Chapter 108
Lower Extremity Arterial Disease
General Considerations
Hasan H. Dosluoglu
Based on a chapter in the seventh edition by John V. White
Chronic lower extremity ischemia due to peripheral arterial disease (PAD) is the most common cause of walking disability seen by vascular specialists. The manifestations of chronic lower extremity ischemia often include pain (Table 108-1) produced by varying degrees of ischemia, ranging from no or atypical leg symptoms to typical exertional muscular pain (intermittent claudication, IC) to ischemic rest pain. Patients may have more than one cause for their extremity pain, making diagnosis and management more difficult (see Chapter 14). The challenge for the vascular specialist is to recognize the presence of lower extremity ischemia, quantify the extent of local and systemic disease, determine the degree of functional impairment related to PAD, identify and control modifiable risk factors, and establish a comprehensive treatment program.
Classification
Claudication
The typical patient with IC experiences calf symptoms ranging from fatigue to aching while walking. Pain or discomfort may also occur in the thigh or buttock. The pain sensation results from ischemic neuropathy involving small unmyelinated A delta and C sensory fibers and a local intramuscular acidosis from anaerobic metabolism enhanced by the release of substance P.1 The symptoms of intermittent claudication are alleviated by a brief period of rest, after which the patient can resume walking. Initially, the symptoms do not occur with regularity; they occur intermittently when walking, and the distance walked before symptoms are noticed is generally similar on different outings. As the process progresses, symptoms occur more frequently and after shorter distances.
Asymptomatic patients with a reduced ankle-brachial index (ABI) but no symptoms may have significant impairment of leg function when tested objectively. Among 460 patients with PAD, 91 had no symptoms; of these, 28 were less active and appeared to control their symptoms through a reduction in walking speed and distance, whereas 63 remained active, walking more than 6 blocks a week.2 When subjected to a 6-minute walking test, however, the 63 active patients performed in a manner similar to claudicants, walking slightly farther but with a slower maximal velocity. Thus some patients may be asymptomatic because of their poor medical condition and functional capacity. McDermott et al3 compared 72 asymptomatic patients with PAD to those with claudication (n = 215) and 292 with no PAD. They found that asymptomatic subjects with PAD had worse functional performance, worse quality of life, and more adverse calf muscle characteristics compared with persons with IC, as well as with the sedentary, asymptomatic, age-matched group of non-PAD persons. This underscores the impact of PAD even in asymptomatic patients who limit their activity to control symptoms or because of other medical illness.
Disease Location
Claudication often results from a single level of arterial disease, such as the iliac artery or the superficial femoral artery, but can result from multilevel disease. Collateral vessels can reconstitute the artery distal to a single site of stenosis or occlusion and provide distal flow. Symptoms of claudication associated with PAD usually manifest in the muscle groups below the hemodynamically significant lesion. Three major patterns of arterial obstruction are possible: inflow disease, outflow disease, and a combination of the two. Inflow disease refers to lesions in the suprainguinal vessels, most commonly the infrarenal aorta and iliac arteries. Occlusive lesions of the infrarenal aorta or iliac arteries commonly lead to buttock and thigh claudication. In men, if the stenoses or occlusions are bilateral and are proximal to the origins of the internal iliac arteries, vasculogenic erectile dysfunction may be present as well (see Chapter 82).
Although buttock and thigh claudication may be the first symptoms, with continued ambulation, these patients may exhibit classic symptoms of intermittent calf claudication resulting from inadequate perfusion of the entire leg while walking. Outflow disease consists of occlusive lesions in the lower extremity arterial tree below the inguinal ligament, from the common femoral artery to the pedal vessels. Superficial femoral artery stenosis or occlusion is the most common lesion associated with intermittent claudication, which leads to calf discomfort with ambulation and relief with rest. No specific thigh or foot symptoms are associated with superficial femoral artery occlusion. Because the deep femoral artery provides collateral circulation to and reconstitution of the popliteal artery, isolated superficial femoral artery occlusion without distal disease is rarely the cause of more advanced forms of ischemia. Popliteal and tibial artery occlusions are more commonly associated with limb-threatening ischemia, owing to the paucity of collateral vascular pathways beyond these lesions. As isolated lesions, they are usually not the cause of IC and become clinically significant in patients with tissue loss. They are typically seen in older adults and in patients with diabetes and end-stage renal disease. Long-term corticosteroid therapy has also been reported to be associated with a distally accentuated, calcifying peripheral atherosclerosis, inducing arterial incompressibility comparable to patients with renal failure or diabetes.4 Patients with a combination of inflow and outflow disease may have widespread symptoms of IC affecting the buttock, hip, thigh, and calf. These symptoms frequently begin in the buttock and thigh and then involve the calf muscles with continued ambulation; however, they may appear in reverse order if the distal disease is more severe than the inflow disease. Severe combined inflow-outflow disease may result in limb-threatening ischemia.
In a review of 400 patients with PAD who underwent a first digital subtraction arteriogram of the lower limbs, Aboyans et al5 found that proximal PAD was associated with greater prevalence of male sex and smoking, whereas more distal PAD was associated with older-age, diabetes, hypertension, and renal failure (P <.05). They found that proximal PAD was associated with a worse prognosis, after adjustments for age, sex, cardiovascular disease, critical leg ischemia, and treatments, but these results need to be confirmed in a more general population of patients with PAD.
Nonatherosclerotic Causes of Claudication
Intermittent claudication in younger individuals may be caused by popliteal artery entrapment syndrome or adventitial cystic disease of the popliteal artery (see Chapter 115), chronic compartment syndrome (see Chapter 163), or kinking or endofibrosis of the iliac arteries. The pain of popliteal entrapment, produced by extrinsic compression of the popliteal artery by the gastrocnemius muscle during leg movement, is similar to that of IC and has the same pathophysiologic mechanism as that associated with PAD.6 Popliteal adventitial cystic disease produces similar symptoms. Chronic compartment syndrome causes exercise-related discomfort only in the anterolateral aspect of the calf. The cellular basis for the anterior compartment muscular pain associated with chronic compartment syndrome is ischemia resulting from diminution of the muscular arteriovenous pressure differential owing to venous congestion and compartment tissue hypertension.7 Iliac artery endofibrosis with kinking is characterized by thickening of vessel intima due to subendothelial accumulation of loose connective tissue containing variable amounts of collagen, elastin, and smooth muscle cells, resulting in progressive stenosis and impaired flow, and has been most commonly described in competitive cyclists. It causes mostly unilateral pain, cramping, or numbness, which may become apparent only at maximal exercise.8 Nonatherosclerotic causes of IC are listed in Box 108-1.
Critical Limb Ischemia
Critical limb ischemia (CLI) is the most severe form of PAD and represents approximately 1% of the total number of patients with PAD.9 The natural history of CLI differs significantly from that of claudication. CLI is associated with a higher risk of limb loss in the absence of revascularization, whereas claudication rarely progresses to the point of requiring amputation. In patients with CLI, the arterioles become maximally vasodilatated and insensitive to vasodilatory stimuli as a result of the chronic exposure to vasorelaxing factors. These dilatated peripheral arterioles have decreased wall thickness and cross-sectional area, leading to edema, which is aggravated by keeping the limb dependent. Chronic ischemia also results in changes in structure and function of endothelial cells, and coupled with platelet activation, leukocyte adhesion result in microthrombi formation in the capillaries. All these changes result in impaired tissue oxygen exchange at the capillary level.10,11
The common major manifestations of CLI are rest pain and ischemic ulceration or gangrene of the forefoot or toes, representing a reduction in distal tissue perfusion below resting metabolic requirements. Rest pain is usually described as a burning sensation or as an uncomfortable coldness or paresthesia of sufficient intensity to interfere with sleep. The ischemic neuropathy in CLI may also cause numbness, and since many patients also have diabetes, it may be difficult to determine how much of the neuropathic changes are caused by ischemia alone.12 The discomfort is worsened by leg elevation, because of the loss of the gravitational pull of blood to the foot; it is relieved by placing the limb in a dependent position, such as dangling it off the side of the bed. In patients with typical ischemic rest pain localized to the forefoot, occurring with elevation and relieved by dependency, the clinical diagnosis is objectively confirmed by hemodynamic measurements such as systolic ankle pressure less than 50 mm Hg, toe pressure less than 30 mm Hg, or ABI less than 0.40. It is important to note that patients with diabetic foot ulcers may have inadequate blood flow for healing even with perfusion levels that exceed these criteria for CLI. In fact, the term CLI was never intended to be applied to patients with diabetes and foot wounds.13
Ischemic ulcers usually represent the effect of repetitive soft tissue trauma, often very mild in degree, with erosion of the overlying skin. Skin repair is hampered by inadequate tissue perfusion, oxygenation, and cellular replication. Arterial ulceration in a nondiabetic patient is characterized by a shallow, nonhealing, pallid erosion of the skin in the distal foot—in a distribution similar to that of rest pain. The pain of such ulcerations, described as aching or burning, is often unremitting and severe and is occasionally refractory to even high-dose oral narcotic analgesics. It is the result of not only chronic, severe ischemic neuropathy but also actual exposure of the sensory nerves in the skin at the site of the ulcer. Diabetic foot ulcerations are broadly divided into ischemic, neuroischemic, and neuropathic ulcers. In recent studies, more than 50% of diabetic foot ulcers are of ischemic or neuroischemic origin.14,15 Therefore ischemia needs to be excluded in all ulcers using objective assessment, since PAD is the most important limiting factor for healing of ischemic or neuroischemic diabetic foot ulcers. In some patients, arterial perfusion may only be decreased to a specific region of the foot (angiosome, see Chapter 116), which may require increasing the flow to that specific angiosome to expedite ulcer healing.16
Ischemic gangrene occurs when resting limb blood flow is insufficient to maintain cellular viability. Tissue death inexorably extends to the junction of threshold blood flow for tissue viability. Initially, the pain may be severe, resulting from not only ischemic neuropathy but also ischemic injury of the skin and subcutaneous sensory nerves, osteomyelitis, and ascending infection. As the course of ischemic necrosis progresses, pain may actually decrease as a result of complete ischemic death of the nerves and other pain-producing tissues. Progression to gangrene occurs in 40% of patients with DM, compared with only 9% in nondiabetic patients with CLI.17
Limb-threatening ischemia usually requires the presence of severe PAD at two or more levels, the additive effects of which severely limit flow through collateral beds and result in profound distal ischemia. The pattern of arterial obstruction often affects sequential vascular beds, such as femoropopliteal and infrapopliteal arteries, but it may affect parallel beds, such as superficial femoral and deep femoral vessels. Both patterns prevent collateralization and reconstitution of the more distal arterial tree. In patients with diabetes, arterial occlusive disease primarily affects the crural and pedal arteries.18
Epidemiology
The prevalence of PAD has been the subject of numerous investigations over the past several decades19–22 The best method of assessing the prevalence of chronic lower extremity arterial occlusive disease is to record the ABI and correlate it with risk factors.
