Chapter 14 Lung Auscultation
Generalities
5 What are lung sounds?
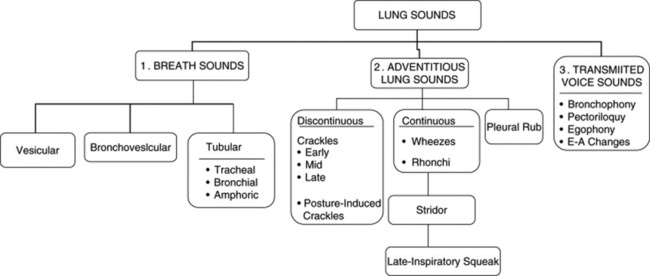
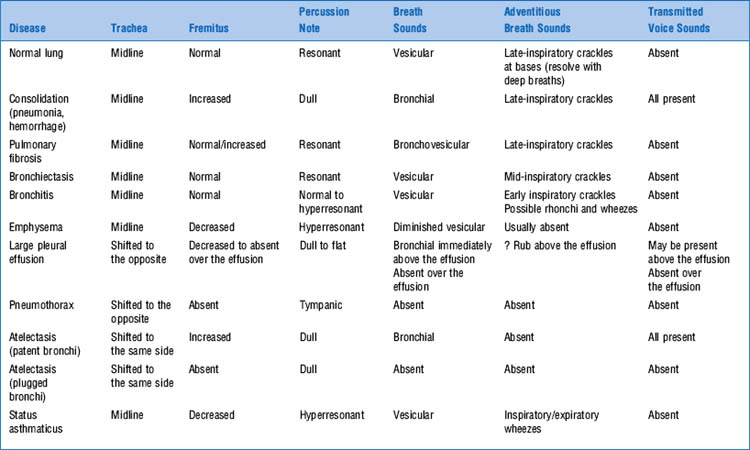
They are sounds generated by the lungs. Transmitted voice sounds are instead sounds generated by the larynx and subsequently transmitted through the lungs (Fig. 14-1).
A. Breath Sounds (Basic Lung Sounds)
9 What are the major types of breath sounds?
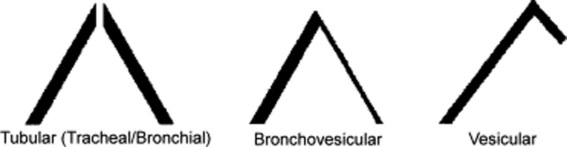
This traditional division is based on the sound’s site of production. Conversely, a more recent classification (based on physical characteristics) reports only two major lung sounds: (1) the normal ones (or vesicular breath sounds) and (2) the abnormal ones (or tubular breath sounds). The latter consist of three subvarieties: tracheal, bronchial, and amphoric. As for the bronchovesicular breath sound, it is in a league of its own and one that should probably be abandoned. (Fig. 14-2)
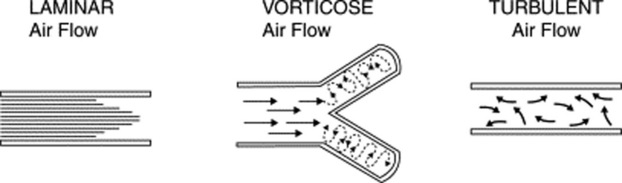
10 What is the air movement responsible for the production of breath sounds?
It depends on the size of the airway. Overall, three types of movement may take place along the tracheobronchial tree (Fig. 14-3). Some are silent and others noisy:
 Laminar airflow is characteristic of small peripheral airways. It is so slow that airflow in the alveoli almost comes to an end. Hence, this is a silent movement.
Laminar airflow is characteristic of small peripheral airways. It is so slow that airflow in the alveoli almost comes to an end. Hence, this is a silent movement.
 Vorticose airflow is a bit faster and typical of medium-sized branching airways. “Branching” separates airflow in different layers with different velocities, whose interaction generates eddies and vortices—all noisy. This air movement is often called mixed (or transitional) because it resembles both laminar and turbulent airflow.
Vorticose airflow is a bit faster and typical of medium-sized branching airways. “Branching” separates airflow in different layers with different velocities, whose interaction generates eddies and vortices—all noisy. This air movement is often called mixed (or transitional) because it resembles both laminar and turbulent airflow.
 Turbulent airflow is very rapid, complex, and typical of large central airways (trachea and major bronchi). Air molecules randomly collide against each other and onto the airway walls. This air movement is characteristically noisy.
Turbulent airflow is very rapid, complex, and typical of large central airways (trachea and major bronchi). Air molecules randomly collide against each other and onto the airway walls. This air movement is characteristically noisy.
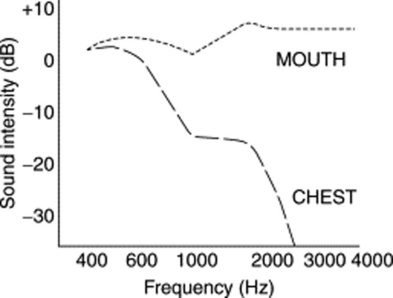
14 What are the acoustic characteristics of breath sounds at the mouth?
The dominant one is the high pitch (and loudness). Hence, sounds at the mouth have much wider frequencies, ranging between 200 and 2000 Hz—very much like white noise (Fig. 14-4).
17 Are there differences in intensity of breath sounds between the various types of airflow obstruction?
Yes (Table 14-2). In fact, the intensity of inspiratory breath sounds at the mouth can help differentiate emphysema from chronic bronchitis or asthma, since in only the last two conditions it directly correlates with (1) increased airway resistance; (2) reduced FEV1 (forced expiratory volume in 1 sec); and (3) reduced peak expiratory flow rate (PEFR). Conversely, in emphysema, inspiratory breath sounds at the mouth are paradoxically quiet and almost silent because emphysema causes no direct narrowing of the bronchi, but only a dynamic expiratory airflow obstruction due to loss of elastic recoil.
| Lung Disease | Breath Sounds | Adventitious Lung Sound |
|---|---|---|
| Pneumonia | Bronchial or absent | Inspiratory crackles |
| Atelectasis | Harsh/bronchial | Late inspiratory crackles |
| Pneumothorax | Absent | None |
| Emphysema | Diminished | Early inspiratory crackles |
| Chronic bronchitis | Normal | Wheezes and crackles |
| Pulmonary fibrosis | Harsh | Inspiratory crackles |
| Congestive heart failure | Diminished | Inspiratory crackles |
| Pleural effusion | Diminished | None |
| Asthma | Diminished | Wheezes |
(From Wilkins R: Lung Sounds. St. Louis, Mosby, 1996.)
22 What are the main characteristics of tubular breath sounds?
26 What are the three characteristics of vesicular breath sounds?
 Low frequency. Hence, soft and muffled, graphically represented by thin respiratory lines.
Low frequency. Hence, soft and muffled, graphically represented by thin respiratory lines.
 Absence of the silent pause between inspiration and expiration
Absence of the silent pause between inspiration and expiration
 Short duration of exhalation because most high frequencies are concentrated in the last two thirds of exhalation and thus totally eliminated by the presence of alveolar air.
Short duration of exhalation because most high frequencies are concentrated in the last two thirds of exhalation and thus totally eliminated by the presence of alveolar air.
