CHAPTER 294 Lumbar Arthroplasty
Total Disk Replacement and Nucleus Replacement Technologies
Mechanical low back pain (LBP) because of lumbar degenerative disk disease (DDD) has been problematic to both diagnose and treat. Nonsurgical treatment is successful in the majority of patients with LBP.1–3 Unfortunately, a significant minority of patients at various stages of the degenerative cascade remain debilitated with diskogenic LBP. Surgical treatment for diskogenic LBP has traditionally focused on fusion with concomitant loss of function of the motion segment.4,5 Traditional lumbar fusion techniques have evolved over the years. The advent of increasingly sophisticated spinal instrumentation and implants have consistently increased fusion rates.6,7 Unfortunately, although fusion rates have approached 100%, clinical success rates have lagged significantly behind.4,8 Arthrodesis of the lumbar three-joint functional spinal unit (anterior disk and two posterior facet joints) results in loss of motion with a known incidence of adjacent level symptomatic degeneration ranging from 10% to 30%.9–15 Spine arthroplasty techniques allow for pain relief by removing presumed pain generators, the annulus and/or nucleus, while maintaining function (i.e. motion with uniform stress distribution) and, thereby, theoretically, protecting adjacent levels.
Partial disk replacement dates back to the 1960s, when Fernstrom implanted stainless steel balls into the cervical and lumbar spine.16 The modern era of lumbar arthroplasty was ushered in when Butner-Janz and Schellnack developed the original Charite artificial disk in the early 1980s.17 In the late 1980s, Marnay developed the Prodisc-L.18 Subsequently, total disk replacement has seen a steady evolution with a proliferation of devices and concomitant refinement in implant design, surgical technique, and instrumentation.
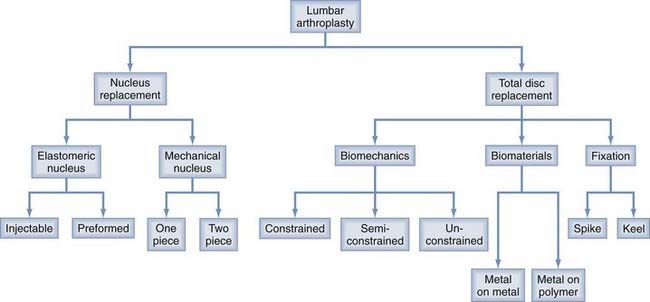
Nucleus replacement devices represent an even more heterogeneous group of devices and are earlier in the developmental process. Functionally, nucleus replacement devices can be divided into two broad classifications: elastomeric and mechanical (Fig. 294-1).16,19–21
The primary indication for both mechanical nucleus replacements and total disk replacement (TDR) is mechanical low back pain because of DDD.5 Elastomeric nucleus replacement can also be used in the post-discectomy setting.21
Generally, both types of nucleus replacement devices represent therapeutic interventions aimed at an early stage in the degenerative cascade (mild to moderate DDD). Advantages of nucleus replacement include minimally invasive and multiple approach options, including anterior retroperitoneal, lateral, and posterior approaches. Limited exposure and annulotomy allow for multiple revision options including TDR and fusion.19,20,22–26 Challenges of nucleus replacement include migration or expulsion risk (devices generally are not fixed to end plates) and subsidence.21,27–29
TDR is generally applicable for more advanced DDD (moderate to severe). Advantages of TDR include more definitive near-total disk removal that addresses pathology involving both the annulus and the nucleus as well as reliable fixation to bony end plates.30–33 Challenges of TDR include implant longevity, failure, and wear debris, as well as more challenging revision strategies (Table 294-1).34–37
TABLE 294-1 Comparison of Nucleus Replacement and Total Disk Replacement
| NUCLEUS REPLACEMENT | TOTAL DISK REPLACEMENT |
|---|---|
| Limited annulotomy; nuclectomy only | Wide annulotomy; near-total discectomy |
| Mild to moderate DDD | Moderate-to-severe DDD |
| Anterior, lateral, posterior approach | Primarily anterior retroperitoneal approach |
| No end plate fixation | End plate fixation |
| Limited device surface area | Large device surface area |
Total Disk Replacement
Total Disk Replacement Devices
The Food and Drug Administration’s (FDA) approval of the Charite Artificial Disc in October 2004 ushered in the era of spinal arthroplasty in the United States.17 Subsequently, Prodisc-L (2006) and the next-generation Charite device, InMotion (2007), received FDA approval.35 Several more devices have followed (Maverick, Kineflex, FlexiCore, and Activ-L) with a number of others in various stages of development (Table 294-2). Cumulatively, these studies have produced a substantial body of level 1 data from prospective randomized studies confirming TDR’s efficacy in the treatment of symptomatic lumbar DDD.17,18,36,37
