CHAPTER 59 Lower intestinal tract disease
Introduction
These close relationships have led to joint working in outpatient clinics and operating lists, and also to the emergence of multidisciplinary meetings. These can be held locally and regionally. They provide education for the members into the understanding of symptoms and treatment within the multidisciplinary field; meetings can be used to plan treatment and joint procedures, as well as for education (Mirnezami et al 2008).
A basic understanding of the anatomy and physiology of the colon, rectum and anus is essential in order to understand the symptoms associated with the posterior compartment (see Chapter 50).
Investigations
A detailed history and examination is often adequate to initiate basic treatment. It is always important to exclude serious pathology, and patients may require full bowel imaging of some sort (colonoscopy, barium enema or computed tomography colonography). In recent years, measurement of patient benefit from treatment has led to the use of incontinence and constipation scores which can be recorded before and after treatment. The Cleveland Clinic Incontinence Score (Table 59.1) is most commonly used.
The Cleveland Clinic Incontinence Score does not include aspects of urgency, but the Vaizey score (Vaizey et al 1999) attempted to address this. Vaizey et al reported that urgency of less than 15 min is not usually a problem; however, when patients are not able to hold for more than 2 min, this has a significant impact on quality of life. A recent study (Cotterill et al 2008) took comments from a panel of seven clinical experts and from patients, and reported five key issues related to anal incontinence. These were unpredictability, toilet locations, coping strategies, embarrassment and restriction of social activities. This group are working on a new instrument to help validate treatments for anal incontinence based around quality of life as well as symptom severity.
Anorectal manometry
Anal manometry is not a single test but a series of measurements used to assess anal sphincter function, rectal sensation, rectoanal reflexes and compliance of the rectum (Kourakalis and Andromanakos 2004). The use of pull-through anal manometry allows assessment of the length of the functioning sphincter, resting pressures give a good estimation of internal sphincter function, and squeeze pressures assess external sphincter function. The patient is usually placed in the left lateral position, and manometry can be performed either using stationery pull-through or continuous pull-through techniques. Many catheters have four or eight channels, and these can therefore record different pressures in different parts of the external sphincter and internal sphincter at different levels. Using vector analysis, it is possible to look at the symmetry of the anal canal pressure, and combining this with anal ultrasonography can show whether there is a structural as well as a functional problem in either of the sphincter muscles. The rectoanal inhibitory reflex is usually assessed during the physiology. Absence of this may indicate a longstanding problem such as Hirschsprung’s disease. Rectal distension is assessed by inflating a balloon within the rectum; measurements are taken when the patient first feels the sensation of rectal filling, the feeling when there is maximum fullness, and then the feeling of urgent desire to defaecate. This can be extended to when the volume is unbearable. If the rectal threshold is low, even with a normal anal pressure, continence may be impaired.
Anal skin sensation is usually assessed by some form of electrical stimulation. Pudendal nerve terminal motor latencies are performed by stimulating the pudendal nerve transanally through the lateral wall of the rectum as the nerve transverses the ischial spine, using a St Mark’s electrode on the tip of the examining finger. The normal delay between stimulation and recording is less than 2.2 ms and a longer delay suggests that there has been damage to the pudendal nerve. A lengthening of the pudendal nerve latency is often associated with poorer outcomes after surgery, and may suggest that the patient’s incontinence is associated with nerve damage. An important outcome measure for sphincter repair is whether there is any pudendal neuropathy, as patients with pudendal neuropathy only have a 10% chance of success compared with 80% in patients without pudendal neuropathy (Laurberg et al 1988).
Ultrasound and dynamic imaging
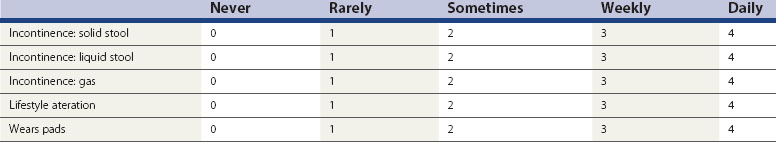
The investigation of choice for structural abnormalities within the anal sphincter is a three-dimensional anal ultrasound. A data set can be captured and then manipulated at a later date to look at structural abnormalities throughout the anal canal (Figure 59.1).
