Chapter 264 Lymphocytic Choriomeningitis Virus
Laboratory Findings
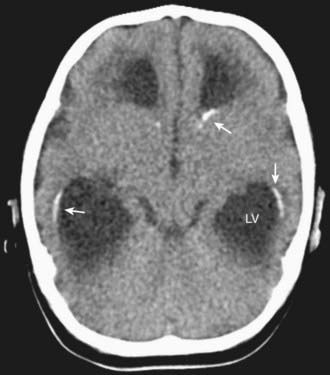
In congenital LCMV infection, laboratory findings in the newborn depend on whether active infection is still present. If the infant still harbors the infection, then examination of the CSF may reveal a lymphocytic pleocytosis. Unlike many other congenital infections, LCMV does not typically induce elevations in liver enzymes, thrombocytopenia, or anemia. In many cases, the most reliably abnormal findings are on the head CT scan, which typically reveals a combination of microencephaly, hydrocephalus, and periventricular calcifications (Fig. 264-1).
Bonthius DJ, Karacay B. Meningitis and encephalitis in children: an update. Neurol Clin. 2002;20:1013-1038.
Bonthius DJ, Nichols B, Harb H, et al. Lymphocytic choriomeningitis virus infection in brain: critical role of host age. Ann Neurol. 2007;62:356-374.
Bonthius DJ, Perlman S. Congenital viral infections of the brain: lessons learned from lymphocytic choriomeningitis virus in the neonatal rat. PLoS Pathogens. 2007;3:e149.
Bonthius DJ, Wright R, Tseng B, et al. Congenital lymphocytic choriomeningitis virus infection: spectrum of disease. Ann Neurol. 2007;62:347-355.
Centers for Disease Control and Prevention. Lymphocytic choriomeningitis virus transmitted through solid organ transplantation—Massachusetts, 2008. MMWR Morb Mortal Wkly Rep. 2008;57:799-800.
Centers for Disease Control and Prevention. Update: interim guidance for minimizing risk for human lymphocytic choriomeningitis virus infection associated with pet rodents. MMWR Morb Mortal Wkly Rep. 2005;54:799-801.
Childs J, Glass G, Korch G, et al. Lymphocytic choriomeningitis virus infection and house mouse (Mus musculus) distribution in urban Baltimore. Am J Trop Med. 1992;47:27-34.
Farmer M, Sebire G. Genetic mimics of congenital lymphocytic choriomeningitis virus encephalitis. Ann Neurol. 2008;64:353-355.
Fischer SA, Graham MB, Kuehnert MJ, et al. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N Engl J Med. 2006;34:2235-2249.
Mets MB, Barton LL, Khan AS, et al. Lymphocytic choriomeningitis virus: an underdiagnosed cause of congenital chorioretinitis. Am J Ophthalmol. 2000;130:209-215.
Wright R, Johnson D, Neumann M, et al. Congenital lymphocytic choriomeningitis virus syndrome: a disease that mimics congenital toxoplasmosis or cytomegalovirus infection. Pediatrics. 1997;100:E9.