Chapter 117
Lower Extremity Amputation
General Considerations
Christian Bianchi, Ahmed M. Abou-Zamzam Jr.,
Based on a chapter in the seventh edition by Wayne W. Zhang and Ahmed M. Abou-Zamzam, Jr.
Major lower extremity amputations continue to be part of all vascular practices, despite the general approach of aggressively attempting to salvage limbs. Though often viewed as a failure of treatment, major amputation should be considered reconstructive, when possible, and a definitive treatment option. The convergence of several important factors, including the increased life expectancy of the population and the epidemics of diabetes and peripheral arterial disease (PAD), suggests that amputations will remain an important issue facing patients and surgeons.
The goal of amputation is to remove all infected, gangrenous, and ischemic tissue and provide the patient with the longest functional limb. Avoidance of repeated amputations and provision of uncomplicated healing of operative sites are crucial for the patient’s optimal recovery and best functional rehabilitation or palliation.
Epidemiology
In the United States, approximately 60,000 major amputations (above the ankle) are performed annually.1–3 In 2003, 115,749 amputations of all types were performed in the United States, which included 55,574 major amputations.1 Diabetes and PAD remain the major risk factors for lower extremity amputation worldwide.4 Studies show that 25% to 90% of all amputations are associated with diabetes.2,4 Patients with diabetes have a 10-fold greater risk of amputation than those without diabetes.3 This association is multifactorial and relates to the presence of neuropathy and infection, as well as the markedly increased prevalence of PAD in this patient population.
Despite the increase in the prevalence of diabetes, current published data support an overall decrease in major amputations.5–8 A review of all Medicare claims from the Centers for Medicare and Medicaid Services between 1996 and 2006 showed a 29% decline.5 Results were not different if above-knee amputations were studied distinctly from below-knee amputations because both decreased in similar magnitude. A report from the National Hospital Discharge Survey and National Health Interview Survey on diabetes prevalence showed the age-adjusted nontraumatic lower extremity major amputation discharge rate per 1000 persons decreased from 11.2 in 1996 to 3.9 in 2008, while rates among persons without diabetes were unchanged.6 These trends were validated in a Scottish national cohort study from 2004 to 2008, where major amputation rates decreased by 40.7% from 1.87 per 1000 in 2004 to 1.11 per 1000 in 2008.8
Variation in Amputation Rates
There is significant regional variation in the performance of amputation around the world, which suggests that factors other than strictly medical issues may affect amputation rates.4–10 A study from the United Kingdom cited significant regional variation in amputation rates and stressed the need for consensus guidelines.9 The Global Lower Extremity Amputation Study Group reported that the highest amputation rate (for a first major amputation) was 44 per 100,000 population per year among Navajo men, and the lowest rate was 2.8 per 100,000 per year in Madrid, Spain.4 These disparities likely result from the influence of socioeconomic issues, the access to care, and the functionalities of health care systems. Physician experience also plays a key role in the selection of amputation as a treatment. In the treatment of critical limb ischemia, surgeon caseload and hospital volume have been shown to affect amputation rates, with low volumes being associated with higher amputation rates.11
A study of a Medicare claims database demonstrated that the number of vascular specialists influences the rate of amputation. A 0.30-fold increase in the number of vascular surgeons per 10,000 Medicare beneficiaries led to a 1.6% reduction in amputations.12 The distribution of vascular surgeons and interventional radiologists in the United States is strongly correlated not only with regional medical needs but also with local climate, education, crime, and transportation. This observation suggests that policies to increase the supply of vascular specialists in underserved areas may reduce regional disparities in amputation rates.12 Patient and health care provider education has also been demonstrated to reduce amputation rates.13 Prompt identification of patients at risk leads to more timely referral, treatment, and intervention.
Effect of Ethnicity and Economic and Social Status
The interaction between race or ethnicity and amputation rates is complex. In certain groups, such as the Native American Navajo population, amputation rates appear to be related to the high incidence of diabetes.4 However, blacks are more likely than whites to undergo amputation as opposed to revascularization, even when controlling for the presence of diabetes.14–16 These differences have been attributed to variations in access to health care, treatment of co-morbidities, and possible physician and patient factors. Insurance status also has an effect on amputation rates. Patients without insurance coverage have higher rates of amputation than those with access to health insurance.16 Data from the National Inpatient Sample documented that 34% of the 691,833 patients presenting with lower extremity ischemia from 1998 to 2002 underwent amputation. The primary amputation rates were significantly higher among people of color and patients with low income and lack of commercial insurance.17 Similar results showed that black patients were 1.7 times more likely to have both primary and repeat amputations than were white or other patients.18
Effect of Revascularization Rates
Trends in the interplay between revascularization and amputation rates are complicated. During a 10-year period, the Mayo Clinic reported a 50% reduction in amputation rates that corresponded to increased rates of lower extremity revascularization by both surgical and endovascular techniques.19 A Finnish study also demonstrated that an increase in revascularization rates correlated with a decrease in major amputation rates in elderly patients.20 A U.S. study of two national (Nationwide Inpatient Sample and National Hospital Discharge Survey) and four state databases demonstrated that both the number of lower extremity revascularizations and the number of major amputations have declined; this has been accompanied by a substantial increase in lower extremity endovascular interventions. From 1998 to 2003, the volume of major amputations decreased 16% regionally1 (New York, California, New Jersey, and Florida) and 25% nationally.5 However, the number of minor amputations increased 4% regionally and 3% nationally. It has been speculated that the improved limb salvage rates are partially explained by the increase in total revascularization procedures (propelled by endovascular interventions attributable to the perceived decrease in morbidity). Earlier endovascular interventions, in less critical lesions, and expanding endovascular procedures in the patient at high risk for open bypass may contribute to the decreased rates of major amputations. Also, failed endovascular procedures may not directly translate into amputations. Other possible factors contributing to lower rates of amputation include the greater awareness of PAD, the presence of clear guidelines for medical management of atherosclerosis, the implementation of risk factor modification, and the availability of improved methods of wound care.
High-risk patients treated with endovascular intervention have superior rates of limb salvage and maintenance of ambulation compared with patients undergoing primary amputation. However, these patients have no better functional benefit than those treated with primary amputation after 1 year.21 The medium-term results of the Bypass versus Angioplasty in Severe Ischemia of the Leg (BASIL) trial indicate that the outcomes of bypass surgery–first and balloon angioplasty–first strategies are similar in terms of amputation-free survival.22
The ideal balance of endovascular and open surgical reconstructions has yet to be determined for lower extremity ischemia. Nationwide Inpatient Sample data suggest that lower extremity revascularizations have reached a plateau of around 140,000; similarly, major amputation may have settled near 60,000 annually.1 The total number of revascularizations may be higher because same-day endovascular procedures might not be included in the Nationwide Inpatient Sample database. The Trans-Atlantic Inter-Society Consensus (TASC) II Working Group documented that the incidence of major amputations varies from 12 to 50 per 100,000 population per year.23 With the aging of the population, future trends in these areas will have a great effect on health care expenditures.
Indications for Amputation
Indications for amputation have traditionally been divided into acute ischemia, chronic ischemia, foot infection, severe traumatic injury, and lower extremity skeletal or soft tissue malignancies. Trauma and malignancy are beyond the scope of this chapter and are not discussed. In the presence of acute ischemia, major amputation is undertaken for irreversible ischemia, for severe ischemia with no revascularization options, or following unsuccessful attempts at revascularization. Amputation for chronic ischemia may be performed owing to failure of revascularization, lack of suitable conduit or target arteries, severe patient co-morbidities, poor functional status, or extensive gangrene or infection such that foot salvage is not possible. Pedal sepsis without ischemia constitutes another major subgroup of patients undergoing amputation; this presentation is extremely common in patients with diabetes and associated neuropathy. Because the underlying indications for amputation frequently overlap, it can be difficult to compare indications for and outcomes of amputations reported in the literature.
