from a failure of the testis to work properly. The normal testis has two functions: synthesis and secretion of testosterone from the Leydig cells and production of sperm from the seminiferous tubules. Deficiency of one or both functions is termed male hypogonadism. This condition can result from a disruption at one or more levels of the hypothalamic-pituitary-gonadal axis. Depending on the stage of development, hypogonadism may have varied manifestations.
2. What are the manifestations of in utero hypogonadism?
In utero androgen deficiency leads to a female phenotype or ambiguous genitalia, most commonly caused by a block in the production of testosterone secondary to congenital testosterone biosynthetic enzyme defects. Rarely, peripheral tissues cannot respond normally to testosterone, thereby resulting in the androgen insensitivity syndromes of testicular feminization (complete) and Reifenstein’s syndrome (incomplete). Other manifestations include micropenis, hypospadias, and cryptorchidism.
3. What are the manifestations of peripubertal hypogonadism?
Childhood androgen deficiency results in delayed, incomplete, or absent pubertal development. Common manifestations include the following:
4. What are the manifestations of hypogonadism in early adulthood?
In early adulthood, a decrease in sperm output (azoospermia or oligospermia) without deficient production of testosterone is common and results in male infertility; thus, infertility is a form of male hypogonadism. A decrease in production of testosterone in adulthood is usually accompanied by a decline in production of sperm. When it is not, the term fertile eunuch (eunuchoid proportions, low levels of luteinizing hormone [LH], low levels of testosterone, normal levels of follicle-stimulating hormone [FSH], and spermatogenesis) is appropriately applied. Libido and/or potency may be diminished.
5. What are the manifestations of hypogonadism in middle to late adulthood?
The most frequent circumstance in which adult hypogonadism occurs is in the middle-aged or senescent man complaining of decreased libido or potency. Semen analysis is rarely performed in these men because they are usually not concerned with fertility. Other findings may include osteoporosis, diminished androgen production, and small prostate. If the onset of hypogonadism is acute, the patient may experience hot flushes and sweats.
6. How is the production of testosterone normally regulated?
LH is episodically secreted from the anterior pituitary in response to pulses of gonadotropin-releasing hormone (GnRH), thus stimulating production of testosterone by Leydig cells. Once testosterone is secreted into the bloodstream, it is bound by sex hormone–binding globulin (SHBG) and albumin. The non–SHBG-bound (or “free”) testosterone provides negative feedback to the hypothalamic-pituitary unit and thus inhibits output of LH. This classic endocrine feedback loop serves to maintain serum testosterone at a predetermined level; if serum testosterone falls below the set point, the pituitary is stimulated to secrete LH, which, in turn, stimulates testicular output of testosterone until serum levels return to the set point. Conversely, if serum testosterone rises above the set point, decreased output of LH results in decreased testicular output of testosterone until serum levels have declined to the set point. Although most automated total testosterone assays are reliable and are generally able to distinguish hypogonadal from eugonadal men, abnormalities in the SHBG level may give falsely low or high total testosterone levels. Equilibrium dialysis is the gold standard for measuring the free testosterone, but it is not commonly available and should be ordered to be performed only in a reliable reference laboratory. Liquid chromatography–mass spectrometry or gas chromatography–mass spectrometry is used by some reference laboratories to measure testosterone. This is a very accurate but expensive method. Analog methods for determining free testosterone are more widely available but are not accurate in the low ranges.
7. What are some conditions associated with decreased or increased serum SHBG levels?
Moderate obesity, nephrotic syndrome, hypothyroidism, and the use of certain medications (notably glucocorticoids and androgenic steroids) decrease SHBG levels and give a low total serum testosterone level, whereas aging, anticonvulsant use, estrogen use, herbal preparations for “prostate health” that contain plant-derived estrogens, hepatic cirrhosis, human immunodeficiency virus (HIV) infection, and hyperthyroidism may all increase SHBG and cause a high total level of testosterone.
