Chapter 78 Lumbar Discectomy
An estimated 12 million Americans suffer from significant degenerative lumbar disc disease. Approximately one million patients per year undergo surgeries, of which about 200,000 to 300,000 are lumbar discectomies.1–5 The combination of tremendous volume and a broad spectrum of standard care, including often vague surgical indications, makes lumbar disc pathology an object of legitimate scrutiny. However, proper patient selection and surgical technique can provide excellent and satisfying results for patients and surgeons alike. Neurosurgeons working in this field should remain scrupulously objective in patient selection, abreast of evidence-based outcomes research, and cautious in the selection of new technology.
Lumbar laminectomy for disc hernia or anular prolapse is one of the most common operations performed by North American spine surgeons; it is also one of the most potentially successful. Lumbar laminectomy for herniated lumbar disc has endured considerable analysis and the test of substantial time since its inception 75 years ago and remains a fundamental part of most spine clinical practice. It is a cost-effective procedure for carefully selected patients, being relatively moderate in cost and providing substantial improvement in quality of life.6
The incidence of lumbar disc hernia peaks between 24 and 45 years of age, with the incidence of surgery most often in patients between 30 to 39 years. Males predominate, at 1.3:1 to 2:1, possibly because of larger mechanical stresses and more tenuous nutritional and waste diffusion through the disc, as will be addressed. Other classically regarded risk factors for disc herniation have included smoking, obesity (BMI >30), sedentary lifestyles, prolonged motor vehicle driving, previous full-term pregnancies, and operating heavily vibrating machinery.1,4,7,8
The Twin Spine Study, a research program investigating various environmental factors involved in the etiology and progression of disc degeneration, looked at differing occupational exposures, driving and whole-body vibration exposure, smoking exposure, anthropomorphic factors, heredity, and the identification of genotypes associated with disc degeneration in monozygotic male twins. Although some environmental factors are relevant, as was mentioned, disc degeneration appears to be determined more significantly by genetics. There is a modest correlation with lifting and smoking but little influence from occupational and leisure-time physical loading activities such as sports and resistance training throughout adulthood. The effect of anthropometric factors, such as body weight and muscle strength, appears to be greater than the effect of occupational physical demands. Routine loading and physical demand may actually have some benefits for the disc, physical inactivity being a greater risk factor.9,10
Terminology of disc pathology is of particular importance in the venture of spine surgery and spine clinical research and has important implications for treatment options. The authors endorse the standardization of nomenclature recommended by the combination of the North American Spine Society, the American Society of Spine Radiology, and the American Society of Neuroradiology.11 Not only do diagnostic radiologists and clinicians need to recognize and utilize the same terms to communicate effectively, but vague or loosely applied terms obscure the results of clinical research. The term hernia specifically defines nucleus and/or end-plate cartilaginous tissues escaping the confines of the anulus and residing outside the apophyseal ring. It might not be possible to radiographically determine an anular defect, so the use of the term hernia is legitimately broadened to refer to displacement of nucleus, cartilage, fragmented apophyseal bone, or fragmented anulus from its normal location to lie beyond the disc space; disruption of the anulus is implied. Use of the term anular bulge or disc bulge defines localized or circumferential prominence of an otherwise radiographically intact anulus, not disconnected from the apophyseal ring. It is also useful to add descriptive terms to further define a hernia, such as protrusion (broad base), extrusion (narrow base or neck), and sequestrum (lack of continuity to the disc of origin). The use of the term rupture is discouraged as inaccurate unless there is indeed a single violent traumatic event that is clearly the origin of a defect in a previously intact anulus and of resultant disc herniation.11
History
A condition that is recognized as sciatica, although not associated with spine abnormality, was described before the time of Alexander the Great.12 In 1779, Pott13 was able to associate deformity of the spinal column with sciatic pain. However, it was Lane14 who described sciatic pain and its origin in a living patient in 1893 and Bailey and Casamajor,15 in 1911, who described a small series, complete with radiographic studies. Also in 1911, Goldthwait,16 who thought herniations of the disc were capable of producing sciatic and low back pain, presented a patient with lumbosacral disc hernia and paraplegia. In 1916, Elsberg17 (who operated on the patients of Bailey and Casamajor and often noted relief after apparently no more than decompressive laminectomy) described it as attributable to a condition of cauda equina radiculitis. Parker and Adson18 in 1925, Putti19 in 1927, Dandy20 in 1929, Mauric21 in 1933, and others attributed sciatic pain to nerve root involvement within the spinal canal and believed that adjacent vertebral structures were responsible. In 1934, Mixter and Barr22 published their milestone paper on the pathology and surgical findings associated with a ruptured nucleus pulposus, not only in the lumbar canal but also in the cervical and thoracic canals, complete with their diagnoses of the condition preoperatively.
The surgical procedure of choice for many of these pioneering surgeons was complete laminectomy, which often provided significant relief. Mixter and Barr favored a hemi-laminectomy approach, as did Love, for the cases of simple herniated disc that were amenable to preoperative localization.23 As experience accumulated, it became apparent that dural incision was unnecessary in most cases.
The complicating effect that developmental lumbar stenosis had on the pathology of disc diseases was appreciated in Verbiest’s24,25 reports from 1949 through 1955. Some of the more serious complications of surgery for lumbar disc hernia can be attributed to lack of preoperative appreciation of this anatomic variation and failure to tailor the procedure accordingly.
Working only with myelography and the power of clinical preoperative and subsequent intraoperative observation, the early surgeons were able to learn much and to steadily improve upon the surgical approach to lumbar disc disease. Currently, with the advantages of improved neurodiagnostic modalities in multiple planes, there is no longer much occasion for “surgical exploration,” and it should not be common to find an intraoperative surprise. Surgeons should be capable not only of making the preoperative diagnosis but also of adhering to a secure surgical plan, one that should accomplish the goal of radicular or cauda equina decompression with minimal risk of complication or injury. Credit for the use of magnification and small incisions, which has become standard care, is given Williams26 and Caspar.27
Outcomes
Relief of radiculopathic leg pain can be expected in the vast majority of patients who are appropriately selected for lumbar discectomy, with a 75% to 90% success rate.2,3,28–34 This variability in reported results, which ranged between 75%2 and nearly 90%3 for good to excellent results, likely is due to data quality, patient selection, short follow-up, and differing definitions of good outcome.2 The Asch paper is significant in that outcomes on over 200 patients who were operated on were prospectively determined by six parameters, including the preoperative ODI (Oswestry Disability Index), and the ODI at 1 and 10 days, 6 weeks, and 6 months and at least 12 months postoperatively. One of the most common causes for poor outcome is the poor definition of selection criteria for surgery, which varies remarkably between communities and countries, as much as fourfold or fivefold. 29,35–37The procedure can be expected, with relative certainty, to relieve radiculopathic leg pain, but relief of back pain cannot be predicted.38–42 Surgery appears to have the least measurable benefit at L5-S1, intermediate benefit at L4-5, and best results at L2-3 or L3-4, unless the conus is involved or cauda equina syndrome experienced. This may relate to the trend of conservatively treated hernias at upper lumbar levels faring worse than hernias at lower levels.43
Recurrent radiculopathy occurs in 5% to 10% of patients,2,35,40,44 which approaches the lifetime incidence of disc surgery.29 Less likely causes for failure include perineurial fibrosis and arachnoiditis. The subject of recurrence is important and is covered in depth later in the chapter.
The long-term outcomes of surgery and conservative treatment are similar, but in the short term, surgery provides the prospect of quicker relief than conservative measures do. 35,38,39,44–49Quicker relief with surgery may translate into reduced economic cost.6,50,51
It is difficult to define preoperative findings that are predictive of success or failure, even in the largest series of patients. Part of the problem arises from the instability of results over time, with as many as 40% of patients crossing over from favorable to unfavorable postoperative groups and vice versa.52 Useful clinical predictors of good outcome from surgery include good underlying health, absence of preoperative comorbidities, absence of previous nonspine surgery or of a workers’ compensation claim, young age, the presence of radicular pain to the foot, positive straight-leg raise without back pain, and reflex asymmetry, in approximately descending order of significance.28,38,39
Risk factors associated with poor outcome include time off work in excess of 3 months, psychosocial problems including poor educational level, smoking, and possibly obesity. Some authors believe that although obesity complicates anesthesia and convalescence, it might not of itself adversely affect outcome.28,53 Smoking is a risk factor for chronic low back pain4,54,55 as well as a risk factor for hernia and poorer outcomes, as has been discussed. Perhaps because of the location of the dorsal root ganglion and because of the difficulty associated with surgically approaching lateral hernias, the probability of good outcome for extraforaminal hernias is not as great as that for paramedian hernia.31,56
Radiology, Indications for Discectomy, and Clinical Correlates
Patient selection is critical to good patient outcomes. Technique may not be as important as patient selection in lumbar discectomy. The patient selection pitfalls to be encountered, recognized, and avoided in lumbar discectomy surgery are numerous. Two of the most common errors are the misinterpretation of leg pain of some other origin as being radiculopathy and correlating back pain with an unrelated neuroimaging finding. The most important determinant in favor of proceeding to surgery should be strict correlation between the distribution of the radicular leg pain and the nerve root compression seen on preoperative imaging studies. Performed carefully and correctly, clinical examination can predict findings of neuroimaging and subsequent surgery approximately 70% to 80% of the time.50,51,57–59
There has been an increasing realization that information about morphology alone is not enough to make a definitive diagnosis. Radiographic prevalence on MRI of abnormalities in an asymptomatic population is the subject of dozens of papers. MRI-documented disc bulge appears present in up to 81%, protrusion in up to 63%, extrusion in up to 24%, dark disc in up to 83%, disc height loss in up to 56%, anular tears in up to 56%, and Schmorl nodes in up to 19% of asymptomatic volunteer individuals. The numbers are similar in patient populations that are chosen without regard to symptoms.60,61 It can be said that the potential for finding false-positive indicators is universal, since they are nearly ubiquitous. As MRI scanning power and resolution improve with time, this problem will only grow larger. Also, clinicians and radiologists have different perspectives and perceptions when reading the same study; clinicians are more focused on clear description of the morphology of a particular pathologic finding than radiologists are.62
Contrasted MRI may occasionally be necessary. MRI with contrast can demonstrate inflammatory change on the periphery of the hernia, which can be of prognostic value. Contrast is useful to help differentiate nerve sheath tumor from disc material, with which it is often confused in the far lateral location, or other tumors and processes with which a hernia may be confused when it has migrated into unusual locations and into the posterior canal.63 In the postoperative setting of recurrent radiculopathic pain, contrast will display epidural scar and inflammation around a nonenhancing retained or recurrent nuclear fragment. Occasionally, it is prudent to augment the MRI with CT. CT will show bone anatomy and important bony subtleties that the MRI might not show or might not show well yet that might be important to surgical planning, such as spondylolysis and apophyseal (limbic) fracture. In the case of apophyseal ring fracture, CT is often the best method of examination, while plain radiographs and MRI might not demonstrate the bone of the apophyseal ring.
The synthesis of a decision to operate should be scrupulously clean and based upon a combination of clinical factors and radiographic findings. If MRI and CT do not provide sufficient explanation for a clinical picture, the second-line alternatives of discography and myelography remain. Proponents of discography claim that, through pain provocation, it can provide the specificity that is missing from the purely morphologic information that CT and MR imaging provide. The specificity of discography, however, is far from clear.64,65 If MR studies fail to provide clear evidence of lumbar disc hernia at the level corresponding with the clinical presentation, myelography may be useful, particularly when no neurologic deficit exists, multiple nerve roots are involved, or a centrally herniated disc affects only a single root.
Not only is there great potential for spine MRI scans and other modern neuroimaging to display abnormal findings and poorly interpreted information that have great potential to lead to inappropriate management, but they can also lead to inappropriate, expensive, and disabling behaviors from the patient. Radiologists must take some responsibility for the way in which their reports are used and interpreted. The addition of epidemiologic data and statements may be worthwhile.66 In an effort to standardize neuroradiologic readings, the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology established a combined task force with recommendations for readings.11
Preoperative clinical history may give clues to the anatomic severity of lumbar disc pathology, whether it is an intact anulus (negative exploration or protruding disc at surgery), a ruptured anulus (subligamentous incarceration), or a completely free disc. Vucetic et al. found that shorter duration of leg pain predicted a ruptured anulus; in their series, a 10-week period of symptoms was found in rupture versus a 50-week period found for an intact anulus. Lack of comorbidity predicted a ruptured anulus, with 18% of patients with rupture having prior medical or psychiatric treatment versus 39% of patients with an intact anulus having prior treatment for other diagnoses. Having had previous nonspine surgery was recorded in 32% of patients with a ruptured anulus, while 55% was recorded in patients with an intact anulus. The two groups differed then not only in disc pathology but also in medical, behavioral, and social factors, which undoubtedly plays a role in surgical outcomes.59
In practice and as a prerequisite for successful surgery, there should be a strong correlation between the pain, neurologic deficit, and preoperative imaging findings, and this rule should be inflexible. Much of the U.S. population obtains information from the Internet, for which there are no standards regarding quality and content, and at least a third of the available information may be of dubious value and/or distorted by potential commercial gain.67 Between the power of modern MRI scanning and the influence of the Internet on patients, contemporary spine surgeons must keep their perspectives scientifically valid and treatment goals clear.
Biology of Disc Degeneration
In the well-hydrated discs of youth, the mechanical stresses that are applied to the vertebral column are borne more upon the center of the end plates; but with desiccation, the loads become transmitted more to the periphery of the vertebral body. Ultimately, the anulus bears more load than it is capable of handling, particularly at the posterolateral segment, and hernia occurs. Nutritional support of the living tissue of the disc is dependent on a number of factors, including size of the disc, changes in the vertebral body end plates from which nutritional support diffuses, age-dependent cell density within the disc, and patterns and levels of physical activity that may encourage or discourage diffusion of nutrients to and waste from and through the tissue.68 The avascular nature of the mature disc also makes it unable to remove and replace degradation products. The mature disc is one of the most sparsely cellular tissues in the body yet with one of the densest extracellular matrices to maintain, dependent on the health of those cells.69
The chemical makeup of various tissues of the disc changes with age, potentially increasing in fragility. Collagen is widely distributed in the body, of various compositions; types I, II, III, VI, and IX are found in the nucleus and anulus in both normal and pathologically degenerated discs, but types III and VI are increased in areas of degeneration. Mutations in at least two collagen IX genes, COL9A2 and COL9A3, have been associated with higher likelihood of hernia, and the presence of childhood hernia implies genetic predisposition outside of environmental factors that are commonly held to be responsible.60,70,71 At a molecular level, collagen cross-links are important to the mechanical stability of the disc, particularly perhaps the anulus. The variety of proteoglycans in the extracellular matrix of the nucleus also change in abundance and structure through life and in their ability to retain hydration of the disc and ability to maintain the electrokinetic environment important to water and nutrient transport. Disc matrix proteins such as fibronectin and elastin throughout the anulus and nucleus also change in age and degeneration.69
The most dramatic changes in degenerating discs occur in the loss of hydrostatic pressure as maintained by the negatively charged proteoglycans, in water content, in cell populations, and consequently in cellular biosynthesis and repair. Mechanical stimuli can elicit different cellular responses from similar cells depending on whether the tissue is of the nucleus and inner anulus or outer anulus. The difference potentially amounts to an anabolic response to mechanical stress in nucleus and inner anulus tissues and a catabolic response in outer anulus tissue.72 Lack of mechanical stimuli or hypomobility of the disc produces changes that may promote degenerative change.73
Apart from the molecular changes, the anulus ages as well at a microstructural level. A significant element in the strength of the anulus comes not only from fiber orientation in alternating lamellae, but also interconnectivity between them.74 With age, decreases in the presence of pyridinoline (by age 65, 50% of that found in younger people) and increases in pentosidine occur within the disc. Decreases in pyridinoline cross-links lead to alterations in the collagen matrix of the disc. Integrity of the anulus deteriorates, perhaps beginning with the translamellar interconnections, leading to delamination of the anulus and ultimately anular tear or fissure. The presence of a tear or fissure does not imply traumatic origin.
