Chapter 68
Lymphedema
Surgical Treatment
Ruediger G.H. Baumeister
The lymphatic system is the least understood part of the vascular system. Lymphatic malformations such as chylous disorders, cystic hygromas, and lymphocysts are rare; acquired disorders such as lymphoceles and chylous effusions are also uncommon. However, local interruption and obstruction of the lymphatic vessels occur frequently from either congenital or acquired causes (see Chapter 66). In developed countries, most acquired lymphatic obstructions are iatrogenic, caused by medical procedures. In developing countries, the most frequent cause of lymphatic obstruction resulting in chronic lymphedema is filariasis.
Vessel interruption is a common pathologic process in the vascular system, and surgical repair that involves bypassing an obstruction is a well-established method in vascular surgery. These principles of surgical treatment have also been applied successfully in the lymphatic system and are the topic of this chapter.
Basis of Surgical Treatment
The development of lymphedema can be described as an imbalance between the lymphatic load—the amount of lymph that has to be cleared from a body part within a given time—and the lymphatic transport capacity—the amount of lymph that can be transported out of a body part within a given time, which is dependent on the number and function of lymphatic vessels and nodes (see Chapter 13).1 Reduced lymphatic flow due to obstruction leads to secondary tissue changes, a process that is not yet fully understood. Lymphatic outflow disorders are manifested mainly with advanced secondary changes and chronic lymphedema, a condition that can be difficult to treat unless the underlying problem of reduced lymphatic outflow is resolved. Historically, secondary tissue changes leading to excess fibrous and adipose tissue were treated solely by excisional procedures, without correcting the underlying cause. However, modern surgical concepts have been developed to attempt to correct the underlying pathophysiologic mechanism to the extent possible.
Enhancing the number of functional lymphatic vessels through surgical reconstruction is the most natural way to address the problem and is especially beneficial when the reconstruction is performed before secondary tissue changes occur. Thus, it is not surprising that the surgical reconstruction of lymphatic vessels in selected patients has yielded excellent results. One obstacle is the small dimension of lymphatic vessels, which can be overcome only with advanced microsurgical approaches. Once secondary tissue changes have evolved, additional efforts may be required. One of the less invasive methods in current use is liposuction.
Because conservative therapy consisting of limb elevation, compression garments, complex decongestive therapy, and compression pump therapy should be the first step (see Chapter 67), the question arises whether and at what time surgery is indicated. If the only purpose of surgery is resection, it is wise to reserve it as a last option. If, however, surgical reconstruction is possible, this procedure should be considered and offered to the patient early in the course of lymphedema.
Historical Perspective
Excisional Operations
Surgical Excision
The most radical excisional approach is the classic operation first described by Charles in 1912.2 It involves complete and circumferential resection of the skin, subcutaneous tissue, and deep fascia, followed by split-skin grafting. However, this procedure is associated with significant complications, and follow-up studies revealed hyperkeratosis, papillomatosis, and ulcerations in the grafted areas.3,4 Modifications of this technique, using the resected skin for grafting and performing the surgery in two stages,5–11 reduced the surgical trauma and the rate of complications.
The techniques of resecting the fascia and subcutaneous tissue and creating skin flaps were based on the proposals of Auchincloss, Fontaine, Homans, and Servelle.12–15 In contrast to the Charles procedure, the flap procedure yields a better cosmetic result, but it requires sufficient healthy skin in the affected extremities. Limited resection of fascia, subcutaneous tissue, and skin was suggested by Sistrunk.16,17
Liposuction
A less invasive way to reduce the amount of subcutaneous tissue is liposuction. It was first described by Illouz as a method for treating lymphedema.18 More recently, Brorson and Svensson demonstrated lasting volume reduction if elastic compression garments are worn after the surgical procedure; this can result in an extremity that is even slimmer than the healthy limb.19
Functional Procedures
Early Techniques
The first attempts to divert lymph from the subcutaneous to the muscular compartment through partial or complete resection of the fascia were described by Lanz and Kondoleon.20–22 Redirection of lymph from the superficial to the deep compartment is also a component of the Thompson method. Thompson resected the fascia along with parts of the subcutaneous tissue, created a flap in two stages, and de-epithelialized the rim of the flap to allow the outflow of lymph. Subsequently, he buried the flap near the deep vessels to facilitate the creation of spontaneous lymphatic anastomoses.23–30
Facilitating spontaneous lympholymphatic anastomoses within the subcutaneous tissue has been the aim of several methods, and a variety of flaps have been created for transposition into the edematous area.31–35 Goldsmith and coworkers proposed the creation of a pedicle from the greater omentum,36,37 and Kinmonth and associates proposed the construction of an enteromesenteric bridge using a pedicle of ileum (denuded of its mucosa) and its mesentery (rich in lymphatics) to drain lymph out of the edematous leg tissue.38
The use of veins for the reconstruction of an interrupted lymphatic system was investigated by Holle and Mandl experimentally and performed in two patients clinically.39,40 Campisi and colleagues reported on a larger series using this technique.41
Modern Techniques
Currently, the most common way to drain lymph from edematous tissue is the construction of connections between the lymphatic (Fig. 68-1) and venous systems in the periphery. The first reports on lymphonodular and lymphovenous anastomoses were provided by Laine and Howard,42 Nielubowicz and Olszewski,43 Rivero and coworkers,44 and Allen and Taylor.45 Degni designed a special needle to facilitate the insertion of lymphatic vessels into veins.46,47 Further improvements were described by O’Brien and colleagues using microsurgical techniques.48,49 In some patients, excisional methods were combined with lymphovenous shunting. A large cohort of patients successfully treated with microsurgical lymphovenous anastomosis was reported by Campisi and associates.50
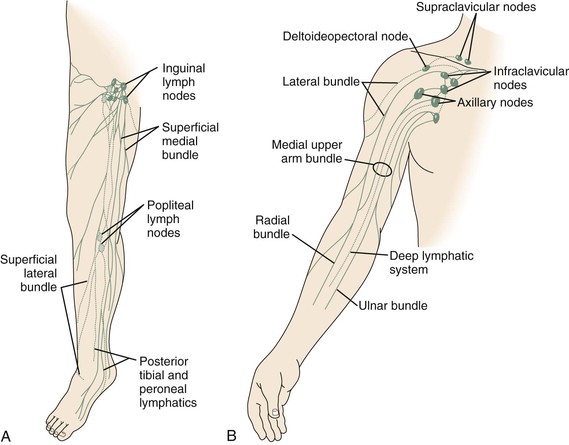
Figure 68-1 A, Superficial lymphatic system of the lower extremity. B, Lymphatic system of the upper extremity. (Courtesy Mayo Foundation.)
However, experimental studies revealed problems with thrombotic occlusion at the site of anastomosis, with a patency of 20% after 5 months of follow-up.51–53 Specific preparations that ensured an undisturbed connection to the venous valve led to an improved patency rate of 44% after 6 months.54,55 Gloviczki and colleagues reported on results of experimental microsurgical end-to-end anastomoses between normal femoral lymph vessels and a tributary of the femoral vein in dogs and noted a 50% patency rate up to 8 months after the operation.56
Direct reconstruction of the lymphatic system became a possibility only after the development of microsurgery. Before that time, it was commonly thought to be impossible to anastomose lymphatic vessels because of their extremely small diameters. Hence, Danese approximated lymphatic vessels as close to each other as possible and waited for spontaneous regeneration. He was able to demonstrate transport of contrast medium (through the lymphatics) with this technique.57 In a patient with lymphedema of the arm, he mobilized two lymphatic channels proximally and distally, approximated them in the axilla, and achieved a reduction in edema.58 Subsequent approaches included interpositioning veins between lymphatic vessels,39–41 implanting microsurgical lymph node grafts,59,60 and implanting free flaps with lymphatic vessels.61,62
The question of optimal reconstruction material has been the subject of two experimental studies. In a series of 14 rats, 100% of the autologous lymphatic grafts were patent (observations made between days 7 and 119 postoperatively), whereas allogeneic lymphatic grafts were patent only until day 21 after transplantation. When lymphatics were replaced by small veins (n = 10), a patency of 70% was observed. Expanded polytetrafluoroethylene implants (n = 10) used as lymphovascular conduits were already thrombosed by day 7.63 In a comparison of lymphatic and venous interpositional autografts in 71 dogs, all 26 lymphatic autografts remained patent up to the end of the observation period at 24 weeks. Of 30 venous interpositional autografts, only 4 were patent after 1 week. None of the lympholymphatic anastomoses with silicone tubing showed patency at any time.64
Acland and Smith were the first to attempt to anastomose lymphatic vessels.65–67 The first successful therapeutic lympholymphatic graft was performed in 1980 by Baumeister in a patient with unilateral lymphedema of the lower extremity.68–71 This followed extensive animal experiments on thoracic duct transplants in rats72 and the treatment of experimental lymphedema in dogs using lymphatic autografts.67
Preoperative Planning
Visualizing Lymphatic Vessels
For vascular surgeons, direct visualization of a single lymphatic vessel would be the ideal technique for preoperative evaluation and planning.
Lymphography
Direct contrast lymphography, using oily contrast medium and invasive administration through dissected lymphatic vessels, was introduced by Kinmonth and greatly advanced our knowledge of the lymphatic system.73 However, owing to the invasiveness of the procedure (and injury to the lymphatic vessels and lymph nodes), it was found to worsen lymphedema and is rarely used today. Indirect contrast lymphography, using a water-soluble contrast medium injected subepidermally, cannot visualize lymphatic vessels as well as direct lymphography and gained only limited use.74 In primary lymphedema, this technique might be used to evaluate whether there are any lymphatic vessels present in the periphery and, if so, whether they might be able to transport lymph toward a proximally performed anastomosis.
