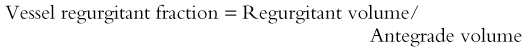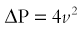Magnetic Resonance Imaging for Congenital Heart Disease
Pulse Sequences
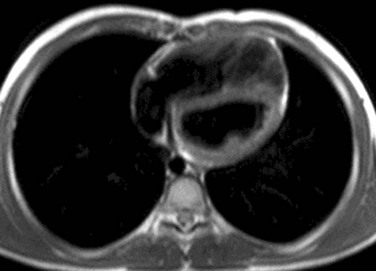
Spin echo and gradient echo sequences are used for cardiac evaluation. Spin echo, or “black blood,” imaging gives excellent contrast resolution between the endocardium or vessel wall and the blood-filled lumen. Double inversion recovery imaging adds additional nonselective and selective 180-degree pulses to further null the blood signal. The double inversion recovery pulse sequence is the current backbone of spin echo morphologic cardiac imaging in teenagers and adults, but it requires that the patient hold his or her breath (Fig. 67-1). More traditional T1-weighted sequences are used in uncooperative or sedated children. T1-weighted sequences are longer but do not require that patients hold their breath.
Blood has a high signal in gradient echo sequences and is the basis for “white blood” or “bright blood” imaging. Balanced SSFP (b-SSFP) is the state-of-the-art gradient echo sequence for bright blood imaging. b-SSFP sequences are faster than earlier gradient echo sequences and have better contrast between myocardium and blood. As with double inversion recovery imaging, b-SSFP is ideally performed with the patient holding his or her breath. Respiratory motion artifacts are minimized in the sedated or uncooperative patient by increasing the number of excitations. This technique is the preferred sequence for evaluating cardiac motion and quantifying cardiac function. Stenosis and turbulent flow appear as a black jet in the white blood on gradient imaging (Fig. 67-2).
Functional Evaluation
MRI is the gold standard for cardiac function assessment and is markedly superior to echocardiography in assessing right ventricular function. A stack of short-axis cine images through the ventricles in end-systole and end-diastole is used to measure ventricular volumes.1 The product of the endocardial cross-section tracing and the section thickness is the section volume. The section volumes are summated to determine end-diastolic and end-systolic volumes for the right and left ventricles. The same images are used to measure the left ventricle mass by performing epicardial and endocardial tracings. The difference in the volumes circumscribed by the epicardium and endocardium is the left ventricle myocardium volume, from which mass is determined.
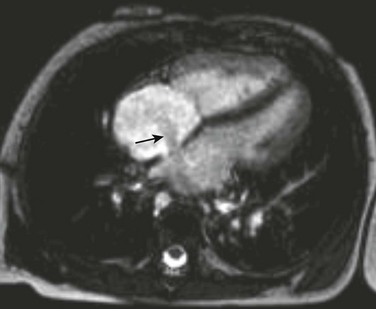
The stroke volumes of the right and left ventricles are the same in the structurally normal heart. Differences in stroke volumes are caused by shunts and valve regurgitation. Using MR, both shunt size and regurgitant fraction can be calculated from the stroke volumes. Shunt size, or the pulmonary to systemic flow ratio (Qp : Qs), is determined by dividing the larger stroke volume by the smaller. The valvular regurgitant fraction is determined by dividing the regurgitant volume, which is the difference of ventricle stroke volumes, by the larger ventricle stroke volume (Fig. 67-3). The regurgitant volume may result from atrioventricular valve or semilunar valve or a combination of both.
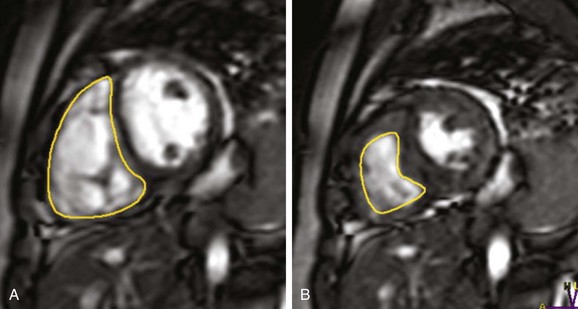
Figure 67-3 Pulmonary regurgitation.
