Chapter 288 Lymphatic Filariasis (Brugia malayi, Brugia timori, and Wuchereria bancrofti)
Clinical Manifestations
Tropical Pulmonary Eosinophilia
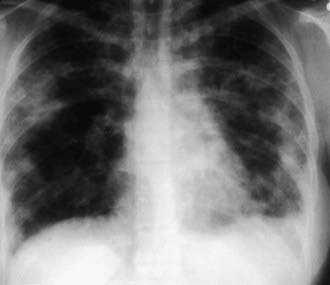
The presence of microfilariae in the body has no apparent pathologic consequences except in persons with tropical pulmonary eosinophilia, a syndrome of filarial etiology in which microfilariae are found in the lungs and lymph nodes but not the bloodstream. It occurs only in individuals who have lived for years in endemic areas. Men 20-30 yr of age are most likely to be affected, although the syndrome occasionally occurs in children. The presentation includes paroxysmal nocturnal cough with dyspnea, fever, weight loss, and fatigue. Rales and rhonchi are found on auscultation of the chest. The x-ray findings may occasionally be normal, but increased bronchovascular markings, discrete opacities in the middle and basal regions of the lung, or diffuse miliary lesions are usually present (Fig. 288-1). Recurrent episodes may result in interstitial fibrosis and chronic respiratory insufficiency in untreated individuals. Hepatosplenomegaly and generalized lymphadenopathy are often seen in children. The diagnosis is suggested by residence in a filarial endemic area, eosinophilia (>2,000/µL), compatible clinical symptoms, increased serum IgE (>1,000 IU/mL), and high titers of antimicrofilarial antibodies in the absence of microfilaremia. Although microfilariae may be found in sections of lung or lymph node, biopsy of these tissues is unwarranted in most situations. The clinical response to diethylcarbamazine (2 mg/kg/dose tid PO for 12-21 days) is the final criterion for diagnosis; the majority of patients improve with this therapy. If symptoms recur, a 2nd course of the anthelmintic should be administered. Patients with chronic symptoms are less likely to show improvement than those who have been ill for a short time.
Kazura JW, Bockarie MJ. Lymphatic filariasis in Papua New Guinea: interdisciplinary research on a national health problem. Trends Parasitol. 2003;19:260-263.
Melrose WD, Durrheim DD, Burgess GW. Update on immunological tests for lymphatic filariasis. Trends Parasitol. 2004;20:255-257.
Molyneux DH. 10 years of success in addressing lymphatic filariasis. Lancet. 2009;373:529-530.
Molyneux DH. Elimination of transmission of lymphatic filariasis in Egypt. Lancet. 2006;367:966-968.
Ottesen EA, Hooper PJ, Bradley M, et al. The global programme to eliminate lymphatic filariasis: health impact after 8 years. PLoS Negl Trop Dis. 2008;2:e317.
Ramzy RMR, El Setouhy M, Helmy H, et al. Effect of yearly mass drug administration with diethylcarbamazine and albendazole on bancroftian filariasis in Egypt: a comprehensive assessment. Lancet. 2006;367:992-999.
Shenoy RK, Suma TK, Kumaraswami V, et al. Antifilarial drugs, in the doses employed in mass drug administrations by the Global Programme to Eliminate Lymphatic Filariasis, reverse lymphatic pathology in children with Brugia malayi infection. Ann Trop Med Parasitol. 2009;103:235-247.
Stolk WA, de Vlas SJ, Habbema JDF. Anti-Wolbachia treatment for lymphatic filariasis. Lancet. 2005;365:2067-2068.
Supali T, Djuardi Y, Pfarr KM, et al. Doxycycline treatment of Brugia malayi-infected persons reduces microfilaremia and adverse reactions after diethylcarbamazine and albendazole treatment. Clin Infect Dis. 2008;46:1385-1393.
Taylor M, Hoerauf A, Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet. 2010;376:1175-1182.
Weil GJ, Kastens W, Susapu M, et al. The impact of repeated rounds of mass drug administration with diethylcarbamazine plus albendazole on bancroftian filariasis in Papua New Guinea. PLoS Negl Trop Dis. 2008;2:e344.