Magnetic Resonance Imaging
Attempts to visualize lymphatic vessels with magnetic resonance imaging (MRI) and subdermally administered contrast medium have been promising. This technique may be useful in the future for preprocedure planning and postoperative assessment of the patency of lymphatic reconstructions.75 For the detection of vascular lymphatic malformations, MRI is extremely valuable both with and without contrast medium.
Lymphoscintigraphy
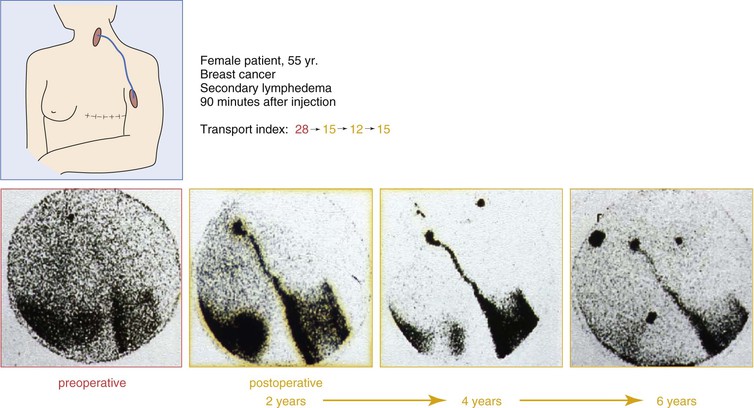
The most important test to evaluate chronic lymphedema and to plan surgical treatment is lymphoscintigraphy. It can be repeated and used for treatment planning as well as for follow-up. It not only evaluates function but also visualizes routes of lymphatic transport. The lymphatic transport index summarizes the findings derived from lymphoscintigraphic studies and allows a semiquantitative evaluation of lymphatic flow without the need for standardized physical movements by the patient. The transport index ranges from 0 for an optimal lymphatic outflow to 45 for no visible transport; normal values are less than 10. It also provides a good basis for follow-up studies and can show lymphatic transport along the route of lymphatic grafts.76,77 In measuring lymph transport at regions of interest, it is critical to standardize the dose of radiopharmaceutical and the physical activity of the patient during the procedure.78
Dye Injection
Another diagnostic tool that can be used to identify lymphatic channels is the subepidermal injection of a vital dye (patent blue dye in Europe; isosulfan blue [Lymphazurin] dye in the United States). Normally, lymphatic transport is visualized in the superficial lymphatic collecting system. In pathologic situations, dermal backflow leads to the pooling of contrast medium within the skin, resulting in a cloudlike appearance. Because allergic reactions have been reported, staining of lymphatic vessels with patent blue or isosulfan blue dye is generally performed during surgery under general anesthesia.
Lymphatic Donor Site Assessment
For lymphatic grafting, it is critical to choose and carefully evaluate the proper harvest site for lymphatic vessels to avoid the development of edema secondary to the procedure. Thus, the donor lower extremity must be evaluated by lymphoscintigraphy before harvesting. During the harvest, the narrowing lymphatic system at the medial aspect of the knee and the groin must be left untouched, and all stained lymphatic vessels other than those used as grafts should be left in place. A study including 80 patients with arm edema showed that when this method was used, the harvest site and the untouched leg were not different in size.71
Patient Risk Assessment
Because this type of surgery is performed in the subcutaneous tissue, the surgical risk is generally low, and the procedure is well tolerated. Peripheral lymphovenous shunting is often performed under local anesthesia and is unproblematic as long as the patient tolerates local anesthetics. Because the application of patent blue or isosulfan blue dye can lead to allergic reactions, it should be used only under general anesthesia. Excisional methods typically involve more surgical trauma and the possibility of greater blood loss. Therefore, it is sometimes advisable to perform large excisional operations in two stages. For surgical intervention within the abdomen and thorax, the usual preoperative risk assessment must be done (see Chapter 31).
Surgery
Autologous Lymphatic Grafting
Indications
Lymphatic vessel grafts can be attempted for the treatment of secondary lymphedema caused by localized obstruction or interruption of lymph vessels and lymph nodes, such as the lymphedema of the arm that develops after breast cancer surgery because of the excision of axillary lymph nodes and possible radiation treatment (see Fig. 68-1B). Patients with unilateral lower limb lymphedema due to the excision of inguinal or pelvic lymph nodes or pelvic irradiation for malignant disease are also potential candidates for such procedures. Transplantation of lymphatic vessels can be attempted in patients with primary lymphedema if it is caused by localized lymphatic obstruction or atresia, such as unilateral atresia of the pelvic or inguinal lymphatic system.
Surgical intervention should be considered only after a trial of conservative therapy. Conservative treatment should be continued for at least 6 months because spontaneous regression has been reported. However, if conservative therapy is unsuccessful during this time frame, reconstruction should be attempted soon to avoid secondary tissue changes. Unfortunately, treatment is often delayed. In my experience, the mean time between the onset of edema and the patient’s presentation for surgery is more than 7 years.
Preoperative Evaluation
Bilateral isotope lymphoscintigraphy is performed in every patient. Both lower extremities are tested to determine a safe harvest site of lymph vessels for the transplant. Only limbs with normal lymphatic transport capacity are used as donors of lymphatic grafts.
In primary lymphedema, indirect lymphography or MRI lymphography is performed in an attempt to visualize lymphatic vessels distal to the occlusion that are suitable for anastomosis. In all cases of lymphedema, malignant disease must be excluded before surgery.
Surgical Technique
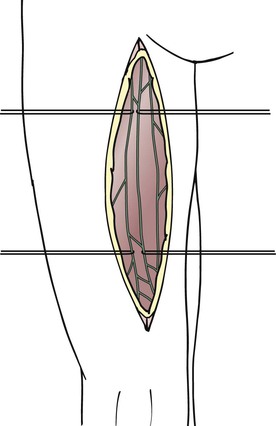
The lymphatic grafts are harvested from the patient’s thigh (Fig. 68-2). The ventromedial lymphatic bundle contains up to 16 lymphatic vessels.79 About one to four lymphatic collectors are dissected in the medial area of the thigh, and great care is taken to spare the lymphatic system where it narrows at the level of the knee and groin. Additional peripheral branches often exist, and these can be dissected as well to create a greater number of peripheral anastomoses.
For free transfer of the graft, a ligature is placed on the lymphatic vessel selected as a graft beneath the inguinal lymph nodes with 6-0 polyglactin 910. One thread is left long to facilitate handling of the graft thereafter. Proximal to the ligature, the lymphatic vessel is transected. At the distal end of the proposed graft, the lymphatic vessel is transected proximal to the level of the knee. The lymphatic vessel beneath the transection site is occluded either by placing a suture or by using coagulation to avoid lymph leakage.
If a transposition procedure is performed, the grafts are transected distally after double ligation and tunneled subcutaneously superior to the pubic symphysis to the contralateral side, where end-to-end lympholymphatic anastomoses are performed. In free transfers as well as in transposition procedures, the graft has to be pulled from one incision to the other (e.g., from the upper arm to the neck or between inguinal regions). To avoid any friction during tunneling, tubes (suction catheters) are placed between the two incisions according to the proposed route of the grafts. Thereafter, the grafts themselves can be pulled through the tubes without tension. After removal of the tubes, the grafts remain undisturbed in place within the subcutaneous tissue.
Arm and Neck.
For arm edema as a result of interventions in the axilla, the grafts are interposed between ascending lymphatic vessels in the upper arm and lymphatic vessels or lymph nodes in the neck (Fig. 68-3). In the upper arm, lymphatic vessels are usually epifascial (if not, they may be located subfascially in proximity to the vessels) and are best sought from an oblique incision made medially and superior to the route of the brachial vessels. The search is performed under the microscope with a medium (3× to 10×) magnification. In the early stages of lymphedema, the lymphatic vessels have a gray, shiny appearance, and the lumen can be seen clearly after transection. As the lymphatic vessels undergo fibrosis in later stages of lymphedema, it becomes more difficult to discriminate between small nerves and fibrous cords. In this case, the final decision about the potential use for grafting can be made only after transection of the structure.
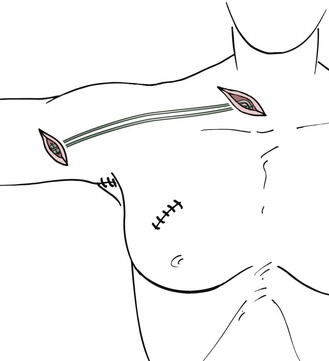
Figure 68-3 Lymphatic grafting in arm edema.
The walls of lymphatic vessels are thinner in the neck than in the arms and legs. Injection of a vital dye in the hair-bearing parietal area above the ear facilitates the search for appropriate vessels. If the lymphatic vessels stain appropriately, recognition is easy. However, suturing in this area is often difficult because of the collapsing, thin-walled vessels. If this is the case, it is also possible to suture the grafts to lymph nodes. A superficial incision is made in the capsule of the node, and the graft is connected with approximately three single interrupted sutures.
To position the grafts between the sites of anastomosis, tubing from a drain is placed in the subcutaneous tissue between the incisions in the upper arm and neck. Subsequently, the grafts are pulled through the wet drain gently and without friction. After removal of the tube, the grafts remain in the subcutaneous tissue free of tension.
Leg.
