Chapter 30 Long-Term Complications and Management
In the modern era, the majority of cardiac surgical patients have brief stays in the intensive care unit (ICU) (<24 hours), and these stays follow a predictable pattern. During this time, most instability and morbidity are attributable to the cardiopulmonary organ systems, bleeding, hypothermia, and the emergence from anesthesia. A small minority of patients, however, have prolonged ICU stays characterized by multisystem complications involving both the cardiac and noncardiac systems. This group of patients consumes a disproportionate number of ICU resources, generates enormous hospital costs, and ultimately has a much worse prognosis (bothin-hospital and long term).1
SEDATION IN THE INTENSIVE CARE UNIT
The major goals of sedation in the ICU are to provide anxiolysis and to improve the patient’s perceptual experience during this physiologically and emotionally stressful period (Box 30-1). Secondarily, sedation reduces the physiologic stress response and attendant cardiovascular work, may facilitate the maintenance of circadian rhythms, and lessens delirium and agitation. These goals are distinct from those associated with analgesia, which are the alleviation of pain through nonpharmacologic and pharmacologic means and to facilitate diagnostic and therapeutic procedures. Although sedation and analgesia are separate therapeutic goals usually provided by individual drugs, there is often synergism between anxiolytic and analgesic drugs; and some newer agents provide elements of both analgesia and anxiolysis, thus blurring the distinction in clinical practice.
BOX 30-1 Sedation
The Society of Critical Care Medicine (SCCM) published guidelines for sedation,2 which emphasize the need for the goal-directed delivery of psychoactive medications. Goal-directed sedation is supported by an increasing body of literature that shows that daily interruption of sedation, intermittent sedation, and sedation protocols all reduce the duration of mechanical ventilation and in some instances decrease ICU length of stay.3
There are several scoring systems available to assess a patient’s degree of sedation in the ICU and facilitate goal-directed therapy (Table 30-1). The Riker Sedation-Agitation Scale (SAS) was the first scale proved to be reliable and valid in critically ill adults. The SAS score is assigned by choosing a score from a seven-item scale that best matches a patient’s behavior. Another scale, the Motor Activity Assessment Scale (MAAS), has seven categories to describe patients’ behavior in response to stimulation. Like the SAS, it has been validated in critically ill adults. Most comparative clinical studies of sedation in critically ill patients have used the Ramsey scale. This scale is a six-point scale of motor activity that ranges from 1 (“patient anxious, agitated or restless, or both”) to 6 (“no response to light glabellar tap or loud auditory stimulus”) (Table 30-2). This scale was originally designed as a research tool but has been used for decades in clinical practice. Although no scientific consensus exists about which level of sedation using the Ramsey scale is optimal, recent literature frequently cites sedation goals of Ramsey 2 to 4, reflecting more realistic levels of sedation as part of goal-directed therapy. Other sedation scales that have been validated in critically ill adults include the Vancouver Interaction and Calmness Scale (VICS), the COMFORT Scale, and the Richmond Agitation-Sedation Scale (RASS). The SCCM’s guidelines do not advocate one specific scoring system. Instead, they advocate defining a specific sedation goal or endpoint for each patient and then regularly assessing and documenting the patient’s level of sedation in response to therapy.
| Awake levels |
Reprinted with permission from Young C, Knudsen N, Hilton A, Reves JG: Sedation in the intensive care unit. Crit Care Med 28:854, 2000.
Sedative Agents
Propofol
Several studies have shown that propofol, compared with midazolam, allows for more rapid weaning of patients from mechanical ventilation, and it is because of this property that it is more commonly used for “fast tracking” cardiac surgical patients.4 When used for sedation, an initial dose of 0.5 to 1.0 mg/kg should be used, followed by an infusion of 25 to 50 μg/kg/min. Because of case reports of mortality in patients who receive excessively high doses of propofol, the maximum dose should probably be 100 μg/kg/min. This propofol infusion syndrome has been reported in patients who have received propofol for a very short period of time, indicating that this may be an idiosyncratic reaction.
Dexmedetomidine
Herr and colleagues conducted a multicenter trial comparing dexmedetomidine and propofol for sedation after coronary artery bypass grafting (CABG).5 In their trial there was no significant difference in time to extubation between groups but the dexmedetomidine patients had significantly reduced use of supplemental analgesics, antiemetics, epinephrine, and diuretics.