8. How is sperm production normally regulated?
The regulation of sperm production is complex and less clearly understood than is the regulation of testosterone production. Both hormonal and nonhormonal factors are important. The Sertoli cells within the seminiferous tubules seem to play an important coordinating role. Sertoli cells respond to FSH by producing inhibin (secreted into the blood) and androgen-binding protein, transferrin, and other proteins (secreted into the seminiferous tubular lumen). Inhibin appears to inhibit the output of FSH from the pituitary gland, thus completing a feedback loop. In theory, if spermatogenesis declines, production of inhibin also should decline; thus the negative feedback effect on the pituitary would be reduced, leading to an increased output of FSH, which would then presumably stimulate spermatogenesis. However, not all aspects of this feedback loop (FSH-inhibin-spermatogenesis) have been verified experimentally. Moreover, spermatogenesis depends on intratesticular production of testosterone mediated by androgen receptors within Sertoli cells. Initiation of spermatogenesis during puberty requires both LH and FSH. However, reinitiation of the process if it is disrupted by exogenous factors (see the following) requires only LH (or human chorionic gonadotropin [hCG]), although FSH may be needed to produce a normal number of sperm.
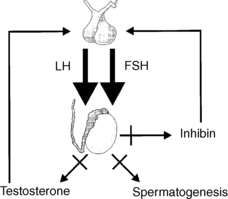
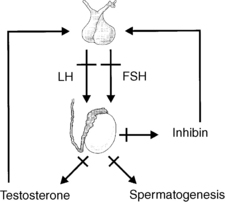
9. What is the difference between primary and secondary hypogonadism?
Failure of testicular function may result from a defect either at the testis or at the hypothalamic-pituitary level. Testicular disorders leading to hypogonadism are termed primary hypogonadism (Fig. 44-1), whereas disorders of hypothalamic-pituitary function leading to hypogonadism are termed secondary hypogonadism (Fig. 44-2). This distinction has therapeutic implications. In men with secondary hypogonadism, fertility can generally be restored with appropriate hormonal treatment. Men with primary hypogonadism have fewer options and more limited success with improvement in fertility. In addition, the evaluation of secondary hypogonadism can reveal a pituitary mass or systemic illness as the underlying cause.
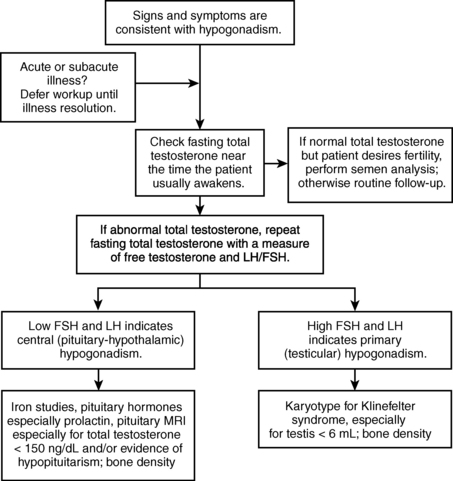
10. What is the initial laboratory workup for hypogonadism?
Primary hypogonadism resulting from a testicular disorder leads to a decline in production of testosterone and sperm, a consequent decrease in the negative feedback effects on the pituitary, and a corresponding increase in serum levels of LH and FSH. Conversely, in secondary hypogonadism resulting from a hypothalamic-pituitary disorder, serum LH and FSH may be subnormal or “inappropriately” normal (explainable, in part, by decreased bioactivity) despite a low testosterone level. A subnormal sperm count and a normal testosterone level with a normal LH and elevated FSH suggest primary hypogonadism with a dysfunction of the seminiferous tubules and sperm production but intact Leydig cell function. An algorithm for the logical evaluation of hypogonadism is shown in Figure 44-3.