| DEVICE MANUFACTURER TYPE | ||
| METAL-ON-POLYMER | ||
| Charite | Depuy Spine | Unconstrained |
| InMotion | Depuy Spine | Unconstrained |
| Prodisc-L | Synthes Spine | Semiconstrained |
| Activ-L | Aesculap | Semiconstrained |
| Mobi-L | LDR Spine | Unconstrained |
| Triumph | Globus | Posterior |
| Physio-L | Nexgen | Elastomeric |
| CAdisc | Ranier Tech | Elastomeric |
| eDisc | Theken | Elastomeric |
| Dynardi | Zimmer | Semiconstrained |
| Freedom | AxioMed | Elastomeric |
| METAL-ON-METAL | ||
| Maverick | Medtronic | Semiconstrained |
| Kineflex | Spinal Motion | Semiconstrained |
| FlexiCore | Stryker Spine | Fully constrained |
| Lateral Disc | NuVasive | Lateral approach |
| TrueDisc PL | Disc Motion Tech | Antenia posterior |
Charite Artificial Disc (Depuy Spine)
Cobalt chrome endplates with an unconstrained, polyethylene floating core and spike fixation. They have a three-piece design with a two-stage application and endplates followed by core placement (Fig. 294-2).
InMotion Artificial Disc (Depuy Spine)
The next-generation Charite device has redesigned end plates and a no-impaction application technique. The three-piece design has a single-stage application. The reconfigured cobalt chrome end plates and spike fixation have the same unconstrained, polyethylene floating core (Fig. 294-3).
Prodisc-L Artificial Disk (Synthes Spine)
The Prodisc-L has cobalt chrome end plates with a semiconstrained, polyethylene core and keel fixation and a three-piece design with a two-stage application (Fig. 294-4).
Maverick Artificial Disk (Medtronic)
The Maverick has a cobalt chrome, metal-on-metal design with semiconstrained ball and socket design with keel fixation (Fig. 294-5).
Kineflex Artificial Disk (Spinal Motion)
The Kineflex features cobalt chrome, metal-on-metal design with a semiconstrained core, a floating core with retention ring and keel fixation, and a three-piece design with a single-stage application (Fig. 294-6).
FlexiCore Artificial Disk (Stryker Spine)
The FlexiCore has a cobalt chrome, metal-on-metal design with a constrained domed core and spike fixation (Fig. 294-7).
Nucleus Replacement
Operative Technique
The surgical approach for nucleus replacement is variable depending on the specific device. Generally, devices can be placed less invasively with multiple approach options. For example, the NUBAC mechanical nucleus and DASCOR elastomeric nucleus can each be placed via an anterolateral retroperitoneal or posterior approach at L5-S1 and a lateral transpsoas approach or posterior approach at L4-5 and above.19–21
Nucleus Replacement Devices
Clinically, indications for nucleus replacement may be separated into two general categories. First, nucleus replacement can be used in select postdiscectomy patients as a largely prophylactic procedure to prevent recurrent disk herniations or progressive DDD.21 It is well documented that a small but significant proportion of patients (approximately 3% to 15%) undergoing standard microdiscectomy will develop recurrent herniations or progressive degenerative changes with symptomatic low back pain.38–40 Iatrogenic changes seen postdiscectomy include loss of disk space height and dessication.41,42 Partial disk replacement in the postdiscectomy setting offers the theoretical benefit of slowing future degenerative changes by maintaining disk space height and normal motion. Similar to TDR, nucleus replacement can also be used in patients with mechanical low back pain caused by mild to moderate DDD.19,21
Functionally, nucleus replacement devices can be divided into two broad classifications: elastomeric and mechanical.19,21 Elastomeric devices can be further subdivided into hydrogel and nonhydrogel replacements. These devices are either preformed or injectable. Injectable nucleus replacements are delivered in liquid form into the nucleotomy void and cure in situ. Preformed nucleus replacements are precured polymers (Table 294-3).19,21,24
Mechanical devices can be subdivided into one- and two-piece designs (Table 294-3).19,21
Elastomeric Nucleus Replacement
The advantages of elastomeric nucleus replacement include the ability to re-create the natural function of the normal nucleus pulposus with uniform stress distribution and shock absorption capability. A central challenge involved in the use of preformed devices is implant extrusion because of their inherently deformable nature. For injectable devices, biocompatibility, long-term durability, and avoidance of leakage are of crucial importance.16,21,22,43
