CHAPTER 19 Magnetic Resonance Imaging Safety
In 2008, MRI safety came under scrutiny by The Joint Commission when a sentinel event alert was issued in the United States because of widespread adverse events. The alert was based on 398 adverse events reported to the U.S. Food and Drug Administration’s (FDA) Manufacturer and User Facility Device Experience Database (MAUDE) over a 10-year period, 9 of which were deaths.1 Burns from significant heating of wires and leads were the most common problem reported at more than 70% of the MRI-related incidents. Adverse events and accidents in the MRI environment happen because of failures in adherence to proper MRI safety procedures, inappropriate use of equipment, outdated safety information, lack of training for individuals working in the MRI environment, and lack of appropriate supervision of the MRI suite.1,2 The safety problems in MRI cannot be taken lightly because the safety issues surrounding MRI are complicated and often unpredictable. The Joint Commission’s recommendations for reducing accidents and injuries in MRI follow the “ACR Guidance Document for Safe MR Practices,”3 and can be found at http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea_38.htm or http://www.acr.org/SecondaryMainMenuCategories/quality_safety/MRSafety/safe_mr07.aspx.
This chapter provides an overview of MRI safety as it pertains to patients with cardiovascular disease, with suggestions of supplemental information and references. MRI safety is an enormous topic that is constantly changing, and a host of Web-based resources are available. Questions of MRI safety need to be evaluated with the most up-to-date information possible, which is why providers need to research the specific devices and scanning circumstances carefully. Box 19-1 lists useful websites for the reader’s reference. MRI safety guidelines are updated on a regular basis. Failure to follow current safety guidelines puts patients at increased risk for injury and, in some instances, results in unnecessary avoidance of clinically necessary MRI examinations.
BOX 19-1
Recommended Websites
GENERAL CONCERNS
Hazards
Individuals who are not educated and trained for safety in the MRI environment cause an added level of danger because they are unfamiliar with the dangers and safety precautions needed to ensure safe operation of equipment in the MRI scanner area. Many accidents are a result of noncompliance with safety guidelines, or of adherence to outdated guidelines. The ECRI Institute, a nonprofit health services research organization, recommends that MRI clinics appoint a safety officer to oversee the establishment, implementation, and maintenance of MRI safety policies and procedures, something The Joint Commission and American College of Radiology (ACR) also recommend as a prudent preventive measure.1,3,4
Magnetic Resonance Imaging Clinic
The physical facilities of an MRI facility play a direct role in safety. Site planning for an MRI system should be done to limit access of non-MRI personnel to the actual MRI scanner room. Only properly screened patients and personnel should be allowed into the MRI scan room, and they should be allowed in the scan room only with the direct supervision of qualified MRI professionals. The ACR has recommended a clinic design safety template that includes control of site access restriction.3 An MRI facility should be considered to be made up of four zones as outlined by the “ACR Guidance Document for Safe MR Practices” (Fig. 19-1).3 Zone I is the area outside the actual MRI facility, and it is open to the general public. This is the area where the general public, patients, health care workers, and MRI center employees enter the MRI facility.
Physics of Electricity and Magnetism
Maxwell’s equations are the building blocks of our understanding of electricity and magnetism. The four equations known as Gauss’ law for electricity, Gauss’ law for magnetism, Faraday’s law, and Ampere-Maxwell law help in understanding many of the important elements of MRI safety.5 The mathematical predictions for safety cannot be calculated easily. Ensuring the safety of a device in a patient undergoing an MRI examination requires knowledge of the device, the specific MRI system, the type of RF coils used, the sequences employed for imaging, the way the patient loads the scanner and transmit RF coil, and whether or not monitoring or gating equipment is also being used. It is nearly impossible to predict all of the possible ways for heat deposition or current induction that may occur with a given implant in a patient—hence the safety dilemma.