37 Are vesicular breath sounds normally heard throughout the chest?
No. Although present over most lung fields of normal patients, vesicular sounds are classically absent in two narrow areas, corresponding anteriorly and posteriorly to the trachea and central bronchi (parascapular areas). Here, the vesicular sounds are replaced by bronchovesicular sounds (see also below, questions 60–62). Outside these narrow areas, presence of distinct vesicular sounds remains a sine qua non for a healthy lung (Fig. 14-6).
41 What is the best bedside predictor for the presence of chronic obstructive lung disease?
A reduction in breath sound intensity (BSI). A total of 32 findings has been said to indicate COPD, with many arguing strongly for its presence (Table 14-3), yet BSI is the single best index of emphysema. Early inspiratory crackles also argue for obstruction (LR, 14.6), but mostly chronic bronchitis. If progressive over time, BSI reduction can help monitor methacholine challenge, even when wheezing is absent. Finally, any two of the following virtually rule in airflow limitation: >70-pack-years of smoking, decreased breath sounds, or history of COPD. Years of cigarette smoking, subjective wheezing, and either objective wheezing or peak expiratory flow rate also predict the likelihood of airflow limitation in males. Although other signs have been linked to obstruction (objective wheezing, barrel chest, positive match test, rhonchi, hyperresonance, and subxiphoid apical impulse), on multivariate analysis only three remain significantly associated with its diagnosis: self-reported history of COPD (LR, 4.4), wheezing (LR, 2.9), and FET >9 seconds (LR, 4.6). Patients with all three have an LR of 33 (ruling in COPD); those with none have an LR of 0.18 (ruling out COPD).
Table 14-3 Accuracy of Bedside Findings for the Evaluation of Obstructive Lung Disease: Likelihood Ratios, Point Estimates, and 95% Confidence Intervals
| Findings | Positive LR(95% CI) | Negative LR(95% CI) |
|---|---|---|
| Subxiphoid cardiac impulse | 7.4 (2.0, 27.1) | 0.9 (0.7, 1.1) |
| Absent cardiac dullness | 11.8 (1.2, 121.4) | 0.9 (0.7, 1.1) |
| Hyperresonance | 5.1 (1.7, 15.6) | 0.7 (0.5, 1.0) |
| Diaphragm excursion <2 cm | 5.3 (0.8, 35.0) | 0.9 (0.7, 1.1) |
| Breath sound intensity <9 | 10.2 (4.6, 22.7) | – |
| Breath sound intensity 10–12 | 3.6 (1.4, 9.5) | – |
| Breath sound intensity 13–15 | 0.7 (0.3, 1.5) | – |
| Breath sound intensity >15 | 0.1 (0, 0.3) | – |
| Forced expiratory time <3 sec | 0.2 (0.1, 0.3) | – |
| Forced expiratory time 3–9 sec | 1.3 (0.5, 2.9) | – |
| Forced expiratory time >9 sec | 4.8 (1.3, 17.6) | – |
| Early crackles, detecting obstructive disease | 14.6 (3.0, 70) | 0.4 (0.1, 1.4) |
| Early crackles, detecting severe obstruction | 20.8 (3.0, 142.2) | 0.1 (0, 0.4) |
| Unforced wheezes, detecting obstructivedisease | 6.0 (2.4, 15.1) | 0.7 (0.6, 1.0) |
| Methacholine wheezes, detecting asthma | 6.0 (1.5, 24.3) | 0.6 (0.4, 0.9) |
| Diminished breath sounds, detecting asthma | 4.2 (1.9, 9.5) | 0.3 (0.1, 0.6) |
(Adapted from McGee S: Evidence-Based Physical Diagnosis. Philadelphia, WB Saunders, 2001.)
42 How can one objectively measure breath sounds’ intensity at the bedside?
By a scoring system originally devised by Pardee:
 Ask the patient to sit up and inspire from residual volume, fast and deep, while at the same time breathing through the mouth. This generates a breath sound as loud as possible.
Ask the patient to sit up and inspire from residual volume, fast and deep, while at the same time breathing through the mouth. This generates a breath sound as loud as possible.
 Auscultate bilaterally over the upper anterior zones, the mid-axillae, and the posterior bases.
Auscultate bilaterally over the upper anterior zones, the mid-axillae, and the posterior bases.
 Quantify the intensity of the inspiratory component of the vesicular breath sounds as: 0, absent; 1, barely audible; 2, faint but definitely audible; 3, normal; 4, louder than normal.
Quantify the intensity of the inspiratory component of the vesicular breath sounds as: 0, absent; 1, barely audible; 2, faint but definitely audible; 3, normal; 4, louder than normal.
 The sum of sound intensities recorded in each area generates the BSI score. This ranges from 0 to 24 (for, respectively, scores of 0 or 4 in each of the six areas).
The sum of sound intensities recorded in each area generates the BSI score. This ranges from 0 to 24 (for, respectively, scores of 0 or 4 in each of the six areas).
 To best use this method, you must disregard superimposed adventitious sounds (rhonchi, wheezes, or crackles), which, since they are louder than the underlying breath sound, would overestimate the BSI.
To best use this method, you must disregard superimposed adventitious sounds (rhonchi, wheezes, or crackles), which, since they are louder than the underlying breath sound, would overestimate the BSI.
44 In addition to airflow obstruction, is there any other process associated with distant breath sounds?
Pneumonia. A reduced BSI with fever and cough is very suggestive of it.
52 What is the cause of this improved transmission?
1. Consolidation (i.e., replacement of alveolar air with a mantle of solidified lung that can better transmit higher frequencies). Consolidation reflects either alveolar collapse or alveolar fluid-filling:
 Alveolar collapse (with patent airways) occurs in pleural effusions, whenever the amount of fluid is large enough to compress the alveoli but too small to compress the airways.
Alveolar collapse (with patent airways) occurs in pleural effusions, whenever the amount of fluid is large enough to compress the alveoli but too small to compress the airways. Alveolar fluid-filling occurs instead in situations of pneumonia (pus in the alveoli), alveolar hemorrhage (blood in the alveoli), or pulmonary edema (serum in the alveoli). In fact, in patients with cough and fever, the presence of bronchial breath sounds argues strongly in favor of pneumonia. Yet, its absence cannot rule it out, since the finding is specific but poorly sensitive.
Alveolar fluid-filling occurs instead in situations of pneumonia (pus in the alveoli), alveolar hemorrhage (blood in the alveoli), or pulmonary edema (serum in the alveoli). In fact, in patients with cough and fever, the presence of bronchial breath sounds argues strongly in favor of pneumonia. Yet, its absence cannot rule it out, since the finding is specific but poorly sensitive.2. Bronchial breath sounds also may be heard in situations of pulmonary fibrosis. This mechanism, however, requires severe fibrosis and tends to be less common than simple consolidation.
61 What are the three acoustic characteristics of bronchovesicular breath sounds?
 Like tubular sounds, they have long and well-preserved expiration (with an I:E ratio of 1:1).
Like tubular sounds, they have long and well-preserved expiration (with an I:E ratio of 1:1).
 Like vesicular sounds, they lack a silent pause between inspiration and expiration.
Like vesicular sounds, they lack a silent pause between inspiration and expiration.
 They are softer/lower-pitched than tubular sounds, but harsher/higher-pitched than vesicular.
They are softer/lower-pitched than tubular sounds, but harsher/higher-pitched than vesicular.