Neuromuscular Blocking Agents
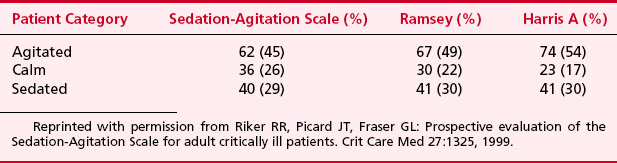
If these medications are used, it cannot be overemphasized that the patient must be adequately sedated before the initiation of the NMBA. Once an adequate degree of sedation (usually to include an analgesic medication such as an opioid) is achieved, the patient is administered a bolus and then a continuous infusion of an NMBA. Although there are several drugs available, the drugs most commonly used in the ICU are the aminosteroidal compounds (pancuronium, vecuronium, and rocuronium) and the benzylisoquinolinium compounds (doxacurium, atracurium, and cisatracurium). Pancuronium and doxacurium are long-acting NMBAs, whereas rocuronium and vecuronium are intermediate-duration medications and atracurium and cisatracurium are short-acting medications, at least when given by bolus. Because these drugs are infused continuously, this attribute is not as important, but it does become important when the medication is discontinued and the physician is assessing the return of the patient’s neuromuscular function. When infusing these medications, a twitch monitor should be used and the physician should strive to achieve a train-of-four of one or two twitches.6 If there are no twitches observed, then the patient may be overdosed and may be at risk for development of acute quadriplegic myopathy syndrome (AQMS), a situation that develops in patients receiving NMBAs in which, when the medication is discontinued, the patient remains flaccid for much longer than would be predicted simply based on pharmacokinetics of the medications that were infused. The etiology of this syndrome is unknown but is most likely secondary to the destruction of myosin by the NMBA or one of its metabolites. Often, it is difficult to differentiate between AQMS and critical illness polyneuropathy, but in the latter profound muscle necrosis as is seen with AQMS would not be expected to occur.
Another way to minimize the likelihood of this syndrome is to institute a daily drug holiday. Not only is this beneficial in decreasing the incidence of AQMS, but in patients receiving opioids and benzodiazepines the incidence of drug withdrawal also decreases with the discontinuation of the medication. When using NMBAs in the ICU, the algorithm as shown in Figure 30-1 is recommended.
INFECTIONS IN THE INTENSIVE CARE UNIT
Intravascular Device-Related Infections
Virtually all adult patients having cardiac surgery are monitored with invasive intravascular devices (IVDs), such as arterial, central venous, and pulmonary artery catheters. Unfortunately, these IVDs are frequently associated with bloodstream infections (BSIs). IVD-related BSIs are associated with an attributable mortality of 12% to 15%, prolonged hospitalization (mean of 7 days), and increased hospital cost of approximately $35,000.7
In an effort to reduce IVD-related BSIs, a Centers for Disease Control and Prevention advisory committee has formulated evidence-based guidelines pertaining to the prevention of IVD-related BSIs. These guidelines are summarized in Box 30-2.8
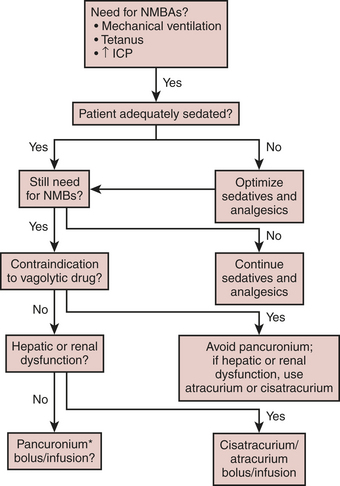
The first clinical decision to make when managing a suspected CVC-related BSI is whether to remove the catheter. This decision is influenced by whether the risk of CVC-related BSI is low, intermediate, or high. Risk, in turn, is determined by the infecting organism and whether the CVC-related BSI is complicated or uncomplicated. Complicated infections are those associated with shock, persistence of positive blood cultures for longer than 48 hours after appropriate antibiotics, CVC-related BSIs associated with septic thrombosis, septic emboli, or deep-seated infections (e.g., endocarditis), or a tunnel or port-pocket infection (Fig. 30-2).
