Chapter 280 Malaria (Plasmodium)
Epidemiology
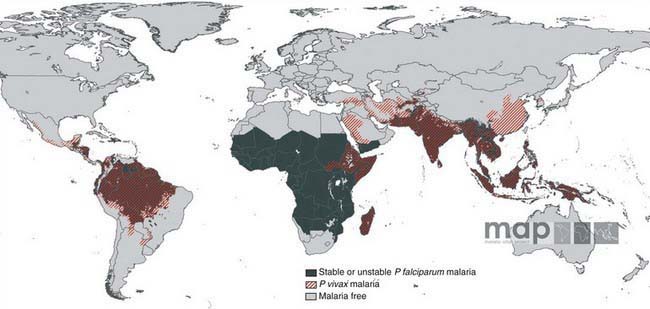
Malaria is a major worldwide problem, occurring in more than 100 countries with a combined population of over 1.6 billion people (Fig. 280-1). The principal areas of transmission are Africa, Asia, and South America. P. falciparum and P. malariae are found in most malarious areas. P. falciparum is the predominant species in Africa, Haiti, and New Guinea. P. vivax predominates in Bangladesh, Central America, India, Pakistan, and Sri Lanka. P. vivax and P. falciparum predominate in Southeast Asia, South America, and Oceania. P. ovale is the least common species and is transmitted primarily in Africa. Transmission of malaria has been eliminated in most of North America (including the USA), Europe, and the Caribbean, as well as Australia, Chile, Israel, Japan, Korea, Lebanon, and Taiwan.
Pathogenesis
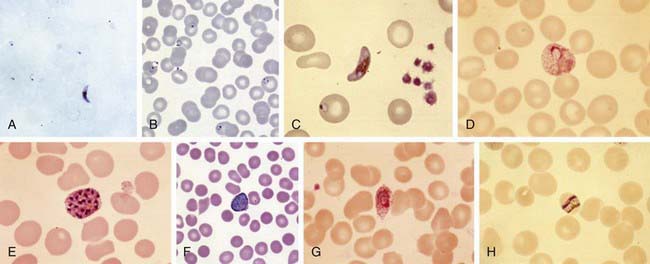
Plasmodium species exist in a variety of forms and have a complex life cycle that enables them to survive in different cellular environments in the human host (asexual phase) and the mosquito (sexual phase) (Fig. 280-2). A marked amplification of Plasmodium, from approximately 102 to as many as 1014 organisms, occurs during a 2-step process in humans, with the 1st phase in hepatic cells (exoerythrocytic phase) and the 2nd phase in the red cells (erythrocytic phase). The exoerythrocytic phase begins with inoculation of sporozoites into the bloodstream by a female Anopheles mosquito. Within minutes, the sporozoites enter the hepatocytes of the liver, where they develop and multiply asexually as a schizont. After 1-2 wk, the hepatocytes rupture and release thousands of merozoites into the circulation. The tissue schizonts of P. falciparum, P. malariae, and apparently P. knowlesi rupture once and do not persist in the liver. There are 2 types of tissue schizonts for P. ovale and P. vivax. The primary type ruptures in 6-9 days, and the secondary type remains dormant in the liver cell for weeks, months, or as long as 5 yr before releasing merozoites and causing relapse of infection. The erythrocytic phase of Plasmodium asexual development begins when the merozoites from the liver penetrate erythrocytes. Once inside the erythrocyte, the parasite transforms into the ring form, which then enlarges to become a trophozoite. These latter 2 forms can be identified with Giemsa stain on blood smear, the primary means of confirming the diagnosis of malaria (Fig. 280-3). The trophozoite multiplies asexually to produce a number of small erythrocytic merozoites that are released into the bloodstream when the erythrocyte membrane ruptures, which is associated with fever. Over time, some of the merozoites develop into male and female gametocytes that complete the Plasmodium life cycle when they are ingested during a blood meal by the female anopheline mosquito. The male and female gametocytes fuse to form a zygote in the stomach cavity of the mosquito. After a series of further transformations, sporozoites enter the salivary gland of the mosquito and are inoculated into a new host with the next blood meal.