11. What are congenital causes of primary hypogonadism?
 Klinefelter’s syndrome (47,XXY and mosaics)
Klinefelter’s syndrome (47,XXY and mosaics)
 Microdeletions of azoospermia factor (AZF) regions of Yp telomere (15% of men with nonobstructive azoospermia; 5% to 10% of those with oligospermia)
Microdeletions of azoospermia factor (AZF) regions of Yp telomere (15% of men with nonobstructive azoospermia; 5% to 10% of those with oligospermia)
 Testosterone biosynthetic enzyme deficiencies (3-beta-hydroxysteroid dehydrogenase, 17-alpha-hydroxylase, or 17-beta-hydroxysteroid dehydrogenase)
Testosterone biosynthetic enzyme deficiencies (3-beta-hydroxysteroid dehydrogenase, 17-alpha-hydroxylase, or 17-beta-hydroxysteroid dehydrogenase)
 Androgen receptor gene mutation (qualitative or quantitative)
Androgen receptor gene mutation (qualitative or quantitative)
 LH receptor mutations (male phenotype, if mild; female phenotype, if severe)
LH receptor mutations (male phenotype, if mild; female phenotype, if severe)
12. What are acquired causes of primary hypogonadism?
 Cancer therapy: chemotherapy (alkylating agents more than cisplatin and carboplatin) and radiation therapy (may be permanent with external radiation; usually transient with radioactive iodine)
Cancer therapy: chemotherapy (alkylating agents more than cisplatin and carboplatin) and radiation therapy (may be permanent with external radiation; usually transient with radioactive iodine)
 Drugs (e.g., ketoconazole, 5-alpha-reductase inhibitors)
Drugs (e.g., ketoconazole, 5-alpha-reductase inhibitors)
 Infiltrative disease (e.g., hemochromatosis)
Infiltrative disease (e.g., hemochromatosis)
 Infections (e.g., HIV [may be multifactorial], mumps orchitis)
Infections (e.g., HIV [may be multifactorial], mumps orchitis)
 Systemic illness (e.g., uremia, cirrhosis); may be multifactorial
Systemic illness (e.g., uremia, cirrhosis); may be multifactorial
13. Is normal aging associated with primary hypogonadism?
Symptomatic hypogonadism, defined by at least three sexual symptoms and a low total testosterone (< 320 ng/dL) and/or low free testosterone (< 64 pg/mL) level, is present in about 2% of men between 40 and 79 years of age. When only biochemical criteria are used, the prevalence is higher (2%–6%) in that age range and 18% to 30% in men more than 70 years old. Multiple cross-sectional studies have noted that older men have mildly reduced levels of total serum testosterone but significantly reduced levels of free testosterone (because of a rise in SHBG with age) compared with younger men. This decline is associated with a rise in LH and FSH, a finding suggesting a primary gonadal cause. Studies have demonstrated an average 1% to 2% decline in total serum testosterone and an even greater reduction in free testosterone (because of elevations in SHBG) per year associated with normal aging. Further complicating the situation is the observation that there has been a population-level decrease in serum testosterone levels in men in the United States since the early 1990s.
14. What are the causes of secondary hypogonadism?
Any disease that affects the hypothalamic-pituitary axis can cause secondary hypogonadism. Involvement of the hypothalamus or pituitary stalk interferes with the secretion of GnRH or the ability of GnRH to communicate with the pituitary. Various anatomic lesions of the pituitary cause secondary hypogonadism by interfering with the release of LH and FSH. Such lesions include benign tumors and cysts, malignant tumors (both primary central nervous system tumors and metastatic tumors from distant sources), vascular aneurysms, infiltrative diseases (e.g., hemochromatosis), pituitary hemorrhage, and pituitary trauma. Certain inflammatory diseases (e.g., sarcoidosis and histiocytosis) can also affect the hypothalamus and pituitary and decrease testosterone production. Congenital disorders, in which output of LH and FSH is impaired, such as Kallmann’s syndrome (see later), also lead to secondary hypogonadism. Both obesity and HIV/acquired immunodeficiency syndrome (AIDS) are associated with secondary hypogonadism as well. Drugs commonly used in the treatment of benign prostatic hypertrophy, the 5-alpha-reductase inhibitors finasteride and dutasteride, are among the iatrogenic causes of hypogonadism. Other drug-related causes include the use of narcotic analgesics and the abuse of anabolic steroids by athletes.
