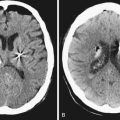
Chapter 34 Low-Grade and High-Grade Gliomas
• Low-grade gliomas (LGGs) account for 15% of primary adult tumors diagnosed, and in adults they mainly occur in supratentorial areas, particularly in the insular and supplementary motor areas.
• LGGs comprise astrocytomas, oligodendrogliomas, and oligoastrocytomas.
• Factors such as age less than 40 years, Karnofsky performance scale (KPS) score of 70 or greater, absence of contrast enhancement, and oligodendrogliomas are associated with a better prognosis for LGGs.
• The extent of surgical resection probably correlates with a better survival for LGGs, but resection must be tempered by their location close to the eloquent area. Motor-sensory mapping, language mapping, and diffusion tensor imaging should be used to assess tumors adjacent to critical regions.
• Postoperative radiotherapy is often used for LGGs and provides improved progression-free survival but does not extend overall survival.
• A better response to chemotherapy is seen in patients with oligodendrogliomas, which exhibit combined 1p/19q loss of heterozygosity.
• High-grade gliomas (malignant gliomas and glioblastoma multiforme [GBM]) may arise in progression from low-grade astrocytic tumors (secondary GBMs) or as de novo lesions (primary GBMs). There are distinct genetic differences between these two groups of tumors.
• Factors such as a younger age group, lower tumor histological grade, and higher KPS score are associated with a better prognosis for high-grade gliomas.
• The extent of resection (absence of class 1 evidence) has a probable influence on survival. Both radiotherapy and temozolomide chemotherapy significantly increase survival time of these patients.
• Advances in molecular markers may open avenues for new therapies.
Low-Grade Gliomas
A Perspective on Management
There are currently a number of considerations involved in the management of patients with low-grade gliomas (LGGs), which, for the purposes of this discussion, will be defined as World Health Organization (WHO) grade II gliomas. Advances in tumor biology, neuroimaging, and treatment paradigms have enabled the neurosurgeon to approach these patients with a better understanding of the disease entity and its natural history. However, many controversial issues remain unanswered. Diagnostic strategies previously considered reliable for LGG patients, including structural magnetic resonance imaging (MRI) and stereotactic biopsy, have more recently been shown to vary substantially with regard to specificity, sensitivity, and sampling error. Surgical management paradigms are also shifting, as mounting evidence now highlights the predictive value of volumetric tumor burden for patient survival, the role of greater extent of resection in reducing malignant transformation rates, and the influence of tumor eloquence in determining resectability. Additionally, LGG-associated seizures are increasingly considered as a key determinant of quality of life, with electrocorticography being used as an effective surgical adjunct in this regard when seizures are medically refractory. Adjuvant therapies are also under renewed scrutiny. Although the utility of radiation therapy is clear, the timing and type of chemotherapy remain somewhat uncertain, as does the role of new molecular therapeutics. Taken together, these new innovations and controversies define the modern era of LGG management. Here we review the current literature in an effort to highlight high-impact developments that are changing our view of LGGs, as well as the pivotal studies that should guide neurosurgeons as they consider these many issues.
Epidemiology
LGGs are not uncommon, representing 15% of all primary adult brain tumors diagnosed each year. They are most frequent among white men and typically affect patients at a younger age than high-grade gliomas (fourth vs. sixth decade of life). Even though LGGs are diffusely distributed along a variety of supratentorial regions, they have a particular predilection for the insula and supplementary motor area. In contrast, LGGs rarely involve the cerebellum, brainstem, or spinal cord, as is commonly found in children. Most patients initially present with relatively good neurological function, and seizures are the most common symptom at presentation (80%). The only definite risk factor for LGG is previous exposure to ionizing radiation.1 Hereditary factors do not play a substantial role in the development of LGGs, although these tumors are more common in patients with neurofibromatosis type 1 and Li-Fraumeni syndrome. From 15% to 20% of individuals with neurofibromatosis type 1 develop LGGs affecting the optic nerves, optic chiasm, and hypothalamus (optic pathway gliomas). Most of these gliomas are classified as WHO grade I tumors, although grade II LGGs can also occur in these locations.2
The etiology of adult LGGs is largely unknown and is thought to be multifactorial; various genetic, infectious, and immunological factors have been implicated. Glioma epidemiology studies have revealed few consistent findings, possibly because of small sample sizes in individual studies and differences between studies in patients, tumor types, and methods of classification. Individual studies generally have lacked samples of sufficient size to examine interactions, but larger consortium efforts have outlined several potential risk factors for gliomagenesis and outcome.1 Data from the Surveillance, Epidemiology, and End Results (SEER) Program indicated that African Americans had similar or poorer survival than whites,3 but those results were adjusted incompletely for important prognostic factors (e.g., age at diagnosis, treatment patterns, and tumor histological types). After adjustment, African Americans had a 40% higher risk of death from low-grade tumors compared with non-Hispanic whites.4 Likewise, risks from specific neurocarcinogens have yet to be identified; however, the continued occurrence of brain tumor clusters leaves open the question of the effect and extent of their exposures. Observations of an association between drinking water and brain tumors suggest that ingestion of an environmental contaminant has an impact,5–7 perhaps from chlorinated sources like chloroethane, a by-product of sewage treatment, or nitrate/nitrite contamination of drinking water supplies.
Recent epidemiological studies have also reported that adults with low- as well as high-grade gliomas are 1.5- to 4-fold less likely than control subjects to have allergies, which ranks the lack of allergies among the most consistent risk factors for glioma reported to date. In addition, an inverse relationship exists between immunoglobulin E (IgE), a biomarker for atopic allergy, and glioma risk. Interestingly, the strongest IgE-glioma association has been observed among the least prevalent allergen—food IgE. Low- and high-grade glioma patients with elevated levels of IgE are associated with an approximately 8 months’ longer survival than individuals with lower or undetectable levels, demonstrating the potential clinical significance of such correlates.8
Classification
Tumor histological type remains the WHO’s current standard for diagnosing glioma grade and subtype. As with all primary brain tumors, gliomas are classified according to their predominant cell type and graded based upon the presence or absence of necrosis, mitotic figures, nuclear atypia, and endothelial cell proliferation. Although grade I and II lesions are both categorized as LGGs, they follow radically different clinical courses and, for the purposes of this review, we will focus only on WHO grade II oligodendrogliomas, astrocytomas, and oligoastrocytomas that occur in adults. Among WHO grade II astrocytomas, cellularity is moderately increased and nuclear atypia is occasional, but mitoses, endothelial proliferation, and necrosis are not present. The prognostic value of defining subcategories of gliomas based upon these features remains unclear; nevertheless three histological subtypes are described: fibrillary, gemistocytic, and protoplasmic neoplastic astrocytes define these subtypes, each embedded in a loosely structured and microcystic tumor matrix. Fibrillary astrocytomas, the most frequent variant, demonstrate low cellularity with minimal nuclear atypia. Neoplastic fibrillary astrocytes are typically seen on a background of loosely structured tumor matrix that is extensively microcystic and expresses the intermediate filament marker, glial fibrillary acidic protein (GFAP), diffusely. Gemistocytic astrocytomas are histologically characterized by plump, glassy, eosinophilic cell bodies of angular shape. These gemistocytes consistently express GFAP, and the presence of abundant, compact glial filaments in the cytoplasm is also evident on electron microscopy. Interestingly, gemistocytic astrocytomas are reportedly more prone to malignant transformation than other histological counterparts, raising the possibility that they are not biologically low-grade gliomas and may belong in the high-grade glioma category. Protoplasmic astrocytomas, the rarest histological subtype, contain small-bodied astrocytes with few processes and scant GFAP expression. Mucoid degeneration and microcystic formation are common characteristics as well.