Endocardial contours are drawn on a short-axis steady-state free precession image of the right ventricle from base to apex in end-diastole (A) and end-systole (B) in a patient with a history of pulmonary stenosis after a pulmonary valvectomy and now with pulmonary regurgitation. The pulmonary regurgitant fraction was calculated to be 37% by right and left ventricular stroke volume differences.
where volume1 is the larger of the two ventricular stroke volumes.
Quantitative phase-contrast imaging uses gradient echo sequences to measure phase shift. Flow quantification using a plane of imaging perpendicular to the vessel or valve of interest allows quantification of blood flow and velocity. Flow quantification gives both magnitude and directional information (Fig. 67-4). Shunt volumes and regurgitant fractions are quantified. Regurgitation is quantified by:
The pressure gradient across a stenosis is calculated using the modified Bernoulli equation:
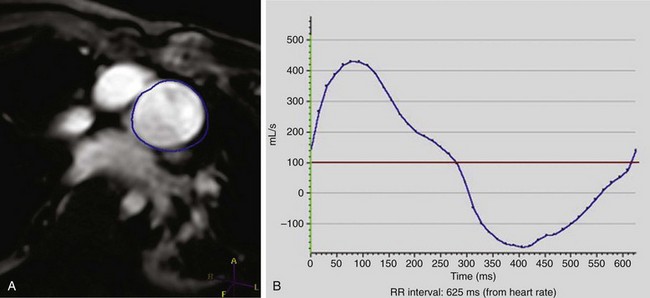
Figure 67-4 Tetralogy of Fallot.
Phase contrast velocity mapping of the pulmonary artery in a 15-year-old with a history of tetralogy of Fallot after patch augmentation of the subpulmonary outflow tract obstruction. The blue region of interest is around the pulmonary outflow tract (A). The flow curve demonstrates moderate pulmonary regurgitation of 34% (B).
where ΔP is the peak instantaneous pressure gradient across the stenosis and v is the velocity across the stenosis measured by flow analysis.
Magnetic Resonance Angiography
Contrast-enhanced magnetic resonance angiography (CE-MRA) is performed after injection of a gadolinium chelate bolus. The technique relies on T1 shortening of blood and requires rapid imaging. A time-of-flight spoiled gradient sequence with a short echo time is used. Conventionally, CE-MRA is not an electrocardiographic-gated technique but ideally is performed as the breath is held. Recently the free-breathing time-resolved CE-MRA technique has proved to be feasible in children with a higher heart rate with use of a keyhole approach with parallel imaging.1
CE-MRA creates a volume data set with high contrast resolution (Fig. 67-5). Three-dimensional volume renderings and multiplanar reformations are created in any plane or view. Overlapping structures are eliminated. Simultaneous imaging of multiple vessels with a single injection is another advantage.
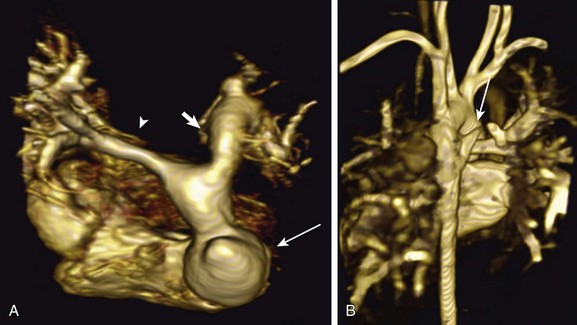
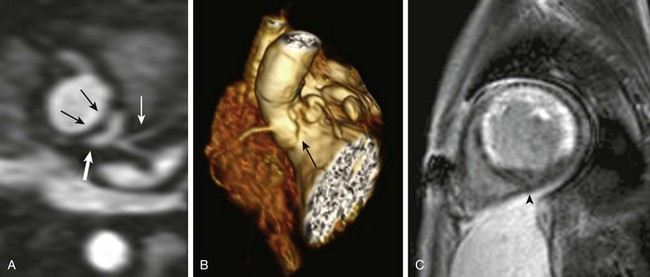
Figure 67-5 Tetralogy of Fallot after patch augmentation for right ventricular outflow tract obstruction.