 These half-way characteristics are all due to the peculiar transmission of these sounds. After being produced by turbulent flow in large and central airways (distal trachea and main bronchi), bronchovesicular sounds cross only a thin mantle of alveolar air before reaching the stethoscope. Hence, in contrast to tracheal breath sounds (which are heard immediately over the neck and thus have no alveolar air to cross), bronchovesicular sounds still have to undergo some physical changes (mostly filtering of high frequencies) before reaching the chest surface. Yet this filtering is not of the same degree as that undergone by vesicular sounds. Hence, their hybrid features.
These half-way characteristics are all due to the peculiar transmission of these sounds. After being produced by turbulent flow in large and central airways (distal trachea and main bronchi), bronchovesicular sounds cross only a thin mantle of alveolar air before reaching the stethoscope. Hence, in contrast to tracheal breath sounds (which are heard immediately over the neck and thus have no alveolar air to cross), bronchovesicular sounds still have to undergo some physical changes (mostly filtering of high frequencies) before reaching the chest surface. Yet this filtering is not of the same degree as that undergone by vesicular sounds. Hence, their hybrid features.
62 What is the clinical significance of bronchovesicular breath sounds?
It depends on location. If heard over the parasternal and parascapular areas (both anteriorly and posteriorly), they are normal. Anywhere else, they indicate pathology—usually early consolidation (with enhanced transmission of high frequencies) or a thin pleural effusion, partially compressing the alveoli but not the bronchi. (Fig. 14-7)
B. Adventitious Lung Sounds
63 What are adventitious lung sounds?
They are extra (i.e., adventitious) sounds that are normally absent in a respiratory cycle but become superimposed on the underlying breath sound (vesicular or bronchial) whenever disease occurs. Theirs is a confusing alphabet soup of rubs, squeaks, crackles, wheezes, stridor, and rhonchi. (Table 14-4)
65 When was the classification of adventitious lung sounds revised?
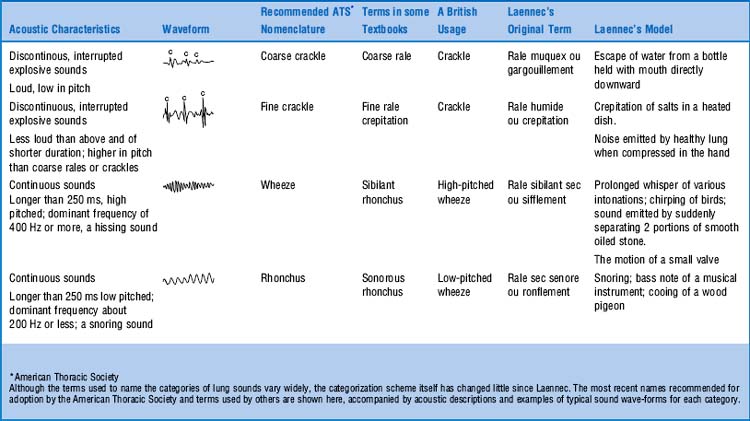
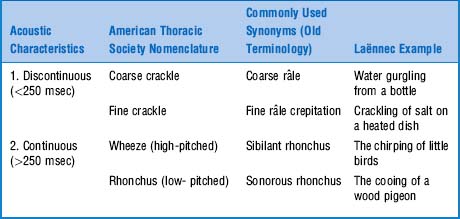
In 1977. The problem, of course, was Laënnec’s terminology and his original “daily life” examples. This was compounded by his inability to even use the term râle at the bedside, since it reminded his patients of the French expression “le râle de la mort” (the death rattle)—that is, the gurgling sound of dying people too ill to clear secretions. To avoid any bedside miscommunication, Laënnec often referred to these sounds as rhonchi, which was the Latin (and less scary) equivalent of rattles. Still, râles and rhonchi meant the same thing to him. This, however, escaped his first English translator, Dr. John Forbes, who decided that rhonchi should only describe long sounds, whereas râles should describe short sounds. Not all translators, however, complied with this rule, thus triggering the beginning of the end for Laënnec’s classification. By the 1970s, the confusion was so bad that Fraser and Pare reported that “every physician seems to have his own classification.” This eventually led to a reclassification by a team of international experts, with recommendations published in 1977, more than 150 years after Laënnec’s original terminology (Table 14-5).
67 So how should crackles be described?
 Depending on their number, as either scanty or profuse
Depending on their number, as either scanty or profuse
 Depending on their predominant frequency, as high-pitched (fine) or low-pitched (coarse)
Depending on their predominant frequency, as high-pitched (fine) or low-pitched (coarse)
 Depending on their amplitude of oscillations, as either faint or loud
Depending on their amplitude of oscillations, as either faint or loud
 Finally, depending on their timing during inspiration, as either early, mid, or late inspiratory
Finally, depending on their timing during inspiration, as either early, mid, or late inspiratory