15. What assessment for congenital hypogonadotropic hypogonadism (CHH) should be done?
The sense of smell in the patient (and his relatives) should be assessed by direct questioning. A quantitative or semiquantitative method is olfactometry. In addition, magnetic resonance imaging (MRI) can be performed to assess the olfactory bulb. If the sense of smell is normal and there does not appear to be a syndromic form of CHH (i.e., Kallmann’s syndrome), then the most common gene abnormalities are GNRHR, KISS1R, GnRH1, TAC3, and TACR3. If the sense of smell is decreased or absent in a man with CHH, then the diagnosis is Kallmann’s syndrome. KAL1 mutations occur in Kallmann’s syndrome, especially in men with mirror movements (bimanual synkinesis), renal agenesis, and an X-linked pattern of inheritance. FGFR1 mutations occur more often in patients with Kallmann’s syndrome and midline abnormalities (e.g., cleft lip or palate, short metacarpals and/or metatarsals), but they can occur in normosmic CHH. FGF8, PROK2, or PROKR2 mutations are more common in Kallmann’s syndrome but can also occur in normosmic CHH.
16. What is the most common pituitary tumor in adults?
The most common pituitary tumor found in adults is a prolactin-secreting adenoma. These tumors primarily cause hypogonadism as a result of local destruction and compression, thus inhibiting the production and release of LH and FSH. Elevated prolactin levels can also interrupt secretion of GnRH, although this is usually of much less significance in men than is the mass effect.
17. How do other pituitary adenomas cause hypogonadism?
Pituitary adenomas that produce growth hormone (acromegaly) or adrenocorticotropic hormone (Cushing’s disease) and nonfunctioning pituitary tumors may similarly cause secondary hypogonadism by their mass effects.
18. What clinical symptoms are seen in male hypogonadism?
Loss of the sperm-producing function of the testis leads to infertility, usually defined as failure of a normal female partner to conceive after 12 months of unprotected intercourse. Loss of the testosterone-producing function of the testis may lead to loss of libido and erectile dysfunction, as well as diminution of secondary sexual characteristics, such as facial and pubic hair, and decrease in testicular volume. Decreased production of testosterone also may cause more generalized symptoms, such as decreased muscle mass and strength, malaise, and fatigue. In boys who develop hypogonadism before sexual maturation, delay or absence of the onset of puberty is typical. Tender gynecomastia is frequently seen in hypogonadism. Numerous nonspecific symptoms are also commonly associated with hypogonadism, such as normochromic normocytic anemia, poor concentration, depressed mood, and increased body fat and body mass index.
19. What questions are most helpful in determining whether a man may have hypogonadism?
When the condition is associated with a low total or free testosterone level, the following questions are useful in establishing a diagnosis of clinical hypogonadism:
20. How does hypogonadism affect bone architecture?
Osteoporosis is now a well-recognized result of both primary and secondary hypogonadism. Trabecular architecture (and bone strength) is even more severely disturbed than bone density in men with hypogonadism. Thus, it is not surprising that hypogonadism is found in up to 30% of men with vertebral fractures. Estradiol that is aromatized from testosterone may be the most important factor in preserving bone architecture and density in both men and women. However, androgen receptors are also found in bone and may explain the sexual dimorphism of bone density.