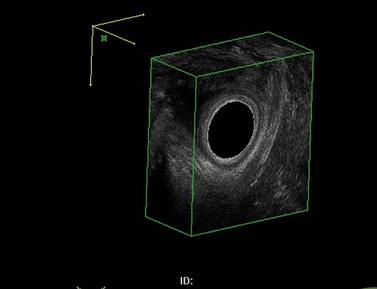
Using the same anal probe transvaginally, it is possible to examine the levator plate and many other anatomical aspects of the pelvic floor. Asymmetry within the levator plate suggests damage during childbirth and often leads to the anus being diverted to the side of the damage (Figure 59.2).
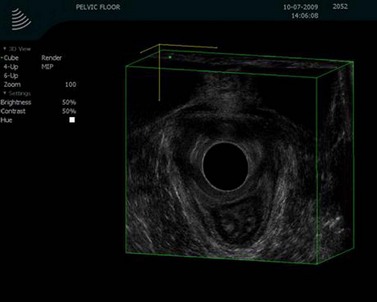
Further assessment of the pelvic floor often requires some form of proctography. In order to assess the whole pelvic floor using defaecating proctography, patients require not only contrast within the rectum but also in the small bowel, vagina and bladder. Defaecatory magnetic resonance imaging (Figure 59.3) can show all these parts of the pelvic floor. There are very few open magnets where a patient can sit and defaecate; therefore, the majority of these studies are performed with the patient lying prone.
It has been shown that magnetic resonance proctography shows increased evidence of other pelvic floor abnormalities over defaecating proctography. However, it underestimates the size of rectoceles and intussusception (Pilkington et al 2009).
Common Conditions and Treatments
Haemorrhoids
Piles often present with a combination of symptoms, including itching, soreness, postdefaecatory leakage and lumps around the anus. It is thought that the sliding anal canal theory proposed by Thompson (1975) is the most likely cause of piles, where Treitz’s muscle is stretched repeatedly resulting in fragmentation of the connective tissue. Haemorrhoids may be small and highly symptomatic, or large and asymptomatic. This is especially so in elderly female patients where normal anal canal pressures are low, and this can result in prolapse but little in the way of symptoms. Several classifications of haemorrhoids have been used over time. Goligher’s classification (Goligher 1976) describes the degree of prolapse and whether these reduce spontaneously (grade 2), require manual reduction (grade 3) or are permanently prolapsed (grade 4). However, although this is good for the description, it does not consider the symptoms of the piles. Work by Lunniss and Mann (2004) has produced a more accurate and useful classification of haemorrhoids, dividing them into non-prolapsing and prolapsing, looking at not only the morphological presentation but also any additional features such as pruritus or pain.
Treatment of haemorrhoids
Estimates of the proportion of the UK population affected with haemorrhoids range from 4.4% to 24.5%. In 2004–2005, approximately 23,000 haemorrhoidal procedures were carried out, and approximately 8000 of these were excision haemorrhoidectomies (National Institute for Health and Clinical Excellence 2007a).
Medical treatment for haemorrhoids
Patients with symptomatic but small internal haemorrhoids are best treated with dietary management; increasing the intake of fluid and fibre is the mainstay of this treatment. The typical symptoms of internal haemorrhoids are rectal bleeding and, sometimes, prolapse; avoiding straining by these dietary methods usually improves the symptoms. Dietary manipulation also improves rectal bleeding alone from grade 1 and grade 2 haemorrhoids, and this has been proven in three randomized controlled trials (Cataldo et al 2005).
Patients with persistent symptoms from grade 1, 2 or 3 haemorrhoids may benefit from an outpatient procedure. The most common procedure used and probably the most effective is haemorrhoidal banding. Other procedures that may be offered include sclerotherapy, infrared coagulation and cryotherapy. All of these treatments are aimed at decreasing the haemorrhoidal tissue volume and fixing the anal cushion back to the rectal wall. A meta-analysis of outpatient treatment suggested that rubber band ligation was the most effective of all outpatient procedures and was associated with a lower recurrence rate (MacRae and McLeod 1995). It is, however, relatively painful and the pain associated with the procedure increases with the number of rubber bands that are used to treat these piles. It is essential that the bands are placed well above the dentate line, or the immediate pain felt from the sensate epithelium is excruciating and patients will need to have these bands removed. Rubber band ligation is associated with a success rate of 65–85%, but follow-up at 5 years shows a relatively high recurrence rate. Following outpatient procedures for haemorrhoids, approximately 2% of patients report pain and less than 1% report urinary retention. Major complications include significant haemorrhage, which may take place immediately or as a secondary haemorrhage between postoperative days 7 and 10.
