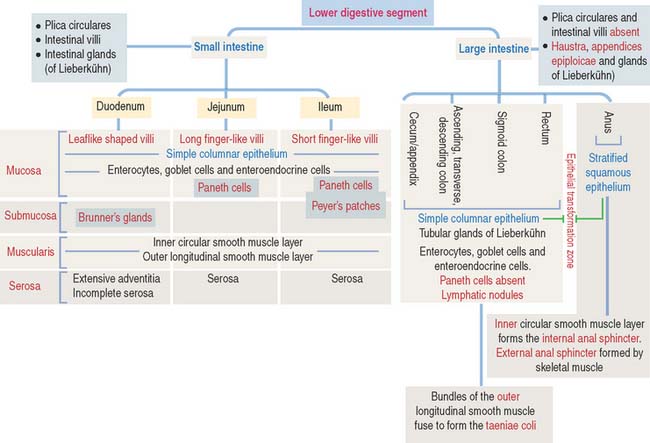
16 LOWER DIGESTIVE SEGMENT
SMALL INTESTINE
The wall of the small intestine consists of four layers (Figures 16-1 to 16-3): (1) the mucosa, (2) the submucosa, (3) the muscularis, and (4) the serosa, or peritoneum. As you will see, histologic differences are seen in the mucosa and submucosa of the three major portions of the small intestine. The muscularis externa and serosa layers are similar.
Intestinal wall
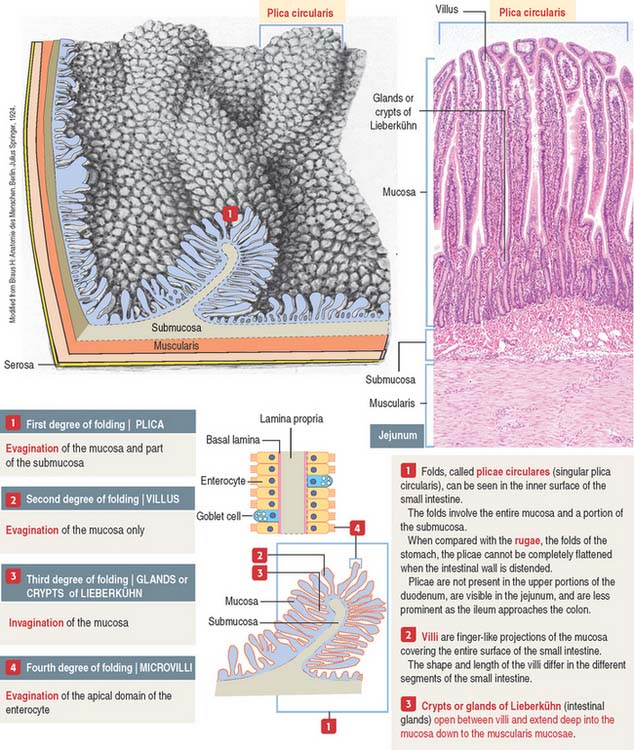
An increase in the total surface of the mucosa reflects the absorptive function of the small intestine. Four degrees of folding amplify the absorptive surface area of the mucosa (see Figure 16-2): (1) the plicae circulares (circular folds; also known as the valves of Kerkring), (2) the intestinal villi, (3) the intestinal glands, and (4) the microvilli on the apical surface of the lining epithelium of the intestinal cells (enterocytes).
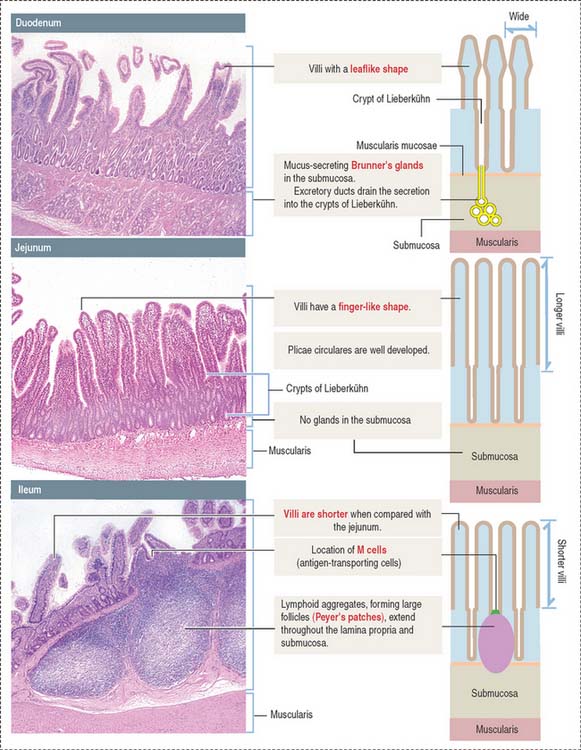
A plica circularis is a permanent fold of the mucosa and submucosa encircling the intestinal lumen.
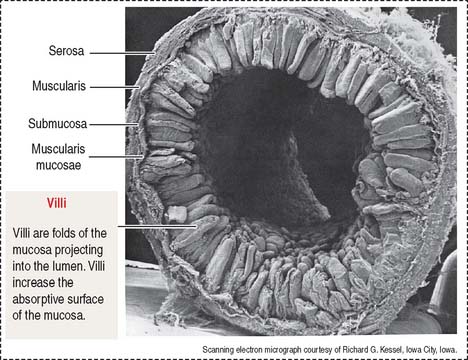
The intestinal villi are finger-like projections of the mucosa covering the entire surface of the small intestine. Villi extend deep into the mucosa to form crypts ending at the muscularis mucosae. The length of the villi depends on the degree of distention of the intestinal wall and the contraction of smooth muscle fibers in the villus core.