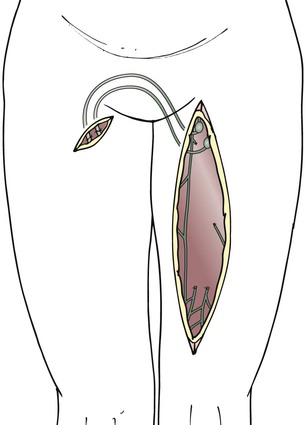
For unilateral edema of the lower extremity, the grafts remain attached to the inguinal lymph nodes, and the distal ends of the grafts are transposed to the opposite thigh with the help of tubing from a drain, which is temporarily interposed between the two incisions at the thigh (Figs. 68-4 to 68-6).
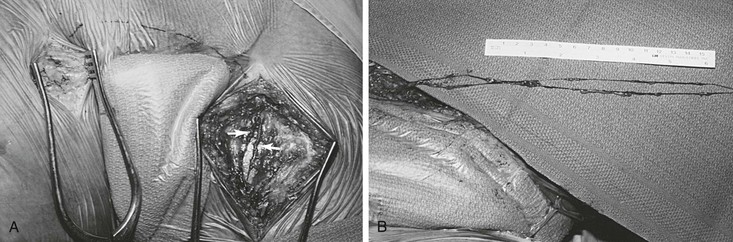
Figure 68-5 A, Exposure of lymph vessels for suprapubic transposition. Note that two major lymph vessels of the left thigh will be used for grafting (arrows). B, Two lymphatic grafts divided at the distal thigh are prepared for grafting. (Courtesy Mayo Foundation.)
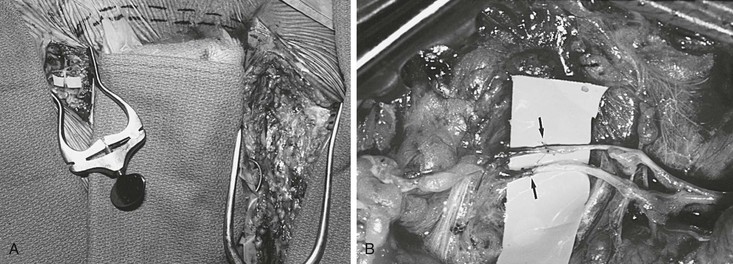
Figure 68-6 A, Completed suprapubic lymph graft with two lympholymphatic anastomoses in the right groin. Dashed line indicates the position of the suprapubic lymphatic grafts. B, Magnified photograph of two end-to-end lympholymphatic anastomoses (arrows) performed with 11-0 interrupted monofilament sutures. (Courtesy Mayo Foundation.)
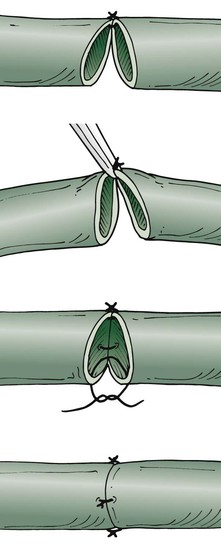
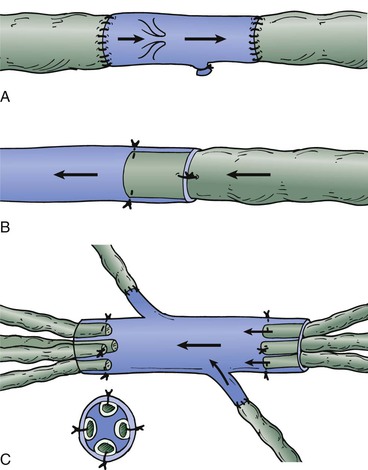
The tension-free technique is used to anastomose the lymphatic vessels under the operating microscope with maximal magnification (Fig. 68-7). The suture opposite the surgeon should be performed first. Because of the fragility of the vessels, the vessels are not turned over. Only the back wall is lifted as far as necessary to place a dorsal stitch. One or two additional single stitches complete the anastomosis. In my experimental studies of suture material, absorbable polyglactin was superior to nonabsorbable material. Currently, 10-0 absorbable suture (polyglactin 910) is the thinnest material available, used on a BV-75-4 needle.
Postoperative Treatment
After surgery, elastic bandages are applied, and an elastic compression garment is prescribed for 6 months, at which time discontinuation of the garment is considered. Antibiotics and erysipelas prophylaxis are given for 6 months because of the reduced immunologic resistance in patients with lymphedema. Low-molecular-weight dextran or 6% hydroxyethyl starch infusions are given for 1 week postoperatively to improve lymph flow.
Results
Results were evaluated by volume estimation based on circumferential measurements along the limb in increments of 4 cm. Further, lymphatic outflow was measured semiquantitatively by the lymphatic transport index based on lymphoscintigraphic studies.76 Direct visualization of the grafts was difficult because, with lymphangiography using water-soluble contrast medium, the lymphatic vessels can generally be visualized only over short distances. However, in several patients, patent grafts could be demonstrated more than 10 years after grafting with this technique.80
In a series of 127 patients with arm edema, a significant volume reduction was achieved, from a mean of 3368 cm3 preoperatively to a mean of 2567cm3 after 8 to 10 days (P < .001). At a mean follow-up of 2.6 years, the mean volume was 2625 cm3 (P < .001). In a group of 8 patients with long-term follow-up of more than 10 years, the mean volume was reduced to 2273 cm3 from a mean preoperative volume of 3004 cm3 (P < .001).
In 81 adult patients with unilateral edema of the lower extremities, the mean preoperative volume of 13,098 cm3 was reduced to a mean of 10,578 cm3 at the time of hospital discharge (P < .001). After a mean follow-up period of 1.7 years, the volume reduction was sustained, with a mean volume of 11,074 cm3 (P < .001). In a group of 12 patients observed for more than 4 years, the volume was reduced to 10,692 cm3 (P < .001).71 The following complications were observed: two cases of erysipelas in the initial group of patients, one lymphocyst at the harvesting site, and swelling of the lower leg after thrombosis in one patient.
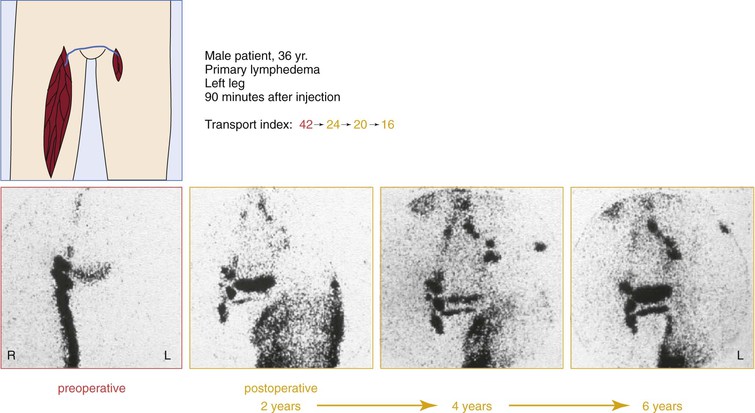
Lymphoscintigraphic studies were performed during a follow-up period of 8 years in 20 patients (12 upper extremities, 8 lower extremities). Of the 20 patients, 17 showed improved lymphatic outflow. In five patients, patent grafts could be demonstrated directly by visualizing the routes of activity.81 Figures 68-8 and 68-9 show lymphoscintigraphic studies along with the transport index for two patients with a follow-up period of 6 years. A quality of life study in 212 patients who had undergone lymphatic grafting showed a significant improvement in the physiologic and the psychologic conditions as well.82
Other Direct Reconstructive Methods
Trevidic and Cormier described a method of directly bridging a lymphatic defect with a free flap.59 Li and colleagues isolated lymphatic vessels within the flap and created lympholymphatic anastomoses on both sides.62 Ho and coworkers used the greater saphenous vein to invaginate the lymphatic vessels and also performed lympholymphatic anastomoses at the peripheral and central ends of the interposition tissue.83 Another attempt to reconstruct a gap in the lymphatic system was described by Becker and associates, consisting of free lymph node grafting with the goal of spreading lymphatic connections peripherally and centrally.60
Another method of reconstructing an interrupted lymphatic system using veins was proposed by Mandl84 and described in a series by Campisi and colleagues41 (Fig. 68-10). The study included 64 patients, 59 with leg lymphedema and 5 with postmastectomy edema. Marked reduction was reported in 40 patients, moderate reduction in 18 patients, and mild reduction in 6 patients.
Conclusions
A prerequisite for all reconstructive methods is a refined microsurgical approach. Lympholymphatic anastomosis appears to be more advantageous than lymphovenous anastomosis; adverse pressure gradients between lymphatic vessels and veins are avoided, and a lympholymphatic anastomosis is likely to heal faster because of the ability of lymphatic vessels to regenerate.85 Lymph’s lower coagulability than blood is another potential advantage of lympholymphatic anastomosis.
In all patients who undergo surgical treatment, an attempt at conservative therapy should have failed. Two or three lymphatic channels seem to be sufficient to restore normal lymphatic transport; successful grafting results in a return of the lymphoscintigraphic transport index to the normal range.
Whether reconstructive surgery can reverse secondary changes that have already occurred in the distal lymphatic channels, subcutaneous tissue, and skin of patients with chronic lymphedema is a challenging question that cannot be answered with generalities. In patients with mild secondary changes, the appearance of the extremity after lympholymphatic grafting can return to close to normal without additional treatment. In patients with more advanced secondary changes, the goal is often not to completely reduce excess volume but to improve the feeling of tension and heaviness; for some patients, reduction of swelling at the wrist and hand alone is a major success. Liposuction can be considered in addition to lymphatic reconstruction to reduce excess volume. Preliminary results show that volumes achievable in the affected extremity are slightly lower than those in the healthy one, without any additional therapy.