Static Magnetic Field Strength
Currently, most clinical scanners for cardiac use are operating at a static magnetic field of 1.5 T or 3 T. Table 19-1 outlines the FDA significant risk recommendations. Magnetic fields have the potential for translational and rotational (or torque) effects. Basically, magnetic field interactions increase as the static magnetic field strength increases. If a device is considered to pose a potential safety concern, it is prudent to consider carefully the potential options for such scanning at the lowest field strength appropriate for the imaging needs (e.g., coil selection, imaging pulse sequence selection). Imaging time is also important because the increase in scan time may prolong the requirements for monitoring a child or a sedated claustrophobic patient. Discussion with the patient on what to expect and the need for the patient to communicate any unusual sensations immediately is imperative. Devices and scanners vary considerably, so many factors must be considered. Substances that are attracted to the magnetic field are often referred to as having ferromagnetic properties. Devices that are ferromagnetic have the potential to interact with the magnetic field in a translational or rotational manner (or both), possibly causing great harm to the patient or equipment or both. The plethora of MRI accident pictures that are available via the Internet provide some idea of the danger of the static magnetic field of the MRI scanner. Many pictures of objects flying into scanners can be found at sites such as Simply Physics (http://www.simplyphysics.com/flying_objects.html).
TABLE 19-1 Static Main Magnetic Field Limit Recommendations by the U.S. Food and Drug Administration*
| Population | Main Static Magnetic Field Greater than (tesla) |
|---|---|
| Adults, children, and infants >1 month old | 8 |
| Neonates (<1 month old) | 4 |
* The FDA considers an MRI scanner to be of significant risk when it is operating above the levels listed.
From United States Food and Drug Administration, Center for Devices and Radiological Health. Guidance for Industry. Criteria for significant risk investigations of magnetic resonance diagnostic devices. Available at: http://www.fda.gov/cdrh/ode/guidance/793.pdf. Accessed June 5, 2008.
Gradient Magnetic Fields
MRI scanners use time-varying magnetic fields called gradients to encode for field of view, slice thickness, and other imaging parameters, and in certain aspects of pulse sequence image contrast. Stronger and faster gradients and more powerful RF transmission coils are being used in MRI scanners.6 The current FDA limit for gradient magnetic field rates of change (dB/dt) varies depending on operating conditions. Significant risk for dB/dt is classified as when painful nerve stimulation or severe discomfort is produced.7 Gradient fields are magnetic fields that are much weaker than the main magnetic field, but they are switched on and off very quickly and, according to Maxwell’s equations, can induce time-varying electrical currents.8 Gradients can induce currents in conductive materials9 and potentiate peripheral nerve stimulation, but are typically not painful on current FDA-approved MRI systems.10 This is a safety concern because these gradient currents have the potential also to induce currents in certain cardiac devices, which could induce arrhythmias.9 Tandri and coworkers8 reported that gradient currents may be strong enough under certain conditions to distort pacing pulses, which is significant enough to interfere with myocardial capture or loss of capture when patients with pacemakers are scanned in MRI. This finding reinforces the need for prudent screening and risk/benefit considerations with patients with any kind of implanted cardiac device.
Radiofrequency Fields
The transmit RF coils of the MRI scanner emit RF energy that is specifically tuned to the tissue of interest at a given static magnetic field strength. RF energy is absorbed by the tissue and released, producing signals that receiver coils can detect. RF energy may cause heating of biologic tissues, however, when the tissues are exposed to excessive RF energy levels. To ensure patient safety, levels of RF energy used for MRI procedures indicated in the units of specific absorption rate (SAR), are recommended by the FDA. Generally, MRI scanners calculate or estimate SAR based on the patient’s weight and report values in W/kg. In 2003, the FDA relaxed the SAR limits as follows: 4 W/kg—whole body for 15 minutes, 3 W/kg—head for 10 minutes, 8 W/kg—peak 1 g of tissue (head or torso) for 15 minutes, 12 W/kg—peak 1 g of tissue (extremities) for 15 minutes.7
SAR increases with increasing static magnetic field strength, flip angle, duty cycle of the equipment, and patient size.11 The SAR experienced at 3 T is approximately four times the absorption rate as 1.5 T,12 making some of the cardiac imaging at 3 T potentially challenging. The SAR can be reduced, however, by modifying parameters such as flip angle, repetition time, slice thickness, imaging matrix, and echo train length, depending on the pulse sequence and the desired image contrast. Parallel imaging schemes have been a successful strategy for SAR reduction at 3 T.