Certain proteinases that are not normally present in the healthy disc also begin to appear in aged discs and are at least partially responsible for the degeneration of the extracellular matrix in the anulus, nucleus, and end plate.75,76 On a macroscopic radiographic level, though, while all the foregoing changes are occurring and fibrous tissue replaces the nuclear mucoid matrix of youth, disc height may yet be preserved, and disc margins remain intact.64
The anatomic composition of disc hernia changes with advancing age and perhaps to some extent with gender. In youth, a particular problem of hernia is involvement of the apophyseal ring. The movement of the hernia and anulus avulses the apophyseal ring into the canal, away from its immature attachment to the vertebral body,77 which often produces more mass effect than will more common nuclear fragments alone. In adolescents and young adults, a hernia is more likely to be composed of nucleus pulposus. As the nucleus becomes more fibrous with age, the percentage of nucleus in the fragments becomes lower, and the likelihood of finding cartilaginous end plate and anulus increases, such that by age 70, a disc hernia is unlikely to contain any nucleus. Women may be found to have higher percentages of cartilaginous end plate than men.78
Back Pain
While the topic of discogenic back pain is not the focus of this chapter, lumbar discectomy is often regarded as treatment for lumbar pain, and the association merits brief review here. Low back pain is a poor indicator for discectomy surgery. Diagnosis of the precise disc among many that might be the source of back pain can be difficult, if not impossible, in most cases because neurologic examinations in patients with only back pain and the absence of radiculopathy provide no direction and because radiologic abnormalities do not necessarily correlate with the source of pain. Something like 85% of patients with low back pain cannot be given a legitimate precise diagnosis of its anatomic origin.44,65 A plain radiographic survey of adults over age 65, examining a cohort of subjects with chronic daily lumbar pain and those without, demonstrates the ubiquity of findings in the discs and facets regardless of pain status. While higher degrees of radiographic severity on plain films may be seen in the pain group as a whole, there is no correlation with the degree of pain experienced.79
An anatomic cause is impossible to establish despite modern neuroimaging. In Western medicine, patients expect and press for plain radiographs, despite the widely known lack of correlation with back pain.66,80 The plain radiographs will often lead to more sensitive imaging studies such as MRI. MRI, in turn, is so sensitive and generally readily accessible that these virtues can in a certain way be looked upon as drawbacks. It is a rare scan that is read as normal or even normal for age, yet it is well known that sizable protrusions and extrusions exist commonly in asymptomatic patients.9,60,61,64 Therefore, interpretation of the neuroimaging studies must be made in the context of good clinical information. There is “an increasing realization that information about morphology alone is not enough to make a definitive diagnosis.”64 It is difficult for the backache patient (and perhaps even the referring doctor) to conceive that radiographs, MRI, and/or CT-myelogram showing pathology have no relationship to the pain. In fact, just the knowledge of pathology can adversely affect outcome.80 If present at multiple levels, the presence of a “dark nucleus” on MRI begins to predict a likelihood of back pain, but the pain generator remains unknown, whether it is the anulus, vertebrae, ligaments, fascia, muscles, or facets.8 The advantage of such sensitivity, of course, is that modern neuroimaging, particularly MRI, has eliminated the need for surgical exploration.
Back surgery on disc hernias as described in this chapter may do little for back pain, and the presence or absence of back pain should have little bearing on the patient’s selection for surgery.42 There are exceptions, however. If a large hernia appears to be responsible for radiographically visible elevation of the posterior longitudinal ligament off the vertebral bodies, particularly in midline,31 discectomy and the resultant relaxation of tension on the ligament might well result in relief of the resultant back pain. If the hernia is large and midline or if the lumbar spinal canal is shallow and resulting central stenosis of the lumbar canal is caused by disc herniation, the patient may develop a reflexive posture of lumbar flexion (the shopping cart position), which results in lumbar fatigue and pain. The symptoms can often be relieved by surgical decompression of the involved motion segment. In the Maine Lumbar Spine Study, an assessment of the predominant symptom, back pain or leg pain, was made, and improvement in back pain was documentable.35 However, it should be made clear to the patient without mechanical instability, facing the prospect of simple single-level surgery for degenerative disc disease, that surgery might have no impact on the lumbar pain. Lumbar pain is manageable by other interventions that are outside the scope of this chapter, such as exercises and other conservative measures, injection treatments, and surgical fusion.81
Radicular Symptoms
Radiculopathic leg pain with straight-leg raise and with Valsalva maneuver is more likely to be positive in herniations of L4-5 and L5-S1, where the compression and irritation are more likely to be at the axilla of the nerve root. The femoral stretch test is more likely to be positive at higher lumbar levels.51 Monoradiculopathic leg pain, or sciatica, is the most useful clinical correlate. It is superior to straight-leg raising, scoliosis, and sensorimotor deficits.82 Leg pain is often more severe in extraforaminal hernia than in intraforaminal or paramedian hernia, perhaps resulting from direct compression of the dorsal root ganglion by the hernia.56
Leg pain is perhaps the most common indicator, and the best indicator, for discectomy. However, conservative measures should be applied for a period of several weeks or longer from onset, if at all possible, prior to consideration for surgery, since long-term outcomes (4 years and more) are similar for conservative and operative care.38,39,46 In practice, however, the time required for spontaneous resolution of radiculopathy to occur may be more than some patients can bear, so pain becomes an important determinant for surgery. It is commonly accepted that the longer pain and numbness exist prior to decompression, the longer they will last following decompression.83 Furthermore, there is a legitimate fear that even permanent deafferentation pain can result from untreated compression.42,84 Inflammation from the disc hernia can adversely affect the nerve root over time and thus affect prognosis.52 Chronic pathologic changes can occur in the nerve root from prolonged compression, and over time (estimated at 3 to 6 months), there may be irreversible neuropathic changes.31,42,46 However, literature review and common clinical experience dictate that there is no consensus on what constitutes an appropriate conservative trial, and there is no consensus on what constitutes the factors leading to irreversible nerve root damage. Solid evidence for the hypothesis that delayed surgery impairs results may be debatable.85 Perhaps this lack of consensus is because there is selection bias to intermediate-level patients without sensorimotor deficit.
The patient’s economic imperatives become an important and valid factor in the selection of surgery in the presence of work disability and the requirement for rapid return to work. Surgery provides more rapid relief than conservative measures do.35 Also, long-term conservative care may ultimately be more expensive than surgery (in properly selected surgical candidates).50
Motor and sensory deficits are surgical indicators. It is practical to observe mild motor weakness and to follow for a period of time if stable. Motor deficit that is not improving, however, may be considered a surgical indicator,42 as should progressively worsening motor deficit31 and, of course, severe motor deficit.85
As will be seen, spontaneous resorption of disc material occurs and should be allowed to progress given the absence of severe motor deficit and the patient’s ability to comply. It is, almost paradoxically, a phenomenon that is likely to be more satisfactory and complete in larger hernias, when there is true extrusion, rather than in simple contained anular prolapse. Extrusion past the anulus marshals the processes of inflammation and phagocytosis of the mass.45,46,86–88
Disc Resorption
The spontaneous resolution of the initially agonizing symptoms of both back and leg pain as well as the radiographic findings of lumbar disc hernia with time is well established.7,35,38,39,41,44,47,75,89–95 Several mechanisms may be involved with the phenomenon of regression of disc hernia. Capillaries invade the hernia, and macrophages derived from monocytes migrate out into the hernia and begin a process of phagocytosis. Macrophages are the most commonly found cell type in both acute and chronic disc herniation. Macrophages contain enzymatic lysosomes, which degrade intracellular collagen and other substances present in disc material after phagocytosis. Macrophages also can secrete lysosomes, promoting the breakdown of extracellular substances such as collagen. Both of these mechanisms are closely involved in the regression of herniation. Apoptosis may occur at a higher rate in free disc fragments, another possible mechanism of absorption.
Macrophage activity itself can be a determinant of pain. There is a statistically significant correlation between histologically observed macrophage infiltration of intraoperative disc specimens and postoperative pain grading; patients who harbor inflammatory histology rate postoperative complaints lower than do patients with no evidence of inflammatory reactions.52,96 The periradicular inflammation that accompanies the hernia characterized by macrophage response also includes an increase in IL-1β and a release of PGE-2.75
Discs that are found to have intense perilesional gadolinium enhancement are more likely to regress spontaneously, the thickness of rim enhancement being a strong determinant of spontaneous resorption. Clinical symptom alleviation occurs concordantly with a faster resorption rate. MRI with contrast can be a useful prognostic tool for identifying patients with herniated nucleus pulposus (HNP)–induced sciatica with a benign natural course.92,93 Ninety-five percent of patients, followed out to 7 years, have decreases in the size of hernia through absorption of the disc. Progression of other disc degenerations occurs in all patients as well.90
Role of Surgery
The association between prolonged delay of treatment and poor outcome has been documented.35,38,39,83,97 In prospective studies of patients having lumbar disc hernia surgery, the duration of leg pain and duration of sick leave are found to be of prognostic value, with leg pain lasting over 8 months predicting a worse outcome, including inability to return to work.83,98
Part of the radicular symptomatology of the disc hernia is due not only to the described inflammatory changes, but also to tethering of and ischemic change in the root resulting from periradicular inflammation. As measured by laser Doppler flow, intraneural flow is improved after discectomy.99,100 Ischemic damage to the root, if present and productive of serious motor deficit, could be expected to have a poor prognosis, better avoided with decompression.
There is good objective evidence that surgery plays a legitimate role in the treatment of acute lumbar radiculopathy from lumbar disc hernia, bringing patients a faster and earlier recovery than would have occurred spontaneously. 35,38,39,44,47–49,101Nearly universally accepted indications for early surgery include significant motor deficit, unmanageable refractory pain persisting for more than 6 to 12 weeks, and of course cauda equina syndrome.7 Practicing neurosurgeons have long observed quick resolution of worrisome motor deficit in surgically treated patients.
Similarly, Peul et al. found that surgery offered short-term benefit. In a randomized prospective trial of early surgery (mean: 2 weeks) versus late surgery (mean: 18 weeks) or conservative treatment, relief of leg pain and recovery was faster in early surgery patients. By 1 year, however, there was a 95% probability of perceived recovery in both the surgically treated cohort and the delayed or conservatively treated cohorts.47 Osterman found similar results at 2 years.49 Longer-term results were found by Weber at 4 years,38,39 the SPORT (Spine Patient Outcomes Research Trial) study at 4 years,44 and the unrandomized but large and comprehensive Maine Lumbar Spine Study at 10 years.35 At 1 year and at 4 years, in the prospective randomized multi-institutional SPORT lumbar disc series, patients who were operated on (in both the randomized and nonrandomized cohorts, without regard for intent to treat) maintained greater improvement (in SF-36 Bodily Pain and Physical Function scales and in the ODI) compared to patients patients who were not operated on, although long-term work status was not significantly benefited, as was mentioned. In the short term (3 months), work status was worse in the former group than in the latter, owing to surgical convalescence.44
In the Maine lumbar spine study, there did appear to be long-term benefit for patients who received surgery compared to hernia patients who did not. Surgical patients reported resolution or substantial improvement (56% vs. 40%, P = .006) and more satisfaction with their current status (71% vs. 56%, P = .002). However, work and disability status at 10 years were comparable among those treated surgically or nonsurgically.35
With regard to work status, these two large studies agree on lack of long-term surgical benefit.35,44 On the other hand, a cost-effectiveness analysis performed in concert with the SPORT series resulted in the demonstration of a cost per quality adjusted year of life of approximately $69,000 in 2008 U.S. dollars, supporting the use of surgery as a cost-effective procedure in the short term and for markedly symptomatic patients.
In the long term, children may do significantly better than adults following lumbar disc surgery; surgery does not appear to lead to chronic complaints of back pain.102
In summary, the ideal patient for discectomy is one with severe, disabling unilateral radiculopathic leg pain without severe sensorimotor loss for whom conservative measures over a period of a few weeks to 2 months have yielded little.42 A poorer recovery can be expected in the presence of severe persistent sensorimotor loss, once pain has remitted or has acquired the burning deafferentation quality suggestive of nerve root damage. Changes that are induced in the course of back pain through discectomy are unpredictable. The relatively soft and poorly defined nature of these indication guidelines has resulted in widely variable rates of surgery: as much as a fourfold or fivefold difference between surgeons and between countries.35–37
Cauda Equina Syndrome
A somewhat separate issue is the cauda equina syndrome. Bladder and bowel sphincter dysfunction and bilateral neurologic deficits are the strongest indicators for surgery. The outcome for the cauda equina syndrome is better if there is unilateral sciatic pain, worse if there is bilateral sciatica, and very poor if there is saddle hypesthesia. Patients with complete perineal anesthesia are at risk for permanent sphincter dysfunction. The mode of onset of symptoms may also be important, the acute onset of symptoms over hours being thought to be a prognosticator of poorer outcome, particularly bladder function, than is a more insidious onset over days or weeks.103
Cauda equina compression often exists in the sensitizing presence of developmental lumbar stenosis and, in the context of this discussion, is the result of acute disc herniation rather than slowly acquired degenerative change such as anular bulge. Unlike with the lesser indications discussed previously, compression of the cauda equina constitutes a medical emergency or urgency and should be relieved as soon as possible after diagnosis.81,103 If the onset of symptoms is abrupt, the symptoms and prognosis for full recovery are worse than if the symptoms are slower in onset,103 and by inference, the urgency for decompression is greater. Other poor prognostic signs for the recovery of sphincter control in cases of cauda equina compression include saddle hypesthesia and bilateral radiculopathic leg pain.
In addition to sensitizing patients to the potential for acute cauda equina syndrome, lumbar stenosis can be responsible for unexpected or false localizing findings in the event of disc hernia, for example, producing footdrop from an L12 level.104 High lumbar hernia also can be symptomatic in the distribution of multiple roots because of their acute angles and the narrow confines of the upper lumbar canal.104–106 These patients might not fare as well as patients who have disc pathology at more caudal levels if sensorimotor deficit is present. Long-term follow-up (average, 81 months) confirmed worse outcome for patients with hernia at L1-2 and L2-3, only 33% of patients reporting an improvement in their economic or functional status, compared with an 88% rate of improvement at L3-4.105 Perhaps this is because when lumbar stenosis is present, conventional discectomy via laminotomy increases the risk of damage to the intracanalicular roots because of the narrow confines of the canal. Particularly in the case of large central and calcified hernia at upper lumbar levels, a generous central canal decompression prior to the manipulation and retraction of the lateral thecal sac must be made. An alternative, if the conus terminates above the level in question, is to retrieve a large or calcific hernia through a transdural approach with repair of the ventral and dorsal dura.106
Technique
Open laminectomy and discectomy work as well as microdiscectomy. The advantages to microdiscectomy are the smaller and more comfortable incision and the shortened hospital stay and diminished trauma to the adjacent motion segments and paraspinous musculature. Microdiscectomy requires the use of magnification. The choice of loupes and headlight or microscope is moot. Results from surgery are the same with both techniques.28,31 Loupes, with the use of a coaxial or near-coaxial headlight, offer the same or nearly the same magnification and the same or nearly the same size incision in the case of microdissection as does the operating microscope. The advantage of the microscope lies in its use by an observer or assistant; the disadvantages are some additional encumbrance and perhaps expense to the patient.
The following discussion assumes that discectomy is the planned end result of the surgery, not to be followed by fusion, in which case restrictions on bone removal would not be as significant. There is insufficient indication for routine spine arthrodesis combined with lumbar disc excision as the primary procedure.107 While primary lumbar fusion may well be successful in disc hernia,108 inclusion criteria should be strict and may include both degenerative and isthmic spondylolisthesis, complete facetectomy, and perhaps degenerative scoliosis.109,110
Primary disc replacement or dynamic stabilization, like primary fusion for HNP, is not standard care; current thought reflects conservatism. Despite early enthusiasm for disc arthroplasty,111 it has been found that there may be only a 0.5% incidence of indication in the overall population with the majority in young patients who averaged 38 years of age. Other nonfusion techniques exist with known results. With criteria for arthroplasty tightly confined and given a small number of potentially eligible patients, there is limited use of this technology.112 Alternatives such as nucleus replacement may ultimately be an option.113
Standard paramedian lumbar disc surgery is estimated to take about 75 to 80 minutes, with an average blood loss of about 65 mL, requirement for blood transfusion being a true rarity.44
Operative Positioning and Patient Preparation
The disc space is avascular and, as a result, less resistant to infection. There is evidence that the risk of disc space infection may be reduced through the use of perioperative antibiotics.114
The preoperative placement of elastic stockings to prevent thromboembolism is recommended.115 A urinary catheter is usually unnecessary unless the procedure is expected to take more than 2 hours.
For most surgeons, the prone position on a frame works well. Often, such devices are inadequate for preventing increased intra-abdominal pressure (and thus increased ventilatory, venous, and cerebrospinal fluid [CSF] pressures) in obese patients. Obese patients are better positioned in such a manner that the abdominal panniculus hangs unimpeded, without bearing any of the patient’s weight (e.g., the knee-chest position). The eyes and facial prominences should be well padded and inspected after the patient is turned to the prone position because ocular pressure, or a combination of pressure and hypotension, can lead to blindness.116 The arms, if held abducted on arm boards, must be well padded over bony prominences and over the ulnar nerves.117 To prevent costoclavicular compression of the brachial plexus, care should be taken to avoid hyperabduction over 90 degrees at the shoulders and shoulder hyperextension. The radial pulse on each side should be felt. Women should bear the weight of the chest on the ventrolateral rib cage (not on the breasts, which should be moved medially). All skin that is in contact with the frame or with pads on the frame should be protected with a layer of linens; skin should not be allowed to touch the bare occlusive surface of vinyl or silicone rubber cushions. The urinary catheter (if placed) is inspected after the patient has been turned. Male genitalia must not be subject to compression between the approximated thighs. All bony prominences of the legs must be padded; in particular, the toes must bear no weight of the foot. A diminished or absent pedal pulse may indicate femoral artery compression. This can be encountered on a conventional frame as well as in the knee-chest position.118 The patient, when positioned, should be stable so that vigorous intraoperative manipulation will not cause movement. Hardware in the room, such as IV stands, light sources, carts, tables, and anesthetic equipment, should be arranged to allow easy access for both radiograph equipment and film cassette holders.