A three-dimensional volume-rendered image of the pulmonary artery and branches (A) from a superior to inferior view. A pseudoaneurysm of the right ventricular outflow tract is seen (arrow). Hypoplasia of the right branch pulmonary artery is present (arrowhead), along with ectasia of the left branch pulmonary artery (short bold arrow). A posterior image of the chest (B) in the same patient shows a major aortopulmonary collateral artery to the upper lobe segment of the right lung (arrow).
Time-resolved CE-MRA has been performed successfully when acquiring angiographic data in various body parts in the pediatric population.2 In contrast to conventional CE-MRA, time-resolved MRA achieves greater temporal resolution.3,4 By oversampling the center of k-space compared with the periphery, portions of k-space are shared to create higher temporal resolution than with spiral k-space ordering. Before contrast material is injected, a mask volume is obtained. The contrast material is then injected and the scan is initiated. The precontrast mask is subtracted from each subsequent volume to obtain a series of maximum intensity projection images obtained over time (Video 67-1).
A respiratory- and cardiac-gated, fat-suppressed, three-dimensional MRA technique (b-SSFP sequence) has been developed for whole-heart imaging that provides high resolution for the evaluation of intracardiac anatomy and coronary arteries (Fig. 67-6 and Video 67-2).5,6 This technique can be used either without or with contrast and is very is helpful in evaluating patients with severe renal dysfunction who cannot undergo testing with gadolinium-chelate contrast agents because of the risk of developing nephrogenic systemic fibrosis.7
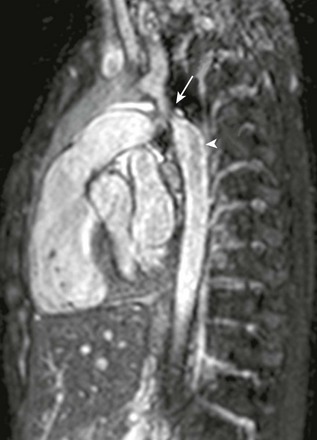
Figure 67-6 Aortic coarctation.
A 3-year-old patient with a recently diagnosed coarctation of the aorta had a preoperative magnetic resonance imaging scan. Per protocol, a nonenhanced three-dimensional magnetic resonance angiogram of the chest was performed. The arrow points to a focal area of high-grade stenosis in the postductal aorta, whereas the arrowhead shows the turbulent flow distal to the coarctation consistent with a hemodynamically significant obstruction (see Video 67-2).
