Magnetic Resonance Safety
The MR environment is the term used to describe the area immediately surrounding and including the MR scanner.1 It is characterized by the three types of electromagnetic fields used to generate images: (1) the strong static magnetic field and associated spatial gradients (fringe field), (2) the smaller time-varying magnetic gradient fields (imaging gradients, measured in kilohertz), and (3) the radiofrequency (RF) magnetic fields (RF pulses, megahertz FM radio band). When performed under the appropriate conditions, no safety risks are inherent to MRI, and millions of MRI examinations are performed each year without incident. However, when not appropriately managed, safety concerns exist for each of the three electromagnetic fields used for MRI (Table 3-1) as well as the associated acoustic noise. Notably, most MR-related injuries and the few fatalities that have occurred primarily were a result of the failure to adhere to MR safety guidelines for the MRI environment, or they occurred because inaccurate or outdated MR safety-related information for biomedical implants and devices was used.2,3 To prevent similar adverse MR safety-related incidents from occurring, it is imperative that the potential safety risks intrinsic to the MR environment be understood and respected by users of this powerful imaging technology (Boxes 3-1 and 3-2).
Table 3-1
| Magnetic Resonance–Related Electromagnetic Field | Mechanism of Interaction | Potential Effects |
| Static magnetic field (T) | Polarization/magnetization | Elevated electrocardiographic T wave and/or transient sensory effects (e.g., vertigo, nausea, phosphenes, and metallic taste) |
| Transient gradient magnetic field (dB/dt) | Induced currents Acoustic noise |
Peripheral nerve stimulation Physiologic stress, anxiety, temporary hearing loss |
| Radio frequency field (specific absorption rate) | Thermal heating | Local burns |
From Bushong SC. Biologic effects of magnetic resonance imaging. In: MR safety, magnetic resonance imaging: physical and biological principles. 3rd ed. St Louis: Mosby; 2003.
Safety Considerations of the Magnetic Resonance Environment
Biologic Effects of Static Magnetic Fields
The majority of MR units in clinical use today operate at main or static magnetic field strengths of 0.2 to 3.0 tesla (T). For comparison, the static field of a 1.5-T scanner is approximately 30,000 times stronger than Earth’s magnetic field (roughly 0.00005 T). The most recent United States Food and Drug Administration (FDA) guidelines state that for adults, children, and infants older than 1 month, diagnostic MRI systems that operate at or below 8 T are considered to be a nonsignificant risk. For neonates, the limit is 4 T.4 In practice, 3 T is the highest field strength in common clinical use. Initially, some concern was expressed that the strong magnetic fields might have irreversible detrimental biologic/health effects in humans, including alterations in cell growth and morphology, cell reproduction and teratogenicity, DNA structure and gene expression, prenatal and postnatal reproduction and development, blood-brain barrier permeability, nerve activity, cognitive function and behavior, cardiovascular dynamics, hematologic indexes, temperature regulation, circadian rhythms, immune responsiveness, and other biologic processes.5–23 However, a comprehensive review of the literature indicates that short-term exposures (of a duration comparable to an MR examination) to high-static magnetic fields produce no appreciable detrimental biologic effects.24,25
Although no lasting adverse effects of short-term exposures to high magnetic field strengths have been reported, several relatively transient reversible biologic effects are known to occur, including electrocardiographic changes and benign sensory effects. These effects have been reported primarily at field strengths greater than 2 T and the sensory effects appear to occur most often when a person’s head is moved rapidly within the static magnetic field. The elevation in electrocardiographic T waves is believed to be due to magnetohydrodynamic phenomena. When an electrically conductive fluid, such as blood, flows within a magnetic field, an electric current is produced, as is a mechanical force opposing the flow. Hence the movement of blood in the magnetic field of the MRI causes a magnetohydrodynamic effect that produces a voltage across the vessel. Typically, the voltage is negligible except in large arteries such as the aorta (on the order of 5 mV/T) and for high blood velocities. Because the peak flow rate occurs during the repolarization phase of the cardiac cycle, the added voltage from the flowing blood manifests as an artifactual elevation of the T wave.26,27 The voltage associated with the magnetohydrodynamic effect is not considered hazardous at the magnetic field strengths that are approved for clinical use. However, at higher field strengths, the possibility exists that the induced potential might exceed 40 mV, which is the threshold for depolarization of cardiac muscle.28
Several short-term, relatively benign sensory effects have been reported at higher field strengths, including vertigo, nausea, headaches, flashing lights (also known as magnetophosphenes), a metallic taste, and/or sensation in dental fillings.29 The magnetophosphenes are believed to be a result of torque upon the rods or cones in the retina imposed by the magnetodynamic resistive forces during quick movements of the head and/or eyes within the magnetic field. Similarly, the vertigo, nausea, and/or headache have been posited to be associated with torque on the hair cells in the semicircular canals (causing disequilibrium). Electrical currents induced in saliva or metallic fillings are believed to account for the incidences of a metallic taste and/or sensations that have been reported.
Interaction of the Main Magnetic Field with Ferromagnetic Objects
5 Gauss Line: Although exposure to high magnetic fields may not inherently be unsafe, failure to adhere to safe practices within the MR environment have led to most of the MR-related accidents and fatalities that have occurred. These incidents have involved the inappropriate and/or inadvertent introduction of metallic objects and or implanted medical devices into the scan room. The term fringe field typically is used to refer to the full spatial extent of the magnetic field gradient associated with an MR magnet. Five gauss (G) (0.0005 T) and below are considered “safe” levels of static magnetic field exposure for the general public.1 The 5 G line identifies the perimeter around an MR scanner within which the static magnetic fields are higher than 5 G; it is the most commonly recognized MR safety policy.26 Safety considerations require that this distance from the magnet be determined and that potentially unsafe areas be identified with appropriate and conspicuous signage. Importantly, the 5G line extends in three dimensions; thus when the MR area is sited, the extent of the fringe field to the floors above and below the magnet must be considered. Notably, the term MR environment also is often used synonymously to refer to the area within the 5 G line. Because of the potential hazards, access to this area must be rigorously controlled and supervised.
Magnetic Field Interactions: Torque and Attractive Forces: The field associated with an MR scanner can be separated into two spatial regions defined by the types of interaction with ferromagnetic objects that predominate in each region. The first region surrounds the magnet isocenter and is contained within the bore of the MRI scanner. The magnetic field in this region essentially is temporally constant and homogeneous in strength. A magnetic object introduced into this region of the static field is subjected to a torque that acts to align it with the magnetic field, just as magnetic material aligns itself with the poles of a permanent bar magnet or a compass needle aligns itself with the earth’s magnetic field.1 Hence any nonspherical metallic object that enters the spatially homogenous static region of the field will be subjected to a rotational force or torque such that its long axis will be aligned with that of the main magnetic field. If metallic implants are not sufficiently anchored by the surrounding tissue (e.g., bone in the case of an orthopedic implant), this rotational motion of metallic implants will cause trauma to the surrounding tissue. The magnitude of these effects depends on the geometry and mass of the object, as well as the characteristics of the MR system’s magnetic field.
In the second region (the fringe field), the strong magnetic field strength drops off rapidly as the distance from the magnet increases, producing a large spatial gradient that typically is greatest in regions immediately adjacent to the magnet. Metallic objects introduced into this magnetic field gradient experience a translational (attractive) and rotational force. The translation will be in the direction of the higher field strength. The strength of the attractive/translational force will depend on the size of the object and its location within the gradient field and will increase rapidly as the object approaches the magnet. As such, the metallic object will be accelerated along the direction of the spatial gradients in the static field and quickly can become a dangerous projectile. Depending on the size and composition of the metallic object, these attractive/translational effects may begin as soon as the object is introduced into the scan room. Once the object enters the uniform field within the bore of the magnet, acceleration will cease and the object will come to a stop.26
The potential hazards associated with the spatial gradients are greatest for high magnetic field strengths and large fringe fields. In general, the forces increase approximately as the square of the field strength but will vary depending on the composition of the object.30 For example, at 3 T compared with 1.5 T, the force on a paramagnetic material (e.g., a stainless steel scalpel) is five times greater, whereas the force on a ferromagnetic object (e.g., a steel wrench) is 2.5 times greater.30 Finally, but importantly, even for a given field strength, the magnitude and footprint of the spatial gradients can vary between magnet configurations. For example, it has been reported that short-bore MR systems have significantly higher spatial gradients than do long-bore scanners, particularly those operating at 3 T.31,32 This finding highlights the multiple nuances and factors that must be considered when evaluating and establishing MR safety guidelines and operating procedures for any object before it may be introduced into a specific MRI environment. The potential risks associated with the introduction of metallic objects into regions of high and or rapidly spatially varying magnetic field strengths cannot be underestimated. It is a common misconception that the magnet is only “on” when the images are being acquired. However, the magnet is always on, and signage to indicate the “Magnet is ON” should be displayed conspicuously on the door to the MR scan room.