Operative procedures for haemorrhoids
Haemorrhoidectomy of any sort should only be offered to patients with large external haemorrhoids or significant prolapse, or those who have been resistant to outpatient procedures. Open haemorrhoidectomy is effective; however, it is associated with pain, infection and other complications, such as incontinence and stenosis of the anal canal. More recently, patients have been able to undertake a less painful form of surgical procedure called a ‘stapled haemorrhoidopexy’. The evidence for this has been reviewed by the National Institute for Health and Clinical Excellence (NICE) (2007a). A stapled haemorrhoidopexy is an intrarectal procedure that aims to reduce the prolapse of haemorrhoidal tissue by excising a band of the prolapse anal mucosal membrane above the dentate line using a specific circular stapling device (PPH03 Ethicon). The procedure is thought to interrupt the blood supply to the haemorrhoids and reduce the potential continuing prolapse of mucosa. The NICE review studied 27 randomized controlled trials of stapled haemorrhoidopexy and found that the procedure was associated with less pain up to 14 days following surgery, shorter wound healing times and significantly less postoperative bleeding. On the basis of the evidence in the literature, it was felt that a stapled haemorrhoidopexy offered benefits over a conventional haemorrhoidectomy in the reduction of short- and medium-term postoperative pain. However, although stapled haemorrhoidopexy is associated with a higher rate of recurrent prolapse, the committee concluded that ‘a stapled haemorrhoidopexy should be recommended as a treatment option for people in whom surgical intervention is considered appropriate for the treatment of prolapsed internal haemorrhoids’.
Anal fissures
Patients with pelvic floor straining and post childbirth often present with anal pain associated with an anal fissure. An anal fissure is a tear running longitudinally in the epithelium of the anal canal, the majority of which lie posteriorly in the midline. Acute fissures are usually superficial, whereas chronic fissures may be associated with secondary changes including a sentinel tag or hypertrophied anal papilla. As with all acute and chronic anal conditions, efforts should be made to identify any precipitating causes or associated diseases (Crohn’s disease or anal carcinoma). A recent consensus statement from the Association of Coloproctology of Great Britain and Ireland covers aspects of diagnosis and treatment (Cross et al 2008). The most common presenting features are pain during defaecation which may last for several hours afterwards, as well as rectal bleeding. The most consistent finding on examination is spasm of the anal canal, and it is uncertain whether this is the result of or due to ischaemia. Many of the management options have been based around reducing the internal anal spasm, but as with many benign anal conditions, one of the most important measures is to increase dietary fibre and adequate fluid intake. The majority of fissures, especially posterior fissures, are associated with a high resting pressure and a low blood flow to the anoderm resulting from this spasm. However, postpartum fissures are usually anterior and may be associated with a rectocele. The scar formation may be associated with ischaemia; however, the resting pressures in these patients are usually low. If there are multiple fissures or the fissures are not in the midline, a differential diagnosis of Crohn’s disease, ulcerative colitis, human immunodeficiency virus and associated infections as well as neoplasia should be considered. An anal fissure may occur at any time in life but is most common between the second and fourth decades, with an equal distribution between men and women and a lifetime incidence of just over 11%. Inspection of the anus will usually reveal the fissure, and it is not usually possible to carry out any further examination due to the pain associated with the fissure.
Treatment of anal fissures
Acute anal fissures are usually treated conservatively with stool softeners and topical analgesics. Recurrence rates are reduced from 68% to 16% after continuing conservative management (Jensen 1987).
Medical therapies
Glyceryl trinitrate
GTN is a widely used drug that works by vasodilating and relaxing smooth muscle. It is suggested that patients should be treated with 0.2% or 0.4% GTN two to three times per day, and healing will occur in approximately 60% of patients in the short term. Unfortunately, the side-effect of severe headaches often precludes usage, although these headaches do wear off with time (Cross et al 2008).
Botulinum toxin
Botulinum toxin works by binding irreversibly to the presynaptic nerve terminals preventing acetylcholine release and resulting in hypotonia and a reduced resting anal pressure. The effects last for 2–3 months until the acetylcholine has reaccumulated in the nerve terminals (Jones et al 2003). The majority of people use 20 units of botulinum toxin, although the dosages reported in the literature vary between 10 and 100 units; healing rates of 40–100% have been reported. Botulinum toxin is given in two doses into the internal sphincter, and 75% of patients will be healed using this treatment.