Oligodendrogliomas occur in the white matter and cortex of the cerebral hemispheres and show a monotonous pattern on low power with occasional nodules of higher cellularity. Unlike WHO grade II astrocytomas, the presence of low mitotic activity, vascular proliferation, and necrosis, including pseudopallisading necrosis, are insufficient by themselves to elevate the grade of WHO grade II oligodendrogliomas. Their nuclei are round and regular, and clear perinuclear halos are present in most paraffin-embedded specimens. This typical “fried egg” appearance is a formalin fixation artifact and is therefore not seen in frozen sections, smears, or rapidly fixed specimens. Oligoastrocytomas are a recognized category of LGGs, but are ill-defined, prone to subjectivity, and based on an unproven concept of dual differentiation of astrocytoma and oligodendroglioma as neoplastic processes.9 Histologically, they are defined by a mixture of cells, some with oligodendroglioma features, and others resembling diffuse astrocytomas. Currently, there are no standardized immunohistochemistry or molecular panels to distinguish oligoastrocytomas from other LGGs.
Clinical Presentation
Patients with LGGs present with signs and symptoms of disease related to direct parenchyma infiltration; local tumor effects due to edema, hemorrhage, or tumor mass; or intracranial hypertension mediated by mass effects or ventricular obstruction. Although the onset of symptoms can be subtle and insidious, when patients become symptomatic, seizure is the most common presenting sign, occurring in up to 80% of cases;10 this is probably due to the superficial localization and low growth rate of the tumor in many cases. Other, less common clinical presentations include headache, lethargy, and personality changes, although these symptoms, caused by raised intracranial pressure, are rare.
Prognostic Factors
Although substantial heterogeneity exists when profiling LGG patient outcome, several clinical factors are known to be predictive. Chief among those is age over 40 years, a predictive factor identified in multivariate analyses from two large, prospective trials.11,12 Age at the time of LGG diagnosis is not only inversely correlated with time to progression, but tumor proliferative index may be higher among those older than 40 years as well.13 Although the biology behind this association is unclear, one possibility is that age-related impairment of DNA repair mechanisms and the resulting acquisition of mutations may promote rapid progression after transformation occurs. Clinical presentation is another strong prognostic factor, as neurologically intact patients presenting with isolated seizures typically have a better performance status and overall prognosis. LGG patients who present with seizures also tend to be younger and have smaller tumors than those without seizure.14
An LGG preoperative prognostic scoring system developed at the University of California at San Francisco (UCSF) assigns a prognostic score based upon the sum of points assigned to the presence of each of the four following factors (1 point per factor): (1) location of tumor in presumed eloquent cortex, (2) Karnofsky performance scale (KPS) score 80 or less, (3) age more than 50 years, and (4) maximum diameter more than 4 cm. Cox proportional hazard modeling was used to confirm that the individual factors were associated with shorter overall survival (OS) and progression-free survival (PFS); and Kaplan-Meier curves estimated OS and PFS for the score groups.15 Low-risk tumors are considered grades 0 or 1, and high-risk tumors are grade 4. This scoring system accurately predicted OS and PFS in a multi-institutional population of LGG patients.16
The median survival for oligodendrogliomas is approximately 15 years, a better prognosis than for astrocytomas, which have a median survival of 10 years.17 Gemistocytic astrocytomas, a subtype of grade II astrocytomas, are more aggressive than predicted by grade.18 Large tumors, nonlobar gliomas, and tumors that cross the midline are associated with a short survival and a high rate of malignant transformation.11 Preoperative tumor burden is also associated with less extensive resection, which in turn portends poorer outcome.19 Proliferative index has also been inversely related with LGG outcome, as has contrast enhancement.20
Recent work in glioma outcomes research also suggests that more unconventional factors may play a role in LGG patient outcome. For example, among patients with nonanaplastic oligodendroglial tumors, younger age and surgical resection versus biopsy were significantly associated with better survival, as expected. Interestingly, however, those patients who were college graduates also showed significantly better survival in age-adjusted comparisons.21 Further consideration of impact of marital status, education, and other social factors in glioma survival may be warranted, as these factors also appear to be significant in predicting high-grade glioma outcome.
Efforts to synthesize LGG risk factors into distinct prognostic classes have led to four categories of patients: (1) younger patients (18-40 years of age) with a good performance status (KPS score ≥ 70) have a median survival of more than 10 years; (2) younger patients with a poor performance status (KPS score < 70) and older patients (>40 years of age) with a good performance status and no contrast enhancement had a median survival of more than 7 years; (3) older patients with a good performance status and with contrast enhancement had a median survival of less than 4 years; and (4) older patients with a poor performance status had a median survival of only 12 months.22 It remains unclear, however, how tumor extent of resection impacts the predictive value of each category following resection because it was not evaluated in that study.
Similarly, the EORTC (European Organization for Research and Treatment of Cancer) developed a prognostic scoring system based on two large, randomized, multicenter trials with more than 600 patients.11,12 In their multivariate analysis, age older than 40 years, astrocytic tumor type, tumor size greater than 6 cm, tumor crossing the midline, and neurological deficit at diagnosis were retained in the model. A favorable prognostic score was defined as no more than two of these adverse factors and was associated with a median survival of 7.7 years. The presence of three to five prognostic factors was associated with a median survival of 3.2 years (95% confidence interval [CI] = 3.0, 4.0).
Diagnostic Imaging
The 1.5-tesla MRI remains the imaging gold standard for noninvasive identification and diagnosis of LGGs, although the emergence of 3-tesla magnets has improved image resolution considerably.23 LGGs are characteristically homogeneously isointense to hypointense on T1-weighted images and hyperintense on T2-weighted images. The epicenters of low-grade astrocytomas are typically within the white matter, whereas oligodendrogliomas can be more superficial and will occasionally expand the adjacent gyrus. Contrast enhancement is uncommon, but more often is seen in oligodendrogliomas (25-50%). Calcifications are apparent in 20% of lesions and are characterized by foci of high T1 and low T2 signals. Vasogenic edema and mass effect are uncommon because of the slow-growing nature of these tumors. Rarely, large LGGs will involve three or more cerebral lobes (gliomatosis cerebri). Diffusion tensor imaging has proved to be an essential adjunct to structural imaging for both preoperative planning and intraoperative neuronavigation. Specification of functional tract deflection around a lesion can not only alter the operative approach, but can dictate the limits of resection. Modalities such as functional magnetic resonance imaging (fMRI) and magnetoencephalography (MEG) can aid in preoperative planning by identifying these functional pathways, although these techniques remain too imprecise for complex functions such as language mapping, as their sensitivity (positron emission tomography [PET], 75%; fMRI, 81%) and specificity (PET, 81%; fMRI, 53%) remain suboptimal.24–27 For the identification of peritumoral language pathways, direct intraoperative stimulation mapping remains the gold standard. Intraoperative MRI is another operating room technology that may impact LGG outcome. Through real-time guidance, it allows for localization of tumors and their margins, and facilitates continuous assessment of surgical progress. Studies of LGG patients who underwent resection in an intraoperative MRI suite report encouraging results in terms of achieving greater extent of resection.28,29
However, using standard structural imaging paradigms, the decision to presume low-grade histological type on the basis of a nonenhancing lesion is a common mistake. For patients with supratentorial mass lesions that exhibit the typical imaging features of LGG, structural MRI has a false positive rate as high as 50% when attempting to predict the histological diagnosis of astrocytoma.30 This risk of anaplasia in MRI nonenhancing lesions increases significantly with patient age.31 Thus, observation of LGGs is not a prudent option, and early tissue diagnosis is essential. These misleading imaging features are likely due to the intrinsic heterogeneity of LGGs, a characteristic evident on physiological MRI such as magnetic resonance spectroscopy (MRS), which can demonstrate pockets of high-grade populations nested within the tumor stroma.32 Thus, stereotactic biopsies should be planned using MRS guidance to target putative high-grade components in nonenhancing tumors.
