Chapter 76A Location of portosystemic shunting
Overview
Esophageal varices develop in patients with portal hypertension, most commonly secondary to hepatic cirrhosis (see Chapter 70A, Chapter 70B ). They occur most frequently in the distal esophagus, although they may be accompanied by gastric varices. Rupture of varices is associated with massive upper gastrointestinal (GI) bleeding with an attendant high mortality rate. Therapy aimed at the prevention and treatment of bleeding varices has included pharmacologic, endoscopic, radiologic, and surgical strategies. All these therapies have evolved technically, and increasing clinical experience has resulted in more accurate definition of the role of each treatment modality. This chapter discusses the appropriate role of surgical shunts for the management of bleeding esophageal varices. An understanding of the role of surgical therapy also requires an understanding of the context in which it is applied, however. The natural history of bleeding esophageal varices is discussed first, followed by a description of the roles of alternative therapies. In current medical practice, it is most appropriate to apply surgical shunts within the context of medical (see Chapter 75A) and endoscopic management (see Chapter 75B), transjugular intrahepatic portosystemic shunts (TIPS; see Chapter 76E), and liver transplantation (see Chapter 97A, Chapter 97B, Chapter 97C, Chapter 97D, Chapter 97E ). Many patients are treated sequentially with more than one modality, and algorithms are presented to help establish the appropriate clinical context for surgical shunt therapy.
Natural History of Esophageal Varices
Esophageal varices may produce massive upper GI bleeding that is difficult to control. Not all varices bleed, and not all patients with cirrhosis or portal hypertension will have esophageal varices develop. Clinical studies have sometimes included control groups without medical intervention, and analysis of these trials has helped define the natural history of esophageal varices. In one series, 46% of 819 patients with biopsy or clinical evidence of cirrhosis and no history of bleeding had esophageal varices by endoscopy (PROVA Study Group, 1991).
Over time, varices may appear, disappear, or change in size depending on alterations in patient physiology. A study of 84 patients with cirrhosis without previous bleeding who were monitored by serial endoscopy over 2 years showed that 31% of patients without varices progressed to large varices over 2 years, whereas in 70% of patients with small varices, the varices enlarged after 2 years (Cales et al, 1990). Dagradi (1972) studied the influence of alcohol on varices in patients with cirrhosis and found that variceal length increased in 65% of patients with cirrhosis who continued to consume alcohol, but it decreased in 80% of patients with cirrhosis who abstained from alcohol. Baker and colleagues (1959) reported that varices regress in 25%, disappear in 32%, and progress in 21% of patients with cirrhosis whose varices are monitored by endoscopy.
Most bleeding episodes in long-term studies occur during the first 1 to 2 years after identification of varices (Baker et al, 1959; Groszmann et al, 1990; Siringo et al, 1994; Triger et al, 1991). Average mortality rates after bleeding from esophageal varices are 23% at 1 year, 34% at 2 years, and 58% at 3 years. Approximately one third of deaths in patients with known esophageal varices are attributable to upper GI bleeding; a larger proportion die as a result of liver failure. The mortality rate directly attributable to variceal hemorrhage is 10% to 17% for cirrhotic patients (Baker et al, 1959; Sauerbruch et al, 1988; Triger et al, 1991). In patients with varices, upper GI bleeding is attributable to variceal hemorrhage in roughly two thirds of patients (Gebhard, 1998). Clinical parameters associated with increased risk of hemorrhage and death from esophageal varices include large varices, those with cherry-red spots (Dagradi, 1972), concurrent gastric varices (Kleber et al, 1991), Child-Turcotte-Pugh (CTP) classification, continued alcohol use (Dagradi, 1972), and infection (Goulis et al, 1999). Death correlates more closely with CTP classification (Merkel et al, 1989) than with any other parameter studied.
Rebleeding and mortality rates markedly increase after varices bleed. Studies have reported rebleeding rates to be 30% within 6 weeks of an initial variceal hemorrhage (Copenhagen Esophageal Varices Sclerotherapy Project [CVESP], 1984; Graham & Smith, 1981) and 60% to 75% within 1 year (Baker et al, 1959; Graham & Smith, 1981). Esophageal varices are the cause of bleeding in approximately 16% of hospital admissions for upper GI bleeding (de Franchis et al, 1991). Mortality rates from all causes within 1 year of initial hemorrhage have been estimated at 40% to 66% (Burroughs et al, 1989; CEVSP, 1984; Graham & Smith, 1981; Le Moine et al, 1992). The risk of dying increases as the interval between initial and second hemorrhage decreases (Gebhard, 1998). If patients survive for more than 12 weeks after a variceal hemorrhage, the risk of rebleeding or dying returns to that of patients who have never bled (Gebhard, 1998).
Pharmacologic Management of Portal Hypertension (See Chapter 75A, Chapter 75B, Chapter 75C )
Prophylaxis
β-Blocker therapy has been studied to test its efficacy in preventing primary variceal hemorrhage in patients with known varices. Nadolol is a nonselective β-blocker, meaning it blocks both β1 and β2 receptors; patients given nadolol were compared with untreated control subjects. Nadolol reduced the incidence of bleeding from 35% ± 3% to 12% ± 3%, and the incidence of fatal bleeds was reduced from 18% ± 3% to 10% ± 2%. There was no difference in overall mortality rate (Poynard et al, 1991). This study is used to support the use of prophylactic β-blockade to prevent a first variceal hemorrhage.
Nitrates are vasodilators whose action is mediated by nitric oxide on vascular smooth muscle. Nitroglycerin decreases portal pressure in patients with cirrhosis when high doses are used (Moreau et al, 1989). In animal studies, nitroglycerin lowered portal pressure 13%, and systemic blood pressure decreased 25%. This drug lowers portal pressure less than systemic pressure. Nitrates in combination with β-blockade may offer prophylaxis against an initial variceal bleed.
Randomized, controlled clinical trials comparing nonselective β-blockers (propranolol or nadolol) with no therapy in cirrhotic patients showed that drug treatment effectively reduced the risk of a first variceal hemorrhage (Poynard et al, 1991). The combination of isosorbide mononitrate and β-blockade further reduces portal pressure and has been shown in three studies to effectively reduce the risk of a first variceal bleed compared with β-blockade alone (Garcia-Pagan et al, 1990; Villanueva et al, 1996; Vorobioff et al, 1993). These investigations have noted, however, the difficult problem of compliance, particularly in patients with alcoholism. In addition, fatigue may be a side effect of therapy with β-blockade, and even more seriously, if patients do bleed, their ability to compensate for blood loss by tachycardia is compromised.
Acute Variceal Hemorrhage
The posterior pituitary hormone vasopressin causes splanchnic arteriolar vasoconstriction, reducing portal blood pressure by approximately 15% when given intra-arterially or intravenously (Chojkier et al, 1979; Huet et al, 1987). Intravenous use is preferred for safety and convenience, and the optimal dose of the drug is 0.3 to 0.4 U/min intravenously. As a result of simultaneous vasoconstrictive effects on the cardiac, mesenteric, and cerebral circulations, the complications increase when doses of 0.5 to 0.7 U/min are administered. It is not necessary to taper the dose; the infusion can be stopped when the therapeutic end point is reached. In a controlled study comparing vasopressin with no therapy, approximately half of the patients on vasopressin stopped bleeding, but this result did not differ from control subjects (Chojkier et al, 1979; Fogel et al, 1982).
Nitrogen is often administered concurrently with vasopressin to reduce the systemic vasoconstrictive effects of vasopressin, and it may further reduce portal pressure. Nitroglycerin infusion begins at 40 µg/min and is titrated to a mean arterial blood pressure of 65 to 75 mm Hg (Gimson et al, 1986).
Octreotide reduces bleeding (D’Amico et al, 1995) and enhances the results of sclerotherapy (Besson et al, 1995). Somatostatin and octreotide are endogenous peptides that act by reducing splanchnic, hepatic, and azygos blood flow (Bosch et al, 1981). Their principal advantage over vasopressin is that they do not cause vasoconstriction of the myocardial and cerebral circulations. Somatostatin and octreotide should be administered continuously at 250 µg/h and increased to 500 µg/h if bleeding continues. Preliminary studies showed that octreotide helped arrest acute variceal bleeding in six of six patients (Thulin et al, 1979; Tyden et al, 1978). Randomized, controlled trials comparing somatostatin or octreotide with vasopressin versus no infusion have shown equivocal results, which suggests that vasopressin and somatostatin have similar efficacy (Burroughs, 1996; Burroughs et al, 1990; Imperiale et al, 1995). Neither vasopressin nor somatostatin has been approved by the U.S. Food and Drug Administration (FDA) for treatment of variceal bleeding, although both agents are commonly used for this purpose (Korula, 1998). A prospective, randomized trial showed equivalence of somatostatin and sclerotherapy in the treatment of acute variceal bleeding (Planas et al, 1994).