Standard Technique and Open Laminotomy (Laminectomy)
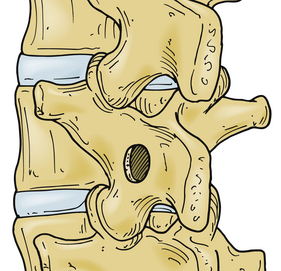
Although the term open laminotomy may imply the absence of visual magnification, some form of low-power magnification is often used. The skin incision for laminotomy for disc herniation is conventionally about three spinous processes in length, 10 to 12 cm for a one-level operation. When performed in this manner, open laminotomy has the advantage of facilitating surgical assistance. Furthermore, illumination is not quite as problematic as with shorter incisions, and the longer incisions have been suggested to be less traumatic to the paraspinous musculature than shorter incisions (that require greater retractor pressure). After the incision has been made, hemostasis is achieved and retractors are placed. As with any incision, tension on the skin retractor should be inspected intermittently throughout the operation because pressure necrosis of the skin edge is a significant source of infectious wound complications. After the skin has been incised, the subcutaneous tissue is divided. This can be accomplished without bleeding or trauma with blunt dissection. The lumbodorsal fascia is then exposed, and for ease of subsequent closure, it is prudent to sweep the subcutaneous fat off a short distance laterally. The fascia is then incised, just lateral to the spinous process, rather than in the midline, which allows the preservation of interspinous ligaments. If the laminotomy is to be performed bilaterally, as in cases of developmental stenosis with superimposed disc hernia, a fascial incision on each side of the spinous processes results in a saved strip, the width of the spinous processes, in the midline, complete with the interspinous ligament.
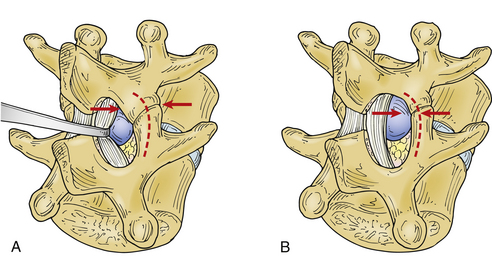
With the field prepared for bone work, the overhanging caudal lip of the rostral lamina can be partially removed, allowing for further exposure of the interlaminar ligamentum flavum. The amount of bone removal is at the surgeon’s discretion. The hernia and the anulus can be accessed with little or no removal of bone if one is comfortable with the amount of force necessary to retract the nerve root medially.26,31,119,120 Wider exposure via laminotomy and medial facetectomy, flush with the medial surface of the caudal pedicle, minimizes the need to mobilize the nerve root aggressively. Paramedian discs at higher lumbar levels represent a slightly greater challenge than those at L4-5 and L5-S1 because of the lamina and facet structure. The spinal canal is smaller in caliber, the lamina and facets descend more caudally, the facets are positioned more medially, and the facet clefts are more sagittally oriented than is the case in more caudal segments. Each of these features not only makes it necessary to remove more bone, but also makes progressive removal more risky to the integrity of the pars and the facet. This anatomy becomes particularly important in addressing intraforaminal disc hernia by the midline approach (see later discussion).
As bone removal proceeds rostrally and laterally, the lateral margin of the removal migrates caudally and laterally, in a fashion that makes the defect appear triangular or lung shaped so that the structural integrity of the pars interarticularis is not compromised. Compromise of the pars by an overly aggressive bone removal can result in a pars interarticularis fracture, either during the course of surgery or during convalescence. More than half the bone of the pars, in its lateral dimension, should be left to avoid fracture121(Fig. 78-1). As was mentioned earlier, this is particularly important at midlumbar segments, where the pars is narrower and the facet cleft is more sagittally oriented than at lower motion segments. Pars fracture isolates the facet, functionally resulting in complete facetectomy, which increases the failure rate due to back pain and instability.28
Alternatively, a minimalist approach can be taken to bone and ligamentum removal as described by Williams.26 This approach does have the disadvantage of reduced visualization and increased traction upon the nerve root during its medial mobilization. While the amount of bone removal is discretionary, as a rule, decompressive removal should be more generous in the case of concomitant developmental stenosis. At times, it can be minimal when the interlaminar space is large.
Perineurial scar has been blamed for postsurgical failure to relieve sciatica. There are many means of dealing with its development, and more discussion will follow. The ligamentum flavum is one such potential source of fibrosis if it is left in large shreds beneath the lamina. The ligamentum is therefore reasonably dealt with in one of two ways: (1) with minimal fenestration of the ligament, leaving its slick inner surface approximated to the nerve root as a natural barrier31,119,120; or (2) with its complete removal from the lateral recess. Because of impaired visualization and mobility of the underlying thecal sac and nerve root sleeve during the surgery and the variable ability to access the lateral recess and its contents with a minimal approach (dependent on the treatment level and concomitant spondylotic enlargement of the facet and lateral recess stenosis), complete flavectomy is usually preferred. With angled curettes, removal from the undersurface of the remaining rostral lamina should be thorough. If lateral ligamentum is left in place completely or partially, its raw surface may lead to a significant postoperative fibroblastic response and scar formation, and its continuation with the medial facet capsule contributes to lateral recess and foraminal stenosis, which may be of significance as the disc space narrows postoperatively and with age.
The location of the foot of the punch or the edge of the curette and the location of the nerve root sleeve should be well perceived by the operating surgeon. It is worth noting that a risk of dural tear is present in every case. If a scar is present, fixing the dura mater to overlying bone, the risk of a dural tear is increased. The chance of tear is also increased in the elderly (particularly elderly women), in whom the dura mater is thin and in whom a noncompliant scar may be present, even in the absence of prior surgery. An inflammatory response to the hernia itself is often present.45,86,88 Naturally occurring adhesion may be present, fixing the dorsal dura to overlying laminar bone and ligamentum flavum. For these reasons and as a basic element of prudent use of a bone punch, the space into which the punch foot will sit should be swept with a blunt instrument such as a Woodson or ball-ended dissector. The geometry of the instrumentation involved is crucial because the dura mater can fold over the foot of a punch or the edge of the curette that is not applied closely to the underside of the bone. This is an error that can be worsened if the dura mater is distended under increased intrathecal pressure; as with epidural venous bleeding, its risk can be reduced by careful positioning, with attention paid to intra-abdominal pressure reduction. Piecemeal bone removal is not slowed by cautious inspection. Caution simply requires keeping the eyes on the target, letting the assistant clean the punch, judiciously appreciating the tactile input, and intermittently sweeping the peripheral undersurface of the bone in the direction of the decompression with a dissector.
A dural tear is a significant problem only if it is not cared for properly. In some cases, dural tears are unavoidable, and their occurrence may even be predictable. It is a problem encountered by the best of surgeons. The risk of a tear is high in the elderly with thin and fragile dura as well as ligamentous adhesions to dura, but it is also a risk if the herniated disc fragment has been present long enough to result in dural adhesions. There is also the possibility of natural dural adhesion and of fibrous bands connecting ventral dura (Hofmann ligaments) to the posterior longitudinal ligament, particularly well developed and of potential surgical consequence in the lumbar canal.122,123
A mistake is to be cavalier about the occurrence of a CSF leak and not to repair the leak properly. Pinhole dural breaches can and often are successfully treated with fibrin glue, DuraSeal, hemostatic gelatin (Gelfoam), other adhesive substances, and/or indirectly with multilayer tight soft tissue closure. However, larger dural tears of more than 1 mm are best managed with convincing primary suture closure, as they risk spontaneously enlarging and producing CSF fistula and symptomatic pseudomeningocele.
If the tear is dorsal or lateral, it will be problematic for the remainder of the case if not attended to. The defect should be protected from further tearing or from aspiration of nerve roots by the placement of Gelfoam and a cottonoid beneath the suction tip over the tear. It should be exposed, with further bone removal, and repaired primarily with suture in a watertight fashion (preferably immediately after its occurrence). Repair can be tedious and time-consuming at a point in the operation at which much remains to be done. The temptation to delay repair until later is often best resisted. If the repair is one of the last tasks after an unusually lengthy procedure, attention might not be paid to the details of adequately repairing the leak. If the arachnoid is also involved and spinal fluid is being lost, thecal sac collapse occurs, and epidural venous bleeding intensifies because of decreased tamponade. This increased bleeding obscures visualization and blood can enter the thecal sac, possibly resulting in arachnoidal adhesions. Other possible results of unrepaired dural tear include radiculopathies with pain and deficit secondary to herniation of nerve roots through the dural defect, symptomatic pseudomeningocele, the possibility of meningitis, and persistent orthostatic headache complaints.124–128 In the modern era, with patients conventionally returning home the same or next day, primary repair becomes standard.
Confines of the field often make primary repair difficult, particularly with modern minimal exposures or tubular retractors, but only rarely is it impossible. The needle is more important than the suture size; a tapered needle not much larger than the suture should be used so as not to leave an excessive pinhole of its own. A commonly used suture is 4-0 or smaller, with 6-0 or 7-0 polypropylene suture (Prolene) being the authors’ preference (more so for BV-1 or BV-175 needle geometry). A new Ethicon (Johnson and Johnson) suture, Hemo-Seal 5-0 Prolene HS, has a sealing hydrophilic coating. The use of a small pituitary instrument to hold the needle and the use of knot pushers can significantly aid the process, even to the extent that dural repair can be effected through a tubular retractor.129
Reinforcement of the durotomy with an onlay of fat, DuraSeal, DuraGen, muscle, fascia, fibrin glue, Gelfoam, Surgicel, polyglactin acid sheet, or mesh,130 collagen mesh, or the like is prudent but might be ineffective in the long run without the underlying primary repair. Fibrin glue is very effective but short lived, lasting only several days. The authors believe that if a trustworthy repair has been provided, it is advantageous to have the patient ambulatory the day of surgery rather than enforcing bed rest; ambulation will expand the thecal sac, redistribute the caudal nerve elements, and preclude epidural bleeding. With good dural repair, only 1.8% of incidental durotomy patients must return to the operating room.131 Additional treatment such as lumbar CSF drainage should be considered second line, again being best used to reinforce a good primary suture line repair.
Repaired primarily, CSF leaks incurred intraoperatively have little or no impact on ultimate surgical outcome.127 Repaired and reinforced carefully, most will heal, but extra consideration should be given host factors that would impair wound healing, such as high CSF pressure anticipated in obesity, connective tissue disorders such as Marfan syndrome and neurofibromatosis, scar tissue, and use of steroids. On occasion, the tear may be located on the ventral aspect of the nerve root sleeve or thecal sac, in which case, while it may be impossible to repair without marked difficulty, it nevertheless does not risk the problems of a dorsal tear and will more than likely tamponade itself.
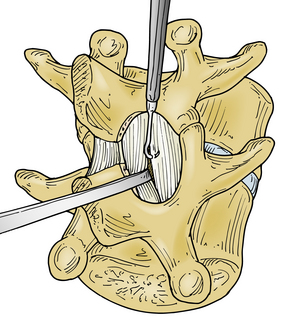
With the medial aspect of the facet joint and capsule partially removed so that the medial surface of the caudal pedicle can be observed and felt, the need for medial retraction on the nerve root to allow visualization of the disc space should be minimized (see Fig. 78-1B). Before retraction instruments are inserted, room ventral to the nerve root and dural sac must be assessed (Fig. 78-2). With a small-caliber aspiration tip (5 or 7 French), blunt hook, or ball-ended dissector, the nerve root can be mobilized medially, and while doing so, the amount of ventral fibrosis or compression can be determined. This palpation should be gentle, and if resistance to mobilization in this manner is met, the surgeon must determine the cause, as well as a solution to the problem of mobilization. The axilla of the nerve root may straddle a sharp focal prominence of the anulus fibrosus. Forcing the nerve root up and over it may invite neural injury. In such a case, the prominence can be trimmed or impacted down, medial to the nerve root in the axilla, following which the nerve root can be mobilized medially and the disc pathology better addressed. Poor ability to mobilize may reflect a nerve root anomaly such as low origin.
Overly aggressive retraction of the nerve root out of the lateral recess is potentially traumatizing to the substance of the root directly, as well as disruptive of root blood flow,99 and can be minimized by partial facet or facet capsule removal. A nerve root retractor with an integral pressure transducer has been described, with the intention of producing retraction which is brief and gentle.132
Conjoined nerve roots, low root origins, and interradicular anastomoses often affect the lower lumbar levels and represent something of a surgical challenge for a number of reasons. They can confuse the surgical anatomy, and if the conjoined root is large and pulled or pushed tightly into the lateral recess by the disc pathology, it can be mistaken for the hernia itself.133 If a low origin from the dura results in a lateral course over the disc space, it can be impossible to safely mobilize to access the disc. Therefore, a thorough decompression of the nerve root may be very difficult to achieve. The presence of a conjoined root should be readily anticipated from modern neuroimaging.
As was previously mentioned, a special problem of hernia in adolescents and young adults is avulsion of the apophyseal ring with the hernia. The bone and firmly attached anulus, in concert with large mass effect, may make complete effective removal doubtful. Surgical options may be limited to wide decompression. Larger exposure is needed to resect the fractured fragments and disc material.77,134,135
To reduce the chances of recurrent disc hernia, all loose nucleus material should be removed. It is neither possible nor desirable to remove all disc material. Overly aggressive curettage with removal of end-plate cartilage and excessive removal of interspace volume can result in a patulous anulus, poor mechanical support of the motion segment, and potential foraminal stenosis or instability. Removal should be limited to loose fragments that are within reach of the anular opening. Approaches range from sequestrectomy alone26,120 to aggressive curettage of the disc space, which may remain the more common procedure.136,137 The topics of discectomy volume and of recurrent disc hernia are covered later in the chapter.
Intraoperative ultrasound138,139 may be useful even when the surgeon believes that removal has been complete. More medially located disc pathology may be difficult to interpret or see.
With the anulus and the cartilaginous end plates being retained, the surgeon must stay focused on an envisioned estimate of the anatomy of the intervertebral space because this is a blind procedure. The majority of vascular and visceral injuries that result from perforation of the ventral anulus occur at L4-5 and L5-S1, although injury can occur at other levels as well.140–146 The firm ventral and lateral anular margins can usually be palpated with the rongeurs, and the depth of penetration can be controlled. It is possible, however, as a result of ventral anular tears,143,147–149 that the anulus fibrosus does not adequately restrain the instruments to blind palpation. Tarlov146 suggests penetrating the anular space to no more than a depth of 1.125 inches and marking the operative instruments at this depth.
Shevlin et al.150 reported a case in which atraumatic passage of a rongeur to an unusual depth was followed by the observation that irrigating fluid then emptied out the ventral anulus; they suggest this as a sign of potential problems, as occurred with their case. During discectomy and shortly thereafter, any sudden vagal or hypovolemic response should be seriously regarded as indicating possible vascular, ureteral, or intestinal injury. Most often, bleeding from the anular space is not noted in major vascular injuries.
Catastrophic problems occurring as a result of perforation through the ventral anulus are possible, even with skilled surgeons. There is an incidence of 1.6 to 17 per 10,000 cases of ventral perforation with vascular or visceral injury.5,146,148,151 Body habitus, the operating surgeon’s experience, patient positioning, and the type of surgical instrumentation used (including the microscope) do not appear to influence the risk. Good outcome is entirely dependent on early recognition and swift appropriate action. However, the mortality rate may still reach 47% with vascular injury. Vascular injuries during lumbar discectomy may of course result in acute life-threatening hemorrhage but also chronic arteriovenous fistula or pseudoaneurysm formation. The majority of vascular injuries associated with lumbar laminectomy are found at the L4-5 and L5-S1 levels and few higher.140
If the anulus fibrosus is simply bulging over a broad area or is partially dislodged from its attachment to the rostral or caudal vertebral lip and does not appear to be torn (permitting expression of the nucleus), it is best left intact. Certainly, if soft disc hernia or prolapse is not seen, the anulus should never be violated. Nerve root decompression can be achieved by removal of overlying bone and ligamentum flavum, allowing the preservation of the motion segment. The decision to violate the posterior longitudinal ligament and anulus can be made with greater assurance by using an intradiscal injection of saline. A small amount of indigo carmine dye, just enough to color the irrigant, and 5 mL of saline are drawn up in a 6- or 10-mL syringe, a 22-gauge spine needle is fitted, and the nuclear space is injected through the anulus. If the irrigant can be observed readily extravasating from the disc space, it can be assumed that the anulus is incompetent and that its contents should, perhaps, be emptied. If the disc accepts only a few milliliters of fluid and no extravasation is observed, the anulus can be assumed to be competent, despite its bulge, and is best left undisturbed.