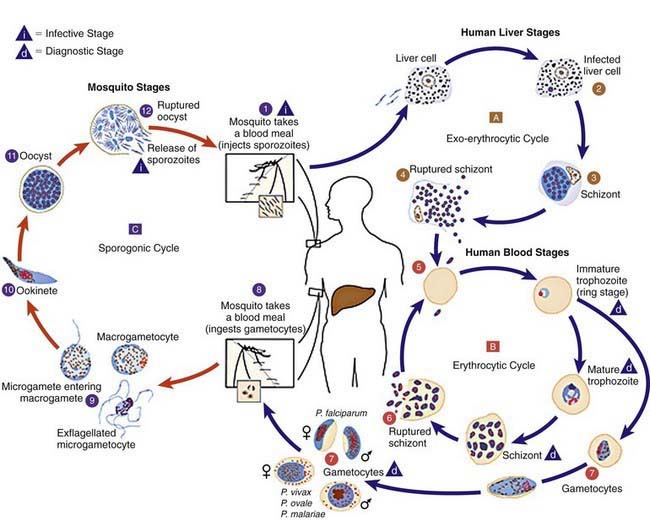
Figure 280-2 Life cycle of Plasmodium spp.
(From Centers for Disease Control and Prevention: Laboratory diagnosis of malaria: Plasmodium spp. [pdf]. www.dpd.cdc.gov/dpdx/HTML/PDF_Files/Parasitemia_and_LifeCycle.pdf. Accessed September 20, 2010.)
Clinical Manifestations
P. falciparum is the most severe form of malaria and is associated with higher density parasitemia and a number of complications. The most common serious complication is severe anemia, which also is associated with other malaria species. Serious complications that appear unique to P. falciparum include cerebral malaria, acute renal failure, respiratory distress from metabolic acidosis, algid malaria and bleeding diatheses (see later section on complications, and Table 280-1). The diagnosis of P. falciparum malaria in a nonimmune individual constitutes a medical emergency. Severe complications and death can occur if appropriate therapy is not instituted promptly. In contrast to malaria caused by P. ovale, P. vivax, and P. malariae, which usually results in parasitemias of less than 2%, malaria caused by P. falciparum can be associated with parasitemia levels as high as 60%. The differences in parasitemia reflect the fact that P. falciparum infects both immature and mature erythrocytes, while P. ovale and P. vivax primarily infect immature erythrocytes and P. malariae infects only mature erythrocytes. Like P. falciparum, P. knowlesi has a 24 hr replication cycle and can also lead to very high density parasitemia.
Diagnosis
The diagnosis of malaria is established by identification of organisms on Giemsa-stained smears of peripheral blood (see Fig. 280-3) or by rapid immunochromatographic assay. Giemsa stain is superior to Wright stain or Leishman stain. Both thick and thin blood smears should be examined. The concentration of erythrocytes on a thick smear is 20-40 times that on a thin smear and is used to quickly scan large numbers of erythrocytes. The thin smear allows for positive identification of the malaria species and determination of the percentage of infected erythrocytes and is useful in following the response to therapy. Identification of the species is best made by an experienced microscopist and checked against color plates of the various Plasmodium species (see Fig. 280-3). Morphologically it is impossible to distinguish P. knowlesi from P. malariae, so polymerase chain reaction (PCR) detection by a reference lab or the CDC is required. Although P. falciparum is most likely to be identified from blood just after a febrile paroxysm, the timing of the smears is less important than their being obtained several times a day over a period of 3 successive days. A single negative blood smear does not exclude malaria. Most symptomatic patients with malaria will have detectable parasites on thick blood smears within 48 hr. For nonimmune persons, symptoms typically occur 1 to 2 days before parasites are detectable on blood smear.