Low-risk CVC-related BSIs can be treated without catheter removal. However, catheters should be removed in low-risk patients with prosthetic heart valves. In intermediate-risk patients, the catheters should be removed and the patients treated with a 10- to 14-day course of antibiotics. In high-risk patients, catheters should be removed and duration of antibiotic use based on the nature of the complication. In deep-seated infections such as septic thrombosis or endocarditis, antimicrobial agents should be administered for 4 to 6 weeks.9
Sternal Wound Infections
Deep and superficial surgical site infections are infrequent but morbid complications after cardiac surgery, with an incidence of 1% to 4% (Box 30-3). Deep sternal infections are defined as those infections involving muscle and fascial layers or any other organ spaces manipulated during the operation or organ involvement. They are associated with a 250% higher mortality than for matched individuals without infection, and postoperative wound infections double the length of hospitalization. A host of preoperative, intraoperative, and postoperative risk factors have been identified for chest wall infections10:
Prosthetic Valve Endocarditis
Prosthetic valve endocarditis (PVE), the infection of a prosthetic heart valve and/or the surrounding cardiac tissues, is a rare but serious source of infection in postoperative cardiac surgical patients.11 The incidence of PVE is between 0.3% and 0.8% after valve replacement surgery. PVE cases can be clustered into two groups according to the time of infection. In early PVE (within 2 months of valve implantation), the valve and sewing ring have not yet endothelialized and hence microorganisms frequently invade the surrounding tissue planes, causing perivalvular abscess and perivalvular leak. In early PVE, the responsible microorganisms are nosocomial pathogens such as staphylococci, gram-negative bacilli, and Candida species that are introduced at the time of surgery or are hematogenously seeded in the immediate postoperative period. The pathophysiology of late PVE probably resembles that of native valve endocarditis; that is, platelet-fibrin thrombi form on the valve leaflet and are then hematogenously seeded during episodes of transient bacteremia. In late PVE, the infecting organisms are usually streptococci, S. aureus, enterococci, and fastidious gram-negative organisms (the HACEK group). Infection appears to occur with equal frequency in both the mitral and aortic position and is exceedingly rare in tricuspid prosthesis (excluding intravenous drug abusers).
In the ICU, cases of early PVE present more dramatically than the often-subtle presentation of either native valve endocarditis or late PVE. The clinical signs that suggest PVE include new or changing murmurs, congestive heart failure, new ECG conduction disturbances, and systemic emboli. In fact, 40% of patients have clinically apparent central nervous system emboli. The diagnosis is confirmed by positive blood cultures and transesophageal echocardiography (TEE). If blood cultures are obtained before antibiotic therapy, more than 90% will be positive. TEE is the diagnostic imaging modality of choice because it has a sensitivity of 82% to 96% versus 17% to 36% with transthoracic echocardiography. TEE also allows the detection of abscesses, fistulas, and perivalvular leaks. The treatment of early PVE involves antibiotics directed at the cultured organism and prompt surgical intervention in cases of complicated PVE. In complicated PVE, survival is improved with both medical and surgical therapy versus with medical therapy alone.12
Systemic Inflammatory Response Syndrome and Sepsis
Sepsis is defined as the clinical syndrome that occurs as the result of an infection (or suspected infection) and an inflammatory response13 (Table 30-3). Severe sepsis is sepsis that is associated with organ dysfunction, hypoperfusion, or hypotension. Septic shock is sepsis-induced hypotension and organ perfusion abnormalities that persist despite fluid resuscitation.
|
Hemodynamic variables
Arterial hypotension† (SBP < 90 mm Hg, MAP < 70, or an SBP decrease > 40 mm Hg in adults or < 2 SDs below normal for age)
|
WBC = white blood cell; SBP = systolic blood pressure; MAP = mean arterial blood pressure; 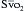 = mixed venous oxygen saturation; INR = international normalized ratio; aPTT = activated partial thromboplastin time.
= mixed venous oxygen saturation; INR = international normalized ratio; aPTT = activated partial thromboplastin time.
* Infection defined as a pathologic process induced by a microorganism.
† 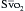 > 70% is normal in children (normal, 75% to 80%), and CI 3.5 to 5.5 is normal in children.
> 70% is normal in children (normal, 75% to 80%), and CI 3.5 to 5.5 is normal in children.
Reprinted with permission from Levy MM, Fink MP, Marshall JC, et al: 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31:1250, 2001.
Sepsis is the leading cause for admission to surgical ICUs and, despite recent advances in therapy, remains the leading cause of mortality in ICUs.14 The mortality rate increases across the inflammatory spectrum from SIRS to septic shock.