Intervertebral disc hernia may be encountered at unusual locations within the canal, either distant from the anulus or hidden intradurally. Immediately upon entering the canal, just under the ligamentum, the surgeon might be met with a dorsally migrated epidural fragment of disc material.152 It is an interesting and occasionally unexpected finding but should not be difficult to remove and trace to its source. More difficult to manage are the rare intradural and intraradicular hernias, which might not be readily apparent either on the MRI or at the tableside. Because of adhesion of the dura to the anulus or posterior longitudinal ligament, a hernia may rarely perforate the dura mater and be located within the thecal sac or within the nerve root itself.153–156 Another problematic location for hernia may be those that have migrated rostrally to lie well rostral to the disc space. The removal of a large amount of lamina may be necessary. There is further discussion of this problem later.
Adhesion can be a consequence of prior surgery, the result of inflammatory changes incurred by the hernia itself, or a natural occurrence as previously. To find the pathology in these cases requires the surgeon to be vigilant for any discrepancies between what is observed in the field and what was observed on the neuroimaging studies and to simply be aware that such conditions exist. It has been postulated that some cases of surgical failure of benefit may indeed be due to such pathology that has gone unrecognized.154
When the ligamentum flavum is aggressively removed, the nerve root decompressed, the fat replaced, and hemostasis obtained, the wound is closed. Irrigating solution is used to flood the wound. A secure, but nonstrangulating, absorbable suture reapproximates the muscle to the midline to eliminate dead space. The fascia is closed in a watertight fashion, and the subcutaneous fat and skin are closed. The surgeon can elect to place a Depo-Medrol–soaked Gelfoam pledget or morphine (Duramorph) over the nerve root before closure or to infiltrate the paraspinous muscle with bupivicaine before closure of the skin.157
Microlaminotomy
Microlaminotomy, or microdiscectomy, is the contemporary gold standard treatment of lumbar disc hernia; the use of the larger open incision is waning. Microdiscectomy was introduced in 1977.27,158 The term microlaminotomy denotes the use of a short skin and fascial incision and, by necessity of the short incision, visual magnification of some sort. To accomplish the task accurately and effectively through a microlaminotomy incision, loupes with a magnification power of at least 3.5, with a strong headlight coaxial with the line of sight, or a binocular operating microscope are required. As with the use of loupes, the use of the microscope has certain advantages and disadvantages.
Microlaminotomy has the advantage of decreased postoperative pain. As a result, the complications of postoperative atelectasis and postoperative temperature elevation may be reduced.27,158,159 The high magnification that is used in its completion encourages gentle tissue handling.
There is no subsequent difference in technique between open laminotomy and microlaminotomy for the remainder of the surgery, other than the use of magnification. It is possible to perform disc surgery using the operating microscope without removing much, if any, bone, and little ligamentum flavum. In fact, when microlaminotomy was first described by Williams,26 this approach was recommended. The focus of the operation, however, should be thorough nerve root decompression and the minimization of the chance for recurrent symptoms, rather than an exercise in leaving the least trace. Without a more or less conventional amount of bone removal, the chances of overlooked pathology and a compromised outcome are increased. It is strongly advised, therefore, that microlaminotomy be performed in the same fashion as one would perform a conventional laminotomy. Results from conventional laminotomy and microlaminotomy are similar.29,101 The theoretical benefit of microdiscectomy is its applicability to outpatient usage.160–162
A third approach is a translaminar approach to a hernia that has migrated rostrally to lie in the proximal foramen medial to and below the pedicle of the rostral level. This procedure has been described and will be discussed in the section on foraminal hernias.163–165
Microendoscopic discectomy, discussed in Chapter 61, is performed through tubular retractors with endoscopic vision. It was introduced in 1997 and has since been demonstrated to be as effective as microsurgical discectomy for treatment of lumbar disc hernia.166–168
Complete Laminectomy
As was mentioned in the section on the cauda equina, voluminous high lumbar hernia may be problematic and may require complete laminectomy, since conventional discectomy via laminotomy increases the risk of damage to the intracanalicular roots because of the narrow confines of the canal. This is particularly true in the case of large central and calcified hernias at upper lumbar levels. A generous central canal decompression prior to the manipulation and retraction of the thecal sac must be made, or a transdural approach with repair of the ventral and dorsal dura.104–106
Lateral and Far Lateral Hernia
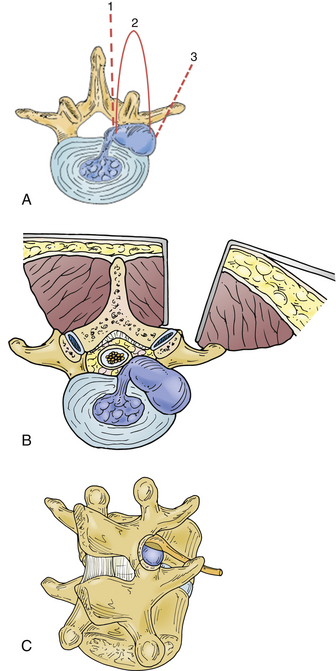
Far lateral disc hernia, with resulting compression of the nerve root in, or lateral to, the intervertebral neuroforamen, occurs in about 10% of all symptomatic anular prolapses or discs.169–180 Extraforaminal hernia is relatively uncommon, accounting for 0.7% to 11.7% of all lumbar hernias.169,170,181–184 Most commonly, far lateral disc herniation occurs at L3-4, L4-5, or higher levels. They occur in about equal numbers at L3-4 and L4-5, about half that at L5-S1, and in small numbers at L1-2 and L2-3. 94,169,170,178,182,183,185,186Lateral hernias occur in older patients more often than the more common dorsolateral hernias do.51,172,187,188 Lateral hernias are more likely to produce sensorimotor deficit.109 As a corollary to its frequency at midlumbar and higher lumbar levels, there is some likelihood that patients with ventral thigh pain and sensory deficit, quadriceps weakness, a positive femoral stretch test, and reduced patellar reflex harbor a far lateral hernia. Recognition of this has been facilitated by use of myelography and postmyelographic CT.189 MRI, particularly the sagittal images, may best demonstrate the pathology.190,191 Radiculopathic pain may be more severe and back pain less severe than that incurred in paramedian disc hernia because of the location of the sensory root ganglion.56,109,192
Preoperative planning is facilitated by classifying the hernia location into one of three areas and judging its accessibility accordingly. First, the lateral hernia may lie within the proximal foramen just at the medial aspect of the pedicle, and in this case can be approached through a modification of the paramedian laminotomy. Second, it may lie in the lateral foramen and must then be approached by means of a lateral facetectomy. Third, it may lie in an extraforaminal location, in which case an extraforaminal or parasagittal approach would be necessary to avoid complete facetectomy. It may be necessary, in a patient with developmental stenosis and therefore unusually large, medially located, and coronal facets to opt for one of the lateral approaches over a more conventional medial facetectomy. Although complete facetectomy has been historically reported to be relatively benign, perhaps more benign at the caudal two levels,172–174 it may result in delayed instability and failure due to chronic back pain. Consequently, complete facetectomy is avoided when possible.28,121
Despite its recognition, it is still difficult to effectively treat a far lateral hernia. The variety of commonly used surgical trajectories is illustrated in Fig. 78-3. The most popular approach is a standard midline incision and interlaminar exposure with medial facetectomy.169,170,172,177,187,188 This approach has the advantages of greater familiarity of the surgeon with surgical anatomy, absence of bleeding, early exposure of the affected nerve root, and the ability to perform discectomy to preclude recurrence. It is most appropriate for a hernia within the proximal foramen. Often, the amount of bone that must be removed to gain exposure to the neuroforamen is greater than that in simple dorsolateral hernias. It helps visualization considerably to tilt the table toward the operator.
On rare occasions, it may be necessary to produce a complete facetectomy in the cases of very large and difficult hernias, such as those including apophyseal ring involvement. While facetectomy may be better tolerated at L5-S1 (where the iliotransverse ligament attaches to L5), partial (medial) facetectomy will nevertheless work best for HNPs at level L5-S1, and complete facetectomy should not be necessary.173 Higher in the vertebral column, in order to preserve the narrow pars, it may be necessary to augment a medial facetectomy approach with an intertransverse approach.109,176
Another option, building on the familiar midline exposure, is the possibility of approaching the hernia in the proximal foramen from an interlaminar approach originating from the contralateral side of the midline and proceeding across the midline and under the facet, minimizing facetectomy.193 Foraminotomy, with some form of partial facetectomy, is still the most popular surgical option, despite the inherent disadvantage of aggressive bone removal.
Using a modification of the midline approach to address a hernia deeper within the lateral foramen, a slightly different combined interlaminar and extralaminar exposure has also been described by Hood,175 in which the muscle is not dissected off the spinous processes but rather incised 1 cm lateral to the midline. The facet joint is exposed, and a drill and punch are used to produce a partial lateral facetectomy through which the rostral and caudal pedicles can be palpated. The nerve root, forced dorsally by the pathologic hernia, lies deep to the facet capsule and ligamentum flavum. It can be mobilized and retracted to address the disc hernia. If necessary, the lateral recess and retained disc material within the spinal canal can then be approached through a standard interlaminar route with a minimum of bone removal, thus maintaining the integrity of the facet joint.
In another variation, in which the affected intraforaminal root is exposed in the lateral recess above the foramen, a standard exposure of the affected root through a routine interlaminar midline approach is performed, one level rostral to the neural foramen (e.g., the L4 nerve root compressed in the L4-5 foramen is exposed at L3-4 through a routine interlaminar approach). The nerve root is then followed a short distance with a small amount of bone removal from the rostral edge of the caudal lamina and facet at this level. The extralaminar approach is then used to deal with the nerve root, now identified and protected under a dissector in the neuroforamen.171
Di Lorenzo et al. and others163–165 have reported on a novel approach to proximal intraforaminal disc hernia, producing a small ovoid window through the pars, sparing an isthmus of bone on both its medial and lateral aspects, leaving the inferior facet connected to the pedicle and lamina (Fig. 78-4). The hole that is produced lies directly over the lateral recess or proximal neural foramen where the sequestrum is believed to lie. The benefits are described as not only being directly over the pathology but also producing no disruption of the ligamentum flavum or joint capsule and hence less epidural fibrosis. It does, however, potentially risk pars fracture. At least 3 mm of bone must be left between the hole and the lateral aspect of the pars. It would be hard to enter and clear the disc, and there may be a higher recurrence rate, since the procedure relies on fragment removal alone. The approach is limited to intraforaminal hernia and those fragments that have migrated rostral from the disc space. If it should become necessary to enlarge the hole, the bone removal ends up being more than would be common in a laminotomy from the rostral or caudal motion segment. This approach might not be appropriate for larger hernias and those with apophyseal ring fracture or calcification. The pars must be intrinsically generously wide enough to permit fenestration without compromising its integrity, and the foramen must be large enough, uninvolved by stenosis, to permit manipulation of the root as well as the hernia.
In the foregoing procedures, the paraspinous musculature is removed from the spinous processes or is dissected by using the column approach, 1 cm lateral to midline. Exposure of the neuroforamen is via a trajectory that is almost directly dorsal. Because a dorsal extraforaminal approach provides the same orientation for viewing the intervertebral foramen as the midline approach, it also can require significant bone resection to allow visualization of the pathology.192
The midline and paramedian routes are popular and effective, given a conventional approach to the anatomy and surgeons’ familiarity. However, they may provide limited access to the intervertebral foramen and the lateral aspect of the vertebral bodies and may require significant bone resection as mentioned. The paramedian, muscle-splitting approach178–180,194,195 has the advantage of sparing the patient the loss of bone and of providing a somewhat more oblique view of the neuroforamen. It is most useful for hernias that are within the lateral foramen or are extraforaminal. A paramedian skin and fascial incision, about 3 cm from the midline (or further lateral at lower levels), is made just over the natural plane groove between the multifidus and longissimus muscles. Descending through the paraspinous musculature between the transverse processes onto the neuroforamen from a lateral orientation, the surgeon is able to locate the lateral facet and its capsule and perhaps be able to remove only a small amount of lateral facet, if necessary (see Fig. 78-3). The medial transverse processes are exposed, the multifidus muscular attachments to the facet are incised, and the intertransverse muscle and ligament are incised. It is then possible to expose the affected nerve root in the neuroforamen, retract it aside, and address the disc hernia. This is not too dissimilar to the lateral extracavitary approach described by Larson et al.196 Transforaminal ligaments in the lumbar intervertebral foramen present in over 80% of foramina may be encountered, which could compromise outcome if not recognized and taken down.197 Although the major advantage is the preservation of the pars interarticularis and the facet joint with little likelihood of instability,181 it has the disadvantages of surgical unfamiliarity, deeper dissection, possibly poorer visualization, difficulty enucleating the disc space, potential injury to the nerve root within the neuroforamen, and dealing with sequestered fragments beneath the posterior longitudinal ligament.172,187 The exposure at the lower levels gained by the muscle-splitting approach can be more difficult to achieve than that at higher levels because of the gradually decreasing room available between the confines of the transverse and accessory processes and the sacral ala.198,199
O’Brien et al. have described a dorsolateral approach that is farther lateral yet, an incision 10 cm lateral to the midline. A basic tenet of the approach is that of following the lateral branch of the dorsal ramus and the terminal branch of the segmental artery. Both landmarks can be found consistently in the intertransverse space running obliquely across the dorsal surface of the caudal transverse process toward the foramen, with the lateral branch of the dorsal ramus continuing as a guide to the postganglionic root.192 Minimal soft tissue dissection removal associated with this approach may help to facilitate rapid postoperative mobilization.
Although most intraforaminal disc hernias can be cared for from the midline approach, the surgeon should be familiar with the paramedian and perhaps far lateral approaches. These approaches are indicated when the nerve root is compressed lateral to the neuroforamen177,192 or when a lateral disc at L4-5 or L5-S1 is encountered. With the availability of these options, there should be little reason to sacrifice the entire facet joint.
Other approaches, such as the ventrolateral retroperitoneal approach and osteoplastic removal and replacement of the pars interarticularis and facet, have been advocated, but they appear to be more complex than warranted, especially with the availability of simpler procedures. Experience has been gained with augmentation of midline and lateral approaches using endoscopy to access the foramen.57,160,186 No matter which surgical approach is taken, it is wise not to displace the nerve root within the foramen aggressively because the dorsal root ganglion within it is sensitive and its manipulation may worsen the symptoms.190,191 The historically reported results of treatment for lateral disc hernia are likely similar to those of more classical dorsolateral hernias in patients of the same age-group.170–173,178,187 However, there may be a lower response rate than that of paramedian disc hernia because of the ganglion issue.31,56
Spinal Cysts
Three types of intraspinal cysts at differing sites have been responsible for radiculopathy: synovial cysts of the facet joint, the ligamentum flavum, and the intervertebral disc. Cysts involved with the intervertebral disc (discal cysts) are rare and uncommonly distinguished from other kinds of lesions given their unfamiliarity. Plain films will likely be unrevealing; myelography and CT myelography will show extradural mass effect compressing the spinal nerve as with lumbar disc herniation. MRI demonstrates spherical extradural mass on low-intensity T1-weighted images and high-signal intensity T2-weighted images, with rim enhancement on contrast. If discography is performed, contrast medium may show communication between the intervertebral discs and cyst, but not necessarily. There may be pathologic differences between discal cysts and those arising from the posterior longitudinal ligament (PLL) or anulus.
Such cysts are probably ganglion cysts of either the anulus fibrosus or PLL. They are typically walled in by fibrous connective tissue without a specific cell lining but with frequent myxoid degeneration, and they frequently contain serous fluid that may be bloody by virtue of even mild trauma.200–202
Prevention of Perineurial Scar Formation
The evidence for and against the concept of symptomatic scar is conflicting in the modern neuroimaging era.203 In the circumstance of recurrent hernia following discectomy, fibrosis around the root certainly affects symptoms and reoperation rate. Fibrosis results in unusual anatomic problems, including intraradicular and intradural herniations as mentioned.153,154 Because of fibrosis and tethering of the dura mater, the presentation of recurrent hernia or prolapse may be polyradicular rather than monoradicular.