Prosthetic Disk Nucleus (Raymedica)
The Prosthetic Disk Nucleus (PDN) consists of a preformed hydrogel core consisting of polyacrylonitrile and polyacrylamide with a polyethylene woven jacket. PDN was first implanted in humans in 1996. PDN remains the most widely studied nucleoplasty device worldwide.16,22,26 PDN is implanted dehydrated and subsequently hydrates and expands. It is designed to absorb up to 80% of its own weight in water. Extrusion risk with the original design led to design and approach modification. Subsequent designs include the PDN Solo and Hydraflex (Fig. 294-9).16
NeuDisc (Replication Medical)
NeuDisc is a compressible preformed hydrogel consisting of an aquacryl polymer reinforced with Dacron mesh. It is implanted dehydrated and expands anisotropically to conform to the nucleotomy defect.21
NuCore Injectable Disc Nucleus (Spine Wave)
The NuCore Injectable Disc Nucleus (IDN) is an injectable nonhydrogel nucleus. It is an rDNA-based synthetic protein copolymer composed of silk and elastin. NuCore has been used in both postdiscectomy and early DDD indications.21
Dascor Prosthetic Intervertebral Nucleus (Disc Dynamics)
Dascor is a constrained, injectable, nonhydrogel nucleus. It is applied as a two-part in situ curable polyurethane and an expandable polyurethane balloon, which is inserted into the disk space after the nucleus has been removed. The balloon is then injected under pressure with the flowable polymer that conforms to the shape and size of the disk space. The flowable polymer cures, creating a firm but pliable implant with shock absorption capability.16,19
Mechanical Nucleus Replacement
The advantages of mechanical nucleus replacement include strength and durability. Challenges include maintaining an even stress distribution and lack of shock absorption. The major weakness revolves around a lack of anchor to the end plates, which predisposes to subsidence and expulsion.20,21,43
NUBAC Disk Arthroplasty Device (Pioneer Surgical)
Nubac is a two-piece, mechanical nucleus. It is composed of polyetheretherketone (PEEK) and is the first PEEK-on-PEEK articulated intradiscal arthroplasty device (Fig. 294-10). The Nubac has a ball-and-socket design with a large surface contact area to distribute stress and theoretically lower the risk of subsidence. Nubac has been implanted through a lateral transpsoas approach at L3-4 and L4-5, as well as anterior retroperitoneal and posterior approaches at L5-S1.19–21
Conclusion
The difficulty in diagnosing and treating mechanical LBP lies in its inherent multifactorial nature. The etiology of LBP can be structural, musculoskeletal, or an underlying disease process (i.e., arthritis or fibromyalgia). Even when identified as primarily due to pathology involving the three-joint structure of the spine, the primary pain generator may be DDD (mild, moderate, severe) and/or degenerative facet disease.44,45 Intuitively, such a heterogenous disease process cannot be adequately addressed with a single surgical procedure. The concept of comparing different surgical techniques and attempting to assess superiority is, at best, a theoretical exercise and, at worst, counterproductive to patient care. A more pragmatic, and potentially successful, approach lies in using a spectrum of surgical treatments to match an inherently heterogenous disease process.
Nucleus replacement remains investigational, but early clinical results have been encouraging.16,20,21,24 PDR may provide the opportunity to address lumbar DDD in a minimally invasive fashion while preserving motion. Specifically, PDR has the potential to address degenerative pathologies more complex than simple herniation, but less advanced than severe DDD. Therefore, nucleus replacement could fill the surgical niche between simple discectomy and TDR or fusion. The potential of nucleus replacement must be tempered by the lack of long-term clinical results.21
TDR represents an emerging treatment alternative to fusion ideally suited to treat isolated moderate to severe DDD without significant facet degeneration. The biomechanics46–51 and clinical efficacy52,53 of TDR devices have been well characterized and the level 1 data body of literature continues to grow. These devices reliably maintain motion while concomitantly decreasing stresses on adjacent levels compared to fusion.54,55
Bao QB, Yuan HA. New technologies in spine: nucleus pulposus replacement. Spine. 2002;27:1245-1247.