Lymphovenous Anastomosis
Indications
Patients with secondary lymphedema of recent onset without previous episodes of cellulitis or lymphangitis are potential candidates for surgical treatment unless they can be managed easily with conservative measures. In the late stage of lymphedema, fibrosis and valvular incompetence of the main lymph vessels develop, the intrinsic contractility of the vessel wall is lost, and interstitial pressure decreases owing to secondary changes in the subcutaneous tissue (see Chapter 13). The chance of successful lymphovenous anastomosis in such limbs is clearly diminished. Because venous hypertension impedes forward flow through the anastomosis, patients with chronic venous insufficiency are not candidates for this operation.
Preoperative Evaluation
Isotope lymphoscintigraphy is usually sufficient for preoperative imaging of the lymphatic system. In ideal candidates, it confirms the presence of dilated infrainguinal lymph vessels with proximal pelvic lymphatic obstruction. Although it does not differentiate between primary and secondary lymphedema, and even though it is primarily a functional study rather than an anatomic one, semiquantitative lymphoscintigraphy with technetium Tc 99m–antimony trisulfide colloid can reliably identify the pattern of lymphatic transport.86 In selected patients, direct contrast lymphangiography can be performed to show the fine details of the lymphatic circulation.
Progress in visualizing superficial lymphatic vessels has been made with the use of indocyanine green fluorescence lymphography. Indocyanine green is injected intradermally. With the help of a near-infrared camera, the lymphatic vessels are detected as blurred linear images, whereas dermal backflow is seen as a spotty image. This facilitates the search for superficial lymphatic vessels appropriate for lymphovenous anastomoses.87
Other preoperative tests include noninvasive venous studies and duplex scanning of the deep veins. Computed tomography (CT) is used in most patients to exclude an underlying mass or malignant tumor. Once surgery has been decided on, the patient is hospitalized for 24 to 48 hours to elevate the extremity in a lymphedema sling and to allow the use of intermittent compression with a pump to decrease the volume of the extremity.
Surgical Technique
Leg.
For lower extremity lymphedema, a transverse incision at the midthigh or a longitudinal incision close to the saphenofemoral junction is performed to allow dissection of the lymphatic of the superficial medial bundle. The greater saphenous vein and any tributaries are also dissected. An attempt is made to visualize the lymph vessels by injecting 5 mL of isosulfan blue dye subcutaneously; half this amount is directed toward the first interdigital space and half toward the area 10 to 15 cm distal to the incision site. Because of lymphatic obstruction, lymph flow even in patent lymphatics may be minimal, and the dye usually is not visible during dissection. With experience, the whitish fluid-filled lymphatics, frequently with vascularized adventitia, can be distinguished from small subcutaneous nerves or fibrotic bands.
If contrast lymphangiography is performed within 24 hours of the operation, the contrast-filled lymphatics are easily identifiable and can be located during the operation with an image intensifier and a C-arm. In some patients, contrast lymphangiography helps avoid many hours of unsuccessful searching for patent lymphatics in the groin. Once the lymphatic vessels and the veins are isolated, a standard microsurgical technique is used to perform an end-to-end anastomosis with six to eight interrupted 11-0 monofilament sutures. The operation is performed with a Zeiss operating microscope with 4× to 40× magnification.
Arm.
For arm lymphedema, the lymphatics are dissected either through a transverse incision at the wrist or in the midcubital fossa or through a longitudinal incision at the medial aspect of the arm, a few centimeters proximal to the elbow. Lymphatics of the superficial medial lymphatic bundle are usually used for anastomoses, which are performed with the midcubital, basilic, or brachial veins or their tributaries in an end-to-side or end-to-end fashion.
Postoperative Treatment
Postoperatively, the limb is wrapped with an elastic bandage and elevated 30 degrees. For the arm, this can be accomplished with two pillows; for the lower extremity, the foot of the bed can be elevated.
Results
Objective evaluation of the long-term effectiveness of lymphovenous anastomosis has been difficult. Decrease in the circumference or volume of the extremity, patient satisfaction, decrease in episodes of cellulitis, and improvement in lymphatic clearance as measured by lymphoscintigraphy have been used as the criteria of success. In a review of 14 patients who underwent lymphovenous anastomosis at the Mayo Clinic, only 5 limbs remained improved at a mean follow-up of 46 months after surgery.88 Improvement was observed in four of seven patients with secondary lymphedema but in only one of seven with primary lymphedema. Improvement in lymphatic clearance from the injection site was the only indirect sign of shunt patency. Therefore, the investigators were unable to provide objective evidence of late patency of the lymphovenous anastomoses in these patients.
A large experience with lymphovenous shunts was reported in Australia. O’Brien and colleagues published a well-documented report with long-term follow-up of 90 patients who underwent lymphovenous anastomoses for chronic lymphedema.89 Although a significant number of patients underwent additional excisional operations, improvement was documented even in those with only lymphovenous anastomoses. Of the latter, 73% had subjective improvement, and 42% had objective long-term improvement. Seventy-four percent of all patients discontinued the use of elastic stockings. Because direct contrast lymphangiography occasionally results in progression of lymphedema owing to chemical lymphangitis or accumulation of contrast material in the lymph nodes due to poor lymphatic transport, this test was not used to document late patency of the anastomoses. Therefore, objective evidence that improvements were caused by patent and functioning lymphovenous anastomoses is still lacking.
The largest experience to date comes from Campisi and colleagues in Italy.90,91 This group treated 665 patients with obstructive lymphedema using microsurgical lymphovenous anastomoses and achieved subjective improvement in 87% of their patients. In the 446 patients available for long-term follow-up, the authors observed volume reduction in 69% and discontinuation of conservative measures in 85%. This is a remarkable result that has not yet been duplicated elsewhere. The authors concluded that microsurgical reconstruction early in the course of lymphedema is more effective because the intrinsic contractility of the lymphatic vessel is maintained and the chance of normalizing the lymph circulation is better before significant chronic inflammatory changes develop in the subcutaneous tissue.
Koshima and coworkers from Japan reported significant improvement of lymphedema at a mean of 3.3 years after lymphovenous anastomoses in 8 of 13 patients.92 However, Vignes and associates failed to confirm the therapeutic benefit of lymphovenous anastomoses in a group of 13 patients (10 with primary and 3 with secondary lymphedema).93 Global assessment of clinical outcome was very good or good in five patients and intermediate in another five, but the operation failed to improve the volume of lower limbs or to reduce the frequency of erysipelas. Variations in performing lymphovenous anastomoses have been described. Mihara and colleagues recommended anastomosis of both the proximal and the distal ends of a transected lymph vessel with a vein or with two tributaries of an adjacent vein, allowing both the anterograde and the retrograde flow of lymph.94
A follow-up study of 237 lymphaticovenous side-to-end anastomoses, performed in 57 patients with lymphedema, showed at least one patent anastomosis in 34 patients by use of indocyanine green fluorescence lymphography after a mean follow-up period of 14 months. In 23 patients, no patent anastomosis was seen. There was no significant difference in limb volume reduction between the two groups.95
In one surgical technique, the so-called lymphaticovenous implantation, the lymph vessel is inserted into the lumen of a bigger vein together with the surrounding fat, including the lymphatic capillaries.96 The same procedure is also used to improve lymphatic drainage of the dermal lymphatics.87
Large series of patients treated for secondary and primary lymphedema by the supermicrosurgical lymphovenous anastomosis and the lymphaticovenous implantation were published by Demirtas and colleagues.97,98 In 40 patients with unilateral primary lymphedema, a mean reduction of 56% of the surplus of volume was seen after a follow-up period of 13.3 months. Whereas only 17% of the patients had a complex physical therapy before surgery, all patients of stage 4 and most patients of stage 3 (according to Campisi’s classification) used custom-made pressure garments after surgery. In 20 patients with unilateral secondary lymphedema, a mean reduction of 60% was seen after a mean follow-up period of 12.8 months; 50% of the patients did not receive complex physical treatment before surgery, and continuous custom-made pressure garments were used in 2 of 5 patients of stage 3 and in all patients of stage 4 lymphedema. There was no significant relationship between the number of microsurgical anastomoses or implantations and the mean reduction in volume.
Resection
Resection involves the removal of the lymphedematous, fibrotic, and frequently sclerotic subcutaneous tissue of the limb. Liposuction is a more recent technique used to accomplish resection. Other open excisions are usually performed during staged procedures. If the skin is diseased and has to be resected, coverage with skin grafts may be necessary.
Liposuction
Surgical Technique.
Brorson and Svensson advocate use of liposuction in patients with postmastectomy lymphedema to reduce the volume of the extremity.19 The rationale behind this treatment is that chronic lymphedema causes hypertrophy of the subcutaneous fat. Removal of the hypertrophied and edematous adipose tissue is performed through 20 to 30 3-mm-long incisions with vacuum aspiration. During the postoperative course, controlled compression therapy (CCT) is administered to decrease bleeding complications and to help reduce the volume of the limb.
Results.
In a report by Brorson and Svensson, preoperative and postoperative arm edema volumes were measured by the water-displacement technique, and lymph transport was assessed by lymphoscintigraphy.99 Twenty-eight patients were prospectively matched and divided into two groups. One group received liposuction combined with CCT, and one group received CCT alone. Liposuction combined with CCT was more effective than CCT alone; the edema reduction figures after 1 year were 104% for liposuction with CCT and 47% for CCT alone (P < .0001). Continued use of compression garments is important to maintain the primary surgical outcome. Liposuction can be useful in patients with no functioning lymphatics, but in others, the destruction of functioning lymphatics and worsening of the edema are possible.