Prevention of Rebleeding After Initial Control
Propranolol was shown by Lebrec and colleagues (1980, 1981) to reduce rebleeding significantly after acute variceal hemorrhage. This effect may be mediated by a decrease in cardiac output (β1-blockade), increased splanchnic arteriolar resistance (β2-blockade), and consequent decrease in portal blood flow (Lebrec et al, 1982) and collateral blood flow via the azygos venous system (Feu et al, 1993). β-Blockade is not widely used in the United States to prevent rebleeding after an episode of variceal hemorrhage because endoscopic sclerotherapy and ligation are preferred, and β-blockade after acute bleeding has not been shown to reduce mortality rate (Pagliaro et al, 1989). Meta-analysis comparing β-blockade with endoscopic sclerotherapy showed a non–statistically significant decrease in pooled relative risk for bleeding in the sclerotherapy group and no difference in mortality rate between the two groups (D’Amico et al, 1995). A randomized, controlled study showed, however, that isosorbide mononitrate (80 mg/day) in combination with nadolol (80 mg/day) was more effective than sclerotherapy in reducing rebleeding (Villanueva et al, 1996), and complications were less frequent in the group treated with drugs (16% vs. 37%). If this study is confirmed by other investigators, pharmacologic therapy may play a larger role than it currently does in the United States in the prevention of rebleeding.
Endoscopic Therapy of Variceal Hemorrhage
Prophylaxis
The use of prophylactic sclerotherapy to prevent a first hemorrhage was studied in three meta-analyses (see Chapter 75A, Chapter 75B, Chapter 75C ; Fardy & Laupacis, 1994; Pagliaro et al, 1989; Van Ruiswyk & Byrd, 1992). One study concluded that paravariceal injection with polidocanol decreased mortality rates (Fardy & Laupacis, 1994). The other two reports found that prophylactic sclerotherapy did not reduce bleeding or mortality rate and concluded that sclerotherapy was not indicated in this setting (D’Amico et al, 1995; Pagliaro et al, 1989; Van Ruiswyk & Byrd, 1992). The largest trial of prophylactic sclerotherapy was the Veterans Affairs (VA) cooperative trial. This trial included 281 patients but was prematurely closed because of excess mortality rate in the sclerotherapy group (VA Cooperative Variceal Sclerotherapy Group [CVSG], 1991). Sclerotherapy prevented variceal hemorrhage but substituted bleeding from sclerotherapy-induced ulceration. This study effectively ended the use of prophylactic sclerotherapy in the United States.
Acute Variceal Hemorrhage
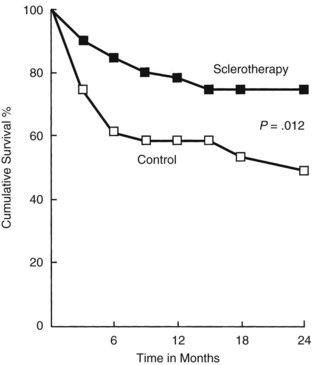
When it became apparent that the once predominant therapy for variceal hemorrhage, emergency surgical shunts, were not improving survival but rather substituting death from liver failure for death from bleeding, endoscopic variceal injection was evaluated as a less invasive therapy. In 1980, a prospective randomized trial with 107 patients from King’s College Hospital showed control of bleeding by sclerotherapy in 57% of 51 treated patients compared with 25% of 56 patients treated medically (MacDougall et al, 1982). Two years later, a follow-up study showed improved patient survival with sclerotherapy compared with controls who received blood transfusions, vasopressin, and a Sengstaken-Blakemore tube when necessary (Fig. 76A.1).
When interpreting this and subsequent trials, it is important to understand that the King’s College trial had more nonalcoholic patients than alcoholic patients (60 vs. 47) and had patients with relatively mild liver failure (74 were CTP class A or B, 33 were class C). The more patients in any study of variceal hemorrhage who are alcoholic or who have CTP class C liver disease, the more difficult it is to show a survival advantage of therapy. Death from bleeding in such patients tends to be replaced by death from liver failure (Block & Reichelderfer, 1998). The VA cooperative study showed no reduction of long-term survival when acute hemorrhage was treated with sclerotherapy (CVSG, 1994).
Sclerotherapy has been shown to stop acute variceal hemorrhage effectively (Gregory, 1990; Westaby et al, 1989). Meta-analysis of 20 trials of emergency sclerotherapy versus a variety of alternative therapies supported the superiority of sclerotherapy with its success rate of 71% to 100%; however, the complication rate was high (18%), and 2.7% patients died as a direct result of sclerotherapy (D’Amico et al, 1995).
Endoscopic variceal ligation (EVL) has been developed as an endoscopic alternative to sclerotherapy, potentially lowering the risk of ulceration and perforation of the esophagus. Seven prospective, randomized, controlled trials compared EVL with endoscopic sclerotherapy (Gimson et al, 1993; Hashizume et al, 1993; Hou et al, 1995; Laine et al, 1993; Lo et al, 1995, 1997; Stiegmann et al, 1992). In all studies, EVL and sclerotherapy were equally effective in controlling active bleeding. Complications were significantly lower with EVL in all studies. No esophageal strictures were seen in patients treated with EVL compared with 5% to 33% of patients treated with sclerotherapy. The development of the multiple-band ligating device, which allows banding without repeatedly reinserting the endoscope, has made this modality of endoscopic control of varices much more attractive, such that it has now become the endoscopic therapy of choice (Laine, 1997).
Prevention of Rebleeding
Although sclerotherapy effectively stops acute variceal bleeding, rebleeding remains a problem, and intermediate (2- to 5-year) survival is not improved in many trials. A confounding variable confusing interpretation of the results in many of these trials is continued alcoholism. Alcohol abstinence for 6 months, CTP class, and aspartate aminotransferase level all were independent predictors of survival in the VA trial (CVSG, 1994). Meta-analyses of trials comparing sclerotherapy with pharmacologic management have shown sclerotherapy to prevent rebleeding more effectively and sometimes improve survival (D’Amico et al, 1995; Infante-Rivard et al, 1989). When EVL was compared with sclerotherapy, rebleeding rates were significantly decreased with EVL in three studies (Gimson et al, 1993; Hou et al, 1995; Lo et al, 1995), and mortality rates were significantly lower in three studies (Hou et al, 1995; Lo et al, 1995; Stiegmann et al, 1992). EVL seems to be at least as effective as sclerotherapy in preventing rebleeding.
Transjugular Intrahepatic Portosystemic Shunt (See Chapter 76E)
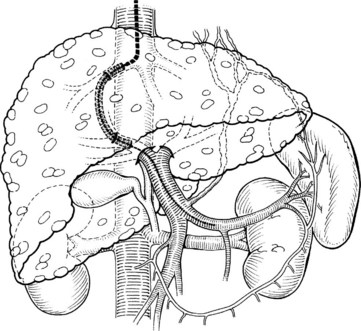
The development and clinical use of TIPS in the treatment of portal hypertension first occurred in the 1990s, and its use for the treatment of variceal hemorrhage has expanded (LaBerge et al, 1992; McCormick et al, 1994). TIPS is used electively far more often than in the emergency setting. Despite the effectiveness of TIPS in abruptly stopping variceal hemorrhage, overall patient mortality rates remain high (Smith & Graham, 1982). Death often is related to multisystem organ failure, progressive liver failure or sepsis, or disseminated intravascular coagulation, reflecting the use of TIPS in patients with end-stage disease. Complications are usually related to the underlying cirrhosis and associated comorbidities. In addition to relieving variceal hemorrhage, TIPS effectively relieves ascites in these patients (Crenshaw et al, 1996; Martin et al, 1993b) because TIPS is functionally a nonselective, side-to-side portacaval shunt. In contrast to all surgical shunts, TIPS creates a shunt to the suprahepatic inferior vena cava (Fig. 76A.2).
TIPS was developed as a minimally invasive procedure performed by radiologists using fluoroscopic imaging to place a noncompressible stent between the portal vein and hepatic vein. Successful TIPS lowers portal pressure, and the procedure is typically well tolerated even in very ill patients. Complications include encephalopathy secondary to portosystemic shunting, shunt stenosis and occlusion, inability to place a TIPS, and intraperitoneal bleeding if the liver capsule is punctured. Mortality rate at 30 days has been reported at 20%, but half of these deaths were unrelated to the procedure itself (Darcy et al, 1993). In patients with CTP class C cirrhosis, a 30-day mortality rate of 67% has been reported (Martin et al, 1993a, 1993b); in a lower risk population of 100 patients, a 30-day mortality rate of 5.3% was reported (Richter et al, 1994).