Prognostic Value of Ankle Brachial Index
ABI results are recommended to be reported with noncompressible values defined as greater than 1.40, normal values 1.00 to 1.40, borderline 0.90 to 0.99, and abnormal 0.90 or less.23 The ABI correlates well with the mortality risk associated with PAD, regardless of whether leg symptoms are present. Feringa et al performed a longitudinal study of 3209 subjects followed for 8 years after recording baseline resting and postexercise ABIs. In this study, lower resting ABI values, lower postexercise ABI values, and a greater drop in resting ABI were associated with a higher incidence of death.24 In a cohort of 6880 unselected subjects ≥65 years old who were monitored for over 5 years in the German Epidemiological Trial on Ankle Brachial Index Study Group,25 836 had asymptomatic PAD (ABI <0.9) and 593 had symptomatic PAD. The composite endpoint of all cause death, myocardial infarction or stroke was similar in symptomatic and asymptomatic patients with PAD, both of which carried significantly higher risk than subjects without PAD. Because 21% of subjects had symptomatic or asymptomatic PAD, the ACCF/AHA 2011 writing group recommended ABI diagnostic screening for patients ≥65 years or for patients ≥50 with a history of smoking or diabetes.23
Prevalence of PAD
Prevalence Based on ABI
In the United States, a comprehensive effort to establish the prevalence of PAD using ABI was undertaken in the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2000,26 involving 9000 individuals 40 years of age or older. ABIs and a complete data set were available for analysis in 2174 participants. The overall prevalence of PAD (defined as an ABI <0.90) was 4.3% (95% confidence interval [CI], 3.1% to 5.5%). Although prevalence was slightly higher in men than in women, the prevalence dramatically increased with age, rising from 0.9% in those younger than 50 years to 14.5% in those 70 years or older (Fig. 108-1). Statistically significant associations between PAD and the common risk factors of hypertension, diabetes, hypercholesterolemia, and smoking were also noted.
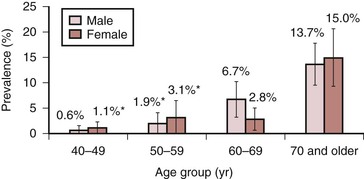
Figure 108-1 Prevalence of peripheral arterial disease by age and gender in adults 40 years and older, United States, 1999-2000 (n = 2174). (Redrawn from Selvin E, et al: Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation 110:738-743, 2004.)
Prevalence Based on Demographics
The relationships of PAD to age, gender, race, and ethnicity have been confirmed by several studies. In the AHA writing group’s meta-analysis,27 the prevalence of PAD was noted to increase with age for both men and women. Using the U.S. census data from 2010, they found that the number of females with PAD was higher among U.S. adults ≥40 years of age. Data adapted from the ABI Collaboration Study, including 480,325 person-years of follow-up of 24,955 men and 23,339 women from the general population who had ABIs measured at baseline and during follow-up, showed that although the ABI correlated with total and cardiovascular mortality rates, which were similar in women compared with men, the risks of morbidity and mortality were increased in women with ABI values either <0.90 or ≥1.40.28
In a primary care setting, 403 patients stratified by race and gender were evaluated with ABIs to determine the prevalence of PAD.29 Study subjects included white, black, and Hispanic women and men. No gender differences were noted, but as with the NHANES data, black women had a significantly greater prevalence of PAD than did white or Hispanic women. A follow-up study using NHANES data from 1999 to 2004 reevaluated the prevalence of PAD in the general population and in ethnic subpopulations.30 Overall, non-Hispanic black men and women (19.2% prevalence) and Mexican American women (19.3% prevalence) had a higher prevalence of PAD than did non-Hispanic white men and women (15.6% prevalence). These studies clearly demonstrate that there is a high prevalence of lower extremity PAD in the United States, affecting an estimated 8 to 12 million people. Further, PAD is now reported to be associated with equal morbidity and mortality and comparable, or possibly higher, cost compared with coronary heart disease and stroke.27,31 The prevalence is higher in some ethnic subpopulations and in those with uncontrolled risk factors, including hypertension, smoking, hypercholesterolemia, diabetes, and renal failure, although in the German Epidemiological Trial,25 48% of patients with asymptomatic PAD were reported to have never smoked, 66% did not have diabetes, and 15% to 16% did not have hypertension or hyperlipidemia. Because of PAD’s high prevalence and substantial mortality risk, even in the absence of symptoms, it is essential to identify and treat patients with PAD. Adding reduced ABI to traditional risk factors increases the sensitivity of the identification of patients with moderate to high risk of cardiovascular mortality.
Prevalence Based on Risk Factors
Hypertension increases the risk of developing symptoms of IC 2.5-fold in men and 3.9-fold in women,32,33 and is present in 55% of patients with PAD.34 The relationship between diabetes and IC has also been well documented.32,33,35 PAD prevalence is 20% to 30% higher in diabetics than in the general population,36 and the risk of developing PAD correlates with the severity and duration of diabetes.37 Patients with diabetes are more likely to have symptomatic PAD, with a 3.5-fold increased risk in men and an 8.6-fold increased risk in women.38 Metabolic syndrome is estimated to be present in at least 25% of the population.39 This syndrome is defined as having three or more of the following: blood pressure elevation (≥130 mm Hg/≥85 mm Hg), triglyceride count ≥150 mg/dL, high-density lipoprotein count ≤50 mg/dL for women or ≤40 mg/dL for men, fasting blood glucose ≥110 mg/dL, and abdominal obesity (BMI ≥30 kg/m2 or waist circumference ≥102 cm in men, ≥88 cm in women). An analysis of data from three National Health and Nutrition Examination Surveys (NHANES, 1999-2004) involving 5376 asymptomatic participants 40 years and older showed that 38% of the population with PAD also had metabolic syndrome, and the prevalence of PAD (ABI <0.9) was 7.7% and 3.3%, respectively in those with and without metabolic syndrome.40
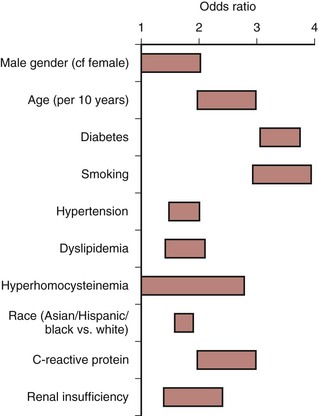
Cigarette smoking is a long-established stimulus for atherosclerosis and increases the risk that PAD will develop in men and women.32,35 A lifetime smoking history exceeding 25 pack years has been reported to be associated with increased risk of PAD (HR 2.72) compared with those who never smoked41; the risk is higher in women than in men, and smoking cessation is associated with substantial risk reduction for development of PAD.42 The severity of arterial occlusive disease is proportional to the number of cigarettes smoked,43 and each additional risk factor independently increases the risk of developing symptomatic PAD (Fig. 108-2).
Natural History
Asymptomatic Disease
Patients with asymptomatic PAD may eventually develop symptoms of claudication or may demonstrate little progression of their disease. The Edinburgh Artery Study found that patients with asymptomatic PAD had no statistically significant drop in ABI over the 5 years of observation.44 Regardless of whether symptoms are present, individuals with PAD, identified by an ABI less than 0.90, have higher morbidity and mortality than age-matched controls with normal ABIs. The risks are inversely related to the amount of physical activity the patient undertakes each day. Evaluating the natural history of 460 patients with ABI-proven PAD, investigators noted that reduced physical activity correlated with increased mortality and cardiovascular events.45 Therefore patients who attempt to control or eliminate their lower extremity PAD symptoms by reducing their walking actually worsen their risk of myocardial infarction (MI), stroke, and death. This asymptomatic group of patients with PAD should be managed medically in the same way as those with symptoms of IC.