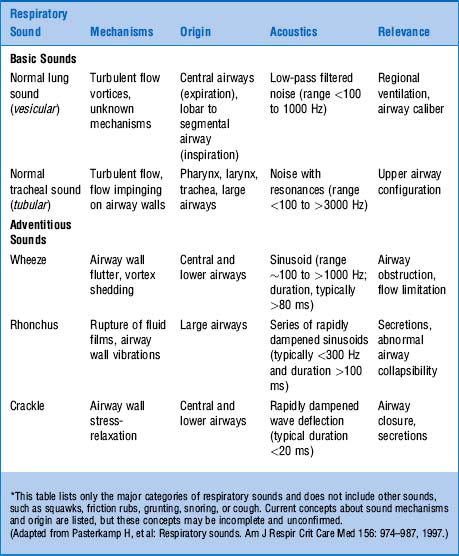
69 How are adventitious lung sounds produced?
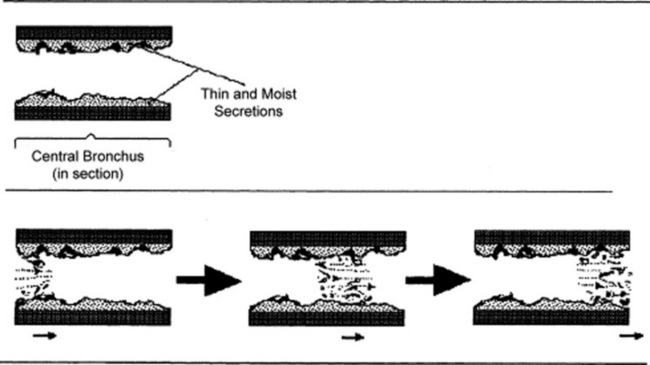
 Rupture of fluid films or bubbles: This is responsible for coarse crackles (discontinuous adventitious lung sounds) and occurs whenever air flows through large central airways coated with thin secretions. The air–fluid interface causes the rupture of fluid films and bubbles, resulting in crackling noises. These are typical of acute and chronic bronchitis and were called by Laënnec “râles gargouillement,” an expression which included the death rattle. (Fig. 14-8)
Rupture of fluid films or bubbles: This is responsible for coarse crackles (discontinuous adventitious lung sounds) and occurs whenever air flows through large central airways coated with thin secretions. The air–fluid interface causes the rupture of fluid films and bubbles, resulting in crackling noises. These are typical of acute and chronic bronchitis and were called by Laënnec “râles gargouillement,” an expression which included the death rattle. (Fig. 14-8)
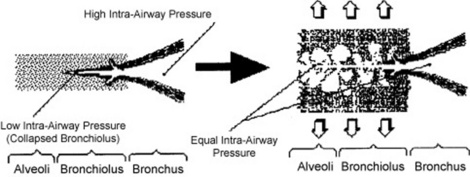
 Sudden equalization of intra-airway pressure: This is responsible for fine crackles, which are also discontinuous adventitious lung sounds, but more typical of pneumonia, pulmonary hemorrhage, pulmonary edema, and pulmonary fibrosis. Sudden equalization of intra-airway pressure occurs whenever small airways that are partially collapsed suddenly “pop” open in inspiration. The partial collapse of distal airways is due to high interstitial pressure, the result of either scarring (pulmonary fibrosis) or fluid (pus, blood, serum). (Fig. 14-9)
Sudden equalization of intra-airway pressure: This is responsible for fine crackles, which are also discontinuous adventitious lung sounds, but more typical of pneumonia, pulmonary hemorrhage, pulmonary edema, and pulmonary fibrosis. Sudden equalization of intra-airway pressure occurs whenever small airways that are partially collapsed suddenly “pop” open in inspiration. The partial collapse of distal airways is due to high interstitial pressure, the result of either scarring (pulmonary fibrosis) or fluid (pus, blood, serum). (Fig. 14-9)
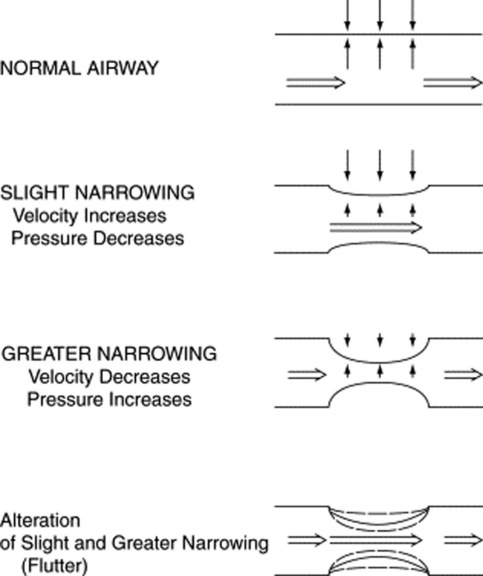
 Fluttering of the airway wall: This is responsible for wheezes, which are instead continuous adventitious lung sounds. Fluttering occurs whenever air flows rapidly through airways that have been narrowed by either bronchospasm or thick secretions/edema. The underlying mechanism is the Bernoulli principle, which also governs the water vacuum pump of many labs. In the case of the pump, it is the water rapidly flowing through a narrow tube that produces a sucking effect (which, in turn, draws-in air through a hole in the tube). In the case of wheezes, however, there is no hole in the airway wall. As a result, air flowing rapidly through a narrow bronchus will simply draw-in the airway wall, thus creating a fluttering and a wheeze (Fig. 14-10).
Fluttering of the airway wall: This is responsible for wheezes, which are instead continuous adventitious lung sounds. Fluttering occurs whenever air flows rapidly through airways that have been narrowed by either bronchospasm or thick secretions/edema. The underlying mechanism is the Bernoulli principle, which also governs the water vacuum pump of many labs. In the case of the pump, it is the water rapidly flowing through a narrow tube that produces a sucking effect (which, in turn, draws-in air through a hole in the tube). In the case of wheezes, however, there is no hole in the airway wall. As a result, air flowing rapidly through a narrow bronchus will simply draw-in the airway wall, thus creating a fluttering and a wheeze (Fig. 14-10).
 Rubbing of inflamed pleural surfaces: This is responsible for the pleural friction rub. The two pleural layers are roughened by inflammation and covered with fibrin, and they grate against each other during respiration. This produces a leather-like sound that is typically inspiratory and expiratory.
Rubbing of inflamed pleural surfaces: This is responsible for the pleural friction rub. The two pleural layers are roughened by inflammation and covered with fibrin, and they grate against each other during respiration. This produces a leather-like sound that is typically inspiratory and expiratory.
(1) Discontinuous Adventitious Lung Sounds
73 What is the underlying breath sound of crackles?
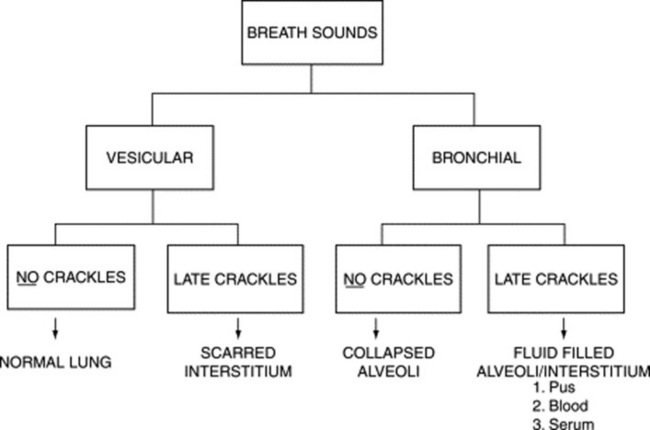
It depends on the type of crackles. For early and mid-inspiratory ones, it is vesicular, whereas for late-inspiratory crackles it is either vesicular or bronchial. This distinction may help differentiate the underlying disease. For example, a process that causes fluid-filling of the interstitium and alveoli (like pneumonia, pulmonary edema, or pulmonary hemorrhage) is much more likely to present with late-inspiratory crackles (due to interstitial fluid) and bronchial breath sounds (due to alveolar fluid). Conversely, scarring of the interstitium (i.e., pulmonary fibrosis) is much more likely to present with late-inspiratory crackles and vesicular breath sounds. This algorithm (Fig. 14-11) is simple but not simplistic and may be useful at the bedside.
74 How do crackles get produced?
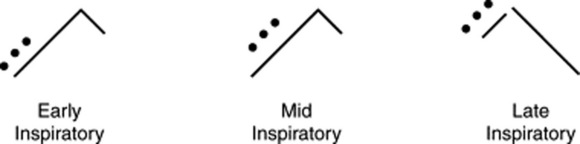
It depends on their timing in the respiratory cycle (Fig. 14-12):
 Early and mid-inspiratory crackles are coarse sounds produced by bubbling of air through thin secretions in large and medium-sized airways (as in, respectively, bronchitis and bronchiectasis). These crackles are superimposed on the underlying breath sound and often clear with coughing. Since they originate in large central airways, they are mostly heard over the central chest, both posteriorly and anteriorly. In addition to air-fluid interface, bronchiectatic crackles also may result from the dilation caused by destruction of the musculoelastic framework. Loss of support causes the airway to collapse in expiration and then suddenly reopen in inspiration, in a to-and-fro cycle that produces mid-inspiratory crackles that do not clear with coughing (since they are not due to secretions).
Early and mid-inspiratory crackles are coarse sounds produced by bubbling of air through thin secretions in large and medium-sized airways (as in, respectively, bronchitis and bronchiectasis). These crackles are superimposed on the underlying breath sound and often clear with coughing. Since they originate in large central airways, they are mostly heard over the central chest, both posteriorly and anteriorly. In addition to air-fluid interface, bronchiectatic crackles also may result from the dilation caused by destruction of the musculoelastic framework. Loss of support causes the airway to collapse in expiration and then suddenly reopen in inspiration, in a to-and-fro cycle that produces mid-inspiratory crackles that do not clear with coughing (since they are not due to secretions).