Impact of Diabetes
The presence of PAD and diabetes, either alone or in combination, contributes to the majority of major nontraumatic lower extremity amputations. Many general classifications, however, overemphasize the role of diabetes and understate the role of concomitant ischemia. Malone reported the indications for amputation as follows: complications of diabetes (60% to 80%), infection without diabetes (15% to 25%), ischemia without infection (5% to 10%), chronic osteomyelitis (3% to 5%), trauma (2% to 5%), and miscellaneous (5% to 10%).24 These general classifications have a certain degree of overlap and mask the true interaction between ischemia and diabetes. This simplistic breakdown does not provide clear insight into the true influence of ischemia or the full potential of revascularization to reduce amputation rates. Also, ischemia may occur in an acute or chronic setting, and because no revascularization is entirely successful, amputation may ultimately follow revascularization.
Impact of Tissue Loss and Anatomy
To understand the continued high number of amputations performed despite aggressive revascularization programs, attempts have been made to more clearly categorize the indications for major amputation.25 When identifying the indications for amputation, it must be recognized that with chronic ischemia, limb loss may ultimately occur despite revascularization. Also, patients may present initially with acutely ischemic limbs that are beyond any hope of salvage. The influence of gangrene and pedal sepsis must also be considered because they have a significant impact on the options for limb salvage. Lastly, patient co-morbidities and ambulatory or functional status influence the decision for amputation.
The TASC II Working Group reported that the rate of primary amputation in chronic critical leg ischemia is approximately 25%. Unreconstructable vascular disease is the most common indication for secondary amputations, which account for nearly 60% of patients.23 To more clearly delineate the reasons for major amputation in an academic vascular practice, a series of 131 consecutive major lower extremity amputations were reviewed, and the indications for amputation clearly classified (Table 117-1). In this setting, more than 50% of patients who underwent amputation had prior attempts at limb salvage via revascularization or had no anatomically feasible revascularization options. This group of patients had exhausted the armamentarium of vascular surgery. Seventeen percent of patients were not considered candidates for aggressive attempts at limb salvage owing to a preexisting nonambulatory status (8%) or the presence of excessive surgical risk (9%). Additionally, 32% of patients had nonsalvageable limbs at presentation attributable to extensive pedal gangrene, pedal sepsis, or a nonviable foot that mandated primary amputation.
Table 117-1
Indications for Major Amputation by Vascular Surgeons
| Indication for Major Amputation | Percentage of Cases (N = 131) |
| Critical limb ischemia with failed revascularization | 39 |
| Extensive pedal gangrene | 15 |
| Unreconstructable arterial anatomy | 11 |
| Overwhelming pedal sepsis | 9 |
| Excessive surgical risk | 9 |
| Nonviable, acutely ischemic foot | 8 |
| Nonambulatory status | 8 |
From Abou-Zamzam AM Jr, et al: Major lower extremity amputation in an academic vascular center. Ann Vasc Surg 17:86-90, 2003.
Similar findings were reported by Nehler and colleagues in a series of 172 major amputations.26 The indications for amputation were critical ischemia in 87% of the patients and complications of diabetic neuropathy without significant ischemia in 13% of the patients. Forty-six patients (30%) had prior bypass failures or amputations despite patent reconstructions, and 10 (6%) had no revascularization options; therefore, 36% had exhausted the resources of revascularization. Eighty-five patients (55%) underwent primary amputation because of severe co-morbidities, poor functional status, extensive necrosis, or a combination of these factors. In another series of 125 major amputations, 38% of procedures were performed following failed revascularizations.27
Impact of Delay in Presentation
The significant role of delay in patient presentation cannot be overstated. The mean time to vascular surgery consultation was 73 days for pedal tissue loss and 27 days for ischemic rest pain in the report by Nehler and colleagues.26 Such delays likely account for the large percentage of primary amputations in their series and underscore the need for patient and physician education. Indeed, in a report by Bailey and coworkers, only 24% of patients with critical limb ischemia were perceived as needing “urgent” vascular consultation, with a mean 8-week duration of symptoms before vascular evaluation.28 Such studies suggest that delayed patient presentation involves not only patient factors but also access issues and physician factors.
Primary Amputation Versus Revascularization
The most important decision in treating critical limb ischemia is the initial determination of whether to attempt limb salvage or proceed with primary major amputation. Despite widespread discussion of great triumphs in revascularization, there is a growing awareness that primary amputation may be the best approach in specific patient subsets. As stated earlier, more than 140,000 lower extremity revascularizations are performed annually in the United States. In recent years, open revascularizations have been partially replaced by endovascular procedures, yet nearly 60,000 major amputations are still being performed each year.1 This implies that the ratio of amputation to revascularization may be close to 1 : 2 nationally. Many specialty centers may have a much lower ratio owing to the filtering effect of referring physicians. Many patients perceived as not being candidates for revascularization are treated locally with amputation and are never referred to these high-volume centers specializing in revascularization.
The ratio of major primary amputation to revascularization differs among facilities and may vary because of surgeon experience and practice protocols. One prospective study demonstrated that 43% of the 224 patients presenting with limb-threatening ischemia were treated by primary amputation, and 57% were treated with revascularization.29 Diabetes mellitus, end-stage renal disease, tissue loss, and poor functional status were all predictors of treatment with amputation as opposed to revascularization.
Groups Benefiting from Primary Amputation
A very reasoned approach to patients presenting with critical limb ischemia is necessary. A thoughtful strategy considering the patient’s co-morbidities, the status of the foot, and the complexity of the required revascularization has been outlined by Nehler and colleagues.30 In a good-risk patient with minimal pedal tissue loss, an aggressive attempt at revascularization should be undertaken with appropriate endovascular or open bypass techniques including the consideration of alternative vein conduits. However, when the patient’s overall health status is poor or the foot lesions are extensive, primary amputation must be considered. Aggressive use of revascularization, particularly endovascular interventions, has resulted in limb salvage attempts in higher risk patients that may have helped to decrease the number of overall major amputations.31 Despite this, secondary amputations are not an uncommon occurrence. In a study that included 358 consecutive patients (412 limbs) with limb loss despite patent endovascular interventions, functional outcomes were compared with the rest of the endovascular-treated group and with those who underwent amputations with patent bypasses (APB). Amputations occurring despite a patent revascularized segment constituted 38% of limb loss in open surgical patients and 80% in endovascular-treated patients (P = .001). Most amputations in the endovascular group were performed within 3 months of the endovascular procedure and the indications were extensive tissue loss or limb dysfunction after radical débridement of infection or gangrene (37%), presentation of recurrent infection (42%), and failure to reverse ischemia (21%).32
The perceived improved outcomes following revascularization compared with amputation have driven the belief that revascularization is always the better option. However, an examination of the data demonstrates that functional outcomes following amputation may not be markedly different from those following revascularization for specific patient subgroups. Taylor and associates analyzed 553 patients who underwent 627 primary major limb amputations.33 In patients younger than 60 years, functional outcomes following below-knee amputation were similar to those of patients undergoing successful revascularization. Such information lends credence to the observation that primary amputation should be considered not a failure of therapy but a viable, important treatment option.26,30,33
Advances in percutaneous revascularization have raised the question of how best to treat the patient considered “unfit” for open revascularization. Taylor and coworkers reported an analysis of 314 patients treated for critical limb ischemia who were unsuitable for open revascularization because of medical, functional, or mental co-morbidities.21 Patients were treated with either percutaneous transluminal angioplasty (PTA) or major amputation. The 131 patients treated with PTA had higher rates of maintenance of ambulation and independent living compared with the 183 patients treated with amputation. However, the PTA group had a higher mortality, and the advantages in ambulation and independent living lasted only 12 months and 3 months, respectively. In the “unfit” patient, endovascular treatment may be no better than primary amputation.
