Chapter 7 Lower Limb Blocks
 Unlike the upper extremity, the entire lower extremity cannot be anesthetized with a single injection.
Unlike the upper extremity, the entire lower extremity cannot be anesthetized with a single injection. The lumbosacral plexus from a functional point of view comprises two distinct entities: the lumbar plexus and the sacral plexus.
The lumbosacral plexus from a functional point of view comprises two distinct entities: the lumbar plexus and the sacral plexus. This functional distinction frequently requires the performance of two separate blocks, increasing the requirement for local anesthetic and hence the likelihood of systemic toxicity.
This functional distinction frequently requires the performance of two separate blocks, increasing the requirement for local anesthetic and hence the likelihood of systemic toxicity. Because of overlying muscle and connective tissue, injections are generally deeper than those required for the upper limb.
Because of overlying muscle and connective tissue, injections are generally deeper than those required for the upper limb. Not surprisingly, ultrasonography has helped increase block success rates, performance times, and onset times for lower limb blocks.
Not surprisingly, ultrasonography has helped increase block success rates, performance times, and onset times for lower limb blocks. Ultrasonography helps identify anatomical variation in individual patients and reduces the incidence of vascular puncture.
Ultrasonography helps identify anatomical variation in individual patients and reduces the incidence of vascular puncture. Nerve imaging can be technically challenging in the lower limb; low-frequency ultrasound probes are needed for deep structures.
Nerve imaging can be technically challenging in the lower limb; low-frequency ultrasound probes are needed for deep structures. Stimulating catheters for continuous femoral nerve block are associated with decreased postoperative visual analog scale (VAS) scores.
Stimulating catheters for continuous femoral nerve block are associated with decreased postoperative visual analog scale (VAS) scores. Lower limb blocks may impair the patient’s ability to mobilize safely, increasing the likelihood of falls and injuries. Patient suitability, type of surgery, and day case or overnight stay all impact the choice of block (or not) to be performed.
Lower limb blocks may impair the patient’s ability to mobilize safely, increasing the likelihood of falls and injuries. Patient suitability, type of surgery, and day case or overnight stay all impact the choice of block (or not) to be performed. Lower limb surgeries frequently require the use of tourniquets; the combination of direct local anesthetic neurotoxicity, tourniquet-induced ischemia, or crush injury, together with patient factors such as diabetes, must be considered on an individual patient basis with regard to the potential for neurological injury.
Lower limb surgeries frequently require the use of tourniquets; the combination of direct local anesthetic neurotoxicity, tourniquet-induced ischemia, or crush injury, together with patient factors such as diabetes, must be considered on an individual patient basis with regard to the potential for neurological injury. The estimated distance from the skin to the lumbar plexus is 8.35 cm (range, 6.1–10.1) in men and 7.1 cm (range, 5.7–9.3) in women.4
The estimated distance from the skin to the lumbar plexus is 8.35 cm (range, 6.1–10.1) in men and 7.1 cm (range, 5.7–9.3) in women.4 The distance from the transverse process to lumbar plexus is consistently less than 2 cm in studies.4
The distance from the transverse process to lumbar plexus is consistently less than 2 cm in studies.4 The femoral nerve frequently divides in the proximal thigh; the practitioner should scan more cephalad if it is difficult to identify.
The femoral nerve frequently divides in the proximal thigh; the practitioner should scan more cephalad if it is difficult to identify. The anterior division of the femoral nerve supplies a motor branch to sartorius muscle. Medial and intermediate cutaneous branches supply the skin of the medial and anterior surfaces of the thigh.
The anterior division of the femoral nerve supplies a motor branch to sartorius muscle. Medial and intermediate cutaneous branches supply the skin of the medial and anterior surfaces of the thigh. The anterior division is most likely to be stimulated first, so the needle should be moved lateral and slightly deeper to stimulate the posterior division.
The anterior division is most likely to be stimulated first, so the needle should be moved lateral and slightly deeper to stimulate the posterior division. The minimum local anesthetic volume to produce successful block in 50% of patients is 14 to 16 mL of 0.5% bupivacaine and ropivacaine, respectively.
The minimum local anesthetic volume to produce successful block in 50% of patients is 14 to 16 mL of 0.5% bupivacaine and ropivacaine, respectively. The distance from the anterior superior iliac spine to the pubic tubercle is consistently 12 to 15 cm.
The distance from the anterior superior iliac spine to the pubic tubercle is consistently 12 to 15 cm. There is a low chance of vascular injury given block anatomy even with the double-pop or “blind” technique.
There is a low chance of vascular injury given block anatomy even with the double-pop or “blind” technique. Asking the patient to cough increases intraabdominal pressure, resulting in retrograde expulsion of local anesthetic from the needle hub and confirming correct fascial plane location.
Asking the patient to cough increases intraabdominal pressure, resulting in retrograde expulsion of local anesthetic from the needle hub and confirming correct fascial plane location. Deposition of local anesthetic in interfascial planes results in a greater than 90% success rate without the need for muscle stimulation as an end point.
Deposition of local anesthetic in interfascial planes results in a greater than 90% success rate without the need for muscle stimulation as an end point. Obturator block reduces opioid consumption and pain scores in patients undergoing major knee surgery.
Obturator block reduces opioid consumption and pain scores in patients undergoing major knee surgery. The posterior approach described by di Benedetto et al38 is associated with a low degree of patient discomfort during block performance for sciatic nerve block in the thigh.
The posterior approach described by di Benedetto et al38 is associated with a low degree of patient discomfort during block performance for sciatic nerve block in the thigh. More proximal approaches to the sciatic nerve are more likely to result in blockade of the posterior cutaneous nerve of the thigh, providing an advantage for procedures above the knee.
More proximal approaches to the sciatic nerve are more likely to result in blockade of the posterior cutaneous nerve of the thigh, providing an advantage for procedures above the knee. Popliteal fossa block anesthetizes the entire leg below the tibial plateau except the skin on the medial aspect of the foot and calf (saphenous nerve).
Popliteal fossa block anesthetizes the entire leg below the tibial plateau except the skin on the medial aspect of the foot and calf (saphenous nerve). If using a blind technique, saphenous paravascular injection greatly increases the chances of a successful block.
If using a blind technique, saphenous paravascular injection greatly increases the chances of a successful block. The saphenous nerve provides sensation to the medial aspect of the ankle and leg and may need to be blocked separately.
The saphenous nerve provides sensation to the medial aspect of the ankle and leg and may need to be blocked separately. If the saphenous nerve cannot be identified using ultrasonography, perivenous ultrasound-guided injection of local anesthetic is associated with a higher success rate than blind injection alone.
If the saphenous nerve cannot be identified using ultrasonography, perivenous ultrasound-guided injection of local anesthetic is associated with a higher success rate than blind injection alone. Epidural spread of local anesthetic is a common side effect with contralateral spread occurring in 9% to 16% of patients.
Epidural spread of local anesthetic is a common side effect with contralateral spread occurring in 9% to 16% of patients. High volumes of local anesthetics are injected into a vascular compartment. If a supplemental or subsequent lower limb block is added, local anesthetic toxicity can become an issue. This has been reported in older patients.
High volumes of local anesthetics are injected into a vascular compartment. If a supplemental or subsequent lower limb block is added, local anesthetic toxicity can become an issue. This has been reported in older patients. The obturator nerve is less frequently anesthetized than the lateral femoral cutaneous nerve when the classical three-in-one block is attempted.