Treatment
P. falciparum Malaria
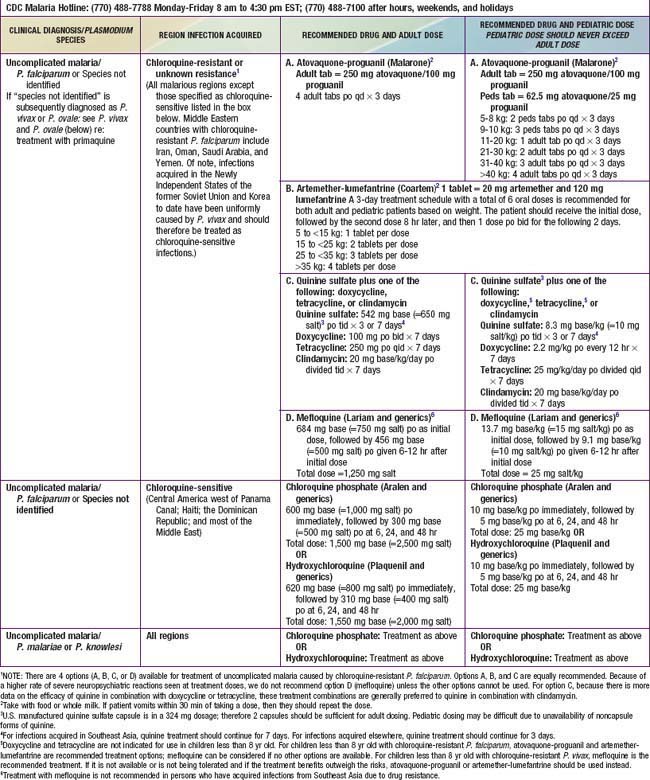
Malarious regions considered chloroquine-sensitive include Central America west of the Panama Canal, Haiti, the Dominican Republic, and most of the Middle East except Iran, Oman, Saudi Arabia, and Yemen. Individuals traveling from areas with chloroquine-susceptible P. falciparum can be treated with chloroquine if they do not have severe malaria. Malaria acquired in P. falciparum areas with chloroquine resistance or where there is any doubt about chloroquine sensitivity after conferring with the CDC should be treated with drugs other than chloroquine (Table 280-2). Intravenous quinidine gluconate (or quinine if outside the USA) should be administered for cases of complicated malaria (see Table 280-2) or patients unable to retain oral medications because of vomiting. These patients should be admitted to the intensive care unit for monitoring of complications, plasma quinidine levels, and adverse effects during quinidine administration. During administration of quinidine, blood pressure monitoring for hypotension and cardiac monitoring for widening of the QRS complex or lengthening of the QTc interval should be performed continuously, and blood glucose monitoring for hypoglycemia should be performed periodically. Cardiac adverse events may require temporary discontinuation of the drug or slowing of the intravenous infusion. Parenteral therapy should be continued until the parasitemia is less than 1%, which usually occurs within 48 hr, and the patient can tolerate oral medication. Quinidine gluconate (USA) or quinine sulfate (other countries) is administered for a total of 3 days for malaria acquired in Africa or South America and for 7 days for malaria acquired in Southeast Asia. Doxycycline, tetracycline, or clindamycin is then given orally to complete the therapeutic course (see Table 280-2). Although there are no data to support the use of sequential quinine and atovaquone-proguanil, the difficulty of maintaining compliance with oral quinine has led many clinicians to complete oral therapy after IV quinine with a complete course of atovaquone-proguanil.
Parenterally administered artesunate or artemether can be substituted for quinine for treatment of severe malaria in children and adults (see Table 280-2). Artesunate is now available on special request from the CDC (770-488-7788) for treatment of severe malaria, but empirical therapy should not be delayed while awaiting delivery of artesunate. Oral and rectal administration of these artemisinin-based antimalarial drugs is effective in treatment of malaria, but such formulations are not indicated or approved in the USA.