Primary patency—that is, patency without radiologic intervention to revise the TIPS—has been reported to be 46% to 85% in the first 3 to 6 months (Richter et al, 1994; Saxon et al, 1993; Sterling & Darcy, 1995) and 27% to 57% at 1 year (Haskal et al, 1994; Malisch et al, 1993; Saxon et al, 1993; Sterling & Darcy, 1995). Primary assisted patency, meaning patency after revision, has been reported to be 85% (Haskal et al, 1994). LaBerge and colleagues (1995) reported shunt stenosis or occlusion in 47% of 90 TIPS patients over a 2-year period.
The newer polytetrafluoroethylene (PTFE)-covered stents are associated with less need for intervention, less shunt dysfunction, and better outcomes (Bureau et al, 2007). If post-TIPS ultrasound shows narrowing or thrombosis of the shunt, patency can be restored by repeat balloon dilation and stenting or by thrombectomy. Color Doppler ultrasound of TIPS is routinely performed at 1- and 6-month intervals after the procedure to evaluate luminal narrowing or increased flow velocity, which would suggest impending thrombosis of the TIPS. The rate of TIPS restenosis or occlusion is higher than the rate of recurrent symptoms because in some patients, occlusion does not produce symptoms. Nevertheless, recurrent variceal hemorrhage occurs in approximately 50% of patients with TIPS stenosis or occlusion. A multicenter trial of TIPS involving 100 patients reported that 16% of patients had rebleeding by 6 months, and five of these were from nonvariceal sources (Coldwell et al, 1995). Similarly, LaBerge and colleagues (1995) reported variceal rebleeding in 32% of 90 patients at 2 years.
Acute Variceal Bleeding and Transjugular Intrahepatic Portosystemic Shunting
TIPS may be used effectively in the control of acute variceal hemorrhage when medical management or endoscopic variceal ligation or both are ineffective. Barton and colleagues (1995) found that TIPS controlled acute variceal bleeding in 91% of patients, whereas Helton and associates (1993) reported control in 17 (74%) of 23 patients. A report by Encarnacíon and colleagues (1995) reported on 65 patients with acute variceal bleeding unresponsive to sclerotherapy or not treated with sclerotherapy because of recurrent massive hemorrhage. Acute bleeding stopped before the TIPS procedure in 26 patients, but not in the other 39 patients. Of the 65 patients with acute bleeding, 64 had successful placement of TIPS, and all these patients stopped bleeding within 3 days. The 30-day survival rate of patients who stopped bleeding before TIPS was 96%, but it was only 69% for patients actively bleeding at the time of TIPS. Survival was also linked to CTP class, with a 30-day survival rate of 91% for class A (n = 2) and class B (n = 32) patients, but survival rate was 71% for class C (n = 31) patients.
When used as primary therapy for acute variceal bleeding, TIPS may reduce treatment failure and mortality rate in high-risk patients. Monescillo and colleagues (2004) reported that in patients defined as high risk by a hepatic venous pressure gradient (HVPG) greater than 20 mm Hg randomized to treatment with TIPS (n = 26) versus no TIPS (n = 26), the no-TIPS group required more transfusions (P = .002), needed more intensive care unit care, had more treatment failures, and had poorer survival (P < .05). The no-TIPS group was treated with β-blockers, variceal banding, or sclerotherapy.
Prevention of Rebleeding by Transjugular Intrahepatic Portosystemic Shunting
TIPS has been used most frequently to prevent recurrent variceal hemorrhage. The results of four large series are shown in Table 76A.1 (Henderson et al, 1998). Rebleeding rates are similar in these series and are approximately 25% at 1 year. Thirty-day mortality rates were 14% to 16% except for the Rössle series (Rössle et al, 1994). Most deaths within 30 days were due to multisystem organ failure, whereas most later deaths were attributable to progressive liver failure. Because TIPS is a nonselective shunt, encephalopathy rates were relatively high at 25%, although in most patients this was not debilitating because it is usually controllable with lactulose, neomycin or rifaximin, and a low-protein, low-ammonia diet.
Table 76A.1 Patency of Transjugular Intrahepatic Portosystemic Shunt for Prevention of Recurrent Bleeding
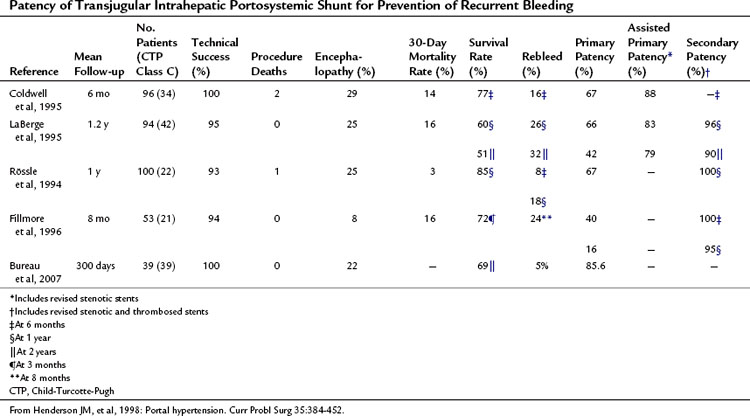
Most episodes of rebleeding after TIPS were related to stenosis or thrombosis of the shunt. In addition, many asymptomatic patients had shunt stenosis or thrombosis detected by ultrasound. Primary patency was 40% to 67% at 1 year, which improved to 79% to 88% with revision of stenotic stents (assisted primary patency). Secondary patency, which is patency after TIPS thrombectomy or revision, was 95% to 100% at 1 year (Coldwell et al, 1995; Fillmore et al, 1996; LaBerge et al, 1995; Rössle et al, 1994).
In the short time since its introduction, TIPS has had a dramatic impact on the treatment of variceal hemorrhage. In addition to its use in preventing variceal hemorrhage, TIPS has the added benefit of often improving overall liver function, as measured by CTP status, and of effectively bridging patients to liver transplantation (Abouljoud et al, 1995; Menegaux et al, 1994; Millis et al, 1995; Odorico, 1998; Suc et al, 1995). Despite the suggestion that TIPS may reduce operative time and blood loss during liver transplantation, data are not yet available to support this contention. Nevertheless, it has been shown that the TIPS procedure effectively prevents rebleeding (D’Amico et al, 1995; Ring et al, 1992).
TIPS has been compared with endoscopic therapy for the long-term prevention of recurrent bleeding. In a meta-analysis of 11 randomized trials, fewer patients rebled after TIPS (19%) than after endoscopic therapy (47%), encephalopathy was more common after TIPS (34%), and TIPS dysfunction developed in 50% of patients overall (Papatheodoridis et al, 1999).
A major recent change in therapy recommendations is that in creating a TIPS, use of expanded PTFE (ePTFE)-covered stents is now preferred. The basis for this recommendation is the decreased need for shunt intervention and a suggestion of better outcomes with covered stents rather than bare ones (Angermayr, et al, 2003). Although TIPS increases the risk of hepatic encephalopathy, prophylactic use of nonabsorbable disaccharides or antibiotics does not reduce this risk and is not recommended (Riggio et al, 2005).
TIPS also has been compared with shunt surgery. The distal splenorenal shunt showed lower rates of rebleeding, encephalopathy, and shunt thrombosis than TIPS, but ascites was less common after TIPS (Khaitiyar et al, 2000). A multi-institutional, randomized trial compared TIPS with the distal splenorenal shunt in CTP class A and B cirrhotic patients (Henderson et al, 2004). Initial analysis of the results showed no significant differences between TIPS and the distal splenorenal shunt in variceal rebleeding, shunt occlusion, and survival. However, of the TIPS patients, 80% required reintervention to maintain shunt patency, and close surveillance was required.
TIPS also has been compared with the small-diameter interposition shunt. In a controlled trial, shunt occlusion, death from hepatic failure, and the need for liver transplantation all were significantly more common after TIPS (Rosemurgy et al, 2000). At the 10-year follow-up report, the small-diameter interposition shunts continued to perform better in terms of shunt occlusion, and survival was also superior in CTP class A and B patients and in those with Model for End-Stage Liver Disease (MELD) scores less than 13 (Rosemurgy et al, 2000). Indications for TIPS supported by current data include 1) continued variceal hemorrhage after sclerotherapy or banding, 2) prevention of rebleeding or treatment of ascites in patients awaiting liver transplantation, and 3) prevention of rebleeding in patients who are not candidates for a surgical shunt or liver transplantation because of expected short survival.