Impact of Female Gender
Women with PAD were reported to experience faster functional decline than men with PAD. McDermott et al46 assessed 380 men and women with PAD using a 6-minute walk test and assessment of mobility disability at baseline and yearly for up to 4 years, and used CT to assess calf muscle characteristics biannually. They found that at 47 months of follow-up, women with PAD were more likely to become unable to walk for 6 minutes continuously, had a higher incidence of mobility loss, and had faster declines in walking capacity compared with men. The more rapid deterioration in women with PAD was attributed to the poorer functional performance and smaller baseline calf muscle mass, resulting in women being closer at baseline to the thresholds for immobility.
Impact on Future Health
The presence of PAD in asymptomatic patients was also found to be a significant risk factor for future disability. In the Cardiovascular Health Study of 4705 participants 65 years of age and older who had ABI measured between 1992 and 1993, lower baseline ABI values were found to be associated with increased risk of late-incident mobility disability and activities of daily living disability during a 6-year follow-up.47 Most recently, Leeper et al assessed 725 PAD patients using a customized symptom-limited ramp treadmill protocol between 1997 and 2011 and found that exercise capacity was the strongest independent predictor of death, with each additional MET achieved being associated with age-adjusted 18% and 20% reductions in all-cause and cardiovascular mortality, respectively (P <.001 for both), surpassing all classical risk factors and all measured exercise tests.48
Intermittent Claudication
Impact on Extremity
The natural history of IC is marked by slow progression to shorter walking distances, but it rarely reaches the level of CLI. Only about one fourth of patients with IC ever deteriorate significantly, and deterioration is most frequent during the first year after diagnosis (6% to 9%) compared with 2% to 3% per annum thereafter.49 This is especially true if risk factors are controlled. Of 224 nondiabetic patients with IC followed for 6 years, only 8% of those who stopped smoking progressed to rest pain, whereas 79% of those who continued to smoke developed signs of CLI.50 Similarly, in a long-term study of 1244 claudicants, only insulin-requiring diabetes, low initial ABI, and high pack-years of smoking predicted progression to ischemic rest pain and ischemic ulceration.51 The risk of major amputation is less than 5% over a 5-year period.44
Quality of Life
Reduced independent mobility and the discomfort imposed by IC profoundly impact a patient’s quality of life. The Short Form (36) Health Survey (SF-36), a generic quality-of-life instrument that includes eight domains to assess physical and emotional function, has been used extensively to document the effect of claudication on quality of life.52 In a study of 68 claudicants, scores in all eight domains were reduced compared with nonclaudicants, especially physical function and role limitations due to emotional impact.53 These findings were extended in a community-based study of 53 patients with documented IC and 327 controls without claudication.54 Using the Rose Intermittent Claudication Questionnaire and the SF-36, the investigators noted reductions in physical function, role limitations due to physical dysfunction, role limitations due to emotional dysfunction, and changes in bodily pain, energy, and general health perception in patients with IC. Only social function and mental health appeared to be unaffected. The adverse impact of IC appears to be directly related to walking ability. Limitations on ambulation give rise to broad physical and emotional effects, as documented in a study of 80 claudicants evaluated with the Walking Impairment Questionnaire, SF-36, ABI, and 6-minute walking test.55 The results of the 6-minute walking test correlated well with quality-of-life scores. Patients with shorter walking distances during the walking test had worse scores in the physical function and role limitations due to physical dysfunction.
Association with Systemic Atherosclerosis
The presence of PAD as documented by an ABI less than 0.90 is also a strong marker for the presence of coronary artery disease (CAD) and cerebrovascular disease (CVD). In the PAD Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) study, which assessed 6979 patients aged 70 years or older or aged 50 to 69 years with diabetes or a history of smoking, symptomatic CAD or CVD was identified in 16% of study subjects with an ABI less than 0.90.56 In the Reduction of Atherothrombosis for Continued Health (REACH) Registry,57 which included an international, prospective cohort of 68,236 patients with either established atherosclerotic arterial disease (CAD, PAD, CVD; n = 55,814) or at least three risk factors for atherothrombosis (n = 12,422), the overall cardiovascular death, MI, or stroke rates in 1 year were 4.5% for patients with CAD, 6.5% for patients with CVD, and 5.4% for patients with PAD. The 3-year MI/stroke/vascular death rates in the 32,247 patients in this registry were significantly higher for patients with symptomatic disease when compared with those with risk factors only (12% vs. 6%, P <0.001).58 In another study, the fate of 2777 male claudicants was documented over a 15-year period, and mortality rates of 42% and 65% at 5 and 10 years, respectively, were noted.59 MI accounted for 66% of the deaths among the 1363 claudicants who died during the study period. The risk of cardiac or cerebrovascular disease increases with lower ABI values, as confirmed by the Atherosclerotic Risk in Communities Study.60
Thus the natural histories of asymptomatic PAD and IC are similar and marked by a significantly elevated risk of fatal cardiac and cerebrovascular events, despite the rather small risk of progression to CLI.
Critical Limb Ischemia
Impact on Extremity
The natural history of CLI is grim; approximately 40% of affected individuals lose their legs and 20% die within 6 months of onset. However, an increasing number of patients with CLI receive some form of active treatment, with over half receiving revascularization, and the amputation rate may be decreasing. An estimation of the primary treatment of CLI patients and their status a year later is shown in Fig. 108-3. Meta-analyses of patients who had popliteal-distal bypass or infrapopliteal angioplasty showed similar limb salvage rates of about 87% at 12 months and 82% at 3 years.61,62 A steady but slow decrease in amputation rates in the last decade has been reported, based on various U.S. national and state databases.63–65 Patients with CLI appear to have a more aggressive form of PAD, with involvement of several segments of the lower extremity arterial tree, especially infrapopliteal vessels. Not all patients with CLI progress through stages of worsening claudication before advancing to the severely ischemic level. In a prospective study on stump healing in 713 below-knee amputations, more than half the patients were noted to have no symptoms 6 months before presenting with CLI that required amputation.66 Because of this unpredictability of development of CLI, interruption of the disease process before the development of CLI is not always possible.
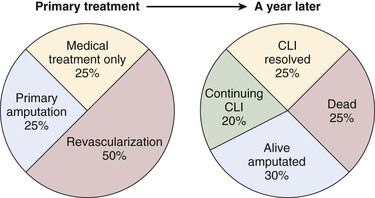
Figure 108-3 The estimate of the initial treatment and status a year later of patients presenting with chronic critical limb ischemia. (Redrawn from Norgren L, et al: TASC II Working Group, Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 45:S11, 2007.)
In several studies of patients with CLI due to unreconstructable arterial occlusive disease, the reported natural history is widely variable, with major amputation rates ranging from 14.3% to 46.4%.67–70 These variable outcomes likely reflect inconsistencies in the definition and application of the term CLI in the initial patient cohorts. The risk of major amputation appears to be inversely proportional to the ABI. In a prospective study of 142 patients harboring 169 severely ischemic limbs with ulceration who could not undergo revascularization, only 15% of patients with an ABI greater than 0.50 required major amputation, whereas 34% of those with an ABI less than 0.50 sustained major limb loss at the end of 1 year.71 This abysmal natural history of CLI propels most vascular specialists to attempt to recommend revascularization to reduce the risk of limb loss.
Quality of Life
The traditional methods of assessing outcomes and quality of care in patients with CLI such as survival and limb salvage is increasingly noted to be inadequate, and functional outcomes, such as maintenance of ambulatory status and independent living status, achievement of healed wound status, avoidance of repeat hospitalizations, and interventions, are proposed as more meaningful parameters in these patients. A disease-specific questionnaire for CLI has not been developed; however, the Vascular Quality of Life (VascuQol) Questionnaire, which was designed as a disease-specific tool for patients with PAD, is accepted to be applicable to patients with CLI.72 Using such questionnaires will enable a more comprehensive assessment and patient-oriented approach to patients presenting with CLI, rather than focusing only on amputation-free survival.
Association with Systemic Atherosclerosis
As would be expected given the systemic nature of atherosclerosis, severe PAD is often associated with advanced coronary artery and cerebrovascular disease. CAD has been estimated to be present in approximately half of patients with CLI.73,74 This strong association results in an exceedingly high mortality from MI and stroke among patients presenting with CLI, significantly higher than those with PAD alone. A review of major series reporting the fate of patients with CLI noted that 26% died within 1 year of diagnosis75 and had 5- and 10-year mortality of 50% and 70%, respectively.74,76,77 In patients with diabetes who are known to have a twofold increased cardiovascular mortality compared with nondiabetics, the development of diabetic foot ulcers is associated with even more significant increase in all-cause and cardiovascular mortality.78 In a meta-analysis of eight studies including 17,830 patients with 81,116 person-years of follow-up, diabetic foot ulcer was found to be associated with an increased risk of all-cause mortality, fatal myocardial infarction, and fatal stroke.79
Impact of Medical Treatment
Aggressive risk modification has not been adequately studied in patients with CLI. In the multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery (PREVENT III) in 1404 patients with CLI, only statin use was found to be associated with improved survival 1 year after revascularization, whereas beta blockers and antiplatelet medication use had no effect on survival.80 In a study of patients with diabetic foot ulcers, aggressive cardiovascular risk management resulted in a decrease of mortality from 48% to 27% following induction of a protocol involving risk factor screening, antiplatelet agent, a statin, an ACE inhibitor, and selective use of beta blockers.81 Given the preponderance of evidence showing benefit of medial management of atherosclerosis, it is logical to extend such treatment to patients with CLI.