Surgery
If these conservative and medical treatments have failed, patients may require surgery. Surgical options include lateral sphincterotomy which permanently divides the internal sphincter, fissurectomy and advancement procedures. Lateral sphincterotomy has a reported cure rate of approximately 85%; however, the downside of this procedure is a significant incontinence problem, with up to 30% of patients having difficulty in controlling wind and at least 3–5% of people suffering from overt incontinence (Garcia-Aguilar et al 1996). If a sphincterotomy is performed, it should be limited to the length of the fissure as this almost certainly reduces the incontinence problems. If the patient has a short anus or has had previous anorectal surgery, extreme caution is advised.
Faecal incontinence
Faecal incontinence is a debilitating symptom or sign which occurs in 1–10% of adults. It is largely a hidden problem due to embarrassment, and may be a result of many contributing factors. Recent NICE guidelines recognized that the treatment of this problem should be patient centred, and will vary considerably between patients according to their lifestyle and culture (National Institute for Health and Clinical Excellence 2007b). One of the recommendations is that people who are reported to have faecal incontinence should be offered care which is managed by appropriate healthcare professionals, and these people should have relevant skills, training and experience. They should also work within an integrated continence service. Groups who are at very high risk of faecal incontinence include frail, older people; people with loose stools from any cause; women following childbirth, especially after a third- or fourth-degree tear; and patients with pelvic organ prolapse or rectal prolapse. Other groups include patients with cognitive impairment or learning difficulties, and those who have undergone pelvic radiotherapy. It is essential to be aware that faecal incontinence is a symptom, not a disease, and there are many contributing factors; there is not usually a primary diagnosis.
Further treatment
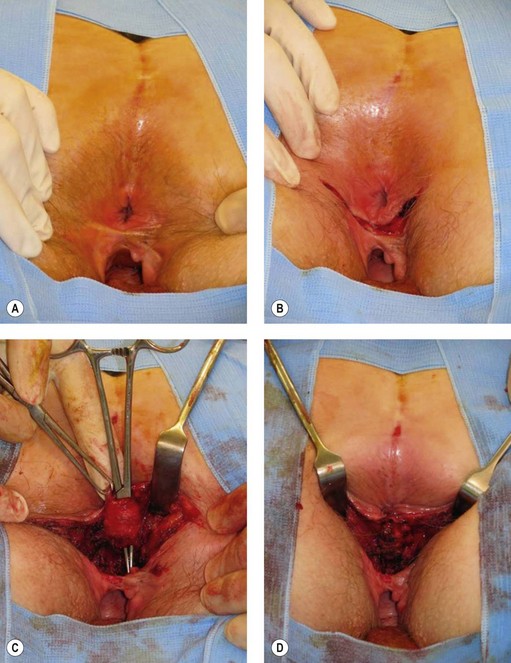
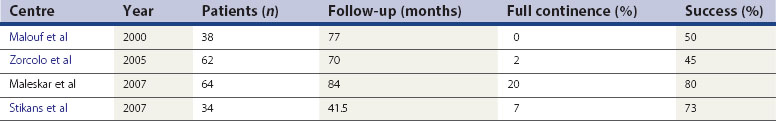
All patients with faecal incontinence who are being considered for surgery should be referred to a specialist colorectal surgeon, who can offer the surgical and non-surgical options and discuss the realistic and likely outcome of any surgical intervention. Patients with a full-length external anal sphincter defect (which can be assessed on three-dimensional ultrasound) which is 90° or greater may be considered for an overlapping sphincter repair. This is done through a transperineal incision (Figure 59.4), and good long-term results at 5 years of 70% improvement should be expected. However, results deteriorate with time (Table 59.2). Patients with a Cleveland Clinic Incontinence Score below 9 should not be offered this as there is also a 15–20% risk that these patients will remain the same or be made worse. If patients have an incomplete internal sphincter defect, pudendal neuropathy, loose stools or irritable bowel, these are likely to decrease the effectiveness of a sphincter repair.
Sacral nerve stimulation
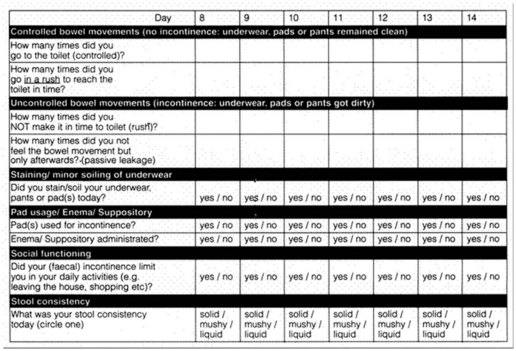
Sacral nerve stimulators have revolutionized the treatment of anal incontinence, and probably work through stimulation of both the external sphincter and the pelvic floor. Patients with significant anal incontinence are asked to keep a 2-week diary (Figure 59.5) about their episodes of incontinence. They have a test or trial of a percutaneous nerve stimulator, placed through S3 (or S2 or S4), and are asked to rediary their bowel movements during the 2 weeks of stimulation.