The muscularis mucosae is the boundary between the mucosa and submucosa (see Figure 16-3). The muscularis consists of inner circular smooth muscle and outer longitudinal smooth muscle. The muscularis is responsible for segmentation and peristaltic movement of the contents of the small intestine (Figure 16-4). A thin layer of loose connective tissue is covered by the visceral peritoneum, a serosal layer lined by a simple squamous epithelium, or mesothelium. The parietal peritoneum covers the inner surface of the abdominal wall.
Microcirculation of the small intestine
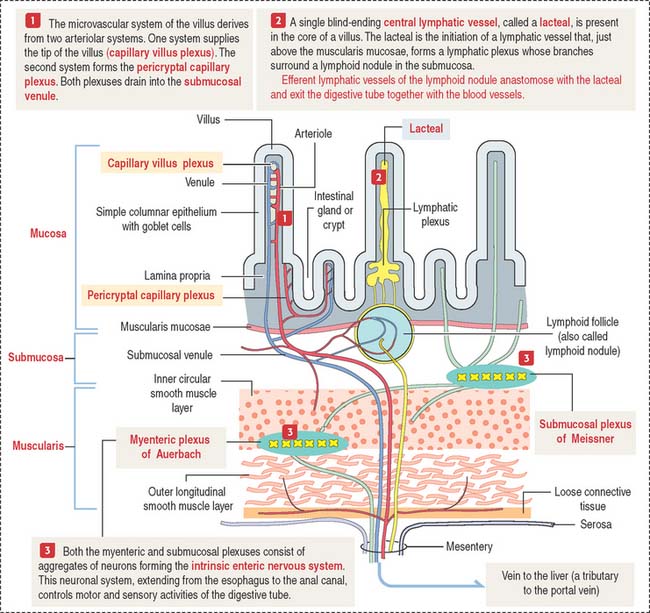
A difference from the microcirculation of the stomach (see Figure 15-8 in Chapter 15, Upper Digestive Segment) is that the intestinal submucosa is the main distribution site of blood and lymphatic flow (see Figure 16-3).
Innervation and motility of the small intestine
Motility of the small intestine is controlled by the autonomic nervous system. The intrinsic autonomic nervous system of the small intestine, consisting of the submucosal plexus of Meissner and myenteric plexus of Auerbach, is similar to that of the stomach (see Figure 15-9 in Chapter 15, Upper Digestive Segment).
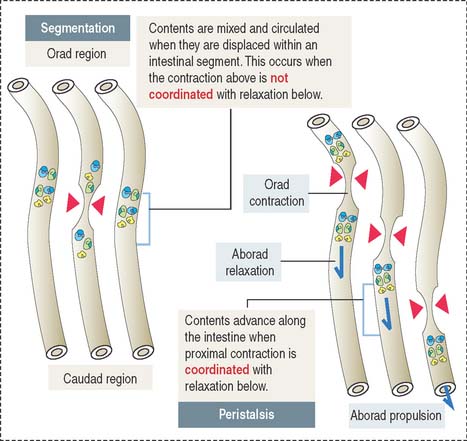
Contraction of the muscularis is coordinated to achieve two objectives (see Figure 16-4): (1) To mix and mobilize the contents within an intestinal segment. This is accomplished when muscular contraction activity is not coordinated and the intestine becomes transiently divided into segments. This process is known as segmentation. (2) To propel the intestinal contents when there is a proximal (orad) contraction coordinated with a distal (aborad; Latin ab, from; os, mouth; away from the mouth) relaxation. When coordinated contraction-relaxation occurs sequentially, the intestinal contents are propelled in an aborad direction. This process is known as peristalsis (Greek peri, around; stalsis, constriction).
Histologic differences between the duodenum, jejunum, and ileum
Each of the three major anatomic portions of the small intestine—the duodenum, jejunum, and ileum—has distinctive features that allow recognition under the light microscope (Figure 16-5).
Villi and crypts of Lieberkühn
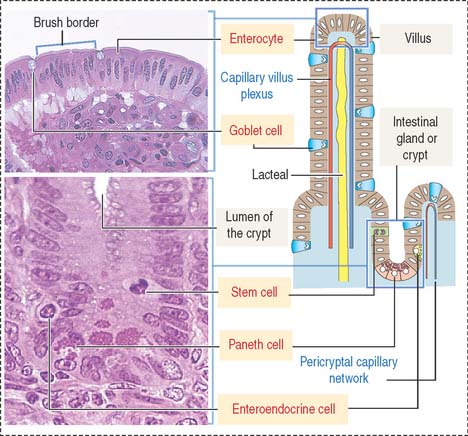
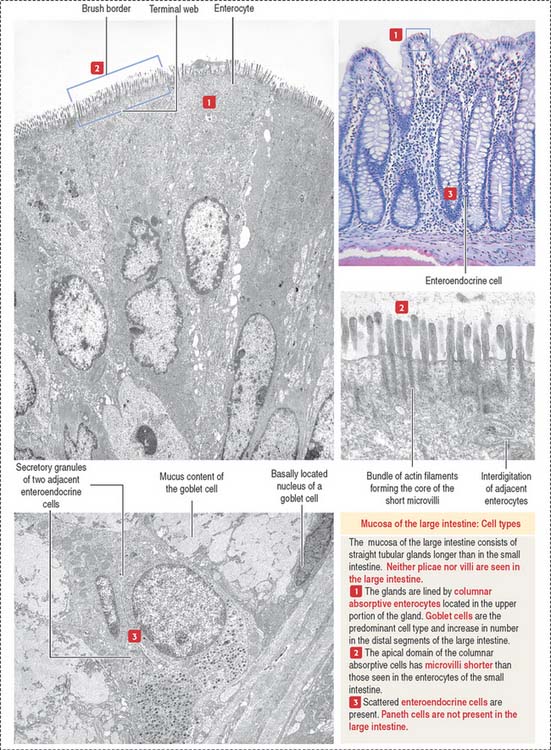
The intestinal mucosa, including the crypts of Lieberkühn, are lined by a simple columnar epithelium containing four major cell types (Figure 16-6): (1) absorptive cells, or enterocytes, (2) goblet cells, (3) Paneth cells, and (4) enteroendocrine cells. Stem cells, Paneth cells, and enteroendocrine cells are found in the crypts of Lieberkühn (see Figure 16-6).