Patients from areas with chloroquine-resistant P. falciparum who have mild to moderate infection, parasitemia less than 1%, no evidence of complications, and no vomiting and who can take oral medication should be given either oral atovaquone-proguanil (Malarone), oral artemether-lumefantrine (Coartem), or oral quinine plus doxycycline, tetracycline, or clindamycin (see Table 280-2). Coartem is approved by the FDA for the treatment of uncomplicated malaria and is an appealing choice because it is highly effective and well-tolerated. Pediatric dosing is well established, but pediatric dispersible tablets, available in some other countries, are not yet available in the USA. Coartem should not be used in children with known QT interval prolongation. Patients who acquire P. falciparum in Thailand, Myanmar, or Cambodia should receive 7 days of quinine therapy if they are prescribed quinine. Mefloquine is contraindicated for use in patients with a known hypersensitivity to mefloquine or with a history of epilepsy or severe psychiatric disorders. Mefloquine is not recommended for persons with cardiac conduction abnormalities but may be administered to persons concurrently receiving β-blockers if they have no underlying arrhythmia. Quinidine or quinine may exacerbate the adverse effects of mefloquine and should generally not be given to patients who have received mefloquine unless there are no other alternatives.
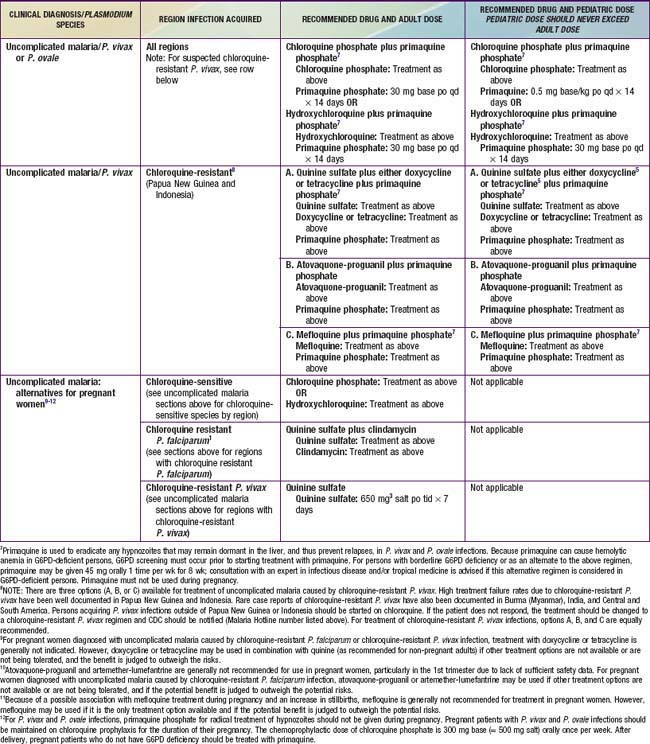
P. vivax, P. ovale, P. malariae, or P. knowlesi Malaria
Uncomplicated infection due to P. vivax, P. ovale, or P. malariae can usually be treated with chloroquine (see Table 280-2). Chloroquine remains the initial drug of choice for P. vivax malaria in the absence of good data on drug alternatives. Indications for using alternative therapy are worsening or new symptoms, persistent P. vivax parasitemia after 72 hours, and possibly acquisition of infection in Oceania or India. Patients with P. vivax or P. ovale malaria should also be given primaquine once daily for 14 days to prevent relapse from the hypnozoite forms that remain dormant in the liver. Some strains may require 2 courses of primaquine. Testing for glucose-6-phosphate dehydrogenase deficiency must be performed before initiation of primaquine, because it can cause hemolytic anemia in such patients. Unfortunately, no alternatives to primaquine currently exist for eradication of the hypnozoite forms of P. vivax or P. ovale. Patients with any type of malaria must be monitored for possible recrudescence with repeat blood smears at the end of therapy because recrudescence may occur more than 90 days after therapy with low-grade resistant organisms. If vomiting precludes oral administration, chloroquine can be given by nasogastric tube. Based on limited evidence, chloroquine plus sulfadoxine-pyrimethamine should be used to treat P. knowlesi infections. For cases of severe malaria due to any Plasmodium species, intravenous quinidine or quinine along with a second drug (clindamycin, doxycycline, or tetracycline) should be used, as for P. falciparum. Patients with any type of malaria must be monitored for possible recrudescence with repeat blood smears at the end of therapy, because recrudescence may occur more than 90 days after therapy with low-grade resistant organisms. For children living in endemic areas, mothers should be encouraged to treat fever with an antimalarial drug. If such children are severely ill, they should be given the same therapy as nonimmune children.