Results suggest that 70–80% of incontinent patients (urge and passive) will benefit from sacral nerve stimulation. If the trial is successful, these patients should be offered a permanent sacral nerve stimulator. This process involves implantation of a battery connected to a tyned wire which is inserted through the sacral foramen under X-ray guidance. The wire is tunnelled back to the battery, which is placed in the buttock or, occasionally, on the abdominal wall. Between 90% and 95% of patients who have at least a 50% improvement in symptoms with a temporary wire have a benefit with the permanent sacral nerve stimulator. There is a risk of infection, lead displacement and non-functioning of the permanent wire. NICE guidelines (National Institute for Health and Clinical Excellence 2004) looked at the results in 266 patients who had a sacral nerve stimulator; complete continence was achieved in 41–75%, and 75–100% of patients experienced a decrease of 50% or more in the number of incontinence episodes. As well as the incontinence, there is an ability to defer defaecation, which was absent previously, and an improvement in general quality of life. Adverse events in the fitting of the permanent implant are low (13%), mostly involving infection (three out of 149), lead migration (seven out of 149) and pain (six out of 149) (National Institute for Health and Clinical Excellence 2004).
Graciloplasty and artificial bowel sphincter
In 2006, NICE looked at a systematic review of 37 studies of graciloplasty and found that 42–85% of patients are continent after the procedure (National Institute for Health and Clinical Excellence 2006). One case series reported a successful outcome in 72% of patients at 5-year follow-up. One of the most common complications of this procedure is wound infection (overall rate of 28%, with 15% requiring hospitalization). Electrical or technical problems also occur in 48% of patients; one of the side-effects of the procedure is problems with evacuation resulting in obstructive defaecation. The procedure is recommended but only if performed in specialist units and in carefully selected patients in whom other treatments have failed.
Artificial anal sphincters were adapted from approved urinary sphincters. They have been associated with high levels of infection and mechanical failure. Recently, in an attempt to avoid these septic complications, Finlay et al (2004) developed a transabdominal artificial bowel sphincter with a control balloon reservoir in the abdominal space. An inflatable cuff keeps the anal canal closed, and the patient presses a control pump in the abdominal wall which deflates the cuff in order to evacuate the bowel. Repressing the button closes the sphincter again by allowing the fluid to return into the cuff. In a series of 12 patients with follow-up of 59 months, 75% of patients had a functioning implant and the continence scores were improved from 16 to 3 after surgery. Three out of 12 patients had to have the device removed because of complications, and it is recommended that this procedure should only be performed under strict audit and clinical governance conditions and in units with specialist interest in faecal incontinence (National Institute for Health and Clinical Excellence 2008a). However, it may be a promising route for patients with severe faecal incontinence.
Injectable bulking agents
Few long-term data are available for the use of bulking agents in passive leakage or incontinence. Injections of a variety of bulking agents (collagen, carbon beads and silicon particles) have been used. They are usually injected submucosally in three or four places, just above the dentate line (where the anal canal is less sensate and therefore less painful), in order to try and reproduce the anal cushions and stop passive leakage. The largest case series has shown that 82 patients had significant continence score improvement in 6–12 months (Tjandra et al 2004). The largest case series showed that the most common complications are pain, minor ulceration and, occasionally, infection, as well as leakage of the substance from the anus. NICE guidance suggests that the evidence is not adequate for bulking agents to be used without special arrangements for consent, audit and research, and these procedures should only be performed in units specializing in faecal incontinence that have the ability to audit these results and inform patients of the potential lack of evidence (National Institute for Health and Clinical Excellence 2007c).