The obturator nerve is less frequently anesthetized than the lateral femoral cutaneous nerve when the classical three-in-one block is attempted. The accuracy of catheter placement for continuous techniques is important, stimulating catheters result in consistently higher success rates. Motor responses at 1 mA (using the catheter) or less are associated with lower median VAS scores.
The accuracy of catheter placement for continuous techniques is important, stimulating catheters result in consistently higher success rates. Motor responses at 1 mA (using the catheter) or less are associated with lower median VAS scores. A motor response may be frequently difficult to illicit and often absent; when a response is elicited below 0.5 mA, intraneural needle placement is likely for this block.
A motor response may be frequently difficult to illicit and often absent; when a response is elicited below 0.5 mA, intraneural needle placement is likely for this block.Lumbar Plexus Block
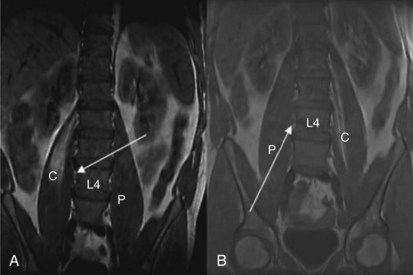
The anterior rami of T12-L4 form the lumbar plexus. The lumbar nerve roots emerge from the intervertebral foramina and immediately enter the body of the psoas muscle; divide into anterior and posterior divisions; and reunite as they course through the muscle to form the ilioinguinal, genitofemoral, lateral femoral cutaneous, femoral, and obturator nerves. The psoas muscle is enclosed in a fascial sheath continuous superiorly with the thoracic fascia, limited medially by the bodies of the lumbar vertebrae and posteriorly by the transverse processes, ligaments, and quadratus lumborum. Many approaches to the psoas compartment have been described, but probably the best known are those of Winnie and Capdevila. Both have an inferior (L4, L5) needle entry point and both result in spread of local anesthetic within the psoas compartment superiorly to L1 (Fig. 7-1).1
Winnie (1974) described the first posterior approach to the lumbar plexus and its subsequent modification with the use of nerve stimulation.2 Bilateral blockade, however, and concurrent epidural and spinal anesthesia occurring with the block led to concerns about its use.3 Capdevila, among others, proposed a number of modifications to the original description, designed to reduce the incidence of bilateral block (reportedly 10% to 20%).4 Capdevila reported a reduction in the incidence of bilateral block to 2%. Mannion et al5 compared both the Winnie and Capdevila approaches and randomized 60 elderly patients having lower limb arthroplasty to either technique for postoperative analgesia. Twelve patients in the Winnie group (40%) and 10 patients in the Capdevila group developed contralateral spread with many having evidence of bilateral thoracic and lumbar dermatomal spread (eight and five, respectively). Postulated reasons for bilateral spread have included (1) puncture of the epidural sleeve of the femoral nerve,6 (2) medial needle orientation leading to direct epidural or subarachnoid spread,4 and (3) spread occurring anterior to the vertebral bodies via areolar connective tissue within the subserous fascial layer as has been previously described to occur in the thorax.7 Gadsden et al8 recently demonstrated evidence of bilateral femoral nerve block or neuraxial anesthesia in six and five of 10 study patients, respectively, when injection pressure exceeded 20 psi. Neither effect occurred in 10 other patients randomized to injection pressure less than 15 psi when 35 mL of mepivacaine was injected. This appears to support the hypothesis that local anesthetic administered under high pressure causes extensive cleaving of tissue planes within the psoas muscle. Presumably, this opens up channels through which local anesthetic approaches the intervertebral foramina.
Clinical Utility
Perhaps more so than any other lower limb block, the clinical utility of a psoas compartment block in an individual patient must be weighed against the potential for the complications already discussed. A retrospective study by Macaire et al9 offers an insight into the incidence of such complications in clinical practice. The study itself involved 42 teams from the United States, Canada, France, Belgium, and Switzerland. In all, 4319 posterior lumbar plexus blocks were reported. Teams declared a 1% to 10% incidence of epidural spread, 11 total spinal anesthetics with one resulting death, 13 intravascular injections with three resulting seizures and one cardiac arrest, four delayed toxic reactions, and 13 incorrect catheter paths. Given the retrospective nature of this report, these figures may well be underestimated.9 A recent meta-analysis evaluated psoas compartment anesthesia for lower extremity surgery.10 Thirty studies were included, and the authors concluded that although psoas compartment block was superior to opioids for postoperative analgesia (providing 13 hours of analgesia after a single-shot block), there was insufficient data to recommend this approach as the sole anesthetic for hip or major knee surgery.10 Conversion rates to general anesthesia were typically in the order of 20% to 25%.
Landmark Technique for Psoas Compartment Block
The Capdevila approach to the psoas compartment block is outlined here because studies in general report lower incidence of bilateral spread. Venous access and standard monitoring (oxygen saturation, electrocardiography, and noninvasive blood pressure monitors) are secured. Because this is a deep block, patient discomfort can be a problem. It is our practice to administer fentanyl 0.5 to 1 µg/kg and 1 to 2 mg of midazolam if required. The patient is positioned in the lateral decubitus position with a slight forward tilt. The side to be blocked is uppermost. Both thighs are flexed, though the foot of the uppermost leg should be positioned over the dependent leg for ease of visibility. The spinous process of L4 is identified as the point at which the intercristal (Tuffier’s) line intersects the vertebral column. The posterior superior iliac spine is identified by palpation. From this point, the practitioner draws a line parallel to the spinous process extending cephalad. The practitioner draws out the intercristal line; this intersects with the line originating at the posterior superior iliac spines. The needle puncture point is at the junction of the lateral one third and medial two thirds of the line joining L4 and the line passing through the posterior superior iliac spine. At a minimum, an 80- to 100-mm insulated stimulating needle is needed for this block, and the stimulating current is set at 1.0 mA, 2 Hz, and 0.1 msec. The needle is oriented perpendicular to the skin and advanced until the transverse process of L4 is encountered. The needle is then directed caudally and advanced no more than 20 mm until a quadriceps muscle twitch is seen.11
Ultrasound-Guided Technique of Psoas Compartment Block
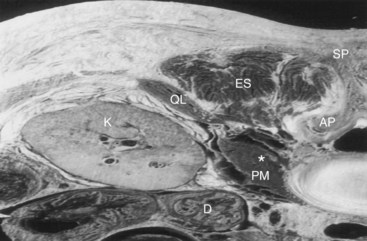
The patient is positioned similar to that used for the landmark approach. A low-frequency 2- to 5-MHz curved array transducer is used. It is our practice to begin by identifying the sacrum and turning the probe through 90 degrees to image the facet joints of the lumbar vertebrae: L3, L4, and L5. The transducer (with the long axis of the probe parallel to the lumbar spine) is moved laterally to identify the transverse processes of L3, L4, and L5. The hyperechoic transverse processes with the resulting acoustic shadow produce a characteristic “trident sign” shown in Fig. 7-2.12 The ultrasound machine settings (focal zones, depth of the scanning plane, and far-field gain) are now optimized. The psoas muscle has a striated appearance; although the lumbar plexus can be identified with ultrasonography, we have found this difficult to achieve in our adult practice.11 Local anesthetic (lidocaine 2%) is used to infiltrate the skin and deeper structures. The stimulating needle is introduced along the long axis of the probe in plane from cephalad to caudad (Fig. 7-3). We believe this approach is less likely to result in damage to structures such as the lower pole of the kidney (Fig. 7-4). The needle tip is angled away from the intervertebral foramina. In performing this block, it is frequently difficult to identify the needle shaft because of the curved array and angle of needle approach. Its position can often only be inferred from tissue movement around the needle when it is rocked side to side or gently probed forward. Entry of the needle into the posterior part of the psoas muscle results in contraction of the psoas muscle seen on the ultrasound screen. The needle is advance slowly to elicit femoral nerve stimulation (patellar twitch) not to be mistaken with hip flexion caused by psoas contraction. After negative aspiration, 10 mL of the local anesthetic solution is slowly injected. Further aspiration after each 10 mL is injected is advisable. It is our practice to use 20 to 30 mL of 0.5% ropivacaine solution. It should be pointed out that in obese patients, because of the technical limitations of ultrasonography, it can be frequently difficult to identify the psoas muscle and associated anatomy.13 It is thus up to the individual practitioner whether to abandon the procedure or attempt to perform it using stimulation alone.