Absorptive intestinal cells, or enterocytes
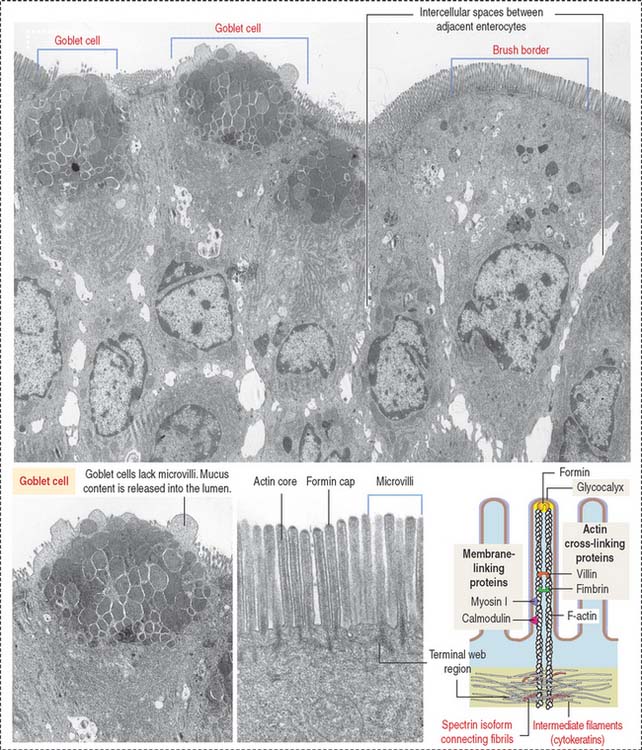
The absorptive intestinal cell or enterocyte has an apical domain with a prominent brush border (also called a striated border), ending on a clear zone, called the terminal web, which contains transverse cytoskeletal filaments. The brush border of each absorptive cell contains about 3000 closely packed microvilli, which increase the surface luminal area 30-fold.
The length of a microvillus ranges from 0.5 to 1.0 μm. The core of a microvillus (Figure 16-7) contains a bundle of 20 to 40 parallel actin filaments cross-linked by fimbrin and villin. The actin bundle core is anchored to the plasma membrane by formin (protein of the cap), myosin I, and the calcium-binding protein calmodulin. Each actin bundle projects into the apical portion of the cell as a rootlet, which is cross-linked by an intestinal isoform of spectrin to an adjacent rootlet. The end portion of the rootlet attaches to cytokeratin-containing intermediate filaments. Spectrin and cytokeratins form the terminal web. The terminal web is responsible for maintaining the upright position and shape of the microvillus and anchoring the actin rootlets.
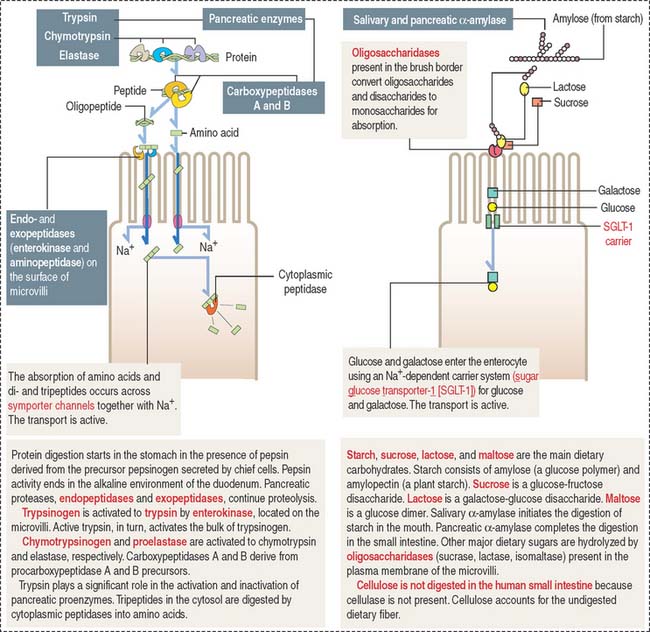
The microvilli, forming a brush border, contain intramembranous enzymes, including lactase, maltase, and sucrase (Figure 16-8). These oligosaccharides reduce carbohydrates to hexoses, which can be transported into the enterocyte by carrier proteins. A genetic defect in lactase prevents the absorption of lactose-rich milk, leading to diarrhea (lactose intolerance). Therefore, the brush border not only increases the absorptive surface of enterocytes but is also the site where enzymes are involved in the terminal digestion of carbohydrates and proteins.