Cryogens
Cryogens are used to create superconducting MRI scanners. Liquid helium is the most common cryogen used in MRI scanners today, but liquid nitrogen is also used in some systems. Generally, only service engineers come in contact with the cryogens; however, if the cryogen system malfunctions, a quench can occur. A quench is the rapid release of the cryogen liquid (in the form of gas) from the MRI scanner that brings the magnetic field strength of the MRI scanner down very quickly. A quench can be problematic if the MRI scan room is built with the door opening inward, and there is not a pressure release mechanism.3 This situation can set up the potential for a pressure lock to occur because helium expands at 754 : 1 as it turns to gas. Helium is also very cold (liquid helium is <−268° C). Ruptured eardrums, frostbite, and asphyxiation are potential adverse consequences to individuals in the scan room if a quench occurs whereby the helium gas escapes into the scan room instead of through the ventilation system.
CARDIAC DEVICES AND Magnetic Resonance Imaging INFORMATION
An increasing number of implantable cardiovascular devices are being made available for patients with cardiovascular disease. Each device has a different composition, mass, geometry, potential for heating and functionality that affects the possible risks associated with MRI procedures. Devices such as cardiac pacemakers, pacing leads, and implantable cardioverter-defibrillators (ICDs) are controversial devices in patients today. Because of the variety and complexity of devices, the safety of implanted devices should never be assumed.3 Patients entering zones III and IV need to have the safety of their implanted devices documented in writing and other external devices carefully tested.
Terminology Issues
The FDA originally adopted the terms MRI safe and MRI compatible in 1997 (Table 19-2). More recently, to try to minimize confusion from the older MRI compatible term, the FDA has adopted new terminology (MRI safe, MRI conditional, and MRI unsafe), developed by the American Society of Testing and Materials, which is based on specified MRI conditions and device interactions (Table 19-3).2,3,14 The overall goal remains the same: to test devices, document the specific findings, and categorize the device for guidance concerning the scanning of patients. Because of the complexity of MRI scanners, RF coils, and devices, it is imperative that the detailed safety information be adequately reviewed by the responsible physicians overseeing the MRI procedure to be as safe as possible with the vast amount of devices implanted in patients today.
TABLE 19-2 Terminology for the Labeling of Items for MRI Purposes*
| Old Terminology | Definition |
|---|---|
| MRI safe† | This term indicates that the device, when used in the MRI environment, has been shown to present no additional risk to the patient, but may affect the quality of the diagnostic information. |
| MRI compatible† | This term indicates that the device, when used in the MRI environment, is MRI safe, and has been shown neither to affect significantly the quality of the diagnostic information nor to have its operations affected by the MRI device. |
* The older terminology from the FDA’s Center for Devices and Radiological Health of 1997 is still in use because the updated, new terminology has not been applied retrospectively.
† The use of the terms MRI compatible and MRI safe without specification of the MRI environment to which the device was tested should be avoided because interpretation of these claims may vary, and they are difficult to substantiate rigorously. Statements such as “intended for use in the MRI environment” or similar claims along with appropriate qualifying information are preferred (i.e., test conditions should be specifically stated).
From United States Food and Drug Administration, Center for Devices and Radiological Health. A primer on medical device interactions with magnetic resonance imaging systems, 1997. Available at: http://www.fda.gov/cdrh/ode/primerf6.html. Accessed June 5, 2008.
TABLE 19-3 Terminology for MRI Implants, Devices, and Labeling from the American Society of Testing and Materials
| New Terminology | Definition |
|---|---|
| MRI safe | An item that poses no known hazards in all MRI environments |
| MRI conditional | An item that has been shown to pose no known hazards in a specified MRI environment with specified conditions of use. Field conditions that define the specified MRI environment include static magnetic field strength, spatial gradient, dB/dt (time varying magnetic fields), radiofrequency fields, and specific absorption rate. Additional conditions, including specific configurations of the item, may be required |
| MRI unsafe | An item that is known to pose hazards in all MRI environments |
From American Society for Testing and Materials (ASTM) International, Designation: F2503-05. Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment. West Conshohocken, PA, ASTM International, 2005.
Cardiac Pacemakers, Implantable Cardioverter-Defibrillators, and Other Devices
Cardiac pacemakers, ICDs, and other electronic devices pose several potential problems to patients relative to the MRI environment. The devices can harm the patients through possible movement of the device, potential to heat or burn the patient, potential to cause arrhythmias, and potential of malfunction or damage occurring to the device.8,15 These problems can lead to harm and even fatalities. Patients with these devices are generally restricted from the MRI environment, but this policy is starting to change.