Next-generation structural MRI technologies have recently focused on preoperatively defining LGGs. Microscopic molecular movement of water in tumor tissue reflects tissue properties that include varying levels of structural alterations, tumor cellularity, and vasogenic edema. Diffusion-weighted MRI (DWI) uses strong gradients to probe the structure of biological tissues at a microscopic level by measuring the brownian motion of water molecules. Acquiring data with gradients in three directions allows the calculation of the apparent diffusion coefficient (ADC), while acquiring data with gradients in six or more directions allows the calculation of the ADC and the fractional anisotropy (FA). Recent work using these emerging imaging paradigms attempted radiographic prediction of specific LGG subtypes. Interestingly, initial attempts demonstrated a significant difference in the ADC and FA values between newly diagnosed patients with grade II oligodendrogliomas and astrocytomas, and patients with the heterogeneous grade II oligoastrocytomas had values that fell in between.33 However, although ADC has been suggested to correlate to cell density in a mixed population of glioma patients, it remains unclear whether this parameter is what drives its correlation with specific LGG subtypes.
The emergence of physiological imaging techniques has indeed added a new dimension to LGG diagnosis and targeting.34 Proton MRS imaging (1H-MRSI) is another emerging modality that identifies the distribution of cellular metabolite levels. Five classes of molecules are generally observed in brain spectra: N-acetylaspartate (NAA); free choline and choline-containing compounds, including phosphocholine and glycerophosphocholine (Cho); creatine and phosphocreatine (Cr); lactate (Lac); and lipid (Lip). Using MRS, typical spectra of LGG include a dominant choline peak (reflecting increased membrane synthesis) with low-intensity N-acetylaspartate (reflecting decreased neuronal elements) and no quantifiable lipid or lactate (suggesting an absence of necrosis or hypoxia, respectively; both features of high-grade gliomas). The choline peak may be associated with cellular density and cellular proliferation, thereby improving selection of targets for biopsy. Normalized creatine/phosphocreatine levels (tCr) of LGGs are a significant prognostic factor for progression-free survival, as well as malignant progression-free survival.35 Newly introduced three-dimensional (3D) techniques allow whole anatomical regions to be quantified metabolically, correlating well with the region of T2 hyperintensity, as well as with tumor extension along white matter tracts.36 Three-dimensional MRS may also have the potential to evaluate the proliferation activity of LGGs and identify potentially more aggressive clinical behavior.37 There is less convincing evidence, however, that MRS is sufficient for monitoring and follow-up of patients with suspected LGG.38 In some instances, MRS can also be used to discriminate radiation necrosis from tumor progression, as well as to monitor treatment progress.39
Among low-grade astrocytomas, measurement of relative cerebral blood volume (rCBV) derived from dynamic susceptibility-weighted perfusion contrast-enhanced MRI (DSC-MRI) correlates well with tumor behavior and patient survival.40 For these tumors, rCBV specifies regional tumor vascularity and expression of vascular endothelial growth factor (VEGF), two critical factors driving tumor growth.41 Most low-grade astrocytomas demonstrate slightly higher rCBV than normal tissue (1.5), with an increase in rCBV (1.75-2.0) indicating the evolution of a more aggressive tumor and often preceding the emergence of enhancement.42 Low-grade astrocytoma rCBV measurements also correlate well with time to progression, raising the possibility that DSC-MRI can predict the risk of transformation.43 In contrast, however, low-grade oligodendrogliomas have a paradoxically high rCBV, confounding the strict reliability of this modality for preoperative assessment. Similar MRI techniques such as quantitative analysis of whole-tumor gadolinium enhancement are also predictive of malignant transformation for likely the same reasons.44
Radiographic quantification of tumor metabolism cannot only identify malignant transformation in LGG,45 but it can also be employed to guide stereotactic biopsies. On positron emission tomography (PET) using fluorodeoxyglucose (18F-FDG), LGGs are hypometabolic, a feature that commonly distinguishes them from high-grade gliomas. In contrast, uptake of radiolabeled amino acids is increased in approximately two thirds of LGGs, and a prognostic role for amino acid PET in LGG has been proposed. O-(2-18F-fluoroethyl)-L-tyrosine (18F-FET) is a new PET tracer that, in contrast to other amino acid tracers, fulfills all requirements for routine clinical application, similar to the widely used 18F-FDG. Accordingly, recent work indicates that baseline amino acid uptake on 18F-FET PET and a diffuse versus circumscribed tumor pattern on MRI are strong predictors for the outcome of patients with LGG.45 A comparable technique using 3′-deoxy-3′-18F-fluorothymidine (FLT), FLT PET, is a useful marker of cellular proliferation that correlates with regional variation in cellular proliferation; however, it is unable to identify the margin of LGGs.46
Surgery and the Value of Extent of Resection
In the past 2 decades, mounting evidence in the literature suggests that a more extensive surgical resection of LGG is associated with a more favorable life expectancy. In addition to providing longer overall survival, more aggressive resections for LGG can also influence the risk of malignant transformation, raising the possibility that a surgical intervention can alter the natural history of the disease.47 These associations are evident not only within the general hemispheric LGG population,19,48 but also for specific LGGs limited to certain subregions, such as insular LGGs.49,50 An overall review of the modern neurosurgical literature reveals 24 studies12,19,20,28,49,51–69 since 1990 that have applied statistical analysis to examine the efficacy of extent of resection in improving survival and delaying tumor progression among LGG patients. Six of these studies included volumetric analysis of extent of resection.12,28,49,57,60,70 Of the nonvolumetric studies, 15 demonstrated evidence supporting extent of resection as a statistically significant predictor of either 5-year survival or 5-year progression-free survival. These studies were published from 1990 to 2009 and most commonly employed a combination of multivariate and univariate analyses to determine statistical significance. Interestingly, of the three nonvolumetric studies that did not support extent of resection as a predictor of patient outcome, none of these reports evaluated progression-free survival, but instead focused solely on 5-year survival.
Special Considerations: Malignant Transformation
Malignant degeneration of LGGs is a special consideration for these patients, as it carries with it a dramatically worse prognosis. Interestingly, the documented incidence of LGG transformation ranges from 17% to 73% in clinical studies published over the previous 15 years,19,20,48,64,67,71–75 suggesting a high level of variability. Defining the timing of this phenomenon is equally elusive, as reported median intervals range from 2.1 to 10.1 years based upon studies published in the past 15 years.19,20,48,67,76,77 Several recent studies have examined malignant transformation in the context of extent of resection, reasoning that the risk of progression increases with tumor burden. In a study of hemispheric LGGs, greater preoperative tumor volume was significantly associated with shorter malignant progression-free survival,19 suggesting that larger tumors at presentation may have an inherently faster growth rate, and thus recur faster in the setting of a gross total resection or continue to grow faster in the setting of a subtotal resection. Tumor growth rates were also studied among 143 consecutive adult LGGs, in which a median survival of 5.16 years was associated with a growth rate of 8 mm per year or more and a median survival of more than 15.0 years was seen with a growth rate less than 8 mm per year.78 Others have also evaluated LGG growth rate, demonstrating that sequential measurement of LGG volume allows accurate determination of growth rates and identification of patients whose tumors are at high risk for early transformation.79 Six-month tumor growth also may help to predict outcome in patients with LGG better than parameters derived from perfusion- or diffusion-weighted MRI.80 Similarly, within the insula, the interval to malignant progression among grade II gliomas is longer in patients who have undergone greater resections.49 As with hemispheric LGGs, the volume of residual tumor in the insula serves as a predictor of malignant transformation. Thus, these studies represent a potential shift in our concept of aggressive glioma resection, as the ability to manipulate the natural history of these tumors makes a case for earlier intervention and argues against the validity of a simple biopsy procedure or a wait-and-watch approach.