Missile/Projectile Effect: The projectile/missile effect, wherein a ferromagnetic object is accelerated toward the isocenter of the magnet, is the most widely recognized and publicized safety hazard associated with MRI. Objects constructed partially or entirely of ferromagnetic materials (e.g., iron, nickel, cobalt, and the rare earth metals chromium, gadolinium, and dysprosium) are strongly attracted to the magnet bore. Steel objects also are highly ferromagnetic, as are some medical grades of stainless steel. Notably, working a metal by machining, molding, and/or bending it, for example, can alter its magnetic properties and, as a result, some forms of nonmagnetic stainless steel can be made magnetic. For this reason, the MR safety of a given metallic object must be evaluated in its final form.33 Although metals such as aluminum, tin, titanium, gold, and lead are not ferromagnetic, objects are rarely made of a single metal (e.g., the ferromagnetic screws used to secure the wheels to the frame of an aluminum cart).33 Consequently, carefully inspecting all objects before introducing them into the MR environment is necessary. If any doubt remains regarding the presence of ferromagnetic material, the object should be checked with a permanent magnet and/or a ferromagnetic wand as a final precautionary measure before it enters the scan room.
As noted previously, within the MR environment, a ferromagnetic object will experience a magnetic pull that increases greatly as it approaches the magnet bore. Depending on the size of the object, the magnetic field strength, and the proximity of the object to the magnet, the attractive force may be so great that it becomes impossible for an individual to continue to hold onto the object. Any individual—whether a patient or a staff member—who is in the path between the object and the center of the magnet (e.g., the patient lying in the magnet bore) can be seriously injured or killed.34 Numerous instances of MR-related accidents involving objects such as scalpels, scissors, oxygen tanks, intravenous line poles, wheelchairs, transport carts, floor buffers, mop buckets, vacuum cleaners, other medical devices, and even firearms have been documented. Any injuries related to ferromagnetic projectiles must be documented; the necessary forms can be accessed on the FDA website.35,36 More comprehensive information regarding these online resources is provided in a later section of this chapter.
Time-Varying Gradient Magnetic Fields
During imaging acquisition, gradient magnetic fields are transiently imposed along the main magnetic field to spatially localize and encode the spins. These gradient magnetic fields are much weaker than the static magnetic field and are generated by gradient coils located inside the magnet bore. Each orthogonal axis (i.e., x, y, and z) has a pair of gradient coils. For a given direction, a linear gradient magnetic field is generated within the main magnetic field by applying electrical current in opposite directions to the coil pair over a short time interval.28 When this gradient magnetic field is applied, the magnetic field intensity changes rapidly, giving rise to a time-varying magnetic field. The rate of change in magnetic field (dB) occurs over time (dt) and usually is measured and reported in units of dB/dt expressed in milliteslas per meter per millisecond (mT/m/msec) or in gauss per centimeter per second (G/cm/sec). During the rise time of the magnetic field, an electrical current can be induced in any electrical conductor (e.g., implanted medical device, wire, human body). Notably, the magnitude of the time-varying magnetic field along each of the three spatial directions (i.e., x, y, and z) is zero at the center of the magnet and increases linearly with increasing distance from the isocenter.
Because the human body is a conductor, the rapidly switching magnetic fields can induce electrical fields and current in a patient (as described by Faraday’s law of induction) that may lead to the stimulation of muscle and nerve tissues.28 The mean threshold levels (measured in tesla per second) for various stimulations are 3600 T/sec for the heart, 900 T/sec for the respiratory system, 90 T/sec for pain, and 60 T/sec for the peripheral nerves. However, the exact values differ significantly among individuals.26 Experience has shown that sufficient dB/dt levels can produce brief muscle twitches and peripheral nerve stimulation (PNS) that is perceptible as a “tingling” or “tapping” sensation. This sensation can become uncomfortable and/or painful as the gradient magnetic field increases to 50% to 100% above perception thresholds.37 For these reasons, threshold sensations, when reported, should not be ignored, because they may readily escalate to uncomfortable levels. Although cardiac stimulation is a concern, studies conducted in dogs have shown that the cardiac stimulation threshold for the most sensitive 1% of the population is 20 times, and the mean defibrillation threshold is 500 times, the energy required for PNS.33 The exceedingly strong and/or rapidly switching gradient magnetic fields necessary to achieve cardiac stimulation threshold levels are more than an order of magnitude greater than those used in commercially available MR systems.37–39
In addition to varying from person to person, stimulation thresholds also depend on gradient direction. Assuming equal dB/dt for each of the three gradient axes, the associated gradient-induced electric fields are highest for the largest perpendicular body cross section. Accordingly, the y gradient has the lowest dB/dt PNS threshold since the x-z cross section of the human body is usually larger than the other cross sections.40,41,41a In addition, the mean PNS threshold for the y gradient was further reduced (by approximately 32%) when the patient’s hands were clasped; the x and z gradient PNS thresholds were not similarly affected.40,41,41a In practice, the potential for PNS is greatest for fast imaging (e.g., echo planar imaging [EPI]) acquisitions, particularly when oblique imaging planes are obtained where the combined contributions of gradients from more than one axis result in a higher effective slew and/or when the readout (i.e., frequency encode) lies along the craniocaudal direction.26
When the threshold for PNS is exceeded, the anatomic site of the stimulation depends on the direction along which the magnetic field gradient is applied. Anatomic sites stimulated by the activation of the x gradients include the bridge of the nose, left side of the thorax, iliac crest, left thigh, buttocks, and lower back. Stimulation sites associated with the application of the y gradient include the scapula, upper arms, shoulder, right side of the thorax, iliac crest, hip, hands, and upper back. For the z gradient, stimulation sites are the scapula, thorax, xiphoid, abdomen, iliac crest, and upper and lower back.37 In addition, it has been noted that the PNS sites typically correspond to bony prominences. Because bone is less conductive than the surrounding tissue, it is believed that current densities are increased in narrow regions of tissue between bone and skin, resulting in lower than expected nerve stimulation thresholds.37
Normal imaging sequences (e.g., conventional spin echo and gradient echo) induce currents of a few tens of milliamperes per square meter, a level that is far below that present in the normal brain and heart tissue. However, as noted previously, PNS is quite possible for the rapid imaging techniques such as EPI and for high-performance gradients. These effects can be controlled by limiting the maximum rate of change in the magnetic field gradients. Early limits imposed by the FDA to prevent PNS were 1 dB/dt at 20 T/sec for pulse durations greater than 120 microseconds.33 However, the increasingly apparent diagnostic benefits and widespread use of EPI, single-shot fast-spin echo, and other fast imaging techniques for routine clinical MR imaging caused the FDA to reevaluate and revise these limits. It is now recognized that although these rapid imaging techniques have the potential to cause PNS, the sensation in itself is not harmful. However, painful stimulation still should be avoided. Thus the current FDA standard is based on the threshold for sensation, rather than a specific numerical value of dB/dt. Specifically, “current FDA guidance limits the time rate of change of magnetic field (dB/dt) to levels which do not result in painful peripheral nerve stimulation.” This policy reflects in part the complexity of modeling and calculating the current distribution in the body associated with the pulsed gradient fields. Correspondingly, dB/dt levels below that resulting in painful stimulation are considered a nonsignificant risk by the FDA. It is important to note that dB/dt is a function of the gradient strength and rise time and not of the static field strength. Thus dB/dt is of equal concern for both lower and higher field scanners.
Clinically, two operating modes for the RF and gradient field levels used for imaging are permissible, normal and first level controlled (Table 3-2). In normal mode, the system output levels are below those that will cause physiologic stress and therefore are appropriate for all subjects. Alternatively, in first level conditional mode, one of the MR system outputs (e.g., dB/dt) may reach a value that may cause physiological stress and thus may not be appropriate for the most medically compromised patients. Operation in this mode requires authorization by appropriate clinical staff and vigilant medical supervision during the examination. Institutional Review Board approval is required for operation above these levels (second level controlled). In practice, the commercial MR systems monitor dB/dt throughout the examination and generate a warning for the specific protocols for which PNS is likely. The PNS limits are derived from the empirical results obtained from clinical trials. For normal operating mode, the dB/dt threshold level is set at 80% of the mean threshold value for PNS stimulation, and it is set at 100% for the first level.30 In addition, the MR operator should remain in constant verbal contact with the patient and instruct him or her to report any tingling, muscle twitching, or painful sensations that occur during scanning. This contact is not possible for infants and for sedated, noncommunicative, or otherwise compromised patients. Operationally, dB/dt can be reduced by increasing the field of view and/or slice thickness, reducing the matrix size, and decreasing receiver bandwidth. In addition, because the y gradient has the lowest stimulation threshold, whenever possible, the most rapidly changing gradient waveform (e.g., read out; i.e., frequency encode in EPI) should be placed along a direction other than y.