Indeed, in those with extensive foot lesions, severe co-morbidities, or very unfavorable anatomy, primary amputation is often the best treatment option.26,30,33 Because of delayed referral, many patients ultimately undergoing amputation present to the vascular surgeon with extensive pedal necrosis, which makes limb salvage unlikely, regardless of the availability of suitable arterial targets for revascularization. End-stage renal disease presents a particularly difficult challenge, and the presence of advanced heel gangrene in this group of patients may best be treated with primary amputation.34,35
Perioperative Evaluation
Although surgeons have traditionally focused on the preoperative evaluation and near-term (usually 30-day) results of amputations, a more lengthy view of the perioperative period can be helpful. For the patient, the overall experience is important and clearly lasts longer than the 30-day postoperative period. A more global view of the patient during the first year may help identify key issues at different points in the patient’s treatment and recovery. A team approach to patient care is useful during all stages of the perioperative period since the results in any series of amputations are directly related to the skill and enthusiasm of the people involved in the program.36 Members of the team include the patient, family, surgeon, podiatrist, physiatrist, therapist, rehabilitation medicine specialist, prosthetist, nurse, social worker, psychologist, peer support group, and case manager. The common goal should be maximal recovery and rehabilitation after limb loss. The team should be flexible since different team members share the leadership and service responsibilities throughout the 12- to 18-month time frame that typically defines the perioperative period.
Perioperative Stages
The postoperative year-long continuum does not separate easily into “stages.” However, for practical purposes, five stages in the treatment of the amputation patient have been described.37
Stage 1.
This is the preoperative stage, which starts with the difficult decision of whether amputation is required. This stage includes an assessment of the vascular status and decisions on attempts to improve circulation. The preoperative evaluation of a patient undergoing major amputation should strive to reduce perioperative complications and mortality. The preoperative evaluation should be rational and expeditious. Evaluation should include the duration and severity of limb ischemia, extent of tissue loss, presence of wound infection, and anatomic considerations of revascularization. An analysis of the patient’s systemic co-morbidities is essential and should be performed systematically. The surgeon typically is the team leader during this stage.
Stage 2.
Once the surgery is completed, the acute hospital postoperative stage begins and is the time the patient spends in the hospital after the amputation. This stage typically ranges from 3 to 10 days. During this time, transition of care to the appropriate rehabilitation experts is appropriate. The surgeon is still involved because treatment for local and systemic complications is important.
Stage 3.
The immediate postacute hospital stage begins with hospital discharge and extends 4 to 8 weeks after surgery. This is the time of recovery from surgery, a time of wound healing, and a time of early rehabilitation. During this phase the surgeon is less involved but uncomplicated wound healing is essential to early rehabilitation.
Stage 4.
The intermediate recovery period is the time of transition from a postoperative strategy to the first formal prosthetic device. It is during this stage that the most rapid changes in limb volume occur, attributable to the beginning of ambulation and prosthetic use. This stage begins with the completed healing of the wound and usually extends 4 to 6 months from the healing date.
Stage 5.
The transition to stable stage is defined as a period of relative limb stabilization although the limb will continue to change to some degree for a period of 12 to 18 months after initial healing. The newer prosthesis will still require occasional adjustments, and visits to the prosthetist will remain relatively frequent until after the first year of prosthetic use. In this stage the patient should move toward social reintegration and higher functional training. The patient should become more empowered and independent from his or her health care practitioner during this period.
Reducing Perioperative Risk
The overall mortality for major amputations is about 8%. Most reports find that mortality is doubled for AKA as compared to BKA patients.38 A recent study of 2911 patients enrolled in the ACS National Surgical Quality Improvement Program (NSQIP) demonstrated a 30-day mortality of 7% for BKAs. Multivariate analysis identified renal insufficiency, cardiac issues, preoperative sepsis, chronic obstructive pulmonary disease (COPD), steroid use, and increased patient age as predictors of mortality.39 The same study identified preoperative sepsis, regular alcohol use, steroid use, cardiac issues, renal disease, and contaminated/infected wounds to be independent predictors of developing postoperative complications. The overall perioperative complication rate was 34%. Local stump complications occurred in nearly 10% of patients. Of note, the incidence of cardiopulmonary, venous thromboembolic, and cerebrovascular events ranged from 0.5% to 2.1%. The low observed incidence is probably due to the current widespread use of beta blockers, statin therapy, and antithrombotic medications in the vascular patient.40,41 A similar study involving 8696 veterans showed that AKA patients were older (69.0 vs. 66.5 years) and suffered a higher mortality (16.5% vs. 9.7%) when compared to BKA patients although both groups had a similar rate of postoperative complications.6 This study found that increased age, increased patient complexity, and admission with acute thromboembolism were predictive of death.6
Perioperative treatment with beta blockade has been beneficial in the general vascular surgery population, but these important studies included few patients undergoing major amputation.40–42 With regards to the need for preoperative cardiac evaluation, guidelines issued in 2007 by the American College of Cardiology and the American Heart Association clarified that its purpose is not to give medical “clearance” but rather to evaluate the patient’s current medical status and cardiac risks over the entire perioperative period.42 These guidelines recommend that no test be performed unless it is likely to influence the patient’s treatment.42
The management of associated hypertension, diabetes, and renal failure should be optimized. When needed, the timing of hemodialysis in relation to surgical intervention is important in managing perioperative fluid and electrolyte balance. An aggressive approach to normalize glucose levels is essential to ensure proper wound healing. Venous thromboembolism (VTE) prophylaxis with either subcutaneous unfractionated heparin or low-molecular-weight heparin appears to be safe and effective.43 The importance of thromboprophylaxis is noteworthy because nearly 17% of all amputation-related deaths are caused by pulmonary emboli.2 Also noteworthy is the propensity of these patients to fall, and fall precautions should be instituted during the early postoperative care period.
Managing Infection
Special consideration should be given to patients presenting with extensive pedal infection necessitating amputation. Aggressive control of infection, surgical extirpation of the source of infection, and administration of adjunctive intravenous antibiotics are the mainstays of treatment. The two-stage approach of guillotine amputation followed by formal amputation several days later has a lower complication rate than single-stage amputation.44 Guillotine amputation is a rapid procedure, but an additional operation to close the amputation stump is necessary.
Another option in patients who are moribund is a physiologic cryoamputation, which can be performed safely at the bedside.45,46 The materials needed to perform cryoamputation are dry ice, a large plastic bag or Styrofoam container, umbilical tape to use as a tourniquet, towels, blankets, adhesive tape, and a heating pad. After parenteral analgesics are administered, umbilical tape is tied around the affected extremity just proximal to the affected area. A large plastic bag is placed over the distal leg and filled with dry ice, circumferentially covering the leg. The dry ice bag is wrapped with blankets and secured with adhesive tape. A heating pad covered with a protective towel is placed around the proximal extremity adjacent to the frost line. The opposite leg should be covered with numerous blankets to avoid collateral injury. The frost line and the frozen limb should be checked periodically by the nursing staff, and dry ice added as needed. This bedside procedure alleviates any time pressures for amputation, controls infection, and allows optimal treatment of associated conditions. If necessary, a cryoamputation can be maintained for several weeks. When the patient’s status improves, he or she may be taken to the operating room for a one-stage amputation.
Planning Rehabilitation
Whenever possible, preamputation evaluation of rehabilitation and prosthetic candidacy should be performed. Centers with dedicated multispecialty teams have much more successful rehabilitation outcomes.47 Understanding the various stages of the perioperative period and adjusting the areas of focus throughout the recovery period may optimize the outcomes. Tempering the patient’s expectations and the timing of prosthetic use and reinforcing the ultimate functional goals are best undertaken by the team members throughout the perioperative period.
Amputation Level Selection
The goals of amputation are (1) to eliminate all infected, necrotic, and painful tissue; (2) to achieve uncomplicated wound healing; and (3) to have an appropriate remnant stump that can accommodate a prosthesis. The length of the residual limb has important implications for rehabilitation. Prosthetic use following major amputation puts an increased energy demand on the patient. Unilateral below-knee amputees require a 10% to 40% increase in energy expenditure for ambulation, and above-knee amputees require 50% to 70% more energy to ambulate.2 This differential may explain why the successful rehabilitation rate is much lower following above-knee amputation (AKA) than below-knee amputation (BKA). Prosthetic use is reportedly 50% to 100% following BKA but only 10% to 30% following AKA.26,47–49 Interestingly, the true rate of ambulation is significantly lower than that of prosthetic use, and it shows a steady attrition in the 5 years following amputation.26,48 Partial foot or toe amputations are minor procedures that preserve the majority of the extremity and allow ambulation without the need for bulky prostheses. Most minor amputations, including toe and ray amputations, lead to minimal increases in energy expenditure and require simple orthotic inserts.