Mapping Functional Pathways
The principle that patient outcome improves with greater extent of resection must be tempered by the potential for functional loss following a radical resection. To this end, MRI neuronavigational techniques not only facilitate greater resection, but embedding of DTI-based tractography can prevent inadvertent resection of adjacent subcortical pathways.81,82 In a recent study of 238 glioma patients randomized to DTI-based imaging versus traditional MRI neuronavigation without DTI, postoperative motor deterioration occurred in 32.8% of control cases, whereas it occurred in only 15.3% of the study cases. Among LGGs in this study, the findings did not impact patient survival, but demonstrated the utility of this technology in maximizing tumor resection while minimizing morbidity. Similarly, for patients with gliomas that are located within or adjacent to the rolandic cortex and, thus, the descending motor tracts, stimulation mapping of cortical and subcortical pathways enables the surgeon to identify these descending motor pathways during tumor removal and achieve an acceptable rate of permanent morbidity in these high-risk functional areas.83–85 In a recent study where 46.1% of LGGs had a complete resection, new immediate postoperative motor deficits were documented in 59.3% of patients in whom a subcortical motor tract was identified intraoperatively and in 10.9% of those in whom subcortical tracts were not observed. However, permanent deficits were observed in 6.5% and 3.5%, respectively.84 In another study of subcortical motor pathways in 294 patients who underwent surgery for hemispheric gliomas, 14 patients (4.8%) had a persistent motor deficit after 3 months. Interestingly, patients whose subcortical pathways were identified intraoperatively were more prone to develop an additional transient or permanent motor deficit (27.5% vs. 13.1%).85 In another study with an 87% gross or subtotal resection rate, the overall neurological morbidity rate was 5% after using cortical motor mapping.86 Thus, collectively the recent literature suggests that intraoperative motor mapping can safely identify corridors for resection, as well as define the limits of tumor resection.
Importantly, prediction of cortical language sites through classic anatomical criteria is inadequate in light of the significant individual variability of cortical organization, the distortion of cerebral topography from tumor mass effect, and the possibility of functional reorganization through plasticity mechanisms. A consistent finding among all cortical language stimulation studies has been significant individual variability.87 Specifically, speech arrest is variably located and can go well beyond the classic anatomical boundaries of Broca’s area for motor speech.87 This variability has also been further confirmed by studies designed to preoperatively predict the location of speech arrest based upon the type of frontal opercular anatomy.88 Similarly, for temporal lobe language sites, the distance from the temporal pole to the area of language function can vary from 3 to 9 cm.89 Neural plasticity mechanisms also introduce an element of unpredictability to functional pathway localization. The capacity for the brain to reorganize itself is critical for the process of functional recovery in patients following central nervous system injury. Interestingly, more progressive lesions such as LGGs also induce large-scale functional reshaping. This reorganization is thought to explain why slow infiltrative LGGs within the eloquent areas often do not induce detectable neurological deficits90 and must be anticipated when reoperating on patients with LGGs within or near functional pathways.91
Furthermore, because functional tissue can be located within the tumor nidus, the standard surgical principle of debulking tumor from within is not always safe.92 In the largest reported series of intraoperative language mapping for gliomas, 4 of 243 surviving patients (1.6%) had a persistent new language deficit at 6 months. Among LGGs, the gross total resection rate was 51.6%, suggesting that intraoperative language mapping remains the most effective technique in maximizing resection while minimizing language morbidity.87 In a recent LGG study designed to identify prognosticators of survival and tumor progression, four variables (areas of eloquence, patient age >50 years, KPS score ≤80, and lesion diameter >4 cm) were predictive of survival on multivariate analysis and were incorporated into a scoring system (UCSF Low-Grade Glioma Scoring System)15 that was externally validated.16 Importantly, the predictive value of tumor eloquence made evident in this study demonstrates the need for mapping functional pathways whenever an LGG is presumed eloquent. Consequently, preliminary evidence suggests that the utilization of intraoperative stimulation mapping in the resection of eloquent LGGs is associated with an improvement in overall survival.
Adjuvant Treatment
The determination of adjuvant treatment of LGG is still challenging and is based mainly on the best definition of prognostic factors. While watchful waiting remains an option for low-risk LGG patients, the optimal treatment has yet to be defined for those at risk of rapid progression and malignant transformation. In a recent prospective trial of 111 RTOG LGG patients, preoperative tumor diameter 4 cm or greater, astrocytoma/oligoastrocytoma histological type, and residual tumor 1 cm or greater according to MRI each was predictive of significantly higher recurrence rates.69 Many clinical trials are evaluating adjuvant treatments stratified by these risk groups.
Chemotherapy
The role of chemotherapy for LGG remains to be defined. Several recent studies have explored upfront treatment (i.e., immediately following resection) of newly diagnosed LGGs with chemotherapy using either procarbazine/CCNU/vincristine (PCV) or temozolomide.93–96 Collectively, these phase II trials provide little conclusive evidence for clinical efficacy in terms of overall survival, although measuring response in the absence of contrast enhancement is a challenge. In select instances, preliminary evidence suggests that temozolomide is associated with improved quality of life, better seizure control, and longer progression-free survival.93,97,98 Furthermore, in a limited trial of 16 low-grade oligodendroglioma patients, data suggesting improved tumor control rates were also reported using PCV chemotherapy.94 However, whether there is a true advantage in treating patients with upfront chemotherapy compared to initial radiotherapy is currently under investigation. Consequently, it remains controversial whether upfront chemotherapy should be offered to LGG patients, although for patients with bulky disease or neurological deficit, this is a reasonable strategy prior to implementation of radiotherapy.
For temozolomide, early data suggest that the best responders, if any, appear to be patients with oligodendrogliomas and mixed oligoastrocytomas. Combined 1p/19q loss of heterozygosity is significantly associated with a higher rate of and longer response to the methylating agent temozolomide.99 The EORTC study 26971 on first-line temozolomide chemotherapy demonstrated this with a reported 50% response rate for recurrent oligodendroglioma patients.100 Dose-intense continuous dosing schedules have also been investigated and were proved to be feasible.101,102 Using a prolonged temozolomide schedule, patients with progressive or recurrent LGGs also have an overall response rate of 30% and a progression-free rate of 56.7%.103
Among PCV chemotherapy trials, the RTOG study 9802 randomized high-risk patients (age >40 or subtotal resection) to postoperative radiotherapy with or without subsequent adjuvant PCV. After stratification by age, histological type, KPS score, and presence/absence of contrast enhancement, patients were randomized to either radiotherapy alone (54 Gy) or radiotherapy followed by six cycles of standard dose PCV. With a median follow-up of more than 4 years, no advantage for the administration of PCV was evident, even in the group of high-risk LGG patients.104 Ongoing clinical trials are evaluating the role of concurrent and adjuvant temozolomide for high-risk patients with LGG.