Bao QB, Yuan HA. Prosthetic disc replacement: the future? Clin Orthop. 2002;394:139-145.
Bertagnoli R, Schonmayr R. Surgical and clinical results with the PDN prosthetic disc nucleus. Eur Spine J. 2002;11:S143-S148.
Bertagnoli R, Karg A, Voigt S. Lumbar partial disc replacement. Orthop Phys Ther Clin N Am. 2005;36:341-347.
Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine. 2005;30:1565-1575.
Brinckmann P, Grootenboer H. Change of disc height, radial disc bulge, and intradiscal pressure from discectomy: an in vitro investigation on human lumbar discs. Spine. 1991;16:641-646.
Carragee EJ. The surgical treatment of disc degeneration: is the race not to the swift? Spine J. 2005;5:587-588.
Coric D, Mummaneni P. Nucleus replacement technologies. J Neurosurg. 2008;8:115-120.
Cunningham BW. Basic scientific considerations in total disc arthroplasty. Spine J. 2004;4:S219-S230.
David T. Long-term results of one-level lumbar arthroplasty: minimum of 10-year follow-up of the CHARITÉ artificial disc in 106 patients. Spine. 2007;32:661-666.
Eck JC, Humphreys SC, Hodges SD. Adjacent-segment degeneration after lumbar fusion: a review of clinical, biomechanical, and radiology studies. Am J Orthop. 1999;28:336-340.
Errico TJ, Gatchel RJ, Schofferman K, et al. A fair and balanced view of spine fusion surgery. Spine J. 2004;4:129S-142S.
Klara PM, Ray CD. Artificial nucleus replacement. Spine. 2002;27:1374-1377.
Geisler FH, Blumenthal SL, Guyer RD, et al. Neurological complications of lumbar artificial disc replacement and comparison of clinical results with those related to lumbar arthrodesis in the literature: results of a multicenter, prospective, randomized investigational device exemption study of Charite intervertebral disc. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves. J Neurosurg Spine. 2004;1:143-154.
Geisler FH, Guyer RD, Blumenthal SL, et al. Patient selection for lumbar arthroplasty and arthrodesis: the effect of revision surgery in a controlled, multicenter, randomized study. J Neurosurg Spine. 2008;8:13-16.
Goel VK, Grauer JN, Patel T, et al. Effects of Charite artificial disc on the implanted and adjacent spinal segments mechanics using a hybrid testing protocol. Spine. 2005;30:2755-2764.
Goins ML, Wimberley DW, Yuan PS, et al. Nucleus pulposus replacement: an emerging technology. Spine J. 2005;5:317S-324S.
Guyer RD, Geisler FH, Blumenthal SL, et al. Effect of age on clinical and radiographic outcomes and adverse events following 1-level lumbar arthroplasty after a minimum 2-year follow-up. J Neurosurg Spine. 2008;8:101-107.
Martino AD, Vaccaro AR, Lee JY, et al. Nucleus pulposus replacement: basic science and indications for clinical use. Spine. 2005;30:S16-S22.
McAfee PC, et al. Revisability of the CHARITE artificial disc replacement: analysis of 688 patients enrolled in the U.S. IDE study of the CHARITE artificial disc. Spine. 2006;31:1217-1226.
Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of lumbar fusion for degenerative disease of the lumbar spine. Part 7: intractable low back pain without stenosis or spondylolisthesis. J Neurosurg Spine. 2005;2:668-670.
Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine. 2007;32:1155-1162.
1 Carragee EJ. The surgical treatment of disc degeneration: is the race not to the swift? Spine J. 2005;5:587-588.
2 Deyo RA, Nachemson A, Mirza SK. Spinal-fusion surgery—the case for restraint. N Engl J Med. 2004;350:722-726.
3 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of lumbar fusion for degenerative disease of the lumbar spine. Part 13: injection therapies for degenerative lumbar spine disease. J Neurosurg Spine. 2005;2:705-713.
4 Errico TJ, Gatchel RJ, Schofferman K, et al. A fair and balanced view of spine fusion surgery. Spine J. 2004;4:129S-142S.