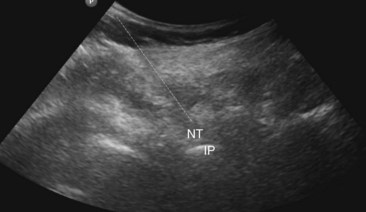
Fig. 7-3 The same sonogram as in Fig. 7-2. The dashed line shows the current needle trajectory and needle tip position (NT) as it approaches the transverse processes (TP) of L4.
Femoral Nerve
The femoral nerve is formed by the dorsal divisions of the anterior rami of the spinal nerves L2 to L4 as they condense within the psoas muscle. (Successful psoas compartment block results in almost 100% incidence of femoral nerve block.) The femoral nerve emerges from the lateral aspect of the psoas muscle at the junction of the middle and lower thirds and courses in the gutter between the psoas and the iliacus muscle lying inferior to the fascia iliaca. It enters the thigh when it passes inferior to the inguinal ligament. It lies deep to fascia lata and iliaca, lateral and at times somewhat posterior to the femoral artery (Fig. 7-5). At this level, the femoral nerve is rarely a single structure; rather, the nerve has rapidly divided into major anterior and posterior divisions. Other branches include the articular branch to the hip joint and branches to the rectus femoris, sartorius, and pectineus muscles. The anterior branches pierce fascia iliaca and supply sensory innervation to the anterior and medial aspects of the thigh. The posterior branches remain deep to fascia iliaca and innervate the quadriceps and the knee joint and terminate as the saphenous nerve, which supplies sensation to the medial aspect of the leg.
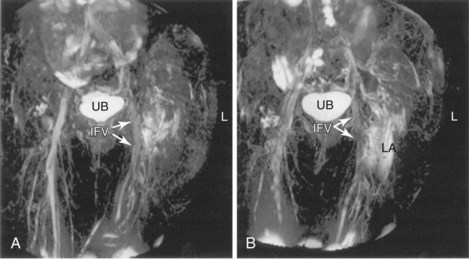
Winnie et al14 (1973) proposed the inguinal paravascular approach to the lumbar plexus (i.e., the three-in-one block), eliciting femoral nerve paresthesia at the level of the inguinal crease and using larger volumes of local anesthetic combined with pressure distal to the needle entry point. They described successful blockade of the femoral, ilioinguinal, and lateral cutaneous nerves simultaneously. Recent work has failed to demonstrate consistent block of all three nerves with high-volume local anesthetic injections. At least partial sensory blockade of the lateral femoral cutaneous nerve is achieved in almost all patients; the obturator nerve, however, is least likely to be blocked. In some series, the incidence of successful block is less than 10% irrespective of volume injected.15,16 This may be because of the medial location of that nerve at the inguinal crease level. Cephalad spread of local anesthetic to the lumbar plexus is limited on magnetic resonance imaging studies and does not appear to consistently occur to the extent postulated earlier by Winnie et al.14 Rather, the injectate spreads lateral, caudal, and slightly medial (Fig. 7-6).17
Ultrasound-guided femoral nerve block results in significantly faster sensory onset times over nerve stimulation alone (onset time, 16 minutes vs. 27 minutes using 20 mL bupivacaine 0.5%).18 Ultrasound guidance also significantly reduces the minimum effective anesthetic volume required for successful nerve block.19 A recent meta-analysis of 13 studies confirmed that regional blocks performed with ultrasonography were more likely to be successful, had faster onset, had on average a 25% longer duration of action, and took marginally less time to perform (1 minute). Perhaps of most importance, ultrasound guidance reduced the risk of vascular puncture by 80% in real terms (relative risk, 0.16; 95% confidence interval 0.05–0.47; P = .001).20 At our institution, we continue to use 20 mL of ropivacaine 0.5% as the standard bolus dose for ultrasound-guided analgesic blocks.
Landmark Technique
The patient is positioned in the supine position. In overweight patients, the lower abdomen may have to be retracted and held out of the field using tape. Venous access and standard monitoring are secured. The site of needle insertion is below the level of the inguinal ligament at the level of the groin crease (easily identified by asking the patient to raise his or her leg 15 degrees or so).22 The femoral pulse is palpated and marked. The insertion point is 1 to 2 cm lateral to the arterial pulsation; the skin is infiltrated with local anesthetic at this point. A 50-mm insulated stimulating needle is used for this block with the stimulating current set at 1.0 mA, 2 Hz, and 0.1 msec. The stimulating needle is advanced at 45 to 60 degrees to the skin in the cephalad direction. Insertion at this level produces the highest rate of needle–femoral nerve contact in cadaver studies.20,21 A distinct loss of resistance is usually felt each time the blunt stimulating needle traverses the fascia layers. A sartorius twitch is frequently the first twitch elicited; the quadriceps twitch (patella dance) is usually elicited by advancing the needle in a more lateral direction and 2 mm deep to the point of original sartorius twitch.22 The current intensity is then reduced; the needle tip position is adequate when quadriceps contraction persists when the current is 0.2 to 0.5 mA. Many believe that a quadriceps twitch is a prerequisite to successful block (New York Society of Regional Anesthesia), but recent evidence appears to contradict this view.23
Ultrasound Technique
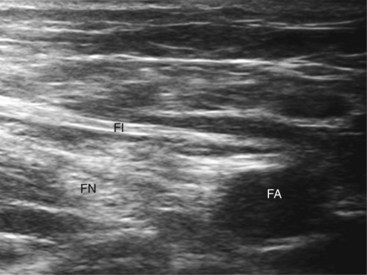
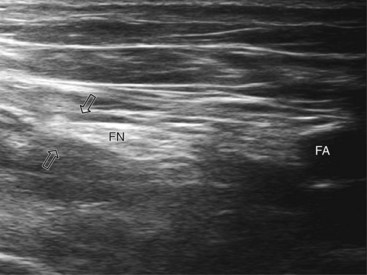
A systematic anatomical survey should first be undertaken. With the patient supine, the leg to be blocked is allowed to rotate slightly lateral. A high-frequency linear ultrasound probe scan is used from medial to lateral and then superficial to deep (at the groin crease). The ultrasound transducer is held perpendicular to the imagined path of the femoral nerve at all times (even a tilt of 10 to 15 degrees can render the femoral nerve isoechoic with the iliopsoas muscle).24 Because of its size in this area, the femoral nerve is often easy to identify and located lateral to the femoral artery (Figs. 7-5 and 7-7). Both lie anterior to the iliopsoas muscle. The femoral nerve is often identified as a triangular or wedge-shaped hyperechoic region lateral to the artery. This wedge is bounded superiorly by fascia iliaca, inferiorly by the iliopsoas fascia, and medially by the artery itself. If the nerve has split into anterior and posterior branches at this point, the posterior division that supplies the quadriceps lies most lateral within the triangle. Often, the femoral artery and profunda femoris artery are visible in the same image; we recommend scanning more cephalad, where imaging of the femoral nerve is often easier when the femoral artery alone is imaged. Despite its size, if the femoral nerve is flat or splits very proximal in the thigh, it can be difficult to image.