 Late-inspiratory crackles are instead fine sounds that occur during the reopening of distal airways that have become partially occluded as a result of high interstitial pressure. Since the two ends of these semi-collapsed bronchioli have different intra-airway pressures (a high central and a low distal), their sudden inspiratory reopening causes rapid intraluminal equalization and, thus, a “pop.” The high interstitial pressure behind them is usually due to either scarring of the interstitium or fluid-filling (such as pus, blood, or serum). Hence, late-inspiratory crackles suggest interstitial fibrosis or interstitial edema (from pneumonia, hemorrhage, or congestive failure).
Late-inspiratory crackles are instead fine sounds that occur during the reopening of distal airways that have become partially occluded as a result of high interstitial pressure. Since the two ends of these semi-collapsed bronchioli have different intra-airway pressures (a high central and a low distal), their sudden inspiratory reopening causes rapid intraluminal equalization and, thus, a “pop.” The high interstitial pressure behind them is usually due to either scarring of the interstitium or fluid-filling (such as pus, blood, or serum). Hence, late-inspiratory crackles suggest interstitial fibrosis or interstitial edema (from pneumonia, hemorrhage, or congestive failure).
79 What should one do at the bedside when confronted with crackles?
 Early crackles originate in large central airways and reflect bronchitis (acute or chronic).
Early crackles originate in large central airways and reflect bronchitis (acute or chronic).
 Mid-inspiratory crackles originate in medium-sized airways and reflect bronchiectasis.
Mid-inspiratory crackles originate in medium-sized airways and reflect bronchiectasis.
 Late crackles arise from peripheral airways and, depending on the patients’ presentation, reflect either fluid in the interstitium or scarring. In asbestos workers, for example, they indicate asbestosis; in patients with fever and cough, they suggest pneumonia; and in cardiac patients, they favor increased left atrial pressure.
Late crackles arise from peripheral airways and, depending on the patients’ presentation, reflect either fluid in the interstitium or scarring. In asbestos workers, for example, they indicate asbestosis; in patients with fever and cough, they suggest pneumonia; and in cardiac patients, they favor increased left atrial pressure.
80 Summarize the characteristics of early, mid-inspiratory, and late-inspiratory crackles.
| Early and Mid-Inspiratory | Late Inspiratory |
|---|---|
| Coarse | Fine |
| Low-pitched | High-pitched |
| Scanty | Profuse |
| Gravity independent | Gravity dependent |
| Do not change with posture | Change with posture |
| Clear with coughing | Do not clear with coughing |
| Well transmitted to the mouth | Poorly transmitted to the mouth |
| Associated with obstruction | Associated with restriction |
83 Are late-inspiratory crackles present in all interstitial lung disease?
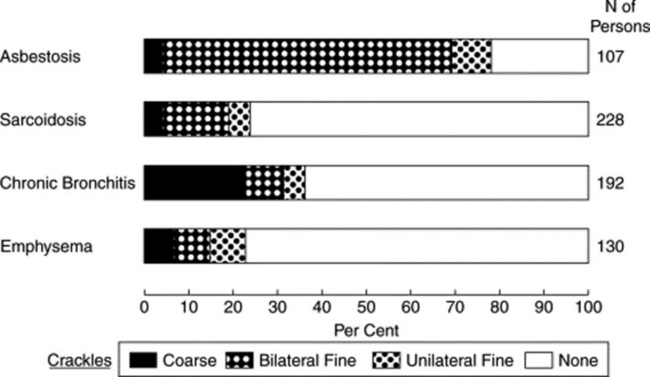
No. Although reported in 65–91% of chronic interstitial pulmonary diseases (including asbestosis or idiopathic pulmonary fibrosis), they occur in only 5–20% of sarcoidosis cases. Fine crackles also are rare in other granulomatous disorders (like miliary tuberculosis, eosinophilic granuloma, or allergic alveolitis), and similarly rare in intra-alveolar processes (only 20% of pulmonary alveolar proteinosis cases). Still, whenever present, sarcoid crackles are basilar too, fine, and late-inspiratory (see Fig. 14-13).
86 Where are asbestosis crackles localized?
Usually at the bases, first centrally (along the mid-axillary lines) and then posterolaterally.
88 Are crackles common in patients with idiopathic pulmonary fibrosis (IPF)?
Yes. In fact, so common (up to 100%) that their absence argues strongly against the diagnosis.
89 Is there a correlation between late-inspiratory crackles and severity of IPF?
Yes. In addition to fewer crackles, milder forms of the disease have crackles that are only late inspiratory and gravity dependent (i.e., limited to the bases in upright patients). As the disease progresses, crackles become paninspiratory (even though still predominant in end-inspiration). They may also persist despite postural changes, eventually extending to higher lung regions. Finally, they may become associated with late-inspiratory squeaks (see below, questions 113 and 114).
90 Can crackles occur in exhalation?
 In obstructive processes (such as bronchitis or bronchiectasis), they tend to be coarse, early expiratory, gravity independent, and profuse. They decrease in number with coughing.
In obstructive processes (such as bronchitis or bronchiectasis), they tend to be coarse, early expiratory, gravity independent, and profuse. They decrease in number with coughing.
 In restrictive processes (such as pulmonary fibrosis or connective tissue disease), they are fine, mid- or late expiratory, gravity dependent, and scanty. They do not resolve with coughing.
In restrictive processes (such as pulmonary fibrosis or connective tissue disease), they are fine, mid- or late expiratory, gravity dependent, and scanty. They do not resolve with coughing.
91 What is the mechanism of production of late-expiratory crackles?
 High interstitial pressure (from interstitial fibrosis, for example) would collapse a small airway.
High interstitial pressure (from interstitial fibrosis, for example) would collapse a small airway.
 The inspiratory traction would snap the airway open, thus creating a “pop” in late inspiration.
The inspiratory traction would snap the airway open, thus creating a “pop” in late inspiration.
 The airway would then recoil in early exhalation, closing its lumen once again. This, in turn, would set up a new reopening, which this time occurs in late exhalation, when the intraluminal air exceeds pressure in the adjacent airways. The reopening produces a late-expiratory crackle.
The airway would then recoil in early exhalation, closing its lumen once again. This, in turn, would set up a new reopening, which this time occurs in late exhalation, when the intraluminal air exceeds pressure in the adjacent airways. The reopening produces a late-expiratory crackle.
(2) Special Problem—Pneumonia
95 What about the presence of diminished breath sounds?
It also can occur in pneumonia, but usually in the setting of a concomitant pleural effusion.
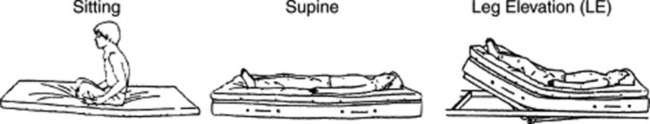
101 What’s the best way to elicit PICs?
By following these steps (Fig. 14-14):
1. Have the patient sit in bed upright for 3 minutes.
2. Listen over the eighth, ninth, and tenth interspaces along the posterior axillary line. Listen for crackles during at least five consecutive breaths. Pay special attention to late inspiration. Make sure that each breath starts from residual volume and ends in total lung capacity.
3. Have the patient assume a supine position, maintaining it for 3 minutes.
4. Listen again by using the same method outlined in step 2.
5. Have the patient elevate both legs passively at an angle of 30 degrees for 3 minutes.
6. Listen again by using the same method outlined in step 2.
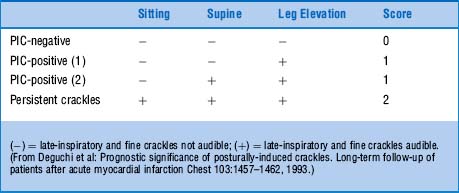
102 How should this maneuver be interpreted?
 If late-inspiratory crackles are absent in all positions (sitting, supine, and leg-elevated), the test is negative, and the patient receives a score of 0.