Because of the unacceptably high mortality rate associated with sepsis and the inflammatory disorders, an international group of experts in sepsis convened in 2003 and launched the “surviving sepsis campaign” with the goal of producing treatment recommendations that could be used to reduce the mortality from sepsis.15 These recommendations were formulated from an evidence-based review of the medical literature and from expert opinion when high-level evidence was absent. They reflect the current “state of the art” in the management of critically ill, septic patients.
Initial Resuscitation (First 6 Hours)
Diagnosis
Antibiotic Therapy
Source Control
Fluid Therapy
Vasopressors
Inotropic Therapy
Corticosteroids
Recombinant Human Activated Protein C (rhAPC)
Blood Product Administration
Mechanical Ventilation of Sepsis-Induced Acute Lung Injury/ARDS
Sedation, Analgesia, and Neuromuscular Blockade
Glucose Control
Renal Replacement
Deep Vein Thrombosis Prophylaxis
HEMATOLOGY
Transfusion
Blood products are frequently transfused into critically ill patients. In a general ICU population, patients receive an average of 0.2 U/day, and this incidence is increased to 1.3 U/day in cardiothoracic ICUs.16 Whereas transfusion is often necessary to either improve oxygen delivery or restore the coagulation system there is a growing body of literature that suggests that transfusion carries substantial risk for postoperative cardiac surgical patients.
Several large studies have identified transfusion as increasing the risk of infection after cardiac surgery (Box 30-4). In fact, in 17 of 19 retrospective studies that were reviewed, transfusion was found to be a significant factor and frequently the best predictor of postoperative infection. Transfusion has been cited as a risk factor for mediastinitis, early bacteremia, pneumonia, increased mortality rate, and length of stay after cardiac surgery. A randomized, controlled trial identified nosocomial pneumonia as the most frequent infection after cardiac surgery and that it only occurred in patients transfused more than 4 units of blood components.
BOX 30-4 Transfusion Strategy in Cardiothoracic Intensive Care Unit
In 1999, Hebert and colleagues17 published a landmark study that has fundamentally altered the approach to transfusion in critically ill patients. Their large, multicenter, randomized, prospective trial of 838 patients admitted to Canadian ICUs found no difference in 30-day mortality between patients assigned to a liberal (hemoglobin: 10 to 12 g/dL) and conservative red blood cell transfusion protocol (hemoglobin: 7 to 9 g/dL). In fact, mortality was lower in less ill patients (APACHE II score ≤ 20) and in younger patients (≤55 years of age). Also, the restrictive strategy resulted in a 54% reduction in red blood cell transfusions. Prior to this study, red blood cell transfusion had been extensively investigated as a component of the now dated paradigm that supranormal oxygen delivery was associated with increased survival in critically ill patients. Hebert and colleagues’ study showed not only that a conservative strategy was associated with no increase in mortality but also that it halved the number of transfused units with its attendant decrease in infectious risk, immunomodulation, and cost.
Acute Renal Failure
Acute renal failure (ARF), like many of the clinical syndromes frequently encountered in the ICU, has been difficult to precisely define.18 If it is agreed that the principal functions of the kidney are to create urine and excrete water-soluble waste products of metabolism, then ARF is the sudden loss of these functions.
Several strategies for preventing perioperative renal failure have been evaluated in cardiac surgical patients. “Renal dose” dopamine has been shown to have no effect on either renal function or mortality after both cardiac and vascular surgery.19 Similarly, the diuretics furosemide and mannitol have demonstrated no renal-protective effects.