Treatment of Budd-Chiari Syndrome with Transjugular Intrahepatic Portosystemic Shunting
Given the advantages of the PTFE-covered stents with regard to patency, this matter was studied in a large series of 221 patients with Budd-Chiari syndrome who were treated with TIPS (133 patients) if they had not responded to anticoagulation treatment (Garcia-Pagan et al, 2008). TIPS is now recommended for patients with Budd-Chiari syndrome who do not improve with anticoagulation therapy.
Treatment of Ascites with Transjugular Intrahepatic Portosystemic Shunting
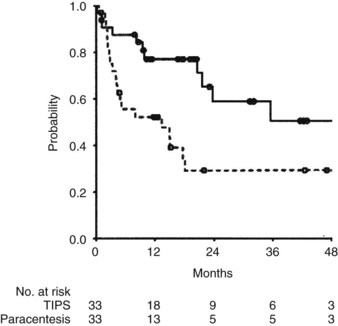
TIPS has been used effectively to relieve ascites in patients refractory to pharmacologic therapy with diuretics. Salerno and associates (2004) reported a multicenter, randomized, controlled trial comparing TIPS (n = 33) with paracentesis plus albumin (n = 33) in patients with CTP class B and C cirrhosis. Survival without liver transplantation was superior in patients treated with TIPS (P = .021; Fig. 76A.3). By multivariate analysis, a higher MELD score and paracentesis independently predicted death. Treatment failure was more common in patients treated with paracentesis, although encephalopathy occurred more commonly in patients receiving TIPS (Salerno et al, 2004).
Surgical Shunts for Bleeding Esophageal Varices (See Chapters 76C and 76D)
Prophylactic Surgery
Early trials of prophylaxis for variceal bleeding compared portacaval shunts with medical therapy. Although bleeding was effectively prevented, survival was not significantly enhanced with surgery because of a marked increase in deaths from accelerated hepatic failure (Grace, 1992). Because only one third of patients with varices eventually bleed, surgery cannot be justified as prophylaxis and is not recommended in this setting.
In a prospective, controlled study to evaluate prophylactic surgery in 112 patients with portal hypertension and esophageal varices, Inokuchi (1984) found the bleeding rates were 19.2% in the medical group and 0% in the surgical group. No difference was reported in the survival rate between the two groups at 2-year follow-up, and prophylactic surgery led to a prevention of esophageal bleeding without any increase in the mortality rates. This is the only study to support a role for prophylactic surgery, but it should be noted that the majority of these patients had posthepatic cirrhosis with reasonably well-preserved liver function.
Acute Variceal Hemorrhage
At most American centers, endoscopic therapy is the first option used to treat bleeding esophageal varices. An exception is the series reported by Orloff and coworkers (1994), who used portacaval shunts as a first-line therapy with excellent results. At most other centers, patients who do not respond to endoscopic variceal ligation are referred for consideration for TIPS or a surgical shunt. Emergency surgical shunts prevent bleeding more effectively than sclerotherapy, but overall mortality rate is equivalent (Cello et al, 1987; D’Amico et al, 1995).
Although nonoperative therapies are useful for initial management of bleeding esophageal varices, if these measures fail to control bleeding, emergency surgery should be promptly considered (Rikkers & Jin, 1994). Emergency surgical shunts normalize portal pressure immediately and effectively control variceal hemorrhage, but emergency surgery has been associated with a mortality rate of 20% to 55% (Cello et al, 1987; D’Amico et al, 1995). The high risk of dying after placement of an emergency shunt is presumably related to the frequent decompensation of liver function and associated comorbidity at the time of an acute bleed. Outcomes correlate with CTP classification rather than with the type of shunt performed. Liver failure and encephalopathy often ensue and are the proximate causes of death associated with emergency surgery in most series.
In choosing which surgical shunt to use for emergency control of bleeding, the portacaval shunt (see Chapter 76B) is an acceptable choice because it effectively decompresses the portal venous system and can usually be rapidly constructed. An end-to-side portacaval shunt is adequate, although patients with ascites should have a side-to-side portacaval shunt to relieve their ascites as well. A series by Orloff and colleagues (1994) showed the usefulness of the portacaval shunt in the emergency setting. Other functional side-to-side shunts, such as the mesocaval shunt (see Chapter 76D) and proximal splenorenal shunt (see Chapter 76C), also effectively decompress the portal vein and relieve esophageal variceal bleeding, and they should be effective for relief of ascites. In contrast to portacaval shunts, these shunts do not require dissection in the porta hepatis and do not complicate future liver transplantation. In appropriately selected patients, a distal splenorenal shunt also may be used in the emergency setting to relieve variceal hemorrhage in patients with a large, patent splenic vein and absent or medically controlled ascites (Rikkers & Jin, 1995).
Prevention of Rebleeding After Initial Control
The role of TIPS versus a surgical shunt for prevention of rebleeding has been recently clarified by a randomized clinical trial comparing the two treatments in patients who did not respond to medical therapy (Henderson et al, 2006). No difference was found in rebleeding rates (5.5% for distal splenorenal shunt [DSRS] vs. 10.5% for TIPS, P value not significant), encephalopathy, or survival. TIPS patients required more interventions, although the study used noncovered stents. TIPS was slightly more cost effective than DSRS at 1 year (Boyer et al, 2008), and the study concluded that the two treatments were of equal efficacy in preventing recurrent variceal hemorrhage.
General Aspects of Nonselective Shunts
The end-to-side portacaval shunt was the first experimental shunt performed in dogs (Konstantinov, 1997). This shunt is the prototype of the nonselective shunt and has been compared in randomized, controlled trials with conventional medical management for the treatment of portal hypertension and its complications (Rikkers et al, 1992). Figure 76A.4 shows combined data of four controlled studies of the portacaval shunt, comparing shunted patients with medically managed patients according to survival. No survival advantage could be shown for shunt patients, although the four studies were all biased in favor of medical management because failures of medical management were crossed over to surgical therapy. Bleeding was effectively stopped in shunt patients, whereas more than 70% of medically treated patients rebled. Encephalopathy occurred in 20% to 40% of shunted patients.
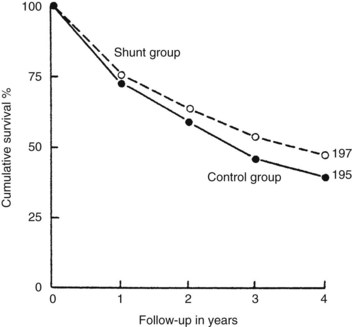
FIGURE 76A.4 Cumulative survival data from four controlled trials of portacaval shunt versus conventional medical management.
(Courtesy HO Conn; from Boyer TD, 1982: Portal hypertension and its complications: bleeding esophageal varices, ascites, and spontaneous bacterial peritonitis. In Zakim D, Boyer TD [eds]: Hepatology: A Textbook of Liver Disease. Philadelphia, Saunders, pp 464-499.)
When the end-to-side portacaval shunt was compared with the side-to-side shunt in a controlled trial, no significant clinical differences were noted between these two shunts (Resnick et al, 1974). The interposition mesocaval shunt (see Chapter 76D), also a nonselective shunt, was studied in a randomized trial comparing it with the direct side-to-side portacaval shunt, and no clinical or hemodynamic differences were evident (Stipa et al, 1981). The same series documented a high graft thrombosis rate after the mesocaval shunt. Nevertheless, the mesocaval shunt avoids dissection in the porta hepatis, which is an advantage for future liver transplant candidates. An additional option is a central or proximal splenorenal shunt with splenectomy. In the current era, indications for a nonselective shunt include an emergency shunt for variceal hemorrhage, an elective shunt in the presence of significant ascites, and treatment of Budd-Chiari syndrome. In some patients not suited for a selective shunt, a nonselective shunt might serve as a long-term bridge to hepatic transplantation, when bleeding is not controlled endoscopically or by TIPS.