21. What laboratory tests help to confirm a suspected diagnosis of male hypogonadism?
The main functions of the testis, production of sperm and production of testosterone, are readily assessed by semen analysis and measurement of serum testosterone, respectively. Normal semen analysis values in men following 2 to 3 days of abstinence are 20 million sperm/mL and more than 60% motility of the sperm. Because sperm density is highly variable from day to day in all men, accurate assessment usually involves several semen analyses done with the same abstinence period each time. The best initial test for testosterone production is measurement of the fasting morning serum total testosterone level. Serum testosterone also varies considerably from moment to moment and from morning to night in response to LH secretion; again, several samples may be needed to establish an accurate measurement. In addition, most testosterone in serum is bound to plasma proteins, particularly SHBG; thus, in patients who have increased or decreased SHBG levels (see earlier) and in those men in whom plasma protein levels may be disrupted, measurement of the physiologically active “free” testosterone may prove informative. Bone density measurement using a dual-energy x-ray absorptiometry (DXA) scan may provide helpful baseline information and assist in deciding whether to provide androgen replacement therapy.
22. What other diagnostic tests are useful in defining the cause of male hypogonadism?
Additional diagnostic testing should be based on clinical suspicion and the results of preliminary testing. For example, in cases of secondary hypogonadism, measurement of serum prolactin and pituitary radiography, preferably MRI with gadolinium, should be done. Computed tomography (CT) of the sella turcica usually detects macroadenomas (> 1.0 cm) but misses many clinically significant microadenomas and is therefore less preferable than MRI. Plain skull or sella turcica films are not adequate for diagnosis. Measurement of other pituitary hormones also may be appropriate to assess either possible tumoral hypersecretion (e.g., Cushing’s disease, acromegaly) or tumor-related hypopituitarism. Visual field testing is indicated if a macroadenoma is present or there is suprasellar extension. Similarly, the initial findings in primary hypogonadism may suggest additional tests. For example, small firm testes, gynecomastia, azoospermia, modestly reduced serum testosterone levels, and high levels of serum LH and FSH in a young man should lead to chromosome analysis to confirm a presumptive diagnosis of Klinefelter’s syndrome. Measurement of serum estradiol levels may be helpful when feminization is prominent clinically, as in secondary hypogonadism related to production of estrogen by testicular or adrenal tumors. If infertility is the primary issue and no hormonal abnormality is found, genetic causes should be investigated. This includes testing for Y chromosome microdeletion syndromes. Testis biopsy rarely provides information that is useful in establishing a specific diagnosis, prognosis, or treatment.
Hermaphrodite refers to someone who has both ovarian and testicular elements in the body. These patients usually have a 46,XX or 46,XX/46,XY karyotype. Such individuals may have an ovary and a testis or an ovotestis. They most often have ambiguous genitalia.
24. What is a pseudohermaphrodite?
Pseudohermaphrodite refers to someone whose external genitalia are not consistent with his or her gonadal sex. A male pseudohermaphrodite, for example, has a 46,XY karyotype and testes but has either ambiguous genitalia or a complete female phenotype. Most often this results from genetic disorders of testosterone biosynthetic enzymes, the androgen receptor, or the 5-alpha-reductase enzyme; the severity of the phenotype depends on the severity of the genetic defect. A female pseudohermaphrodite, in contrast, has a 46,XX karyotype and ovaries but has ambiguous external genitalia. The most common cause of this is congenital adrenal hyperplasia, which results in virilization of the female fetus in utero.
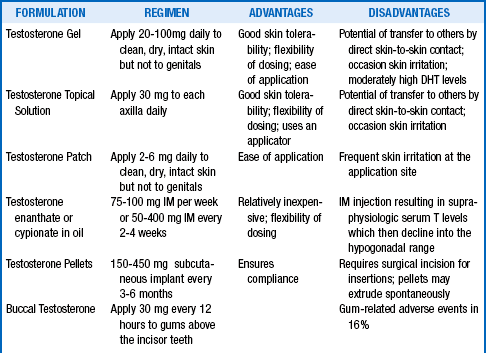
25. How do you treat hypogonadism?
Testosterone deficiency is easily treated with testosterone replacement therapy (TRT) (Table 44-1). An alternative approach to TRT is oral clomiphene citrate, which blocks estrogen feedback on the hypothalamic-pituitary axis and thereby increases LH and FSH secretion, with a resultant increase in testosterone production. In general, the treatment goal for TRT in primary hypogonadism is to provide sufficient testosterone doses to normalize the serum LH, which may take normal to high-normal serum testosterone levels. For patients with secondary hypogonadism, the goal is a serum total testosterone level in the midnormal range. The treatment goal in elderly men is a low-normal to midnormal range serum testosterone without regard to the LH level.