Radiation Therapy
Radiation therapy is often used postoperatively for many LGG patients. The EORTC study 22845 revealed an advantage for immediate postoperative radiotherapy in terms of progression-free survival (5.3 years compared with 3.4 years), but not for overall survival, among LGG patients.105 The factors that influence the timing of radiation therapy relate to speed of progression and the possibility of late toxicity to normal brain. Higher doses of radiation (>45-50 Gy) have also failed to demonstrate an improved outcome and are associated with increased late toxicity.11 In an ongoing international study (EORTC 22033–26033), LGG patients with high-risk disease or with progressive tumors are randomized between primary radiotherapy or primary chemotherapy with low-dose temozolomide for up to 1 year (12 cycles). In addition to clinical factors, these patients are stratified according to 1p/19q status and their tissue will also be surveyed for other molecular markers of interest in the hopes of generating a predictable profile for radiation response. Beyond fractionated radiotherapy, stereotactic radiosurgery has also been raised as a possibility for treating LGGs, although no data exist to support this strategy beyond small, retrospective case series.106–110
High-Grade Gliomas
Pathogenesis
Glioblastoma multiforme (GBM) may arise through two distinct pathways of neoplastic progression. Tumors that progress from lower grade (II or III) astrocytic tumors, termed secondary or type 1 GBMs, typically display both well-differentiated and poorly differentiated foci. Secondary GBMs develop in younger patients (fifth to sixth decade), with time to progression from lower-grade lesions ranging from months to decades. In contrast, primary type 2 GBMs develop in older individuals (sixth to seventh decade), have short clinical histories (less than 3 months), and arise de novo without any evidence of a lower-grade precursor. Primary and secondary GBMs also harbor distinct molecular genetic abnormalities: Primary GBMs are characterized by relatively high frequencies of EGFR amplification, PTEN deletion, and CDKN2A (p16) loss, whereas secondary GBMs often contain TP53 mutations, especially those involving codons 248 and 273 or G:C → A:T mutations at CpG sites.111
Pathology
Even within the conventional GBM category, the cellular composition is heterogeneous and may include fibrillary, gemistocytic, and occasional giant cells. Neoplastic fibrillary astrocytes contain enlarged, elongated to irregularly shaped, hyperchromatic nuclei, scant cytoplasm, and variable glial fibrillary acidic protein (GFAP)—immunoreactive processes that form a loose, fibrillary matrix. Such tumors may also harbor mucin-rich microcystic spaces. Because of cross-sectioning of spindled or cylindrical nuclei, neoplastic fibrillary astrocytes may also appear to have occasional rounded nuclear profiles, raising the differential diagnosis of high-grade oligodendroglial neoplasms (WHO grade III anaplastic oligodendroglioma and WHO grade III anaplastic mixed oligoastrocytoma). Nevertheless, a significant fraction of cells should have classic cytological appearance (e.g., round uniform cells with sharp nuclear membranes and bland chromatin) before one invokes an oligodendroglial component in the diagnosis.112
Multimodal Management
In general, high-grade gliomas (HGGs) have a poor prognosis, are rapidly progressive, and are resistant to therapy. Their infiltrating nature means they cannot be completely excised and the majority will recur within 2 cm of their original location. Median survival is around 1 year for GBM, 2 years for anaplastic astrocytoma (AA), and 5 years for anaplastic oligodendroglioma (AO). Management is based around symptomatic relief and increasing survival. The first option is surgery, which is usually required in some form for histological diagnosis. This may entail a biopsy or more likely aggressive resection. Radiotherapy is the treatment with the greatest evidence base for effect and this is now part of standard management, resulting in an increase in median survival from 3 to 4 months to 9 to 10 months.113 The other principal therapy is glucocorticosteroids, which have an important role in the reduction of peritumoral edema and can produce a marked improvement in neurological symptoms and survival by themselves.
Chemotherapy has been used as part of initial therapy as either single-agent or multiagent regimens to try to maximize penetration through the blood-brain barrier and tumor responsiveness. A meta-analysis of chemotherapy in HGG has demonstrated an improvement in survival with PCV chemotherapy (HR [hazard ratio] 0.85 CI [confidence interval] 0.78 to 0.92, P < 0.0001) with an overall improvement of 2 months in median survival to 12 months. It is not clear whether the gain in survival reflects a useful period of good quality of life. In grade III gliomas two recent randomized controlled trials did not demonstrate an increase in survival with PCV.114,115 Temozolomide is effective as primary therapy for GBM. It prolongs survival and time to progression without a significant risk of early adverse events. It appears to be most effective in young and fit patients with GBM who have had debulking surgery. These results are based on two randomized controlled trials of 703 patients in total.116,117 Temozolomide is also effective as therapy for recurrent disease, where it increases time to progression without an increase in adverse events. There are still some reservations with these data as the trials were not blinded or placebo controlled, while quality of life data could be further expanded upon.
The Value of Extent of Resection
Microsurgical resection remains a critical therapeutic modality for HGGs. However, there remains no general consensus in the literature regarding the efficacy of extent of resection in improving patient outcome. With the exception of WHO grade I tumors, gliomas are difficult to cure with surgery alone, and the majority of patients will experience some form of tumor recurrence.
For all HGGs, the identification of universally applicable prognostic factors and treatment options remains a great challenge. Among the many tumor- and treatment-related parameters, only patient age and tumor histological type have been identified as reliable predictors of patient prognosis, although functional status can also be statistically significant. While the importance of glioma resection in obtaining tissue diagnosis and to alleviate symptoms is clear, a lack of class I evidence prevents similar certainty in assessing the influence of extent of resection. In fact, despite significant advances in brain tumor imaging and intraoperative technology during the past 15 years, the effect of glioma resection in extending tumor-free progression and patient survival remains unknown.
Thus, beyond establishing the histological diagnosis and decompressing tumor mass effect, microsurgical resection of glioblastomas remains controversial in value. However, in the past decade, mounting evidence suggests that surgical extent of resection is associated with better glioblastoma patient survival. Although these data have helped establish a fragile, and frequently debated, consensus that glioblastoma resection improves patient outcome, the impracticality of conducting a randomized clinical trial limits our ability to quantify the value of greater tumor resection.
Among the 16 HGG studies demonstrating a statistically significant survival benefit with greater extent of resection, this prognostic factor was determined through univariate analysis in 11 studies. Additionally, multivariate analysis confirmed that extent of resection was a significant patient survival prognostic factor in 12 studies. Of note, three studies demonstrated conflicting statistical results between univariate and multivariate analyses of patient survival, although it was the authors’ interpretation in all three series that their findings supported extent of resection as a predictor of survival. Time to tumor progression was another commonly used endpoint in extent of resection studies. However, only five studies demonstrated statistical significance through either univariate or multivariate analysis. Overall, however, our analysis of these 28 HGG studies strongly suggested an implicit benefit in terms of patient survival with greater extent of resection. Accordingly, the mean survival following gross-total versus subtotal resection in the HGG studies differed by nearly 3 months.
It is clear that a lack of class I evidence prevents the establishment of convincing criteria guiding either low- or high-grade glioma extent of resection. While careful analysis reveals a growing correlation between greater extent of resection and patient survival, it remains each practitioner’s responsibility to determine if the magnitude and quality of evidence are sufficient to influence practice standards. In our practice, extent of resection is based on the functional nature of the tissue, not on its perceived biological aggressiveness.
Many critical questions remain unanswered in the literature regarding the value of extent of resection for HGGs. It is unclear whether the emerging correlation between aggressive glioma resection and survival holds true for both first-time and recurrent operations. The effect of extent of resection for different ages and histological subtypes must also be studied, although at least one study has addressed the former. Additionally, the question of the impact of surgical resection on patient survival must be asked in the context of current prognostic factors (e.g., 1p/19q and MGMT-methylation status) as well as in the context of specific adjuvant therapy regimens. Future studies linking extent of resection and outcome should use these markers as stratification factors in the analysis. Similarly, the efficacy of adjuvant therapies must also be studied in the context of extent of resection.