Table 3-2
| Operating Mode | Conditions/Qualifications |
| Normal mode | Will not cause stress; suitable for all patients |
| First level controlled mode | May cause stress; requires medical supervision and positive action by operator to enter |
| Second level controlled mode | Institutional Review Board approval required |
From Center for Devices and Radiological Health, Food and Drug Administration. FDA guidelines for magnetic resonance equipment safety (website): http://www.aapm.org/meetings/02AM/pdf/8356-48054.pdf. Accessed July 25, 2012.
Radiofrequency Fields
One of the primary safety concerns in MRI is tissue heating. The conductivity of tissue allows the absorption of RF energy, which is transformed into heat as a result of resistive losses.42,43 The heating is greatest at the periphery of the body, and thus the skin is the most prone to this effect.30 When thermally challenged, the body responds by thermoregulatory mechanisms and attempts to dissipate the heat by means of convection, conduction, radiation, and evaporation. If these attempts are not completely successful, the heat accumulates and is stored, resulting in a rise in local tissue and/or systemic core temperatures,42–44 which has the potential to produce physiological changes.28 For humans, the typical skin temperatures are about 33° C, whereas core temperatures are about 37° C. Experience has shown that when the body is subjected to significant RF power levels, it immediately attempts to dissipate the heat load through vasodilatation of the blood vessels of the skin, with the skin approaching core temperature. This response mechanism allows the body to reduce heat fairly rapidly and typically is evidenced by flushing of the skin, which in itself is not harmful and usually subsides within several hours.
The dose measure used to describe this energy absorption or heat dose is the specific absorption rate (SAR). SAR is defined in the International Electrotechnical Commission standard as the amount of RF power (measured in watts) absorbed per patient mass in kilograms (W/kg). The MR system software calculates an SAR for each acquisition and enforces the limits established by the FDA. Because SAR depends on patient weight, it is important that an accurate value be entered during the patient registration procedure. The amount of RF energy that is absorbed is dependent on the frequency (as determined by the static magnetic field strength), the RF coil used, the volume (size), composition (conductivity), and configuration of the exposed tissue and the duty cycle and type of RF pulses applied, as well as other factors.42–46 Notably, SAR increases with the square of the frequency (and hence magnetic field strength) and patient size.30 Consequently, for a given pulse sequence, doubling the field strength (e.g., 1.5 vs. 3 T) results in a factor of four increase in SAR. SAR can be minimized by decreasing the power and/or duty cycle of the RF pulses. In practice, this minimization can be accomplished by increasing the repetition time, reducing the number of slices, and, when feasible, reducing the RF flip angle (because SAR is proportional to the square of the flip angle). In addition, for fast-spin echo and EPI acquisitions, SAR also can be reduced by increasing the interecho spacing and/or reducing the echo train length (for a fixed repetition time).
A person’s ability to respond to the thermal challenge and effectively dissipate the heat is influenced by the rate at which the energy is deposited and the duration of the exposure. Underlying health conditions such as cardiovascular disease, hypertension, diabetes, fever, old age, obesity, or a compromised ability to perspire can impair a person’s ability to tolerate the thermal challenge.47–51 In addition, certain medications such as diuretics, β-blockers, calcium channel blockers, amphetamines, muscle relaxants, and sedatives can alter the body’s thermoregulatory responses significantly, with some medications actually having a synergistic effect with respect to tissue heating caused by RF exposure.3 Finally, the ability of the subject to dissipate heat also is affected by the ambient temperature, relative humidity, and airflow in the MR scan room.
To prevent excessive heat stress and/or local tissue damage, the FDA has established guidelines to limit allowable whole-body SAR levels to those that produce no more than a 1° C increase in tissue and/or core temperature beyond the normal 37° C.28 As with dB/dt, two levels of clinical operation are possible for SAR. Note, however, that only the whole-body SAR limit is increased, from 2 W/kg to 4 W/kg, in the advancement from normal to first level controlled operation. The SAR limit for normal and first level controlled operating modes for the head is 3.2 W/kg (1° C maximum temperature increase) averaged over head mass and 10 W/kg temperature over any 10 g of tissue for the torso and extremities.52 These SAR limits correspond to time-averaged values over a 6-minute interval. Exceptions to the specified FDA guidelines include infants and pregnant women, for whom the FDA recommends that the limits be reduced by a factor of two. Similarly, for patients with thermoregulatory compromise (e.g., cardiovascular impairment, cerebral vascular impairment, and diabetes), the FDA recommends a whole-body SAR limit of 1.5 W/kg.33 In addition, the SAR limits are reduced when the ambient temperature rises above 24° C (75° F) or if the humidity exceeds 60%.
Interaction with Other Devices
Additional safety concerns associated with RF irradiation exist when objects made from conductive materials that have an elongated shape or are looped, such as electrodes, monitor leads, guidewires, and certain types of catheters (e.g., catheters with thermistors or other conducting components), are present in the magnet bore.38,53–58 The rapidly changing magnetic field associated with the RF can induce an electromotive force in the conductor and, hence, current flow, which, because of electrical resistance, will lead to heating of the conductor. If the conductor is in contact with the skin, burns can result. Incidents of first-, second- and third-degree burns have been reported.3,26 Notably, maximum current induction occurs when the plane of the conducting loop is perpendicular to that of the changing magnetic field. Inductive heating also can occur when the active-decoupling circuitry associates with a receive-only coil fails. In addition, because the body is a conductor, inadvertent conductive loops can arise in the absence of any external conductors, when, for example, the patient’s hands or calves are in contact to form a closed loop. In such cases, heating can occur at the high-resistance skin-to-skin contact point, which can result in local redness and blistering. To minimize the possibility of RF burns, all conductors (e.g., leads and coils) that are not being used should be removed from the magnet bore. In addition, the integrity of all conductors that must remain in the magnet bore should be verified before the MR examination, and these cables, leads, and/or wires should be kept as straight as possible and run down the center of the bore. In addition, the patient should be thermally and/or electrically isolated (e.g., using pads and/or sheets or blankets) from direct contact with all conductors and all RF body transmitters, as well as surface coils, and skin to skin contact should be avoided. The MR operator must be extra vigilant to mitigate the potential heating risks for infants and sedated or other noncommunicative patients. Any metallic object in or on the patient may absorb RF energy, resulting in excessive heating that may lead to thermal damage of surrounding tissue; the potential for this effect is greatest at higher field strengths.28,33
Acoustic Noise
The predominant and most widely recognized source of MRI-related acoustic noise is the time-varying gradient magnetic field applied during MR imaging. The rapid pulsed currents within the gradient coils in the presence of the static magnetic field create strong Lorentz forces that produce a torque on the coil itself, causing it to move and/or vibrate against its mounting. This movement, analogous to that of the diaphragm of a loudspeaker, creates the loud chirping, tapping, knocking, and banging noises heard by the subject during MR imaging.3,28 Sound levels for MRI are measured in A-weighted decibels (dBA), which takes the frequency response of the human auditory system into account. As a reference, normal conversation is approximately 60 dBA (www.osha.gov), and a difference of 6 dBA represents a doubling of sound intensity. Sound pressure levels (SPL) of 81 to 117 dBA are common for standard 1.5 T MRI examinations59 and can be as high as 130 dBA for high-speed acquisitions such as EPI and techniques that use nontraditional (i.e., non-Cartesian) k-space trajectories, such as propeller, radial, and spiral sequences. Although many factors influence the intensity of the MR-related acoustic noise (e.g., gradient coil design, gradient amplitude and slew rate, size of patient, location of patient in magnet bore), higher field strength systems tend to be louder.
MRI-related acoustic noise may lead to simple annoyance, difficulties in verbal communication, heightened anxiety, and temporary or potentially permanent hearing loss for both the patient and other persons in or near the MR scan room.60–72 In addition, the noise may cause confusion and or extreme anxiety in more vulnerable patients such as children and elderly persons and in persons with psychiatric disorders.60,62,73 MRI-related noise also has been reported to produce alterations in physiologic parameters in neonates, which may be problematic in light of their immature cardiac physiology and cerebrovascular regulation.64 Furthermore, the acoustic noise levels may cause discomfort to sedated patients, and certain drugs are known to increase hearing sensitivity.63 Because exposure duration is one of the most influential factors in determining the effect of noise on hearing, it is unlikely that the noise levels experienced during the relatively short MR examinations will have a long-term negative effect on hearing.74,75 It is the short-term effects of MR-related acoustic noise that are the primary concern.