Scar tethering the roots could theoretically inhibit the normal sliding movements and could cause pain, numbness, and weakness.204 Rationally, there would seem to be some impact of both intradural arachnoidal adhesions and extradural scar upon outcome, simply because physiologically, the root sleeves and elements of the cauda equina move with leg and body motion. On the other hand, recent randomized controlled trials showed that neither scar nor the use of the scar-inhibiting barrier Adcon-L had an impact on outcome.101,205,206
Determinants of scar formation include soft tissue traumatization and blood left behind in the operative field. Therefore, surgical minimization and cleanliness can be expected to improve outcomes, and these are reasonable and naturally accepted tenets. Gelatin sponge has been reported to increase scar formation. Therefore, the manufacturer recommends its removal from the field after hemostasis is achieved. Urokinase has been used in an animal model to break down the small amount of blood that invariably remains or accumulates after surgery. This has reduced scar formation and lends credence to the theory of blood products being at least partially responsible.207
Epidural fat is the principal barrier to scar. It is different from subcutaneous fat, being semifluid without as much connective tissue, allowing roots to move freely in the healthy state.208 A precept of technically expert surgery is that epidural fat should be treated gingerly and left as intact as possible.3
A number of substances have been proposed to reduce scar formation in addition to clean technique. One of the most popular measures is the use of epidural steroids, in use for the last 30 years or so. Epidural methylprednisolone or dexamethasone is commonly used and has been shown in animal models to reduce the epidural scar.157,209,210 Steroids also have an effect on the disturbed, unmyelinated pain-transmitting C-fibers, which are the most sensitive to the inflammatory milieu of the disc hernia.98 A combination of systemic and epidural corticosteroids may diminish such damage and improve results with respect to both resolution of pain and preservation of sensation in both the short term and long term.211
Free fat grafts taken from the subcutaneous space or paraspinous tissue have also been used for the last 30 years. Fat grafts can be placed in the interlaminar defect dorsally and may prevent the formation of a dorsal scar or “laminectomy membrane” but cannot be placed circumferentially around the nerve root, including its ventral surface. Literature review shows that fat grafts have not been definitively proven to help, 101,204,212,213but there can be little down side to the application of a small pledget of fat into the interlaminar defect.
In the early postoperative period (within 6 weeks after surgery), the MRI signal intensity of grafted fat decreases, being lower than that of normal subcutaneous fat, but recovers to normal by 1 year after surgery. The total amount of grafted fat used is reduced, but it is alive and remodeled along the shape of the dura mater. There is a remodeling of the grafted fat, which is effective in protecting the spinal nerve. Reoperation with histology on grafted patients shows that grafted fat changes, showing reduction in size of the fat globules, as compared with normal fat tissue.56,214
Minimizing disruption of the ligamentum flavum may reduce scar formation,3,204,215 thereby theoretically relieving the susceptibility to symptomatic tethering and recompression given subsequent anular prolapsed or small recurrent hernia, and facilitating reoperation if necessary.215 In one method of leaving the deep ligamentum flavum and its smooth inner surface, the bony periphery of the interlaminar space is enlarged cephalad, the ligamentum flavum is thinned to paper thickness starting caudally, and then a lateral slit is produced entering the lateral recess.
Now of only historical significance, carbohydrate polymer in gelatin, Adcon-L, had proven efficacy in the reduction of scar formation around the nerve root, as shown by both animal histologic and human radiographic studies, when placed following discectomy.216–218 However, there was noted increased incidence of postoperative CSF leakage, and the product was taken off the market.219,220 Hyaluronic acid has also been used in animal trials213 and is used in other surgical fields for the purpose of discouraging scar formation, though it has not been used clinically in laminectomy patients. Both agents inhibit the initial influx of inflammatory cells and the ingrowth of fibroblasts, and both agents are biodegradable. Combination carboxymethylcellulose with polyethylene oxide gel preparations are commercially available in Europe and elsewhere, but are not currently available for distribution in the United States. Used in a variety of surgical applications to reduce scar formation, the gel has been used in a randomized controlled trial in microdiscectomy. Blinded outcome assessments at intervals of up to 3-year follow-up demonstrated benefit. The biologically inactive gel does not discourage the formation of scar, but rather encourages its adhesion to dura through a barrier effect.221
The interposition of bone wax, silicone rubber, or Dacron sheeting and of fascial graft has also been used; none of these can be recommended, however. Common to the use of these materials is a lack of proven efficacy in the prevention of postoperative residual radiculopathy in large trials.101
Recurrence, Loss of Disc Height, and Discectomy Technique
Recurrence of hernia, should it occur, usually occurs within 1 or 2 years of surgery.2,44,215,222,223 As time passes, the recurrence rate approaches the natural history likelihood that any patient may develop disc hernia. In the Maine lumbar spine study, while at the end of 10 years, 25% of surgical patients had undergone at least one additional lumbar spine operation and 25% of the nonsurgical patients also had at least one lumbar spine operation.35
There is broad variability in the reported rate of disc recurrence requiring surgery (as a result of recurrent hernia or secondary degenerative change and stenosis) amongst several series, ranging between 3% and 19.4%. 30,35,40,224,225This may be the result of several factors, including differences in the definition of a recurrence, confusion with other entities such as foraminal stenosis and segmental instability, differences in technique, differences in postoperative follow-up, and differences in postoperative studies and management.226,227 When it occurs, recurrent hernia usually occurs within the first 1 or 2 years postoperatively and ultimately approaches the likelihood of hernia in the general patient population.44,223 The SPORT trial followed 798 surgical patients prospectively for 4 years and found an overall reoperation rate for recurrent hernia of 6% by 1 year, 8% by 2 years, 9% by 3 years, and 10% by 4 years, but half were at the previous level of operation.44
Patients in the SPORT study who underwent second operations scored worse outcomes than single-surgery patients and had three times the disability rate, at 8 years. While the outcome for truly recurrent soft disc hernia is as good or nearly as good as initial surgery,226 the outcome for patients who were reoperated on without the finding of recurrent disc is worse than the outcome of reoperation for recurrent disc at the same level.222 Operating on scar alone results in the poorest outcomes of potential postoperative radiculopathic entities and is considered futile, and repeat back surgery on an “exploratory” basis is not warranted in any situation and most likely will lead only to further disability228 and possibly to further scar, instability, and failed back syndrome.
Recurrent disc herniation is mainly found at the first reintervention, the rate of epidural fibrosis and instability increasing with each iteration of repeat surgery along with a consequent decline in long-term improvement.229 Therefore, a firm clinical diagnosis with strong radiculopathic symptoms and signs in concert with firm concordant radiographic findings should be standard practice.
Height loss after disc hernia and discectomy is pertinent, since it can produce foraminal stenosis, anular bulging, and lateral recess stenosis with delayed onset of recurrent radiculopathy in the same or rostral root distribution. Disc hernia itself may89 or may not lead to disc space height loss,230 although it is not known with certainty, since there are no long-term studies of this particular issue in patients who did not undergo surgery. Discectomy resulted in a mean 18% loss of height at 3 months and a 26% loss of height at 2 years in a recent prospective study that measured the clinical outcomes and obtained repetitive CT imaging of the lumbar spine at close intervals for a period of 2 years.227 The study was designed to find the variables that are responsible for recurrent disc hernia. Loss of disc space height following hernia and hernia surgery is well appreciated; most patients lose about 25% of disc space height. The effect of disc space height loss on long-term outcomes has been examined, and a correlation with low back pain was found, but there is limited evidence that back pain is strongly correlated with disc space collapse.40 Disc space height loss correlates with enlargement of the end plates and with loss of lumbar flexibility.75 There may be better evidence that radiculopathy from foraminal stenosis may be a long-delayed result.
It has been stated that bulging anulus should be decompressed rather than violated, in the effort to reduce the chance of recurrence. Most patients with sciatica in fact have contained disc bulge rather than expressed sequestrated disc fragments. As a result, simple sequestrectomy is likely infrequently performed.137
Aggressive emptying of the disc space reduces recurrence, according to McGirt et al. in a prospective study specifically addressing the question of recurrence.227 The percentage of disc material removed does correlate with loss in disc space height, but disc space height loss does not correlate with back pain, leg pain, ODI, or SF-36 at 2 years, although in the longer term, it may.227 In a prospective outcomes study including five institutions and 2-year follow-up, it was found that those patients with recurrence (10.2%) had less disc volume removed (13% of the disc volume) compared to the patients without recurrence (28% of disc volume removed).227 Other researchers have also found a relationship between extent of discectomy and potential for recurrent hernia or at least a relationship between preserved disc height and risk of recurrence.40 In a comprehensive meta-analysis of the literature on the subject, it has been shown that conservative discectomy may result in a shorter operative time, quicker return to work, and decreased incidence of long-term recurrent low back and leg pain but with an increased incidence of recurrent disc herniation compared to aggressive discectomy.227,231
Aggressive discectomy has its down side, however, with resulting greater disc space height loss likely producing more postoperative back pain at 3 years than with sequestrectomy alone. This has been shown by Barth et al. in a prospective study of the issue and by others.3,232–234 Barth did not find recurrence to be higher after conservative discectomy in their series.
According to McGirt et al., 11 of 108 patients suffered recurrent disc hernia (10.2%) at an average of 10.5 months postoperatively. The size of the anular defect and the amount of disc material left at the original surgery were associated with an increased risk of recurrence.227 The same correlation between the size of the anular defect and the risk of recurrence and between the amount of disc removed and the risk of recurrence has been found by others.235,236
As was mentioned, recurrent radiculopathic pain may be due to traction injury of a root fixed in perineurial or arachnoidal scar, or it may be due to recurrent or residual disc hernia or prolapsed anulus. When radiculopathic leg pain reappears or does not remit, repeat neuroimaging is indicated. Optimally, this would be a high-quality MRI without and with gadolinium enhancement. The finding of perineurial scar only is neither a surgical complication nor an indication for revision surgery. However, lateral recess stenosis, anular prolapse, foraminal stenosis, and recurrent or residual disc herniation are all secondary complications that can be responsible for symptoms and are potentially benefitted by revision surgery. The management of repeat or incompletely treated nerve root compression is similar to that of an otherwise untreated hernia, with the exception that there is a variable amount of scar around the nerve root. The fibrosis can make anatomic planes difficult to discern and dissect and can result in unusual anatomic problems, such as intraradicular and intradural herniations, as was mentioned in the section on technique. Also, because of fibrosis and tethering of the dura mater, the presentation may be polyradicular rather than monoradicular. The anatomy is easier to understand if the bony exposure is made wider and fresh dura is identified around the periphery of the exposure.
A time-honored belief was that recurrent hernia required fusion. Fusion is not routinely required in patients undergoing repeat laminectomy and discectomy for recurrent disc herniation. In the absence of objective evidence of spinal instability, recurrent disc herniation may be adequately treated by repeat lumbar laminectomy and discectomy alone.237
There is little reason to proceed with fusion unless the motion segment is demonstrably unstable. Of course, the addition of fusion increases the likelihood of its own complications and reoperation rate.225 Therefore, an initial recurrent disc hernia or secondary stenosis can be treated with conventional laminotomy or microlaminotomy, with good results.228,237,238 In the case of multiple recurrences, because of the implication of the role of instability, repetitive revision may indicate the need for thorough decompression and fusion.229 With the need for repetitive revisions, poorer results are encountered.224
Management of Complications
In either the prone or the lateral decubitus position, air embolism can occur, though in neither position is it considered to be enough of a risk to warrant invasive line placement. However, if a Doppler monitor is placed, it provides the earliest diagnosis. With paradoxical embolism across a patent foramen ovale, a decrease in arterial carbon dioxide pressure, a decrease in end-tidal carbon dioxide pressure, an increase in arterial carbon dioxide pressure, and subsequent hypotension and tachycardia may all be observed. Proper management consists of ceasing nitrous oxide administration, flooding the field with saline, waxing the bony surfaces, and repositioning the patient quickly to lower the field in relation to the heart.239
The incidence of dural tears may be 1.6%3,240 to 4%,241 with an incidence of 3% in the SPORT trial.44 Perhaps the number is more, since ventral tears that are not noted intraoperatively would not be detected. Outcomes studies demonstrate no long-term sequelae when patients are satisfactorily repaired primarily during initial surgery.127,240–242 The hazard of a dural tear lies not with the nuisance meningeal injury that is sustained, recognized, and repaired adequately at the time of surgery, but rather with injury that is not recognized at surgery, is not adequately repaired, or is acquired postoperatively.
In such cases, hydrostatic pressure and superimposed events such as Valsalva and motion are likely generating or aggravating factors.243 Long-term postoperative symptoms such as headache reduce daily activity and impair postoperative progress, such that a longer duration of inability to work and long-term functional limitation may be engendered.242 Delayed or inadequately repaired durotomy risks wound dehiscence and CSF leakage or symptomatic pseudomeningocele. Should they occur, the dura should be repaired as quickly as possible following surgery.124–126242
Pseudomeningocele can cause recurrent back and leg pain, the latter being the result of nerve root herniation into the extradural sac.125 Good results, with resolution of symptoms by primary closure of the dural defect, can be expected. Ventral dural tear may be a consequence of dural adhesion to the PLL or anulus, in this way perhaps similar to transdural HNP. Generally, ventral dural tear does not require suture closure; however, symptomatic herniation of nerve roots through a ventral defect into the empty disc cavity has been described.243
If a spontaneous, unrecognized, or inadequately repaired durotomy complicates the wound postoperatively, an attempt at the use of an autologous blood patch or fibrin glue patch in the treatment of postoperative pseudomeningocele or fistula, if small, is reasonable and potentially successful. The use of closed lumbar drainage for several days placed at a distance from the original incision is also effective.128 The decision to reoperate and repair the defect is elective and dependent on symptoms in the case of pseudomeningocele but is more urgent in the event of high-volume or persistent fistula, since the soft tissue becomes compromised and has the potential for becoming infected quickly.
A rare complication, cerebellar hemorrhagic venous infarction, has been reported as resulting from durotomy with intraoperative loss of CSF. It is possible that downward cerebellar displacement, or “sag,” causes transient stretch occlusion of superior cerebellar veins draining in the rostral direction into the deep venous system. This may cause intracerebellar hemorrhage in patients with insufficient venous collaterals. Remote cerebellar hemorrhage should be considered in a case of neurologic deterioration following lumbar durotomy.244
Injury of abdominal organs or viscera occurs in 1.6 to 17 per 10,000 cases.148 Effective management of visceral or vascular injury requires prompt recognition of its occurrence. Mortality approaches 100% in cases of untreated vascular or visceral injuries.148 In some reported cases of bowel and ureteral injury, visceral tissue was found in the disc specimen that was submitted for examination. As was mentioned in the section on technique, injury may be suspected intraoperatively because of hypotension, the unexplained egress of irrigation fluid through the anulus and out of the field, or persistent bleeding up through the anulus that is not explained by bone or epidural bleeding. There appear to be no common risk factors predisposing patients to this type of hazard, apart from ventral anular tear. A sound knowledge of retroperitoneal anatomy is useful (Fig. 78-5). Even with treatment, mortality of these injuries is high, at nearly 50%; therefore, if suspected, the injury demands immediate attention.148 Unfortunately, the initial lack of clinical signs of injury usually accounts for any delay that is encountered, and in a majority of cases, the surgeon fails to recognize perforation of the anulus or ligament and intra-abdominal injury. In convalescence, persistent flank or abdominal pain, ileus, hypotension, and fever may all signal intra-abdominal injury and should be investigated. Plain abdominal radiographs, CT, intravenous pyelogram, angiography, and abdominal ultrasound may all be necessary.
A patient may be sensitized to major vascular injury through one of two or three anatomic variations. Since the anterior longitudinal ligament provides a barrier to the passage of an instrument through the anulus into the retroperitoneum, a high aortic bifurcation results in the iliac vessel lying lateral to the protection of the ligament. A spondylotic ridge may result in attenuation of the ligament.147 Also, peridiscal inflammation, resulting in fibrosis, can result in adhesion of a large vessel to the anulus. The left iliac artery is the most commonly injured named vessel. Arterial injury is at times large enough, and hypotension is rapid enough, that there might not be time for angiography or scanning. In such cases, immediate laparotomy is indicated purely on clinical suspicion. If the vessel is small and the patient is stable, perhaps having presented with hemorrhage from the disc space, angiography is useful in determining its location prior to laparotomy.245 There may be an initial lack of clinical signs of vascular injury until significant blood loss has occurred and the patient is in danger of vascular collapse, particularly in healthy young patients with significant cardiovascular reserve (if a small vessel such as a medial sacral vessel has been injured).
Bowel motility is slowed by the use of general anesthesia, but it can also result from the surgery itself, and ileus can affect both the large and small bowel. Usually, it is not a significant problem, but it can last several days and can require nasogastric suction.246 A more severe form of impaired bowel motility—pseudo-obstruction of the colon (Ogilvie syndrome)—is a life-threatening complication that is characterized by massive cecal distention that can lead to perforation. Bowel sounds are present from areas of active motility, but cecal distention and tenderness are also present. Decompression by colonoscopy is the treatment of choice unless rupture has already occurred.247
If disc interspace infection is present, the superficial wound is often well healed, with the nidus of infection contained in the disc space. Marked persistent spastic back pain, marked sedimentation rate elevation, and elevated C-reactive protein levels are clinical and laboratory indicators of disc interspace infection. In these cases, discitis should be assumed until it has been ruled out.248 The presence of unusually severe pain in the postoperative period, even if the wound is well healed, should prompt a workup for disc interspace infection because superficial evidence of wound infection is unusual. MRI is a very sensitive indicator and can show evidence of infection early in its course. Patients with sedimentation rates of over 45 mm/min and C-reactive protein levels higher than 2.5 mg/L on the fifth or sixth postoperative day are suspected of having disc interspace infections. Although the temperature and peripheral white blood cell count may be elevated, this is not always the case. If disc interspace infection is present, antibiotics should be appropriate for the infectious agent as proven by open or CT-guided biopsy or by peripheral blood culture (which is positive in 25% of cases). Intravenous antibiotics should be administered for a period of 6 weeks. If there is reason to suspect involvement of adjacent vertebral bone, by MRI or CT or by progressive deformity, intravenous antibiotics should be continued for 6 to 8 weeks, followed by oral antibiotics for another 8 weeks. Immobilization is generally recommended.
