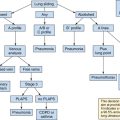
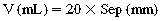Lung ultrasound in mechanically ventilated patients
Recruitment/positive end-expiratory pressure (PEEP)
Recruitment maneuvers play an integral role in the management of patients with severe lung injury. Key to the success of a recruitment maneuver is identification of patients with potentially recruitable lung units. However, the amount of potentially recruitable lung varies widely within this patient population, from negligible to upward of 50% of lung weight.1 Identifying who will and will not benefit from such maneuvers is crucial because there are risks to maintaining these high airway pressures, even if done transiently, including hemodynamic effects, pneumothoraces, and unintended airway trauma.
High-resolution computed tomography (CT) of the chest and evaluation of static pressure-volume loops on modern ventilators have been used to assess the effectiveness of lung recruitment. However, each of these has potential limitations because the improvements in oxygenation may not be immediately evident.2 Point-of-care lung ultrasound offers a noninvasive, portable, easily reproducible method of identifying potential recruitable lung parenchyma. The normal and abnormal ultrasonographic appearance of lung parenchyma has been well described in previous chapters. The degree of lung aeration can easily be identified at the bedside. This bedside assessment can be categorized into four discrete ultrasonographic patterns.3 These patterns include (1) horizontal A-lines, representing normal lung aeration; (2) multiple vertical B-lines that are regularly spaced, representing a moderate loss of lung aeration; (3) coalescence of closely spaced B-lines, representing severe loss of lung aeration; and finally, (4) gross collapse. With this simple classification, researchers have developed a prediction model to better define how much an individual patient may benefit from attempts at lung recruitment (Table 23-1). The lung re-aeration score is simply a tool that helps quantify the amount of lung recruited (in milliliters) postrecruitment. This scoring system has been validated against traditional pressure-volume loop analysis.3 Each lung is systematically examined in six lung regions (upper anterior, posterior, and lateral; lower anterior, posterior, and lateral). The prerecruitment degree of lung aeration is then categorized as normal (N), moderate loss of aeration (B1), severe loss of aeration (B2), or completely consolidated (C) in each of these regions. A score of 1, 3, or 5 is then assigned to the change in the appearance of the lung postrecruitment. For example, an area of lung that went from being completely consolidated (C) to normal (N) would be assigned 5 points, whereas an area of lung that went from completely consolidated (C) to moderate loss of aeration (B1) would be assigned 3 points. The lung aeration score is calculated by the sum of scores from each of the six lung regions. A lung aeration score of +8 predicts a recruitable lung volume of greater than 600 mL, with the application of moderate amounts of PEEP.4 Identification of patients who will minimally benefit (lung aeration score <8) from such maneuvers is tantamount in avoiding unnecessary and potential harmful attempts at future recruitment.
TABLE 23-1
Ultrasound Lung Re-aeration Score
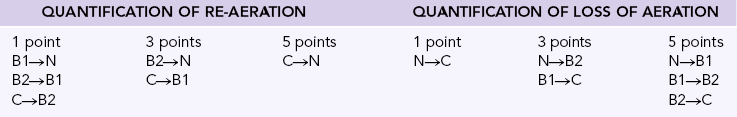
Modified from Bouhemad B, Brisson H, Le-Guen M, et al: Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment, Am J Respir Crit Care Med 183(3):341-347, 2001.
The “lung pulse” has also been advocated to identify potentially recruitable lung units.5 Sliding of the pleura with respiration in normal lungs prevents ultrasonographic perception of cardiac oscillation. When visualized lung is atelectatic, the motion of the patient’s beating heart is transmitted through the collapsed lung and perceived as cyclic motion, termed the lung pulse. Abolishment of this sign and the resumption of normal lung sliding postrecruitment maneuver can be an early sign of successful reexpansion of collapsed lung.5
The duration and level of peak airway pressure attained during recruitment is variable between studies and institutions. This may be because each collapsed lung unit has a unique time constant, and a “one-size-fits-all protocol” may not be appropriate to all patients. The use of real-time lung ultrasound allows the operator direct visualization of the lung during recruitment, thus yielding real-time feedback and allowing customization of both the peak pressure and duration of the maneuver (Figure 23-1, Video 23-1![]() ).3,4,6
).3,4,6
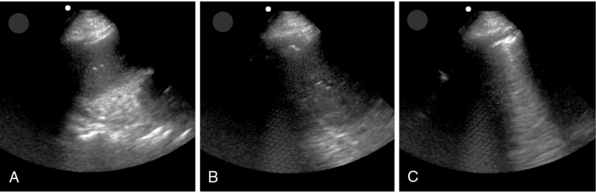
Figure 23-1 Point-of-care lung ultrasonogram during a recruitment maneuver. Lung tissue can be seen to progress from initial lung atelectasis (A) to improving atelectasis (B) and eventually visualization of the pleural interface and comet-tail artifacts (C) as the atelectatic lung expands.