Staged Subcutaneous Excision beneath Flaps
Surgical Technique.
The technique described by Miller and colleagues is most popular in the United States.100–103 The excision is performed in two or sometimes three stages, starting medially during the first operation. A bloodless field is obtained with a pneumatic tourniquet. An incision is made along the ankle beginning 1 cm posterior to the medial malleolus and extending proximally into the midthigh. Flaps approximately 1.5 cm thick are elevated anteriorly and posteriorly to the midsagittal plane in the calf, with less extensive dissection in the thigh and ankle. All subcutaneous tissue beneath the flaps is then removed, but the sural nerve is preserved. The deep fascia of the calf is incised over the tibia and resected, sparing the fascia at the knee and ankle to preserve joint integrity. Redundant skin, about 4 to 10 cm wide, is then resected; suction catheters are placed, and the wound is closed in a single layer with 4-0 nylon. In suturing around the ankle and knee, portions of the deep fascia are included in the suture to fix the skin flaps deeply and to ensure contour. The extremity is immobilized with a posterior splint and elevated. The patient is kept on bed rest for 9 days and mobilized afterward wearing tightly wrapped elastic bandages to prevent seroma formation and to promote optimal healing and contour. This regimen is continued for 3 weeks postoperatively.
The second stage, consisting of lateral excision, is performed 3 months later in a similar fashion. During lateral excision, the sensory branches of the peroneal nerve are identified and carefully preserved.
Results.
Staged excision underneath skin flaps offers the most reliable improvement of all debulking operations while minimizing the likelihood of postoperative complications. Miller and colleagues reported the long-term results in 38 patients with chronic lymphedema after staged subcutaneous excisions performed beneath skin flaps.103 Thirty patients had marked and durable reduction in extremity size with improved function. Episodes of cellulitis were reduced or eliminated. No difference in long-term results was seen in patients with primary versus secondary lymphedema. This series had a remarkably low rate of skin complications (four patients) and a low rate of late disease progression (six patients).
The clinical benefit of excisional operations is directly related to the amount of subcutaneous tissue removed. Patients are susceptible to recurrences and should continue to wear elastic compression stockings. Although good volume reduction is achievable with most of these procedures, prolonged hospitalization, poor wound healing, long surgical scars, sensory nerve loss, and residual edema of the foot and ankle can be significant problems. Because of these potential complications, these procedures are generally offered only to patients with significant, disabling lymphedema that is not responding to medical measures. Staged skin and subcutaneous excision beneath skin flaps appears to provide the most durable result with the lowest rate of complications.
Primary Chylous Disorders
Chylous disorders are characterized by an accumulation of chyle in abnormal areas of the body. Chylous ascites, chylothorax, and chylocutaneous fistula may be caused by a malignant tumor (most frequently lymphoma) or by trauma to the mesenteric lymphatics or the thoracic duct, which may also occur during vascular surgical procedures. Primary chylous disorders are usually caused by congenital lymphangiectasia or megalymphatics, which in some patients are associated with obstruction of the thoracic duct. In patients with lymphangiectasia and lymphatic valvular incompetence, chyle may reflux into the lower extremities, perineum, or genitalia.99–106 Depending on the site of the dilated lymphatics and the site of the chylous leak, these patients may also have protein-losing enteropathy, chylous ascites, chylothorax, chylopericardium, or reflux of chyle into the lungs and tracheobronchial tree.99–116 The mean age of 35 patients (15 male, 20 female) treated surgically for primary chylous disorders at the Mayo Clinic was 29 years and ranged from 1 day to 81 years.108 The patients presented with lower limb edema (54%), dyspnea (49%), scrotal or labial edema (43%), and abdominal distention (37%). The cause was primary lymphangiectasia in 66%, yellow nail syndrome in 11%, lymphangioleiomyomatosis in 9%, and other disorders in 18%.108
Medical treatment is aimed at decreasing the production of chyle by means of a medium-chain triglyceride diet or occasionally by parenteral nutrition. Repletion of proteins and calcium lost with chyle is as important as strengthening of the body’s defense mechanism because lymphocytes and important immunoglobulins are also wasted in these patients. Only surgical treatment can provide long-term improvement and occasionally cure by ligation of the incompetent retroperitoneal lymph vessels and oversewing of the site of the lymph leak. Attempts to reconstruct the obstructed thoracic duct by the creation of thoracic duct–azygos vein or internal jugular vein anastomoses have been reported.106,117–119
Lower Extremity and Genitalia
Many patients with lymphangiectasia and reflux of chyle have unilateral lower extremity lymphedema. The main discomfort for these patients, however, is the intermittent or continuous discharge of chyle from cutaneous vesicles in the lower extremity or the genitalia. The first five patients known to have suffered from this rare condition were described in 1949 by Servelle and Deysson.110
The preoperative evaluation of patients with chylous reflux into the lower extremity or genitalia should include lymphoscintigraphy (Fig. 68-11A). However, contrast lymphangiography is the definitive test to confirm the diagnosis and to localize the dilated retroperitoneal lymphatics and, frequently, the site of lymph leak. MRI with contrast enhancement may provide more precise information in the future.
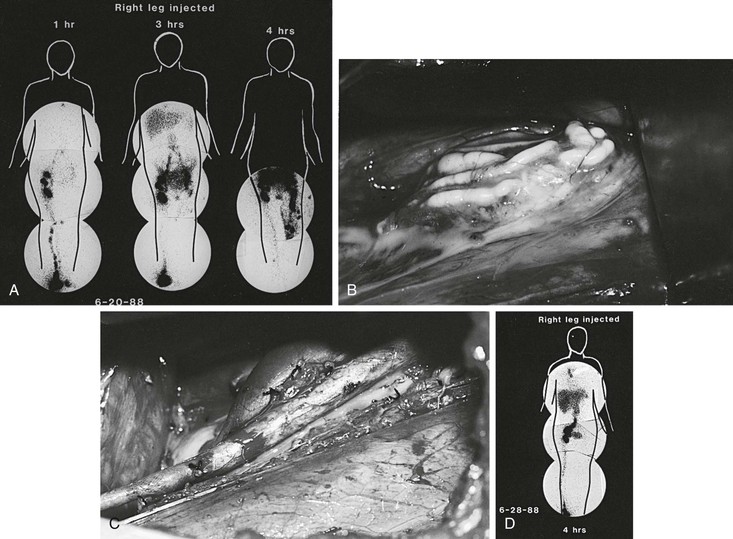
Figure 68-11 A, Right lower extremity lymphoscintigram in a 16-year-old girl with lymphangiectasia and severe reflux of chyle into the genitalia and left lower extremity. Injection of the isotope into the right foot reveals reflux into the pelvis at 3 hours and into the left lower extremity at 4 hours. B, Intraoperative photograph reveals dilated, incompetent retroperitoneal lymphatics in the left iliac fossa containing chyle. C, Radical excision and ligation of the lymph vessels were performed. In addition, two lymphovenous anastomoses were created between two dilated lymphatics and two lumbar veins. D, Postoperative lymphoscintigram reveals no evidence of reflux at 4 hours. The patient has no significant reflux 4 years after surgery. (From Gloviczki P, et al: Noninvasive evaluation of the swollen extremity: experiences with 190 lymphoscintigraphic examinations. J Vasc Surg 9:683, 1989.)
Surgical Treatment
The only effective technique to control the reflux of chyle and its drainage through skin vesicles in the perineum, labia, scrotum, or lower extremity is radical excision and ligation of the incompetent retroperitoneal lymph vessels. Gloviczki and coworkers used the technique of Servelle and performed the entire reflux operation in two stages through flank incisions by the retroperitoneal approach.108 Four hours before the procedure, the patient ingested 60 g of butter and 8 oz of whipped cream. The fatty meal allowed ready visualization of the retroperitoneal lymphatics during exploration (Fig. 68-11B to D). Ligation of the lymph vessels should be done with the utmost care to avoid tearing or avulsion of the lymphatics, resulting in residual leaks or rupture. In recent years, sclerotherapy of the dilated lymphatics has been added to ligation to increase the efficacy of the operation. Tetracycline solution, 500 to 1000 mg diluted in 20 mL of normal saline, is injected directly into the dilated retroperitoneal lymph vessels to provoke obstructive lymphangitis.
As reported by Molitch and associates, percutaneous CT- or MRI-guided cannulation of these dilated lymphatics may be possible, and sclerotherapy to decrease reflux can be performed repeatedly if necessary.112 Lymphovenous anastomoses with the dilated lymphatics can also be performed. Reflux of blood into the dilated and incompetent lymphatics can occur, however. A competent valve on the venous side completely avoids reflux and increases the chance of successful lymphatic drainage.113,117
Results
The largest group of patients studied was reported by Servelle, who operated on 55 patients with chylous reflux into the lower extremity or genitalia and reported a durable benefit in most patients.105 In Kinmonth and Cox’s series of 19 patients who underwent ligation of the retroperitoneal lymphatics for chylous reflux to the limbs and genitalia, permanent cure was achieved in 5 patients, and alleviation of symptoms—frequently after several operations—occurred in 12 patients.106 No improvement or failure was noted in only two cases. Noel and coworkers reviewed the results of 35 patients with primary chylous disorders treated during a 24-year period.113 Twenty-one patients (60%) underwent 27 surgical procedures. Nineteen procedures were performed for chylous ascites or reflux; 10 of these patients (53%) underwent resection of retroperitoneal lymphatics with or without sclerotherapy, 4 (21%) had lymphovenous anastomoses or saphenous vein interposition grafts, 4 (21%) had peritoneovenous shunts, and 1 (5%) had a hysterectomy for periuterine lymphangiectasia. All patients improved initially, but 29% had a recurrence of symptoms at a mean of 25 months (range, 1 to 43 months). Three patients with leg swelling had postoperative lymphoscintigraphy confirming improved lymphatic transport and diminished reflux.