Diagnosis
History and Physical Examination
Conducting a complete history and physical examination of patients with PAD is important, and focus on the legs, as well as systemic risk factors (see Chapter 14), is essential. Vasculogenic and neurogenic claudication must be differentiated, as must different causes for leg ulcers, and other nonvascular etiologies for leg symptoms). Many patients with PAD have been increasingly recognized as having either atypical leg symptoms (such as leg muscle symptoms that are present at rest and with exercise) or are insufficiently active to produce typical symptoms. A latent phase that is difficult to detect as part of a routine clinical history also seems to occur during the systemic atherosclerotic process. Women may be more likely than men to present with atypical leg symptoms, and they may be more likely to be assessed as asymptomatic. In the Walking and Leg Circulation Study (WALCS) cohort of 460 participants, atypical exertional leg symptoms were twice more likely to be reported by the 187 women as compared with the men.82
Risk Factor Assessment
Atherosclerosis is a pathologic process related to human aging. A stepwise increase in the incidence of IC occurs with each passing decade of age (Fig. 108-4). Many other risk factors seem to accelerate the development and growth of atherosclerotic lesions (Box 108-2). The classic risk factors, including hypertension, diabetes mellitus, hyperlipidemia, chronic renal insufficiency, and cigarette smoking, as well as other less frequently recognized factors, must be identified and defined. It is essential to control modifiable risk factors to slow the progression of atherosclerosis and enhance the benefits of any eventual vascular intervention.
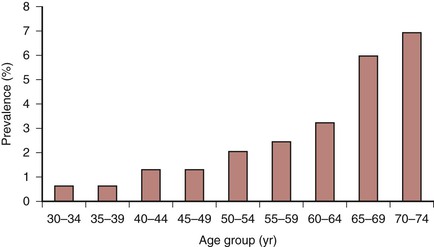
Figure 108-4 Weighted mean prevalence of intermittent claudication (symptomatic peripheral arterial disease) in large population-based studies. (Redrawn from Norgren L, et al: TASC II Working Group, Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 45:S7A, 2007.)
Suspicion of unrecognized and uncontrolled risk factors for accelerated atherosclerosis, such as hyperhomocysteinemia or hypercoagulability, should be raised in cases characterize by the absence of commonly recognized risk factors, a sudden symptom onset (especially in younger individuals), or a more rapidly progressive form of PAD. McCully examined the autopsy results of 194 consecutive patients and correlated the extent of atherosclerosis with serum cholesterol and other risk factors.83 The mean serum cholesterol of patients who died of complications from arterial occlusive disease was not extremely high (187 mg/dL [4.84 mmol/L]); 65% had a total serum cholesterol level less than 200 mg/dL (5.18 mmol/L), and 92% had a total serum cholesterol level less than 250 mg/dL (6.48 mmol/L). In 66% of patients with severe systemic atherosclerosis, elevated serum cholesterol, hypertension, and diabetes were absent. This study strongly supports the effort to actively search for other, less common risk factors.
Hyperhomocysteinemia
An elevated homocysteine level, which is often not measured during routine health assessments, may increase the patient’s likelihood of developing PAD nearly sevenfold.84 A meta-analysis of more than 3000 patients in 14 cross-sectional and prospective studies showed that patients with PAD had homocysteine levels that were 4.31 mmol/L (ranging from 0.70 to 10.46) higher than those of controls without PAD.85 Unfortunately, folate supplementation has not been found to benefit patients with elevated homocysteine levels in any randomized study, and it was found to be detrimental in subgroups with higher (>12 mmol/L) levels.86
Hypercoagulable States
Hypercoagulable states are more common in patients who require vascular reconstruction for the treatment of lower extremity arterial occlusive disease.87 In a cross-sectional study of 181 claudicants, 110 CLI patients and 210 controls, Sartori et al88 found that fibrinogen was higher in patients with CLI compared with those with claudication and controls; homocysteine and FVIII were higher in patients with PAD than in controls, but were similar in patients with CLI and claudication; the prevalence of lupus anticoagulant increased in patients with CLI compared with those with claudication and controls; and the prevalence of FII 20210A allele was higher in patients with CLI compared with those with claudication and controls. These data suggest that the presence of two or more thrombophilic risk factors raise the likelihood of PAD being more severe, justifying the need for larger longitudinal studies. Although our understanding of these additional risk factors has increased in the last decade, the question as to whether controlling these factors will ultimately improve outcomes is still unresolved.
Diagnostic Studies
Hematologic Studies
At initial presentation, a patient with manifestations of PAD should undergo a battery of basic hematologic studies to characterize risk factors and identify end-organ involvement (Box 108-3). The hemoglobin and hematocrit levels yield potential information about blood hemorheology and other forms of distal perfusion inhibitors, such as secondary polycythemia from cardiopulmonary disease. Elevated platelet counts may suggest the risk of thrombotic occlusions. A fasting blood glucose or hemoglobin A1c level is an important test for all patients who initially present with PAD because diabetes is such a significant risk factor for claudication and more advanced forms of ischemia. Increased serum creatinine levels may indicate the presence of intrinsic renal disease, especially in the presence of diabetes. Nutritional assessment by measuring serum albumin and prealbumin levels should be considered especially in patients with CLI.
Lipid Profile
A fasting lipid profile, consisting of total cholesterol, high-density lipoprotein, low-density lipoprotein, and triglyceride concentration, is an important part of patient screening and risk stratification (see Chapter 29). Lipid abnormalities may underlie the progression of atherosclerosis. Although the impact of elevated cholesterol or low-density lipoproteins on the course of atherosclerosis has thus far been more clearly defined in patients with coronary artery disease than in those with PAD, it is likely that lipids accelerate PAD as well.89 The impact of diabetes on the progression of atherosclerosis may be worsened in the setting of lipid abnormalities.90 Careful lipid control may reduce the risk of coronary, cerebral, and peripheral artery morbidity and mortality.91
C-Reactive Protein
An increasing body of evidence suggests that atherosclerosis is an inflammatory process with an elevation in inflammatory markers (see Chapter 26). Of the many markers, high-sensitivity C-reactive protein (CRP) is the leading biomarker for clinical application because of its relatively long half-life and stability, and it should be obtained to evaluate the patient’s inflammatory status. CRP has a strong correlation with a reduced ABI. CRP levels were evaluated in 370 patients with an ABI less than 0.90 and compared with levels in 231 patients with an ABI greater than 0.90.92 Levels of this inflammatory marker were associated with ABI in patients with cardiac and cerebrovascular disease. CRP was not associated with ABI in patients without arterial occlusive disease in these vascular beds (P = .026). In a prospective cohort of PAD patients undergoing lower extremity vein bypass surgery, the mean CRP was 12 mg/L, which was an independent predictor of 5-year all-cause mortality, even after controlling for lipid levels and other risk factors.93 CRP was shown to have a significantly inverse correlation over time and predicted progression of PAD over 12 years in the Edinburgh Artery Study.94 Thus this easily measured inflammatory marker serves as an indicator not only of worsening lower extremity arterial occlusive disease but also of increased risk of cardiac and cerebrovascular disease.
Hypercoagulable States
An evaluation for hypercoagulable states should be undertaken when such a condition is suspected clinically on the basis of prior thrombotic events or a familial history (see Chapter 38). Despite the plethora of tests available for the specific diagnosis of hypercoagulable states, the best screening test is a carefully performed patient history. Random thrombotic events without a specific cause should raise the suspicion of a clotting disorder. Hypercoagulable states can be identified in a significant proportion of patients with arterial occlusive disease.87 When such a condition is suspected, a broad range of testing may be required (Box 108-4).
Homocysteine
Patients who develop manifestations of PAD at an early age, without other identifiable risk factors, should have a plasma homocysteine level documented (see Chapter 26). High levels of homocysteine indicate hyperhomocysteinemia, which may accelerate atherosclerosis through a variety of mechanisms.95,96 High levels of this amino acid may be toxic to endothelial cells and reduce their ability to generate and release nitric oxide. Excessive concentrations of homocysteine also may promote medial smooth muscle cell proliferation and arterial wall inflammation and increased levels of plasminogen activator inhibitor. As a result, arterial wall atherosclerotic plaque formation may be increased and thromboresistance decreased. Patients with hyperhomocysteinemia may develop clinically apparent vascular disease and coronary artery occlusive disease at a young age in the absence of other risk factors.97
The relationship between increased levels of homocysteine and vascular disease in older patients is not as well defined. Taylor et al evaluated homocysteine levels in 214 patients with symptomatic arterial occlusive disease and tracked ABIs over time.98 They found a more rapid progression of occlusive disease in patients with elevated homocysteine levels, after correction for other variables.