In January 2008, the FDA granted approval to begin the first clinical trials on a pacemaker system designed for safe use within an MRI scanner.16 The pacemaker system (EnRhythm MRI SureScan; Medtronic, Minneapolis, MN) has gone through extensive design steps to improve the safety and efficacy of the cardiac pacemaker. Many physicians have been insisting for many years that pacemakers made with the newer technologies in recent years are safe; however, no manufacturer currently claims MRI safety. It has been reported that patients with pacemakers can safely undergo MRI scanning under certain highly specific and controlled conditions.17 There are many potential problems associated with pacemakers that relate to such issues as the reed switches, lead lengths, circuitry, batteries, capacitors, types of functions, and types of patient therapies that must be considered. According to the recommendations from the American Heart Association Scientific Statement of 2007, only sites with expertise in cardiac MRI and electrophysiology should be attempting to scan patients with pacemakers and ICDs.9 The risk/benefit ratio must clearly show the clinical benefit to the patient.
Pacemaker leads are also problematic, even when the pulse generator is detached. The problem is that long lengths of any wire can potentially cause heating, which may be excessive, and cardiac excitation.9,18 Leads are often wound up with the tip of the lead still embedded in the myocardium. The amount of heating varies according to the size, shape, and composition of the metal; the location in the body; the location of the implant with respect to the RF coil; and the amount of SAR deposition.18 Leads that are fractured pose a higher risk for excessive heating. The American Heart Association Scientific Statement in 2007 recommends that retained leads follow the same guidelines as pacemakers and ICDs.9 Clinical studies are unavailable on fractured leads; MRI scanning in patients with damaged or fractured transvenous leads is discouraged.
Insertable Loop Recorders
Insertable loop recorders (ILR), also known as event monitors, are subcutaneously implanted devices used to monitor patients with unexplained syncope continuously. One ILR (Reveal; Medtronic, Minneapolis, MN) has been tested and has not shown adverse effects to the patient, but data retrieved from the recorder after scanning showed artifacts on the recorded ECG data retrieved from the ILR device.19 There is also a theoretical risk of the electromagnetic fields from the MRI scanner compromising the data on the ILR.9 Data on the ILR should be retrieved before the MRI examination. The ILR is considered to be MRI conditional.
Prosthetic Heart Valves
Prosthetic heart valves are made from various metals and biomaterials, and come in a wide variety of designs. Some valves may not contain any metal, and some may be made only of metal. Many heart valves have been tested at 1.5 T and 3 T. Many of the tested heart valves have been found to have measurable magnetic field interactions at 1.5 T.2 The blood flow and beating of the heart exerted on the valve in vivo have been found to be greater, however, than the forces of the magnetic field at 1.5 T.20 In a study of excised heart valves, it was measured that the forces necessary to dislodge sutures in a heart valve were greater than those found at 4.7 T.21 Similarly, the forces necessary to cause valve dehiscence were found to be clinically insignificant at exposures of 4.7 T.
The Lenz effect theorizes significant electromotive force when metal heart valves interact in the MRI environment. The heart valves can theoretically create, in themselves, a little magnetic field that could oppose the static magnetic field of the MRI scanner, and possibly inhibit the opening and closing of the heart valve.22 The potential for the hazards with heart valves are at this point theoretical only because serious adverse events have not been reported with heart valves at 1.5 T.22 The Lenz effect increases linearly with field strength, so the potential for adverse interactions increases with field strength. In any case, patients with metallic heart valves should be monitored more closely because of the potential for heart valves to have magnetic field interactions at any field strength.
Stents
Many types of stents are available for cardiovascular and other applications. Most of these stents are made of 316L stainless steel or nitinol, but other materials, such as tantalum, MP35N, titanium, platinum, Elgiloy, and cobalt chromium, are also used.2 Most of these stents are nonferromagnetic or weakly ferromagnetic. If the stent is nonferromagnetic, an MRI procedure may be performed immediately after implantation.9 There may be a potential problem with stents that are overlapped as two or more devices. Overlapped stents or long length stents may be able to heat up substantially in the MRI environment because of current induction from RF power deposition, but this is a theoretical hazard. One key concept to remember is that new stents are being developed on a regular basis, and that not all stents are made in the United States under rigorous FDA guidelines. With the increase in globalization of health care and medical tourism, a growing number of patients in the United States are getting devices that may be available only in other countries. Obtaining specific stent information before performing an MRI examination on a patient is necessary to optimize patient safety.