Conclusions
For the neurosurgeon, treatment of gliomas represents an opportunity to intervene early and impact outcome. Advances in molecular markers, diagnostic imaging, operative techniques and technologies, and adjuvant therapies have collectively pushed the envelope toward improved quality of life and survival. Stereotactic biopsy remains a source of sampling error, but can be enhanced through metabolic imaging. Extent of resection has been increasingly shown to correlate with improved outcome, as well as with better seizure control and reduced malignant transformation rates. Recent advances in our molecular understanding of the disease may open new avenues for novel therapy, including mTOR inhibitors currently in trial. Nevertheless, significant challenges remain; chief among them are the substantial clinical and biological heterogeneity that still exists within gliomas. Such limitations not only emphasize the need for further investigation into the tumor subsets, but also the value of clinical studies examining the forces shaping tumor recurrence and transformation.
Abrey L.E., Childs B.H., Paleologos N., et al. High-dose chemotherapy with stem cell rescue as initial therapy for anaplastic oligodendroglioma: long-term follow-up. Neuro-Oncology. 2006;8:183-188.
Athanassiou H., Synodinou M., Maragoudakis E., et al. Randomized phase II study of temozolomide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme. J Clin Oncol. 2005;23:2372-2377.
Chaichana K.L., McGirt M.J., Laterra J., et al. Recurrence and malignant degeneration after resection of adult hemispheric low-grade gliomas. J Neurosurg. 2009;111(2):203-210.
Claus E.B., Black P.M. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973-2001. Cancer. 2006;106:1358-1363.
Karim A.B., Afra D., Cornu P., et al. Randomized trial on the efficacy of radiotherapy for cerebral low-grade glioma in the adult: European Organization for Research and Treatment of Cancer Study 22845 with the Medical Research Council study BRO4: an interim analysis. Int J Radiat Oncol Biol Phys. 2002;52:316-324.
Please go to expertconsult.com to view the complete list of references.
1. Bondy M.L., Scheurer M.E., Malmer B., et al. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008;113:1953-1968.
2. Gutmann D.H. Using neurofibromatosis-1 to better understand and treat pediatric low-grade glioma. J Child Neurol. 2008;23:1186-1194.
3. Barnholtz-Sloan J.S., Sloan A.E., Schwartz A.G. Relative survival rates and patterns of diagnosis analyzed by time period for individuals with primary malignant brain tumor, 1973-1997. J Neurosurg. 2003;99:458-466.
4. Claus E.B., Black P.M. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973-2001. Cancer. 2006;106:1358-1363.
5. Barbone F., Delzell E., Austin H., Cole P. Exposure to epichlorohydrin and central nervous system neoplasms at a resin and dye manufacturing plant. Arch Environ Health. 1994;49:355-358.
6. Barregard L., Sallsten G., Jarvholm B. Mortality and cancer incidence in chloralkali workers exposed to inorganic mercury. Br J Ind Med. 1990;47:99-104.
7. Wesseling C., Pukkala E., Neuvonen K., et al. Cancer of the brain and nervous system and occupational exposures in Finnish women. J Occup Environ Med. 2002;44:663-668.
8. Wiemels J.L., Wilson D., Patil C., et al. IgE, allergy, and risk of glioma: update from the San Francisco Bay Area Adult Glioma Study in the temozolomide era. Int J Cancer. 2009;125:680-687.
9. Davis F.G., Malmer B.S., Aldape K., et al. Issues of diagnostic review in brain tumor studies: from the Brain Tumor Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2008;17:484-489.
10. Ruiz J., Lesser G.J. Low-grade gliomas. Curr Treat Options Oncol. 2009;10:231-242.
11. Karim A.B., Afra D., Cornu P., et al. Randomized trial on the efficacy of radiotherapy for cerebral low-grade glioma in the adult: European Organization for Research and Treatment of Cancer Study 22845 with the Medical Research Council Study BRO4: an interim analysis. Int J Radiat Oncol Biol Phys. 2002;52:316-324.
12. Karim A.B., Maat B., Hatlevoll R., et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36:549-556.
13. Fisher B.J., Naumova E., Leighton C.C., et al. Ki-67: a prognostic factor for low-grade glioma? Int J Radiat Oncol Biol Phys. 2002;52:996-1001.
14. Chang E.F., Potts M.B., Keles G.E., et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108:227-235.
15. Chang E.F., Smith J.S., Chang S.M., et al. Preoperative prognostic classification system for hemispheric low-grade gliomas in adults. J Neurosurg. 2008;109:817-824.
16. Chang E.F., Clark A., Jensen R.L., et al. Multi-institutional validation of the University of California at San Francisco low-grade glioma prognostic scoring system. Clinical article. J Neurosurg. 2009;111:203-210.
17. Bauman G., Fisher B., Watling C., et al. Adult supratentorial low-grade glioma: long-term experience at a single institution. Int J Radiat Oncol Biol Phys. 2009;75:1401-1407.
18. Krouwer H.G., Davis R.L., Silver P., Prados M. Gemistocytic astrocytomas: a reappraisal. J Neurosurg. 1991;74:399-406.
19. Smith J.S., Chang E.F., Lamborn K.R., et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338-1345.
20. Lote K., Egeland T., Hager B., et al. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J Clin Oncol. 1997;15:3129-3140.
21. Wrensch M., Rice T., Miike R., et al. Diagnostic, treatment, and demographic factors influencing survival in a population-based study of adult glioma patients in the San Francisco Bay Area. Neuro-Oncology. 2006;8:12-26.
22. Bauman G., Lote K., Larson D., et al. Pretreatment factors predict overall survival for patients with low-grade glioma: a recursive partitioning analysis. Int J Radiat Oncol Biol Phys. 1999;45:923-929.
23. Pamir M.N., Ozduman K., Dincer A., et al. First intraoperative, shared-resource, ultrahigh-field 3-tesla magnetic resonance imaging system and its application in low-grade glioma resection. J Neurosurg. 2010;112(1):57-69.
24. FitzGerald D.B., Cosgrove G.R., Ronner S., et al. Location of language in the cortex: a comparison between functional MR imaging and electrocortical stimulation. AJNR Am J Neuroradiol. 1997;18:1529-1539.
25. Herholz K., Reulen H.J., von Stockhausen H.M., et al. Preoperative activation and intraoperative stimulation of language-related areas in patients with glioma. Neurosurgery. 1997;41:1253-1260. discussion 1260-1262
26. Carpentier A., Pugh K.R., Westerveld M., et al. Functional MRI of language processing: dependence on input modality and temporal lobe epilepsy. Epilepsia. 2001;42:1241-1254.
27. Giussani C., Roux F.E., Ojemann J., et al. Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery. 2010;66(1):113-120.
28. Claus E.B., Horlacher A., Hsu L., et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103:1227-1233.
29. Black P.M., Alexander E.3rd, Martin C., et al. Craniotomy for tumor treatment in an intraoperative magnetic resonance imaging unit. Neurosurgery. 1999;45:423-431. discussion 431-433
30. Kondziolka D., Lunsford L.D., Martinez A.J. Unreliability of contemporary neurodiagnostic imaging in evaluating suspected adult supratentorial (low-grade) astrocytoma. J Neurosurg. 1993;79:533-536.
31. Barker F.G.2nd, Chang S.M., Huhn S.L., et al. Age and the risk of anaplasia in magnetic resonance-nonenhancing supratentorial cerebral tumors. Cancer. 1997;80:936-941.
32. Dowling C., Bollen A.W., Noworolski S.M., et al. Preoperative proton MR spectroscopic imaging of brain tumors: correlation with histopathologic analysis of resection specimens. AJNR Am J Neuroradiol. 2001;22:604-612.
33. Khayal I.S., McKnight T.R., McGue C., et al. Apparent diffusion coefficient and fractional anisotropy of newly diagnosed grade II gliomas. NMR Biomed. 2009;22:449-455.