After successful recruitment of atelectatic lung, it is important to ensure that the recruited lung does not derecruit after the sustained high airway pressure is removed. Arbitrarily increasing the PEEP postrecruitment maneuver without confirmation that reexpansion of collapsed lung was successful can have detrimental effects on other areas of the lung. When the increased PEEP is not transmitted to the atelectatic lung, the parts of the lung that were nonatelectatic are now exposed to higher end-expiratory pressures, potentially resulting in overdistention and worsening V/Q mismatch. In theory, direct visualization of the lung would be of benefit for the setting of “best” PEEP, but further research is needed to validate its use routinely.
Screening for complications of mechanical ventilation
As well described in Chapter 21, lung ultrasound is both a sensitive and specific method of identifying pneumothoraces. Critically ill patients undergoing mechanical ventilation are at high risk of developing a pneumothorax from invasive procedures (e.g., central line placement, thoracentesis) or simply from the mode of ventilation.7 This is a very dangerous occurrence because mortality has been reported as being up to 91% if a tension pneumothorax occurs on a ventilator. In patients with a high clinical suspicion of pneumothorax, awaiting a chest radiograph or CT scan to confirm or refute its presence can delay intervention, which may have detrimental effects on the patient. However, blind needle decompression or chest tube placement in these critically ill patients with tenuous respiratory status, although sometimes lifesaving, can have severe consequences if the diagnosis is incorrect. As well, even the complication rate of tube thoracostomy tubes is much greater than clinicians typically perceive. The speed and accuracy of diagnosis without having to transport the patient outside a monitored setting makes ultrasound an attractive alternative to other methods used to identify a pneumothorax.
The difficult-to-wean patient
Prolonged mechanical ventilation has been associated with a number of serious complications.8 Patients who are difficult to wean from mechanical ventilation make up 20% to 30% of intubated patients in an intensive care unit (ICU).9 The etiology of liberation failure is complex and multifactorial. It is important to undertake a systematic evaluation of these difficult-to-wean patients in order to identify potentially reversible causes that may be impairing weaning. Point-of-care ultrasound can be an effective tool in the evaluation of such patients.
Evaluation of the pleural space
Pleural effusions can compromise chest wall compliance and lead to compressive atelectasis. This can ultimately lead to prolongation of mechanical ventilation, especially in those patients whose respiratory status is tenuous.10 Ultrasonography has both diagnostic and therapeutic implications in the management of pleural effusions. First, identifying the volume of effusion can help predict the potential benefit of fluid removal. Balik, et al11 have developed simple formulas to aid in the quantification of pleural fluid:
Ultrasound also allows dynamic visualization of the pleural space, intercostal spaces, and hemidiaphragms, which helps to ensure safe entrance into an appropriate location.12 Real-time visualization of the needle/introducer tip can help in minimizing complications through inadvertent puncture of lung parenchyma or other structures.
Evaluation of diaphragmatic weakness/excursion
Ventilator-induced diaphragmatic dysfunction is a common complication of mechanical ventilation9,13 and likely contributes to patients’ failure to liberate from the ventilator. Measurement of diaphragm function with nerve conduction studies and fluoroscopic evaluation is complex and impractical. In recent years, lung ultrasound has been evaluated as a noninvasive bedside method of assessing diaphragmatic function.14 Diaphragmatic excursion, measured by M-mode ultrasonography, can be used adjunctively with the rapid-shallow breathing index (RSBI) to aid in the identification of those patients at high risk of postextubation distress15 as well as in the workup of patients who require prolonged mechanical ventilation.