Failure of an amputation to heal is multifactorial. Much emphasis has been placed on assessing blood flow at the level of the amputation to predict wound healing. However, failure may be caused not just by ischemia but also by infection, hematoma, or trauma (falls). This explains why no single test can predict with 100% accuracy the ability of an amputation to heal or, conversely, its inability to heal. Most tests are better at predicting wound healing than failure to heal. Thus, using any single criterion may lead to unnecessarily proximal amputation.
The importance of optimizing level selection is underlined by the need to revise BKAs to AKAs in 15% to 25% of patients.2,25,26,50 This revision rate is frequently accompanied by a perioperative mortality of greater than 5%.2 Such events also lead to increased patient anxiety and fear of repeated, more proximal amputations.
Objective Testing and Clinical Judgment
The drive to maximize limb length in amputees and to minimize the need for revisions has led to a search for the optimal modality for selecting an amputation level. Physical findings (pulses, skin quality, extent of foot ischemia or infection, skin temperature), noninvasive hemodynamic tests (segmental arterial pressures, Doppler waveforms, toe pressures), invasive anatomic tests (angiographic scoring systems), and physiologic tests (skin blood flow, skin perfusion pressure, muscle perfusion, transcutaneous oxygen measurements) have all been extensively investigated.
Physical Findings
Physical examination is the essential first step in determining the level of amputation. The extent of gangrene and infection dictates the maximal length attainable. In this evaluation, the presence of dependent rubor should be considered analogous to gangrene because this tissue is ischemic. The presence of pulses should be accurately assessed. The presence of a palpable pulse immediately proximal to a proposed amputation level predicts successful healing in nearly 100% of patients undergoing either major or minor amputation.51,52 However, the absence of a pulse does not necessarily lead to failure of wound healing; therefore, sole reliance on the presence of a pulse leads to unnecessarily proximal amputations.
Using “clinical judgment,” which incorporates physical findings and consideration of the patient’s overall status, yields wound healing rates of 80% in BKAs and 90% in AKAs. Wagner and colleagues found that objective data may supplement clinical judgment but not replace it; more distal amputations were achieved with clinical judgment than with sole reliance on objective examinations.53 Experience is important in the determination of amputation level; therefore, this decision should not be relegated to junior surgeons or trainees.
Skin Temperature Measurements
The subjective interpretation of skin temperature as a guide for amputation is not reliable. However, several investigators have demonstrated that objective, direct skin temperature measurement may predict amputation healing with an accuracy of 80% to 90%.2,53,54 In a study comparing several noninvasive techniques, direct skin temperature measurement at the level of amputation with a threshold of 90° F demonstrated the best accuracy.53 Special attention must be paid to room temperature, and comparison with a normal contralateral extremity can be helpful.
Ankle and Toe Pressure Measurements
The use of noninvasive hemodynamic tests has been extensively evaluated. Frequently employed tests are segmental arterial pressures, Doppler waveforms, and toe pressures. Absolute ankle pressures greater than 60 mm Hg can predict the healing of BKAs with an accuracy of 50% to 90%. Calf pressures and thigh pressures have shown similar reliability.2 However, Wagner and colleagues found that Doppler-derived pressures at the thigh, popliteal, calf, and ankle levels are less reliable than clinical judgment in predicting the healing of BKAs.53 This inaccuracy may be due in part to the high prevalence of diabetes in this population, making measured pressures less reliable because of medial calcinosis.
The ankle-brachial index (ABI) should always be obtained, regardless of the presence of a palpable pulse. Marston and colleagues reported on the role of ABI in predicting the need for amputation in a cohort of high-risk patients with critical limb ischemia treated with meticulous wound care but without revascularization. In patients with an ABI less than 0.5, 28% and 34% of limbs required amputation at 6 and 12 months, respectively, versus 10% and 15% of limbs in patients with an ABI greater than 0.5 (P = .01).55
The use of toe pressures has been advocated as being predictive of forefoot amputation healing. Vitti and associates demonstrated universal failure of minor amputations in patients with diabetes and toe pressures less than 38 mm Hg.56 However, there was no similar threshold value in patients without diabetes, limiting the usefulness of this parameter.
Arteriography
Invasive testing with arteriography has been investigated as a means of determining amputation level, but the correlation between arteriographic findings and healing potential has been poor. Dwars and coworkers found that angiographic scores did not correlate with amputation healing.52 In fact, in their report, angiographic patency tended to be greater in limbs with failed or delayed healing than in limbs with successful healing.
Radioisotope Scans, Scintigraphy, and Skin Perfusion Pressure
All physiologic tests attempt to predict wound healing based on tissue perfusion or oxygen delivery at the proposed level of amputation. One technique of measuring skin blood flow involves injecting an intradermal isotope (xenon-133 or iodine-125) and then calculating blood flow by measuring the isotope washout rate using nuclear medicine scanning devices.24,57 Malone and associates initially reported excellent results with xenon-133 clearance, with an accuracy of 92% to 97%.58 However, in a follow-up report, this same group found that the overlap in values between patients with healed and failed amputations made this test too unreliable.57
Sarikaya and coworkers used technetium (Tc) 99m sestamibi scintigraphy to predict the healing of extremity amputation based on deep tissue perfusion.59 Perfusion to the ischemic limb was evaluated preoperatively based on the Tc 99m sestamibi uptake pattern. Nonviable tissue in the extremity was suggested by a clear-cut edge of perfused muscle. The most distal level of amputation was determined above the nonviable tissue. In their 25 patients, the proposed level of amputation based on physical examination and Doppler study was changed to a lower level after Tc 99m sestamibi scintigraphy in 65% of cases, and all amputation wounds healed.
Skin perfusion pressure is another physiologic test to determine amputation level. This test involves a scintigraphic technique in which intracutaneous iodine-123 is injected at different amputation levels. External pressure is applied, and by measuring the washout of isotope, the skin perfusion pressure is determined. A level less than 20 mm Hg was predictive of wound failure in 89% of amputations, and a reading greater than 20 mm Hg predicted healing in 99% of amputations.52 Skin perfusion pressure can also be measured by laser Doppler velocimetry, thus avoiding the need for isotopes and markedly simplifying the test.
Skin fluorescence employs a Wood’s or ultraviolet light following the intravenous injection of fluorescein dye. A qualitative determination of regional blood flow is used to determine the level of amputation. Success rates in predicting healing have ranged from 86% to 100%.24 As a result of the wide availability of these tools and the needless requirement for radioisotope, this technique is more accessible than scintigraphic techniques. However, the fluorescein technique may be more affected by the presence of inflammation and cellulitis than are scintigraphic techniques.