The radiographic changes of disc interspace infection are often delayed. Optimally, the presence of infection should be recognized before its radiographic appearance. Plain radiographs do not show a change until 6 weeks after the onset of infection, until there is erosion of the subchondral cortical bone. The most sensitive radiographic study early in the course of infection is the MRI scan, which shows (1) a diffuse decrease in the T1 signal from vertebral body bone adjacent to the disc as a result of edema fluid in the marrow; (2) blurring of the margins between vertebral bone, cartilaginous end plates, and disc; and (3) an increased T2 signal in the area of inflammation, particularly in the disc. There may be swelling of paravertebral soft tissues and, with gadolinium infusion, epidural contrast enhancement. Bone scanning and indium-labeled leukocyte scanning are also quite sensitive and positive relatively early in the course of infection.
Staphylococcus aureus can produce, in addition to abscess formation and recurrent neurologic deficit on the basis of space-occupying mass effect, exotoxins with local and distant effects. Toxic shock syndrome is attributable to this, with vascular collapse and encephalopathy. Reportedly, local staphylococcal wound infection after lumbar laminectomy can even result in a cauda equina syndrome, without a compressive mass effect.249
Postoperative Course and Postoperative Care
Classically, the first symptom to improve following successful surgery is the pain of radiculopathy, typically followed by improvement in motor function, and finally by resolution of sensory loss.7,38,39 Sensory loss may be permanent, however, persisting at 10-year follow-up in 35% of Weber’s patients.
Impairment of back muscle function due to months of inactivity before surgery may be a factor contributing to prolonged and suboptimal outcome. Surgery itself only superimposes further damage on lumbar paraspinous musculature and does not, by itself, improve function. In a prospective randomized controlled trial of supervised exercise therapy addressing patients who had undergone microdiscectomy, Dolan et al. found that a 4-week exercise program focused on improving strength and endurance of the back and abdominal muscles and mobility of the spine and hips, initiated at 6 weeks postoperatively, provided clear benefit. Outcome measures that improved in the exercise group included paraspinous surface electromyography, pain, disability, back muscle endurance, and lumbar and hip mobility.250,251
In another prospective randomized trial of similar design, significant benefit following lumbar disc hernia surgery was demonstrated by a decrease in disability and pain scores at 6 months and 12 months in a group that was randomized to training starting at 4 weeks postoperatively.252 There is no evidence that activity restriction improves outcomes or avoids complications after lumbar surgery.235,252
Dewing et al. reported the outcomes of lumbar discectomy in a population of young, active military personnel, finding not only that 84% were capable of unrestricted return to active service, but that those who did best were special operations personnel and aviators.253 There is actually little evidence that aggressive physical activity is harmful to disc tissue, whether it is in a degenerative, normal, or postoperative state. In fact, immobilization has been determined to be harmful to the disc, likely through its adverse effect on the diffusion of nutrients and the maintenance of disc tissue homeostasis.73
Recurrent symptoms may result from, or be exacerbated by, poor bone and ligamentous healing. Bone healing might not be as important to outcome in discectomy as it would be in attempted fusion, but it is nevertheless significant in bone remodeling postoperatively. Physiologic bone remodeling is important for the reacquisition of normal pars strength and therefore to long-term results and return to preoperative activities. Systemic factors that have been demonstrated to inhibit bone healing and likely to interfere with optimum outcome include smoking, malnutrition, diabetes, rheumatologic conditions, and osteoporosis. Additionally, courses of steroids, nonsteroidal medications, and cytotoxic medications in the perioperative period are to be avoided, particularly the first 2 postoperative weeks.254 It is suggested that postoperative patients engage in and maintain daily reconditioning, including low-impact aerobic exercises, such as walking and swimming and active range-of-motion exercises.
Conclusions
Surgical discectomy is a cost-effective solution to the problem of short-term pain and disability in carefully selected patients harboring a symptomatic lumbar disc hernia refractory to conservative management.6,44 In the short term, up to a year postoperatively, the benefits are clear.
Atlas S.J., Keller R.B., Wu Y.A., et al. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the Maine lumbar spine study. Spine (Phila Pa 1976). 2005;30(8):927-935.
Battié M.C., Videman T., Kaprio J., et al. The Twin Spine Study: contributions to a changing view of disc degeneration. Spine J. 2009;9(1):47-59.
Epstein N. Foraminal and far lateral lumbar disc herniations: surgical alternatives and outcome measures. Spinal Cord. 2002;40(10):491-500.
Gibson J.N., Waddell G. Surgical interventions for lumbar disc prolapse: updated Cochrane Review. Spine (Phila Pa 1976). 2007;32(16):1735-1747.
Weber H. The natural history of disc herniation and the influence of intervention. Spine (Phila Pa 1976). 1994;19(19):2234-2238.
Weinstein J.N., Lurie J.D., Tosteson T.D., et al. Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976). 2008;33(25):2789-2800.
1. AANS: American Association of Neurological Surgeons (AANS) Talking Points. 2009.
2. Asch H.L., Lewis P.J., Moreland D.B., et al. Prospective multiple outcomes study of outpatient lumbar microdiscectomy: should 75 to 80% success rates be the norm? J Neurosurg. 2002;96:34.
3. Koebbe C.J., Maroon J.C., Abla A., et al. Lumbar microdiscectomy: a historical perspective and current technical considerations. Neurosurg Focus. 2002;13:E3.
4. Mattila V.M., Saarni L., Parkkari J., et al. Early risk factors for lumbar discectomy: an 11-year follow-up of 57,408 adolescents. Eur Spine J. 2008;17:1317.
5. Ramirez L.F. Thisted R: Complications and demographic characteristics of patients undergoing lumbar discectomy in community hospitals. Neurosurgery. 1989;25:226.
6. Malter A.D., Larson E.B., Urban N., Deyo R.A. Cost-effectiveness of lumbar discectomy for the treatment of herniated intervertebral disc. Spine (Phila Pa 1976). 1996;21:1048.
7. Baldwin N.G. Lumbar disc disease: the natural history. Neurosurg Focus. 2002;13:1.
8. Luoma K., Riihimäki H., Luukkonen R., et al. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976). 2000;25:487.
9. Battié M.C., Videman T., Kaprio J., et al. The Twin Spine Study: contributions to a changing view of disc degeneration. Spine J. 2009;9:47.
10. Videman T., Battié M.C., Parent E., et al. Progression and determinants of quantitative magnetic resonance imaging measures of lumbar disc degeneration: a five-year follow-up of adult male monozygotic twins. Spine (Phila Pa 1976). 2008;33:1484.
11. Fardon D.F., Milette P.C. Combined Task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology: nomenclature and classification of lumbar disc pathology: recommendations of the Combined Task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine (Phila Pa 1976). 2001;26:E93.
12. Ehni G.J. Effects of certain degenerative diseases of the spine, especially spondylosis and disc protrusion, on the neural contents, particularly in the lumbar region: historical account. Mayo Clinic Proc. 1975;50:327.
13. Pott P. Remarks on that kind of palsy of the lower limbs which is frequently found to accompany a curvature of the spine, and is supposed to be caused by it, together with its method of cure. Med Classics. 1936;1:281.
14. Lane W.A. Spondylolisthesis associated with progressive paraplegia: laminectomy. Lancet. 1893;1:991.
15. Bailey P., Casamajor L. Osteo-arthritis of the spine as a cause of compression of the spinal cord and its roots: with reports of five cases. J Nerv Ment Dis. 1911;38:588.
16. Goldthwait J. The lumbo-sacral articulation: an explanation of many cases of “lumbago,” “sciatica,” and paraplegia. Boston Med Surg J. 1911;164:365.
17. Elsberg C.A. Diagnosis and treatment of surgical diseases of the spinal cord and its membranes. Philadelphia: WB Saunders; 1916.
18. Parker H.L., Adson A.W. Compression of the spinal cord and its roots by hypertrophic osteo-arthritis. Surg Gynecol Obstet. 1925;41:1.
19. Putti V. New conceptions in the pathogenesis of sciatic pain. Lancet. 1927;2:53.
20. Dandy W. Loose cartilage from intervertebral disc simulating tumor of the spinal cord. Arch Surg. 1929;19:660.
21. Mauric G. Le disque intervertebral: physiologie, pathologie, et indications therapeutiques. Paris: Masson & Cie; 1933.
22. Mixter W., Barr J.S. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 211, 1934.
23. Love J.G. Removal of protruded intervertebral discs without laminectomy. Proc Staff Meet Mayo Clin. 1939;14:800.
24. Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg [Br]. 230, 1954.
25. Verbiest H. Further experiences on the pathological influence of a developmental narrowness of the bony canal. J Bone Joint Surg [Br]. 576, 1955.
26. Williams R.W. Microlumbar discectomy: a conservative surgical approach to the virgin herniated lumbar disc. Spine (Phila Pa 1976). 1978;3:175.
27. Caspar W. A new surgical procedure for lumbar disc herniation causing less tissue damage through a microsurgical approach. In: Wullenweber R., Brock M., Hamer J., editors. Advances in neurosurgery. Berlin: Springer Verlag; 1977:p 74.
28. Abramovitz J.N., Neff S.R. Lumbar disc surgery: results of the Prospective Lumbar Discectomy Study of the Joint Section on Disorders of the Spine and Peripheral Nerves of the American Association of Neurological Surgeons and the Congress of Neurological Surgeons. Neurosurgery. 1991;29:301.
29. Daneyemez M., Sali A., Kahraman S., et al. Outcome analyses in 1072 surgically treated lumbar disc herniations. Minim Invasive Neurosurg. 1999;42:63.
30. Davis R.A. A long-term outcome analysis of 984 surgically treated herniated lumbar discs. J Neurosurg. 1994;80:415.
31. McCulloch J.A. Focus issue on lumbar disc herniation: macro- and microdiscectomy. Spine (Phila Pa 1976). 1996;21:45S.
32. Nashold B.S., Hrubek Z. Lumbar disc disease: a twenty year followup study. St. Louis: CV Mosby; 1971.
33. Pappas C.T., Harrington T., Sonntag V.K. Outcome analysis in 654 surgically treated lumbar disc herniations. Neurosurgery. 1992;30:862.
34. Spurling R.G., Grantham E.G. The end-results of surgery for ruptured intervertebral discs: a follow-up study of 327 cases. J Neurosurg. 1949;6:57.
35. Atlas S.J., Keller R.B., Wu Y.A., et al. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the Maine lumbar spine study. Spine (Phila Pa 1976). 2005;30:927.
36. Larequi-Lauber T., Vader J.P., Burnand B., et al. Appropriateness of indications for surgery of lumbar disc hernia and spinal stenosis. Spine (Phila Pa 1976). 1997;22:203.
37. Keller R.B., Atlas S.J., Soule D.N., et al. Relationship between rates and outcomes of operative treatment for lumbar disc herniation and spinal stenosis. J Bone Joint Surg [Am]. 1999;81:752.
38. Weber H. Lumbar disc herniation: a controlled, prospective study with ten years of observation. Spine. 1983;8:131.
39. Weber H. The natural history of disc herniation and the influence of intervention. Spine (Phila Pa 1976). 1994;19:2234.
40. Yorimitsu E., Chiba K., Toyama Y., Hirabayashi K. Long-term outcomes of standard discectomy for lumbar disc herniation: a follow-up study of more than 10 years. Spine (Phila Pa 1976). 2001;26:652.
41. Thomas K.C., Fisher C.G., Boyd M., et al. Outcome evaluation of surgical and nonsurgical management of lumbar disc protrusion causing radiculopathy. Spine (Phila Pa 1976). 2007;32:1414.
42. Errico T.J., Fardon D.F., Lowell T.D. Open discectomy as treatment for herniated nucleus pulposus of the lumbar spine. Spine J. 2003;3(Suppl):45.
43. Lurie J.D., Faucett S.C., Hanscom B., et al. Lumbar discectomy outcomes vary by herniation level in the Spine Patient Outcomes Research Trial. J Bone Joint Surg [Am]. 2008;90:1811.
44. Weinstein J.N., Lurie J.D., Tosteson T.D., et al. Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976). 2008;33:2789.
45. Ito T., Yamada M., Ikuta F., et al. Histologic evidence of absorption of sequestration-type herniated disc. Spine (Phila Pa 1976). 1996;21:230.
46. Ito T., Takano Y., Yuasa N. Types of lumbar herniated disc and clinical course. Spine (Phila Pa 1976). 2001;26:648.
47. Peul W.C., van Houwelingen H.C., van den Hout W.B., et al. Surgery versus prolonged conservative treatment for sciatica. N Engl J Med. 2007;356:2245.
48. Greenfield K., Nelson R.J., Findlay G.D., et al. Microdiscectomy and conservative treatment for lumbar disc herniation with back pain and sciatica: a randomised clinical trial. 30th Annual Meeting of the International Society for the Study of the Lumbar Spine. Vancouver: British Columbia; 2003.
49. Osterman H., Seitsalo S., Karppinen J., Malmivaara A. Effectiveness of microdiscectomy for lumbar disc herniation: a randomized controlled trial with 2 years of follow-up. Spine (Phila Pa 1976). 2006;31:2409.
50. Vroomen P.C., de Krom M.C., Knottnerus J.A. When does the patient with a disc herniation undergo lumbosacral discectomy? J Neurol Neurosurg Psychiatry. 2000;68:75.
51. Vroomen P.C., de Krom M.C., Wilmink J.T. Pathoanatomy of clinical findings in patients with sciatica: a magnetic resonance imaging study. J Neurosurg. 2000;92:135.
52. Woertgen C., Rothoerl R.D., Brawanski A. Influence of macrophage infiltration of herniated lumbar disc tissue on outcome after lumbar disc surgery. Spine (Phila Pa. 2000;1976)(25):871.
53. Andreshak T.G., An H.S., Hall J., Stein B. Lumbar spine surgery in the obese patient. J Spinal Disord Tech. 1997;10:376.
54. Leboeuf-Yde C. Smoking and low back pain: a systematic literature review of 41 journal articles reporting 47 epidemiologic studies. Spine (Phila Pa 1976). 1999;24:1463.
55. Fogelholm R.R., Alho A.V. Smoking and intervertebral disc degeneration. Med Hypotheses. 2001;56:537.
56. Ohmori K., Kanamori M., Kawaguchi Y., et al. Clinical features of extraforaminal lumbar disc herniation based on the radiographic location of the dorsal root ganglion. Spine (Phila Pa 1976). 2001;26:662.
57. Frank E. Removal of a lateral disc herniation with malleable endoscopic forceps: technical note. Neurosurgery. 1997;41:311.
58. Stankovic R., Johnell O., Maly P., Willner S. Use of lumbar extension, slump test, physical and neurological examination in the evaluation of patients with suspected herniated nucleus pulposus: a prospective clinical study. Man Ther. 1999;4:25.
59. Vucetic N., de Bri E., Svensson O. Clinical history in lumbar disc herniation: a prospective study in 160 patients. Acta Orthop Scand. 1997;68:116.
60. Battié M.C., Videman T., Parent E. Lumbar disc degeneration: epidemiology and genetic influences. Spine (Phila Pa 1976). 2004;29:2679.
61. Jensen M.C., Brant-Zawadzki M.N., Obuchowski N., et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69.
62. Lurie J.D., Doman D.M., Spratt K.F., et al. Magnetic resonance imaging interpretation in patients with symptomatic lumbar spine disc herniations: comparison of clinician and radiologist readings. Spine (Phila Pa 1976). 2009;34:701.
63. Carvi y Nievas M.N. Hoellerhage HG: Unusual sequestered disc fragments simulating spinal tumors and other space-occupying lesions. J Neurosurg Spine. 2009;11:43.
64. Jarvik J.G., Deyo R.A. Imaging of lumbar intervertebral disc degeneration and aging, excluding disc herniations. Radiol Clin North Am. 2000;38:1255.
65. Deyo R.A., Weinstein J.N. Low back pain. N Engl J Med. 2001;344:363.
66. Roland M., van Tulder M. Should radiologists change the way they report plain radiography of the spine? Lancet. 1998;352:229.
67. Greene D.L., Appel A.J., Reinert S.E., Palumbo M.A. Lumbar disc herniation: evaluation of information on the internet. Spine (Phila Pa 1976). 2005;30:826.
68. Urban J.P., Smith S., Fairbank J.C. Nutrition of the intervertebral disc. Spine (Phila Pa 1976). 2004;29:2700.
69. Roughley P.J. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976). 2004;29:2691.
70. Matsui H., Terahata N., Tsuji H., et al. Familial predisposition and clustering for juvenile lumbar disc herniation. Spine (Phila Pa 1976). 1992;17:1323.