Rectal prolapse surgery for full-thickness rectal prolapse
A full-thickness rectal prolapse is an extremely distressing and uncomfortable condition. It occurs most frequently in elderly ladies, and the quality of life of these patients can be severely compromised. Occasionally, there are complications such as gangrene and perforation, but the majority of patients have symptoms of a heavy feeling, mucus leakage, bleeding and recurrent prolapse through the anus. This may be associated with other pelvic floor prolapses such as uterine prolapse. In younger age groups, full-thickness rectal prolapse may be associated with previous eating disorders, and although the cause is not known, it is suspected that this may be due to a problem of collagen formation. The only possible curative treatment for a full-thickness rectal prolapse is surgery, but if a patient is relatively asymptomatic, surgery can be avoided and patients can have their constipation treated with a stool softener. Surgical interventions are based on either ‘chopping off the prolapse’ or ‘hitching it up’. When hitching it up (rectopexy), a resection of the sigmoid may also be undertaken if there is associated constipation. A recent Cochrane analysis (Tou et al 2008) looked at a series of five different types of intervention, with the outcome measures being morbidity, length of stay and mortality. Unfortunately, the number of studies available was extremely small, and most of the randomized controlled trials included few patients. Twelve studies were included in this work with a total of 380 participants. Patients who are fit should be offered a laparoscopic or open rectopexy; these are associated with lower recurrence than a perineal procedure. Frail and unfit patients should have a perineal procedure (i.e. Altemeier operation or Delorme’s procedure), the results of which suggest a recurrence rate of 25–40%. However, the morbidity and mortality associated with perineal procedures is low, and since many of these patients are over 80 years of age, this may make the recurrence rates relatively acceptable. If any form of prolapse is associated with a gynaecological prolapse, a combined procedure should be undertaken.
Altemeier operation
The design of this operation is to resect any prolapsing or redundant bowel and to attempt to recreate the pelvic floor by plicating the levators and reconnecting the sigmoid colon back to the anus with a coloanal anastomosis. However, there is no fixation of the rectum back up to the sacrum. The recurrence rate is approximately 16%, in comparison with 5% or less for an abdominal procedure (Tou et al 2008).
Delorme’s procedure
The alternative perineal procedure is the Delorme’s procedure, where the mucosa is resected off the bowel muscle tube. The bowel wall is then plicated and the mucosa of the anal canal is reanastomosed to the musosa high up within the anal canal. It is associated with a relatively high recurrence rate (up to 37%) (Tou et al 2008).
Chronic constipation
Rome criteria for constipation
Constipation is defined as the presence of two or more of the following symptoms:
Constipation occurs in up to 30% of the population (Garrigues et al 2004), and females have a higher incidence than males. Physical exercise and a high fibre diet are protective against constipation. Constipation can be divided into slow transit constipation, obstructive defaecation syndrome and irritable bowel syndrome (IBS).
Simple causes of constipation include a low fibre diet, dementia, depression and eating disorders. There are metabolic causes such as hypothyroidism, hypokalaemia, hypercalcaemia and diabetes mellitus (McCallum et al 2009). Neurological problems can include multiple sclerosis and Parkinson’s disease, and there are a variety of drug-related causes for constipation. Patients with painful anorectal conditions such as fissures, haemorrhoids, fistulas etc. may develop constipation secondary to their painful anus. The majority of cases of constipation can be treated with basic dietary advice. Patients should sit on the toilet with their feet raised so that they sit in a semi-squatting position; this opens up the anorectal junction and allows them to defaecate more effectively. The use of abdominal massage and exercises which can be provided through a specialist nurse practitioner can help with the process of defaecation. Patients should ensure that they are well hydrated and take adequate amounts of fluid as well as exercise. Fibre may be beneficial, although patients with constipation related to IBS may find that their abdominal pain is increased with their intake of fibre. Preferred laxatives include movicol and magnesium hydroxide which do not rely on gut stimulation. However, stimulant laxatives are sometimes of use in patients who have poor colonic motility, especially secondary to opiates (McCallum et al 2009).
Slow transit constipation
Slow transit constipation is often idiopathic or may be associated with a neuropathy or recent or previous pelvic surgery. Patients with a megacolon associated with slow transit constipation and a normal functioning rectum may do well with a subtotal colectomy and ileorectal anastomosis; satisfaction rates of up to 90% have been reported (Lubowski et al 1996). Segmental resection appears to be unsatisfactory, and those patients who have an associated megarectum may be better treated with a ileoanal pouch (Gladman et al 2005). More recently, there have been some promising results using sacral nerve stimulators in patients with slow transit and combined causes of constipation. This seems to work by retrograde stimulation of the parasympathetic and sympathetic nerve chain.