34. Chang S.M., Nelson S., Vandenberg S., et al. Integration of preoperative anatomic and metabolic physiologic imaging of newly diagnosed glioma. J Neurooncol. 2009;92:401-415.
35. Hattingen E., Raab P., Franz K., et al. Prognostic value of choline and creatine in WHO grade II gliomas. Neuroradiology. 2008;50:759-767.
36. Pirzkall A., Nelson S.J., McKnight T.R., et al. Metabolic imaging of low-grade gliomas with three-dimensional magnetic resonance spectroscopy. Int J Radiat Oncol Biol Phys. 2002;53:1254-1264.
37. Guillevin R., Menuel C., Duffau H., et al. Proton magnetic resonance spectroscopy predicts proliferative activity in diffuse low-grade gliomas. J Neurooncol. 2008;87:181-187.
38. Reijneveld J.C., van der Grond J., Ramos L.M., et al. Proton MRS imaging in the follow-up of patients with suspected low-grade gliomas. Neuroradiology. 2005;47:887-891.
39. Schlemmer H.P., Bachert P., Henze M., et al. Differentiation of radiation necrosis from tumor progression using proton magnetic resonance spectroscopy. Neuroradiology. 2002;44:216-222.
40. Law M., Oh S., Johnson G., et al. Perfusion magnetic resonance imaging predicts patient outcome as an adjunct to histopathology: a second reference standard in the surgical and nonsurgical treatment of low-grade gliomas. Neurosurgery. 2006;58:1099-1107.
41. Maia A.C.Jr., Malheiros S.M., da Rocha A.J., et al. MR cerebral blood volume maps correlated with vascular endothelial growth factor expression and tumor grade in nonenhancing gliomas. AJNR Am J Neuroradiol. 2005;26:777-783.
42. Danchaivijitr N., Waldman A.D., Tozer D.J., et al. Low-grade gliomas: do changes in rCBV measurements at longitudinal perfusion-weighted MR imaging predict malignant transformation? Radiology. 2008;247:170-178.
43. Caseiras G.B., Chheang S., Babb J., et al. Relative cerebral blood volume measurements of low-grade gliomas predict patient outcome in a multi-institution setting. Eur J Radiol. 2010;73(2):215-220.
44. Tofts P.S., Benton C.E., Weil R.S., et al. Quantitative analysis of whole-tumor Gd enhancement histograms predicts malignant transformation in low-grade gliomas. J Magn Reson Imaging. 2007;25:208-214.
45. Floeth F.W., Pauleit D., Sabel M., et al. Prognostic value of O-(2-18F-fluoroethyl)-l-tyrosine PET and MRI in low-grade glioma. J Nucl Med. 2007;48:519-527.
46. Price S.J., Fryer T.D., Cleij M.C., et al. Imaging regional variation of cellular proliferation in gliomas using 3′-deoxy-3′-[18F]fluorothymidine positron-emission tomography: an image-guided biopsy study. Clin Radiol. 2009;64:52-63.
47. Sanai N., Berger M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753-764.
48. Chaichana K.L., McGirt M.J., Laterra J., et al. Recurrence and malignant degeneration after resection of adult hemispheric low-grade gliomas. J Neurosurg. 2009;111(2):203-210.
49. Sanai N., Polley M.Y., Berger M.S. Insular glioma resection: assessment of patient morbidity, survival, and tumor progression. J Neurosurg. 2010;112(1):1-9.
50. Simon M., Neuloh G., von Lehe M., et al. Insular gliomas: the case for surgical management. J Neurosurg. 2009;110:685-695.
51. North C.A., North R.B., Epstein J.A., et al. Low-grade cerebral astrocytomas. Survival and quality of life after radiation therapy. Cancer. 1990;66:6-14.
52. Philippon J.H., Clemenceau S.H., Fauchon F.H., Foncin J.F. Supratentorial low-grade astrocytomas in adults. Neurosurgery. 1993;32:554-559.
53. Rajan B., Pickuth D., Ashley S., et al. The management of histologically unverified presumed cerebral gliomas with radiotherapy. Int J Radiat Oncol Biol Phys. 1994;28:405-413.
54. Ito S., Chandler K.L., Prados M.D., et al. Proliferative potential and prognostic evaluation of low-grade astrocytomas. J Neurooncol. 1994;19:1-9.
55. Nicolato A., Gerosa M.A., Fina P., et al. Prognostic factors in low-grade supratentorial astrocytomas: a uni-multivariate statistical analysis in 76 surgically treated adult patients. Surg Neurol. 1995;44:208-221. discussion 221-223
56. Whitton A.C., Bloom H.J. Low grade glioma of the cerebral hemispheres in adults: a retrospective analysis of 88 cases. Int J Radiat Oncol Biol Phys. 1990;18:783-786.
57. Shibamoto Y., Kitakabu Y., Takahashi M., et al. Supratentorial low-grade astrocytoma. Correlation of computed tomography findings with effect of radiation therapy and prognostic variables. Cancer. 1993;72:190-195.
58. Scerrati M., Roselli R., Iacoangeli M., et al. Prognostic factors in low grade (WHO grade II) gliomas of the cerebral hemispheres: the role of surgery. J Neurol Neurosurg Psychiatry. 1996;61:291-296.
59. Peraud A., Ansari H., Bise K., Reulen H.J. Clinical outcome of supratentorial astrocytoma WHO grade II. Acta Neurochir (Wien). 1998;140:1213-1222.
60. van Veelen M.L., Avezaat C.J., Kros J.M., et al. Supratentorial low grade astrocytoma: prognostic factors, dedifferentiation, and the issue of early versus late surgery. J Neurol Neurosurg Psychiatry. 1998;64:581-587.
61. Bauman G., Pahapill P., Macdonald D., et al. Low grade glioma: a measuring radiographic response to radiotherapy. Can J Neurol Sci. 1999;26:18-22.
62. Nakamura M., Konishi N., Tsunoda S., et al. Analysis of prognostic and survival factors related to treatment of low-grade astrocytomas in adults. Oncology. 2000;58:108-116.
63. Shaw E., Arusell R., Scheithauer B., et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20:2267-2276.
64. Yeh S.A., Ho J.T., Lui C.C., et al. Treatment outcomes and prognostic factors in patients with supratentorial low-grade gliomas. Br J Radiol. 2005;78:230-235.
65. Johannesen T.B., Langmark F., Lote K. Progress in long-term survival in adult patients with supratentorial low-grade gliomas: a population-based study of 993 patients in whom tumors were diagnosed between 1970 and 1993. J Neurosurg. 2003;99:854-862.
66. Leighton C., Fisher B., Bauman G., et al. Supratentorial low-grade glioma in adults: an analysis of prognostic factors and timing of radiation. J Clin Oncol. 1997;15:1294-1301.
67. McGirt M.J., Chaichana K.L., Attenello F.J., et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63:700-707. author reply 707-708
68. Rezvan A., Christine D., Christian H., et al. Long-term outcome and survival of surgically treated supratentorial low-grade glioma in adult patients. Acta Neurochir (Wien). 2009;151(11):1359-1365.
69. Shaw E.G., Berkey B., Coons S.W., et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J Neurosurg. 2008;109:835-841.
70. Smith J.S., Chang E.F., Lamborn K.R., et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338-1345.
71. Dirks P.B., Jay V., Becker L.E., et al. Development of anaplastic changes in low-grade astrocytomas of childhood. Neurosurgery. 1994;34:68-78.
72. Lunsford L.D., Somaza S., Kondziolka D., Flickinger J.C. Survival after stereotactic biopsy and irradiation of cerebral nonanaplastic, nonpilocytic astrocytoma. J Neurosurg. 1995;82:523-529.