Although other authors failed to identify a similar impact,100 because treatment of hyperhomocysteinemia with the oral administration of folate and other vitamins and nutrients is relatively simple, many vascular specialists believe that evaluation for this potential cause of accelerated atherogenesis should be undertaken.101
Cardiac and Cerebrovascular Evaluation
The systemic nature of atherosclerosis has a significant impact on all vascular beds to a greater or lesser extent. The presence of coronary artery and cerebrovascular disease must be assessed in all patients with a new onset of manifestations of PAD who have not undergone such studies.
Cardiac Disease
Patients undergoing peripheral vascular surgery are at high risk (>5% likelihood) of having a perioperative MI, and they frequently manifest more than one of the clinical predictors of MI, heart failure, or death (Box 108-5). The evaluation of patients for cardiac disease should be directed toward identifying the presence of disease and determining its severity (See Chapter 39). This evaluation can be done most effectively in a stepwise manner. The guidelines for patient assessment developed by the American Heart Association and the American College of Cardiology provide a framework for this aspect of patient care.102 Algorithms for the perioperative management of cardiovascular disease are based on clinical markers, functional capacity, and surgery-specific risk (Fig. 108-5). Resting left ventricular function alone is not a specific indicator of perioperative MI.103
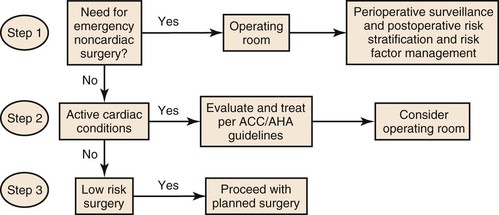
Figure 108-5 Cardiac evaluation for noncardiac surgery based on active clinical conditions, known cardiovascular disease, or cardiac risk factors in patients aged 50 years or older. For clinical risk factors, see Box 108-2. For active clinical conditions, see Box 108-5. ACC/AHA, American College of Cardiology/American Heart Association. (Redrawn from Fleisher LA, et al: Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [Writing Committee to revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery]. J Am Coll Cardiol 50:1707-1732, 2007.)
Cerebrovascular Disease
Patients with lower extremity ischemia also have an increased incidence of carotid artery stenosis. Araki et al104 conducted a cross-sectional study in 543 patients with PAD and 314 control subjects using CT scans and carotid duplex exams. The authors found the prevalences of carotid artery stenosis of ≥70% and ≥50% to be higher in patients with PAD than in controls (5.2% vs. 0.6%, 17.6% vs. 3.8%, respectively, P <0.01); they also found the incidence of cerebral infarcts and lacunar infarcts to be higher in patients with PAD than in controls (15.0% vs. 9.8%, 41.0% vs. 13.4%, respectively, P <0.05). Thus we recommend that presenting patients or patients with progressive PAD should undergo carotid artery duplex imaging, and conversely, patients with cerebrovascular disease should be screened for the presence of PAD.
Risk Stratification for Patients with Critical Limb Ischemia
Considering the high risk of early and late cardiovascular complications following revascularizations (especially open procedures), patients with CLI present a particularly challenging group to treat. In the multicenter randomized Bypass versus Angioplasty in Severe Ischemia of the Leg (BASIL) Trial, the patients who had bypass initially and were alive with an intact limb for more than 2 years lived longer than those who initially had angioplasty.105 In an effort to stratify these patients for risk of early and late mortality and amputation, as well as to identify those who are unlikely to survive to benefit from aggressive revascularization, a variety of scoring systems were developed and validated, including the following: Finland National Vascular (FINNVASC),106 Prevention of Infrainguinal Vein Graft Failure (PREVENT) III,107 and Bypass versus Angioplasty in Severe Ischemia of the Leg (BASIL) scoring systems.108 Patients were classified based on risk factors identified on multivariate analysis (Table 108-2). All three of these scoring systems were independently validated and can be used to predict amputation-free survival and to help in the decision making for planning treatment modality for patients with CLI.107,109,110
Table 108-2
The Variables, Output Methods, and Early and Late Outcomes for Low-, Medium-, and High-Risk Groups in Finland Vascular (FINNVASC); Prevention of Infrainguinal Vein Graft Failure (PREVENT) III (Modified); and Bypass Versus Angioplasty in Severe Ischemia of the Leg (BASIL) Scoring Systems
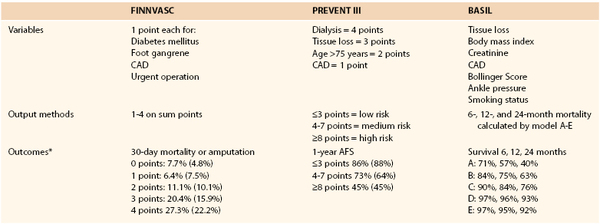
* Percentages in parentheses show validation data set.
AFS, Amputation-free survival; CAD, coronary artery disease.
Exclusion of Associated Aneurysms
A significant body of information supports screening patients with PAD for the presence of infrarenal abdominal aortic aneurysms. Barba et al111 performed abdominal ultrasound in 1166 consecutive patients with chronic limb ischemia and found abdominal aortic aneurysms (>3.0 cm) in 13%, which was more prevalent in men (13.6%) than in women (4.1, P = 0.02), and only 1.5% had abdominal aortic aneurysms >5 cm. The prevalence increased with age, being 5.4% in younger (<55) men, 14.8% in men between 65 and 74 years of age, and 17.1% in men and 10.3% in women over age 75. In a Swedish study of 5924 patients undergoing duplex imaging for the evaluation of stenoses and aortic aneurysms,112 the prevalence of aneurysms was 7.3% in men older than 60 years with occlusive disease of a major artery (carotid, renal, or lower extremity), compared with 4.0% in the absence of such stenoses. Although the aneurysms detected in each of these studies were generally below the threshold for intervention, the low risk of screening patients with clinically significant PAD seems to justify doing so, especially in men older than 60.
Vascular Laboratory and Imaging Studies
The decision to recommend surgical or percutaneous intervention for a patient with lower extremity arterial occlusive disease is based on many factors, including symptoms, degree of functional impairment, comorbid conditions, and location and severity of occlusive lesions. In addition, the anatomic pattern of the disease may have a significant impact on the type of procedure that can be used to improve distal perfusion. A clear understanding of the extent of PAD is required before a therapeutic plan can be established.
Vascular Laboratory
Disease Severity.
In most patients with lower extremity ischemia, the initial vascular laboratory measurement of segmental arterial pressure and the calculation of ABI are sufficient to identify the presence of arterial occlusive disease and localize the segment involved. Pressures and pressure gradients are not sufficient indicators of patency and occlusion because of the variable presence of calcium within the arterial walls of patients with PAD. A high or even supranormal ABI can be recorded in patients with severe calcific arterial occlusive disease, commonly seen in diabetics and dialysis-dependent patients. Pressures and indices must be correlated with pulse volume recording and Doppler waveform analysis. Toe pressures, transcutaneous oxygen pressure (tcPO2), and various skin perfusion pressure techniques have been proposed to assess global and regional foot perfusion to detect and quantify the presence and hemodynamic impact of arterial occlusive disease.113
For patients with palpable pulses but disproportionately disabling symptoms or those capable of undergoing an exercise therapy program, exercise testing in the vascular laboratory can be helpful.114 Commonly, after the recording of ankle pressures at rest, a patient walks at 3.5 km/hr on a treadmill at a 12% incline until the onset of claudication-like symptoms. At that time, ankle pressures are measured again. A more than 20% decrease in ankle pressures for more than 3 minutes after the cessation of exercise indicates vascular claudication.115 No decrease or a small decrease in pressure after exercise suggests a nonvascular cause of symptoms, even in the presence of decreased peripheral pulses. Other regimens measure the distance walked per unit time or maximal walking distance. Although each of these methods has proponents, perhaps the most important factor is the use of a consistent methodology to follow patients. The combination of ankle pressure and exercise response can also be used to classify the patient’s degree of ischemia (Table 108-3).
Table 108-3
Stages of Chronic Limb Ischemia
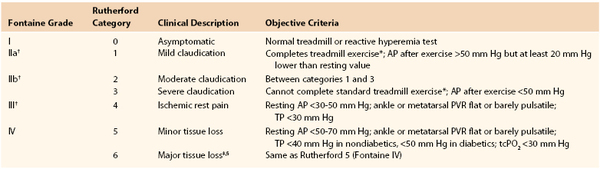
* Five minutes at 2 miles per hour on a 12% incline.
† Grades II and III correspond to critical limb ischemia.
‡ Nonhealing ulcer or focal gangrene with diffuse pedal ischemia.
§ Extending above transmetatarsal level, or foot no longer salvageable.
AP, Ankle pressure; PVR, pulse volume recording; tcPO2, transcutaneous oxygen; TP, toe pressure.
From Rutherford RB, et al: Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 26(3):517-538, 1997; and Norgren L, et al: TASC II Working Group, Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 45:S34, 2007.