Budd-Chiari syndrome (see Chapter 77) with ascites, abdominal pain, and portal hypertension is an indication for a side-to-side portacaval shunt. A side-to-side shunt is necessary because the portal vein (PV) serves as the major efferent conduit in this syndrome. If the disease is fulminant, or if cirrhosis has developed secondary to longstanding hepatic venous occlusion, liver transplantation is a preferable option. If liver transplantation is not anticipated, a side-to-side portacaval shunt may be the ideal procedure. Often the caudate lobe enlarges after occlusion of the major hepatic veins because the caudate lobe communicates directly with the vena cava and may become the major route of venous outflow from the liver. Massive hypertrophy of the caudate lobe may prevent a side-to-side portacaval shunt from being technically possible because of caudal expansion of the caudate lobe, which is interposed between the PV and the inferior vena cava (IVC) and prevents their side-to-side anastomosis. A mesocaval shunt may be technically more feasible in this setting.
General Aspects of Distal Splenorenal Shunt
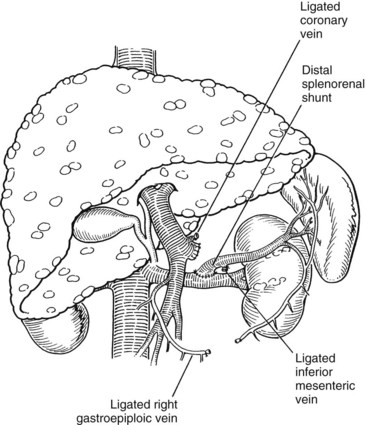
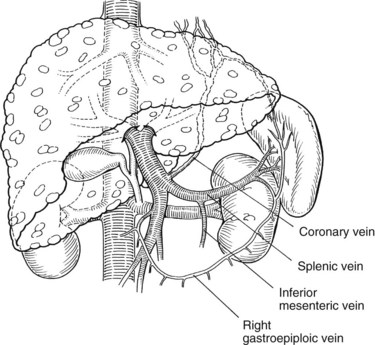
Warren and colleagues (1967) introduced the distal splenorenal shunt (see Chapter 76C) with the goal of preserving hepatopetal blood flow in the PV while decompressing esophageal varices. The distal splenic vein is anastomosed to the left renal vein, and collateral vessels are ligated, such as the coronary and gastroepiploic veins connecting the superior mesenteric and gastrosplenic components of the splanchnic venous circulation (Fig. 76A.5). This procedure compartmentalizes the portal venous circulation into a high-pressure superior mesenteric venous system to perfuse the liver and a decompressed gastrosplenic venous system to avoid variceal bleeding (Knechtle, 1998).
In patients with advanced ascites, the distal splenorenal shunt is contraindicated because lymphatics are transected during the dissection of the left renal vein and the liver continues to have elevated sinusoidal pressures. In such patients, the distal splenorenal shunt may worsen ascites rather than relieve it. Warren’s claim that the operation effectively accomplishes its goal of preserving hepatic function better than nonselective shunts remains controversial. Controlled trials have shown decreased portosystemic encephalopathy after the distal splenorenal shunt. Henderson and colleagues (1983) showed that portal flow is maintained in most patients with nonalcoholic cirrhosis and noncirrhotic portal hypertension, but portal flow rapidly collateralizes to the shunt in patients with alcoholic cirrhosis, particularly if alcohol consumption continues. Failure to ligate the coronary vein (CV) results in early loss of hepatopetal portal flow.
Six of seven controlled trials comparing the distal splenorenal shunt with nonselective shunts have evaluated alcoholic cirrhotic patients and were summarized by Jin and Rikkers (1991). None of these trials showed a survival advantage of either procedure. Four of the seven trials showed less encephalopathy after a selective shunt, whereas the other trials showed no difference in encephalopathy rates. Rebleeding rates did not differ between the two shunt groups, although one trial noted a higher rate of rebleeding after distal splenorenal shunt.
When the distal splenorenal shunt was compared with repeated endoscopic therapy, rebleeding was less frequent with selective shunts, but hepatic portal perfusion was better maintained by sclerotherapy. Encephalopathy rates were similar in both groups (Henderson et al, 1990; Rikkers et al, 1993). These two studies suggest that sclerotherapy effectively controls initial bleeding, but patients who do not respond to sclerotherapy should promptly undergo surgery. Another indication for surgery, rather than sclerotherapy, is poor access to advanced medical care. Such patients benefit from an initial selective shunt, rather than long-term sclerotherapy, because the latter requires multiple visits to a medical center.
Partial Shunt
The partial shunt proposed by Sarfeh and colleagues (1986) is a means of decompressing varices while preserving hepatic portal perfusion. An 8- or 10-mm PTFE graft is interposed between the PV and IVC. A prospective randomized trial of partial (8-mm diameter) and nonselective (16-mm diameter) interposition portacaval shunts showed a lower frequency of encephalopathy after the partial shunt, but similar survival was reported after both types of shunts (Sarfeh & Rypins, 1994). The largest series of partial shunts was reported by Rosemurgy and colleagues (2007) and includes 170 patients older than 18 years. Small-diameter H-graft shunts were performed in patients who did not respond to endoscopic variceal ablative therapy (56% alcoholic, 44% nonalcoholic); 38% were CTP class A or B and 62% were CTP class C. Variceal bleeding after shunt placement was very uncommon (2%). Actual survival was superior to that predicted by MELD scores but did parallel the degree of hepatic reserve. Small-diameter H-graft shunting was encouraged in those patients who were neither eligible nor suitable for liver transplantation (Rosemurgy et al, 2007).
Types of Shunts: Technical Aspects
Portacaval Shunt (See Chapter 76B)
A side-to-side portacaval shunt is performed through a transverse upper abdominal incision. The common bile duct is encircled and retracted to the patient’s left. If a replaced right hepatic artery arises from the superior mesenteric artery, this also needs to be encircled and retracted to the left, which makes the exposure of the PV more difficult. The PV is dissected and encircled with a vessel loop, and the IVC is dissected and encircled with a vessel loop between the lower edge of the liver and the right renal vein. Partially occluding vascular clamps are placed on the IVC and PV, and the two are approximated. A venotomy is made longitudinally in each vein approximately 2 cm in length, and stay sutures of 6-0 polypropylene are placed at the corners. These stay sutures are tied down, and the anastomosis is performed with a running technique. Vascular clamps are removed and the wound is checked for hemostasis. The completed anastomosis is shown in Fig. 76A.6.
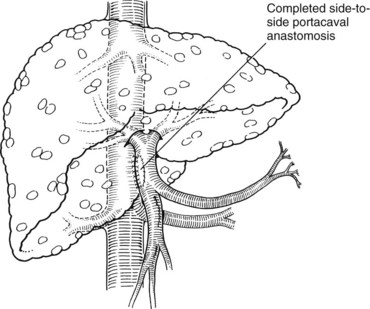
FIGURE 76A.6 The completed anastomosis normalizes portal pressures and results in hepatofugal blood flow through the shunt.
(From Knechtle SJ, 1998: Surgical shunts for portal hypertension. In Knechtle SJ [ed]: Portal Hypertension: A Multidisciplinary Approach to Current Clinical Management. Armonk, NY, Futura, pp 175-202.)
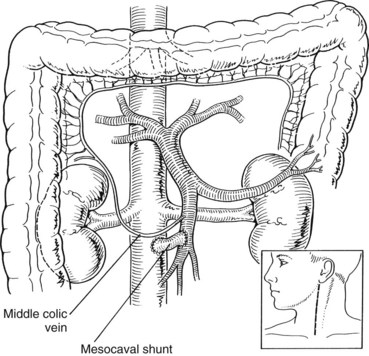
Mesocaval Shunt (See Chapter 76D)
When using a jugular vein conduit, the left neck is generally preferred because the left jugular vein is often slightly longer than the right jugular vein (Fig. 76A.7). An incision similar to a carotid endarterectomy incision is made along the anterior border of the sternocleidomastoid muscle. The platysma muscle is divided, and the jugular vein is identified. Branches are doubly ligated and divided, and the jugular vein is dissected free from the clavicle to the mastoid. It is ligated with silk ties proximally and distally, and the graft segment is excised and placed in sterile saline until it is used. Next, the neck wound is closed, and the proximal end of the jugular vein graft is anastomosed end-to-side to the SMV with running 6-0 polypropylene (see Fig. 76A.7). The distal end of the graft is anastomosed end-to-side to the IVC also with running 6-0 polypropylene. Partial occlusion clamps are placed on the SMV and IVC during construction of the anastomoses; after completion, the clamps are removed and shunt flow is assessed with an electromagnetic or ultrasonic flowmeter. Flow should be 1 to 2 L/min, and significantly lower flow rates should prompt inspection of the graft for technical problems.