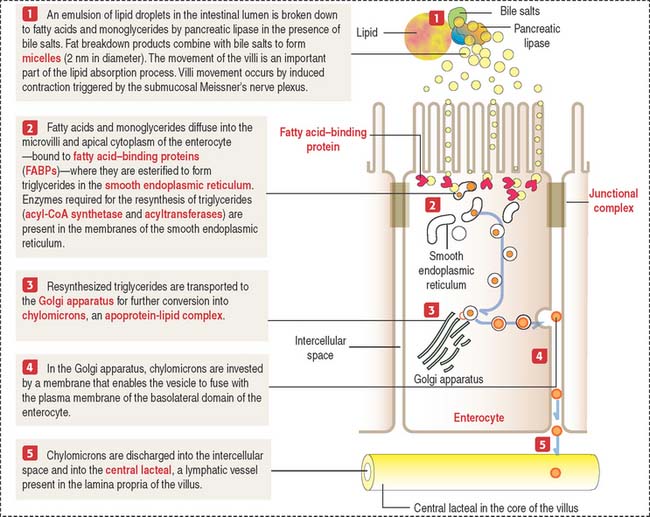
The absorption of lipids involves the enzymatic breakdown of dietary lipids into fatty acids and monoglycerides, which can diffuse across the plasma membrane of the microvilli and the apical plasma membrane of the enterocyte. Details of the process of fat absorption are depicted in Figure 16-9.
Goblet cells
Goblet cells are columnar mucus-secreting cells scattered among enterocytes of the intestinal epithelium (see Figure 16-7).
Goblet cells have two domains: (1) a cup- or goblet-shaped apical domain containing large mucus granules that are discharged on the surface of the epithelium and (2) a narrow basal domain, which attaches to the basal lamina and contains the rough endoplasmic reticulum in which the protein portion of mucus is produced. The Golgi apparatus, which adds oligosaccharide groups to mucus, is prominent and situated above the basally located nucleus.
Enteroendocrine cells
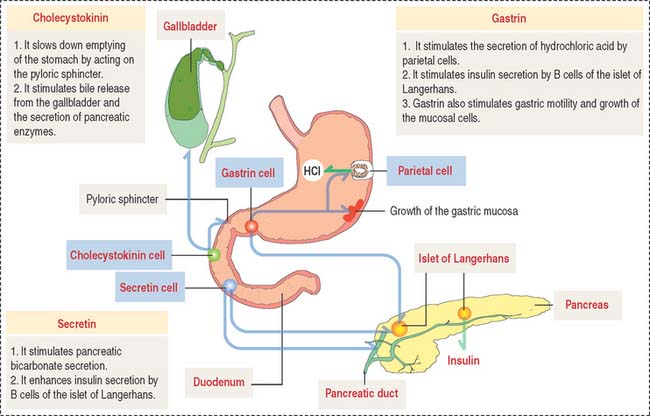
We have already studied the structural and functional features of enteroendocrine cells in the stomach (see Chapter 15, Upper Digestive Segment). As in the stomach, enteroendocrine cells secrete peptide hormones controlling several functions of the gastrointestinal system. The location and function of gastrin-, secretin-, and cholecystokinin-secreting cells are summarized in Figure 16-10.
PROTECTION OF THE SMALL INTESTINE
The large surface area of the gastrointestinal tract, about 200 m2 in humans, is vulnerable to resident microorganisms, called microbiota, and potentially harmful microorganisms and dietary antigens. We discussed in Chapter 15, Upper Digestive Segment, the role of the mucus blanket in the protection of the surface of the stomach during Helicobacter pylori infection. In the small and large intestines, goblet cells secrete mucin glycoproteins assembled into a viscous gel-like blanket limiting direct bacterial contact with enterocytes. A lack of mucin glycoprotein 2 (MIC2) causes spontaneous intestinal inflammation.
Intestinal tight junction barrier
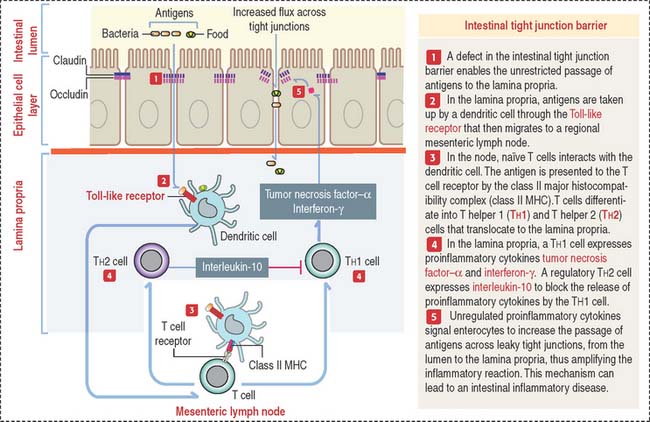
A minor tight junction barrier defect can allow bacterial products or dietary antigens to cross the epithelium and enter the lamina propria. Antigens can bind to Toll-like receptor of dendritic cells. Dendritic cells migrate to a local mesentery lymph node and the antigen is presented to naïve T cells by the major histocompatibility complex to determine their differentiation into T helper 1 (Th1) and T helper 2 (Th2) cells that relocate to the lamina propria (Figure 16-11). Th1 cells produce the proinflammarory cytokines tumor necrosis factor and interferon-γ. Th2 cells downregulate the proinflammatory activity of Th1 cells by secreting interleukin-10. If the mucosa immune cell activation response proceeds unchecked, proinflammatory cytokines will continue enhancing further leakage across the tight junction barrier, a condition leading to intestinal chronic inflammatory diseases.