71. Varlotta G.P., Brown M.D., Kelsey J.L., Golden A.L. Familial predisposition for herniation of a lumbar disc in patients who are less than twenty-one years old. J Bone Joint Surg [Am]. 1991;73:124.
72. Setton L.A., Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg [Am]. 2006;88:52.
73. Stokes I.A., Iatridis J.C. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine (Phila Pa 1976). 2004;29:2724.
74. Schollum M.L., Robertson P.A., Broom N.D. ISSLS prize winner: microstructure and mechanical disruption of the lumbar disc anulus: part I: a microscopic investigation of the translamellar bridging network. Spine (Phila Pa 1976). 2008;33:2702.
75. Martin M.D., Boxell C.M., Malone D.G. Pathophysiology of lumbar disc degeneration: a review of the literature. Neurosurg Focus. 2002;13:E1.
76. Gruber H.E., Hanley E.N. Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine (Phila Pa 1976). 1998;23:751.
77. Chang C.H., Lee Z.L., Chen W.J., et al. Clinical significance of ring apophysis fracture in adolescent lumbar disc herniation. Spine (Phila Pa 1976). 2008;33:1750.
78. Willburger R.E., Ehiosun U.K., Kuhnen C., et al. Clinical symptoms in lumbar disc herniations and their correlation to the histological composition of the extruded disc material. Spine (Phila Pa 1976). 2004;29:1655.
79. Hicks G.E., Morone N., Weiner D.K. Degenerative lumbar disc and facet disease in older adults: prevalence and clinical correlates. Spine (Phila Pa 1976). 2009;34:1301.
80. Kendrick D., Fielding K., Bentley E., et al. Radiography of the lumbar spine in primary care patients with low back pain: randomised controlled trial. BMJ. 2003;322:400.
81. Borenstein D.G. Epidemiology, etiology, diagnostic evaluation, and treatment of low back pain. Curr Opin Rheumatol. 2001;13:128.
82. Albeck M.J. A critical assessment of clinical diagnosis of disc herniation in patients with monoradicular sciatica. Acta Neuruchir (Wien). 1996;138:40.
83. Ng L.C., Sell P. Predictive value of the duration of sciatica for lumbar discectomy. A prospective cohort study. J Bone Joint Surg [Br]. 2004;86:546.
84. Nachemson A.L. Advances in low-back pain. Clin Orthop Relat Res. 1985;200:266.
85. Eysel P., Rompe J.D., Hopf C. Prognostic criteria of discogenic paresis. Eur Spine J. 1994;3:214.
86. Hall H. Surgery: indications and options. Neurol Clin. 1999;17:113.
87. Maigne J.Y., Rime B., Deligne B. Computed tomographic follow-up study of forty-eight cases of nonoperatively treated lumbar intervertebral disc herniation. Spine (Phila Pa 1976). 1992;17:1071.
88. Ozaki S., Muro T., Ito S., Mizushima M. Neovascularization of the outermost area of herniated lumbar intervertebral discs. J Orthop Sci. 1999;4:286.
89. Matsubara Y., Kato F., Mimatsu K., et al. Serial changes on MRI in lumbar disc herniations treated conservatively. Neuroradiology. 1995;37:378.
90. Masui T., Yukawa Y., Nakamura S., et al. Natural history of patients with lumbar disc herniation observed by magnetic resonance imaging for minimum 7 years. J Spinal Disord Tech. 2005;18:121.
91. Saal J.A., Saal J.S., Herzog R.J. The natural history of lumbar intervertebral disc extrusions treated nonoperatively. Spine (Phila Pa 1976). 1990;15:683.
92. Autio R.A., Karppinen J., Niinimäki J., et al. Determinants of spontaneous resorption of intervertebral disc herniations. Spine (Phila Pa 1976). 2006;31:1247.
93. Sakai T., Tsuji T., Asazuma T., et al. Spontaneous resorption in recurrent intradural lumbar disc herniation. Case report. J Neurosurg Spine. 2007;6:574.
94. Rust M.S., Olivero W.C. Far lateral disc herniations: the results of conservative management. J Spinal Disord. 1999;12:138.
95. Kobayashi S., Meir A., Kokubo Y., et al. Ultrastructural analysis on lumbar disc herniation using surgical specimens: role of neovascularization and macrophages in hernias. Spine (Phila Pa 1976). 2009;34:655.
96. Rothoerl R.D., Woertgen C., Brawanski A. Pain resolution after lumbar disc surgery is influenced by macrophage tissue infiltration: a prospective consecutive study on 177 patients. J Clin Neurosci. 2002;9:633.
97. Fisher C., Noonan V., Bishop P., et al. Outcome evaluation of the operative management of lumbar disc herniation causing sciatica. J Neurosurg. 2004;100:317.
98. Nygaard O.P., Kloster R., Solberg T. Duration of leg pain as a predictor of outcome after surgery for lumbar disc herniation: a prospective cohort study with 1-year follow up. J Neurosurg. 2000;92:131.
99. Hida S., Naito M., Kubo M. Intraoperative measurements of nerve root blood flow during discectomy for lumbar disc herniation. Spine (Phila Pa 1976). 2003;28:85.
100. Kobayashi S., Suzuki Y., Asai T., Yoshizawa H. Changes in nerve root motion and intraradicular flow during intraoperative femoral nerve stretch test. J Neurosurg. 2003;99:298.
101. Gibson J.N., Waddell G. Surgical interventions for lumbar disc prolapse: updated Cochrane Review. Spine (Phila Pa 1976). 2007;32:1735.
102. Durham S.R., Sun P.P., Sutton L.N. Surgically treated lumbar disc disease in the pediatric population: an outcome study. J Neurosurg. 2000;92:1.
103. Kostuik J.P., Harrington I., Alexander D., et al. Cauda equina syndrome and lumbar disc herniation. J Bone Joint Surg [Am]. 1986;68:386.
104. Hidalgo-Ovejero A.M., García-Mata S., Martínez-Grande M., et al. L5 root compression caused by degenerative spinal stenosis of the L1-L2 and L2-L3 spaces. Spine (Phila Pa 1976). 1998;23:1891.
105. Sanderson S.P., Houten J., Errico T., et al. The unique characteristics of “upper” lumbar disc herniations. Neurosurgery. 2004;55:385.
106. Choi J.W., Lee J.K., Moon K.S., et al. Transdural approach for calcified central disc herniations of the upper lumbar spine. Technical note. J Neurosurg Spine. 2007;7:370.
107. Chhabra M.S., Hussein A.A., Eisenstein S.M. Should fusion accompany lumbar discectomy? A medium-term answer. Clin Orthop Relat Res. 1994;301:177.
108. Glassman S.D., Carreon L.Y., Djurasovic M., et al. Lumbar fusion outcomes stratified by specific diagnostic indication. Spine J. 2009;9:13.
109. Epstein N.E. Foraminal and far lateral lumbar disc herniations: surgical alternatives and outcome measures. Spinal Cord. 2002;40:491.
110. Martin B.I., Mirza S.K., Comstock B.A., et al. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine (Phila Pa 1976). 2007;32:382.
111. Wenger M., Markwalder T. A novel surgical treatment of lumbar disc herniation in patients with long-standing degenerative disc disease. J Neurosurg Spine. 2005;2:515.
112. Chin K.R. Epidemiology of indications and contraindications to total disc replacement in an academic practice. Spine J. 2007;7:392.
113. Coric D., Mummaneni P.V. Nucleus replacement technologies. J Neurosurg Spine. 2008;8:115.
114. Haines S.J. Systemic antibiotic prophylaxis in neurological surgery. Neurosurgery. 1980;6:355.
115. Ferree B.A. Deep venous thrombosis following lumbar laminotomy and laminectomy. Orthopedics. 1994;17:35.
116. Katz D.M., Trobe J.D., et al. Ischemic optic neuropathy after lumbar spine surgery. Arch Ophthalmol. 1994;112:925.
117. Parks B.J. Postoperative peripheral neuropathies. Surgery. 1973;74:348.
118. Aschoff A., Steiner-Milz H., Steiner H.H. Lower limb compartment syndrome following lumbar discectomy in the knee-chest position. Neurosurg Rev. 1990;13:155.
119. Song J., Park Y. Ligament-sparing lumbar microdiscectomy: technical note. Surg Neurol. 2000;53:592.
120. Wenger M., Mariani L., Kalbarczyk A., Gröger U. Long-term outcome of 104 patients after lumbar sequestrectomy according to Williams. Neurosurgery. 2001;49:329.
121. Rosen C., Rothman S., Zigler J., Capen D. Lumbar facet fracture as a possible source of pain after lumbar laminectomy. Spine (Phila Pa 1976). 1991;16:S234.
122. Blikra G. Intradural herniated lumbar disc. J Neurosurg. 1969;31:676.
123. Wadhawani S., Loughenbury P., Soames R. The anterior dural (Hofmann) ligaments. Spine (Phila Pa 1976). 2004;29:623.
124. Lee K.S., Hardy I.M.II. Postlaminectomy lumbar pseudomeningocele: report of four cases. Neurosurgery. 1992;30:131.
125. Nishi S., Hashimoto N., Takagi Y., Tsukahara T. Herniation and entrapment of a nerve root secondary to an unrepaired small dural laceration at lumbar hemilaminectomies. Spine (Phila Pa 1976). 1995;20:2576.
126. Tsuji H., Handa N., Handa O., et al. Postlaminectomy ossified extradural pseudocyst: case report. J Neurosurg. 1990;73:785.
127. Wang J.C., Bohlman H.H., Riew K.D. Dural tears secondary to operations on the lumbar spine: management and results after a two-year-minimum follow-up of eighty-eight patients. J Bone Joint Surg [Am]. 1998;80:1728.
128. Couture D., Branch C.L. Spinal pseudomeningocoeles and cerebrospinal fluid fistulas. Neurosurg Focus. 2003;15(6):E6.
129. Chou D., Wang V.Y., Khan A.S. Primary dural repair during minimally invasive microdiscectomy using standard operating room instruments. Neurosurgery. 2009;64:356.
130. Sugawara T., Itoh Y., Hirano Y., et al. Novel dural closure technique using polyglactin acid sheet prevents cerebrospinal fluid leakage after spinal surgery. Neurosurgery. 2005;57:290.
131. Khan M.H., Rihn J., Steele G., et al. Postoperative management protocol for incidental dural tears during degenerative lumbar spine surgery. Spine (Phila Pa 1976). 2006;31:2609.
132. Feltes C., Fountas K., Davydov R., et al. Effects of nerve root retraction in lumbar discectomy. Neurosurg Focus. 2002;13:E6.
133. Prestar F.J. Anomalies and malformations of lumbar spinal nerve roots. Minim Invasive Neurosurg. 1996;39:133.
134. Martínez-Lage J.F., Fernández Cornejo V., López F., Poza M. Lumbar disc herniation in early childhood: case report and literature review. Childs Nerv Syst. 2003;19:258.
135. Mendez J.S., Huete I.L., Tagle P.M. Limbus lumbar and sacral vertebral fractures. Neurol Res. 2002;24:139.
136. Cenic A., Kachur E. Lumbar discectomy: a national survey of neurosurgeons and literature review. Can J Neurol Sci. 2009;36:196.
137. Arts M.P., Peul W.C., Koes B.W., Thomeer R.T., for the Hague Spine Intervention Prognostic Study (SIPS) Group. Management of sciatica due to lumbar disc herniation in the Netherlands: a survey among spine surgeons. J Neurosurg Spine. 2008;9:32.
138. Aoyama T., Hida K., Akino M., et al. Detection of residual disc hernia material and confirmation of nerve root decompression at lumbar disc herniation surgery by intraoperative ultrasound. Ultrasound Med Biol. 2009;35:920.
139. Koivukangas J., Tervonen O. Intraoperative ultrasound imaging in lumbar disc herniation surgery. Acta Neuroschir (Wien). 1989;98:47.
140. Bashkoff E., Gadaleta D., Moccio C. Postlaminectomy aortic pseudoaneurysm. J Spinal Disord Tech. 1992;5:219.
141. Brewster D.C., May A.R., Darling R.C., et al. Variable manifestations of vascular injury during lumbar disc surgery. Arch Surg. 1979;114:1026.
142. De Saussure R. Vascular injury coincident to disc surgery. J Neurosurg. 1959;16:222.
143. Shaw E.D., Scarborough J.T., Beals R.K. Bowel injury as a complication of lumbar discectomy: a case report and review of the literature. J Bone Joint Surg [Am]. 1981;63:478.
144. Smith D.W., Lawrence B.D. Vascular complications of lumbar decompression laminectomy and foraminotomy: a unique case and review of the literature. Spine (Phila Pa 1976). 1991;16:387.
145. Smith E.B., DeBord J.R., Hanigan W.C. Intestinal injury after lumbar discectomy. Surg Gynecol Obstet. 1991;173:22.
146. Tarlov E.C. Major vascular injury secondary to spine surgery. E.C. Tarlov. Park Ridge, IL: American Association of Neurological Surgeons, 1991.
147. Ezra E., Richenberg J.L., Smellie W.A. Major vascular injury during lumbar laminectomy. J R Soc Med. 1996;89:108P.
148. Goodkin R., Laska L.L. Vascular and visceral injuries associated with lumbar disc surgery: medicolegal implications. Sub Neurol. 1998;49:358.
149. Leavens M.E., Bradford F.K. Ruptured intervertebral disc: report of a case with a defect in the anterior annulus fibrosus. J Neurosurg. 1953;10:544.
150. Shevlin W.A., Luessenhop A.J., Fox J.L., McCullough D.C. Perforation of the anterior annulus during lumbar discectomy: case report. J Neurosurg. 1973;38:514.
151. Oppel F., Schramm J., Schirmer M., et al. Results and complicated course after surgery for lumbar disc herniation. In: Wullenweber R., Brock M., Hamer J., et al, editors. Advances in neurosurgery. New York: Springer-Verlag; 1977:6.
152. Robe P., Martin D., Lenelle J., Stevenaert A. Posterior epidural migration of sequestered lumbar disc fragments: report of two cases. J Neurosurg. 1999;90:264.
153. Schisano G., Franco A., Nina P. Intraradicular and intradural lumbar disc herniation: experiences with nine cases. Surg Neurol. 1995;44:536.
154. Suzer T., Tahta K., Coskun E. Intraradicular lumbar disc herniation: case report and review of the literature. Neurosurgery. 1997;41:956.
155. Cliappetta P., Delfini R., Cantore G.P. Intradural lumbar disc hernia: description of Three Cases. Neurosurgery. 1981;8:104.
156. Peyser E., Harari A. Intradural rupture of lumbar intervertebral disk: report of two cases with review of the literature. Surg Neurol. 1977;8:1977.
157. Glasser R.S., Knego R.S., Delashaw J.B., Fessler R.G. The perioperative use of corticosteroids and bupivacaine in the management of lumbar disc disease. J Neurosurg. 1993;78:383.
158. Caspar W., Campbell B., Barbier D.D., et al. The Caspar microsurgical discectomy and comparison with a conventional standard lumbar disc procedure. Neurosurgery. 1991;28:78.
159. Kelly R.E., Dinner M.H., Lavyne M.H., Andrews D.W. The effect of lumbar disc surgery on postoperative pulmonary function and temperature: a comparison study of microsurgical lumbar discectomy with standard lumbar discectomy. Spine (Phila Pa 1976). 1993;18:287.
160. Ahn Y., Lee S.H., Lee J.H., et al. Transforaminal percutaneous endoscopic lumbar discectomy for upper lumbar disc herniation: clinical outcome, prognostic factors, and technical consideration. Acta Neurochir (Wien). 2009;151:199.
161. Bookwalter J.W.III, Busch M.D., Nicely D. Ambulatory surgery is safe and effective in radicular disc disease. Spine (Phila Pa 1976). 1994;19:526.
162. Tomaras C.R., Blacklock J.B., Parker W.D., et al. Outpatient surgical treatment of cervical radiculopathy. J Neurosurg. 1997;87:41.
163. Papavero L., Langer N., Fritzsche E., et al. The translaminar approach to lumbar disc herniations impinging the exiting root. Neurosurgery. 2008;62(3 Suppl 1):173-177. discussion 177–178
164. Soldner F., Hoelper B.M., Wallenfang T., Behr R. The translaminar approach to canalicular and cranio-dorsolateral lumbar disc herniations. Acta Neurochir (Wien). 2002;144:315.
165. DiLorenzo N., Porta F., Onnis G., et al. Par interarticularis fenestration in the treatment of foraminal lumbar disc herniation: a further surgical approach. Neurosurgery. 1998;42:87.
166. Schizas C., Tsiridis E., Saksena J. Microendoscopic discectomy compared with standard microsurgical discectomy for treatment of uncontained or large contained disc herniations. Neurosurgery. 2005;57:357.
167. Perez-Cruet M.J., Foley K.T., Isaacs R.E., et al. Microendoscopic lumbar discectomy: technical note. Neurosurgery. 2002;51:S129.