Transcutaneous Oxygen Measurements
Transcutaneous oxygen measurement is a completely noninvasive method that may be used to select an amputation level. A small sensor is placed on the skin in the area of interest. By heating the sensor and skin to 44° C, local skin hyperemia results in decreased flow resistance and arteriolarization of capillary blood. The partial pressure of oxygen measured transcutaneously (tcPO2) approximates the true arterial oxygen pressure in the area of interest.60 The sensors can be placed anywhere on the body, and readings are given in millimeters of mercury (mm Hg). Absolute readings can be recorded, or readings in areas of interest can be indexed to a reference site (often the chest). The probes are small and atraumatic, and multiple sites can be tested simultaneously, depending on the machine. Readings in the supine position are more predictive than measurements in the dependent position or during supplemental oxygen breathing.61 The values recorded are reliable and show an acceptable day-to-day variability in repeat measurements.62
Transcutaneous oxygen levels have an accuracy of 87% to 100% in predicting wound healing.2,51,57,63 Malone and coworkers reported no amputation failures in patients with tcPO2 measurements greater than 20 mm Hg and universal failure when tcPO2 readings were less than 20 mm Hg.57 Unfortunately, other investigators have not confirmed any consistent absolute tcPO2 threshold.2,51,53 Some authors have reported a useful tcPO2 threshold of 30 mm Hg, whereas others have reported 16 mm Hg as a cutoff value. In general, tcPO2 readings greater than 40 mm Hg are associated with healing and readings less than 20 mm Hg are associated with failure.60,63 The lack of a consistent minimal level is likely due to the fact that nutrient blood flow may be present even in the setting of tcPO2 readings of 0 mm Hg. The tcPO2 reading may be artificially low in the setting of infection, inflammation, or edema, and repeat measurements are advised once such processes have resolved. Incidentally, in addition to modest utility in predicting wound healing, tcPO2 measurement has accuracy in predicting outcome following revascularization. Increases in tcPO2 values of greater than 30 mm Hg following revascularization are predictive of a successful clinical outcome.63
In direct comparisons with segmental pressures and skin blood flow, tcPO2 has been the most accurate predictor of wound healing.57 This applies not only to major amputation but also to forefoot amputation.28,64 Transcutaneous oxygen measurements are also more accurate than fluorescein dye injections.28, In addition, tcPO2 has several advantages over other tests in terms of ease of measurement, reproducibility, and simple instrumentation that can be readily introduced into any vascular laboratory.
Yamada and colleagues studied 211 patients with 403 ischemic limbs using skin perfusion pressure, toe pressure, ankle pressure, and tcPO2 measurements.65 The correlations between these methods demonstrate that the combination of skin perfusion pressure and toe pressure can accurately predict wound healing. However, the combination of skin perfusion pressure and tcPO2 did not result in a more accurate prediction. A meaningful approach to the accurate determination of amputation level should therefore include a combination of physical findings, clinical judgment, and objective testing.51–53,57,60
Technique Selection
Whether skin temperature, skin blood flow or perfusion, or transcutaneous oxygen measurements are used depends on local experience and availability. Measurement of tcPO2 is easily incorporated into a noninvasive vascular laboratory, requires minimal equipment and training, and is reliable and reproducible. For these reasons, our preferred objective test is tcPO2, with a threshold value of 30 mm Hg. However, strict utilization of a single objective method, rather than taking all available clinical data into account, leads to unnecessarily proximal amputations and denies patients the best opportunity for successful rehabilitation. The various methods of predicting wound healing and their accuracies are shown in Table 117-2.65
Table 117-2
Prediction of Wound Healing by Noninvasive Vascular Studies65

* ABP, Ankle blood pressure; SPP, skin perfusion pressure; TBP, toe blood pressure; tcPO2, transcutaneous oxygen pressure.
Rehabilitation Considerations
In the United States there are approximately 1.6 million people living with limb loss. Vascular disease accounts for the majority (82%) of limb loss hospital discharges. It is projected that the number of people living with the loss of a limb will more than double by the year 2050 to 3.6 million.38,66 Rehabilitation is crucial for maximizing the functional outcome of these patients. The significant physical and psychological changes following major amputation make rehabilitation a complex process. Integrated rehabilitation requires an interdisciplinary team that incorporates members from surgery, internal and family medicine, psychiatry, physical therapy, occupational therapy, prosthetics, social services, nursing, nutrition, and recreational therapy.
As mentioned earlier in this chapter, greater energy expenditure is required for ambulation in patients with higher levels of amputation. Energy expenditure and ambulatory rates at various amputation levels are shown in Table 117-3.67 Many studies have shown that BKA patients have a significantly greater chance to achieve ambulation than AKA patients. In general, the goal is the preservation of maximal limb length at which a healed stump wound can be achieved.
Table 117-3
Energy Expenditure and Ambulation Rate at Various Amputation Levels
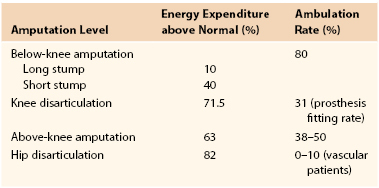
From Tang PCY, et al: Let them walk! Current prosthesis options for leg and foot amputees. J Am Coll Surg 206:548, 2008.
Preoperative evaluation and amputation level selection should always include postoperative rehabilitation considerations. Preoperative ambulatory ability, co-morbidities, age, and mental status affect rehabilitation potential. Patients with limited preoperative ambulatory function, age older than 70 years, dementia, end-stage renal disease, and advanced coronary artery disease perform poorly following amputation.33
A comprehensive treatment plan should be developed at the beginning of rehabilitation and updated frequently based on the patient’s condition. A dedicated team approach clearly improves the ultimate outcome for amputees.47,64 One report documented that successful rehabilitation following major amputation increased from 69% before the institution of a coordinated team approach to 100% after the development of such a team.47
Medical assessment and treatment should be targeted to optimize the patient’s overall condition. Addressing co-morbidities increases the patient’s ability to participate in the rehabilitation process. Pain management is essential to ensure that amputees can actively participate in intensive rehabilitation and prosthetic training. Assessment and modification of health risk factors can improve long-term functional outcome. All patients should be encouraged to exercise daily to improve their performance of activities of daily living.
Amputation Wound Dressings
An important aspect of rehabilitation is the uncomplicated healing of the remnant limb. The role that early wound dressings play in ultimate wound healing and rehabilitation is unclear and deserves special mention. Currently, no consensus exists on the most effective postoperative management strategy for individuals who undergo major amputation. As a result of the longer residual limb and functional knee, there are more dressing options for BKA than AKA. Most practitioners elect a soft dressing following a standard AKA. Typical postoperative dressings and management strategies following BKA include soft gauze dressings with an elastic wrap68–70; thigh-level rigid plaster dressings without an immediate prosthesis; thigh-level rigid plaster dressings with an immediate postoperative prosthetic (IPOP)71; short, removable rigid plaster dressings72,73; and prefabricated pneumatic postoperative prosthesis (prefabricated pneumatic IPOP). A soft gauze dressing with a mild compression wrap is the most widely used dressing following below-knee amputation. Patients remain nonambulatory until the fitting of a prosthesis 4 to 6 weeks after surgery. There are concerns that this type of dressing may delay effective physical therapy and rehabilitation.
Data from controlled trials have found that for soft dressing strategies the uncomplicated healing rates, postoperative pain, prosthetic use, and mortality were not significantly different when compared with other types of dressings.68,74,75 However, the data from controlled studies have shown that thigh-level rigid dressings lead to significantly shorter rehabilitation times than soft dressings when measured as time to initial gait training.76–80 An alternative technique known as immediate postoperative prosthesis (IPOP) placement, proposed by Berlemont in the 1950s, has been documented to improve both primary healing and rehabilitation rates.80 An IPOP, which is applied at the initial operation, combines a rigid postoperative dressing with a temporary prosthesis and has been advocated for the early rehabilitation of nonischemic amputees. Concerns from surgeons include lack of familiarity with the prosthesis, fear of placing a hard cast on a potentially compromised remnant limb, and the inability to inspect the wound frequently.81 Schon and colleagues found that significantly fewer patients with a prefabricated pneumatic IPOP had postoperative complications (16%) when compared with patients with soft gauze dressings (65%).74
Prosthesis Selection and Training
Prosthetic training plays an important role in rehabilitation. An appropriate durable prosthesis should be prescribed based on the individual’s situation. Advanced prosthetic technology provides amputees with more choices. New functional designs and ultralight materials help amputees live independently. Various lower extremity prostheses are commercially available for patients following AKA and BKA (Figs. 117-1 and 117-2).67
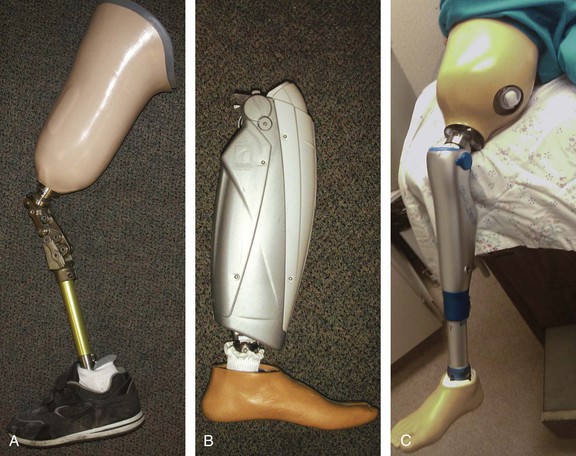
Figure 117-1 Above-knee prostheses. A, Standard mechanical knee with swing control. B, “Intelligent” transfemoral microprocessor-controlled prosthesis (without socket). C, Patient with “intelligent” above-knee prosthesis seated on an examination table.