The FDA indicates that MR-related acoustic noise levels must be below the level of concern established by pertinent federal regulatory or other recognized standard-setting organizations.76 Guidelines for acceptable acoustic noise levels associated with MRI are based on Occupational Safety and Health Administration (OSHA) guidelines for industrial workers, which recognize that the risk is a function of its intensity and the duration of exposure.26 OSHA standards stipulate that to avoid hearing damage, the peak unweighted SPL cannot exceed 140 dB28 and the A-weighted root mean square SPL cannot exceed 99 dBA, with hearing protection in place. Because the acoustic noise level for many sequences can exceed 99 dBA, the MR manufacturer must recommend that it is necessary for the MR system operator to provide the patient with hearing protection.3 Hearing protection should also be given to all other persons who will be present in the scan room during the MR exam.
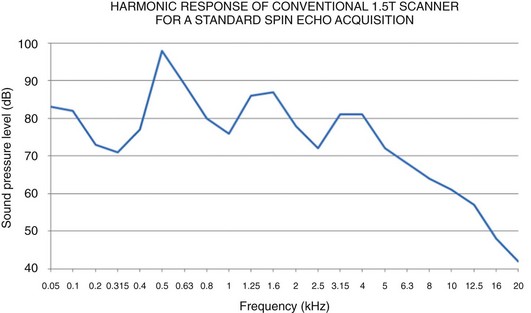
The Noise Reduction Rating (NRR) is the standard for hearing protection devices established by the Environmental Protection Agency. For a given noise attenuator, the NRR value is the mean attenuation value (in dB) for the device minus two standard deviations, corresponding to the minimum noise reduction achieved by 98% of the population. For MR examinations, adequate hearing protection can be most easily and effectively accomplished with earplugs (≈29 to 32 dBA NRR) and/or headphones (≈29 to 49 dBA for adults and 7 to 12 dBA mini muffs for infants). Notably, the NRR reported for earplugs will not be realized if they are improperly inserted or do not fit snugly, as often may be the case for young pediatric patients. Although the combined use of earplugs and headphones do not produce additive effects in hearing protection per se, they can increase the NRR by 5 to 6 dB (equivalent to approximately half the sound intensity) for frequencies ≤2000 Hz (http://www.biac.duke.edu/research/safety/tutorial.asp). At frequencies higher than 2000 Hz, hearing protection is limited by bone conduction, and the combined use of headphones and earplugs is less beneficial. Nevertheless, the harmonic response of MR systems for a wide spectrum of imaging protocols contains a significant amount of energy at or below 2000 Hz so that combined use is still advisable (Fig. 3-1). MR manufacturers are actively working to further decrease noise levels by using methods such as quieter MR sequences, active noise cancellation filters, acoustic hoods,59 and vacuum packing.73
Claustrophobia
Many persons find the confinement of the MRI bore at least somewhat disconcerting, particularly when a head coil is used. For some persons, this discomfort is extreme and can result in anxiety that may escalate into panic. In these cases, sedation is required to perform the MR examination. Claustrophobic reactions in the scanner typically are characterized by a fear of suffocation and/or a fear that something will happen while they are confined.77 Operationally, experience suggests that this reaction can be minimized by maintaining frequent verbal contact with the individual throughout the examination and sufficient airflow (e.g., a bore fan) to reduce heat and mitigate the fear of suffocation. In addition, the individual also should be provided with an emergency panic notification device (e.g., a pneumatic squeeze bulb) to allow him or her to summon help at any time throughout the examination. In fact, such a device should be provided to all nonsedated communicative patients as a means of notifying the MR operator immediately upon any sign of discomfort, including local heating or tugging of ferromagnetic objects (e.g., a belt or shoes).
Medical Implants, Devices, and Other Metallic Potential Hazards
Passive and Electrically Active Medical Devices
The MR environment may be unsafe for persons with certain medical implants or devices that are made out of ferromagnetic materials. For passive implants (i.e., any medical device that serves its function without the supply of power, such as aneurysm clips, shunts, or stents) made of ferromagnetic materials, the primary concern is movement and/or dislodgement,31,32,35,36,54,78–87 although excessive heating of the implant is also possible (Table 3-3). Several incidents have occurred in which metallic implants were mistakenly introduced into the MR environment and were dislodged within or from the body, resulting in blindness or death.26 For electrically active implants such as deep brain or vagal nerve stimulators and drug pumps, excessive heating at the lead tips and associated tissue damage is the primary safety concern.56,88–90 Device malfunction, device heating, induced currents, and movement and/or dislodgement also are potential hazards (Table 3-3).
Table 3-3
Magnetic Resonance Environment Medical Device Concerns
| Component of Magnetic Resonance Environment | Medical Device Concern | Potential Adverse Effect |
| Static magnetic field (always on) | Rotational force (torque) on object Translational force on object |
Trauma to tissue as object rotates to align with main field Tissue damage as object accelerates toward magnet bore (projectile/missile effect) |
| Gradient magnetic field (pulsed during imaging) | Induced currents due to dB/dt | Device malfunction or failure |
| Radio frequency field (pulsed during imaging) | Radio frequency–induced currents resulting in heating Electromagnetic interference—active |
Patient burns (thermal and electrical) Device malfunction, induced noise (monitoring devices) |
From Food and Drug Administration.Primer on medical device interactions with magnetic resonance imaging, US Food and Drug Administration (FDA) Systems. Available at http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm107721.htm. Accessed July 25, 2012.
When it is known that a patient has an implant (passive or active), the implant should be considered a contraindication for MRI until documented proof of its MR compatibility and safety is verified. The information must correspond to the exact model and serial number of the device, which should be recorded in the patient’s medical record. If such information is not available, the radiologist or MR technologist should consult the implanting or monitoring physician. Once the device is identified, the associated device documentation typically is available online, posted on the device manufacturer’s website. The device labeling should be carefully reviewed for information regarding behavior in the MR environment specific to the exact MR scanner and set of imaging conditions to be used to perform the examination. Salient MR-related information present in the labeling includes system parameters such as field strength(s), magnet types and manufacturers, RF transmit coils, system software versions and imaging conditions (e.g., RF power levels and gradient slew rates [dB/dt]). In addition, when appropriate, information regarding the configuration of the device itself, such as lead length and routing, that were evaluated and reported in the device labeling must also be considered. Note that the safety information for any implantable medical devices (passive or active) and medical equipment applies only to the magnetic field strengths, MR system configurations, RF coils, and/or imaging conditions evaluated. Safety at 1.5 T does not necessarily imply safety at 3 T, and vice versa. The consequences of failing to strictly adhere to the product information regarding MR safety can be catastrophic to the patient (Fig. 3-2).88,91
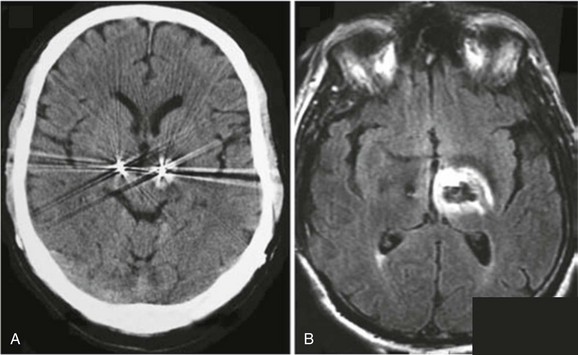
Figure 3-2 Adverse event due to failure to adhere to medical device product guidelines.
Tissue damage (hemorrhage on computerized tomography [CT] [A] and edema on magnetic resonance imaging [MRI]) as a result of radiofrequency heating at the deep brain stimulator (DBS) lead tip that occurred during a routine MR examination of the lumbar spine. The CT and MRI images of the brain were acquired immediately after a lumbar spine MR scan performed on a 1.0 T MR scanner using the body coil to transmit and a surface coil to receive, and a relatively specific absorption rate (SAR) aggressive imaging protocol. Alternatively, the DBS device manufacturers specified the MR conditions under which MR imaging can be accomplished safely for patients implanted with this device as a 1.5 T scanner using a Transmit receive head coil and head SAR levels at or below 0.1 W/kg. (From Henderson JM, Tkach JA, Phillips M, et al. Permanent neurological deficit related to magnetic resonance imaging in a patient with implanted deep brain stimulation electrodes for Parkinson’s disease: case report. Neurosurgery. 2005;57:1063.)