If late-inspiratory crackles are absent in all positions (sitting, supine, and leg-elevated), the test is negative, and the patient receives a score of 0.
 If late-inspiratory crackles are absent in the sitting position but become audible in the supine or leg-elevated position, the test is positive for PICs, and the patient receives a score of 1.
If late-inspiratory crackles are absent in the sitting position but become audible in the supine or leg-elevated position, the test is positive for PICs, and the patient receives a score of 1.
 If late-inspiratory crackles are already present in the sitting position and continue throughout supine and leg-elevated changes, they are persistent, and the patient receives a score of 2.
If late-inspiratory crackles are already present in the sitting position and continue throughout supine and leg-elevated changes, they are persistent, and the patient receives a score of 2.
108 What are monophonic and polyphonic CALs?
CALs that can be compared to either a solo singer (monophonic wheezes) or a polyphonic choir.
 Monophonic wheezes consist of single or multiple notes starting and ending at different times. A good example is the rhonchus produced by a tumor almost completely occluding a bronchus.
Monophonic wheezes consist of single or multiple notes starting and ending at different times. A good example is the rhonchus produced by a tumor almost completely occluding a bronchus.
 Polyphonic wheezes contain instead several notes, all starting and ending at the same time, like a chord. These can occur in healthy people at the end of a forceful exhalation, but are usually more typical of asthma, where narrowing of multiple airways produces a real polyphonic choir: the so-called “concertus asthmaticus.” Because fluttering of large airway walls is diffuse in asthma, CALs occur throughout the lung fields (see expiratory polyphonic CALs in question 113).
Polyphonic wheezes contain instead several notes, all starting and ending at the same time, like a chord. These can occur in healthy people at the end of a forceful exhalation, but are usually more typical of asthma, where narrowing of multiple airways produces a real polyphonic choir: the so-called “concertus asthmaticus.” Because fluttering of large airway walls is diffuse in asthma, CALs occur throughout the lung fields (see expiratory polyphonic CALs in question 113).
110 How are wheezes produced?
 Airways would have to be 4–8 feet long, which is the necessary length for producing some of the lowest-pitched wheezes of asthma. Instead, the bronchial tree is <1 foot long.
Airways would have to be 4–8 feet long, which is the necessary length for producing some of the lowest-pitched wheezes of asthma. Instead, the bronchial tree is <1 foot long.
 The frequency of a wheeze generated by a particular airway would stay constant throughout respiration (while instead it differs by as much as 1 octave between inspiration and expiration).
The frequency of a wheeze generated by a particular airway would stay constant throughout respiration (while instead it differs by as much as 1 octave between inspiration and expiration).
 The pitch of a wheeze would change when breathing a mixture of helium and oxygen (just as the pitch of an organ pipe would rise when blown with helium). Instead, the pitch is constant.
The pitch of a wheeze would change when breathing a mixture of helium and oxygen (just as the pitch of an organ pipe would rise when blown with helium). Instead, the pitch is constant.
111 What is the physical principle behind this mechanism?
It is the Bernoulli principle, which centers on a local drop in intra-airway pressure whenever air flows at high velocity through narrowed pipes. The faster the flow at point of constriction, the lower the local pressure. Eventually, the drop in pressure becomes severe enough to collapse the airway. Since the collapse also reduces flow, the airway reopens and the fluttering cycle starts anew, repeating itself indefinitely (see Fig. 14-5). Thus, wheezes are not produced by vibration of air (as in organ pipes or woodwind instruments), but by vibration (fluttering) of airway walls. This is facilitated by fast jets of air, forced by high expiratory pressures through tightly compressed airways.
113 How are CALs classified?
 Expiratory polyphonic CALs are multiple musical tones that occur only in exhalation. Each component has constant frequency and similar duration, but all have a high-pitched hissing quality that earns them the name of wheezes. Although typical of mild asthma, they also may be heard in healthy subjects at the end of a forced exhalation. Because each tone has its own frequency, their summation generates a polyphonic sound, like the one of a chorus. Since they reflect widespread alteration of airway mechanics, they are present throughout the lung fields. (Fig. 14-15)
Expiratory polyphonic CALs are multiple musical tones that occur only in exhalation. Each component has constant frequency and similar duration, but all have a high-pitched hissing quality that earns them the name of wheezes. Although typical of mild asthma, they also may be heard in healthy subjects at the end of a forced exhalation. Because each tone has its own frequency, their summation generates a polyphonic sound, like the one of a chorus. Since they reflect widespread alteration of airway mechanics, they are present throughout the lung fields. (Fig. 14-15)
 Random monophonic CALs are single or multiple musical tones of various frequency and duration that occur randomly throughout respiration. They are produced by fluttering of central airways, narrowed by bronchospasm or inflammation. They have a high-pitched hissing quality and are often referred to as multiple monophonic wheezes. They are typical of severe asthma (status asthmaticus), where they occur throughout the chest. Like expiratory polyphonic CALs, they can occur in exhalation, though they more typically tend to cover the entire respiratory cycle. Yet, in contrast to stridor and squeaks, they are never limited to inspiration.(Fig. 14-16)
Random monophonic CALs are single or multiple musical tones of various frequency and duration that occur randomly throughout respiration. They are produced by fluttering of central airways, narrowed by bronchospasm or inflammation. They have a high-pitched hissing quality and are often referred to as multiple monophonic wheezes. They are typical of severe asthma (status asthmaticus), where they occur throughout the chest. Like expiratory polyphonic CALs, they can occur in exhalation, though they more typically tend to cover the entire respiratory cycle. Yet, in contrast to stridor and squeaks, they are never limited to inspiration.(Fig. 14-16)
 Fixed monophonic CALs are single musical tones of constant frequency and long duration that are generated by the vibration of a large and partially obstructed bronchus because of tumor, inflammation, secretions, or a foreign body. They are low pitched and snoring and often are referred to as rhonchi. Change in posture may soften or eliminate them. Still, a localized and persistent rhonchus should arouse suspicion, since it can be the earliest and only abnormal finding of an endobronchial lesion. Auscultatory site usually reflects the location of the process.
Fixed monophonic CALs are single musical tones of constant frequency and long duration that are generated by the vibration of a large and partially obstructed bronchus because of tumor, inflammation, secretions, or a foreign body. They are low pitched and snoring and often are referred to as rhonchi. Change in posture may soften or eliminate them. Still, a localized and persistent rhonchus should arouse suspicion, since it can be the earliest and only abnormal finding of an endobronchial lesion. Auscultatory site usually reflects the location of the process.
 Sequential inspiratory CALs are also called late-inspiratory squeaks, or squawks (in John Earis’ 1982 description). They are produced by the reopening of partially collapsed airways and thus are common in interstitial lung diseases (especially at the bases), where they coexist with late-inspiratory crackles. Except in the case of squeaks, the airway has a very irregular lumen, usually due to regenerating mucosa (as in bronchiolitis obliterans). It’s this irregularity (and the resulting narrowing) that is responsible for the high-pitched and squeaky characteristics of the sound. Hence, air passing through a newly reopened but still partially narrowed airway (because of the irregular lumen) will cause both a crackle (as the airway opens abruptly) and a wheeze (as the air rushes through the narrowing). Squeaks can be simple (monophonic) or multiple (polyphonic), musical, and of various duration and frequency. Most often, they are single, short, high pitched, and resembling a late-inspiratory wheeze. Laënnec called it “le cri d’un petit oiseau” (the chirp of a little bird).