With the patient in the supine position, a low-frequency ultrasound probe is placed in the anterior axillary line. Time motion (M) mode is used to measure diaphragmatic movement during inspiration and expiration. An excursion distance of less than 10 mm or paradoxic diaphragmatic movement is suggestive of diaphragmatic dysfunction (Figure 23-2).13 This evaluation can provide static measurements for diagnostic purposes and dynamic measurements, which can be helpful for following the progression or regression of the patient.
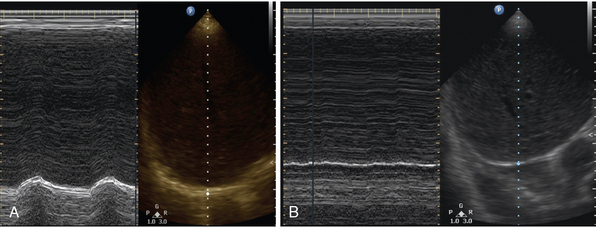
Figure 23-2 Point-of-care lung ultrasonogram of diaphragmatic excursion in a patient with normal diaphragmatic excursion (A) and diaphragmatic paralysis (B). Time motion (M) mode can be seen in the left panel, with the corresponding B-mode image on the right. (Courtesy Dr. Dimitrios Karakitsos.)
Evaluation of lung parenchyma
Lung ultrasound allows the operator to distinguish different forms of parenchymal lung problems. As outlined in previous chapters, the identification of B-lines can be indicative of loss of lung aeration or interstitial and alveolar edema,2,16,17 whereas “hepatitization” of lung tissue along with “dynamic air bronchograms” is suggestive of dense consolidation and may prompt consideration of therapeutic bronchoscopy to reexpand these consolidated areas to facilitate ventilator weaning.18,19
The lung re-aeration score, as described above, can also have a role in identifying those patients who are at high risk of postextubation failure.16 Because the lung re-aeration score is useful in quantifying the amount of volume gained with a recruitment maneuver, it can also help quantify the amount of volume lost with reduction in ventilatory support. The lungs are examined systematically before and then during a spontaneous breathing trial. A lung re-aeration score of greater than 17 (see Table 23-1) during a spontaneous breathing trial suggests significant derecruitment and is predictive of postextubation distress, whereas a lung re-aeration score (LAS) less than 12 may predict successful extubation.16
Evaluation of cardiac function
Mechanical ventilation has a number of beneficial effects on the physiology of the weakened heart.19 Positive pressure ventilation decreases preload by increasing intrathoracic pressure, thus reducing venous return to the right heart. The elevation in intrathoracic pressure also augments afterload by reducing the transmural pressure gradient of the left heart. With removal of mechanical ventilation, both preload and afterload are increased, along with an increase in oxygen consumption caused largely by the increased work of breathing placed on the respiratory muscles.9 Subclinical myocardial ischemia is an underappreciated cause of the difficult-to-wean patient. In these patients, ultrasound can be used to dynamically assess the effects of weaning ventilation on the cardiac function of the heart. This “stress test” can potentially reveal patients who will develop myocardial ischemia with weaning.
Lung ultrasound with alternative forms of ventilation
Advances in technology and critical care medicine have brought along alternative forms of mechanical ventilation, termed nonconventional mechanical ventilation. One such form of ventilation is high-frequency oscillatory ventilation (HFOV). First used in the 1960s, this alternative form of ventilation uses constantly maintained, high mean airway pressures to prevent derecruitment. Recent advances in lung protective ventilation in the treatment of ARDS have increased the use of HFOV in this subset of patients.20 Although it still remains mainly a rescue therapy, the results of ongoing studies of early acute lung injury/ARDS may make its use more mainstream.