Peyer’s patches
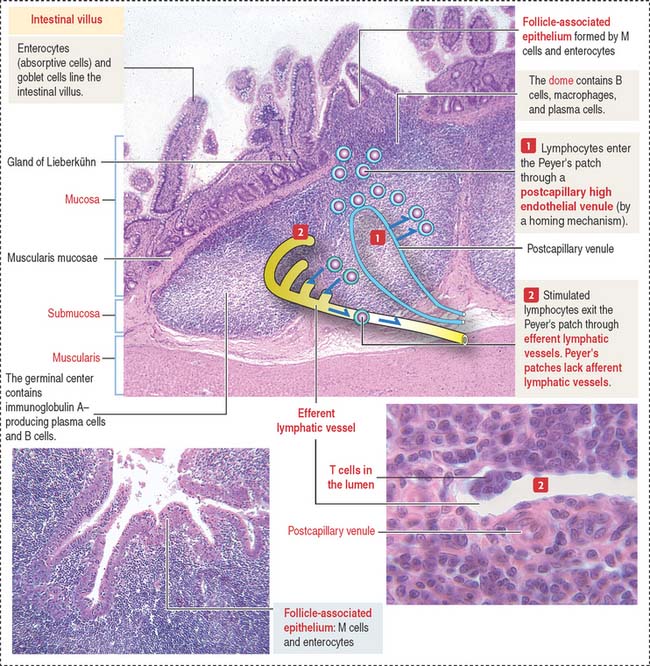
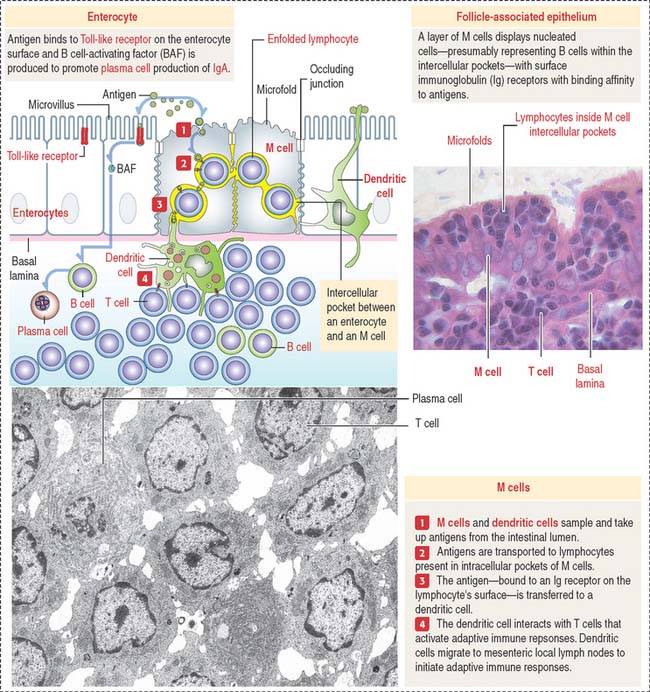
Peyer’s patches—the main component of the gut-associated lymphoid tissue (GALT)—are specialized lymphoid follicles found in the intestinal mucosa and part of the submucosa (see Box 16-A). A Peyer’s patch displays two main components (Figure 16-12): (1) a dome and (2) a germinal center.
Box 16-A Development of Peyer’s patches
Peyer’s patches are lined by the follicle-associated epithelium (FAE), consisting of M cells and enterocytes—both derived from stem cells present in the intestinal glands. Antigens in the intestinal lumen activate Toll-like receptors, expressed by enterocytes (see Box 10-A in Chapter 10, Immune-Lymphatic System), leads to the production of B cell-activating factor and cytokines that stimulate the production of immunoglobulin (Ig) A by plasma cells.
The dome separates the Peyer’s patch from the overlying surface epithelium and contains B cells expressing all immunoglobulin isotypes, except IgD. The germinal center contains IgA-positive B cells, CD4+ T cells, and antigen-presenting cells (dendritic cells). A few plasma cells are present in the Peyer’s patches.
The main components of the FAE are the M cell (Figure 16-13), a specialized epithelial cell that takes up antigens into protease (cathepsin E)-containing vesicles, and the dendritic cell, extending cytoplasmic processes across epithelial tight junctions. Antigens are transported by transcytosis to adjacent intercellular spaces and presented to immunocompetent cells (B cells).
Clinical significance: Targeting mucosal vaccine vectors to M cells
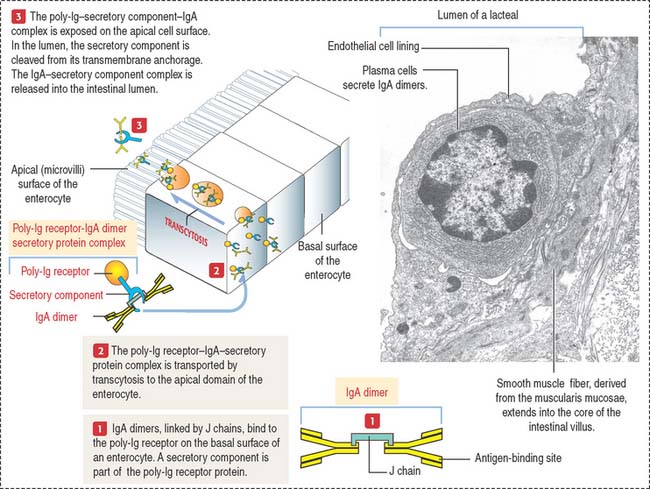
This host defense strategy can eventually lead to the production of secretory IgA dimer (Figure 16-14) and proteins from Paneth cells (Figure 16-15) to clear the mucosal surface of pathogens.
Plasma cells and secretory IgA dimer
IgA molecules secreted by plasma cells are transported from the lamina propria to the intestinal lumen by a transcytosis mechanism consisting of the following steps (see Figure 16-14): (1) IgA is secreted into the lamina propria as a dimeric molecule associated with a joining peptide, called the J chain. (2) The IgA dimer binds to a specific receptor, called the poly-immunoglobulin (poly-Ig) receptor, expressed on the basolateral surfaces of the intestinal epithelial cell. The poly-Ig receptor has an attached extracellular secretory component. (3) The IgA–poly-Ig receptor–secretory component complex is internalized and transported across the cell to the apical surface of the epithelial cell (transcytosis). (4) At the apical surface, the complex is cleaved enzymatically and the IgA-secretory component complex is released into the intestinal lumen. The secretory component protects the dimeric IgA from proteolytic degradation. (5) IgA antibodies prevent the attachment of bacteria or toxins to epithelial cells. (6) Excess IgA dimers diffuse from the lamina propria into the bloodstream and are excreted into the intestinal lumen via the bile.