5 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of lumbar fusion for degenerative disease of the lumbar spine. Part 7: intractable low back pain without stenosis or spondylolisthesis. J Neurosurg Spine. 2005;2:668-670.
6 Mummaneni PV, Haid RW, Rodts GE. Lumbar interbody fusion: state of the art technical advances. J Neurosurg Spine. 2004;1:24-30.
7 Ozgur BM, Aryan HE, Pimenta L, et al. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435-443.
8 Wang J, Mummaneni PV, Haid RW. Current treatment strategies for the painful lumbar motion segment: posterolateral fusion versus interbody fusion. Spine. 2005;30:S33-S43.
9 Eck JC, Humphreys SC, Hodges SD. Adjacent-segment degeneration after lumbar fusion: a review of clinical, biomechanical, and radiology studies. Am J Orthop. 1999;28:336-340.
10 Etebar S, Cahill DW. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J Neurosurg. 1999;90:163-169.
11 Frymoyer JW, Hanley EN, Howe J, et al. A comparison of radiographic findings in fusion and nonfusion patients ten or more years following lumbar disc surgery. Spine. 1979;4:435-440.
12 Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine. 1988;13:375-377.
13 Lehmann TR, Spratt KF, Tozzi JE, et al. Long-term follow-up of lower lumbar fusion patients. Spine. 1987;12:97-104.
14 Schlegel JD, Smith JA, Schleusener RL. Lumbar motion segment pathology adjacent to thoracolumbar, lumbar, and lumbosacral fusions. Spine. 1996;21:970-981.
15 Weinhoffer SL, Guyer RD, Herbert M, et al. Intradiscal pressure measurements above an instrumented fusion. A cadaveric study. Spine. 1995;20:526-531.
16 Goins ML, Wimberley DW, Yuan PS, et al. Nucleus pulposus replacement: an emerging technology. Spine J. 2005;5:317S-324S.
17 Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine. 2005;30:1565-1575.
18 Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine. 2007;32:1155-1162.
19 Bao QB, Yuan HA. New technologies in spine: nucleus pulposus replacement. Spine. 2002;27:1245-1247.
20 Bao QB, Yuan HA. Prosthetic disc replacement: the future? Clin Orthop. 2002;394:139-145.
21 Coric D, Mummaneni P. Nucleus replacement technologies. J Neurosurgl Spine. 2008;8:115-120.
22 Bertagnoli R, Schonmayr R. Surgical and clinical results with the PDN prosthetic disc nucleus. Eur Spine J. 2002;11:S143-S148.
23 Bertagnoli R, Vazquez RJ. The anterolateral transpsoatic approach (ALPHA): a new technique for implanting prosthetic disc-nucleus devices. J Spinal Disord. 2003;16:398-404.
24 Bertagnoli R, Karg A, Voigt S. Lumbar partial disc replacement. Orthop Phys Ther Clin N Am. 2005;36:341-347.
25 Klara PM, Ray CD. Artificial nucleus replacement. Spine. 2002;27:1374-1377.
26 Shim CS, Lee SH, Park CW, et al. Partial disc replacement with the PDN prosthetic disc nucleus device: early clinical results. J Spinal Disord Tech. 2003;16:324-330.
27 Bertagnoli R, Sabatino CT, Edwards JT, et al. Mechanical testing of a novel hydrogel nucleus replacement implant. Spine J. 2005;5:672-681.
28 Fraser RD, Ross ER, Lowery GL, et al. AcroFlex design and results. Spine J. 2004;4:S245-S251.
29 Martino AD, Vaccaro AR, Lee JY, et al. Nucleus pulposus replacement: basic science and indications for clinical use. Spine. 2005;30:S16-S22.
30 Cunningham BW. Basic scientific considerations in total disc arthroplasty. Spine J. 2004;4:S219-S230.
31 David T. Long-term results of one-level lumbar arthroplasty: minimum of 10-year follow-up of the CHARITÉ artificial disc in 106 patients. Spine. 2007;32:661-666.
32 Geisler FH, Guyer D, Blumenthal SL, et al. Effect of previous surgery on clinical outcome following 1-level lumbar arthroplasty. J Neurosurg Spine. 2008;8:108-114.
33 Lemaire JP, Carrier H, Sariali el H, et al. Clinical and radiological outcomes with the Charite artificial disc: a 10-year minimum follow-up. J Spinal Disord Tech. 2005;18:353-359.