At our institution, we use a stimulating needle to confirm that the target structure is the femoral nerve. The stimulating needle can be positioned using an in-plane or out-of-plane approach. In the out-of-plane approach, the image is centered on the femoral nerve and the stimulating needle is introduced through the skin at an almost perpendicular angle parallel to the ultrasound beam. The needle tip is visualized as a white dot. A distinct loss of resistance is felt as the needle traverses fascia lata and then fascia iliaca. At this point, caution is needed as the needle tip is directly over the nerve itself. The needle is advanced in millimeter increments until a motor response is elicited if the twitch is sartorius and then orient the needle more lateral. The local anesthetic solution or injection of dextrose 5% solution (hypoechoic) should bring the nerve (hyperechoic) into better view as the femoral triangle is distended (Fig. 7-7). For successful block, the solution must be deposited inferior to the fascia iliaca and not extend superior to and medial to the femoral artery. The appearance of the femoral nerve itself after injection of the local anesthetic exhibits significant variability, depending on patient, body habitus, and imaging equipment (Figs. 7-7 and 7-8 illustrate this).
Iliacus Fascia Block
Sharrock25 (1989) described a three-in-one block after an attempted block of the lateral femoral cutaneous nerve of the thigh, sparking interest in other approaches to the three-in-one block outside of those required to stimulate the femoral nerve itself. Medial and lateral spread of local anesthetic beneath the plane of the fascia iliaca is the anatomical basis for this block. Not surprisingly, the obturator nerve (most medial nerve of the lumbar plexus) is less likely to be blocked than if the traditional three-in-one approach is used.26 Capdevila randomized 100 patients to either block in a prospective randomized controlled trial.26 Sensory block of the femoral, obturator, and lateral cutaneous nerve was achieved in 88%, 38%, and 90%, respectively, of those having fascia iliaca block versus 90%, 52%, and 62%, respectively, of those having three-in-one block.26 Similar efficacy has been demonstrated in children.27 Dolan and colleagues28 compared traditional “blind” fascia iliaca block to ultrasound-guided deposition of local anesthetic beneath the fascia iliaca. Ultrasound-guided block resulted in 82% of patients having complete loss of sensation on the anterior, lateral, and medial aspects of the thigh. Sensory inhibition increased to 95% (versus 60%) of cases on the medial aspect of the thigh when using ultrasonography. It would seem that both femoral and fascia iliaca block are approximately equivalent. Fascia iliaca block has the advantage of reducing the potential for direct needle trauma to the femoral nerve and artery.
Technique of Fascia Iliaca Block
The landmarks for this block are the anterior superior iliac spine, pubic tubercle, and inguinal ligament. The patient is positioned in the supine position. Dalens et al27 describe drawing a line on the skin connecting the anterior superior iliac spine to pubic tubercle. This line (usually 12 cm in adults) is divided into thirds. At the junction of the lateral and medial two thirds, a second line is drawn perpendicular to and intersecting the line joining anterior superior iliac spine and pubic tubercle. One cm along this line is the insertion point. A blunt needle (an 18-gauge Tuohy needle being a good choice) is inserted perpendicular to the skin at this point. The femoral artery and nerve are 3 to 4 cm medial, so no muscle stimulation end point is sought. A “pop” or give is felt as the needle passes through the fascia lata, and a second loss of resistance is felt as it passes through the fascia iliaca.27 The local anesthetic should inject easily (similar to injecting into the epidural space), and repeated aspiration should be performed. Asking the patient to cough increases intraabdominal pressure, resulting in retrograde expulsion of local anesthetic from the needle hub. This confirms correct position beneath the fascia iliaca. Because this is a compartment block, a large volume of injectate is important, with most authors using a volume of at least 30 mL.26,28
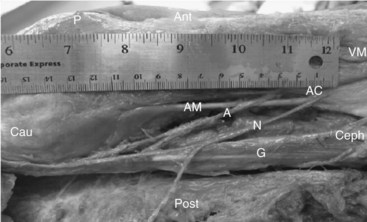
Obturator Nerve Block
The obturator nerve enters the leg through the craniomedial part of the obturator foramen. It divides into an anterior branch, which passes between the adductor longus and brevis muscles and a posterior branch between the adductor brevis and magnus muscles, although this is somewhat of an oversimplification (Fig. 7-9). The division of the obturator nerve into anterior and posterior divisions within the obturator canal exhibits quite a degree of variability. The anterior branch is initially located in the plane between pectineus and adductor brevis muscles. As it courses down the canal, its course is between adductor longus and brevis muscles.29 The obturator nerve is motor to the adductor, obturator, and gracilis muscles. The anterior branch provides sensation to a limited area on the medial aspect of the knee. The posterior branch often supplies part of the knee joint. The adductor muscles also receive variable innervation from the femoral and sciatic nerves; thus, successful obturator block does not imply complete absence of adduction. Obturator nerve blocks have been used in patients with cancer and osteoarthritis for pain control. The addition of an obturator nerve block to a femoral three-in-one block for analgesia after total knee arthroplasty is controversial and is currently not recommended for routine use.30 Studies involving small numbers of participants have produced contradictory results.31,32 This may reflect interindividual variation in the dermatomal innervation of the anterior branch of the obturator nerve; 32% of patients in one series were reported as having no cutaneous distribution.33 Small numbers of patients and the omission of ultrasonography make interpreting these studies difficult. It is not our routine practice to include an obturator nerve block as part of the multimodal analgesic regimen used at our institution, but it must be acknowledged that a small number of patients do complain of troublesome medial aspect knee pain following knee arthroplasty.
Technique of Obturator Nerve Block
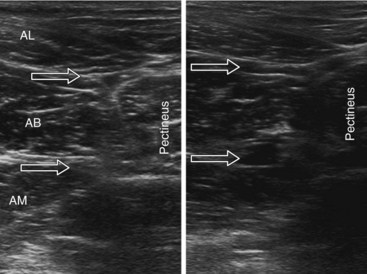
Selective obturator nerve block has been described as “both difficult and uncomfortable.”34 The use of ultrasonography and published ultrasonographic descriptions have made the process of the block itself easier. Visualizing the branches of the obturator nerve individually can be difficult. Alternatively, deposition of local anesthetic in the fascial planes between the (1) adductor longus and brevis and (2) adductor brevis and magnus will result in a high probability of successful block with minimal time required.35 Multiple muscle planes are traversed, making this a deep block with the use of sedation advisable. Ten mL of local anesthetic (5 mL in each plane) is sufficient.