Irritable bowel syndrome
At least two of the following should also be associated:
Patients do not need investigation with invasive tests, but should have a full blood count, erythrocyte sedimentation rate, C-reactive protein (CRP) and antibody testing for coeliac disease. Patients should only be referred for secondary care if they have associated symptoms of unintentional or unexplained weight loss, associated rectal bleeding, a family history of any bowel or ovarian cancer, or are over 60 years of age with a change in bowel habit lasting more than 6 weeks with looser and/or more frequent stools. Additional ‘red flags’ are anaemia, abdominal masses, rectal masses or raised CRP (National Institute for Health and Clinical Excellence 2008b).
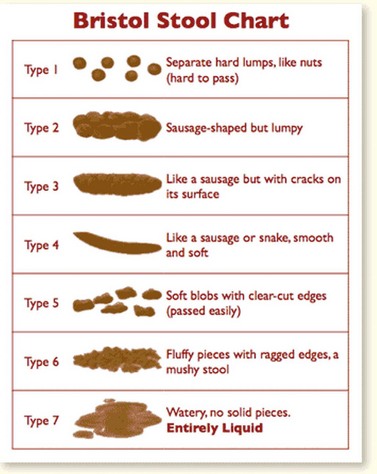
Patients should be treated for their IBS symptoms by self-help; this means looking at lifestyle, physical activity, diet and symptom-targeted medications. An essential part of treatment of IBS is assessing diet and nutrition (this includes fibre intake, which should usually be reduced). Insoluble fibre, especially wheat fibre, seems to exacerbate patients’ symptoms by creating more wind; if more fibre is needed in order to treat constipation-related IBS, patients should eat foods high in soluble fibre (e.g. oat fibre). Other general dietary advice includes taking small meals regularly, eating meals slowly, avoiding missing meals and drinking at least eight cups of fluid per day, especially non-caffeinated drinks or water. Caffeine intake should be restricted, as should the intake of alcohol or fizzy drinks. Fresh fruit should be limited to three portions per day, and if IBS is associated with diarrhoea, artificial sweeteners should be avoided, as should diabetic insulin products. Other management can be by pharmacological intervention, and the decision regarding which drugs should be used should be tiered to the predominant symptom. If pain is a problem, patients should be offered antispasmodic agents. For patients with constipation, laxatives should be given but fybogel and lactulose should be avoided because of their wind and bloating aspects. If patients have diarrhoea-associated IBS, loperamide is the first-choice antimotility agent, and patients should be advised to adjust the dosage of laxative or antimotility agent to aim for a well-formed stool (type 4 on Bristol stool chart; Figure 59.6) (National Institute for Health and Clinical Excellence 2008b).
Second-line treatment, tricylic antidepressants, may be used for pain relief, starting with an extremely low dose (5–10 mg) of amitryptyline, taken once at night. A selective serotonin reuptake inhibitor may be used if a tricylic antidepressant is ineffective. Patients should be warned that side-effects of dry mouth and drowsiness may occur with these medications. Patients should not be referred to hospital or followed-up except on an annual basis unless symptoms change. NICE has published a full report of treatment options (National Institute for Health and Clinical Excellence 2008b).
Summary
KEY POINTS
Cataldo P, Ellis CN, Gregorcyk S, et al. Practice parameters for the management of hemorrhoids (revised). Diseases of the Colon and Rectum. 2005;48:189-194.
Cotterill N, Norton C, Avery KNL, Abrams P, Donovan JL. A patient-centred approach to developing a comprehensive symptom and quality of life assessment of anal incontinence. Diseases of the Colon and Rectum. 2008;51:82-87.
Cross KLR, Massey EJD, Fowler AL, Monson JRT. The management of anal fissure: ACPGBI position statement. Colorectal Disease. 2008;10(Suppl 3):1-7.
Finlay IG, Richardson W, Hajivassiliou CA. Outcome after implantation of a novel prosthetic anal sphincter in humans. British Journal of Surgery. 2004;91:1485-1492.
Garcia-Aguilar J, Belmonte C, Wong WD, Lowry AC, Madoff RD. Open vs. closed sphincterotomy for chronic anal fissure: long term results. Diseases of the Colon and Rectum. 1996;39:440-443.
Garrigues V, Gálvez C, Ortiz V, Ponce M, Nos P, Ponce J. Prevalence of constipation: agreement among several criteria and evaluation of the diagnostic accuracy of qualifying symptoms and self-reported definition in a population-based survey in Spain. American Journal of Epidemiology. 2004;159:520-526.