Disease Location.
Identifying the anatomic locations of hemodynamically significant lesions is crucial in planning intervention in either the claudicant or, especially, the patient with CLI. Currently, color-guided duplex imaging, gadolinium-enhanced magnetic resonance imaging, computed tomographic angiography, and intra-arterial subtraction angiography) are the most frequently used imaging modalities for the delineation of arterial anatomy. These modalities are discussed in detail in their respective chapters (see Chapters 16, 19, 22, and 23).
Imaging Modality Selection
Patients with borderline renal function, especially those with diabetes, present a special challenge to the vascular specialist. Because of the risks of renal failure associated with iodinated contrast agents116 and nephrogenic systemic fibrosis induced by gadolinium,117 the decision to proceed to advanced imaging of lower extremity inflow and outflow vessels for the planning of intervention can be problematic. Selective catheterization and angiogram using diluted contrast at the popliteal artery or even infrapopliteal arteries allow excellent imaging even of the pedal arteries using minimal iodinated contrast volumes. Improvements in image processing have also renewed the interest in carbon dioxide angiography, which has no adverse effect on renal function, although it may be difficult to clear in patients with severe chronic obstructive pulmonary disease.118 However, small supplemental doses of iodinated contrast averaging between 10 and 40 mL may be needed to better define the arterial anatomy in certain situations.119
The optimal choice of arterial imaging studies depends on the type of anticipated intervention. Visser et al performed a Markov analysis to determine the best testing strategies for the evaluation of claudicants.120 Using test sensitivity, incidence and type of complications associated with the test, implications of a missed lesion, and the cost of overtreatment based on test results, the authors evaluated the cost-effectiveness of duplex imaging, MRA, and digital subtraction conventional angiography. They found that if treatment considerations were limited to angioplasty in patients suspected of having suitable lesions, MRA was more cost-effective than conventional angiography. Likewise, digital subtraction angiography proved superior to duplex ultrasound and MRA if surgery was anticipated. Although the difference in overall cost of these diagnostic modalities was small (<$1800 lifetime costs), the results of the study indicate that the pretreatment evaluation of claudicants is generally simple, and the need for multiple imaging studies is uncommon.
A comprehensive systematic review comparing duplex ultrasound, MRA, and computed tomography for the diagnosis of lower extremity ischemia concluded that contrast-enhanced MRA has better overall accuracy than the other imaging modalities when evaluating the entire arterial segment from abdominal aorta to foot.121 As noted by Visser et al, this systematic review also confirmed that when a single arterial segment of the leg above or below the knee is to be evaluated, two-dimensional time-of-flight MRI is the most cost-effective study.
Although the vascular specialist has the ability to visualize specific portions of the arterial tree with increasing detail and ease, diagnostic tests beyond the standard vascular laboratory assessment should be reserved for patients in whom a percutaneous or open intervention is planned.
Treatment
The decision of when and how to treat IC or CLI can be difficult (see Chapter 109). Nonetheless, in view of the high risk associated with systemic atherosclerosis, all patients should attempt to control cardiovascular risk factors and implement risk-reduction strategies to decrease the risk of MI and stroke. However, based on a recent analysis of data from the National Health and Nutrition Examination Survey (NHANES) between 1999 to 2004, statin use was reported only in 30%, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use in 25%, and aspirin use in only 36% of patients aged ≥40 with PAD, corresponding to 5.0 million adults with PAD not taking statins, 5.4 million not taking ACEI/ARB, and 4.5 million not taking aspirin. The use of multiple preventive therapies was associated with 65% lower all-cause mortality in patients with PAD without known cardiovascular disease.122
Antiplatelet Therapy
The mainstay of cardiovascular risk reduction is antiplatelet therapy (see Chapter 35). Numerous studies have demonstrated that aspirin in doses ranging from 75 to 325 mg/day significantly lowers the risk of MI and stroke in patients with symptomatic PAD123,124; however, the benefit of aspirin in asymptomatic PAD patients is less clear.125–127 Clopidogrel is a suitable alternative to aspirin for risk reduction in patients with symptomatic PAD. In very-high-risk patients who are not considered at increased risk of bleeding, a combination of aspirin and clopidogrel may be beneficial.23 A statistically significant benefit, documented by a reduction in MI, stroke, or death, was noted in patients with symptomatic lower extremity ischemia treated with aspirin and clopidogrel compared with those who received aspirin and placebo.128
Smoking Cessation
Smoking cessation is also critical for reducing atherosclerotic risk and is central to the medical management of patients with PAD. Smoking is associated with progression of atherosclerosis, an increased incidence of death due to coronary artery disease, and accelerated graft failure after lower extremity revascularization.129,130 Smoking cessation reduces death from coronary heart disease, lower extremity interventions, and amputation rates in both men and women (see Chapter 27).129–131
Treatment of Hyperlipidemia
The treatment of hyperlipidemia with a statin to achieve a low-density lipoprotein level less than 100 mg/dL (2.59 mmol/L) is recommended for all patients with PAD (<70 mg/dL in those who are at very high risk of ischemic events) to reduce the risk of MI. This recent recommendation from the Adult Treatment Panel III of the National Cholesterol Education Program is based on the fact that patients with lower extremity ischemia are at high or very high risk of cardiac events (see Chapter 29).132
Treatment of Hypertension
Control of hypertension to achieve a systolic blood pressure less than 140 mm Hg and a diastolic pressure less than 90 mm Hg (less than 130/80 mm Hg in those with diabetes or renal insufficiency) should be implemented (see Chapter 30). TASC II guidelines consider ACEI and thiazide diuretics first-line therapy for patients with PAD to reduce the risk of cardiovascular events.74 β-Adrenergic blockers are also an excellent class of drugs for this purpose, especially in those with concomitant coronary artery disease due to their cardioprotective effects. Although there has been theoretical concern that a reduction in systolic pressure might worsen symptoms of lower extremity ischemia, this does not appear to occur. A meta-analysis of six major studies addressing this issue concluded that beta blockade does not reduce walking distance or worsen the pain of IC133
Treatment of Diabetes and Other Risk Factors
Careful management of diabetes is also essential to reduce the likelihood of adverse cardiovascular events and the progression of PAD (see Chapter 28). Other risk factors, such as dietary indiscretion and inactivity, should be identified and addressed. Each patient with chronic lower extremity ischemia must have a comprehensive treatment plan for the control of risk factors as soon as the diagnosis of PAD is established. Once this is done, the patient will be better prepared for any subsequent intervention that might be required for limb salvage and improvement in walking ability and quality of life.
Selected Key References
Diehm C, Allenberg JR, Pittrow D, Mahn M, Tepohl G, Haberl RL, Darius H, Burghaus I, Trampisch HJ. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. [German Epidemiological Trial on Ankle Brachial Index Study Group] Circulation. 2009;120:2053–2061.
Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JFACC/AHA. 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. J Am Coll Cardiol. 2007;50:1707–1732.
Garg P, Tian L, Criqui MH, Ferrucci L, Guralnik JM, Tan J, McDermott MM. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation. 2006;114:242–248.
Hirsch AT, Allison MA, Gomes AS, Corriere Ma, Duval S, Ershow AG, Hiatt WR, Karas RH, Lovell MB, McDermott MM, Mendes DM, Nussmeier NA, Treat-Jacobson D. A call to action: women and peripheral artery disease: a scientific statement from the American Heart Association. [American Heart Association Council on Peripheral Vascular Disease; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology; Council on Epidemiology and Prevention] Circulation. 2012;125:1449–1472.
Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ, White J, White RA, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654.
Hooi JD, Stoffers HE, Kester AD, Rinkens PE, Kaiser V, van Ree JW, Snotterus JA. Risk factors and cardiovascular diseases associated with asymptomatic peripheral arterial disease. The Limburgh PAOD study. Scand J Prom Health Care. 1998;16:177–182.
Leeper NJ, Myers J, Zhou M, Nead KT, Syed A, Kojima Y, Caceres RD, Cooke JP. Exercise capacity is the strongest predictor of mortality in patients with peripheral arterial disease. J Vasc Surg. 2013;57:728–733.
Weatherley BD, Nelson JJ, Heiss G, Chambless LE, Sharrett AR, Nieto FJ, Folsom AR, Rosamond WD. The association of the ankle-brachial index with incident coronary heart disease: the Atherosclerotic Risk in Communities (ARIC) study, 1987-2001. BMC Cardiovasc Disord. 2007;7:3.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Sluka KA. Pain mechanisms involved in musculoskeletal disorders. J Orthop Sports Phys Ther. 1996;24:240–254.
2. McDermott MM, et al. Leg symptoms in peripheral arterial disease. JAMA. 2001;286:1599–1606.
3. McDermott MM, et al. Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation. 2008;117:2484–2491.
4. Willenberg T, et al. Impact of long-term corticosteroid therapy on the distribution pattern of lower limb atherosclerosis. Eur J Vasc Endovasc Surg. 2010;39:441.
5. Aboyans V, et al. The general prognosis of patients with peripheral arterial disease differs according to the disease localization. J Am Coll Cardiol. 2010;55:898–903.