The patient is positioned in the supine position with the leg externally rotated 5 to 10 degrees in slight abduction. The medial groin and proximal thigh is exposed. A linear 5- to 10-MHz ultrasound probe is used. A systematic survey is performed, and the femoral artery and vein are identified. The probe is then moved medially along the inguinal crease to identify the fascial planes of the pectineus and adductor muscles. The block needle can be introduced in or out of plane. An 80-mm block needle or 22-gauge spinal needle can be used. The needle tip is first advanced into the plane between the adductor longus and brevis muscles. After negative aspiration, 1 to 2 mL of solution is injected to confirm needle tip position. Interfascial spread should result in separation of the target muscles; 5 mL in total is deposited (Fig. 7-9). The needle is then further advanced into the plane between adductor brevis and magnus, and the procedure is repeated. Sinha and colleagues35 have recently reported a 93% success rate with this technique (defined as a 50% reduction in strength of adductor muscle contraction).
Sciatic Nerve Block
Multiple approaches to the sciatic nerve have been described. The nerve is commonly blocked at the gluteal, proximal or mid-thigh, and popliteal fossa levels. Historically, lower approaches or those inferior to the gluteus muscle (i.e., in the thigh) were described as “subgluteal.” It is our impression that this type of approach (i.e., proximal and mid-thigh) are increasingly referred to as infragluteal approaches. We describe a traditional landmark- and stimulator-only approach and an ultrasound-guided approach at the gluteal level. The ultrasound-guided approach discussed here is more frequently referred to as the “subgluteal” approach in the context of the recent ultrasound-guided literature.36,37 Distal sciatic nerve block at the popliteal fossa is also described. As with many other ultrasound-guided blocks, ultrasonography has recently been shown to reduce the minimum effective local anesthetic volume required for successful block.36
Landmark Approach to the Sciatic Nerve
The first sciatic nerve block was described by Victor Pauchet in 1920. Winnie modified this approach in 1975. Most recently, di Benedetto et al38 described a posterior approach to the sciatic nerve that resulted in decreased needle to nerve depth, increased patient satisfaction, and success compared with the classic approach described by Pauchet and later Labat.39 We describe the approach of di Benedetto here as the nerve stimulator–only technique. However, the ultrasound technique described here, which is very much different in terms of needle entry point, results in a final needle tip position conceptually closer to that approach described by Labat39 and Mansour and Benetts.40
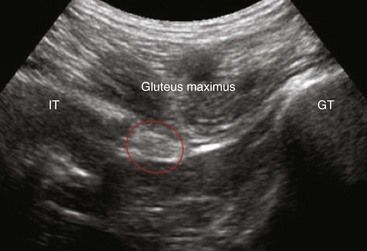
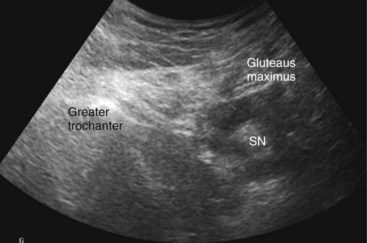
The patient is positioned on the side with that to be blocked uppermost. Standard monitoring is established, and intravenous (IV) access is secured. Adequate sedation using midazolam, fentanyl, or both is important for this block. The thigh is flexed 30 degrees or so on the block side, and the dependent leg is kept straight (Sims position). The greater trochanter is identified and marked as the most lateral bony landmark. The ischial tuberosity is identified by palpating inferiorly and medially from the greater trochanter. The position of this bony landmark is also marked. A line is drawn between the two points. At the midpoint of this line, a second line is drawn perpendicularly in a caudad direction. Four cm along this line is the needle insertion point. At this level, the sciatic nerve is more superficial with only the inferior and thinning aspect of the gluteus maximus muscle overlying it. A skin depression can be palpated and usually seen in the groove between biceps femoris laterally and the more medial semitendinosus muscles. In overweight patients, the skin can be depressed at this point using the middle and index fingers of the nondominant hand; this reduces the distance between the skin and nerve. The stimulating needle is inserted at right angles to the skin plane and advanced (1.5 mA, 2 Hz, 0.1 msec). Sciatic nerve stimulation is typically observed at a depth of 45 ± 13 mm in subjects weighing 73 ± 12 kg.38
Ultrasound Technique of Sciatic Nerve Block
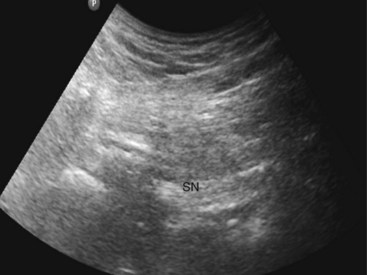
The gluteus maximus muscle is identified (Fig. 7-10). The sciatic nerve generally lies in a plane inferior to the gluteus maximus muscle or fascia and anterior to the quadratus femoris muscle fascia (Fig. 7-10). After the possible location of the target structure is identified, the resolution settings are optimized so that the target image is optimized. The sciatic nerve can be difficult to image both because of the depth and characteristic of the nerve. At this level, the nerve can often only be definitively identified using stimulation; its ultrasound appearance is a hyperechoic broad, often flat, structure. An 8- to 10-cm stimulating needle is used with the current set at 2.0 mA. The lattermost skin to the probe is anesthetized with local anesthetic, and deeper structures in the needle path (subcutaneous tissue and gluteus maximus muscle) are also infiltrated with local anesthetic. Although this is an in-plane technique, the needle tip can be difficult to identify as the needle approaches the target structure because of the steep angle between the ultrasound beam and needle shaft. If bone is contacted, the needle tip has most likely contacted the ischium; withdraw and angle the stimulating needle more laterally. Often, the first twitches obtained are from the gluteus muscle; twitches of the calf muscles or foot indicate appropriate sciatic stimulation. The stimulating current is decreased and the current intensity noted at which the motor twitch disappears. Recent work demonstrates that an intraneural needle tip position is highly likely in the distal sciatic nerve when an end point for stimulation of a muscle twitch at 0.2 to 0.4 mA is sought (100% intraneural),41 although this does not imply long-term neurologic damage.42 Perhaps more worrisome are the results of animal and human sciatic nerve studies; significant subsets demonstrate absent muscle twitches despite intraneural needle tip position and high stimulating currents (>1.0 mA).41,43 For this reason, we recommend using high stimulating currents to merely identify the target structure. The current intensity is reduced to exclude intraneural needle tip position. Our main end point is the ultrasonographic appearance of injectate pushing away the sciatic nerve from the needle tip and coming to envelop it circumferentially (Figs. 7-11 and 7-12).
Sciatic Nerve Block: Popliteal Approach
Technique of Popliteal Block
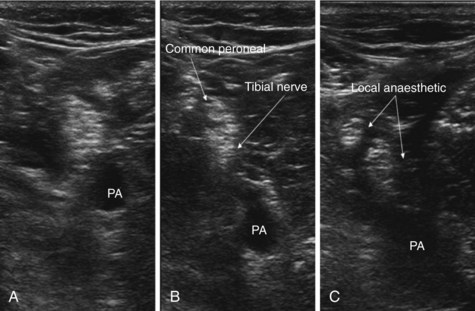
The patient is positioned prone or semiprone. Standard monitoring and IV access are secured. If possible, the patient is asked to flex the leg to identify the tendon of biceps femoris laterally and the tendons of semimembranosus and semitendinosus medially. The popliteal crease is easily identified. The level at which the sciatic nerve divides into its two components (the tibial and common peroneal nerves) is variable. Anatomical studies demonstrate this division occurs at a range of 50 to 120 mm proximal to the popliteal crease.44 At 8 cm proximal to the crease, the nerves typically lie 3 cm medial to the biceps tendon.