There is currently considerable controversy over whether men with age-associated hypogonadism should be treated with TRT. Although some short-term studies have demonstrated treatment benefits, long-term large studies are lacking and are needed to clarify the criteria for treatment, as well as the risks and benefits associated with TRT in this population. One study in older men with hypogonadism and impaired mobility was stopped early because of an increase in cardiovascular events. Some older men with testosterone deficiency are unconcerned about sexual function and may not desire TRT. In testosterone-deficient men of any age, osteopenia or osteoporosis and/or reduced hematopoiesis may be indications for TRT even in the absence of decreased libido or erectile dysfunction, although low bone mass may be more safely improved with a bisphosphonate. Testosterone preparations are currently designated as schedule III drugs by the Anabolic Steroid Control Act because of their potential for abuse by athletes and others.
26. What are the potential adverse effects of TRT?
Gynecomastia and acne are rare symptoms that may occur in the first few months after initiating TRT; these side effects may resolve with continued treatment, although temporary dose reductions may be helpful. Abnormalities of liver function tests are uncommon with currently used injectable and transdermal preparations, but they can be seen with seldom-used oral preparations. A testosterone-induced increase in hematocrit is common, especially when testosterone injections are used, although clinically significant polycythemia is quite rare unless the drug is being abused. TRT may also precipitate or worsen sleep apnea; marked increases in hematocrit may be a clue to this side effect. Skin reactions are commonly seen in patients using the transdermal patch and are occasionally, but much less frequently, seen with the gels. In boys who have not yet gone through puberty, the rapid increase in serum testosterone after initial treatment may lead to considerable psychological difficulties and physically aggressive behavior; initiating treatment with smaller doses may be helpful. TRT has no adverse effect on lipid profiles compared with eugonadal men, but overtreatment can lead to several lipid abnormalities, including decreases in high-density lipoprotein cholesterol level. There does not appear to be a significant increase in cardiovascular disease associated with physiologic TRT, and some studies have even suggested a treatment benefit. However, patients with class III or IV heart failure should be given TRT cautiously.
27. Does TRT affect the prostate in older men?
In older men, TRT effects on the prostate must be considered, including the possibility of precipitating urinary retention secondary to testosterone-induced prostate enlargement. Short-term studies have not shown any histologic or gene expression effects of TRT. However, prostate volume increases with long-term TRT to a level comparable to eugonadal men without any significant associated increases in symptoms, urine flow rates, or residual volumes. Individual men may experience voiding symptoms along with this enlargement, which they should be advised to monitor. Although TRT with a scrotal patch or gel (but not a nonscrotal patch) increases dihydrotestosterone more than testosterone and it is the former that stimulates the prostate, it is advisable to perform a digital rectal examination (DRE) of the prostate and monitor prostate-specific antigen (PSA) in middle-aged and older men before and annually while they are receiving any TRT. Although no compelling evidence indicates that TRT causes prostate carcinoma, the potential for testosterone stimulation of occult prostate carcinoma growth exists. Men with an elevated PSA level or an abnormal DRE should be evaluated further, potentially including a prostate biopsy, before initiation of TRT.
28. How does one treat deficient sperm production in primary hypogonadism?
In men with primary hypogonadism, as manifested by elevated levels of serum FSH, there seems to be no effective pharmacologic treatment for increasing the sperm count. Anatomic lesions, such as varicoceles and ejaculatory duct obstructions, can be corrected surgically, but improvement in spermatogenesis may not result. If one plans to use a medication that is known to cause hypogonadism (e.g., cancer chemotherapeutic agents), it may be desirable to cryopreserve semen specimens before treatment, provided that treatment is not unduly delayed.