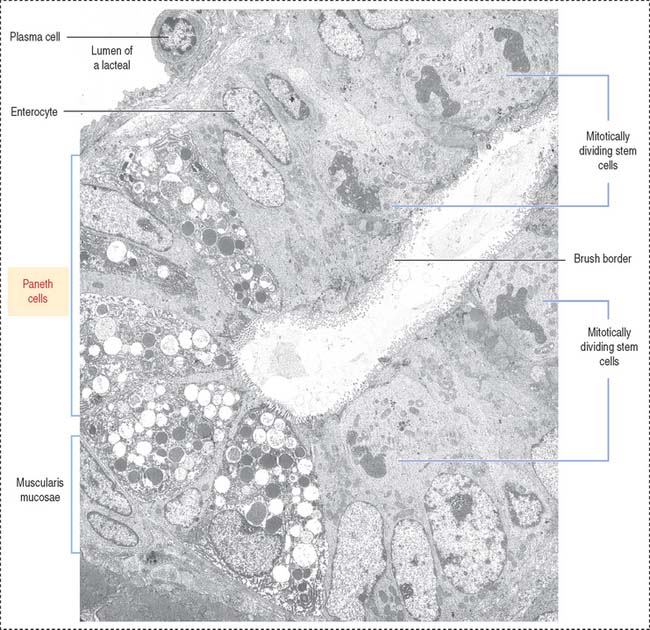
Paneth cells
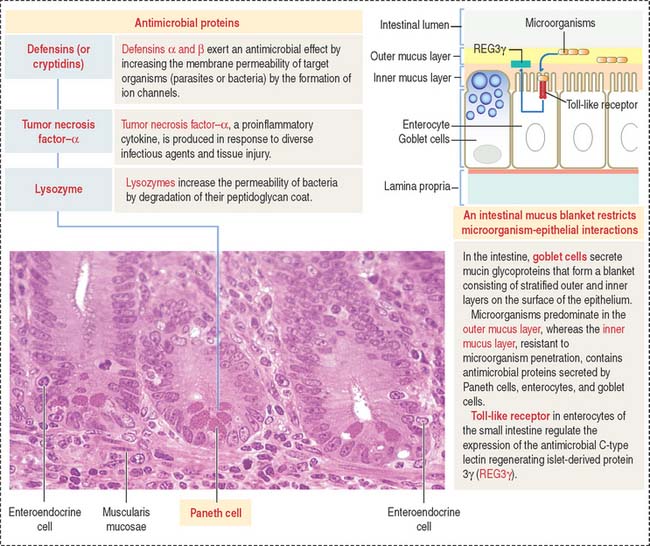
Paneth cells are present at the base of the crypts of Lieberkühn and have a lifetime of about 20 days. The pyramid-shaped Paneth cells have a basal domain containing the rough endoplasmic reticulum. The apical region contains numerous protein granules (see Figure 16-15. Figure 16-16).
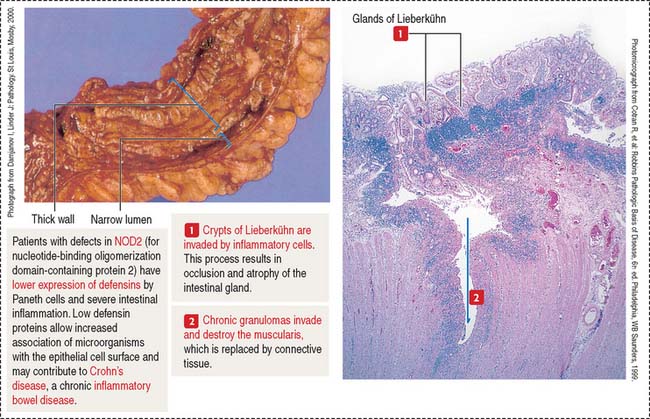
The three major products contained in the granules of Paneth cells are (1) TNF-α, (2) lysozyme, and (3) a group of proteins known as defensins or cryptidins. The expression of a subset of antimicrobial proteins is controlled by bacterial signals. For example, Toll-like receptor in enterocytes control the expression of the antimicrobial C-type lectin REG3γ (for regenerating islet-derived protein 3γ) in the small intestine. NOD2 (for nucleotide-binding oligomerization domain-containing protein 2) controls the expression of defensins.
Clinical significance: Inflammatory bowel diseases
Crohn’s disease is a chronic inflammatory process involving the terminal ileum but is also observed in the large intestine. Inflammatory cells (neutrophils, lymphocytes, and macrophages) produce cytokines that cause damage to the intestinal mucosa (Figure 16-17).
As discussed (see Figure 16-11), cytokines produced by helper T cells within the intestinal mucosa cause a proinflammatory response that characterizes inflammatory bowel disease. In Crohn’s disease, type 1 helper cells (Th1 cells) produce TNF-α and interferon-γ. Because TNF-α is a proinflammatory cytokine, antibodies to this cytokine are being administered to patients with Crohn’s disease to attenuate proinflammatory activity.