MAGNETIC RESONANCE IMAGING CONTRAST AGENTS
Gadolinium (Gd)-chelate contrast agents are the primary contrast agents used in cardiovascular MRI and are cleared by the kidney. For individuals with normal renal function, Gd contrast agents are considered non-nephrotoxic when given intravenously at FDA-approved doses.23 In 2000, findings of nephrogenic systemic fibrosis were reported, however, in patients who had received Gd-chelate contrast agents. Nephrogenic systemic fibrosis is found almost exclusively in patients with severe renal impairment and acidosis.24 At this time, the FDA and the ACR recommend that patients with glomerular filtration rate less than 30 mL/min/1.73 m2 should not receive Gd-chelate contrast agents. Currently, the FDA recommends that patients on dialysis be dialyzed immediately after receiving Gd-chelate contrast agents because they are dialyzable. More detailed recommendations are discussed in Chapter 18; see also the FDA and ACR websites (http://www.fda.gov/cder/drug/infopage/gcca/qa_200705.htm and http://www.acr.org/SecondaryMainMenuCategories/quality_safety/MRSafety/recommendations_gadolinium-based.aspx).
SEDATION
The Joint Commission requires that institutions have a plan of care for all sedation/anesthesia performed for procedures. The ACR follows the recommendations of The Joint Commission for their practice guidelines for adult sedation/analgesia, which can be found at http://www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/iv/adult_sedation.aspx. The guidelines for sedation/anesthesia are designed to assist in the safe administration of sedation/analgesia and monitoring in the radiology department. Regardless of the sedation/anesthesia state, qualified personnel must be available, and patients must be monitored throughout the MRI examination. Sedation is considered to be a continuous spectrum; the health care provider must always be qualified to be able to reverse a patient if the patient unexpectedly slips into a deeper level of sedation than that intended.25 Patient management procedures before and after the MRI examination must also be taken into consideration.
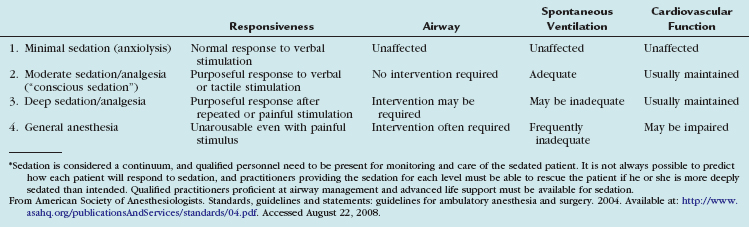
There are four generally accepted levels of sedation/analgesia according to the American Society of Anesthesiologists and The Joint Commission, ranging from light sedation to general anesthesia (Table 19-4).1,26 Each level requires increasing level of qualifications for the personnel administering and monitoring the patients. The ACR recommends that patients should not be discharged until level of consciousness, vital signs, and motor function return to acceptable levels as assessed by health care personnel, and that written discharge instructions be given to the patient and family.27 Discharge instructions should include possible adverse effects of the medication, a physician contact number, and warning not to drive for at least 12 hours.27
Pediatric patients represent a special challenge in cardiovascular MRI. Similar to adult patients, it is best if the pediatric patient is not sedated for cardiac studies. It is unreasonable, however, to expect a young child to hold still for a long MRI study. MRI sites that perform pediatric MRI must carefully develop a detailed protocol for the sedation of the pediatric patient. The American Academy of Pediatrics and the American Academy of Pediatric Dentistry have emphasized that children are different when it comes to sedation.28 A child’s respiratory system is vulnerable to sedative medications, and a child can easily slip from a state of sedation to anesthesia during a procedure.29–31 Children are at risk for complications during sedation, such as apnea, hypoventilation, laryngospasm, and cardiopulmonary impairment, even under carefully monitored conditions.31,32 Experienced teams that are able to rescue pediatric patients when they slip into unintended levels of sedation must be available. The American Academy of Pediatrics, American Academy of Pediatric Dentistry, and ACR all agree that the specific guidelines established by the American Society of Anesthesiologists for the personnel and delivery of sedation/analgesia should be followed.3,27,28
ACOUSTIC NOISE AND HEARING PROTECTION