Although theoretically reducing volutrauma and atelectrauma, the high mean airway pressures of HFOV result in a substantial risk of barotrauma. However, making the diagnosis of a pneumothorax during HFOV can be extremely challenging. Classic physical examination findings of asymmetric breath sounds are limited by the background noise of the ventilator and the lack of true breath sounds with HFOV. The greatest strength of lung ultrasound in this situation lies in its ability to rule out rather than rule in a pneumothorax. The visualization of comet-tail artifacts or normal lung sliding implies the apposition of the parietal and visceral pleura and essentially rules out a pneumothorax at that probe position with close to 100% negative predictive value.21–23
There have been some theoretic concerns that because of the extremely small and rapid tidal breaths used during HFOV (3-12 per second), lung sliding may be absent or undetectable even in the absence of pneumothoraces. Only a single case study has reported on the use of lung ultrasound in this patient population.7 In that case, normal lung sliding was visualized on one hemithorax in a patient on HFOV and was absent on the opposite side (Figure 23-3![]() , Videos 23-2 and 23-3), where a pneumothorax was later confirmed by detection of a lung point (Figure 23-4
, Videos 23-2 and 23-3), where a pneumothorax was later confirmed by detection of a lung point (Figure 23-4![]() , Video 23-4). However, depending on the power and frequency settings (which determine tidal volumes), lung sliding may still be subtle or undetectable, and its absence should not be relied upon as the sole sign for diagnosis of pneumothorax. There have been no reports of lung ultrasound in other alternative forms of ventilation, but it seems like an ideal method for the evaluation for pneumothoraces in the prone positioned patient.
, Video 23-4). However, depending on the power and frequency settings (which determine tidal volumes), lung sliding may still be subtle or undetectable, and its absence should not be relied upon as the sole sign for diagnosis of pneumothorax. There have been no reports of lung ultrasound in other alternative forms of ventilation, but it seems like an ideal method for the evaluation for pneumothoraces in the prone positioned patient.
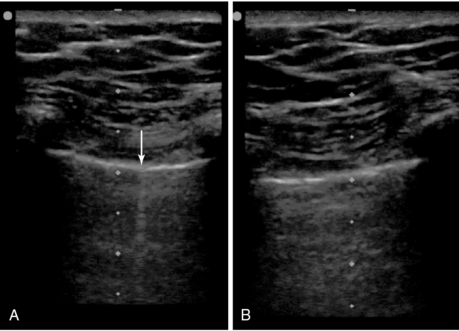
Figure 23-3 Point-of-care lung ultrasonogram of the right lung (A) of a patient receiving high-frequency oscillatory ventilation (HFOV), confirming the absence of a pneumothorax by the presence of lung sliding (not shown) and comet-tail artifacts (arrow), and of the left lung (B), suggesting the presence of a pneumothorax by the absence of lung sliding and comet-tail artifacts. (Republished with permission of the Journal of Ultrasound in Medicine: From Gillman LM, Kirkpatrick AW: Lung sonography as a bedside tool for the diagnosis of a pneumothorax in a patient receiving high-frequency oscillatory ventilation, J Ultrasound Med 29:997-1000, 2010. Permission conveyed through the Copyright Clearance Center.)
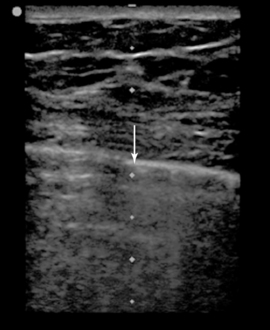
Figure 23-4 Point-of-care lung sonogram of the left lung of a patient receiving high-frequency oscillatory ventilation (HFOV), confirming the presence of a pneumothorax by the presence of a lung point (arrow). (Republished with permission of the Journal of Ultrasound in Medicine: From Gillman LM, Kirkpatrick AW: Lung sonography as a bedside tool for the diagnosis of a pneumothorax in a patient receiving high-frequency oscillatory ventilation, J Ultrasound Med 29:997-1000, 2010. Permission conveyed through the Copyright Clearance Center.)
Lung ultrasound represents an ideal tool for rapid, repeated, and noninvasive evaluation of the critically ill, mechanically ventilated patient. From the initiation of the mechanical ventilation to guiding recruitment and evaluating for complications and assessment of weaning, its role extends throughout a patient’s ICU stay.