Perfusion and Delayed Enhancement
First-pass contrast-enhanced perfusion images, with or without vasodilators, provide assessment of myocardial perfusion reserve. Perfusion imaging has limited application in children. Delayed enhanced images provide information about abnormal myocardium in both ischemic and nonischemic cardiomyopathies and can be used frequently in pediatric protocols for cardiac MR.8 Stress imaging to detect decreased coronary flow is assessed by cine spoiled gradient echo sequences or SSFP sequences. The ventricular function at rest is compared with the regional function during pharmacologic stress. Infarction is always seen in the subendocardium, and delayed hyperenhancement is seen in ischemia (Fig. 67-7 and Video 67-3). Myocardial perfusion is acquired by rapid imaging during multiple cardiac cycles, which allows visualization of a contrast bolus through the heart. Perfusion is delayed with the decreased appearance of contrast in a myocardial territory distributed by a stenosed artery. Delayed enhancement is assessed 10 minutes after the injection of gadolinium. Depending on the extent of delayed enhancement and specific patterns of distribution, a distinction can be made between different nonischemic causes of cardiomyopathies. For example, hypertrophic cardiomyopathy shows predominantly patchy rather than confluent late enhancement and is localized predominantly to the interventricular septum. Nonischemic dilated cardiomyopathy occurs as a result of several causes and shows a variable pattern of late enhancement. This late enhancement is shown to have two distinct patterns: patchy with mid wall late enhancement or transmural or subendocardial late enhancement that cannot be distinguished from the ischemic heart disease. Arrhythmogenic right ventricular cardiomyopathy may show late enhancement in regions of fibrofatty infiltration of the right ventricular wall (see Chapter 80).9,10
Protocols
Because MRI examinations contain a large number of images, an organized segmental approach is necessary for image interpretation. The morphologic assessment includes determining atrial, bronchial, and abdominal situs. The cardiac situs and segments, including the atrioventricular and ventriculoarterial connections, are evaluated (see Chapter 63). The number and connections of the pulmonary veins are noted. Septal defects, pulmonary artery anomalies, aortic anomalies, shunts, and valvular and vascular stenoses or regurgitations are assessed.
Extracardiac abnormalities of the aorta and pulmonary artery are evaluated with a combination of spin echo imaging and CE-MRA. Like other forms of angiography, CE-MRA provides intraluminal structural information, or lumenograms. A spin echo imaging sequence complements CE-MRA by allowing visualization of the vessel wall. Aortic and pulmonary valve stenosis can result in poststenotic dilation that is well imaged by CE-MRA. Progressive dilation of the ascending aorta associated with Marfan syndrome or aortic valve stenosis can be difficult to measure by echocardiography, but it is easily imaged with CE-MRA. Vascular rings are well delineated by CE-MRA but require additional spin echo imaging to identify the degree of associated airway compression. Sagittal spin echo imaging of the trachea may be helpful. Coarctation of the aorta and associated collateral arteries are identified by CE-MRA, but flow analysis is helpful in determining the associated pressure gradient. Collateral flow in the descending aorta can be quantified by measuring flow in the aorta proximal to the coarctation and at the diaphragm.11,12
Postoperative Imaging
MRI of the heart is performed more commonly in children after surgery than before surgery for preoperative diagnosis. Palliative procedures such as Blalock-Taussig shunts, central shunts, cavopulmonary shunts, and Fontan procedures for treatment of complex congenital heart disease with single ventricle physiology are well evaluated by a combination of SSFP cardiac imaging and a CE-MRA of the great vessels. Stenoses, aneurysms, and collateral flow can be evaluated, but care must be taken in planning CE-MRA because streaming is associated with possible imaging pitfalls.13 The shunts can be studied for patency or stenosis by CE-MRA. Shunt flow is quantified by flow analysis. Flow patterns in the superior vena cava and inferior vena cava can be analyzed to determine the flow (e.g., whether the target of continuous flow is present versus reduced or even physically reversed through the circuit) of Fontan conduits.
Cardiac evaluation in the child with right ventricle dilation resulting from pulmonary regurgitation is among the most common indications for cardiac MRI following tetralogy of Fallot repair or pulmonary stenosis that has been treated by disruption of the pulmonary valve (see Chapter 76). Cardiac MRI can provide essential quantitative data and prognostic information to cardiothoracic surgeons that echocardiography and cardiac computed tomography or catheterization cannot provide.14,15
Chung, T. Magnetic resonance angiography of the body in pediatric patients: experience with a contrast-enhanced time-resolved technique. Pediatr Radiol. 2005;35:3–10.
Krishnamurthy, R, Slesnick, T, Taylor, M, et al. Free breathing high temporal resolution time resolved contrast enhanced MRA (4D MRA) at high heart rates using keyhole SENSE CENTRA in congenital heart disease. J Cardiovasc Magn Reson. 2011;i12(suppl 1):O31.
Lim, R, Srichai, M, Lee, V. Non-ischemic causes of delayed myocardial hyperenhancement on MRI. AJR Am J Roentgenol. 2007;188:1675–1681.