MRI scanners are noisy. Patients complain often about the banging noises the MRI systems make. The noise may be very loud, may interfere with patient-operator communication, may increase patient anxiety, may interfere with functional MRI responses to stimuli, and may be annoying to patients and health care workers.33,34 The loud noises made by the MRI scanners have the potential for temporary hearing loss, and patients need to be given hearing protection. In clinical tests performed at 0.35 T, the noise generated by the coils of the scanner were loud enough to cause a temporary hearing loss of more than 15 dB in 5 of 14 patients who were scanned in the scanner without hearing protection.33
Noise is measured as the sound pressure level. The FDA rates an MRI scanner as having significant risk if it generates peak unweighted sound pressure levels greater than 140 dB. Hearing loss is multifaceted, but generally the FDA recommends hearing protection for dB levels greater than 85 for more than 15 minutes of exposure. Many MRI systems operate at dB levels greater than 100. Passively reducing the acoustic noise through the use of earplugs or earmuffs can reduce the noise level by 25 to 29 dB (earplugs) and 31 to 38 dB (earmuffs),35 reducing the impact on hearing. Some patients may be much more sensitive to acoustic noise than others, so attention to the patient’s needs (e.g., using earplugs and earmuffs) may help, or trying to minimize total scan time may be considered. Because sound travels through the body, totally eliminating the sound to the patient is impossible. Educating the patient as to what to expect during the study is helpful for compliance and patient satisfaction.
STRESS TESTING AND SAFETY IN MAGNETIC RESONANCE IMAGING
The most commonly used medications for cardiac MRI stress are adenosine and dobutamine. Adenosine is a vasodilator that is used for myocardial perfusion studies.36,37 Adenosine is well tolerated and has a half-life of only 10 seconds. For this reason, adenosine is considered a safer drug of choice for perfusion stress testing in MRI. Another vasodilator that has been used for perfusion is dipyridamole.38 Although inexpensive, dipyridamole has a longer half-life than adenosine, requiring a longer monitoring period. Dipyridamole is also much more likely to require theophylline to be used to normalize the patient’s response. Caution must be used because theophylline is a shorter acting drug than dipyridamole, so the patient must be counseled and monitored about having recurrence of the vasodilator symptoms.
Dobutamine is a positive inotrope that is used for the wall motion studies.39 Stress perfusion can also be achieved in dobutamine patients if atropine is added to the medication protocol. Dobutamine is a much riskier drug to use for stress than adenosine. A 12-lead ECG must be performed before and after the procedure to monitor for arrhythmias. The effects of the dobutamine start quickly, with the patient often needing an increased dose during the scanning. Caution must be used because dobutamine’s half-life is 2 minutes with the effects lasting in some cases for 20 or 30 minutes. Dobutamine may precipitate or exacerbate ventricular ectopy. Severe hypotension and ventricular tachycardia can occur with dobutamine, so the crash cart and fluid expanders must be readily available in the MRI suite during these procedures. To help the patient recover from dobutamine stress faster, β-blockers may be given. In all stress procedures, the patient must be assessed by a physician or nurse practitioner before discharge. For additional information on cardiac stress imaging, see Chapter 26.
CONCLUSION
American Academy of PediatricsAmerican Academy of Pediatric DentistryCoté CJ, Wilson S. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Pediatrics. 2006;118:2587-2602.
Faris OP, Shein M. Food and Drug Administration perspective: magnetic resonance imaging of pacemaker and implantable cardioverter-defibrillator patients. Circulation. 2006;114:1232-1233.
Kanal E, Barkovich AJ, Bell C, et al. ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188:1447-1474.
Lee VS, Hecht EM, Taouli B, et al. Body and cardiovascular MR imaging at 3.0 T. Radiology. 2007;244:692-705.
Levine GN, Gomes AS, Arai AE, et al. Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization. Circulation. 2007;116:2878-2891.
Roguin A, Schwitter J, Vahlhaus C, et al. Magnetic resonance imaging in individuals with cardiovascular implantable electronic devices. Europace. 2008;10:336-346.