Distal Splenorenal Shunt (See Chapter 76C)
The splenic vein dissection is generally the most challenging aspect of the procedure and may be particularly difficult in patients with pancreatic fibrosis from chronic pancreatitis. The splenic vein is dissected medially to its confluence with the SMV and laterally to a point that allows the vein to be brought down to the renal vein without kinking or tension. If splenopancreatic dissection is a goal of the procedure, the dissection continues all the way to the spleen such that all pancreatic tributaries are ligated and divided (Fig. 76A.8).
The left renal vein is dissected next, and adjacent lymphatics are ligated to avoid the complication of chylous ascites. The left adrenal vein is ligated and divided to assist in mobilization of the left renal vein. The gonadal vein and descending lumbrical veins are left intact to give additional venous outflow to the shunt. If a circumaortic left renal vein is present with a small anterior branch, decompression may be compromised. In this case, anastomosis directly to the IVC should be considered (i.e., a distal splenocaval shunt) (Atta, 1992). A vessel loop is placed around the left renal vein, and an adequate length of splenic vein should be dissected such that when it is divided at the PV, it can reach the left renal vein easily without tension.
Identification and ligation of the CV is an essential component of the operation. It is preferable to ligate the CV at its junction with the PV or splenic vein. The CV also can be ligated at the superior border of the pancreas, just before it extends along the lesser curvature of the stomach. The CV is generally large and attenuated and must be ligated if the shunt is to be selective. Blood flow through the completed shunt (see Fig. 76A.5) can be measured with a flowmeter and is generally 300 to 1000 mL/min.
Devascularization Procedures for Bleeding Esophageal Varices
Surgical devascularization procedures were developed with the intent of disconnecting varices from the hypertensive portal venous system, decreasing the risk of variceal hemorrhage (see Chapter 75C). In contrast to most shunts, these procedures avoid encephalopathy by preserving hepatic portal perfusion. The gold standard of devascularization procedures has become the Sugiura procedure, which consists of thoracic esophageal transection and devascularization followed weeks later by laparotomy to control bleeding from esophageal varices (Sugiura & Futagawa, 1973). The Sugiura procedure differs from other devascularization procedures in that extensive esophageal and gastric devascularization is performed in a manner that preserves the venous collaterals connecting the CV to the azygos system, which discourages varices from reforming. The initial report by Sugiura and Futagawa (1973) included 276 patients and described an operative mortality rate of 4.3% and reoccurrence of varices in 2.3% after follow-up of 1 to 10 years. Actuarial survival was 83%. Survival according to CTP classification was 95% for class A, 87% for class B, and 57% for class C. Survival was better after an elective procedure than after an emergency operation.
The outstanding results achieved in the Japanese series have not been duplicated elsewhere. Many surgeons outside Japan have used modifications of the Sugiura procedure to control bleeding esophageal varices, particularly in patients with extensive mesenteric venous thrombosis or in those with a previous failed surgical shunt. Orozco and colleagues (1992) reported a 10-year experience with the elective Sugiura procedure using a one-stage transabdominal approach. Mortality rate correlated with CTP class. The Toronto experience with the modified Sugiura procedure was reported by Dagenais and colleagues in 1994 and included a 22% operative mortality rate when the procedure was used in the emergency setting; the 5-year survival rates were 100% for CTP class A, 43% for class B, and 25% for class C patients. In a series of 32 patients undergoing transabdominal esophagogastric devascularization for variceal bleeding, 11 of the 12 patients without liver disease survived more than 10 years. The other 20 patients with cirrhosis had a 5-year survival rate of 51% (Jin & Rikkers, 1996). This experience suggests that esophagogastric devascularization is an effective alternative to shunt surgery, particularly in patients whose underlying condition is diffuse splanchnic venous thrombosis in the absence of liver disease. The only prospective, randomized clinical trial comparing the Sugiura procedure with selective or total shunts was performed in patients with bilharzial cirrhosis and bleeding varices (da Silva et al, 1986). This trial concluded that patients treated with devascularization were more likely to survive longer without encephalopathy compared with patients treated with a shunt (da Silva et al, 1986; Raia et al, 1991).
Liver Transplantation for Bleeding Esophageal Varices
Definitive therapy for patients with advanced liver failure (CTP class B or C) is liver transplantation (see Chapter 97A, Chapter 97B, Chapter 97C, Chapter 97D, Chapter 97E ). Variceal hemorrhage is the most common clinical manifestation of portal hypertension to prompt liver transplant evaluation. The acute management of variceal hemorrhage in liver transplant candidates begins with endoscopic diagnosis and endoscopic variceal ligation where feasible, in combination with pharmacologic therapy, TIPS, or both for failures of endoscopic management. In view of the strong correlation between CTP class and long-term outcome, seen with virtually every form of therapy for bleeding varices, compelling evidence suggests that liver transplantation should be the treatment of choice for such patients with advanced liver disease. In the current era, each of the treatments discussed so far, including surgical shunts, should be used in a complementary fashion, and many patients require sequential application of the various modalities. Naturally, liver transplantation is the ultimate solution to cirrhosis and end-stage liver failure, when this is the underlying cause of portal hypertension and variceal hemorrhage.
Because of the disparity between the number of patients awaiting liver transplantation and the supply of donor livers, transplantation is generally accompanied by an unpredictable waiting period that varies depending on the supply of donor livers. Average waiting times for liver transplantation in the United States vary from 6 months to more than 2 years, depending on blood type (U.S. Department of Health and Human Services, 2003). Operative morbidity and mortality associated with liver transplantation correlate with the preoperative condition of the recipient. For this reason, it is advisable to use alternative measures to arrest variceal hemorrhage and to optimize the medical condition of the patient before proceeding to liver transplantation. Liver transplant programs in the United States preclude active alcoholics from liver transplantation and require at least a 6-month period of abstinence. This therapy generally is unavailable to active alcoholics with variceal hemorrhage, and such patients may need to be considered for surgical shunts. Because of immediate normalization of portal pressure, liver transplantation is effective therapy for bleeding esophageal varices resulting from underlying portal hypertension (Ewaga et al, 1994).
A portacaval shunt or surgical shunt involving dissection in the porta hepatis makes subsequent liver transplantation more technically difficult. If liver transplantation is anticipated after a surgical shunt, a shunt should be performed outside the porta hepatis whenever possible. Distal splenorenal and mesocaval shunts are preferred shunts for such patients (Knechtle et al, 1994; Shaked & Busuttil, 1991). A surgical shunt is an attractive means of controlling variceal hemorrhage in a patient who may not need liver transplantation for several years. Most patients eligible for liver transplantation with CTP class C cirrhosis require transplantation in the short term and are managed more appropriately with TIPS as a bridge to liver transplantation. TIPS may significantly improve the CTP class and may reduce morbidity for patients awaiting liver transplantation (Fig. 76A.9; Odorico, 1998).
Meso-Rex Shunt for Portal Vein Stenosis after Liver Transplantation
Especially in children, extrahepatic PV stenosis may occur after liver transplantation, and it is generally seen many months or years after surgery. Often it manifests as portal hypertension, with GI bleeding from the Roux-en-Y, worsening splenomegaly, or even encephalopathy (Chiu & Superina, 2006; Superina et al, 2006). This problem, limiting PV blood flow to the liver and creating portal hypertension, can be addressed with a PV or SMV to left PV shunt graft, termed the Meso-Rex shunt. Autologous vein is generally used to create such a shunt, which relieves portal hypertension and restores physiologic blood flow to the liver (Bambini et al, 2000).
Extrahepatic Portal Vein Thrombosis
Experience in the treatment of variceal bleeding as a result of PV thrombosis indicates three possible treatment options. Expectant medical management of each acute bleeding episode is tempting because the bleeding is generally well tolerated when the liver is normal. This approach may be appropriate in infants because bleeding tends to be self-limited, and half of these patients outgrow their variceal bleeding without therapy. Endoscopic variceal ablation and surgery should certainly be considered in the adult population, in whom the mortality rate for variceal hemorrhage approaches 20% over time with recurrent bleeding. It must be remembered that the same normal liver that allows patients to tolerate conservative management of bleeding also makes them excellent candidates for more aggressive treatment. Typically, the acute episode of variceal bleeding can be managed by resuscitation, vasopressin or octreotide infusion, and then endoscopic variceal ablation (Howard et al, 1988; Warren et al, 1988).