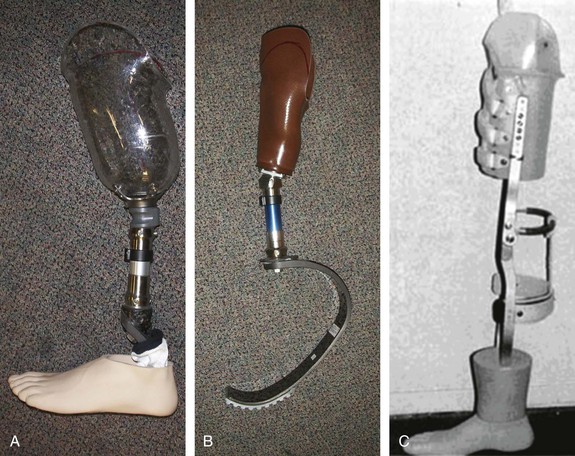
Figure 117-2 Below-knee prostheses. A, Standard prosthesis with solid ankle and cushioned heel foot. B, Dynamic, energy-conserving athletic prosthesis. C, Below-knee prosthesis for patients with wound difficulties.
The advantages and disadvantages of different types of prostheses and the indications for their use depend on the individual patient and should be fully explored by the experienced prosthetist.67 For example, microprocessor-controlled above-knee prostheses are now typical of the high-technology products (see Fig. 117-1B). These “intelligent” prostheses can change the orifice size, based on different walking speeds, to allow the appropriate shin swing time. The shin includes numerous sensors to accumulate biomechanical data, such as vertical loading amplitude and sagittal knee movement, to determine the direction and angular acceleration of the artificial knee joint. A software analysis system can optimize prosthetic characteristics through a process of data sampling and calculations up to 60 times in a 1.2-second gait cycle.67 A patient wearing one of these prostheses can easily step up and sit on an examination table (see Fig. 117-1C).
Different types of prostheses are available to meet the needs of amputees with different amputation levels, physical conditioning, and exercise demands.67 In general, persons of smaller stature with a slow to moderate cadence are better suited to smaller, simpler, and lighter hydraulic swing-control prostheses. Taller, more active ambulators benefit from larger, more powerful hydraulic swing-control prostheses.82 Below-knee prostheses are generally much more lightweight than above-knee prostheses. The below-knee prostheses vary and include standard passive ankle designs, energy-conserving ankles, and open-type sockets that can accommodate slowly healing stumps (see Fig. 117-2).
Reasonable goals must be set for each patient. Many patients may not walk independently, but many wear prostheses, and the large majority return to their home environments. Although all patients may be offered the eventual fitting of a prosthesis, only a fraction will actively walk with their prostheses. In a recent series of major amputations, 29% of patients were able to walk outside the home with a prosthesis, 42% used a prosthetic limb, and 92% of patients were able to return to community living.26
Functional Outcome
Results of lower extremity amputation are discussed in detail in Chapter 118. However, the assessment of functional performance following major amputation is a complex issue that influences decision making, so it is also addressed here. The most important factors that influence functional outcome are age, preoperative functional status, co-morbidities, mental status, amputation level, stump healing status, unilateral versus bilateral amputation, and postoperative rehabilitation.
Impact of Amputation Level and Co-Morbidities
Among amputees with healed stumps, 80% of those with BKAs can achieve ambulation, compared with only 38% to 50% of patients with AKAs.83 Taylor and coworkers, in a large consecutive single institutional series, documented that patients older than 70 years had a 3-fold greater chance of not wearing a prosthesis, a 3.1-fold greater chance of death, a 2.3-fold greater chance of being nonambulatory, and a 4-fold greater chance of losing functional independence at 1 year compared with patients younger than 50 years.33 Patients aged 70 years or older and those with limited preoperative ambulatory ability, dementia, end-stage renal disease, and advanced coronary artery disease all had a significantly higher chance of experiencing death, nonambulatory status, and loss of functional independence and never using a prosthesis. AKA patients who were not ambulatory preoperatively had a 10-fold greater chance of not wearing a prosthesis and twice the chance of death at 1 year. Amputees with dementia were 2.4 times less likely to wear a prosthesis. Patients with advanced co-morbidities, including coronary artery disease, end-stage renal disease, and severe chronic obstructive pulmonary disease, also had a greater chance of not ambulating following amputation.33
Through-knee amputation is not commonly performed because of poor soft tissue coverage and a higher risk of wound complications. However, in patients with adequate residual skin and subcutaneous tissue, through-knee amputation is an option. Through-knee amputees are able to achieve higher normal and maximal walking speeds with lower relative energy expenditure than above-knee amputees. In elderly patients or those with significant co-morbidities preventing them from postoperative ambulation, through-knee amputation allows earlier weight bearing with a lower risk of wound complications compared with BKA.67
Minor amputations, such as digital, ray, transmetatarsal, and midfoot amputations, are considered limb salvage procedures. Patients with digital or ray amputation may walk with little additional energy expenditure. Partial foot amputation requires less ambulation energy than more proximal amputation. Pinzur and associates demonstrated that energy demands after midfoot, Syme’s, below-knee, through-knee, and above-knee amputations are directly related to the level of amputation.84
Impact of Age
Patients younger than 60 years with well-controlled co-morbidities who were ambulating preoperatively can anticipate an ambulatory rate of 70%, a survival rate of 80%, and an independent living rate of 90% at 1 year following major amputation.33 A Canadian study showed that although older patients had more co-morbidities at admission, they benefited as much as younger people from an intensive rehabilitation program with a comparable length of stay.85 However, younger amputees continued to improve after 3 months, whereas older patients tended to plateau. In selected patients who developed stump wound complications but had good perceived healing potential, a “redo” BKA yielded better functional outcomes than conversion to an AKA.86 Integrative and vocational rehabilitation combined with newly designed prostheses allow high-level amputation patients to achieve independent lifestyles. Interdisciplinary management should be employed in the care of all amputees. Preoperative and postoperative optimization of a patient’s medical condition, intensive rehabilitation, meticulous wound care, pain management, and proper prosthesis fitting all significantly improve the patient’s ultimate functional outcome.
Fate of the Contralateral Limb after Amputation
Following a major amputation, many patients worry about the fate of their remaining limb. Patients surviving more than 3 years after the first amputation have a significant chance of needing a contralateral amputation.87 Of amputees with diabetes, 15% to 35% lose their remaining leg within 5 years.88 The presence of renal failure is a particularly poor prognostic indicator of second limb amputation.25 These facts underscore the need for diligence in protecting and surveying the remaining limb. Continued efforts focused on patient education and preventive foot care are essential. Periodic evaluation and management of PAD in the contralateral limb after unilateral amputation is important for the long-term functional outcome of these patients.
Depression after Amputation
As in other areas of vascular surgery, the successful completion of the operation is only the beginning of the patient’s recovery. Although the surgeon may initially focus on the surgical wounds, other considerations are unique to the recovery of an amputee. Depression is common following major amputation, especially in younger amputees.89 The loss of a limb has been compared to the loss of a spouse in terms of magnitude. Although the feeling of bereavement abates during the first year, many amputees experience a continued sense of loss extending beyond 1 year.90 In the recovery period, positive outcomes should continually be stressed, and clinical screening with appropriate medical therapy for depression should be considered. Amputees with significant depression may become malnourished owing to loss of appetite; malnutrition may delay healing, contribute to the development of pressure ulcers, and seriously affect the patient’s overall recovery.