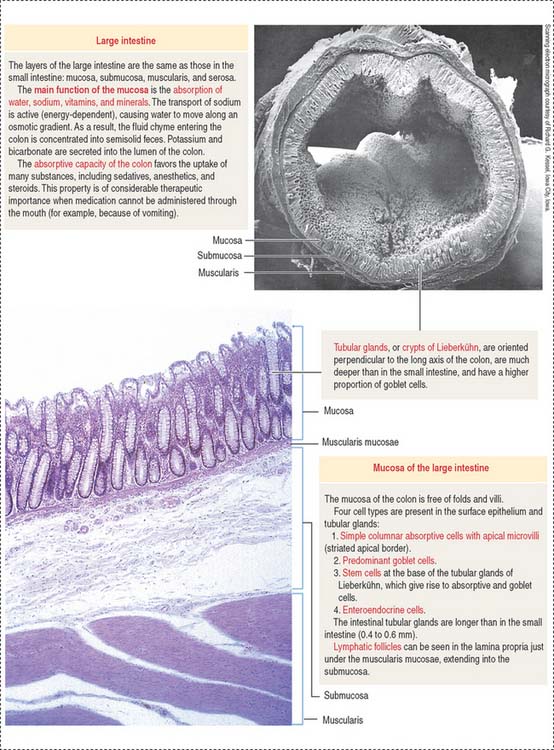
LARGE INTESTINE
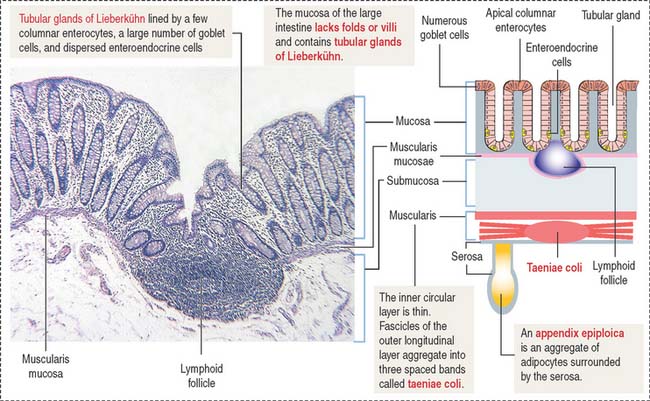
Plicae circulares and intestinal villi are not found beyond the ileocecal valve. Numerous openings of the straight tubular glands or crypts of Lieberkühn are characteristic of the mucosa of the colon (Figure 16-18).
The lining of the tubular glands of the colon consists of the following (Figures 16-19 and 16-20):
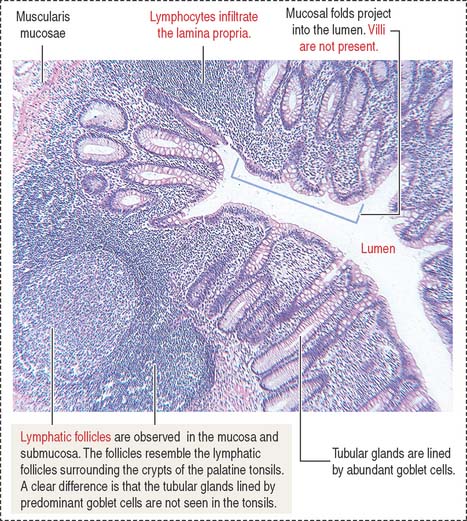
The appendix (Figure 16-21) is a diverticulum of the cecum and has layers similar to those of the large intestine. The characteristic features of the appendix are the lymphoid tissue, represented by multiple lymphatic follicles, and lymphocytes infiltrating the lamina propria. Lymphatic follicles extend into the mucosa and submucosa and disrupt the continuity of the muscularis mucosae.
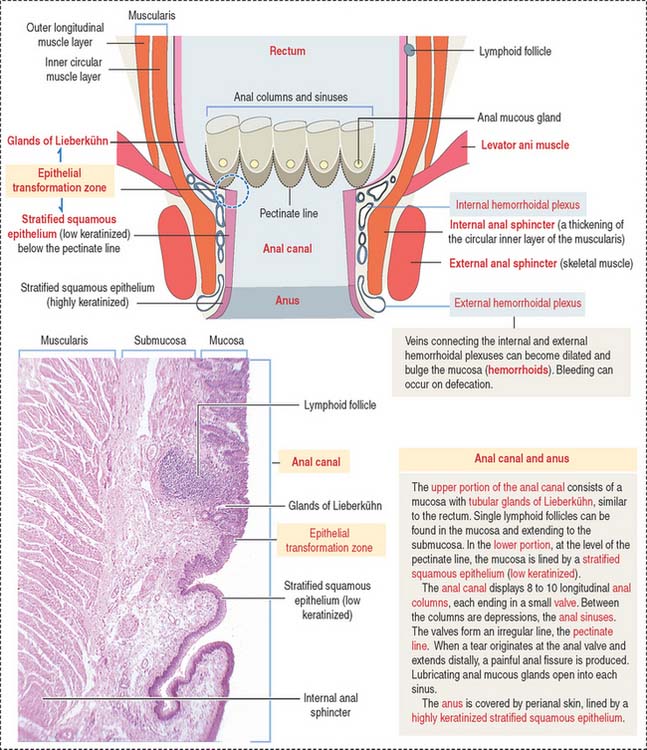
The rectum, the terminal portion of the intestinal tract, is a continuation of the sigmoid colon. The rectum consists of two parts: (1) the upper part, or rectum proper, and (2) the lower part, or anal canal. The mucosa is thicker, with prominent veins, and the crypts of Lieberkühn are longer (0.7 mm) than in the small intestine and lined predominantly by goblet cells. At the level of the anal canal, the crypts gradually disappear and the serosa is replaced by an adventitia.
The anal canal extends from the anorectal junction to the anus (Figure 16-22). A characteristic feature of the mucosa of the anal canal are 8 to 10 longitudinal anal columns. The base of the anal columns is the pectinate line. The anal columns are connected at their base by valves, corresponding to transverse folds of the mucosa. Small pockets, called anal sinuses, or crypts, are found behind the valves. Anal mucous glands open into each sinus.