Complications of P. Falciparum Malaria
WHO has identified 10 complications of P. falciparum malaria that define severe malaria (see Table 280-1). The most common complications in children are severe anemia, impaired consciousness (including cerebral malaria), respiratory distress (due to metabolic acidosis), multiple seizures, prostration, and jaundice.
Prevention
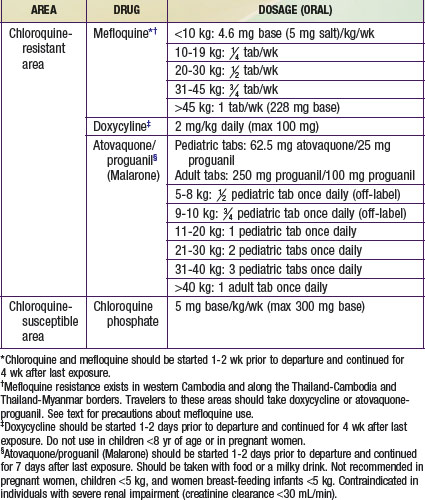
Chemoprophylaxis is necessary for all visitors to and residents of the tropics who have not lived there since infancy, including children of all ages (Table 280-3). Health care providers should consult the latest information on resistance patterns before prescribing prophylaxis for their patients. Chloroquine is given in the few remaining areas of the world free of chloroquine-resistant malaria strains. In areas where chloroquine-resistant P. falciparum exists, atovaquone-proguanil, mefloquine, or doxycycline may be given as chemoprophylaxis. Atovaquone-proguanil is generally recommended for shorter trips (up to 2 wk), since it must be taken daily. Pediatric tablets are available and are generally well tolerated, although the taste is sometimes unpleasant to very young children. For longer trips, mefloquine is preferred, as it is given only once a week. Mefloquine does not have a pediatric formulation and has an unpleasant taste that usually requires that the cut tablet be “disguised” in another food, such as chocolate syrup. Mefloquine should not be given to children if they have a known hypersensitivity to mefloquine, are receiving cardiotropic drugs, have a history of convulsive or certain psychiatric disorders, or travel to an area where mefloquine resistance exists (the borders of Thailand with Myanmar and Cambodia, the western provinces of Cambodia, and the eastern states of Myanmar). Atovaqoune-proguanil is started 1-2 days before travel, and mefloquine is started 2 wk before travel. It is important that these doses are given, both to allow therapeutic levels of the drugs to be achieved and also to be sure that the drugs are tolerated. Doxycycline is an alternative for children over 8 yr of age. It must be given daily and should be given with food. Side effects of doxycycline include photosensitivity and vaginal yeast infections. Provision of medication for self-treatment is controversial, but it can be considered in individuals who refuse to take prophylaxis or will be in very remote areas without accessible medical care. Provision of medication for self-treatment of malaria should be done in consultation with a travel medicine specialist, and the medication provided should be different than that used for prophylaxis.
Table 280-3 CHEMOPROPHYLAXIS OF MALARIA FOR CHILDREN
Aceng JR, Byarugaba JS, Tumwine JK. Rectal artemether versus intravenous quinine for the treatment of cerebral malaria in children in Uganda: randomized clinical trial. Br Med J. 2005;330:334-337.
Bejon P, Lusingu J, Olutu A, et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359:2521-2532.
Bottieau E, Clerinx J, Colebunders R, et al. Selective ambulatory management of imported falciparum malaria: a 5-year prospective study. Eur J Clin Microbiol Infect Dis. 2007;26:181-188.
Centers for Disease Control and Prevention. Malaria surveillance—United States, 2008. MMWR. 2010;59:1-15.