Successful rehabilitation can decrease the severity and incidence of depression following amputation.91 Social discomfort, the appearance of the prosthesis, and individual coping skills should be addressed to aid in the recovery process.92–94 The psychological impact of the loss of independence may be minimized by aggressive, focused rehabilitation that openly addresses the concerns of the amputee. Dealing with these issues requires the participation of psychologists, nurses, and geriatric and rehabilitation specialists.
Selected Key References
Abou-Zamzam AM Jr, Gomez NR, Molkara A, Banta JE, Teruya TH, Killeen JD, Bianchi C. A prospective analysis of critical limb ischemia: factors leading to major primary amputation versus revascularization. Ann Vasc Surg. 2007;21:458.
Li Y, Burrows NR, Gregg EW, Albright A, Geiss LS. Declining rates of hospitalization for nontraumatic lower extremity amputation in the diabetic population aged 40 years or older: U.S., 1988-2008. Diabetes Care. 2012;35:273.
Nehler MR, Hiatt WR, Taylor LM Jr. Is revascularization and limb salvage always the best treatment for critical limb ischemia? J Vasc Surg. 2003;37:704.
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45S:S5.
Excellent review of the current data and approach to PAD..
Nowygrod R, Egorova N, Greco G, Anderson P, Gelijns A, Moskowitz A, McKinsey J, Morrissey N, Kent KD. Trends, complications, and mortality in peripheral vascular surgery. J Vasc Surg. 2006;43:205.
Tang PCY, Ravji K, Key JJ, Mahler DB, Blume PA, Sumpio B. Let them walk! Current prosthesis options for leg and foot amputees. J Am Coll Surg. 2008;206:548.
Succinct review of prostheses available for amputees..
Taylor SM, Kalbaugh CA, Blackhurst DW, Hamontree SE, Cull DL, Messich HS, Robertson RT, Langan EM 3rd, York JW, Carsten CG 3rd, Snyder BA, Jackson MR, Youkey JR. Preoperative clinical factors predict postoperative functional outcomes after major lower limb amputation: an analysis of 553 consecutive patients. J Vasc Surg. 2005;42:227.
Yamada T, Ohta T, Ishibashi H, Sugimoto I, Iwata H, Takahashi M, Kawanishi J. Clinical reliability and utility of skin perfusion pressure measurement in ischemic limbs—comparison with other noninvasive diagnostic methods. J Vasc Surg. 2008;47:318.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Nowygrod R, et al. Trends, complications, and mortality in peripheral vascular surgery. J Vasc Surg. 2006;43:205.
2. DeFrang RD, et al. Basic data related to amputations. Ann Vasc Surg. 1991;5:202.
3. Feinglass J, et al. Rates of lower-extremity amputation and arterial reconstruction in the United States, 1979 to 1996. Am J Public Health. 1999;89:1222.
4. Group TG. Epidemiology of lower extremity amputation in centres in Europe, North America and East Asia. The Global Lower Extremity Amputation Study Group. Br J Surg. 2000;87:328.
5. Goodney PP, et al. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50:54.
6. Li Y, et al. Declining rates of hospitalization for nontraumatic lower extremity amputation in the diabetic population aged 40 years or older: U.S., 1988-2008. Diabetes Care. 2012;35:273.
7. Kennon B, et al. Reduced incidence of lower extremity amputations in people with diabetes in Scotland: a nationwide study. Diabetes Care. 2012;35:2588.
8. Schofield CJ, et al. Decreasing amputation rates in patients with diabetes—a population-based study. Diabet Med. 2009;26:773.
9. Connelly J, et al. Variation in clinical decision making is a partial explanation for geographical variation in lower extremity amputation rates. Br J Surg. 2001;88:529.
10. Kazmers A, et al. Major lower extremity amputation in Veterans Affairs medical centers. Ann Vasc Surg. 2000;14:216.
11. Kantonen I, et al. Factors affecting the results of surgery for chronic critical leg ischemia—a nationwide survey. Finnvasc Study Group. J Vasc Surg. 1998;27:940.
12. Ho V, et al. Physician supply, treatment, and amputation rates for peripheral arterial disease. J Vasc Surg. 2005;42:81.
13. Bruckner M, et al. Project LEAP of New Jersey: lower extremity amputation prevention in persons with type 2 diabetes. Am J Manag Care. 1999;5:609.
14. Huber TS, et al. Impact of race on the treatment for peripheral arterial occlusive disease. J Vasc Surg. 1999;30:417.
15. Resnick HE, et al. Diabetes mellitus and nontraumatic lower extremity amputation in black and white Americans: the National Health and Nutrition Examination Survey Epidemiologic Follow-up Study, 1971-1992. Arch Intern Med. 1999;159:2470.
16. Tunis SR, et al. Variation in utilization of procedures for treatment of peripheral arterial disease. A look at patient characteristics. Arch Intern Med. 1993;153:991.
17. Eslami MH, et al. The adverse effects of race, insurance status, and low income on the rate of amputation in patients presenting with lower extremity ischemia. J Vasc Surg. 2007;45:55.
18. Feinglass J, et al. Racial differences in primary and repeat lower extremity amputation: results from a multihospital study. J Vasc Surg. 2005;41:823.
19. Hallett JW Jr, et al. Impact of arterial surgery and balloon angioplasty on amputation: a population-based study of 1155 procedures between 1973 and 1992. J Vasc Surg. 1997;25:29.
20. Eskelinen E, et al. Infrapopliteal bypass reduces amputation incidence in elderly patients: a population-based study. Eur J Vasc Endovasc Surg. 2003;26:65.
21. Taylor SM, et al. A comparison of percutaneous transluminal angioplasty versus amputation for critical limb ischemia in patients unsuitable for open surgery. J Vasc Surg. 2007;45:304.
22. Adam DJ, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925.
23. Norgren L, et al. TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45S:S5.
24. Malone JM. Lower extremity amputations. Moore WS. Vascular surgery: a comprehensive review. WB Saunders: Philadelphia; 2002:875.
25. Abou-Zamzam AM Jr, et al. Major lower extremity amputation in an academic vascular center. Ann Vasc Surg. 2003;17:86.
26. Nehler MR, et al. Functional outcome in a contemporary series of major lower extremity amputations. J Vasc Surg. 2003;38:7.
27. Amirhamzeh MMR. The decision to amputate. Surgery Rounds Feb. 2003;86.
28. Bailey CM, et al. A 1 year prospective study of management and outcome of patients presenting with critical lower limb ischaemia. Eur J Vasc Endovasc Surg. 2003;25:131.
29. Abou-Zamzam AM, et al. A prospective analysis of critical limb ischemia: factors leading to major primary amputation versus revascularization. Ann Vasc Surg. 2007;21:458.
30. Nehler MR, et al. Is revascularization and limb salvage always the best treatment for critical limb ischemia? J Vasc Surg. 2003;37:704.
31. Dosluoglu HH, et al. Does preferential use of endovascular interventions by vascular surgeons improve limb salvage, control of symptoms and survival in patients presenting with critical limb ischemia? Am J Surg. 2006;192:572–576.
32. Khan MU, et al. Predictors of limb loss despite a patent endovascular- treated arterial segment. J Vasc Surg. 2009;49:1440.
33. Taylor SM, et al. Preoperative clinical factors predict postoperative functional outcomes after major lower limb amputation: an analysis of 553 consecutive patients. J Vasc Surg. 2005;42:227.
34. Edwards JM, et al. Limb salvage in end-stage renal disease (ESRD). Comparison of modern results in patients with and without ESRD. Arch Surg. 1998;123:1164.
35. Leers SA, et al. Realistic expectations for pedal bypass grafts in patients with end-stage renal disease. J Vasc Surg. 1998;28:976.