Pearls and highlights
• Through direct visualization of the lung, ultrasound can be used to identify patients with recruitable lung parenchyma and in real time can guide and personalize recruitment maneuvers.
• Ultrasound can be incorporated into a comprehensive approach to the difficult-to-wean patient by systematically evaluating for and potentially guiding treatment of parenchymal, pleural, intraabdominal, and cardiac disease, and of diaphragmatic dysfunction.
• Lung ultrasound can potentially be used in the identification of pneumothoraces as a complication of nonconventional modes of ventilation such as HFOV.
References
1. Gattinoni, L, Caironi, P, Cressoni, M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006; 354(17):1775–1786.
2. Luecke, T, Corradi, F, Pelosi, P. Lung imaging for the titration of mechanical ventilation. Curr Opin Anesthesiol. 2012; 25(2):131–140.
3. Bouhemad, B, Brisson, H, Le-Guen, M, et al. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med. 2011; 183(3):341–347.
4. Cavaliere, F, Biasucci, D, Costa, R, et al, Chest ultrasounds to guide manual reexpansion of a postoperative pulmonary atelectasis: a case report. Minerva Anestesiol. 2011;77(7):750–753.
5. Stefanidis, K, Dimopoulos, S, Nanas, S. Basic principles and current applications of lung ultrasonography in the intensive care unit. Respirology. 2001; 16(2):249–256.
6. Gardelli, G, Feletti, F, Gamberini, E, et al. Using sonography to assess lung recruitment in patients with acute respiratory distress syndrome. Emerg Radiol. 2009; 16(3):3.
7. Gillman, L, Kirkpatrick, A. Lung sonography as a bedside tool for the diagnosis of a pneumothorax in a patient receiving high-frequency oscillatory ventilation. J Ultrasound Med. 2010; 29(6):4.
8. Scheinhorn, D, Hassenpflug, M, Votto, J, et al, Post-ICU mechanical ventilation at 23 long-term care hospitals: a multicenter outcomes study. Chest. 2007;131(1):85.
9. Heunks, LM, van der Hoeven, JG, Clinical review: the ABC of weaning failure—a structured approach. Crit Care. 2010;14(6):245.
10. Kupfer, Y, Seneviratne, C, Chawla, K, et al. Chest tube drainage of transudative pleural effusions hastens liberation from mechanical ventilation. Chest. 2011; 139(3):519–523.
11. Balik, M, Plasil, P, Waldauf, P, et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006; 32(2):318–321.
12. Maslove, D, Chen, BT, Wang, H, Kuschner, WG. The diagnosis and management of pleural effusions in the ICU. J Int Care Med. 2011; 28(1):24–36.
13. Boussuges, A, Gole, Y, Blanc, P, Diaphragmatic motion studied by M-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135(2):391–400.
14. Bouhemad, B, Zhang, M, Lu, Q, Rouby, JJ, Clinical review: bedside lung ultrasound in critical care practice. Crit Care. 2007;11(1):205.
15. Kim, WY, Suh, HJ, Hong, S-B, et al, Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med. 2011;39(12):2627–2630.
16. Soummer, A, Perbet, S, Brisson, H, et al, Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit Care Med. 2012;40(7):2064–2072.
17. Lichtenstein, D, Mezière, G, Seitz, J, The dynamic air bronchogram: a lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest. 2009;135(6):1421–1425.
18. Gillman, L, Kirkpatrick, A, Portable bedside ultrasound: the visual stethoscope of the 21st century. Scand J Trauma Resusc Emerg Me. 2012; 20:18.
19. Rocco, M, Carbone, I, Morelli, A, et al, Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand. 2008;52(6):776–784.
20. , Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308.
21. Lichtenstein, D, Mezière, G, Biderman, P, Gepner, A, et al, The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26(10):1434–1440.
22. Rowan, K, Kirkpatrick, A, Liu, D, et al, Traumatic pneumothorax detection with thoracic US: correlation with chest radiography and CT—initial experience. Radiology. 2002;225(1):210–214.
23. Ball, CG, Hameed, SM, Evans, D, et al. Canadian Trauma Trials Collaborative. Occult pneumothorax in the mechanically ventilated trauma patient. Can J Surg. 2003; 46(5):373–379.