Several comprehensive reviews of the topic of MR safety and implants have been published,3,78,92–96 and more than 1200 objects have been tested for MR safety, with more than 200 evaluated at 3 T or higher.97 This MR safety information is readily available to the public as published reports, compiled lists online (www.MRIsafety.com), and in the Reference Manual for Magnetic Resonance Safety, Implants and Devices, a publication that is compiled and updated annually.98
Other Metallic Potential Hazards
Some body piercing jewelry is ferromagnetic, and certain tattoos and permanent cosmetics may contain irregularly shaped iron oxide or other metal-based pigments and therefore may be of concern. In these instances, mild to moderate movement or displacement in the MR environment may occur that, in the case of the tattoos and/or permanent cosmetics, may result in localized swelling and/or irritation. In addition, when the jewelry or pigments are contained within the transmit RF coil, a risk of localized heating also exists. For these reasons, patients and/or individuals should be informed of the potential risks. Body jewelry should be removed before entering the MR environment. If removal is not possible and the individual chooses to proceed, the jewelry should be stabilized (e.g., with application of adhesive tape or a bandage) and, if it is contained within the transmit RF coil, it should be wrapped with gauze or tape to insulate it from underlying skin.97 Similarly, for tattoos and permanent cosmetics, if any concern exists that heating may occur, an ice pack or cold compress can be applied to the site as a precautionary measure. Adverse events associated with tattoos and/or permanent cosmetics are relatively infrequent, and when they do occur, they are relatively minor and transient.35,36,97,99 In the opinion of the FDA, “The risks of avoiding an MRI when your doctor has recommended one are likely to be much greater than the risks of complications from an interaction between the MRI and tattoo or permanent makeup. Instead of avoiding an MRI, individuals who have tattoos or permanent makeup should inform the radiologist or technician of this fact in order to take appropriate precautions, avoid complications, and assure the best results.”3,100
A comprehensive and updated list of reported adverse MR safety-related incidents can be accessed online from the Manufacturer and User Facility Device Experience Database (MAUDE; available at www.fda.gov/cdrh/maude.html)36 and the Medical Device Report (available at www.fda.gov/CDRH/mdrfile.html),35 both of which are compiled and maintained by the FDA Center for Devices and Radiological Health.97
Magnetic Resonance “Safety” Labeling
In response to the severity of the potential hazards of the presence of ferromagnetic objects in the MR environment, the FDA has developed and implemented a well-defined and stringent evaluation and labeling convention. Medical equipment, devices, and/or implants are now classified as “MR safe,” “MR unsafe,” and “MR conditional.”101,102 These terms replace the previous MR-related evaluation and labeling convention of “MR safe” and “MR compatible.” In the prior labeling convention, “MR safe” was applied to devices and implants that, when present and/or used in the MR environment, presented no additional risk to the patient or any other individual but may, however, have affected the diagnostic quality of the images. “MR compatible” implied that in addition to being “MR safe,” the device had no significant effect on the diagnostic quality of the images, nor was its operation affected by the MR system.
The replacement of these previous designations and definitions by “MR safe,” “MR unsafe,” and “MR conditional” was deemed necessary because the earlier designations were confusing and the terms often were used inappropriately. To clarify and prevent misuse, the new set of terms and associated icons was developed (Fig. 3-3).101–103 “MR safe” and/or “MR unsafe” designates an item (e.g., a medical device, equipment, or implant) as unequivocally safe or unsafe in any or all MRI environments (e.g., field strength, magnet type, and RF coils) and imaging conditions (e.g., RF power levels and gradient slew/switching rates [dB/dt]) and for all configurations of device/object use. “MR safe” objects include nonconducting, nonmetallic, and/or nonmagnetic items such as plastic or gauze tape. “MR unsafe” items include any magnetic object such as a pair of ferromagnetic/metal scissors, scalpel, wrench, or cleaning bucket.97

Figure 3-3 International magnetic resonance safety labeling icons.
U.S. Food and Drug Administration (FDA) labeling criteria (developed by American Society for Testing and Materials International) for portable objects (e.g., medical devices, implants, medical equipment, oxygen tanks, housekeeping equipment, and tools) that may or may not enter the magnetic resonance (MR) environment. Square/green “MR safe” labels correspond to objects that are totally nonreactive, triangular/yellow labels correspond to objects with “MR conditional” status, and round/red labels identify an object as being “MR unsafe.” The use of the new labeling convention has been adopted by the FDA and applied to items prospectively (but not retrospectively), beginning approximately in August 2005. (From ASTM International. Standard practice for marking medical devices and other items for safety in the magnetic resonance environment (designation F203-5), West Conshohocken, PA: ASTM International; 2005.
Alternatively, “MR conditional” indicates an item that has no known hazards for very specific combination(s) of MR environment and imaging conditions. When appropriate, additional conditions of use of the item (e.g., routing of leads associated with a neurostimulation system) also are specified. For MR conditional items, the testing must include the assessment of the magnetically induced displacement force and torque, RF heating, and other potential hazards such as thermal injury, induced currents and voltages, electromagnetic compatibility, and neurostimulation. In addition, the function and operational status of the item during and after exposure to the MR environment must be evaluated, along with its impact on the MR system itself (e.g., image artifact). The salient results of this testing should be documented in the product’s labeling, and any parameters that affect or alter the safety of the item should be noted and the potential influence described.97 The original and complete definition of the new terms and testing conditions are provided in the American Society for Testing and Materials International document.97,101,103 Notably, the use of the new labeling convention has only been applied to items prospectively, beginning approximately as of August 2005.97 Because it has not been applied by the FDA retrospectively, the labeling for many currently used items may still adhere to the prior definitions of and labeling as “MR safe” and “MR compatible.”
Magnetic Resonance Safety, Facility Operation, and Patient Care Guidelines
Magnetic Resonance Safety Risks and Considerations
In light of the extreme severity of the potential hazards associated with the improper management of the MR environment, all hospital personnel who may have reason to be in the MR department, patients, and patient family members must be appropriately educated and screened for metallic objects and/or electrically active devices on or within their person well before entering the scan room. The education must include information about the behavior of metallic objects in static magnetic fields and the associated hazards.97 The importance and necessity of these measures are highlighted by the fact that the majority of adverse MR safety incidents have been due either to deficiencies in and/or failure to enforce screening or MR environment access control procedures and practices, resulting in contraindicated personal items and other potentially problematic objects entering the MR scan room.35,36
Magnetic Resonance Safety Education and Screening
Because of the danger of inappropriately introducing ferromagnetic materials into the MR environment, the following educational and screening procedures have been implemented for facilities and apply to both patient and nonpatient populations. Access to areas that exceed the 5 G line must be vigilantly restricted and supervised at all times.26 It is important to remind all persons entering the MR facility that the magnet is always “on,” even when no examinations are being performed. Screening involves both written documentation and a verbal review. The written screening questionnaire is designed to elicit information about the presence of a medical device, implant, or other ferromagnetic object within or on the individual and/or of the existence of an underlying condition (e.g., pregnancy or disability) that may require special consideration. Once completed, an oral interview is conducted to verify the information reported, and the individual is given the opportunity to express concerns and have any remaining questions about the examination answered before entrance into the MR environment is finally allowed.
The education and screening must be performed by a health care worker specially trained in MR safety who has a comprehensive appreciation and understanding of the potential hazards of the MR environment and procedures and is familiar with the information and implications of the contents of the screening forms for patients and other individuals. Because some of the questions included on the patient screening form may not be relevant for nonpatients, separate screening forms for the patient and nonpatient groups have been compiled. Template screening forms for patients (English and Spanish versions) and a screening form for nonpatient individuals can be downloaded for use at www.MRI safety.com.93,94
The fact that a patient has had a previous MR examination without incident does not ensure the safety of current or future studies. For example, the patient may have undergone a surgical procedure and/or experienced an accident involving a metallic foreign body in the interim that now makes him or her ineligible to enter the MR environment. The exact conditions (e.g., static magnetic field strength of the MR system, RF coil used, orientation of the patient, body part evaluated, or orientation of a metallic implant or object) also can alter the safety profile substantially.96,104,105 Therefore comprehensive screening must be conducted each time any person prepares to enter the MR environment.
With respect to pregnancy, in 1991, the Safety Committee of the Society for Magnetic Resonance Imaging issued a document entitled “Policies, Guidelines, and Recommendations for MR Imaging Safety and Patient Management,”3,106 which stated that “MR imaging may be used in pregnant women if other non-ionizing forms of diagnostic imaging are inadequate or if the examination provides important information that would otherwise require exposure to ionizing radiation (e.g., fluoroscopy, computed tomography). Pregnant patients should be informed that, to date, there has been no indication that the use of clinical MR imaging during pregnancy has produced deleterious effects.” This policy was adopted by the American College of Radiology (ACR) and remains the current “standard of care”; it is applicable to MR systems operating at static magnetic field strengths up to and including 3 T.3 Because no deleterious effects of MR imaging exposure on the fetus have been documented during any stage of development, pregnant women can undergo MRI examinations at any point during their pregnancy.