Shellock FG. Reference Manual for Magnetic Resonance Safety, Implants, and Devices: 2008 Edition. Los Angeles: Biomedical Research Publishing Group; 2008.
Shellock FG, Spinazzi A. MRI safety update 2008—parts 1 and 2. AJR Am J Roentgenol. 2008;191:1129-1139. 1140-1149
Shinbane JS, Colletti PM, Shellock FG. MR in patients with pacemakers and ICDs: defining the issues. J Cardiovasc Magn Reson. 2007;9:5-13.
Zhuo J, Gullapalli RP. AAPM/RSNA physics tutorial for residents: MR artifacts, safety, and quality control. RadioGraphics. 2006;26:275-297.
1 The Joint Commission Preventing accidents and injuries in the MRI suite Issue 38, February 14, 2008. Available at: http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea_38.htm Accessed June 5, 2008
2 Shellock FG. Reference Manual for Magnetic Resonance Safety, Implants, and Devices: 2008 Edition. Los Angeles: Biomedical Research Publishing Group; 2008.
3 Kanal E, Barkovich AJ, Bell C, et al. ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188:1447-1474.
4 ECRI. Hazard report: patient death illustrates the importance of adhering to safety precautions in magnetic resonance environments. Health Devices. 30, 2001.
5 Maxwell JC 2nd ed, A Treatise on Electricity and Magnetism, Vol 1. Oxford, Clarendon Press, 1881.
6 Shellock FG, Crues JV. MR procedures: biological effects, safety, and patient care. Radiology. 2004;232:635-652.
7 United States Food and Drug Administration, Center for Devices and Radiological Health Guidance for Industry. Criteria for significant risk investigations of magnetic resonance diagnostic devices Available at: http://www.fda.gov/cdrh/ode/guidance/793.pdf Accessed June 5, 2008
8 Tandri H, Zviman MM, Wedan SR, et al. Determinants of gradient field-induced current in a pacemaker lead system in a magnetic resonance imaging environment. Heart Rhythm. 2008;5:462-468.
9 Levine GN, Gomes AS, Arai AE, et al. Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization. Circulation. 2007;116:2878-2891.
10 Schaefer DJ. Bioeffects of MRI and patient safety. In: Bronskill MJ, Sprawls P, editors. The Physics of MRI: 1992 AAPM Summer School Proceedings. Woodbury, NY: American Association of Physics in Medicine, 1993.
11 Zhuo J, Gullapalli RP. AAPM/RSNA physics tutorial for residents: MR artifacts, safety, and quality control. RadioGraphics. 2006;26:275-297.
12 Lee VS, Hecht EM, Taouli B, et al. Body and cardiovascular MR imaging at 3.0 T. Radiology. 2007;244:692-705.
13 United States Food and Drug Administration, Center for Devices and Radiological Health A primer on medical device interactions with magnetic resonance imaging systems, 1997 Available at: http://www.fda.gov/cdrh/ode/primerf6.html Accessed June 5, 2008
14 American Society for Testing and Materials (ASTM) International, Designation: F2503-05. Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment. West Conshohocken, PA: ASTM International; 2005.
15 Shinbane JS, Colletti PM, Shellock FG. MR in patients with pacemakers and ICDs: defining the issues. J Cardiovasc Magn Reson. 2007;9:5-13.
16 Medtronic FDA grants Medtronic approval to begin clinical trial of first pacemaker system designed for safe use in MRI machines: innovations in pacing technology advance access to non-invasive diagnostics Available at: http://wwwp.medtronic.com/Newsroom/NewsReleaseDetails.do?itemId=1201527823548&lang=en_US Accessed June 5, 2008
17 Sommer T, Naehle CP, Yang A, et al. Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 tesla in the presence of cardiac pacemakers in non-pacemaker-dependent patients: a prospective study with 115 examinations. Circulation. 2006;114:1285-1292.
18 Bassen H, Kainz W, Mendoza G, et al. MRI-induced heating of selected thin wire metallic implants—laboratory and computational studies—findings and new questions raised. Minim Invasive Ther Allied Technol. 2006;15:76-84.