As discussed previously, intraneural injection is common when the block is performed by stimulation only. For this reason alone, we recommend the routine use of ultrasonography for this block together with the fact that ultrasound guidance increases the chance of a successful popliteal block over stimulation alone (studies show conflicting results when assessing whether the time taken to perform the block is reduced).45–47
A linear 5- to 7-MHz ultrasound probe is used. The probe is placed 8 to 10 cm proximal to the popliteal crease, with the probe perpendicular to the presumed path of the sciatic nerve scan lateral to medial. The popliteal artery is an easily identifiable first landmark. The sciatic nerve usually lies in close proximity and superficial to the artery (Fig. 7-13, A). The practitioner scans in a caudad direction following the nerve observing for its bifurcation into the common peroneal (lateral) and tibial nerve (usually larger) component (Fig. 7-13, B). After the bifurcation is identified, the direction of scanning is reversed. The injection point is immediately after the bifurcation. A 22-gauge stimulating needle is introduced along the long axis (in plane approach) of the probe. Sala-Blanch et al48 found that despite clear evidence of needle to nerve proximity, 16 of 37 patients required stimulating currents greater than 1.0 mA to demonstrate any motor response, four of 37 patients had no motor response with current intensity less than 2.0 mA, and only 17 of 37 patients demonstrated a motor response with a current intensity less than 0.5 mA. Robarts et al,49 reporting on 24 patients, found a motor response could only be elicited at 0.2 to 0.4 mA on intraneural needle placement in 20 patients. In the remaining four, no motor response could be obtained with a stimulating current of 1.5 mA despite intraneural placement. Reassuringly, few patients are reported to develop postoperative neurologic deficits as a result. In view of these findings, it is not our practice to persist in approximating the needle tip to the nerve solely to achieve a motor response. Rather, we carefully inject the first 1 mL of local anesthetic solution, observing for intraneural swelling (>15% increase in nerve diameter) and patient discomfort. In the absence of both, we continue to inject, observing the spread of local anesthetic around the structure. After injection, separation of the two sciatic nerve components should be seen (Fig. 7-13, C). A total of 20 to 30 mL of local anesthetic solution is required for adequate block.
Saphenous Nerve Block
The saphenous nerve is the terminal branch of the posterior division of the femoral nerve and provides sensation to the medial aspect of the leg. This includes the anteromedial, posterolateral, and medial aspect of the leg as far distal as the medial malleolus. The recent literature has seen a resurgence of interest in this block.50–52 The availability of ultrasonography offers the potential of more reproducible and reliable blocks over the older landmark technique, which had a high failure rate.53 Both are described here.
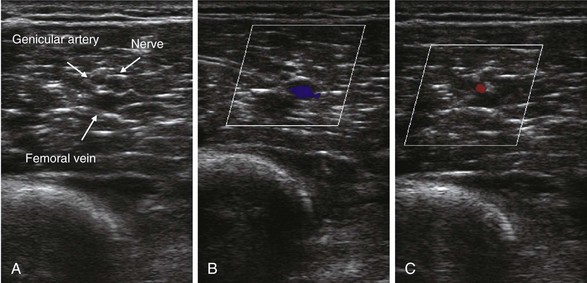
Contemporary anatomical studies describe the course of the saphenous nerve in the thigh.51 It separates from the femoral nerve in the proximal thigh and accompanies the femoral artery through the adductor canal. Within the canal and more distal along its course, it accompanies the descending genicular artery (Fig. 7-14) and emerges alongside it. The descending genicular artery, which supplies the knee itself, arises from the femoral artery just prior to where the femoral artery passes deep to the tendon of adductor magnus (vasoadductor membrane) to enter the popliteal fossa.
The median distance from the proximal patella to the distal end of the adductor canal is 10.25 cm with a range of 7.5 to 11.5 cm in cadaver studies.51 The saphenous nerve bifurcates into the infrapatellar (which reflects downward) and sartorial branches. This bifurcation occurs at a median distance of 2.7 cm (range, 2.1–3.4) cephalad to the proximal border of the patella and 6.6 cm (range, 5.0–9.0) deep to the medial border of the patella (Fig. 7-15).51
Landmark Technique of Saphenous Nerve Block
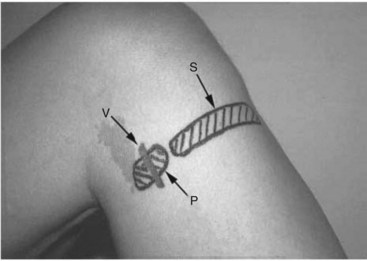
The patient is positioned in the supine position with the knee elevated and leg flexed to 45 degrees. The practitioner palpates for the tibial tuberosity and medial head of gastrocnemius muscle. A blind subcutaneous infiltration is made to raise a “wheal” between both points. The skin may need to be lifted off the tibia, and the needle is inserted parallel to the bone (5 to 7.5 mL of local anesthetic and a 25-gauge needle). Two or three needle insertions may be required. The success rates reported for this classical approach are typically 30% to 40%.54 De Mey et al54 report improved success rates (100%) when a modified landmark paravascular technique is used. Cadaveric dissection (n = 5) demonstrated a constant relationship between the saphenous vein and nerve. The relationship between nerve and vein was found to be similar in 10 dissections with the nerve being found just medial (within 1 cm) and posterior to the saphenous vein.
De Mey et al54 performed saphenous nerve block with 100% success rate when local anesthetic was infiltrated or fanned near the vein. A tourniquet around the thigh was used to first identify the vein.54 Fig. 7-16 demonstrates the more posterior position of the saphenous injection site. Comfort et al55 also report 100% successful saphenous nerve block using nerve stimulation at this level, although the time taken to successfully identify the saphenous nerve and perform the block was an average of 11 minutes.55
Ultrasound Technique of Saphenous Nerve Block
The patient is positioned in the supine position. Standard monitoring and IV access are secured. A 5- to 7-MHz linear ultrasound probe is used. The ultrasound probe is positioned on the medial surface of the distal thigh 5 to 10 cm proximal to the patella. The probe should be in a transverse orientation with respect to the path of the sartorius muscle. The practitioner scans from lateral to medial to identify the neurovascular bundle. The saphenous nerve may appear hyper- or hypoechoic and is often surrounded by a rim of perineural fat (part of the subsartorial fat pad). Color Doppler ultrasonography is often required to help distinguish the descending genicular artery from the saphenous nerve (Fig. 7-14). A 22-gauge needle is introduced in plane from lateral to medial in the fascial plane between the sartorius and vastus medialis muscles. Five to 10 mL of local anesthetic is injected around the nerve.