Magnetic Resonance Facility Operating Procedure Guidelines
In recognition and acknowledgement of the appreciable potential hazards and severe consequences of failure to adhere to safety precautions associated with the MR environment, the ACR formed the Blue Ribbon Panel on MR Safety. First convened in 2001, the panel reviewed and refined the current MR safety practices and guidelines and established new ones when appropriate. The results of this first review were published in 2002106 and became the de facto industry standards for safe and responsible practice in both clinical and research MR environments. These guidelines were reviewed and updated in May 2004107 and again in 2006-2007, incorporating recommendations and feedback from the MR community.95 The results of the 2006–2007 review were published as the “ACR Guidance Document for Safe MR Practices: 2007.”95
The ACR outlines well-defined methodologies and procedures to ensure and enforce safe and restricted exposure and access to the MR environment most appropriate to the existing state of the MR technology, recognizing that this is continuously evolving. Specifically, in their 2007 publication, the ACR stated that the ACR MR Safe Practice Guidelines document was intended to be used as a template for MR facilities to follow in the development of their own individualized MR safety program.95 In addition, the ACR recommended that once established, each site should regularly review, reevaluate, and update its safety program as the field of MR, and MR safety, continues to evolve. The ACR also recommended that each site name an MR medical director whose responsibilities include ensuring that MR safe practice guidelines are established and maintained as current and appropriate for the site.95
One of the recommendations is the separation of the general MR facility into four zones of increasing potential MR-related danger.95 Zone One is to include all areas that are freely accessible to the general public, such as outside areas surrounding a freestanding MRI facility or the corridor in an imaging department. Zone Two is defined as the area controlled and supervised by the MR personnel, such as the reception area and patient preparation area. Zone Three is the area where there is potential for injury from ferromagnetic objects and equipment. Access to this zone must be vigilantly and strictly controlled and limited by MR personnel. These areas include the operator control room, computer room, and/or any areas immediately adjacent to the MR scan room. Finally, Zone Four is the MR scan room itself. This zone must be clearly marked with warning signs and notification of high magnetic field strengths within this area, including a sign that the magnet is always on. Access to Zone Four is strictly limited to persons with a demonstrated need to be there (i.e., patients and medical personnel), and then only after comprehensive education about the MR environment and a rigorous screening.
MR safety (Journal of Magnetic Resonance Imaging special edition), J Magn Reson Imaging 12(1), 2000.
Hornak, JP. The basics of MRI. Available at http://www.cis.rit.edu/htbooks/mri/, 2012. Accessed July 25
Shellock FG, ed. Magnetic resonance procedures: health effects and safety. Boca Raton, FL: CRC Press, 2001.
Stafford, RJ. Physics of MRI safety. Available at http://www.aapm.org/meetings/amos2/pdf/59-17207-59975-979.pdf, 2012. Accessed July 25
Bushong, SC. Magnetic resonance imaging: physical and biological principles, 3rd ed. St Louis: Mosby; 2003.
Danger! Flying objects! is an illustrative gallery from the educative “Simply Physics” site, an MRI portal created by Dr Moriel NessAiver, PhD. The gallery, which is available at http://simplyphysics.com/flying_objects.html (accessed July 27, 2012), depicts the dangers resulting from common hospital-based ferromagnetic medical equipment.
ECRI Institute is an independent, nonprofit organization that researches the best approaches to improving the safety, quality, and cost-effectiveness of patient care. ECRI is designated an Evidence-Based Practice Center by the U.S. Agency for Healthcare Research and Quality and is listed as a federal Patient Safety Organization by the U.S. Department of Health and Human Services. The organization has a Website at https://www.ecri.org (accessed July 27, 2012).
MRIsafety.com is available at http://www.mrisafety.com/ (accessed July 27, 2012). This site, developed and maintained by Dr. F.G. Shellock, provides up-to-date information on MRI safety-related topics. Impressively, the latest information regarding screening patients with implants, materials, and medical devices is provided. A key feature of the site is The List, a searchable database that contains more than 1200 implants and devices, including more than 200 objects tested at 3 T for MRI safety. Moreover, the site includes a summary section that is a presentation of more than 100 peer-reviewed articles on MRI bioeffects and safety. Other features include a downloadable Pre-MRI Screening form and safety information.
ReviseMRI.com is designed principally as a revision aid but also may be used as an educational resource. Contents include a detailed question and answer section, Web-based animated tutorials, interactive learning tools, and links to resources for further reading in common textbooks and online for nearly every question and answer posed. The site can be accessed at http://www.revisemri.com/questions/safety (accessed July 27, 2012).
The Institute for Magnetic Resonance Safety, Education and Research is a multidisciplinary professional organization headed by Director Dr. Frank Shellock. It focuses on information and research on magnetic resonance (MR) safety, while “promoting awareness, understanding, and communication of MR safety issues through education and research.” The Web site is available at http://www.imrser.org/ (accessed July 27, 2012) and has useful sections, including MRI Safety Guidelines and MR Safety Papers.
The U.S. Food and Drug Administration has a Center for Devices and Radiological Health, which is an integral part of the Department of Health & Human Services. Online documents available include “A Primer on Medical Device Interactions with Magnetic Resonance Imaging Systems” at http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm107721.htm (accessed July 27, 2012) and “MRI Safety” at http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm135362.htm (accessed July 27, 2012).
Two important MRI accident databases derived from the Database of Medical Device Related Accidents and Events are available from Maude Accidents Database (http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.cfm; accessed July 27, 2012) and the UK Medical Devices Agency (http://www.mhra.gov.uk/index.htm#page=DynamicListMedicines; accessed July 27, 2012).
medicalphysicsweb, which was launched in 2006, is a unique site for the medical physics community. It provides in-depth analysis and incisive commentary on the fundamental research, emerging technologies, and clinical applications that underpin the dynamic disciplines of medical physics and biomedical engineering. It can be accessed at http://medicalphysicsweb.org/ (accessed July 27, 2012).
References
1. Center for Devices and Radiologic Health, Food and Drug Administration. A primer on medical device interactions with magnetic resonance imaging systems. Available at http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm107721.htm, 2012. [Accessed July 25].
2. Chaljub, G, Kramer, LA, Johnson, RF, 3rd., et al. Projectile cylinder accidents resulting from the presence of ferromagnetic nitrous oxide or oxygen tanks in the MR suite. AJR Am J Roentgenol. 2001;177:27–30.
3. Shellock, FG, Crues, JV. MR procedures: biologic effects, safety, and patient care. Radiology. 2004;232:635–652.
4. Center for Devices and Radiologic Health, Food and Drug Administration. Criteria for significant risk investigations of magnetic resonance diagnostic devices. Available at http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM072688.pdf, 2012. [Accessed July 25].
5. Geard, CR, Osmak, RS, Hall, EJ, et al. Magnetic resonance and ionizing radiation: a comparative evaluation in vitro of oncogenic and genotoxic potential. Radiology. 1984;152:199–202.
6. Heinrichs, WL, Fong, P, Flannery, M, et al. Midgestational exposure of pregnant BALB/c mice to magnetic resonance imaging conditions. Magn Reson Imaging. 1988;6:305–313.
7. Hong, CZ, Shellock, FG. Short-term exposure to a 1.5 tesla static magnetic field does not affect somato-sensory-evoked potentials in man. Magn Reson Imaging. 1990;8:65–69.
8. Schenck, JF, Dumoulin, CL, Redington, RW, et al. Human exposure to 4.0-Tesla magnetic fields in a whole-body scanner. Med Phys. 1992;19:1089–1098.
9. Kangarlu, A, Burgess, RE, Zhu, H, et al. Cognitive, cardiac, and physiological safety studies in ultra high field magnetic resonance imaging. Magn Reson Imaging. 1999;17:1407–1416.
10. Shellock, FG. Biological effects and safety aspects of magnetic resonance imaging. Magn Reson Q. 1989;5:243–261.
11. Brody, AS, Embury, SH, Mentzer, WC, et al. Preservation of sickle cell blood-flow patterns during MR imaging: an in vivo study. AJR Am J Roentgenol. 1988;151:139–141.
12. Tenforde, T, Budinger, T. Biological effects and physical safety aspects of NMR imaging and in vivo spectroscopy. In: Thomas S, Dixon R, eds. NMR in medicine: instrumentation and clinical applications. New York: American Association of Physicists in Medicine, 1986.
13. Vogl, TJ, Paulus, W, Fuchs, A, et al. Influence of magnetic resonance imaging on evoked potentials and nerve conduction velocities in humans. Invest Radiol. 1991;26:432–437.
14. Weiss, J, Herrick, RC, Taber, KH, et al. Bio-effects of high magnetic fields: a study using a simple animal model. Magn Reson Imaging. 1992;10:689–694.
15. Yuh, WT, Fisher, DJ, Shields, RK, et al. Phantom limb pain induced in amputee by strong magnetic fields. J Magn Reson Imaging. 1992;2:221–223.