36. McKeever FM. Editorial. Am J Surg. 1972;124:133.
37. Clinical Standards of Practice on postoperative management of the lower extremity. J Prosthet Ortho. 2004;16(3S):6.
38. Aulivola B, et al. Major lower extremity amputation: outcome of a modern series. Arch Surg. 2004;139:395.
39. Belmont PJ, et al. Risk factors for 30-day postoperative complications and mortality after below-knee amputation study of 2,911 patients from the National Surgical Quality Improvement Program. J Am Coll Surg. 2011;213:370–378.
40. Mangano DT, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group [published erratum appears in N Engl J Med 336(14):1039, 1997]. N Engl J Med. 1996;335:1713.
41. Poldermans D, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. N Engl J Med. 1999;341:1789.
42. Fleisher LA, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:1971.
43. Bani-Hani M, et al. Deep venous thrombosis after arterial surgery: a literature review. Eur J Vasc Endovasc Surg. 2008;36:565.
44. Fisher DF Jr, et al. One-stage versus two-stage amputation for wet gangrene of the lower extremity: a randomized study. J Vasc Surg. 1988;8:428.
45. Hunsaker RH, et al. Dry ice cryoamputation: a twelve-year experience. J Vasc Surg. 1985;2:812.
46. Winburn GB, et al. Current role of cryoamputation. Am J Surg. 1991;162:647.
47. Malone JM, et al. Rehabilitation for lower extremity amputation. Arch Surg. 1981;116:93.
48. McWhinnie DL, et al. Rehabilitation outcome 5 years after 100 lower-limb amputations. Br J Surg. 1994;81:1596.
50. Keagy BA, et al. Lower extremity amputation: the control series. J Vasc Surg. 1986;4:321.
51. Ballard JL, et al. A prospective evaluation of transcutaneous oxygen measurements in the management of diabetic foot problems. J Vasc Surg. 1995;22:485.
52. Dwars BJ, et al. Criteria for reliable selection of the lowest level of amputation in peripheral vascular disease. J Vasc Surg. 1992;15:536.
53. Wagner WH, et al. Noninvasive determination of healing of major lower extremity amputation: the continued role of clinical judgment. J Vasc Surg. 1988;8:703.
54. Spence VA, et al. Amputation of the ischemic limb: selection of the optimum site by thermography. Angiology. 1981;32:155.
55. Marston WA, et al. Natural history of limbs with arterial insufficiency and chronic ulceration treated without revascularization. J Vasc Surg. 2006;44:108.
56. Vitti MJ, et al. Wound healing in forefoot amputations: the predictive value of toe pressure. Ann Vasc Surg. 1994;8:99.
57. Malone JM, et al. Prospective comparison of noninvasive techniques for amputation level selection. Am J Surg. 1987;154:179.
58. Malone JM, et al. The “gold standard” for amputation level selection—xenon-133 clearance. J Surg Res. 1981;30:449.
59. Sarikaya A, et al. Predictive value of (99m)Tc-sestamibi scintigraphy for healing of extremity amputation. Eur J Nucl Med Mol Imaging. 2006;33:1500.
60. Ballard JL, et al. Transcutaneous oxygen tension: principles and application. Abu-Rhama AF, et al. Noninvasive vascular diagnosis. Springer: New York; 2000:403.
61. Scheffler A, et al. A comparative analysis of transcutaneous oximetry (tcPO2) during oxygen inhalation and leg dependency in severe peripheral arterial occlusive disease. J Vasc Surg. 1992;16:218.
62. Jorneskog G, et al. Day-to-day variability of transcutaneous oxygen tension in patients with diabetes mellitus and peripheral arterial occlusive disease. J Vasc Surg. 2001;34:277.
63. Bunt TJ, et al. TcPO2 as an accurate predictor of therapy in limb salvage. Ann Vasc Surg. 1996;10:224.
64. Gibbons GW. Lower extremity bypass in patients with diabetic foot ulcers. Surg Clin North Am. 2003;83:659.
65. Yamada T, et al. Clinical reliability and utility of skin perfusion pressure measurement in ischemic limbs—comparison with other noninvasive diagnostic methods. J Vasc Surg. 2008;47:318.
66. Ziegler-Graham K, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422.
67. Tang PCY, et al. Let them walk! Current prosthesis options for leg and foot amputees. J Am Coll Surg. 2008;206:548.
68. Mooney V, et al. Comparison of postoperative stump management: plaster vs. soft dressings. J Bone Joint Surg Am. 1971;53A:241–249.
69. Isherwood PA, et al. Pressure measurements beneath below-knee amputation stump bandages: elastic bandaging, the Puddifoot dressing and a pneumatic bandaging technique compared. Br J Surg. 1975;62:982.
70. Mueller MJ. Comparison of removable rigid dressings and elastic bandages in preprosthetic management of patients with below-knee amputations. Phys Ther. 1982;62:1438.
71. Burgess EM, et al. The management of lower extremity amputations: surgery, immediate postsurgical prosthetic fitting, rehabilitation. Bulletin TR 10–16. US Government Printing Office; 1969.
72. Wu Y, et al. An innovative removable rigid dressing technique for below-the-knee amputation. J Bone Joint Surg Am. 1979;61:724–729.
73. Wu Y, et al. Scotchcast PVC interim prosthesis for below-knee amputees. Bull Prosthet Res. 1981;10–36.
74. Schon LC, et al. Benefits of early prosthetic management of transtibial amputees: a prospective clinical study of a prefabricated prosthesis. Foot Ankle Int. 2002;23:509–514.
75. Baker WH, et al. The healing of below-knee amputations. A comparison of soft and plaster dressings. Am J Surg. 1977;133:716.
76. Nicholas GG, et al. Evaluation of use of the rigid dressing in amputation of the lower extremity. Surg Gynecol Obstet. 1976;143:398.
77. Cohen SI, et al. The deleterious effect of immediate postoperative prosthesis in below knee amputation for ischemic disease. Surgery. 1974;76:992.
78. Barber GG, et al. A prospective study of lower limb amputations. Can J Surg. 1983;26:339.
79. Kane TJ, et al. The rigid versus soft postoperative dressing controversy: a controlled study in vascular below-knee amputees. Am Surg. 1980;46:244.
80. Walsh TL. Custom removable immediate postoperative prosthesis. J Prosthet Orthot. 2003;15:158.
81. Michael JW. Modern prosthetic knee mechanisms. Clin Orthop Relat Res. 1999;361:39.
82. Francis W, et al. Mobility after major limb amputation for arterial occlusive disease. Prosthet Orthot Int. 1987;11:85.
83. Robinson K. Amputation in vascular disease. Ann Royal Coll Surg Engl. 1980;62:87.
84. Pinzur MS, et al. Energy demands for walking in dysvascular amputees as related to level of amputation. Orthopedics. 1992;15:1033.
85. Gosselin S, et al. Outcomes during and after inpatient rehabilitation: comparison between adults and older adults. J Rehabil Med. 2008;40:55.
86. Stasik CN, et al. Functional outcome after redo below-knee amputation. World J Surg. 2008;32:1823.
87. Stewart CP, et al. Lower-limb amputee survival. Prosthet Orthot Int. 1992;16:11.
88. Dormandy J, et al. Major amputations: clinical patterns and predictors. Semin Vasc Surg. 1999;12:154.
89. Frank RG, et al. Psychological response to amputation as a function of age and time since amputation. Br J Psychiatry. 1984;144:493.
90. Parkes CM. Psycho-social transitions: comparison between reactions to loss of a limb and loss of a spouse. Br J Psychiatry. 1975;127:204.
91. Schubert DS, et al. Decrease of depression during stroke and amputation rehabilitation. Gen Hosp Psychiatry. 1992;14:135.
92. Donovan-Hall MK, et al. Engagement in activities revealing the body and psychosocial adjustment in adults with a trans-tibial prosthesis. Prosthet Orthot Int. 2002;26:15.
93. Gallagher P, et al. Psychological adjustment and coping in adults with prosthetic limbs. Behav Med. 1999;25:117.
94. Rybarczyk BD, et al. Social discomfort and depression in a sample of adults with leg amputations. Arch Phys Med Rehabil. 1992;73:1169.