If bleeding becomes recurrent, evaluation of the portal, mesenteric, and splenic veins is performed with visceral angiography to include venous phases and/or magnetic resonance angiography. If the spleen is in situ and the splenic vein is patent, the patient is an excellent candidate for the distal splenorenal shunt, although central splenorenal shunts and mesocaval shunts can also be used if the SMV is also open. If the splenic vein is also occluded or if the spleen is absent, further endoscopic variceal ablation should be attempted. If this fails, exploration is performed. In rare cases, a shuntable vein can be found, such as the CV (a coronary caval shunt). Most often, if the spleen has not already been removed at a previous operation, usually for thrombocytopenia, a gastric devascularization with splenectomy is required. Recurrent variceal hemorrhage after devascularization can occur in 20% to 30% of patients, which requires further endoscopic variceal ablation but rarely esophagogastrectomy (Galloway & Henderson, 1990).
Present Role of Surgical Shunts
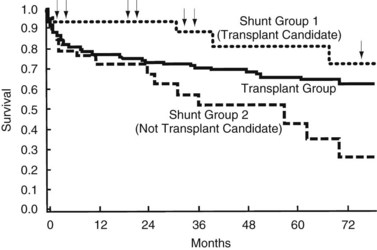
A shunt may often serve as a long-term bridge to liver transplantation in patients who are deemed to be acceptable candidates for liver transplantation. In a report comparing liver transplantation, shunts (82% distal splenorenal) in future candidates for liver transplantation, and shunts in patients without the future prospect of liver transplantation, the operative mortality rate was best in the two shunt groups (5% and 7% vs. 19% in liver transplant recipients). This finding was attributed to more advanced disease in the liver transplant cohort. The patients not considered for liver transplantation were excluded because of active alcoholism or advanced age. Kaplan-Meier survival analysis showed better survival in shunt patients who were transplant candidates—seven of 44 patients had progressed to liver transplantation—than either the liver transplant group or the shunt group without prospective liver transplantation during the first 5 years of follow-up. However, by 10 years no difference was reported between the groups (Fig. 76A.10). These results suggest that patients with CTP class A or early B cirrhosis, who are not actively drinking and are not too elderly or medically unfit, benefit from shunt surgery most. Because it is complicated less frequently by encephalopathy and because it has an excellent long-term patency rate, a distal splenorenal shunt is a good choice for such patients. If they progress to end-stage liver failure, they can be salvaged with transplantation (Rikkers et al, 1997). During the period studied, distal splenorenal shunts and nonselective shunts were more protective against rebleeding than was esophagogastric devascularization (Rikkers et al, 1997).
Consistent with the aforementioned observations, surgical shunts generally are reserved for patients with good hepatic reserve and variceal bleeding. Excellent results are achieved in this setting (Knechtle et al, 1999).
An algorithm summarizing current decision making in the management of variceal hemorrhage is shown in Figure 76A.11. Patients are divided into those potentially eligible for a liver transplant versus ineligible patients. The sequential use of various modalities is illustrated. The current use of procedures performed for variceal hemorrhage suggests that although banding continues to be used frequently, the use of TIPS has dramatically increased, as has liver transplantation. Nonselective shunts are rarely used today, and selective shunts are performed in a highly select group of patients.
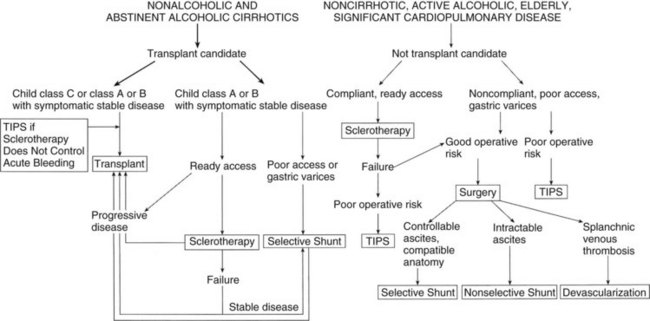
FIGURE 76A.11 Algorithm for definitive therapy of variceal hemorrhage. TIPS, transjugular intrahepatic portosystemic shunt.
(From Rikkers LF, 1995: Portal hypertension. In Levine BA, et al [eds]: Current Practice of Surgery, vol 3. New York, Churchill Livingstone, pp 1-22.)
During the past 25 years, liver transplantation and TIPS have evolved into effective therapies and have substantially affected management of this problem and indirectly changed the risk status (CTP class) of patients undergoing shunts. Analysis of this group of patients showed that in recent years the CTP class has progressively improved, and the need for emergency surgery has declined. The use of nonselective shunts declined because of the development of effective alternatives, such as EVL and TIPS, and because advanced liver failure with ascites was managed by liver transplantation, sometimes preceded by TIPS. Consequently, less risky patients were selected to undergo elective shunts for treatment of variceal bleeding. The incidence of postoperative encephalopathy decreased, and long-term (10-year) survival improved, especially because shunt patients could be salvaged if they developed liver failure postoperatively (Rikkers, 1998).
Another role for portosystemic surgical shunts is in children with variceal hemorrhage after endoscopic therapy. Excellent results have been achieved in children with distal splenorenal shunts and mesocaval shunts to prevent bleeding (Botha et al, 2004).
Abouljoud MS, et al. A comparison of treatment with transjugular intrahepatic portosystemic shunt or distal splenorenal shunt in the management of variceal bleeding prior to liver transplantation. Transplantation. 1995;59:226-229.
Angermayr B, et al. Survival in patients undergoing transjugular intrahepatic portosystemic shunt: ePTFE-covered stentgrafts versus bare stents, for Vienna TIPS study group. Hepatology. 2003;38:1043-1050.
Atta HM, et al. Selective splenocaval shunt. Arch Surg. 1992;126:582-585.
Baker LA, et al. The natural history of esophageal varices: a study of 115 cirrhotic patients in whom varices were diagnosed prior to bleeding. Am J Med. 1959;26:228-237.
Bambini DA, et al. Experience with the Rex shunt (mesenterico-left portal bypass) in children with extrahepatic portal hypertension. J Pediatr Surg. 2000;35:13-18.
Barton RE, et al. TIPS: short- and long-term results: a survey of 1750 patients. Semin Interv Radiol. 1995;12:364-367.
Besson I, et al. Sclerotherapy with or without octreotide for acute variceal bleeding. N Engl J Med. 1995;333:555-560.
Block KP, Reichelderfer M. Endoscopic therapy of variceal hemorrhage. In: Knechtle, SJ, editor. Portal Hypertension: A Multidisciplinary Approach to Current Clinical Management. Armonk, NY: Futura; 1998:27-55.
Bosch J, et al. Effects of somatostatin on hepatic and systemic hemodynamics in patients with cirrhosis of the liver: comparison with vasopressin. Gastroenterology. 1981;80:518-525.
Botha JF, et al. Portosystemic shunts in children: a 15-year experience. J Am Coll Surg. 2004;199:179-185.
Boyer TD, et al. Cost of preventing variceal rebleeding with transjugular intrahepatic portal systemic shunt and distal splenorenal shunt. J Hepatol. 2008;48:407-414.
Bureau C, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular portosystemic shunts: long-term results of a randomized multicentre study. Liver Int. 2007;27:742-747.
Burroughs AK. Double blind RCT of 5 day octreotide versus placebo associated with sclerotherapy for trial failures. International Octreotide Varices Study Group. Hepatology. 1996;24:352A.
Burroughs AK, et al. Cirrhotics with variceal hemorrhage: the importance of the time interval between admission and the start of analysis for survival and rebleeding rates. Hepatology. 1989;9:801-807.
Burroughs AK, et al. Randomized, double-blind, placebo-controlled trial of somatostatin for variceal bleeding: emergency control and prevention of early variceal rebleeding. Gastroenterology. 1990;99:1388-1395.
Cales P, et al. Incidence of large oesophageal varices in patients with cirrhosis: application to prophylaxis of first bleeding. Gut. 1990;31:1298-1302.
Cello JP, et al. Endoscopic sclerotherapy versus portacaval shunt in patients with severe cirrhosis and acute variceal hemorrhage: long-term follow-up. N Engl J Med. 1987;316:11-15.
Chiu B, Superina RA. Encephalopathy caused by a splenorenal shunt can be reversed by performing a mesenteric-to-left portal vein bypass. J Pediatr Surg. 2006;41:1177-1179.
Chojkier M, et al. A controlled comparison of continuous intraarterial and intravenous infusions of vasopressin in hemorrhage from esophageal varices. Gastroenterology. 1979;77:540-546.
Coldwell DM, et al. Multicenter investigation of the role of transjugular intrahepatic portosystemic shunt in management of portal hypertension. Radiology. 1995;196:335-340.
Copenhagen Esophageal Varices Sclerotherapy Project. Sclerotherapy after first variceal hemorrhage in cirrhosis: a randomized multicenter trial. N Engl J Med. 1984;311:1594-1600.