16. Prasad, N, Wright, DA, Ford, JJ, et al. Safety of 4-T MR imaging: study of effects on developing frog embryos. Radiology. 1990;174:251–253.
17. Prasad, N, Bushong, SC, Thornby, JI, et al. Effect of nuclear magnetic resonance on chromosomes of mouse bone marrow cells. Magn Reson Imaging. 1984;2:37–39.
18. Cooke, P, Morris, PG. The effects of NMR exposure on living organisms. II. A genetic study of human lymphocytes. Br J Radiol. 1981;54:622–625.
19. Schwartz, JL, Crooks, LE. NMR imaging produces no observable mutations or cytotoxicity in mammalian cells. AJR Am J Roentgenol. 1982;139:583–585.
20. Tyndall, DA, Sulik, KK. Effects of magnetic resonance imaging on eye development in the C57BL/6J mouse. Teratology. 1991;43:263–275.
21. McRobbie, D, Foster, MA. Pulsed magnetic field exposure during pregnancy and implications for NMR foetal imaging: a study with mice. Magn Reson Imaging. 1985;3:231–234.
22. Brockway, JP, Bream, PR, Jr. Does memory loss occur after MR imaging? J Magn Reson Imaging. 1992;2:721–728.
23. Besson, JA, Foreman, EI, Eastwood, LM, et al. Cognitive evaluation following NMR imaging of the brain. J Neurol Neurosurg Psychiatry. 1984;47:314–316.
24. Schenck, JF. Health effects and safety of static magnetic fields. In: Shellock FG, ed. Magnetic resonance procedures: health effects and safety. Boca Raton, Fla: CRC Press, 2001.
25. Schenck, JF. Safety of strong, static magnetic fields. J Magn Reson Imaging. 2000;12:2–19.
26. Price, RR. The AAPM/RSNA physics tutorial for residents. MR imaging safety considerations. Radiological Society of North America. Radiographics. 1999;19:1641–1651.
27. Chung, SM. Safety issues in magnetic resonance imaging. J Neuroophthalmol. 2002;22:35–39.
28. Zhuo, J, Gullapalli, RP. AAPM/RSNA physics tutorial for residents: MR artifacts, safety, and quality control. Radiographics. 2006;26:275–297.
29. Schenck, JF. Health and physiological effects of human exposure to whole-body four-tesla magnetic fields during MRI. Ann N Y Acad Sci. 1992;649:285–301.
30. Zaremba, L. FDA guidelines for magnetic resonance equipment safety. Presented at the Annual Meeting of the American Association of Physicists in Medicine, Montreal, July 16, 2002. Available at http://www.aapm.org/meetings/02AM/pdf/8356-48054.pdf, 2012. [Accessed July 25].
31. Shellock, FG, Tkach, JA, Ruggieri, PM, et al. Aneurysm clips: evaluation of magnetic field interactions and translational attraction by use of “long-bore” and “short-bore” 3.0-T MR imaging systems. AJNR Am J Neuroradiol. 2003;24:463–471.
32. Shellock, FG, Tkach, JA, Ruggieri, PM, et al. Cardiac pacemakers, ICDs, and loop recorder: evaluation of translational attraction using conventional (“long-bore”) and “short-bore” 1.5- and 3.0-Tesla MR systems. J Cardiovasc Magn Reson. 2003;5:387–397.
33. Duke-UNC Brain Imaging and Analysis Center. MRI safety tutorial (website). http://www.biac.duke.edu/research/safety/tutorial.asp, 2012. [Accessed July 25].
34. ECRI Institute. Patient death illustrates the importance of adhering to safety precautions in magnetic resonance environments. Available at http://www.bic.mni.mcgill.ca/~mferre/fmri.html/hazard_MRI_080601.pdf, 2012. [Accessed July 25].
35. Center for Devices and Radiologic Health, Food and Drug Administration. MDR data files (website). http://www.fda.gov/MedicalDevices/Safety/ReportaProblem/ucm124073.htm, 2012. [Accessed July 25].
36. Center for Devices and Radiologic Health, Food and Drug Administration. Manufacturer and user facility device experience database (MAUDE). Available at http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/PostmarketRequirements/ReportingAdverseEvents/ucm127891.htm, 2012. [Accessed July 25].
37. Schaefer, DJ, Bourland, JD, Nyenhuis, JA. Review of patient safety in time-varying gradient fields. J Magn Reson Imaging. 2000;12:20–29.
38. Nyenhuis, JA, Kildishev, AV, Bourland, JD, et al. Heating near implanted medical devices by the MRI RF-magnetic field. IEEE Transact Magn. 1999;35:4133–4135.
39. Bourland, JD, Nyenhuis, JA, Schaefer, DJ. Physiologic effects of intense MR imaging gradient fields. Neuroimaging Clin North Am. 1999;9:363–377.
40. Schaefer, DJ. Safety aspects of switched gradient fields. Magn Reson Imaging Clin North Am. 1998;6:731–748.
41. Cohen, MS, Weisskoff, RM, Rzedzian, RR, et al. Sensory stimulation by time-varying magnetic fields. Magn Reson Med. 1990;14:409–414.
41a. Schaer, DJ, Bourland, JD, Nyenhuis, JA. Review of patient safety in time-varying gradient fileds. J Magn Res Imaging. 2000;12:20–29.
42. Schaefer, DJ. Safety aspects of radiofrequency power deposition in magnetic resonance. Magn Reson Imaging Clin North Am. 1998;6:775–789.
43. Shellock, FG. Radiofrequency energy-induced heating during MR procedures: a review. J Magn Reson Imaging. 2000;12:30–36.
44. Schaefer, DJ. Health effects and safety of radiofrequency power deposition associated with magnetic resonance procedures. In: Shellock FG, ed. Magnetic resonance procedures: health effects and safety. Boca Raton, Fla: CRC Press, 2001.
45. Gordon, CJ. Normalizing the thermal effects of radiofrequency radiation: body mass versus total body surface area. Bioelectromagnetics. 1987;8:111–118.
46. National Council on Radiation Protection and Measurements. Biological effects and exposure criteria for radiofrequency electromagnetic fields. Bethesda, MD: National Council on Radiation Protection and Measurements; 1986.
47. Rowell, LB. Cardiovascular aspects of human thermoregulation. Circ Res. 1983;52:367–379.
48. Drinkwater, BL, Horvath, SM. Heat tolerance and aging. Med Sci Sports. 1979;11:49–55.
49. Kenney, WL. Physiological correlates of heat intolerance. Sports Med. 1985;2:279–286.
50. Barany, FR. Abnormal vascular reactions in diabetes mellitus; a clinical physiological study. Acta Med Scand Suppl. 1955;304:1–129.
51. Buskirk, ER, Lundegren, H, Magnusson, L. Heat acclimatization patterns in obese and lean individuals. Ann N Y Acad Sci. 1965;131:637–653.
52. International Electrotechnical Commission. Medical electrical equipment Part 2-33: Particular requirements for the basic safety and essential performance of magnetic resonance equipment for medical diagnosis. Geneva: International Electrotechnical Commission; 2010.
53. Smith, CD, Nyenhuis, JA, Kildishev, AV. Health effects of induced electrical currents: implications for implants. In: Shellock FG, ed. Magnetic resonance procedures: health effects and safety. Boca Raton, FL: CRC Press, 2001.
54. Shellock, FG. Magnetic resonance safety update 2002: implants and devices. J Magn Reson Imaging. 2002;16:485–496.
55. Chou, CK, McDougall, JA, Can, KW. Absence of radiofrequency heating from auditory implants during magnetic resonance imaging. Bioelectromagnetics. 1995;16:307–316.
56. Rezai, AR, Finelli, D, Nyenhuis, JA, et al. Neurostimulation systems for deep brain stimulation: in vitro evaluation of magnetic resonance imaging-related heating at 1.5 tesla. J Magn Reson Imaging. 2002;15:241–250.
57. Finelli, DA, Rezai, AR, Ruggieri, PM, et al. MR imaging-related heating of deep brain stimulation electrodes: in vitro study. AJNR Am J Neuroradiol. 2002;23:1795–1802.
58. Shellock, FG, Hatfield, M, Simon, BJ, et al. Implantable spinal fusion stimulator: assessment of MR safety and artifacts. J Magn Reson Imaging. 2000;12:214–223.
59. Nordell, A, Lundh, M, Horsch, S, et al. The acoustic hood: a patient-independent device improving acoustic noise protection during neonatal magnetic resonance imaging. Acta Paediatr. 2009;98:1278–1283.
60. McJury, M. Acoustic noise and magnetic resonance procedures. In: Shellock FG, ed. Magnetic resonance procedures: health effects and safety. Boca Raton, FL: CRC Press, 2001.