6. Levien LJ. Popliteal artery entrapment syndrome. Semin Vasc Surg. 2003;16:223–231.
7. Turnipseed WD. Diagnosis and management of chronic compartment syndrome. Surgery. 2002;132:613–617.
8. Peach G, et al. Endofibrosis and kinking of the iliac arteries in athletes: a systematic review. Eur J Vasc Endovasc Surg. 2012;43:208–217.
9. Dormandy JA, et al. The TransAtlantic Inter-Society Consensus on the management of peripheral arterial disease. J Vasc Surg. 2000;31:S1–S296.
10. Coats P, et al. Marriage of resistance and conduit arteries breeds critical limb ischemia. Am J Physiol Heart Circ Physiol. 2005;288:H1044–H1050.
11. Tang GL, et al. The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia. J Vasc Surg. 2005;41:312–320.
12. Weinberg DH, et al. Chronic ischemic monomelic neuropathy from critical limb ischemia. Neurology. 2001;57:1008–1012.
13. Jamieson C. The definition of critical ischaemia of a limb. Br J Surg. 1982;69(Suppl):S1.
14. Prompers L, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50:18–25.
15. Jeffcoate WJ, et al. Assessing the outcome of the management of diabetic foot ulcers using ulcer-related and person related measures. Diabetes Care. 2006;29:1784–1787.
16. Söderström M, et al. Angiosome-targeted infrapopliteal endovascular revascularization for treatment of diabetic foot ulcers. J Vasc Surg. 2012;57:427–435.
17. Kannel WB. Risk factors for atherosclerotic cardiovascular outcomes in different arterial territories. J Cardiovasc Risk. 1994;1:333–339.
18. Graziani L, et al. Vascular involvement in diabetic subjects with ischemic foot ulcer:a new morphologic categorization of disease severity. Eur J Vasc Endovasc Surg. 2007;33:453–460.
19. Kannel WB, et al. Intermittent claudication: incidence in the Framingham Study. Circulation. 1970;41:875–883.
20. Criqui MH, et al. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71:510–515.
21. Fowkes FG, et al. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20:384–392.
22. Hirsch AT, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): A Collaborative Report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease)—Summary of Recommendations. J Vasc Interv Radiol. 2006;17:1383–1397.
23. Rooke TW, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society for Vascular Medicine, and Society for Vascular Surgery. [American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society for Vascular Medicine; Society for Vascular Surgery] J Vasc Surg. 2011;54:e32–e58.
24. Feringa HH, et al. The long-term prognostic value of resting and postexercise ankle-brachial index. Arch Intern Med. 2006;166:529–535.
25. Diehm C, et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. [German Epidemiological Trial on Ankle Brachial Index Study Group] Circulation. 2009;120:2053–2061.
26. Selvin E, et al. Prevalence of and risk factors for peripheral arterial disease in the Unites States: Results from the National Health and Nutrition Examination Survey 1999-2000. Circulation. 2004;110:738–743.
27. Hirsch AT, et al. A call to action: women and peripheral artery disease: a scientific statement from the American Heart Association. [American Heart Association Council on Peripheral Vascular Disease; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology; Council on Epidemiology and Prevention] Circulation. 2012;125:1449–1472.
28. Ankle Brachial Index Collaboration, Fowkes FG, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208.
29. Collins TC, et al. Gender and peripheral arterial disease. J Am Board Fam Med. 2006;19:132–140.
30. Ostchega Y, et al. Prevalence of peripheral arterial disease and risk factors in persons aged 60 and older: data from the National Health and Nutrition Examination Survey 1999-2004. J Am Geriatr Soc. 2007;55:583–589.
31. Mahoney EM, et al. One-year costs in patients with a history of or at risk for atherothrombosis in the United States. [REACH Registry Investigators] Circ Cardiovasc Qual Outcomes. 2008;1:38–45.
32. Kannel WB, et al. Update on some epidemiological features of intermittent claudication. J Am Geriatr Soc. 1985;33:13–18.
33. Fowkes GR, et al. Smoking, lipids, glucose intolerance, and blood pressure as risk factors for peripheral atherosclerosis compared with ischemic heart disease in the Edinburgh Artery Study. Am J Epidemiol. 1992;135:331–340.
34. Lane DA, et al. Treatment of hypertension in peripheral arterial disease. Cochrane Database Syst Rev. 2009 [CD003075] .
35. Murabito JM, et al. Intermittent claudication: a risk profile from the Framingham Heart Study. Circulation. 1997;96:44–49.
36. Marso SP, et al. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47:921–929.
37. Wattanakit K, et al. Risk factors for peripheral arterial disease incidence in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2005;180:389–397.
38. Kannel WB, et al. Update on some epidemiologic features of intermittent claudication: the Framingham Study. J Am Geriatr Soc. 1985;33:13–18.
39. Ford ES, et al. Prevalence of metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359.
40. Sumner AD, et al. The relationship of peripheral arterial disease and metabolic syndrome prevalence in asymptomatic US adults 40 years and older: results from the National Health and Nutrition Examination Survey (1999-2004). J Clin Hypertens (Greenwich). 2012;14:144–148.
41. Conen D, et al. Smoking, smoking cessation, [corrected] and risk for symptomatic peripheral artery disease in women: a cohort study. Ann Intern Med. 2011;154:719–726.
43. Powell JT, et al. Risk factors associated with the development of peripheral arterial disease in smokers: a case control study. Atherosclerosis. 1997;129:41–48.
44. Leng GC, et al. Incidence, natural history, and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1996;25:1172–1181.
45. Garg P, et al. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation. 2006;114:242–248.
46. McDermott MM, et al. Women with peripheral arterial disease experience faster functional decline than men with peripheral arterial disease. J Am Coll Cardiol. 2011;57:707–714.
47. Brach JS, et al. Incident physical disability in people with lower extremity peripheral arterial disease: the role of cardiovascular disease. [for the Cardiovascular Health Study Research Group] J Am Geriatr Soc. 2008;56:1037–1044.
48. Leeper NJ, et al. Exercise capacity is the strongest predictor of mortality in patients with peripheral arterial disease. J Vasc Surg. 2013;57:728–733.
49. Dormandy J, et al. The natural history of claudication: risk to life and limb. Semin Vasc Surg. 1999;12:123–137.
50. Jonason J, et al. Factors of prognostic importance for subsequent rest pain in patients with intermittent claudication. Acta Med Scand. 1985;218:27–33.
51. Aquino R, et al. Natural history of claudication: long-term serial follow-up study of 1244 claudicants. J Vasc Surg. 2001;34:962–970.
52. deVries M, et al. Comparison of generic and disease-specific questionnaires for the assessment of quality of life in patients with peripheral arterial disease. J Vasc Surg. 2005;41:261–268.
53. Bosch JL, et al. The relationship between descriptive and valuational quality-of-life measures in patients with intermittent claudication. Med Decis Making. 1996;16:217–225.
54. Dumville JC, et al. The health-related quality of life of people with peripheral arterial disease in the community: the Edinburgh Artery Study. Br J Gen Prac. 2004;54:826–831.
55. Izquierdo-Porrera AM, et al. Relationship between objective measures of peripheral arterial disease severity to self-reported quality of life in older patients with intermittent claudication. J Vasc Surg. 2005;41:625–630.
56. Hirsch AT, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324.
57. Steg PG, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. [REACH Registry Investigators] JAMA. 2007;297:1197–1206.
58. Alberts MJ, et al. Three-year follow-up and event rates in the international Reduction of Atherothrombosis for Continued Health Registry. [Reduction of Atherothrombosis for Continued Health Registry Investigators] Eur Heart J. 2009;30:2318–2326.
59. Muluk SC, et al. Outcome events in patients with claudication: a 15-year study in 2777 patients. J Vasc Surg. 2001;33:251–258.
60. Weatherley BD, et al. The association of the ankle-brachial index with incident coronary heart disease: the Atherosclerotic Risk in Communities (ARIC) study, 1987-2001. BMC Cardiovasc Disord. 2007;7:3.
61. Romiti M, et al. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg. 2008;47:975–981.
62. Albers M, et al. Meta-analysis of alternate autologous vein bypass grafts to infrapopliteal arteries. J Vasc Surg. 2005;42:449–455.
63. Hong MS, et al. Emerging national trends in the management and outcomes of lower extremity peripheral arterial disease. Ann Vasc Surg. 2011;25:44–54.
64. Egorova NN, et al. An analysis of the outcomes of a decade of experience with lower extremity revascularization including limb salvage, lengths of stay, and safety. J Vasc Surg. 2010;51:878–885.
65. Goodney PP, et al. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50:54–60.
66. Dormandy J, et al. Prospective-study of 713 below-knee amputations for ischemia and the effect of a prostacyclin analog on healing. Br J Surg. 1994;81:33–37.
67. Schuler JJ, et al. Efficacy of prostaglandin E1 in the treatment of lower extremity ulcers secondary to peripheral vascular occlusive disease: results of a prospective randomized, double-blind multi-center clinical trial. J Vasc Surg. 1984;1:160–170.
68. Telles GS, et al. Prostaglandin E1 in severe lower extremity ischaemia: a double-blind controlled trial. Br J Surg. 1984;71:506–508.