Clinical significance: Hirschsprung’s disease
We discussed in Chapter 8, Nervous Tissue, that during formation of the neural tube, neural crest cells migrate from the neuroepithelium along defined pathways to tissues, where they differentiate into various cell types. One destination of neural crest cells is the alimentary tube, where they develop the enteric nervous system. The enteric nervous system partially controls and coordinates the normal movements of the alimentary tube that facilitate digestion and transport of bowel contents.
The large intestine, like the rest of the alimentary tube, is innervated by the enteric nervous system receiving impulses from extrinsic parasympathetic and sympathetic nerves and from receptors within the large intestine.
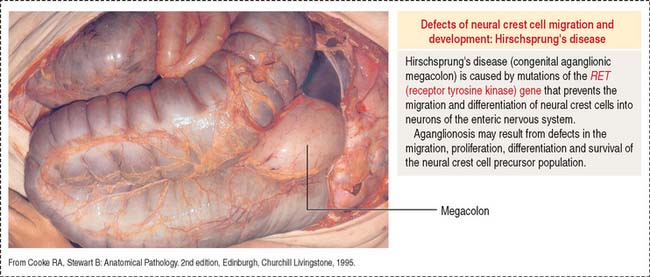
Delayed transit through the colon leads to severe constipation. An abnormal form of constipation is seen in Hirschsprung’s disease (congenital megacolon) caused by the absence of the enteric nervous system in a segment of the distal colon (Figure 16-23). This condition, called aganglionosis, is the result of an arrest in the migration of cells from the neural crest, the precursors of the intramural ganglion cells of the plexuses of Meissner and Auerbach.
Aganglionosis is caused by mutations affecting the RET gene encoding receptor tyrosine kinase. RET signaling is required for the formation of Peyer’s patches (see Box 16-A), for the migration of neural crest cells into the distal portions of the large intestine and for differentiation into neurons of the enteric nervous system.
Clinical significance: Familial polyposis gene and colorectal tumorigenesis
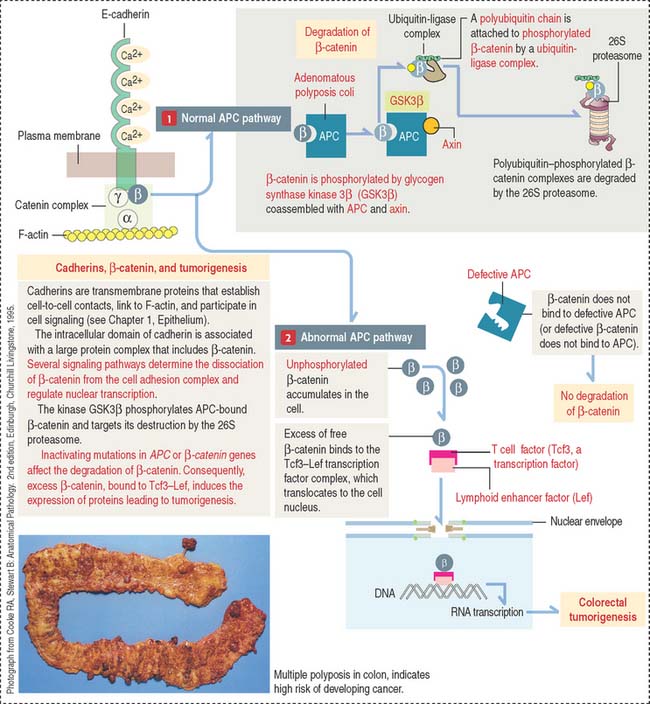
Colorectal tumors develop from a polyp, a tumoral mass that protrudes into the lumen of the intestine. Some polyps are non-neoplastic and are relatively common in persons 60 years and older. Polyps can be present in large number (100 or more) in familial polyposis syndromes such as familial adenomatous polyposis and the Peutz-Jeghers syndrome. Familial polyposis is determined by autosomal dominant mutations, in particular in the APC (adenomatous polyposis coli) gene. Mutations in the APC gene have been detected in 85% of colon tumors, indicating that, as with the retinoblastoma (Rb) gene, the inherited gene is also important in the development of the sporadic form of the cancer.
The APC gene encodes APC protein with binding affinity to microtubules and β-catenin, a molecule associated with a catenin complex linked to E-cadherin (discussed in Chapter 1, Epithelium) and also a component of nuclear transcription complexes.
When β-catenin is not part of the catenin α, β, γ complex, free β-catenin interacts with DNA binding proteins of a family of transcription factor proteins called T cell factor–lymphoid enhancer factor (Tcf3-Lef) to form a transactivator complex that stimulates transcription of immediate gene targets (Figure 16-24).
The APC gene is also a major regulator of the Wnt pathway, a signaling system expressed during early development and embryogenesis (see Chapter 3, Cell Signaling). The Wnt pathway has an important function in the development of neural crest–derived cells. Wnt proteins can inactivate GSK3β, prevent the phosphorylation of β-catenin, and abrogate its destruction by the 26S proteasome. Consequently, an excess of β-catenin translocates to the cell nucleus to affect gene transcription.
A defective β-catenin pathway can overexpress the microphthalmia-associated transcription factor (MITF). We discussed in Chapter 11, Integumentary System, the significance of MITF in the survival and proliferation of melanoma cells.
Lower Digestive Segment
Enteroendocrine cells produce gastrin, secretin, and cholecystokinin. Their distribution and function of enteroendocrine cells are summarized in Essential Concepts in Chapter 15, Upper Digestive Segment.
The rectum, the terminal portion of the large intestine and a continuation of the sigmoid colon, consists of two regions: (1) the upper region, or rectum proper, and (2) the lower region, or anal canal, which extends from the anorectal junction to the anus.