73. Janny P., Cure H., Mohr M., et al. Low grade supratentorial astrocytomas. Management and prognostic factors. Cancer. 1994;73:1937-1945.
74. Piepmeier J., Christopher S., Spencer D., et al. Variations in the natural history and survival of patients with supratentorial low-grade astrocytomas. Neurosurgery. 1996;38:872-878. discussion 878-879
75. Afra D., Osztie E. Histologically confirmed changes on CT of reoperated low-grade astrocytomas. Neuroradiology. 1997;39:804-810.
76. Shafqat S., Hedley-Whyte E.T., Henson J.W. Age-dependent rate of anaplastic transformation in low-grade astrocytoma. Neurology. 1999;52:867-869.
77. Kreth F.W., Warnke P.C., Ostertag C.B. Low grade supratentorial astrocytomas: management and prognostic factors. Cancer. 1994;74:3247-3248.
78. Pallud J., Mandonnet E., Duffau H., et al. Prognostic value of initial magnetic resonance imaging growth rates for World Health Organization grade II gliomas. Ann Neurol. 2006;60:380-383.
79. Rees J., Watt H., Jager H.R., et al. Volumes and growth rates of untreated adult low-grade gliomas indicate risk of early malignant transformation. Eur J Radiol. 2009;72:54-64.
80. Brasil Caseiras G., Ciccarelli O., Altmann D.R., et al. Low-grade gliomas: six-month tumor growth predicts patient outcome better than admission tumor volume, relative cerebral blood volume, and apparent diffusion coefficient. Radiology. 2009;253:505-512.
81. Talos I.F., Zou K.H., Kikinis R., Jolesz F.A. Volumetric assessment of tumor infiltration of adjacent white matter based on anatomic MRI and diffusion tensor tractography. Acad Radiol. 2007;14:431-436.
82. Wu J.S., Zhou L.F., Tang W.J., et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007;61:935-948. discussion 948-949
83. Duffau H., Capelle L., Sichez J., et al. Intra-operative direct electrical stimulations of the central nervous system: the Salpetrière experience with 60 patients. Acta Neurochir. 1999;141:1157-1167.
84. Carrabba G., Fava E., Giussani C., et al. Cortical and subcortical motor mapping in rolandic and perirolandic glioma surgery: impact on postoperative morbidity and extent of resection. J Neurosurg Sci. 2007;51:45-51.
85. Keles G.E., Lundin D.A., Lamborn K.R., et al. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg. 2004;100:369-375.
86. Duffau H., Capelle L., Sichez J., et al. Intra-operative direct electrical stimulations of the central nervous system: the Salpetrière experience with 60 patients. Acta Neurochir (Wien). 1999;141:1157-1167.
87. Sanai N., Mirzadeh Z., Berger M.S. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18-27.
88. Quinones-Hinojosa A., Ojemann S.G., Sanai N., et al. Preoperative correlation of intraoperative cortical mapping with magnetic resonance imaging landmarks to predict localization of the Broca area. J Neurosurg. 2003;99:311-318.
89. Ojemann G., Ojemann J., Lettich E., Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316-326.
90. Duffau H. Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol. 2005;4:476-486.
91. Robles S.G., Gatignol P., Lehericy S., Duffau H. Long-term brain plasticity allowing a multistage surgical approach to World Health Organization Grade II gliomas in eloquent areas. J Neurosurg. 2008;109:615-624.
92. Skirboll S.S., Ojemann G.A., Berger M.S., et al. Functional cortex and subcortical white matter located within gliomas. Neurosurgery. 1996;38:678-684. discussion 684-685
93. Brada M., Viviers L., Abson C., et al. Phase II study of primary temozolomide chemotherapy in patients with WHO grade II gliomas. Ann Oncol. 2003;14:1715-1721.
94. Stege E.M., Kros J.M., de Bruin H.G., et al. Successful treatment of low-grade oligodendroglial tumors with a chemotherapy regimen of procarbazine, lomustine, and vincristine. Cancer. 2005;103:802-809.
95. Buckner J.C., Gesme D.Jr., O’Fallon J.R., et al. Phase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: efficacy and associations with chromosomal abnormalities. J Clin Oncol. 2003;21:251-255.
96. Hoang-Xuan K., Capelle L., Kujas M., et al. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22:3133-3138.
97. Pace A., Vidiri A., Galie E., et al. Temozolomide chemotherapy for progressive low-grade glioma: clinical benefits and radiological response. Ann Oncol. 2003;14:1722-1726.
98. Quinn J.A., Reardon D.A., Friedman A.H., et al. Phase II trial of temozolomide in patients with progressive low-grade glioma. J Clin Oncol. 2003;21:646-651.
99. Kaloshi G., Benouaich-Amiel A., Diakite F., et al. Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology. 2007;68:1831-1836.
100. van den Bent M.J., Taphoorn M.J., Brandes A.A., et al. Phase II study of first-line chemotherapy with temozolomide in recurrent oligodendroglial tumors: the European Organization for Research and Treatment of Cancer Brain Tumor Group Study 26971. J Clin Oncol. 2003;21:2525-2528.
101. Khan R.B., Raizer J.J., Malkin M.G., et al. A phase II study of extended low-dose temozolomide in recurrent malignant gliomas. Neuro-Oncology. 2002;4:39-43.
102. Brock C.S., Newlands E.S., Wedge S.R., et al. Phase I trial of temozolomide using an extended continuous oral schedule. Cancer Res. 1998;58:4363-4367.
103. Tosoni A., Franceschi E., Ermani M., et al. Temozolomide three weeks on and one week off as first line therapy for patients with recurrent or progressive low grade gliomas. J Neurooncol. 2008;89:179-185.
104. Baumert B.G., Stupp R. Low-grade glioma: a challenge in therapeutic options: the role of radiotherapy. Ann Oncol. 2008;19(Suppl 7):217-222.
105. van den Bent M.J., Afra D., de Witte O., et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985-990.
106. Heppner P.A., Sheehan J.P., Steiner L.E. Gamma knife surgery for low-grade gliomas. Neurosurgery. 2005;57:1132-1139.
107. Hadjipanayis C.G., Kondziolka D., Flickinger J.C., Lunsford L.D. The role of stereotactic radiosurgery for low-grade astrocytomas. Neurosurg Focus. 2003;14:e15.
108. Kida Y., Kobayashi T., Mori Y. Gamma knife radiosurgery for low-grade astrocytomas: results of long-term follow up. J Neurosurg. 2000;93(Suppl 3):42-46.
109. Kihlstrom L., Lindquist C., Lindquist M., Karlsson B. Stereotactic radiosurgery for tectal low-grade gliomas. Acta Neurochir Suppl. 1994;62:55-57.
110. Barcia J.A., Barcia-Salorio J.L., Ferrer C., et al. Stereotactic radiosurgery of deeply seated low grade gliomas. Acta Neurochir Suppl. 1994;62:58-61.
111. Ohgaki H., Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445-1453.
112. Miller C.R., Perry A. Glioblastoma. Arch Pathol Lab Med. 2007;131:397-406.
113. Walker M.D., Strike T.A., Sheline G.E. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725-1731.
114. Cairncross G., Berkey B., Shaw E., et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707-2714.
115. Abrey L.E., Childs B.H., Paleologos N., et al. High-dose chemotherapy with stem cell rescue as initial therapy for anaplastic oligodendroglioma: long-term follow-up. Neuro-Oncology. 2006;8:183-188.
116. Athanassiou H., Synodinou M., Maragoudakis E., et al. Randomized phase II study of temozolomide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme. J Clin Oncol. 2005;23:2372-2377.
117. Stupp R., Mason W.P., van den Bent M.J., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996.