Varaprasathan, GA, Araoz, PA, Higgins, CB, et al. Quantification of flow dynamics in congenital heart disease: applications of velocity-encoded cine MR imaging. Radiographics. 2002;22(4):895–905.
Vasanawala, SS, Chan, FP, Newman, B, et al. Combined respiratory and cardiac triggering improves blood pool contrast-enhanced pediatric cardiovascular MRI. Pediatr Radiol. 2011;41(12):1536–1544.
References
1. Vasanawala, SS, Chan, FP, Newman, B, et al. Combined respiratory and cardiac triggering improves blood pool contrast-enhanced pediatric cardiovascular MRI. Pediatr Radiol. 2011;41(12):1536–1544.
2. Chung, T. Magnetic resonance angiography of the body in pediatric patients: experience with a contrast-enhanced time-resolved technique. Pediatr Radiol. 2005;35:3–10.
3. Krishnamurthy, R, Slesnick, T, Taylor, M, et al. Free breathing high temporal resolution time resolved contrast enhanced MRA (4D MRA) at high heart rates using keyhole SENSE CENTRA in congenital heart disease. J Cardiovasc Magn Reson. 2011;12(suppl 1):O31.
4. Giesel, F, Runge, V, Kirchin, M, et al. Three-dimensional multiphase time-resolved low-dose contrast-enhanced magnetic resonance angiography using TWIST on a 32-channel coil at 3T: a quantitative and qualitative comparison of a conventional gadolinium chelate with a high relaxivity agent. J Comput Assist Tomogr. 2010;34(5):678–683.
5. Miyazaki, M, Sugiura, S, Tateishi, F, et al. Non-contrast-enhanced MR angiography using 3D ECG-synchronized half-Fourier fast spin echo. J Magn Reson Imaging. 2000;12(5):776–783.
6. Kato, S, Kitagawa, K, Ishida, N, et al. Assessment of coronary artery disease using magnetic resonance coronary angiography: a national multicenter trial. J Am Coll Cardiol. 2010;56(12):983–991.
7. Weinreb, JC, Abu-Alfa, AK. Gadolinium-based contrast agents and nephrogenic systemic fibrosis: why did it happen and what have we learned? J Magn Reson Imaging. 2009;30(6):1236–1239.
8. Lim, R, Srichai, M, Lee, V. Non-ischemic causes of delayed myocardial hyperenhancement on MRI. AJR Am J Roentgenol. 2007;188:1675–1681.
9. Gottlieb, I, Macedo, R, Bluemke, D, et al. Magnetic resonance imaging in the evaluation of non-ischemic cardiomyopathies: current applications and future perspectives. Heart Fail Rev. 2006;11:313–323.
10. Cummings, K, Bhalla, S, Javidan, C, et al. A pattern-based approach to assessment of delayed enhancement in no-ischemic cardiomyopathy at MR imaging. Radiographics. 2009;29:89–103.
11. Steffens, JC, Bourne, MW, Sakuma, H, et al. Quantification of collateral blood flow in coarctation of the aorta by velocity encoded cine magnetic resonance imaging. Circulation. 1994;90(2):937–943.
12. Varaprasathan, GA, Araoz, PA, Higgins, CB, et al. Quantification of flow dynamics in congenital heart disease: applications of velocity-encoded cine MR imaging. Radiographics. 2002;22(4):895–905. [discussion 905-906].
13. Miki, H. Pitfalls in diagnosing cerebrovascular diseases using magnetic resonance angiography. Brain Nerve. 2010;62(5):477–488.
14. Grothoff, M, Hoffmann, J, Lehmkuhl, L, et al. Time course of right ventricular functional parameters after surgical correction of tetralogy of Fallot determined by cardiac magnetic resonance. Clin Res Cardiol. 2011;100(4):343–350.
15. Oosterhof, T, van Straten, A, Vliegen, HW, et al. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116(5):545–551.