168. Parikh K., Tomasino A., Knopman J., et al. Operative results and learning curve: microscope-assisted tubular microsurgery for 1- and 2-level discectomies and laminectomies. Neurosurg Focus. 2008;25:E14.
169. Abdullah A.F., Ditto E.W.III, Byrd E.B., Williams R. Extreme lateral lumbar disc herniations: clinical syndrome and special problems of diagnosis. J Neurosurg. 1974;41:229.
170. Abdullah A.F., Wolber P.G., Warfield J.R., Gunadi I.K. Surgical management of extreme lateral lumbar disc herniations: review of 138 cases. Neurosurgery. 1988;22:648.
171. Donaldson W.F.III, Star M.J., Thorne R.P. Surgical treatment for the far lateral herniated lumbar disc. Spine (Phila Pa 1976). 1993;18:1263.
172. Epstein N.E. Evaluation of varied surgical approaches used in the management of 170 far-lateral lumbar disc herniations: indications and results. J Neurosurg. 1995;83:648.
173. Garrido E., Connaughton P.N. Unilateral facetectomy approach for lateral lumbar disc herniation. J Neurosurg. 1991;74:754.
174. Hazlett J.W., Kinnard P. Lumbar apophyseal process excision and spinal instability. Spine (Phila Pa 1976). 1982;7:171.
175. Hood R.S. Far lateral lumbar disc herniations. Neurosurg Clin North Am. 1993;4:117.
176. Jane J.A., Haworth C.S., Broaddus W.C., et al. A neurosurgical approach to far-lateral disc herniation: technical note. J Neurosurg. 1990;72:143.
177. Lejeune J.P., Hladky J.P., Cotten A., et al. Foraminal lumbar disc herniation: experience with 83 patients. Spine (Phila Pa 1976). 1994;19:1905.
178. Maroon J.C., Kopitnik T.A., Schulhof L.A.A., et al. Diagnosis and microsurgical approach to far-lateral disc herniation in the lumbar spine. J Neurosurg. 1990;72:378.
179. Schlesinger S.M., Fankhauser H., de Tribolet N. Microsurgical anatomy and operative technique for extreme lateral lumbar disc herniations. Acta Neurochir (Wien). 1992;118:117.
180. Pirris S.M., Dhall S., Mummaneni P.V., Kanter A.S. Minimally invasive approach to extraforaminal disc herniations at the lumbosacral junction using an operating microscope: case series and review of the literature. Neurosurg Focus. 2008;25:1.
181. Siebner H.R., Faulhauer K. Frequency and specific surgical management of far lateral lumbar disc herniations. Acta Neurochir (Wien). 1990;105:124.
182. Tessitore E., de Tribolet N. Far-lateral lumbar disc herniation: the microsurgical transmuscular approach. Neurosurgery. 54, 2004.
183. Porchet F., Chollet-Bornand A., de Tribolet N. Long-term follow up of patients surgically treated by the far-lateral approach for foraminal and extraforaminal lumbar disc herniations. J Neurosurg. 1999;90:59.
184. Eichholz K.M., Hitchon P.W. Far lateral lumbar disc herniation. Contemp Neurosurg. 2003;25:1.
185. Darden B.V., Wade J.F., Alexander R., et al. Far lateral disc herniations treated by microscopic fragment excision. Techniques and results. Spine (Phila Pa 1976). 1995;20:1500.
186. Foley K.T., Smith M.M., Rampersaud Y.R. Microendoscopic approach to far-lateral lumbar disc herniation. Neurosurg Focus. 1999;7:E5.
187. Epstein N.E., Epstein J.A., Carras R., Hyman R. Far lateral lumbar disc herniations and associated structural abnormalities: an evaluation in 60 patients of the comparative value of CT, MRI, and Myelo-CT in diagnosis and management. Spine (Phila Pa 1976). 1990;15:534.
188. Lanzino G., Shaffrey C.I., Jane J.A. Surgical treatment of lateral lumbar herniated discs. In: Rengachary S.S., Wilkins R.H., editors. Neurosurgical operative atlas. Park Ridge, IL: American Association of Neurological Surgeons; 1999:243.
189. Kornberg M. Extreme lateral lumbar disc herniations: clinical syndrome and computed tomography recognition. Spine (Phila Pa 1976). 1987;12:586.
190. Jenis L.G., An H.S. Spine update: lumbar foraminal stenosis. Spine (Phila Pa 1976). 2000;25:389.
191. Jenis L.G., An H.S., Gordin R. Foraminal stenosis of the lumbar spine: a review of 65 surgical cases. Am J Orthop. 2001;30:205.
192. O’Brien M.F., Peterson D., Crockard H.A. A posterolateral microsurgical approach to extreme-lateral lumbar disc herniation. J Neurosurg. 1995;83:636.
193. Pal D., Tyagi A.K. Interlaminar approach for excision of lateral lumbar disc herniation: technical note. Spine (Phila Pa 1976). 2006;31:E114.
194. Faust S.E., Ducker T.B., VanHassent J.A. Lateral lumbar disc herniations. J Spinal Disord Tech. 1992;5:97.
195. Wiltse L.L., Bateman J.G., Hutchinson R.H., Nelson W.E. The paraspinal sacrospinalis-splitting approach to the lumbar spine. J Bone Joint Surg [Am]. 919, 1968.
196. Larson S.J., Holst R.A., Hemmy D.C., Sances A.Jr. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45:628.
197. Min J.H., Kang S.H., Lee J.B., et al. Anatomic analysis of the transforaminal ligament in the lumbar intervertebral foramen. Neurosurgery. 2005;57:37.
198. Gioia G., Mandelli D., Capaccioni B., et al. Surgical treatment of far lateral lumbar disc herniation: identification of compressed root and discectomy by lateral approach. Spine (Phila Pa 1976). 1999;24:1952.
199. Reulen H.J., Muller A., Ebeling U. Microsurgical anatomy of the lateral approach to extraforaminal lumbar disc herniations. Neurosurgery. 1996;39:345.
200. Nabeta M., Yoshimoto H., Sato S., et al. Discal cysts of the lumbar spine. Report of five cases. J Neurosurg Spine. 2007;6:85.
201. Chiba K., Toyama Y., Matsumoto M., et al. Intraspinal cyst communicating with the intervertebral disc in the lumbar spine: discal cyst. Spine (Phila Pa 1976). 2001;26:2112.
202. Marshman L.A., Benjamin J.C., David K.M., et al. “Disc cysts” and “posterior longitudinal ligament ganglion cysts”: synonymous entities? Report of three cases and literature review. Neurosurgery. 2005;57:E818.
203. Almeida D.B., Prandini M.N., Awamura Y., et al. Outcome following lumbar disc surgery: the role of fibrosis. Acta Neurochir (Wien). 2008;150:1167.
204. Ozer A.F., Oktenoglu T., Sasani M., et al. Preserving the ligamentum flavum in lumbar discectomy: a new technique that prevents scar tissue formation in the first 6 months postsurgery. Neurosurgery. 2006;59:126.
205. Rönnberg K., Lind B., Zoega B., et al. Peridural scar and its relation to clinical outcome: a randomised study on surgically treated lumbar disc herniation patients. Eur Spine J. 2008;17:1714.
206. Richter H.P., Kast E., Tomczak R., et al. Results of applying ADCON-L gel after lumbar discectomy: the German ADCON-L study. J Neurosurg. 2001;95:179.
207. Ceviz A., Arslan A., Ak H.E., Inalöz S. The effect of urokinase in preventing the formation of epidural fibrosis and/or leptomeningeal arachnoiditis. Surg Neurol. 1997;47:124.
208. Wolfram-Gabel R., Beaujeux R., Fabre M., et al. Histologic characteristics of posterior lumbar epidural fatty tissue. J Neuroradiol. 1998;23:19.
209. Foulkes G.D., Robinson J.S.Jr. Intraoperative dexamethasone irrigation in lumbar microdiskectomy. Clin Orthop Relat Res. 261:224, 1990.
210. Hinton J.L.Jr., Warejcka D.J., Mei Y., et al. Inhibition of epidural scar formation after lumbar laminectomy in the rat. Spine (Phila Pa 1976). 1995;20:564.
211. Lundin A., Magnuson A., Axelsson K., et al. Corticosteroids perioperatively diminishes damage to the C-fibers in microscopic lumbar disc surgery. Spine (Phila Pa 1976). 2005;30:2362.
212. MacKay M.A., Fischgrund J.S., Herkowitz H.N., et al. The effect of interposition membrane on the outcome of lumbar laminectomy and discectomy. Spine (Phila Pa 1976). 1995;20:1793.
213. Songer M.N., Rauschning W., Carson E.W., Pandit S.M. Analysis of peridural scar formation and its prevention after lumbar laminotomy and discectomy in dogs. Spine (Phila Pa 1976). 1995;20:571.
214. Kanamori M., Kawaguchi Y., Ohmori K., et al. The fate of autogenous free-fat grafts after posterior lumbar surgery: part 2. Magnetic resonance imaging and histologic studies in repeated surgery cases. Spine (Phila Pa 1976). 2001;26:2264.
215. Park Y.K., Kim J.H., Chung H.S. Outcome analysis of patients after ligament-sparing microdiscectomy for lumbar disc herniation. Neurosurg Focus. 2002;13(2):E4.
216. Brotchi J., Pirotte B., De Witte O., Levivier M. Prevention of epidural fibrosis in a prospective series of 100 primary lumbo-sacral discectomy patients: follow-up and assessment at re-operation. Neurol Res. 1999;21:S47.
217. Fischgrund J.S. Perspectives on modern orthopaedics: use of Adcon-L for epidural scar prevention. J Am Acad Orthop Surg. 2000;8:339.
218. Robertson J.T., Maier K., Anderson R.W., et al. Prevention of epidural fibrosis with ADCON-L in presence of a durotomy during lumbar disc surgery: experiences with a pre-clinical model. Neurol Res. 1999;21:S61.
219. Hieb L.D., Stevens D.L. Spontaneous postoperative cerebrospinal fluid leaks following application of antiadhesion barrier gel: case report and review of the literature. Spine (Phila Pa 1976). 2001;26:748.
220. Le A.X., Rogers D.E., Dawson E.G., et al. Unrecognized durotomy after lumbar discectomy: a report of four cases associated with the use of ADCON-L. Spine (Phila Pa 1976). 2001;26:115.
221. Assietti R., Mora A., Brayda-Bruno M. Use of carboxymethylcellulose/polyethylene oxide gel in microdiscectomy with interlaminectomy: a case series comparison with long-term follow-up. Spine (Phila Pa 1976). 2008;33:1762.
222. Vik A., Zwart J.A., Hulleberg G., Nygaard O.P. Eight year outcome after surgery for lumbar disc herniation: a comparison of reoperated and not reoperated patients. Acta Neurochir (Wien). 2001;143:607.
223. Kim M.S., Park K.W., Hwang C., et al. Recurrence rate of lumbar disc herniation after open discectomy in active young men. Spine (Phila Pa 1976). 2009;34:24.
224. Keskimäki I., Seitsalo S., Osterman H., Rissanen P. Reoperations after lumbar disc surgery: a population-based study of regional and interspecialty variations. Spine (Phila Pa 1976). 2000;25:1500.
225. Malter A.D., McNeney B., Loeser J.D., Deyo R.A. 5-year reoperation rates after different types of lumbar spine surgery. Spine (Phila Pa 1976). 1998;23:814.
226. Papadopoulos E.C., Girardi F.P., Sandhu H.S., et al. Outcome of revision discectomies following recurrent lumbar disc herniation. Spine (Phila Pa 1976). 2006;31:1473.
227. McGirt M.J., Eustacchio S., Varga P., et al. A prospective cohort study of close interval computed tomography and magnetic resonance imaging after primary lumbar discectomy: factors associated with recurrent disc herniation and disc height loss. Spine (Phila Pa 1976). 2009;34:2044.
228. Carroll S.E., Wiesel S.W. Neurologic complications and lumbar laminectomy: a standardized approach to the multiply-operated lumbar spine. Clin Orthop Relat Res. 284:14, 1992.
229. Fritsch E.W., Heisel J., Rupp S. The failed back surgery syndrome: reasons, intraoperative findings, and long-term results: a report of 182 operative treatments. Spine (Phila Pa 1976). 1996;21:626.
230. Holodny A.I., Kisza P.S., Contractor S., Liu W.C. Does a herniated nucleus pulposus contribute significantly to a decrease in height of the intervertebral disc? Quantitative volumetric MRI. Neuroradiology. 2000;42:451.
231. McGirt M.J., Ambrossi G.L., Datoo G., et al. Recurrent disc herniation and long-term back pain after primary lumbar discectomy: review of outcomes reported for limited versus aggressive disc removal. Neurosurgery. 2009;64:338.
232. Balderston R.A., Gilyard G.G., Jones A.A., et al. The treatment of lumbar disc herniation: simple fragment excision versus disc space curettage. J Spinal Disord. 1991;4:22.
233. Barth M., Diepers M., Weiss C., Thomé C. Two-year outcome after lumbar microdiscectomy versus microscopic sequestrectomy: part 2: radiographic evaluation and correlation with clinical outcome. Spine (Phila Pa 1976). 2008;33:273.
234. Barth M., Weiss C., Thomé C. Two-year outcome after lumbar microdiscectomy versus microscopic sequestrectomy: part 1: evaluation of clinical outcome. Spine (Phila Pa 1976). 2008;33:265.
235. Carragee E.J., Han M.Y., Yang B., et al. Activity restrictions after posterior lumbar discectomy. A prospective study of outcomes in 152 cases with no postoperative restrictions. Spine (Phila Pa 1976). 1999;24:2346.
236. Carragee E.J., Spinnickie A.O., Alamin T.F., Paragioudakis S. A prospective controlled study of limited versus subtotal posterior discectomy: short-term outcomes in patients with herniated lumbar intervertebral discs and large posterior annular defect. Spine (Phila Pa 1976). 2006;31:653.
237. Herron L. Recurrent lumbar disc herniation: results of repeat laminectomy and discectomy. J Spinal Disord Tech. 1994;7:161.
238. Suk K.S., Lee H.W., Moon S.H., Kim N.H. Recurrent lumbar disc herniation: results of operative management. Spine (Phila Pa 1976). 2001;26:672.
239. Albin M.S., Ritter R.R., Pruett C.E., Kalff K. Venous air embolism during lumbar laminectomy in the prone position: report of three cases. Anesth Analg. 1991;73:346.
240. Cammisa F.P., Girardi F.P., Sangani P.K., et al. Incidental durotomy in spine surgery. Spine (Phila Pa 1976). 2000;25:2663.
241. Jones A.A., Stambough J.L., Balderston R.A., et al. Long-term results of lumbar spine surgery complicated by unintended incidental durotomy. Spine (Phila Pa 1976). 1989;14:443.
242. Saxler G., Krämer J., Barden B., et al. The long-term clinical sequelae of incidental durotomy in lumbar disc surgery. Spine (Phila Pa 1976). 2005;30:2298.
243. Kothbauer K.F., Seiler R.W. Transdural cauda equina incarceration after microsurgical lumbar discectomy; case report. Neurosurgery. 2000;47:1449.
244. Friedman J.A., Ecker R.D., Piepgras D.G., Duke D.A. Cerebellar hemorrhage after spinal surgery: report of two cases and literature review. Neurosurgery. 2002;50:1361.
245. Kiev J., Dupont J.R., Kerstein M.D. Injury of a medial sacral vessel during lumbar laminectomy. Ann Vasc Surg. 1996;10:63.
246. Price S.J., Buxton N. Adynamic ileus complicating lumbar laminectomy: a report of two cases. Br J Neurosurg. 1998;12:162.
247. Feldman R.A., Karl R.C. Diagnosis and treatment of Ogilvie’s syndrome after lumbar spinal surgery: report of three cases. J Neurosurg. 1992;76:1012.
248. Schulitz K.P., Assheuer J. Discitis after procedures on the intervertebral disc. Spine (Phila Pa 1976). 1994;19:1172.
249. Arend S.M., Steenmeyer A.V., Mosmans P.C., et al. Postoperative cauda syndrome caused by Staphylococcus aureus. Infection. 1993;21:248.
250. Dolan P., Greenfield K., Nelson R.J., Nelson I.W. Can exercise therapy improve the outcome of microdiscectomy? Spine (Phila Pa 1976). 2000;25:1523.
251. Ostelo R.W., de Vet H.C., Waddell G., et al. Rehabilitation after lumbar disc surgery. Cochrane Database Syst Rev. 2008;CD003007:209.
252. Danielsen J.M., Johnsen R., Kibsgaard S.K., Hellevik E. Early aggressive exercise for postoperative rehabilitation after discectomy. Spine (Phila Pa 1976). 2000;25:1015.
253. Dewing C.B., Provencher M.T., Riffenburgh R.H., et al. The outcomes of lumbar microdiscectomy in a young, active population: correlation by herniation type and level. Spine (Phila Pa 1976). 2008;33:33.
254. Kalfas I.H. Principles of bone healing. Neurosurg Focus. 2001;10:1.