Chylous Ascites
Chylous ascites usually results from intraperitoneal rupture of the mesenteric or retroperitoneal lymphatics or from exudation of chyle into the peritoneal cavity.113 The evaluation of such patients should include CT or MRI to exclude abdominal malignant disease. The diagnosis of lymphangiectasia is confirmed by bipedal contrast lymphangiography. Paracentesis is both diagnostic and therapeutic.
Surgical Treatment
If conservative measures fail and ascites returns, abdominal exploration should be performed after a fatty meal, as described previously. If chylous ascites is due to primary lymphangiectasia, abdominal exploration may reveal ruptured lymphatics, which can be oversewn. Some patients develop large chylous cysts, which should be excised (see Fig. 68-11). If the condition is associated with protein-losing enteropathy and the disease is localized to a segment of the small bowel, the bowel segment should be resected. The outcome of the operation is usually good if a well-defined abdominal fistula is found. However, if the mesenteric lymphatic trunks are fibrosed, aplastic, or hypoplastic and exudation of chyle is the main source of the ascites, the prognosis is poor, and recurrence is frequent.
Results
Browse and coworkers treated 45 patients with chylous ascites.107 The age at presentation ranged from 1 to 80 years (median, 12 years). Thirty-five patients had an abnormality of the lymphatics (primary chylous ascites); in the remaining 10, ascites was secondary to other conditions, principally non-Hodgkin’s lymphoma (6 patients). Surgery (fistula closure, bowel resection, or insertion of a peritoneovenous shunt) was performed in 30 patients. Closure of a retroperitoneal or mesenteric fistula, when present, was the most successful operation, curing 7 of 12 patients. In those patients who develop chylous ascites from iatrogenic trauma, frequently after aortic reconstructions, a short period of conservative management is justified. If chylous ascites reaccumulates, reoperation with ligation of the fistula is the most effective treatment. Campisi et al reported on 12 patients with a mean follow-up of 5 years; there was 1 death after 1 year, 3 mild recurrences, and 1 major recurrence that was treated.120
Results with peritoneovenous shunts have been mixed; patency is usually judged by the recurrence of ascites. In Browse and coworkers’ experience with nine peritoneovenous shunt placements, all occluded within 3 to 6 months after insertion.107 Noel and colleagues used the LeVeen shunt with good results, although one of four patients developed symptomatic superior vena cava syndrome owing to thrombosis around the shunt.113
Chylothorax
As with chylous ascites, the most frequent cause of chylothorax is trauma or malignant disease.118 Primary lymphatic disorders that cause chylothorax include lymphangiectasia with or without thoracic duct obstruction. However, chylothorax may also result from chylous ascites passing through the diaphragm. In these patients, the chylothorax is cured when the chylous ascites is controlled. Preoperative lymphangiography should be performed in these patients because it may localize the site of the chylous fistula or document occlusion of the thoracic duct (Fig. 68-12). Thoracentesis usually is not effective in curing the disease, and chyle that leaks from the thoracic duct or one of the large intercostal, mediastinal, or diaphragmatic collaterals reaccumulates. Injection of tetracycline is frequently ineffective because it is diluted by the leaking chyle.
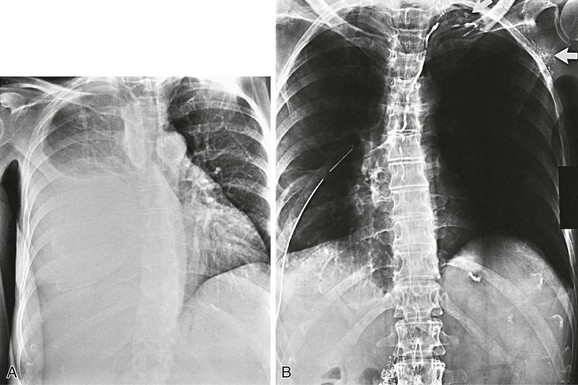
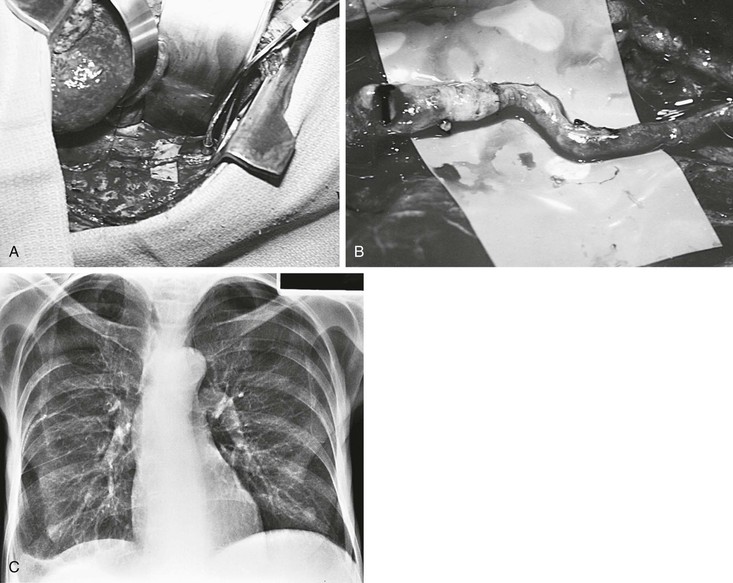
Figure 68-12 A, Right chylothorax in a 63-year-old woman. B, Bipedal lymphangiography confirms thoracic duct obstruction at the base of the neck. Note contrast medium in the supraclavicular and left axillary lymphatics (arrows).
Surgical Treatment
The best treatment of chylothorax is surgical pleurodesis with excision of the parietal pleura and prolonged pleural suction.114,118 After the patient has eaten a fatty meal, thoracotomy or video-assisted thoracoscopy is performed, and the leaking lymphatics are oversewn or clipped. This is followed by pleurodesis. In the Mayo Clinic series, eight procedures for chylothorax consisted of thoracotomy with decortication and pleurodesis (four patients), ligation of the thoracic duct (three patients), and resection of a thoracic duct cyst (one patient), with excellent early results in all patients.113
Thoracic Duct Reconstruction
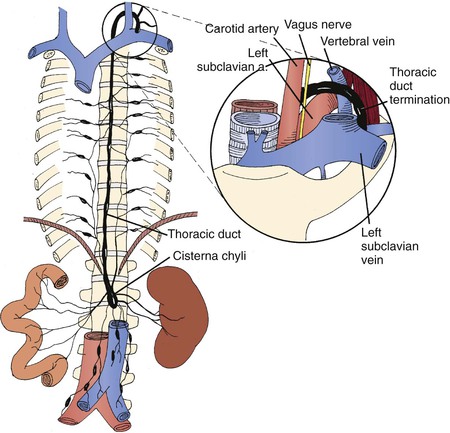
If occlusion of the cervical or upper thoracic duct (Fig. 68-13) is the cause of lymphangiectasia and reflux of chyle into the pleural or peritoneal cavity, thoracic duct–azygos vein anastomosis can be attempted to reconstruct the duct and to improve lymphatic transport. Preoperative imaging of the duct with contrast pedal lymphangiography is important because occlusion of the entire duct precludes anastomoses.
The operation is performed through a right posterolateral thoracotomy, and the anastomosis between the lower thoracic duct and the azygos vein is performed in an end-to-end fashion with 8-0 or 10-0 nonabsorbable interrupted sutures and magnification with loupes or an operating microscope. Only a few patients undergoing this operation have been described.106,118 Both patients operated on by Gloviczki’s group121 had good immediate patency, and excellent flow of chyle was observed through the anastomosis intraoperatively (Fig. 68-14). Although neither had postoperative contrast lymphangiography, recurrent chylothorax (the main indication for the procedure) ultimately resolved in both. Browse et al reported two successes in three patients who underwent thoracic duct reconstruction.118 Kinmonth et al performed this procedure in two patients and concluded that the anastomosis alone is not effective for decompressing the thoracic duct; ligation of the abnormal mediastinal lymphatics and oversewing of the sites of lymphatic leak are also necessary.106
Other Treatment Options
A prospective study from Cope and colleagues described 11 patients with primary and secondary chylothorax treated with percutaneous catheterization and embolization of the thoracic duct; their 45% technical success rate suggests a role for percutaneous intervention.114 Silk and associates115 and, more recently, Engum and colleagues116 reported on the use of pleuroperitoneal shunts in children, with good results.
Selected Key References
Baumeister RGH, Frick A. The microsurgical lymph vessel transplantation. Handchir Mikrochir Plast Chir. 2003;35:201.
Survey of technique and results in lymphatic grafting.
Brorson H. Liposuction of arm lymphoedema. Handchir Mikrochir Plast Chir. 2003;35:225.
Survey of technique and results in liposuction treatment of lymphedema.
Campisi C, Boccardo F, Tacchella M. Reconstructive microsurgery of lymph vessels: the personal method of lymphatic-venous-lymphatic (LVL) interpositioned grafted shunt. Microsurgery. 1995;16:161.
Survey of lymphatic-venous-lymphatic shunts.
Miller TA, Wyatt LE, Rudkin GH. Staged skin and subcutaneous excision for lymphedema: a favorable report of long-term results. Plast Reconstr Surg. 1998;102:1486.