Centers for Disease Control and Prevention. Simian malaria in a U.S. traveler—New York, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:229-232.
Centers for Disease Control and Prevention. Health information for international travel, 2010. wwwn.cdc.gov/travel/content/yellowbook/home-2010.aspx. Accessed September 20, 2010
Cox-Singh, Davis K, Lee T, et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008;46:165-171.
Crawley J, Chu C, Mtove G, et al. Malaria in children. Lancet. 2010;375:1468-1478.
Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455-467.
Feachem RGA, Phillips AA. Malaria: 2 years in the fast lane. Lancet. 2009;373:1409-1410.
Gimnig JE, Slutsker L. House screening for malaria control. Lancet. 2009;374:854-955.
Goldfarb DM, Gaboury I, Dayneka N, et al. Protocol for management of imported pediatric malaria decreases time to medication administration. Pediatr Infect Dis J. 2009;28:810-813.
Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurologic outcome of cerebral malaria. Lancet Neurol. 2005;4:827-840.
John CC, Bangirana P, Byarugaba J, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122:e92-e99.
Krause G, Schoneberg I, Altmann D, et al. Chemoprophylaxis and malaria death rates. Emerg Infect Dis. 2006;12:447-451.
Lesko CR, Arguin PM, Newman RD. Congenital malaria in the United States. Arch Pediatr Adolesc Med. 2007;161:1062-1067.
Maitland K. Antimicrobials in children admitted to hospital in malaria endemic areas. BMJ. 2010;340:822-823.
Maitland K, Nadel S, Pollard AJ, et al. Management of severe malaria in children: proposed guidelines for the United Kingdom. Br Med J. 2005;331:337-343.
Maroushek SR, Aguilar EF, Stauffer W, et al. Malaria among refugee children at arrival in the United States. Pediatr Infect Dis J. 2005;24:450-452.
McGready R. Intermittent preventive treatment of malaria in infancy. Lancet. 2009;374:1478-1480.
Moorthy V, Smith PG, Kieny MP. A vaccine against malaria: a substantial step forward. Lancet. 2009;373:1411-1422.
Nadjm B, Amos B, Mtove G, et al. WHO guidelines for antimicrobial treatment in children admitted to hospital in an area of intense Plasmodium falciparum transmission: prospective study. BMJ. 2010;340:848.
Nosten FH. Pyronaridine-artesunate for uncomplicated falciparum malaria. Lancet. 2010;375:1413.
O’Meara W, Bejon P, Mwangi TW, et al. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;372:1555-1562.
Oramasionwu GE, Wooten SH, Edwards MS. Epidemiologic features impacting the presentation of malaria in children in Houston. Pediatr Infect Dis J. 2010;29:28-32.
Reyburn H. New WHO guidelines for the treatment of malaria. BMJ. 2010;341:161-162.
Reyburn H, Mbatia R, Drakeley C, et al. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA. 2005;293:1461-1470.
Roestenberg M, McCall M, Hopman J, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361:468-477.
Slutsker L, Newman RD. Malaria scale-up progress: is the glass half-empty or half full? Lancet. 2009;373:11-12.
South East Asian Quinine Artesunate Malaria Trial Group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomized trial. Lancet. 2005;366:717-724.
Staedke SG, Mwebaza N, Kamya MR, et al. Home management of malaria with artemether-lumefantrine compared with standard care in urban Ugandan children: a randomized controlled trial. Lancet. 2009;373:1623-1630.
Stäger K, Legros F, Krause G, et al. Imported malaria in children in industrialized countries, 1992–2002. Emerg Infect Dis. 2009;15:185-191.
Taylor SM, Molyneux ME, Simel DL, et al. Does this patient have malaria? JAMA. 2010;304(18):2048-2056.
von Seidlein L, Deen JL. Pre-referral rectal artesunate in severe malaria. Lancet. 2009;373:522-523.
White NJ. Artemisinin resistance—the clock is ticking. Lancet. 2010;376:2051-2052.