61. Brummett, RE, Talbot, JM, Charuhas, P. Potential hearing loss resulting from MR imaging. Radiology. 1988;169:539–540.
62. Quirk, ME, Letendre, AJ, Ciottone, RA, et al. Anxiety in patients undergoing MR imaging. Radiology. 1989;170:463–466.
63. Laurell, GF. Combined effects of noise and cisplatin: short- and long-term follow-up. Ann Otol Rhinol Laryngol. 1992;101:969–976.
64. Philbin, MK, Taber, KH, Hayman, LA. Preliminary report: changes in vital signs of term newborns during MR. AJNR Am J Neuroradiol. 1996;17:1033–1036.
65. Counter, SA, Olofsson, A, Borg, E, et al. Analysis of magnetic resonance imaging acoustic noise generated by a 4.7 T experimental system. Acta Otolaryngol. 2000;120:739–743.
66. Price, DL, De Wilde, JP, Papadaki, AM, et al. Investigation of acoustic noise on 15 MRI scanners from 0.2 T to 3 T. J Magn Reson Imaging. 2001;13:288–293.
67. Shellock, FG, Ziarati, M, Atkinson, D, et al. Determination of gradient magnetic field-induced acoustic noise associated with the use of echo planar and three-dimensional, fast spin echo techniques. J Magn Reson Imaging. 1998;8:1154–1157.
68. Strainer, JC, Ulmer, JL, Yetkin, FZ, et al. Functional MR of the primary auditory cortex: an analysis of pure tone activation and tone discrimination. AJNR Am J Neuroradiol. 1997;18:601–610.
69. Ulmer, JL, Biswal, BB, Mark, LP, et al. Acoustic echoplanar scanner noise and pure tone hearing thresholds: the effects of sequence repetition times and acoustic noise rates. J Comput Assist Tomogr. 1998;22:480–486.
70. Bandettini, PA, Jesmanowicz, A, Van Kylen, J, et al. Functional MRI of brain activation induced by scanner acoustic noise. Magn Reson Med. 1998;39:410–416.
71. Goldman, AM, Gossman, WE, Friedlander, PC. Reduction of sound levels with antinoise in MR imaging. Radiology. 1989;173:549–550.
72. Hurwitz, R, Lane, SR, Bell, RA, et al. Acoustic analysis of gradient-coil noise in MR imaging. Radiology. 1989;173:545–548.
73. McJury, M, Shellock, FG. Auditory noise associated with MR procedures: a review. J Magn Reson Imaging. 2000;12:37–45.
74. Miller, LE, Keller, AM. Regulation of occupational noise. In: Harris CM, ed. Handbook of noise control. New York: McGraw-Hill, 1979.
75. Melnick, W. Hearing loss from noise exposure. In: Harris CM, ed. Handbook of noise control. New York: McGraw-Hill, 1979.
76. Zaremba, LA. FDA guidance for magnetic resonance system safety and patient exposures: current status and future considerations. In: Shellock FG, ed. Magnetic resonance procedures: health effects and safety. Boca Raton, FL: CRC Press, 2001.
77. Harris, LM, Robinson, J, Menzies, RG. Evidence for fear of restriction and fear of suffocation as components of claustrophobia. Behav Res Ther. 1999;37:155–159.
78. Shellock, FG. Magnetic resonance procedure: health effects and safety. Boca Raton, FL: CRC Press; 2001.
79. Shellock, FG. Biomedical implants and devices: assessment of magnetic field interactions with a 3.0-Tesla MR system. J Magn Reson Imaging. 2002;16:721–732.
80. Kangarlu, A, Shellock, FG. Aneurysm clips: evaluation of magnetic field interactions with an 8.0 T MR system. J Magn Reson Imaging. 2000;12:107–111.
81. Yuh, WT, Hanigan, MT, Nerad, JA, et al. Extrusion of eye socket magnetic implant after MR imaging: potential hazard to patient with eye prosthesis. J Magn Reson Imaging. 1991;1:711–713.
82. Fagan, LL, Shellock, FG, Brenner, RJ, et al. Ex vivo evaluation of ferromagnetism, heating, and artifacts of breast tissue expanders exposed to a 1.5-T MR system. J Magn Reson Imaging. 1995;5:614–616.
83. Shellock, FG. MR imaging and cervical fixation devices: evaluation of ferromagnetism, heating, and artifacts at 1.5 Tesla. Magn Reson Imaging. 1996;14:1093–1098.
84. Shellock, FG, Shellock, VJ. Cardiovascular catheters and accessories: ex vivo testing of ferromagnetism, heating, and artifacts associated with MRI. J Magn Reson Imaging. 1998;8:1338–1342.
85. Kanal, E, Shellock, FG. Aneurysm clips: effects of long-term and multiple exposures to a 1.5-T MR system. Radiology. 1999;210:563–565.
86. Kanal, E, Shellock, FG, Lewin, JS. Aneurysm clip testing for ferromagnetic properties: clip variability issues. Radiology. 1996;200:576–578.
87. Klucznik, RP, Carrier, DA, Pyka, R, et al. Placement of a ferromagnetic intracerebral aneurysm clip in a magnetic field with a fatal outcome. Radiology. 1993;187:855–856.
88. Rezai, AR, Finelli, D, Rugieri, P, et al. Neurostimulators: potential for excessive heating of deep brain stimulation electrodes during magnetic resonance imaging. J Magn Reson Imaging. 2001;14:488–489.
89. Shellock, FG, Cosendai, G, Park, SM, et al. Implantable microstimulator: magnetic resonance safety at 1.5 Tesla. Invest Radiol. 2004;39:591–599.
90. Baker, KB, Nyenhuis, JA, Hrdlicka, G, et al. Neurostimulation systems: assessment of magnetic field interactions associated with 1.5- and 3-Tesla MR systems. J Magn Reson Imaging. 2005;21:72–77.
91. Henderson, JM, Tkach, J, Phillips, M, et al. Permanent neurological deficit related to magnetic resonance imaging in a patient with implanted deep brain stimulation electrodes for Parkinson’s disease: case report. Neurosurgery. 2005;57:E1063. [discussion E1063].
92. Shellock, FG. Magnetic resonance bioeffects, safety, and patient management. In: Edelman R, Hesselink J, Zlatkin M, et al, eds. Clinical magnetic resonance imaging. Philadelphia: Saunders, 2005.
93. Shellock, FG. Welcome to MRI safety.com. Available at http://www.mrisafety.com/, 2012. [Accessed July 25].
94. Institute for Magnetic Resonance Safety, Education, and Research. Available at www.IMRSER.org, 2012. [Accessed July 25].
95. Kanal, E, Barkovich, AJ, Bell, C, et al. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188:1447–1474.
96. Sawyer-Glover, AM, Shellock, FG. Pre-MRI procedure screening: recommendations and safety considerations for biomedical implants and devices. J Magn Reson Imaging. 2000;12:510.
97. Shellock, FG, Spinazzi, A. MRI safety update 2008: part 2, screening patients for MRI. AJR Am J Roentgenol. 2008;191:1140–1149.
98. Shellock, FG. Reference manual for magnetic resonance safety, implants, and devices: 2011 ed. Los Angeles: Biomedical Research Publishing; 2011.
99. Tope, WD, Shellock, FG. Magnetic resonance imaging and permanent cosmetics (tattoos): survey of complications and adverse events. J Magn Reson Imaging. 2002;15:180–184.
100. Food and Drug Administration. Tattoos and permanent makeup. Available at http://www.fda.gov/cosmetics/productandingredientsafety/productinformation/ucm108530.htm, 2012. [Accessed July 25].
101. ASTM International. Standard practice for marking medical devices and other items for safety in the magnetic resonance environment. Conshohocken, PA: ASTM International; 2005.
102. Woods, TO. Standards for medical devices in MRI: present and future. J Magn Reson Imaging. 2007;26:1186–1189.
103. Shellock, FG, Woods, TO, Crues, JV, 3rd. MR labeling information for implants and devices: explanation of terminology. Radiology. 2009;253:26–30.
104. Sawyer-Glover, A, Shellock, FG. Pre-magnetic resonance procedure screening. In: Shellock FG, ed. Magnetic resonance procedures. Boca Raton, FL: CRC Press, 2001.
105. Kanal, E, Borgstede, JP, Barkovich, AJ, et al. American College of Radiology White Paper on MR Safety. AJR Am J Roentgenol. 2002;178:1335–1347.
106. Shellock, FG, Kanal, E. Policies, guidelines, and recommendations for MR imaging safety and patient management, SMRI Safety Committee. J Magn Reson Imaging. 1991;1:97–101.
107. Kanal, E, Borgstede, JP, Barkovich, AJ, et al. American College of Radiology White Paper on MR Safety: 2004 update and revisions. AJR Am J Roentgenol. 2004;182:1111–1114.