69. Lepantalo M, et al. Outcome of unreconstructed chronic critical leg ischaemia. Eur J Vasc Endovasc Surg. 1996;11:153–157.
70. Norgren L, et al. A stable prostacyclin analog (Iloprost) in the treatment of ischaemic ulcers of the lower limb: a Scandinavian-Polish placebo controlled randomized multi-centre study. Eur J Vasc Endovasc Surg. 1990;4:463–467.
71. Marston WA, et al. Natural history of limbs with arterial insufficiency and chronic ulceration treated without revascularization. J Vasc Surg. 2006;44:108–114.
72. Morgan MBF, et al. Developing the vascular quality of life questionnaire: a new disease-specific quality of life measure for use in lower limb ischemia. J Vasc Surg. 2001;33:679–687.
73. Feringa HH, et al. A prognostic risk index for long-term mortality in patients with peripheral arterial disease. Arch Intern Med. 2007;167:2482–2489.
74. Norgren L, et al. TASC II Working Group. Inter-Society Consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45:S5–S67.
75. Wolfe JN, et al. Critical and subcritical ischaemia. Eur J Vasc Endovasc Surg. 1997;13:578–582.
76. Nehler MR, et al. Is revascularization and limb salvage always the treatment for critical limb ischemia? J Cardiovasc Surg. 2004;45:177–184.
77. Watelet J, et al. Femoropopliteal bypass: in situ or reversed vein grafts? Ten-year results of a randomized prospective study. Ann Vasc Surg. 1997;11:510–519.
78. Preis SR, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009;119:1728–1735.
79. Brownrigg JR, et al. The association of ulceration of the foot with cardiovascular and all-cause mortality in patients with diabetes: a meta-analysis. Diabetologia. 2012;55:2906–2912.
80. Schanzer A, et al. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg. 2008;47:774–781.
81. Young MJ, et al. Improved survival of diabetic foot ulcer patients 1995-2008: possible impact of aggressive cardiovascular risk management. Diabetes Care. 2008;31:2143–2147.
82. McDermott MM, et al. Sex differences in peripheral arterial disease: leg symptoms and physical functioning. J Am Geriatr Soc. 2003;51:222–228.
83. McCully KS. Atherosclerosis, serum cholesterol and the homocysteine theory: a study of 194 consecutive autopsies. Am J Med Sci. 1990;299:217–221.
84. Boushey CJ, et al. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. JAMA. 1995;274:1049–1057.
85. Khandanpour N, et al. Homocysteine and peripheral arterial disease: systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2009;38:316–322.
86. Miller ER 3rd, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517–527.
87. Ray SA, et al. Hypercoagulable states in patients with leg ischemia. Br J Surg. 1994;81:811–814.
88. Sartori M, et al. Thrombophilic risk factors and peripheral arterial disease severity. Thromb Haemost. 2010;104:71–77.
89. Hirsch AT, et al. Undertreatment of dyslipidemia in peripheral arterial disease and other high-risk populations: an opportunity for cardiovascular disease reduction. Vasc Med. 2002;7:323–331.
91. Leng GC, et al. Lipid-lowering for lower limb atherosclerosis. Cochrane Database Syst Rev. 2000;(2).
92. McDermott MM, et al. Relation of levels of hemostatic factors and inflammatory markers to the ankle brachial index. Am J Cardiol. 2003;92:194–199.
93. Owens CD, et al. An integrated biochemical prediction model of all-cause mortality in patients undergoing lower extremity bypass surgery for advanced peripheral artery disease. J Vasc Surg. 2012;56:686–695.
94. Tzoulaki I, et al. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation. 2005;112:976–983.
95. Guilland JC, et al. Hyperhomocysteinemia: an independent risk factor or a simple marker of vascular disease? Pathol Biol. 2003;51:101–110.
96. Cook JW, et al. Homocysteine and arterial disease: experimental mechanisms. Vasc Pharmacol. 2002;38:293–300.
97. Assanelli D, et al. Premature arterial and venous events in three families: effects of folate levels and MTHFR mutation mediated by family/generation and homocysteine level. Thromb Res. 2002;105:109–115.
98. Taylor LM, et al. The association of elevated plasma homocyst(e)ine with progression of symptomatic peripheral arterial disease. J Vasc Surg. 1991;13:128–136.
99. Deleted in proofs.
100. Valentine RJ, et al. Lipoprotein (a), homocysteine, and hypercoagulable state in young men with premature peripheral atherosclerosis: a prospective, controlled analysis. J Vasc Surg. 1996;23:53–63.
101. Tofler GH, et al. Association between increased homocysteine levels and impaired fibrinolytic potential: potential mechanism for cardiovascular risk. Thromb Hemost. 2002;88:799–804.
102. Fleisher LA, et al. Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [Writing Committee to revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery]. J Am Coll Cardiol. 2007;50:1707–1732.
103. Pasternack PF, et al. The value of the radionuclide angiogram in the prediction of perioperative myocardial infarction in patients undergoing lower extremity revascularization procedures. Circulation. 1985;72:1113–1117.
104. Araki Y, et al. Prevalence and risk factors for cerebral infarction and carotid artery stenosis in peripheral arterial disease. Atherosclerosis. 2012;223:473–477.
105. Bradbury AW, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: a survival prediction model to facilitate clinical decision making. J Vasc Surg. 2010;51(5 Suppl):52S–68S.
106. Biancari F, et al. Risk-scoring method for prediction of 30-day postoperative outcome after infrainguinal surgical revascularization for critical lower-limb ischemia: a Finnvasc registry study. World J Surg. 2007;31:217–225.
107. Schanzer A, et al. Risk stratification in critical limb ischemia: derivation and validation of a model to predict amputation-free survival using multicenter surgical outcomes data. J Vasc Surg. 2008;48:1464–1471.
108. Bradbury AW, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: a survival prediction model to facilitate clinical decision making. J Vasc Surg. 2010;51(5 Suppl):52S–68S.
109. Moxey PW, et al. The BASIL survival prediction model in patients with peripheral arterial disease undergoing revascularization in a university hospital setting and comparison with the FINNVASC and modified PREVENT scores. J Vasc Surg. 2013;57:1–7.
110. Arvela E, et al. Finnvasc score and modified Prevent III score predict long-term outcome after infrainguinal surgical and endovascular revascularization for critical limb ischemia. J Vasc Surg. 2010;52:1218–1225.
111. Barba A, et al. Detection of abdominal aortic aneurysm in patients with peripheral artery disease. Eur J Vasc Endovasc Surg. 2005;30:504–508.
112. Alund M, et al. Selective screening for abdominal aortic aneurysm among patients referred to the vascular laboratory. Eur J Vasc Endovasc Surg. 2008;35:669–674.
113. Yamada T, et al. Clinical reliability and utility of skin perfusion pressure measurement in ischemic limbs—comparison with other noninvasive diagnostic methods. J Vasc Surg. 2008;47:318–323.
114. Gahtan V. The noninvasive laboratory. Surg Clin North Am. 1998;78:507–518.
115. Evaluation of claudication. Vascular Diagnosis. Elsevier, Inc.: Philadelphia; 2004.
116. Parfrey PS, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989;320:143–149.
117. Othersen JB, et al. Nephrogenic systemic fibrosis after exposure to gadolinium in patients with renal failure. Nephrol Dial Transplant. 2007;22:3179–3185.
118. Back MR, et al. Angiography with carbon dioxide (CO2). Surg Clin North Am. 1998;78:575–591.
119. Moos JM, et al. Safety of carbon dioxide digital subtraction angiography. Arch Surg. 2011;146:1428–1432.
120. Visser K, et al. Pretreatment imaging workup for patients with intermittent claudication: a cost-effectiveness analysis. J Vasc Interv Radiol. 2003;14:53–62.
121. Collins R, et al. Duplex ultrasonography, magnetic resonance angiography, and computerized tomography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ. 2007;334:1257.
122. Pande RL, et al. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011;124:17–23.
123. Antiplatelet Trialists. Collaborative review of randomized trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81–106.
124. Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86.
125. Fowkes FG, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303:841–848.
126. Belch J, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. [Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh] BMJ. 2008;337:a1840.
127. Critical Leg Ischaemia Prevention Study (CLIPS) Group, Catalano M, et al. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. J Intern Med. 2007;261:276–284.
128. Bhatt DL, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CARISMA trial. J Am Coll Cardiol. 2007;49:1982–1988.
129. Kabir Z, et al. Coronary heart disease deaths and decreased smoking prevalence in Massachusetts, 1993-2003. Am J Public Health. 2008;98:1468–1469.
130. Willigendael EM, et al. Smoking and the patency of lower extremity bypass grafts: a meta-analysis. J Vasc Surg. 2005;42:67–74.
131. Kenfield SA, et al. Smoking and smoking cessation in relation to mortality in women. JAMA. 2008;299:2037–2047.
132. Grundy SM, et al. Implications of recent clinical trials for the National Cholesterol Education Program. Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–732.
133. Radack K, et al. Beta-adrenergic blocker therapy does not worsen intermittent claudication in subjects with peripheral arterial disease. A meta-analysis of randomized controlled trials. Arch Intern Med. 1991;151:1705–1707.