ELECTROLYTE ABNORMALITIES
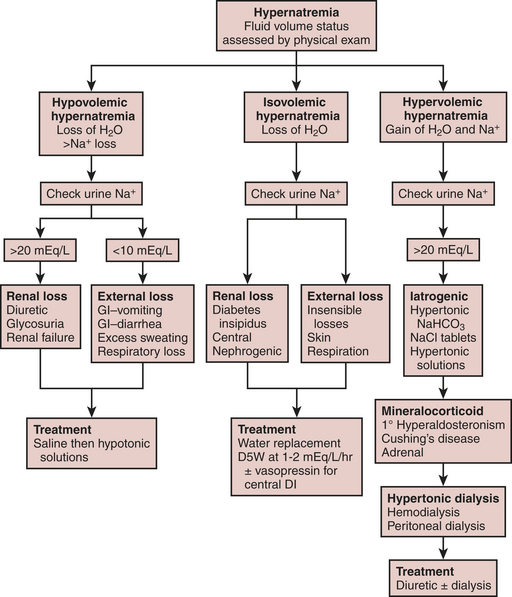
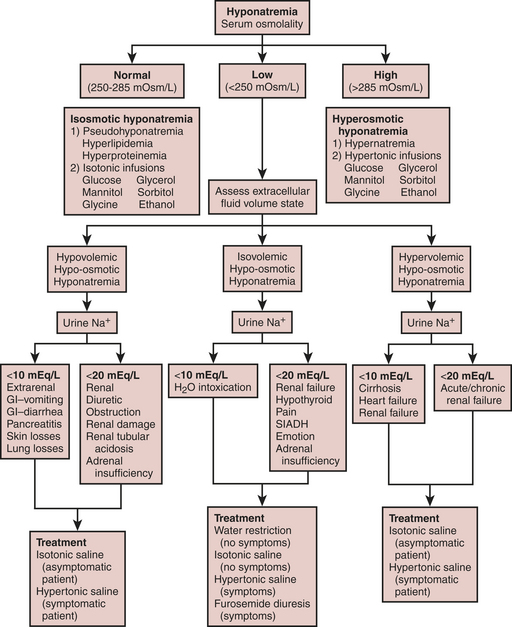
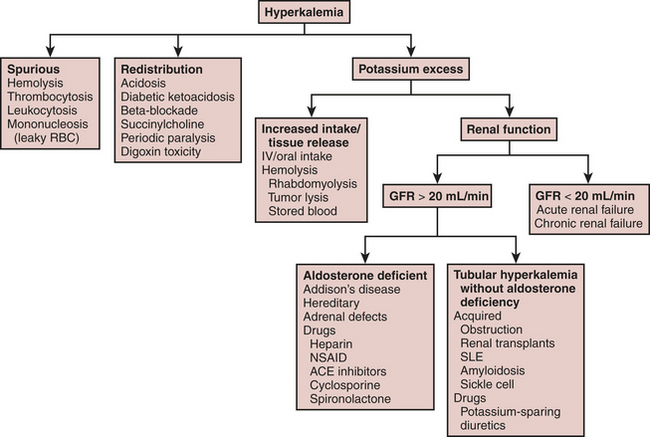
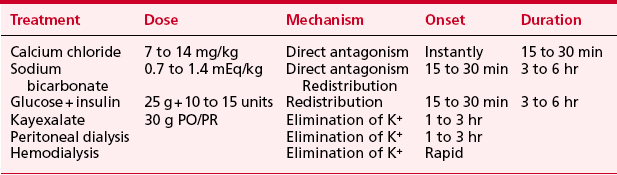
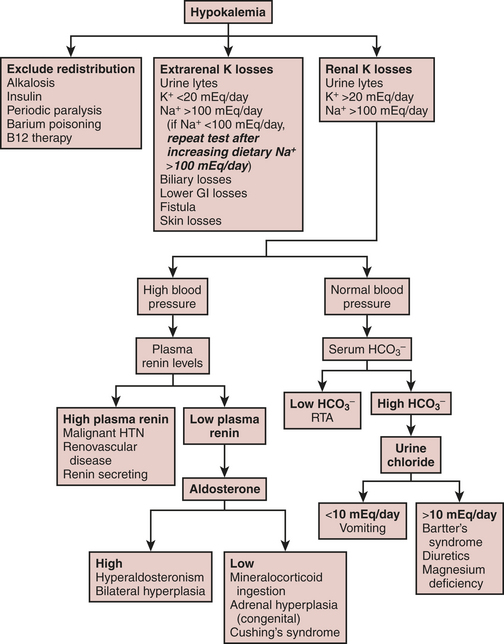
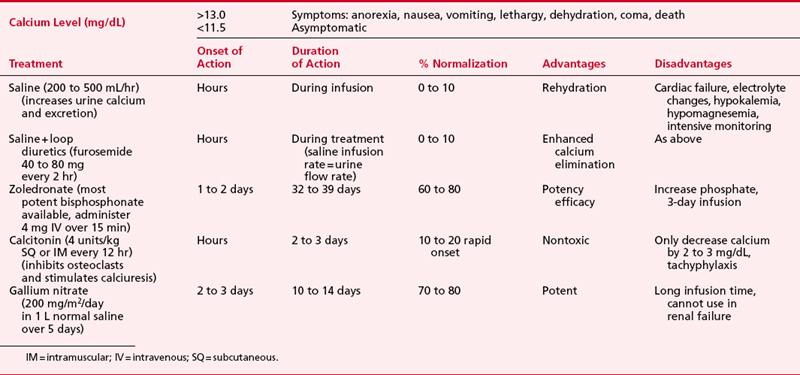
Fluid and electrolyte abnormalities are common after cardiac surgery. Diagnosis and treatment algorithms are shown in Figures 30-3 and 30-4 for hypernatremia and hyponatremia, Figure 30-5 and Table 30-4 for hyperkalemia, Figure 30-6 for hypokalemia, and Table 30-5 for hypercalcemia. Hypomagnesemia is common; and the underlying cause should be identified, if possible, and then treated with an intravenous infusion of 0.1 to 0.2 mEq/kg/day or via the oral route at 0.4 mEq/kg/day. Close monitoring is necessary with treatment of any electrolyte disorder.