Sequential inspiratory CALs are also called late-inspiratory squeaks, or squawks (in John Earis’ 1982 description). They are produced by the reopening of partially collapsed airways and thus are common in interstitial lung diseases (especially at the bases), where they coexist with late-inspiratory crackles. Except in the case of squeaks, the airway has a very irregular lumen, usually due to regenerating mucosa (as in bronchiolitis obliterans). It’s this irregularity (and the resulting narrowing) that is responsible for the high-pitched and squeaky characteristics of the sound. Hence, air passing through a newly reopened but still partially narrowed airway (because of the irregular lumen) will cause both a crackle (as the airway opens abruptly) and a wheeze (as the air rushes through the narrowing). Squeaks can be simple (monophonic) or multiple (polyphonic), musical, and of various duration and frequency. Most often, they are single, short, high pitched, and resembling a late-inspiratory wheeze. Laënnec called it “le cri d’un petit oiseau” (the chirp of a little bird).
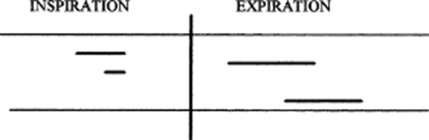
115 How are CALs graphically represented?
By bars, in a way very similar to the representation of musical notes:
 The pitch of the CALs is graphically indicated by the height of the bar (i.e., the vertical location of the bar above the respiratory cycle; the higher the pitch, the higher the bar and vice versa).
The pitch of the CALs is graphically indicated by the height of the bar (i.e., the vertical location of the bar above the respiratory cycle; the higher the pitch, the higher the bar and vice versa).
 The loudness is represented by the thickness of the bar (thicker bars, louder sounds).
The loudness is represented by the thickness of the bar (thicker bars, louder sounds).
 The duration is represented by the length of the bar (the longer the bar, the longer the sound).
The duration is represented by the length of the bar (the longer the bar, the longer the sound).
 Finally, the timing of the CALs is represented by their location within the respiratory cycle. CALs may be early or late expiratory, and even panrespiratory (i.e., throughout the cycle).
Finally, the timing of the CALs is represented by their location within the respiratory cycle. CALs may be early or late expiratory, and even panrespiratory (i.e., throughout the cycle).
123 What are then the acoustic characteristics of a resolving asthma attack?
A decrease in frequency of the highest-pitched wheeze component, and a decrease in Tw/Ttot.
124 Summarize the time course of status asthmaticus based on auscultation.
 In mild asthma, the site of dynamic obstruction is in the central airways. The bronchial fluttering generates random monophonic wheezes that are loud and well transmitted, both upward (to the mouth) and downward (to the chest wall).
In mild asthma, the site of dynamic obstruction is in the central airways. The bronchial fluttering generates random monophonic wheezes that are loud and well transmitted, both upward (to the mouth) and downward (to the chest wall).
 In worse asthma, the site of dynamic obstruction is instead peripheral. Because airflow in small airways is too slow to cause major fluttering, the sounds generated will be random monophonic wheezes, barely audible at the chest wall and too weak to reach the mouth.
In worse asthma, the site of dynamic obstruction is instead peripheral. Because airflow in small airways is too slow to cause major fluttering, the sounds generated will be random monophonic wheezes, barely audible at the chest wall and too weak to reach the mouth.
 Finally, in status asthmaticus, the airflow obstruction is due to a combination of bronchial edema, bronchospasm, and mucous plugging. These changes start in the central airways and cause at first only expiratory, random, monophonic CALs (i.e., wheezes). Subsequently, however, the site of airway fluttering moves gradually toward the periphery of the tracheobronchial tree, where airflow rate is too low to generate adequate vibration. Hence, the chest of patients with advanced status asthmaticus may become paradoxically silent.
Finally, in status asthmaticus, the airflow obstruction is due to a combination of bronchial edema, bronchospasm, and mucous plugging. These changes start in the central airways and cause at first only expiratory, random, monophonic CALs (i.e., wheezes). Subsequently, however, the site of airway fluttering moves gradually toward the periphery of the tracheobronchial tree, where airflow rate is too low to generate adequate vibration. Hence, the chest of patients with advanced status asthmaticus may become paradoxically silent.
128 What is the differential diagnosis of wheezes?
It is a rather broad one. Overall, wheezes are more frequent in asthma than COPD. Still, the old saying, “Not all that wheezes is asthma” is an important reminder that we should always exclude other etiologies before concluding that a wheezy patient is indeed asthmatic (Table 14-8). In a large epidemiologic study, for example, wheezes were present in 25% of the population, whereas the prevalence of asthma was only 7%. Among the extrathoracic causes of wheezing, vocal cord dysfunction is a particularly common one. And, of course, stridor is important, too.
| Infections (croup, whooping cough, laryngitis, tracheobronchitis) |
| Laryngomalacia, tracheomalacia, or bronchomalacia |
| Laryngeal or tracheal tumors |
| Tracheal stenosis |
| Vocal cord dysfunction |
| Foreign body aspiration |
| Large airway compression or stenosis |
| Asthma |
| Chronic obstructive pulmonary disease |
| Bronchorrhea states (such as chronic bronchitis, cystic fibrosis, bronchiectasis) |
| Bronchiolitis obliterans* |
| Interstitial fibrosis * |
| Hypersensitivity pneumonitis* |
| Pulmonary edema |
| Forced expiration in normal subjects |
* Conditions that tend to be associated with a late-inspiratory squeak (or squawk).
139 How can one separate pleural from pericardial rubs?
By asking patients to hold their breath. If the rub persists, it is more likely to be pericardial.
143 What are the physical characteristics of a rub?
The hallmark is a series of short, loud, creaky, and high-frequency sounds. These can be visualized as tall “spikes” superimposed on the underlying breath sound (Fig. 14-17). Hence, the soundwave of a rub is very similar to that of a crackle (although the rub tends to span throughout inspiration and expiration, whereas crackles predominate in inspiration). The frequencies of these spikes are medium to high pitched, but not as high as those of crackles. Still, rubs are well perceived by the human ear and appear loud. Their inspiratory components usually have higher intensity and sometimes may be the only audible sounds. Finally, the rub is characterized by what Forgacs defines as the “mirror image effect,” which refers to the apparent reverse sequence of the expiratory component of the rub as compared with the inspiratory component.
C. Transmitted Voice Sounds
145 What are the most important transmitted voice sounds?
 Bronchophony (Greek for “sound of the bronchi”) indicates a clear voice sound—at least as clear as a voice sound heard over the bronchi or larynx (in bronchophony, however, this sound is heard over chest areas that are remote from either the bronchi or larynx). The words spoken by the patient remain nonetheless unintelligible; only the sound becomes clear and loud.
Bronchophony (Greek for “sound of the bronchi”) indicates a clear voice sound—at least as clear as a voice sound heard over the bronchi or larynx (in bronchophony, however, this sound is heard over chest areas that are remote from either the bronchi or larynx). The words spoken by the patient remain nonetheless unintelligible; only the sound becomes clear and loud.