Survey of technique and results in staged excisions for lymphedema.
Noel AA, Gloviczki P, Bender CE, Whitley D, Stanson AW, Deschamps C. Treatment of symptomatic primary chylous disorders. J Vasc Surg. 2001;34:785.
Survey of lymphedema in chylous disorders.
O’Brien BM, Sykes PJ, Threlfall GN, Browning FC. Microlymphaticovenous anastomosis for obstructive lymphedema. Plast Reconstr Surg. 1977;60:197.
Survey of technique and results in lymphovenous anastomosis.
Weiss M, Baumeister RGH, Hahn K. Post-therapeutic lymphedema: scintigraphy before and after autologous lymph vessel transplantation—8 years of long-term follow-up. Clin Nucl Med. 2002;27:788.
Long-term controlled study of lymphatic grafting.
Weissleder H, Schuchhardt C. Lymphedema: diagnosis and therapy. Viavital: Essen; 2008.
Concise information on lymphedema.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Foeldi M, et al. Foeldi’s textbook of lymphology. Elsevier Urban & Fischer: München; 2006.
2. Charles RH. Elephantiasis scroti. A system of treatment. Churchill: London; 1912:504. Latham A, English TC. A system of treatment. vol 3.
3. Deutschmann W, et al. Long-term results in the surgical treatment of lymphedema. Chir Plast. 1980;5:109.
4. Pierer H. Die radikale Resektion beim chronischen Lymphödem. Langenbecks Arch Chir. 1969;325:113.
5. Barinka L. Our experience with the treatment of lymphedema of the lower limb. Acta Chir Plast. 1967;9:218.
6. Barinka L. Employment of dermatome-super in the treatment of lower limb lymphedema. Acta Chir Plast. 1968;10:223.
7. Barinka L. The technique of the excisional operation of the lower extremity using a new instrument. Clodius L. Lymphedema. Thieme: Stuttgart; 1977.
8. Cobbett J. Small vessel anastomoses. Br J Plast Surg. 1967;20:16.
9. Clodius L. Conservative management of postmastectomy lymphedema. Ther Ggw. 1979;118:229.
10. Mowlen R. The treatment of lymphedema. Br J Plast Surg. 1948;1:48.
11. Vediayev JP, et al. Simplified method for elaboration of tissues excised for replantation during radical operations of elephantiasis. Acta Chir Plast. 1978;20:4.
12. Auchincloss H. New operation for elephantiasis. Puerto Rico J Publ Health Trop Med. 1930;6:1949.
13. Fontaine R, et al. Die operative Therapie des Lymphödems. Langenbecks Arch Chir. 1969;325:89.
14. Homans J. The treatment of elephantiasis of the legs. N Engl J Med. 1936;215:1099.
15. Servelle M. Chirurgie der Elephantiasis. Foeldi M. Erkrankungen des Lymphsystems. Witzstrock: Baden-Baden; 1971.
16. Sistrunk WE. Further experiences with the Kondoleon operation for elephantiasis. JAMA. 1918;71:800.
17. Sistrunk WE. Certain modifications of the Kondoleon-operation for elephantiasis. Ann Surg. 1927;85:185.
18. Illouz YG. Body sculpturing by lipoplasty. Churchill Livingstone: Edinburgh; 1989.
19. Brorson H, et al. Complete reduction of lymphoedema of the arm by liposuction after breast cancer. Scand J Plast Reconstr Surg Hand Surg. 1997;31:137.
20. Lanz O. Eröffnung neuer Abflusswege bei Stauung im Bauch und unteren Extremitäten. Zentralbl Chir. 1911;38:153.
21. Kondoleon E. Die chirurgische Behandlung der elefantiastischen Ödeme durch eine neue Methode der Lymphableitung. Munch Med Wochenschr. 1912;59:2726.
22. Kondoleon E. Operative Behandlung der elefantiastischen Ödeme. Zentralbl Chir. 1912;30:1022.
23. Thompson N. Surgical treatment of chronic lymphedema in lower limb. BMJ. 1962;2:1566.
24. Thompson N. Surgical treatment of primary and secondary lymphoedema of the extremities by lymphatic transposition. Proc R Soc Med. 1965;38:1026.
25. Thompson N. Buried dermal flap operations for chronic lymphoedema of the extremities. Surg Clin North Am. 1967;47:245.
26. Thompson N. The late results of operative treatment of chronic lymphedema of the extremities using the buried dermis flap procedure. J Cardiovasc Surg. 1968;92:93.
27. Thompson N. Buried dermal flap operation for chronic lymphedema of the extremities. Plast Reconstr Surg. 1970;45:541.
28. Bohmert H, et al. Die operative Therapie des Armlymphödems. Ergebnisse der Angiologie, Bd 6. Thieme: Stuttgart; 1973.
29. Bohmert H, et al. Zur Behandlung des Lymphödems mit einer modifizierten Methode nach Thompson. Ther Ggw. 1974;113:842.
30. Bunchmann HH II, et al. Long term results with the use of the buried skin flap in lymphedemas. Clodius L. Lymphedema. Thieme: Stuttgart; 1977.
31. Grenzmann M, et al. Die lymphovenösen Anastomosen. Rofo. 1968;109:564.
32. Gillies HD, et al. Treatment of lymphoedema by plastic operation. BMJ. 1935;1:96.
33. Mukherji M. Surgical treatment of filarial elephantiasis of legs by the lymph-bearing flap. Clodius L. Lymphedema. Thieme: Stuttgart; 1977.
34. Smith JW, et al. Selection of appropriate surgical procedures in lymphedema. Introduction of the hinged pedicle. Plast Reconstr Surg. 1962;30:10.
35. Wynn SA. Surgical approach to postmastectomy lymphoedematous extremity. Arch Surg. 1955;70:418.
36. Goldsmith HS, et al. Relief of chronic lymphedema by omental transposition. Am Surg. 1967;166:573.
37. Goldsmith HS. Long term evaluation of omental transposition for chronic lymphedema. Ann Surg. 1974;180:847.
38. Kinmonth JB, et al. Relief of lymph obstruction by use of mesentery and ileum. Br J Surg. 1978;65:829.
39. Holle J, et al: Überbrückung eines Lymphgefässdefektes mittels Veneninterposition—eine experimentelle Studie Vortr a.d. 2. Jahrestagung der deutschsprachigen Arbeitsgemeinschaft für Mikrochirurgie der peripheren Nerven und Gefaesse, Hamburg, 1979.
40. Mandl H, et al: Experimentelle Untersuchungen zur Mikrochirurgie der Lymphgefässe [Abstr XVIII]. Wissenschaftliche Tagung der GV-SOLAS, Lausanne, 1980, pp 28.5–30.5.
41. Campisi C, et al. Reconstructive microsurgery of lymph vessels: the personal method of lymphatic-venous-lymphatic (LVL) interpositional grafted shunt. Microsurgery. 1995;16:161.
42. Laine JB, et al. Experimental lymphatico-venous anastomosis. Surg Forum. 1963;14:111.
43. Nielubowicz J, et al. Surgical lymphovenous shunts for decompression of secondary lymphoedema. J Cardiovasc Surg. 1966;7(Suppl 2):262–267.
44. Rivero OR, et al. Experimental lympho-venous communications. Br J Plast Surg. 1976;20:124.
45. Allen PJ, et al. The use of free lymph-node grafts as a method of producing peripheral lymphovenous shunts. Br J Surg. 1968;55:385.
46. Degni M. New technique of lymphatic-venous anastomosis for the treatment of lymphedema. J Cardiovasc Surg (Turin). 1978;19:577.
47. Fox U, et al. Surgical treatment of chronic oedema of the leg. Proc R Soc Med. 1950;43:1054.
48. O’Brien BM. Microlymphaticovenous surgery for obstructive lymphedema. Aust N Z J Surg. 1977;47:284.
49. O’Brien BM, et al. Role of microlymphaticovenous surgery in obstructive lymphedema. Clin Plast Surg. 1978;5:293.
50. Campisi C, et al. Microsurgery for treatment of peripheral lymphedema: long-term outcome and future perspectives. Microsurgery. 2007;27:333.
51. Calnan JS, et al. The natural history of lymph node to vein anastomosis in the dog. Br J Plast Surg. 1967;20:134.
52. Calnan JS, et al. Attempts to create anastomoses between lymphatics and veins at peripheral sites. Br J Surg. 1968;55:385.
53. Calnan JS, et al. The late results of lymph node to vein anastomosis in the dog. J Cardiovasc Surg (spec issue). 1968.
54. Firica A, et al. Alleviation of experimental lymphedema by lymphovenous anastomosis in dogs. Am Surg. 1972;37:409.
55. Yamada Y. The study on lymphatic venous anastomosis in lymphoedema. Nagoya J Med Sci. 1969;32:1.
56. Gloviczki P, et al. The natural history of microsurgical lymphovenous anastomoses: an experimental study. J Vasc Surg. 1986;4:148.
57. Danese G, et al. Postmastectomy lymphedema. Surg Gynecol Obstet. 1965;129:797.
58. Danese C, et al. A model of chronic postsurgical lymphedema in dog’s limbs. Surgery. 1968;64:814.
59. Trevidic P, et al. Free axillary lymph node transfer. Progress in lymphology. Excerpta Medica: Paris; 1992. Cluzan RV. Progress in lymphology. vol XIII.
61. Trevidic P, et al. Limb radionuclide lymphoscintigraphy prior and after a lymphatic bypass using an axillary flap. Lymphology. 1998;31(Suppl):605.