34 Hallab N, Link HD, McAfee PC. Biomaterial optimization in total disc arthroplasty. Spine. 2003;28:S139-S152.
35 Punt IM, et al. Complications and reoperations of the SB Charite lumbar disc prosthesis: experience in 75 patients. Eur Spine J. 2008;17:36-42.
36 Geisler FH, Blumenthal SL, Guyer RD, et al. Neurological complications of lumbar artificial disc replacement and comparison of clinical results with those related to lumbar arthrodesis in the literature: results of a multicenter, prospective, randomized investigational device exemption study of Charite intervertebral disc. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves. J Neurosurg Spine. 2004;1:143-154.
37 Geisler FH, Guyer RD, Blumenthal SL, et al. Patient selection for lumbar arthroplasty and arthrodesis: the effect of revision surgery in a controlled, multicenter, randomized study. J Neurosurg Spine. 2008;8:13-16.
38 Lowell TD, Errico TJ, Fehlings MG, et al. Microdiskectomy for lumbar disk herniation: a review of 100 cases. Orthopedics. 1995;18:985-990.
39 Smorgick Y, Floman Y, Millgram MA, et al. Mid- to long-term outcome of disc excision in adolescent disc herniation. Spine J. 2006;6:380-384.
40 Fritzell P, Hagg O, Wessberg P, et al. 2001 Volvo Award Winner in Clinical Studies: lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish lumbar spine study group. Spine. 2001;26:2521-2532.
41 Brinckmann P, Grootenboer H. Change of disc height, radial disc bulge, and intradiscal pressure from discectomy: an in vitro investigation on human lumbar discs. Spine. 1991;16:641-646.
42 Meakin JR, Huins DW. Effect of removing the nucleus pulposus on the deformation of the annulus fibrosus during compression of the intervertebral disc. J Biomech. 2000;33:575-580.
43 Yorimitsu I, Chiba K, Toyama Y, et al. Long-term outcomes of standard discectomy for lumbar disc herniation: a follow-up of more than 10 years. Spine. 2001;26:652-657.
44 Rodts GE, Mummaneni PV. Discogenic back pain: the case for surgery. Clin Neurosurg. 2004;51:277-280.
45 Sheehan JM, Shaffrey CI, Jane JA. Degenerative lumbar stenosis: the neurosurgical perspective. Clin Orthop Rel Res. 2001;384:61-74.
46 Cunningham BW, Gordon JD, Dmitriev AE, et al. Biomechanical evaluation of total disc replacement arthroplasty: an in vitro human cadaveric model. Spine. 2003;28:S110-S117.
47 Cunningham BW, et al. Distribution of in vivo and in vitro ROM following 1-level arthroplasty with the CHARITÉ artificial disc compared with fusion. J Neurosurg Spine. 2008;8:7-12.
48 Goel VK, Grauer JN, Patel T, et al. Effects of Charite artificial disc on the implanted and adjacent spinal segments mechanics using a hybrid testing protocol. Spine. 2005;30:2755-2764.
49 Hitchon PW, Eichholz KM, Barry C, et al. Biomechanical studies of an artificial disc implant in the human cadaveric spine. J Neurosurg Spine. 2005;2:339-343.
50 Moumene M, Geisler FH. Comparison of biomechanical function at ideal and varied surgical placement for two lumbar artificial disc implant designs: mobile-core versus fixed core. Spine. 2007;32:1840-1851.
51 O’Leary P, Nicolakis M, Lorenz MA, et al. Response of Charite total disc replacement under physiologic loads: prosthesis component motion patterns. Spine J. 2005;5:590-599.
52 Guyer RD, Geisler FH, Blumenthal SL, et al. Effect of age on clinical and radiographic outcomes and adverse events following 1-level lumbar arthroplasty after a minimum 2-year follow-up. J Neurosurg Spine. 2008;8:101-107.
53 McAfee PC, et al. Revisability of the CHARITE artificial disc replacement: analysis of 688 patients enrolled in the U.S. IDE study of the CHARITE artificial disc. Spine. 2006;31:1217-1226.
54 Huang RC, Tropiano P, Marna T, et al. Range of motion and adjacent level degeneration after lumbar total disc replacement. Spine J. 2006;6:242-247.
55 Panjabi M, Malcolmson G, Teng E, et al. Hybrid testing of lumbar CHARITE discs versus fusions. Spine. 2007;32:959-966.