Gladman MA, Scott SM, Lunniss PJ, Williams NS. Systematic review of surgical options for idiopathic megarectum and megacolon. Annals of Surgery. 2005;241:562-574.
Goligher JC. Cryosurgery for haemorrhoids. Diseases of the Colon and Rectum. 1976;19(3):213-218.
Jensen SL. Maintenance therapy with unprocessed bran in the prevention of acute anal fissure recurrence. Journal of the Royal Society of Medicine. 1987;80:296-298.
Jones OM, Moore JA, Brading AF, Mortenesen NJ. Botulinum toxin injection inhibits myogenic tone and sympathetic nerve function in the porcine internal anal sphincter. Colorectal Disease. 2003;5:552-557.
Kourakalis G, Andromanakos N. Evaluating patients with anorectal incontinence. Surgery Today. 2004;34:304-312.
Laurberg S, Swash M, Henry MM. Delayed external sphincter repair for obstetric tear. British Journal of Surgery. 1988;75:786-788.
Lubowski DZ, Chen FC, Kennedy ML, King DW. Results of colectomy for severe slow transit constipation. Diseases of the Colon and Rectum. 1996;39:23-29.
Lunniss PJ, Mann CV. Classification of internal haemorrhoids: a discussion paper. Colorectal Disease. 2004;6:226-232.
MacRae HM, McLeod RS. Comparison of haemorrhoidal treatment modalities. A meta-analysis. Diseases of the Colon and Rectum. 1995;38:687-694.
Malouf AJ, Norton CS, Engel AF, Nicholls RJ, Kamm MA. Long-term results of overlapping anterior anal-sphincter repair for obstetric trauma. The Lancet. 2000;355:260-265.
Maslekar S, Gardiner AB, Duthie GS. Anterior anal sphincter repair for fecal incontinence: good long term results are possible. Journal of the American College of Surgeons. 2007;204:40-46.
McCallum IJD, Ong S, Mercer-Jones M. Chronic constipation in adults. BMJ (Clinical Research Ed.). 2009;338:763-766.
Mirnezami A, Pilkington S, Monga A, Nugent KP. Multidisciplinary team meetings for pelvic floor disorders. Colorectal Disease. 2008;10:413.
National Institute for Health and Clinical Excellence. Sacral Nerve Stimulation for Faecal Incontinence. IPG99. London: NICE; 2004.
National Institute for Health and Clinical Excellence. Stimulated Graciloplasty for Faecal Incontinence. IPG159. London: NICE; 2006.
National Institute for Health and Clinical Excellence. Stapled Haemorrhoidopexy for the Treatment of Haemorrhoids. TA128. London: NICE; 2007.
National Institute for Health and Clinical Excellence. Faecal Incontinence: the Management of Faecal Incontinence in Adults. CG49. London: NICE; 2007.
National Institute for Health and Clinical Excellence. Injectable Bulking Agents for Faecal Incontinence. IPG210. London: NICE; 2007.
National Institute for Health and Clinical Excellence. Transabdominal Artificial Bowel Sphincter Implantation for Faecal Incontinence. IPG276. London: NICE; 2008.
National Institute for Health and Clinical Excellence. Irritable Bowel Syndrome in Adults: Diagnosis and Management of Irritable Bowel Syndrome in Primary Care. CG61. London: NICE; 2008.
Pilkington SA, James A, Monga A, Dewbury K, Nugent K. Comparing the diagnostic findings of dynamic MRI proctography and evacuating barium proctography for the assessment of patients with obstructive defaecation. Colorectal Disease. 2009;11:662.
Stikans C, Pilkinton SA, Nugent. Secondary repair of obstetric anal sphincter injury (OASIS). Colorectal Disease. 2009;11:663.
Thompson WHF. The nature of haemorrhoids. British Journal of Surgery. 1975;62:542-552.
Tjandra JJ, Lim JF, Hiscock R, Rajendra P. Injectable silicone biomaterial for fecal incontinence caused by internal anal sphincter dysfunction is effective. Diseases of the Colon and Rectum. 2004;47:2138-2146.
Tou S, Brown SR, Malik AJ, Nelson RL. Surgery for complete rectal prolapse in adults. Cochrane Database of Systematic Reviews Issue 4; 2008.
Vaizey CJ, Carapeti E, Cahill JA, Kamm MA. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44:77-80.
Zorcolo L, Covotta L, Bartolo DC. Outcome of anterior sphincter repair for obstetric injury: comparison of early and late results. Diseases of the Colon and Rectum. 2005;48:524-531.