 Pectoriloquy (Latin for “the voice of the chest”) indicates clear and intelligible words over the chest of patients either whispering (whispered pectoriloquy) or speaking (spoken pectoriloquy).
Pectoriloquy (Latin for “the voice of the chest”) indicates clear and intelligible words over the chest of patients either whispering (whispered pectoriloquy) or speaking (spoken pectoriloquy).
 Egophony (Greek for “the voice of the goat”) is the goat-like and bleating sound produced by the patient’s voice when heard over an area of consolidation.
Egophony (Greek for “the voice of the goat”) is the goat-like and bleating sound produced by the patient’s voice when heard over an area of consolidation.
146 Is there any magic word that one should ask patients to say in order to elicit these sounds?
Not really. In the United States, we ask them to say “ninety-nine” (which is the same number used to elicit tactile fremitus). In Italy (smaller country), “thirty-three” is the number of choice. As Sapira points out, “ninety-nine” is nothing more than the translation of the German “neun und neunzig” (akin to the onomatopeic “oink-oink”), which actually changed the physical characteristics of the original sound, making it possibly less fit for the task. An alternative is to ask the patient to say, “one, two, three.” Still, for eliciting abnormally transmitted voice sounds, any sound will do (except, of course, for eliciting E-to-A changes; see below, questions 151–155).
148 Are the bronchi of patients with abnormally transmitted voice sounds open or closed?
Open. If closed, there would be no sounds transmission across the chest.
1 Baughman RP, Loudon RG. Quantitation of wheezing in acute asthma. Chest. 1984;86:718-722.
2 Baughman RP, Loudon RG. Sound spectral analysis of voice-transmitted sound. Am Rev Respir Dis. 1986;134:167-169.
3 Baughman RP, Loudon RG. Stridor: Differentiation from asthma or upper airway noise. Am Rev Respir Dis. 1989;139:1407-1409.
4 Baughman RP, Shipley RT, Loudon RG, Lower EE. Crackles in interstitial lung disease: Comparison of sarcoidosis and fibrosing alveolitis. Chest. 1991;100:96-101.
5 Bohadana AB, Peslin R, Uffholtz H. Breath sounds in the assessment of airflow obstruction. Thorax. 1978;33:345.
6 Bohadana AB, Kopferschmitt-Kubler MC, Pauli G, et al. Breath sound intensity in patients with airway provocation challenge test positive by spirometry but negative for wheezing. Respiration. 1994;61:274-279.
7 Brenner BE, Abraham E, Simon RR, et al. Position and diaphoresis in acute asthma. Am J Med. 1983;74:1005-1009.
8 Cugell DW. Lung sounds: Classification and controversies. Semin Respir Med. 1985;6:210-219.
9 Deguchi F, Hirakawa S, Gotoh K, et al. Prognostic significance of posturally induced crackles. Long-term follow-up of patients after recovery from acute myocardial infarction. Chest. 1993;103:1457-1462.
10 Earis JE, March K, Pearson MG, Ogilvie CM. The inspiratory “squawk” in extrinsic allergic alveolitis and other pulmonary fibroses. Thorax. 1982;37:923-926.
11 Epler GR, Carrington CB, Gaensler EA, et al. Crackles (râles) in the interstitial pulmonary disease. Chest. 1978;73:333-339.
12 Forgacs P, Nathoo AR, Richardson HD. Breath sounds. Thorax. 1971;26:288-295.
13 Forgacs P. Crackles and wheezes. Lancet. 1967;2:203-205.
14 Forgacs P. Lung Sounds. London: Bailliere Tindall, 1978;34.
15 Forgacs P. The functional basis of pulmonary sounds. Chest. 1978;3:399-405.
16 Gavriely N, Nassan M, Cugell W, Rubin AH. Respiratory health screening using pulmonary function tests and lung sound analysis. Eur Respir J. 1994;7:35-42.
17 Godfrey S, Edwards RH, Campbell EJ, et al. Repeatability of physical signs in airways obstruction. Thorax. 1969;24:4-9.
18 Hidalgo HA, Wegmann MJ, Waring WW, et al. Frequency spectra of breath sounds in childhood. Chest. 1991;100:992-1002.
19 Holleman DRJr, Simel DL. Does clinical examination predict airflow limitation? JAMA. 1995;273:313-319.
20 King DK, Thompson BT, Johnson DC. Wheezing on maximal forced exhalation in the diagnosis of atypical asthma: Lack of sensitivity and specificity. Ann Intern Med. 1989;110:451-455.
21 Lal S, Ferguson AD, Campbell EJ, et al. Forced-expiratory time: A simple test for airway obstruction. BMJ. 1964;1:814.
22 LeBlanc P, Macklem PT, Ross WR, et al. Breath sounds and distribution of ventilation. Am Rev Respir Dis. 1970;102:10-16.
23 Marini JJ, Pierson DJ, Hudson LD, Lakshminarayan S. The significance of wheezing in chronic airflow obstruction. Am Rev Respir Dis. 1979;120:1069-1072.
24 Martell JA, Lopez JG, Harker JE, et al. Pulsus paradoxus in acute asthma in children. J Asthma. 1992;29:349-352.
25 McFadden ER, Kiser R, DeGroot WJ. Acute bronchial asthma: Relations between clinical and physiologic manifestations. N Engl J Med. 1973;388:221-224.
26 McGee S. Evidence-Based Physical Diagnosis. Philadelphia: Saunders, 2001.
27 Meslier N, Charbonneau G, Racineuz JL. Wheezes. Eur Respir J. 1995;8:1942-1948.
28 Murphy RL, Gaensler EA, Holford SK, et al. Crackles in the detection of asbestosis. Am Rev Respir Dis. 1984;129:375-379.
29 Nath AR, Capel LH. Lung crackles in bronchiectasis. Thorax. 1980;35:694-699.
30 Nath AR, Capel LH. Inspiratory crackles: Early and late. Thorax. 1974;29:223-227.
31 Pardee NE, Martin CJ, Morgan EH. A test of the practical value of estimating breath sound intensity. Breath sounds related to measured ventilatory function. Chest. 1976;70:341-344.
32 Pasterkamp H, Kraman SS, Wodicka GR, et al. Respiratory sounds. Am J Respir Crit Care Med. 1997;156:974-987.
33 Rundell KW, Spiering BA. Inspiratory stridor in elite athletes. Chest. 2003;123:468-474.
34 Scheider IC, Anderson AE. Correlation of clinical signs with ventilatory function in obstructive lung disease. Ann Intern Med. 1965;62:477-485.
35 Schilling RSF, Hughes JP, Dingwall I, et al. Disagreement between observers in an epidemiologic study of respiratory disease. BMJ. 1995;1:65.
36 Shim CS, Williams MH. Relationship of wheezing to the severity of obstruction in asthma. Arch Intern Med. 1983;143:890-892.
37 Shirai F, Kudoh S, Shi A, et al. Crackles in asbestos workers. Br J Dis Chest. 1981;75:383-396.
38 Straus SE, McAlister FA, Sackett DL, et al. CARE-COAD2 Group. Clinical Assessment of the Reliability of the Examination-Chronic Obstructive Airways Disease: Accuracy of history, wheezing, and forced expiratory time in the diagnosis of chronic obstructive pulmonary disease. J Gen Intern Med. 2002;17:684-688.
39 Thatcher RE, Kraman SS. The prevalence of auscultatory crackles in subjects without lung disease. Chest. 1982;81:672-674.