29. How does one treat deficient sperm production in secondary hypogonadism?
The outlook is much less pessimistic with secondary hypogonadism, particularly if the condition developed after puberty. Treatment with gonadotropins (hCG with or without added FSH) may be successful in restoring production of sperm, as well as testosterone. The pretreatment size of the testis is often a clue to prognosis; larger testis size is associated with a better outcome. Production of testosterone and sperm in men with secondary hypogonadism also may be enhanced with pulsatile administration of GnRH via a portable infusion pump, provided that the pituitary retains the capability to make gonadotropins. Treatment with gonadotropins or GnRH tends to be both costly and prolonged.
30. What alternative is available to men with hypogonadism who do not respond to therapy with an increase in spermatogenesis?
In men with primary or secondary hypogonadism who have not responded to specific therapy when appropriate and who have preservation of some germ cells in either ejaculate or testis, intracytoplasmic sperm injection (ICSI) may offer some hope, although at a high financial cost. The prognosis for successful ICSI is dependent on the site and extent of microdeletions on the Y chromosome. If microdeletions are found, the patient should be counseled about the possibility of transmittal to his male child. Microsurgical testicular sperm extraction (micro-TESE) is a surgical method for harvesting sperm. Fertility options that should be discussed also include donor sperm and adoption.
31. What are the advantages and disadvantages of the various forms of androgen replacement therapy?
32. What parameters should be monitored in men receiving TRT?
The following should be determined at baseline, at 3 months after initiation of TRT, and then followed at least yearly, once the patient is stabilized:
 Prostate size by digital rectal examination
Prostate size by digital rectal examination
 Development of gynecomastia, acne, or edema
Development of gynecomastia, acne, or edema
 Serum testosterone levels in all forms of treatment
Serum testosterone levels in all forms of treatment
 Serum dihydrotestosterone levels in patients receiving scrotal patches or gel
Serum dihydrotestosterone levels in patients receiving scrotal patches or gel
 Development of or worsening of sleep apnea
Development of or worsening of sleep apnea
 Bone mineral density (at baseline and at 1- to 2-yearly intervals)
Bone mineral density (at baseline and at 1- to 2-yearly intervals)
33. In what conditions is testosterone therapy absolutely or relatively contraindicated?
Adamopoulos, DA, Lawrence, DM, Vassilopoulos, P, et al. Pituitary-testicular relationships in mumps orchitis and other viral infections. BMJ. 1978;1:1177.
Armory, JK, Wang, C, Swerdloff, RS, et al. The effect of 5 reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J Clin Endocrinol Metab. 2007;92:1659–1665.
Araujo, AB, Esche, GR, Kupelian, V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92:4241–4247.
Bagatell, CJ, Bremner, WJ, Androgens in men. uses and abuses. N Engl J Med 1996;334:707–714.
Baker, HWG, Burger, HF, DeKretser, DM, et al. Changes in the pituitary-testicular system with age. Clin Endocrinol. 1976;5:349.
Basaria, S, Coviello, AD, Travison, TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122.
Bannister, P, Handley, T, Chapman, C, Losowsky, MS, Hypogonadism in chronic liver disease. impaired release of luteinising hormone. BMJ 1986;293:1191.
Bhasin, S, Cunningham, GR, Hayes, FJ, et al, Testosterone therapy in adult men with androgen deficiency syndromes. an Endocrine Society Clinical practice guideline. J Clin Endocrinol Metab 2006;91:1995–2010.
Bhasin, S. Approach to the infertile man. J Clin Endocrinol Metab. 2007;92:1995–2004.
Byrne, M, Nieschlag, E. Testosterone replacement therapy in male hypogonadism. J Endocrinol Invest. 2003;26:481–489.
Castro-Magana, M, Bronsther, B, Angulo, MA. Genetic forms of male hypogonadism. Urology. 1990;35:195.
Dada, R, Gupta, NP, Kucheria, K. Molecular screening for Yq microdeletion in men with idiopathic oligospermia and azoospermia. J Biosci. 2003;28:163–168.