Ankle Block
The ankle block is easily performed and is a safe, relatively low-risk procedure for both surgical anesthesia and postoperative analgesia. Previously, in the era of the landmark-only approach to the ankle block, many patients required rescue analgesia when the block was used for postoperative analgesia, and yet more anesthetists believed their success rate to be “medium” or “low.”56–58
Ultrasound Approach to the Ankle Block
We routinely use ultrasonography for both the sural and posterior tibial injections, but we rely on traditional landmark techniques for blockade of the other three nerves. Ultrasonography can be used to identify the dorsalis pedis artery; however, the deep peroneal nerve can be difficult to identify. Antonakakis et al59 report being unable to identify the nerve in seven of 18 subjects. In the same study, ultrasonography was not shown to improve the success rate of deep peroneal nerve block and led to a longer time to perform the block. However, Benzon et al60 point out that the nerve is frequently more lateral than expected, emphasizing the need for light pressure when applying the ultrasound probe. This is so as not to compress the artery because it is used as the landmark. They reported very high success rates for combined ultrasonography and stimulator-guided block in volunteers.60
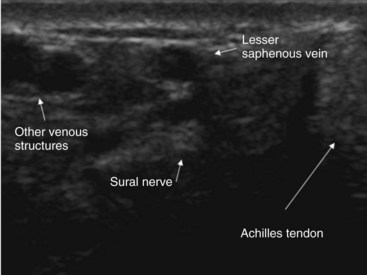
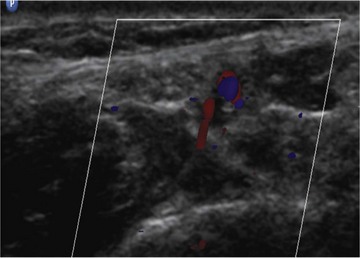
Ultrasonography improves the success rate of the sural and tibial nerve block.61,62 We recommend using a 2.5-cm “hockey stick” high-frequency linear ultrasound probe simply because of the limited size of the scanning surfaces. The sural nerve can frequently be difficult to identify (Fig. 7-17). However, the sural nerve is consistently found slightly medial or posterior to the saphenous vein (Fig. 7-17).59 If the structure cannot be definitively identified, perivenous ultrasound-guided injection of local anesthetic is associated with a higher success rate and more dense blocks rate than blind injection alone (94% vs. 56%).61
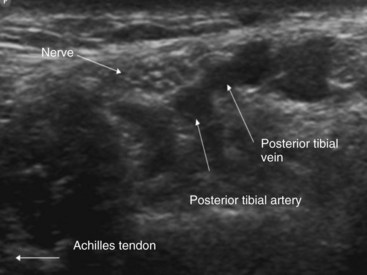
The tibial nerve is also best imaged in the short-axis view. The practitioner begins by scanning around the medial malleolus moving toward the Achilles tendon. The area contains several vascular structures, and color Doppler ultrasonography is often required to confirm the nature of anechoic structures (Fig. 7-18). The tibial nerve frequently appears as a honeycomb structure and can be differentiated from the flexor hallucis tendon simply by asking the patient to flex the great toe; the tendon should thus move (Fig. 7-19). The needle is introduced in plane from lateral to medial, and the practitioner injects circumferentially around the nerve. Although ultrasound-guided tibial nerve block takes longer to perform (159 vs. 79 seconds) and is associated with more needle redirections, its use is associated with a faster onset and more complete blocks at all times.61
1 Mannion S, Barrett J, Kelly D, et al. A description of the spread of injectate after psoas compartment block using magnetic resonance imaging. Reg Anesth Pain Med. 2005;30:567-571.
2 Winnie AP, Ramamurthy S, Durani Z, Radonjic R. Plexus blocks for lower surgery: new answers to old problems. Anesthesiol Rev. 1974;1:11-16.
3 Auroy Y, Benhamou D, Bargues L, et al. Major complications of regional anesthesia in France: the SOS Regional Anesthesia Hotline Service. Anesthesiology. 2002;97:1274-1280.
4 Capdevila X, Macaire P, Dadure C, et al. Continuous psoas compartment block for postoperative analgesia after total hip arthroplasty: new landmarks, technical guidelines and clinical evaluation. Anesth Analg. 2002;94:1606-1613.
5 Mannion S, O Callaghan S, Walsh M, et al. Approaches for psoas compartment block. Anesth Analg. 2005;101:259-264.
6 Dalens B, Tanguy A, Vanneuville G. Lumbar plexus block in children: a comparison of two procedures in 50 patients. Anesth Analg. 1998;67:750-758.
7 Karmakar MK, Kwok WH, Kew J. Thoracic paravertebral block: radiological evidence of contralateral spread anterior to the vertebral bodies. Br J Anaesth. 2000;84:263-265.
8 Gadsden JC, Lindenmuth DM, Hadzic A, et al. Lumbar plexus block using a high pressure injection leads to contralateral epidural spread. Anesthesiology. 2008;109:683-688.
9 Macarie P, Gaertner E, Choquet O. Le Bloc du Plexus lombaire est-il dangereux? In: Evaluation et traitement de la douleur. SFAR 2002. Paris: Elsevier and SFAR; 2002:37-50.
10 Touray ST, de Leeeuw MA, Zuurmond WWA, Perez RSGM. Psoas compartment block for lower extremity surgery: a meta-analysis. Br J Anaesth. 2008;101:750-760.
11 Awad IT, Duggan EM. Posterior lumbar plexus block. Anatomy, approaches and techniques. Reg Anesth Pain Med. 2005;30:143-149.
12 Karmakar MK, Ho AM, Li X, et al. Ultrasound-guided lumbar plexus block through the acoustic window of the lumbar ultrasound trident. Br J Anaesth. 2008;100:533-537.
13 Kirchmair L, Entner T, Wissel J, et al. A study of the paravertebral anatomy for ultrasound-guided posterior lumbar plexus block. Anesth Analg. 2001;93:477-481.
14 Winnie AP, Ramamurthy S, Durrani Z. The inguinal paravascular technic of lumbar plexus anesthesia: the “3-in-1 block.”. Anesth Analg. 1973;52:989-996.
15 Lang SA, Yip RW, Chang PC, et al. The femoral nerve block revisited. J Clin Anesth. 1993;5:292-296.
16 Seegerger MD, Urwyler A. Paravascular lumbar plexus block: block extension after femoral nerve stimulation and injection of 20 vs 40 ml mepivacaine 10 mg/ml. Acta Anaesthesiol Scand. 1995;39:769-773.
17 Marhofer P, Nasel C, Sitzwohl C, Kapral S. Magnetic resonance imaging of the distribution of local anesthetic during the three-in-one block. Anesth Analg. 2000;90:119-124.
18 Marhofer P, Schrogendorfer K, Koinig H, et al. Ultrasonographic guidance improves sensory block and onset time of three-in-one blocks. Anesth Analg. 1997;85:854-857.
19 Casati A, Baciarello M, Di Cianni S, et al. Effects of Ultrasound guidance on the minimum effective anaesthetic volume required to block the femoral nerve. Br J Anaesth. 2007;98:823-827.
20 Abrahams MS, Aziz MF, Fu RF, Horn JL. Ultrasound guidance compared with electrical stimulation for peripheral nerve block: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2009;102:408-417.
21 Vloka DJ, Hadzic A, Drobnik L, et al. Anatomical landmarks for femoral nerve block: a comparison of four needle insertion sites. Anesth Analg. 1999;89:1467-1470.
22 Nader A, Malik K, Kendall MC, et al. Relationship between ultrasound imaging and eliciting motor response during femoral nerve stimulation. J Ultrasound Med. 2009;3:345-350.
23 Anns J, Awad I, McCartney C, et al. An interim analysis of femoral nerve block anatomical insertion point: a prospective, randomised, double-blind controlled trial. Reg Anesth Pain Med. 2008;33(5):e106.
24 Soong J, Schafhalter-Zoppoth I, Gray AT. The importance of transducer angle on ultrasound visibility of the femoral nerve. Reg Anesth Pain Med. 2005;30:505.