62. Li S, et al. Microvascular transfer of a “lymphatic-bearing” flap in the treatment of obstructive lymphedema. Plast Reconstr Surg. 2008;121:150e.
63. Saumweber DM, et al. Experiences in experimental reconstructive microsurgery of lymphatics. Progress in lymphology. Excerpta Medica: Amsterdam; 1988. Partsch H. Progress in lymphology. vol XI.
64. Yuwono HS, et al. Comparison of lymphatic and venous interpositional autografts in experimental microsurgery of the canine lymphatics. Plast Reconstr Surg. 1990;85:752.
65. Acland RD, et al. Experimental lymphatico-lymphatic anastomoses, abstract book VII. International Congress of Lymphology: Florence; 1979.
66. Cordeiro AK, et al. Transplantations of lymphatic ducts, preliminary and experimental report, abstract book VII. International Congress of Lymphology: Florence; 1979.
67. Baumeister RGH, et al. Transplantation of lymph vessels on rats as well as first therapeutic application on the experimental lymphedema of the dog. Eur Surg Res. 1980;12(Suppl 21):7.
68. Baumeister RGH, et al. Experimental basis and first application of clinical lymph vessel transplantation of secondary lymphedema. World J Surg. 1981;5:401.
69. Baumeister RGH, et al. Treatment of lymphedema by microsurgical lymphatic grafting: what is proved? Plast Reconstr Surg. 1990;85:64.
70. Baumeister RGH, et al. 10 years experience with autogenous microsurgical lymph vessel transplantation. Eur J Lymphol. 1991;6:62.
71. Baumeister RGH, et al. The microsurgical lymph vessel transplantation. Handchir Mikrochir Plast Chir. 2003;35:202.
72. Baumeister RGH, et al. Homologous and autologous experimental lymph vessel transplantation: initial experience. Int J Microsurg. 1981;3:19.
73. Kinmonth JB. The lymphatics. Edward Arnold: London; 1972.
74. Partsch H. Practical aspects of indirect lymphography and lymphoscintigraphy. Lymphat Res Biol. 2003;1:71.
75. Lohrmann C, et al. High-resolution MR-lymphangiography in patients with primary and secondary lymphedema. AJR Am J Roentgenol. 2006;187:556.
76. Kleinhans E, et al. Evaluation of transport kinetics in lymphoscintigraphy: follow-up study in patients with transplanted lymphatic vessels. Eur J Nucl Med. 1985;10:349.
77. Weiss M, et al. Planning and monitoring of autologous lymph vessel transplantation by means of nuclear medicine lymphography. Handchir Mikrochir Plast Chir. 2003;35:210.
78. Weissleder H, et al. Examination methods in lymphedema. Viavital: Essen; 2008.
79. Kubik S. Zur klinischen Anatomie des Lymphsystems. Verh Anat Ges. 1975;69:109.
80. Baumeister RGH, et al. Microsurgical lymphatic grafting: first demonstration of patent grafts by indirect lymphography and long term follow-up studies. Lymphology. 1994;27(Suppl):787.
81. Weiss M, et al. Posttherapeutic lymphedema: scintigraphy before and after autologous lymph vessel transplantation—8 years long-term follow up. Clin Nucl Med. 2002;27:788.
82. Springer S, et al. Changes in quality of life of patients with lymphedema after lymphatic vessel transplantation. Lymphology. 2011;44:65–71.
83. Ho LCY, et al. Microlymphatic bypass in the treatment of obstructive lymphoedema of the arm: case report of a new technique. Br J Plast Surg. 1983;36:350.
84. Mandl H. Experimentelle Untersuchungen zur mikrochirurgischen Rekonstruktion von Lymphgefäßdefekten. Plast Chir. 1981;5:70.
85. Danese C, et al. Experimental anastomosis of lymphatics. Arch Surg. 1962;84:24.
86. Cambria RA, et al. Noninvasive evaluation of the lymphatic system with lymphoscintigraphy: a prospective, semiquantitative analysis in 386 extremities. J Vasc Surg. 1993;18:773.
87. Furukawa H, et al. Microsurgical lymphaticovenous implantation targeting dermal lymphatic backflow using indocyanine green fluorescence lymphography in the treatment of postmastectomy lymphedema. Plast Reconstr Surg. 2011;127:1804–1811.
88. Gloviczki P, et al. Microsurgical lymphovenous anastomosis for treatment of lymphedema: a critical review. J Vasc Surg. 1988;7:647.
89. O’Brien BM, et al. Long-term results after microlymphaticovenous anastomoses for the treatment of obstructive lymphedema. Plast Reconstr Surg. 1990;85:562.
90. Campisi C, et al. Long-term results after lymphatic-venous anastomoses for the treatment of obstructive lymphedema. Microsurgery. 2001;21:135.
91. Campisi C, et al. Lymphedema and microsurgery. Microsurgery. 2002;22:74.
92. Koshima I, et al. Long-term follow-up after lymphaticovenular anastomosis for lymphedema in the leg. J Reconstr Microsurg. 2003;19:209.
93. Vignes S, et al. Quantitative evaluation and qualitative results of surgical lymphovenous anastomosis in lower limb lymphedema [in French]. J Mal Vasc. 2003;28:30.
94. Mihara M, et al. Antegrade and retrograde lymphatico-venous anastomosis for cancer-related lymphedema with lymphatic valve dysfunction and lymphatic varix. Microsurgery. 2012;32:580–584.
95. Maegawa J, et al. Outcomes of lymphaticovenous side-to-end anastomosis in peripheral lymphedema. J Vasc Surg. 2012;55:753–760.
96. Yamamoto Y, et al. Microsurgical lymphaticovenous implantation for the treatment of chronic lymphedema. Plast Reconstr Surg. 1998;101:157–161.
97. Demirtas Y, et al. Supermicrosurgical lymphaticovenular anastomosis and lymphaticovenous implantation for treatment of unilateral lower extremity lymphedema. Microsurgery. 2009;29:609–618.
98. Demirtas Y, et al. Comparison of primary and secondary lower-extremity lymphedema treated with supermicrosurgical lymphaticovenous anastomosis and lymphaticovenous implantation. J Reconstr Microsurg. 2010;26:137–143.
99. Brorson H, et al. Liposuction combined with controlled compression therapy reduces arm lymphedema more effectively than controlled compression therapy alone. Plast Reconstr Surg. 1998;102:1058.
100. Heffel DF, et al. Excisional operations for chronic lymphedema. Rutherford RB. Vascular surgery. ed 5. WB Saunders: Philadelphia; 2000:2153–2159.
101. Miller TA. Surgical management of lymphedema of the extremity. Plast Reconstr Surg. 1975;56:633.
102. Miller TA. Surgical approach to lymphedema of the arm after mastectomy. Am J Surg. 1984;148:152.
103. Miller TA, et al. Staged skin and subcutaneous excision for lymphedema: a favorable report of long-term results. Plast Reconstr Surg. 1998;102:1486.
104. Kinmonth JB. Chylous diseases and syndromes, including references to tropical elephantiasis. Kinmonth JB. The lymphatics: surgery, lymphography and diseases of the chyle and lymph systems. ed 2. Edward Arnold: London; 1982:221–268.
105. Servelle M. Congenital malformation of the lymphatics of the small intestine. J Cardiovasc Surg. 1991;32:159.
106. Kinmonth JB, et al. Protein losing enteropathy in lymphedema: surgical investigation and treatment. J Cardiovasc Surg. 1975;16:111.
107. Browse NL, et al. Aetiology and treatment of chylous ascites. Br J Surg. 1992;79:1145.
108. Gloviczki P, et al. The surgical treatment of lymphedema caused by chylous reflux. Bartos V, Davidson JW. Advances in lymphology, Proceedings of the 8th International Congress of Lymphology, Montreal, 1981. Czechoslovak Medical Press: Prague; 1982:502–507.
109. Sanders JS, et al. Chyloptysis (chylous sputum) due to thoracic lymphangiectasia with successful surgical correction. Arch Intern Med. 1988;148:1465.
111. Browse NL. The surgery of lymphedema. Veith FJ. Current critical problems in vascular surgery. Quality Medical Publishing: St. Louis; 1989:408–409.
112. Molitch HI, et al. Percutaneous sclerotherapy of lymphangiomas. Radiology. 1995;194:343.
113. Noel AA, et al. Treatment of symptomatic primary chylous disorders. J Vasc Surg. 2001;34:785.
114. Cope C, et al. Management of chylothorax by percutaneous catheterization and embolization of the thoracic duct: prospective trial. J Vasc Interv Radiol. 1999;10:1248.
115. Silk YN, et al. Chylous ascites and lymphocyst management by peritoneovenous shunt. Surgery. 1991;110:561.
116. Engum SA, et al. The use of pleuroperitoneal shunts in the management of persistent chylothorax in infants. J Pediatr Surg. 1999;34:286.
117. Burnand GK, et al. Principles of surgical treatment. Browse N, Burnand GK, Mortimer PS. Diseases of the lymphatics. Arnold: London; 2003:179–204.
118. Browse NL, et al. Management of chylothorax. Br J Surg. 1997;84:1711.
119. Melduni RM, et al. Reconstruction of occluded thoracic duct for treatment of chylopericardium: a novel surgical therapy. J Vasc Surg. 2008;48:1600–1602.
120. Campisi C, et al. Diagnosis and management of primary chylous ascites. J Vasc Surg. 2006;43:1244.
121. Gloviczki P, et al. Surgical treatment of chronic lymphedema and primary chylous disorders. Rutherford RB. Vascular surgery. ed 6. 2005:2428–2445.