Gambineri, A, Pelusi, C, Vicennati, V, Pagotto, U, Pasquali, R. Testosterone in ageing men. Expert Opin Investig Drugs. 2001;10:477–492.
Griffin, JL, Wilson, JD. The syndromes of androgen resistance. N Engl J Med. 1980;302:198.
Gromoll, J, Eiholzer, U, Nieschlag, E, Simoni, M, Male hypogonadism caused by homozygous deletion of exon 10 of the luteinizing hormone (LH) receptor. differential action of human chorionic gonadotropin. J Clin Endocrinol Metab 2000;85:2281.
Guo, CY, Jones, TH, Eastell, R. Treatment of isolated hypogonadotropic hypogonadism effect on bone mineral density and bone turnover. J Clin Endocrinol Metab. 1997;82:658–665.
Harman, SM, Metter, EJ, Tobin, JD, et al, Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 2001;86:724–731.
Hayes, FJ, Seminara, SB, Crowley, WF. Hypogonadotropic hypogonadism. Endocrinol Metab Clin North Am. 1998;27:739–763.
Hopps, CV, Mielnik, A, Goldstein, M. Detection of sperm in men with Y chromosome microdeletions on the AZFa, AZFb and AZFc regions. Hum Reprod. 2003;18:1660–1665.
Hsueh, WA, Hsu, TH, Federman, DD. Endocrine features of Klinefelter’s syndrome. Medicine. 1978;57:447.
Kalyani, RR, Gavini, S, Dobs, AS. Male hypogonadism in systemic disease. Endocrinol Metab Clin North Am. 2007;36:333–348.
Kidd, GS, Glass, AR, Vigersky, RA. The hypothalamic-pituitary-testicular axis in thyrotoxicosis. J Clin Endocrinol Metab. 1979;48:798–802.
Layman, LC. Hypogonadotropic hypogonadism. Endocrinol Metab Clin North Am. 2007;36:283–296.
Lee, PA, O’Dea, LS. Primary and secondary testicular insufficiency. Pediatr Clin North Am. 1990;37:1359.
Lieblich, JM, Rogol, AD, White, BJ, Rosen, SW, Syndrome of anosmia with hypogonadotropic hypogonadism (Kallman syndrome). clinical and laboratory studies in 23 cases. Am J Med 1982;73:506.
Marks, LS, Mazer, NA, Mostaghel, E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism. JAMA. 2006;296:2351–2361.
Matsumoto, AM, Bremner, WJ. Endocrinology of the hypothalamic-pituitary-testicular axis with particular reference to the hormonal control of spermatogenesis. Baillieres Clin Endocrinol Metab. 1987;1:71.
Rhoden, EL, Morgentaler, A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med. 2004;350:482–492.
Schwartz, ID, Root, AW. The Klinefelter syndrome of testicular dysgenesis. Endocrinol Metab Clin North Am. 1991;20:153.
Seminara, SB, Hayes, FJ, Crowley, WF, Jr., Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann’s syndrome). pathophysiological and genetic considerations. Endocr Rev 1998;19:521.
Silveira, LF, MacColl, GS, Bouloux, PM. Hypogonadotropic hypogonadism. Semin Reprod Med. 2002;20:327–338.
Snyder, PJ, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2670.
Swerdloff, RS, Wang, C, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500–4510.
Szulc, P, Munoz, F, Claustrat, B, et al, Bioavailable estradiol may be an important determinant of osteoporosis in men. the MINOS study. J Clin Endocrinol Metab 2001;86:192.
Tenover, JL. Male hormone replacement therapy including andropause. Endocrinol Metab Clin North Am. 1998;27:969–987.
Whitcomb, RW, Crowley, WF. Male hypogonadotropic hypogonadism. Endocrinol Metab Clin North Am. 1993;22:125.
Wu, FCW, Tajar, A, Beynon, JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–135.
Young, J. Approach to the male patient with congenital hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2012;97:707–718.