25 Sharrock NE. Inadvertent “3-in-1” block following injection of the lateral femoral cutaneous nerve of the thigh. Anesth Analg. 1989;59:887-888.
26 Capdevila X, Biboulet P, Bouregba M, et al. Comparison of the three-in-one and fascia iliaca compartment blocks in adults: clinical and radiographic analysis. Anesth Analg. 1998;86:1039-1044.
27 Dalens B, Vanneuville G, Tanguy A. Comparison of the fascia iliaca block with the 3-in-1 block in children. Anesth Analg. 1989;69:705-713.
28 Dolan J, Williams A, Murney E, et al. Ultrasound guided fascia iliaca block: a comparison with the loss of resistance technique. Reg Anesth Pain Med. 2008;33:526-531.
29 Saranteas T, Paraskeuopoulos T, Alevizou A, et al. Identification of the obturator nerve divisions and subdivisions in the inguinal region: as study with ultrasound. Acta Anaesthesiol Scand. 2007;51:1404-1406.
30 Fischer HBJ, Simanski CJP, Sharp C, et al. A procedure-specific systematic review and consensus recommendations for postoperative analgesia following total knee arthroplasty. Anaesthesia. 2008;63:1105-1223.
31 Kardash K, Hickey D, Tessler MJ, et al. Obturator versus femoral nerve block for analgesia after total knee arthroplasty. Anesth Analg. 2007;105:853-858.
32 Macalou D, Trueck S, Meuret P, et al. Postoperative analgesia after total knee replacement: the effect of an obturator nerve block added to the femoral 3-in-1 nerve block. Anesth Analg. 2004;99:251-254.
33 Helayel PE, Da Conceicao DB, Pavei P, et al. Ultrasound-guided obturator nerve block: a preliminary report of case series. Reg Anesth Pain Med. 2007;32:221-226.
34 Macrae WA, Coventry DM. Lower limb blocks. In: Wildsmith JAW, Armitage EN, McClure JH, editors. Principles and practice of regional anaesthesia. London: Churchill Livingstone; 1987:219.
35 Sinha SK, Abrams JH, Houle TT, Weller RS. Ultrasound-guided obturator nerve block: an interfascial injection approach without nerve stimulation. Reg Anesth Pain Med. 2009;34:261-264.
36 Danelli G, Ghisi D, Fanelli A, et al. The effects of ultrasound guidance and neurostimulation on the minimum effective analgesic volume of mepivacaine 1.5% required to block the Sciatic nerve using the subgluteal approach. Anesth Analg. 2009;109:1674-1678.
37 Karmakar MK, Kwok WH, Ho AM, et al. Ultrasound-guided sciatic nerve block: description of a new approach at the subgluteal space. Br J Anaesth. 2007;98:390-395.
38 di Benedetto P, Bertini L, Casati A, et al. A new posterior approach to the sciatic nerve block: a prospective, randomized comparison with the classic posterior approach. Anesth Analg. 2001;93:1040-1044.
39 Labat G. Regional anesthesia: its technic and clinical application. Philadelphia: WB Saunders; 1923.
40 Mansour NY, Benetts FE. An observational study of combined continuous lumbar plexus and single-shot sciatic nerve blocks for post-knee surgery analgesia. Reg Anesth. 1996;21:287-291.
41 Robards C, Hadzic A, Somasundaram L, et al. Intraneural injection with low-current stimulation during popliteal sciatic nerve block. Anesth Analg. 2009;109:673-677.
42 Sala Blanch X, Lopez AM, Carazo J, et al. Intraneural injection during nerve stimulator-guided sciatic nerve block at the popliteal fossa. Br J Anaesth. 2009;102:855-861.
43 Tsai TP, Vuckovic I, Dilberovic F, et al. Intensity of the stimulating current may not be a reliable indicator of intraneural needle placement. Reg Anesth Pain Med. 2008;33:207-210.
44 Vlodka JD, Hadzic A, April EW, et al. Division of the sciatic nerve in the popliteal fossa and its possible implications in the popliteal nerve blockade. Anesth Analg. 2001;92:215-217.
45 Perlas A, Brull B, Chan VW, et al. Ultrasound guidance improves the success of sciatic nerve block at the popliteal fossa. Reg Anesth Pain Med. 2008;33:259-265.
46 Dufour E, Quennesson P, Van Robais Al, et al. Combined ultrasound and neurostimulation guidance for popliteal sciatic nerve block: a prospective randomised comparison with neurostimulation alone. Anesth Analg. 2008;106:1553-1558.
47 Danelli G Fanellie A, Ghisi D, et al. Ultrasound Vs nerve stimulation multiple injection technique for posterior popliteal sciatic nerve block. Anaesthesia. 2009;64:638-642.
48 Sala Blanch X, Lopez AM, Carazo J, et al. Intraneuronal injection during nerve stimulator-guided sciatic nerve block at the popliteal fossa. Br J Anaesth. 2009;102:855-861.
49 Robarts C, Hadzic A, Somasundaram L, et al. Intraneural injection with low-current stimulation during popliteal sciatic nerve block. Anesth Analg. 2009;109:673-677.
50 Manickam B, Perlas A, Duggan E, et al. Feasibility and efficacy of ultrasound-guided block of the saphenous nerve in the adductor canal. Reg Anesth Pain Med. 2009;4(6):578-580.
51 Horn JL, Pitsch T, Salinas F, Benninger B. Anatomic basis for the ultrasound-guided approach for saphenous nerve blockade. Reg Anesth Pain Med. 2009;34:486-489.
52 Krombach J, Gray AT. Sonography for saphenous nerve block near the adductor canal. Reg Anesth Pain Med. 2007;32:369-370.
53 Taboada M, Lorenzo D, Oliveira J, et al. Comparison of 4 techniques for internal saphenous nerve block. Rev Esp Anesthesiol Reamin. 2004;51:509-514.
54 De Mey JCJ, Deruyck LJ, Cammu GMD, et al. A paravenous approach for the saphenous nerve block. Reg Anesth Pain Med. 2001;26:504-506.
55 Comfort V, Lang S, Yip R. Saphenous nerve anaesthesia—a nerve stimulator technique. Can J Anaesth. 1996;43:852-857.
56 Rudkin GE, Rudkin HK, Dracopoulos GC. Ankle block success rate: a prospective analysis of 1000 patients. Can J Anaesth. 2005;52:209-210.
57 Mc Leod DH, Wong D, Himat V, et al. Lateral popliteal sciatic nerve block compared with ankle block for analgesia following foot surgery. Can J Anaesth. 1995;42:765-770.
58 Rudkin GE, Micallef TA. Impediments to the use of ankle block in Australia. Anaesth Intensive Care. 2004;32:368-371.
59 Antonakakis JG, Scalzo DC, Jorgenson AS, et al. Ultrasound does not improve the success rate of deep peroneal nerve block at the ankle. Reg Anesth Pain Med. 2010;35:217-221.
60 Benzon HT, Sekhadia M, Benzon HA, et al. Ultrasound-assisted and evoked motor response stimulation of the deep peroneal nerve. Anesth Analg. 2009;109:2022-2024.
61 Kirsten R, Sites BD, Chinn CD, et al. Ultrasound improves the success rate of a sural nerve block at the ankle. Reg Anesth Pain Med. 2009;34:24-28.
62 Redborg KE, Antonakakis JG, Beach ML, et al. Ultrasound Improves the success rate of tibial nerve block at the ankle. Reg Anesth Pain Med. 2009;34:256-260.