19 Gimbel JR, Zarghami J, Machado C, et al. Safe scanning, but frequent artifacts mimicking bradycardia and tachycardia during magnetic resonance imaging (MRI) in patients with an implantable loop recorder (ILR). Ann Noninvasive Electrocardiol. 2005;10:404-408.
20 Soulen RL, Budinger TF, Higgins CB. Magnetic resonance imaging of prosthetic heart valves. Radiology. 1985;154:705-707.
21 Edwards MB, Ordidge RJ, Hand JW, et al. Assessment of magnetic field (4.7 T) induced forces on prosthetic heart valves and annuloplasty rings. J Magn Reson Imaging. 2005;22:311-317.
22 Condon B, Hadley DM. Potential MR hazard to patients with metallic heart valves: the Lenz effect. J Magn Reson Imaging. 2000;12:171-176.
23 Prince MR, Arnoldus C, Frisoli JK. Nephrotoxicity of high-dose gadolinium compared with iodinated contrast. J Magn Reson Imaging. 1996;6:162-166.
24 United States Food and Drug Administration, Center for Devices and Radiological Health Information on gadolinium-containing contrast agents Available at: http://www.fda.gov/Cder/Drug/infopage/gcca/default.htm Accessed June 10, 2008
25 American Society of Anesthesiologists Standards, guidelines and statements: guidelines for ambulatory anesthesia and surgery. 2004 Available at: http://www.asahq.org/publicationsAndServices/standards/04.pdf Accessed August 22, 2008
26 American Society of Anesthesiologists Standards, guidelines and statements: continuum of depth of sedation: definition of general anesthesia and levels of sedation/analgesia Available at: http://www.asahq.org/publicationsAndServices/standards/20.pdf Accessed June 5, 2008
27 American College of Radiology ACR practice guideline for adult sedation/analgesia 2005. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/iv/adult_sedation.aspx Accessed June 5, 2008
28 American Academy of PediatricsAmerican Academy of Pediatric DentistryCoté CJ, Wilson S, Work Group on Sedation. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Pediatrics. 2006;118:2587-2602.
29 Dial S, Silver P, Bock K, Sagy M. Pediatric sedation for procedures titrated to a desired degree of immobility results in unpredictable depth of sedation. Pediatr Emerg Care. 2001;17:414-420.
30 Motas D, McDermott NB, VanSickle T, et al. Depth of consciousness and deep sedation attained in children as administered by nonanaesthesiologists in a children’s hospital. Paediatr Anaesth. 2004;14:256-260.
31 American Academy of Pediatrics Committee on Drugs. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: addendum. Pediatrics. 2002;110:836-838.
32 Coté CJ, Notterman DA, Karl HW, et al. Adverse sedation events in pediatrics: a critical incident analysis of contributing factors. Pediatrics. 2000;105(4 Pt 1):805-814.
33 Brummett RE, Talbot JM, Charuhas P. Potential hearing loss resulting from MR imaging. Radiology. 1988;169:539-540.
34 Bandettini PA, Jesmanowicz A, Van Kylen J, et al. Functional MRI of brain activation induced by scanner acoustic noise. Magn Reson Med. 1998;39:410-416.
35 Ravicz ME, Melcher JR, Kiang NY. Acoustic noise during functional magnetic resonance imaging. J Acoust Soc Am. 2000;108:1683-1696.
36 Nagel E, Klein C, Paetsch I, et al. Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation. 2003;108:432-437.
37 Gudmundsson P, Winter R, Kitlinski M, et al. Real-time perfusion adenosine stress echocardiography versus myocardial perfusion adenosine scintigraphy for the detection of myocardial ischemia in patients with stable coronary artery disease. Clin Physiol Funct Imaging. 2006;25:32-38.
38 Picano E, Sicari R, Varga A. Dipyridamole stress echocardiography. Cardiol Clin. 1999;17:481-499.
39 Nagel E, Lehmkuhl HB, Bocksch W, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation. 1999;99:763-770.

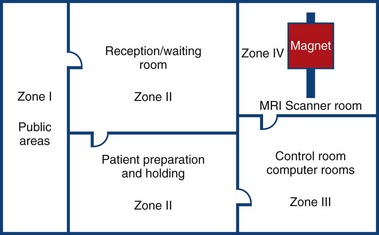
 FIGURE 19-1
FIGURE 19-1