SUMMARY
1. Williams M.R., Wellner R.B., Hartnett E.A., et al. Long-term survival and quality of life in cardiac surgical patients with prolonged intensive care unit length of stay. Ann Thorac Surg. 2002;73:1472.
2. Nasraway S.A., Jacobi J., Murray M.J., Lumb P.D. Sedation, analgesia, and neuromuscular blockade of the critically ill adult: Revised clinical practice guidelines for 2002. Crit Care Med. 2002;30:117.
3. Kress J.P., Pohlman A.S., O’Conner M.F., Hall J.B. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471.
4. Myles P.S., Buckland M.R., Weeks A.M., et al. Hemodynamic effects, myocardial ischemia, and timing of tracheal extubation with propofol-based anesthesia for cardiac surgery. Anesth Analg. 1997;84:12.
5. Herr D.L., Sum-Ping S.T., England M. ICU sedation after coronary artery bypass graft surgery: Dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003;17:576.
6. Murray M.J., Cowen J., DeBlock H., et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2002;30:142.
7. Alberti C., Brun-Buisson C., Burchardi H., et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108.
8. Garland J.S., Henrickson K., Maki D.G. The 2002 Hospital Infection Control Practices Advisory Committee Centers for Disease Control and Prevention Guideline for Prevention of Intravascular Device-Related Infection. Pediatrics. 2002;110:1009.
9. RaadII, Hanna H.A. Intravascular catheter-related infections. New horizons and recent advances. Arch Intern Med. 2002;162:871.
10. Hollenbeak C.S., Murphy D.M., Koenig S., et al. The clinical and economic impact of deep chest surgical site infections following coronary artery bypass graft surgery. Chest. 2000;118:397.
11. Edwards M.B., Ratnatunga C.P., Dore C.J., Taylor K.M. Thirty-day mortality and long-term survival following surgery for prosthetic endocarditis: A study from the UK heart valve registry. Eur J Cardiothorac Surg. 1998;14:156.
12. Gordon S.M., Serkey J.M., Longworth D.L., et al. Early onset prosthetic valve endocarditis: The Cleveland Clinic experience 1992-1997. Ann Thorac Surg. 2000;69:1388.
13. Levy M.M., Fink M.P., Marshall J.C., et al. SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250.
14. Hotchkiss R.S., Karl I.E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138.
15. Dellinger R.P., Carlet J.M., Masur H., et al. Surviving sepsis campaign guidelines for the management of severe sepsis and septic shock. Crit Care Med. 2004;32:858.
16. Leal-Noval S.R., Rincón-Ferrari M.D., García-Curiel A., et al. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest. 2001;119:1461.
17. Hebert P.C., Wells G., Blajchman M.A., et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators: Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409.
18. Bellomo R., Kellum J.A., Ronco C. Defining acute renal failure: Physiologic principles. Intensive Care Med. 2004;30:33.
19. Lassnigg A., Donner E., Grubhofer G., et al. Lack of renoprotective effects of dopamine and furosemide during cardiac surgery. J Am Soc Nephrol. 2000;11:97.
20. Bellomo R., Ronco C. Continuous renal replacement therapy in the intensive care unit. Intensive Care Med. 1999;25:781.

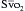 > 70%
> 70%