Crenshaw WB, et al. Severe ascites: efficacy of the transjugular intrahepatic portosystemic shunt in treatment. Radiology. 1996;200:185-192.
Dagenais M, et al. Experience with radical esophagogastric devascularization procedures (Sugiura) for variceal bleeding outside of Japan. World J Surg. 1994;18:222-228.
Dagradi AE. The natural history of esophageal varices in patients with alcoholic liver cirrhosis: an endoscopic and clinical study. Am J Gastroenterol. 1972;57:520-540.
D’Amico G, et al. The treatment of portal hypertension: a meta-analytic review. Hepatology. 1995;22:332-354.
Darcy M, et al. Efficacy and complications of transjugular intrahepatic portosystemic shunts. Radiology. 1993;189(Suppl):227.
da Silva LC, et al. A randomized trial for the study of the elective surgical treatment of portal hypertension in mansonic schistosomiasis. Ann Surg. 1986;204:148-153.
de Franchis R, et al. Prophylactic sclerotherapy in high-risk cirrhotics selected by endoscopic criteria: a multicenter randomized controlled trial. Gastroenterology. 1991;101:1087-1093.
Encarnacíon CE, et al. Transjugular intrahepatic portosystemic shunt placement for variceal bleeding: predictors of mortality. J Vasc Interv Radiol. 1995;6:687-694.
Ewaga H, et al. Liver transplantation for uncontrollable variceal bleeding. Am J Gastroenterol. 1994;89:1823-1826.
Fardy JM, Laupacis A. A meta-analysis of prophylactic endoscopic sclerotherapy for esophageal varices. Am J Gastroenterol. 1994;89:1938-1948.
Feu F, et al. Reduction of variceal pressure by propranolol: comparison of the effects on portal pressure and azygos blood flow in patients with cirrhosis. Hepatology. 1993;18:1082-1089.
Fillmore DJ, et al. Transjugular intrahepatic portosystemic shunt: midterm clinical and angiographic follow-up. J Vasc Interv Radiol. 1996;7:255-261.
Fogel MR, et al. Continuous intravenous vasopressin in active upper gastrointestinal bleeding: a placebo controlled trial. Ann Intern Med. 1982;96:565-569.
Galloway JR, Henderson JM. Management of variceal bleeding in patients with extrahepatic portal vein thrombosis. Am J Surg. 1990;160:122-126.
Garcia-Pagan JC, et al. Enhancement of portal pressure reduction by the association of isosorbide mononitrate to propranolol administration in patients with cirrhosis. Hepatology. 1990;11:230-238.
Garcia-Pagan JC, et al. TIPS for Budd–Chiari syndrome: long-term results and prognostic factors in 124 patients. Gastroenterology. 2008;135:808-815.
Gebhard RL. Natural history of esophageal varices. In: Knechtle, SJ, editor. Portal Hypertension: A Multidisciplinary Approach to Current Clinical Management. Armonk, NY: Futura; 1998:1-8.
Gimson AE, et al. A randomized trial of vasopressin plus nitroglycerin in the control of acute variceal hemorrhage. Hepatology. 1986;6:410-413.
Gimson AE, et al. Randomised trial of variceal banding ligation versus injection sclerotherapy for bleeding oesophageal varices. Lancet. 1993;342:391-394.
Goulis J, Patch D, Burroughs AK. Bacterial infection in the pathogenesis of variceal bleeding. Lancet. 1999;353:139-142.
Grace ND. Prevention of initial variceal hemorrhage. Gastroenterol Clin North Am. 1992;21:149-161.
Graham D, Smith JL. The course of patients after variceal hemorrhage. Gastroenterology. 1981;80:800-809.
Gregory PB. Sclerotherapy for actively bleeding esophageal varices in male alcoholics with cirrhosis: results of a randomized, multicenter clinical trial. VA Cooperative Variceal Sclerotherapy Group. Gastroenterology. 1990;98:A53.
Groszmann RJ, et al. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology. 1990;99:1401-1407.
Hashizume M, et al. Endoscopic ligation of esophageal varices compared with injection sclerotherapy: a prospective randomized trial. Gastrointest Endosc. 1993;39:123-126.
Haskal ZJ, et al. Transjugular intrahepatic portosystemic shunt stenosis and revision: early and midterm results. AJR Am J Roentgenol. 1994;163:439-444.
Helton WS, et al. Critical appraisal of the angiographic portacaval shunt (TIPS). Am J Surg. 1993;165:566-571.
Henderson JM, et al. Hemodynamic differences between alcoholic and nonalcoholic cirrhotics following distal splenorenal shunt-effect on survival? Ann Surg. 1983;198:325-334.
Henderson JM, et al. Endoscopic variceal sclerosis compared with distal splenorenal shunt to prevent recurrent variceal bleeding in cirrhosis: a prospective, randomized trial. Ann Intern Med. 1990;112:262-269.
Henderson JM, et al. Portal hypertension. Curr Probl Surg. 1998;35:384-452.
Henderson JM, et al. DSRS or TIPS for refractory variceal bleeding: a prospective randomized controlled trial. Hepatology. 2004;40(Suppl 1):725A.
Henderson JM, et al. Distal spenorenal shunt versus transjugular intrahepatic portal systemic shunt for variceal bleeding: a randomized trial. Gastroenterology. 2006;130:1643-1651.
Hou MC, et al. Comparison of endoscopic variceal injection sclerotherapy and ligation for the treatment of esophageal variceal hemorrhage: a prospective randomized trial. Hepatology. 1995;21:1517-1522.
Howard ER, Stringer MD, Mowat AP. Assessment of injection sclerotherapy in the management of 152 children with esophageal varices. Br J Surg. 1988;75:404-408.
Huet PM, et al. Hemodynamic effects of vasopressin in cirrhotic patients. In: Lebrec, D, Blei, AT. Vasopressin Analogues and Portal Hypertension. Paris: John Libbey Eurotext; 1987:83-94.
Imperiale TF, et al. A meta-analysis of somatostatin versus vasopressin in the management of acute esophageal variceal hemorrhage. Gastroenterology. 1995;109:1289-1294.
Infante-Rivard C, et al. Role of endoscopic variceal sclerotherapy in the long-term management of variceal bleeding: a meta-analysis. Gastroenterology. 1989;96:1087-1092.
Inokuchi K. Prophylactic portal nondecompression surgery in patients with esophageal varices: an interim report. Cooperative Study of Portal Hypertension of Japan. Ann Surg. 1984;200:61-65.
Jin GL, Rikkers LF. Selective variceal decompression: current status. HPB Surg. 1991;5:1-15.
Jin G, Rikkers LF. Transabdominal esophagogastric devascularization as treatment for variceal hemorrhage. Surgery. 1996;120:641-649.
Khaitiyar JS, et al. Transjugular intrahepatic portosystemic shunt versus distal splenorenal shunt: a comparative study. Hepatogastroenterology. 2000;47:492-497.
Kleber G, et al. Prediction of variceal hemorrhage in cirrhosis: a prospective follow-up study. Gastroenterology. 1991;100:1332-1337.
Knechtle SJ. Surgical shunts for portal hypertension. In: Knechtle, SJ, editor. Portal Hypertension: A Multidisciplinary Approach to Current Clinical Management. Armonk, NY: Futura; 1998:175-202.
Knechtle SJ, et al. Portal hypertension: surgical management in the 1990s. Surgery. 1994;116:687-695.
Knechtle SJ, et al. Surgical portosystemic shunts for treatment of portal hypertensive bleeding: outcome and effect on liver function. Surgery. 1999;126:708-711.
Konstantinov IE. Eck-Paulov shunt: the 120th anniversary of the first vascular anastomosis. Surgery. 1997;121:640-645.
Korula J. Medical management of portal hypertension. In: Knechtle, SJ, editor. Portal Hypertension: A Multidisciplinary Approach to Current Clinical Management. Armonk, NY: Futura; 1998:9-26.
LaBerge JM, et al. Transjugular intrahepatic portosystemic shunts: preliminary results in 25 patients. J Vasc Surg. 1992;16:258-267.
LaBerge JM, et al. Two-year outcome following transjugular intrahepatic portosystemic shunt for variceal bleeding: results in 90 patients. Gastroenterology. 1995;108:1143-1151.
Laine L. Management of actively bleeding esophageal varices. Gastrointest Endosc. 1997;46:83.
Laine L, et al. Endoscopic ligation compared with sclerotherapy for the treatment of bleeding esophageal varices. Ann Intern Med. 1993;119:1-7.
Lebrec D, et al. Propranolol: a medical treatment for portal hypertension? Lancet. 1980;2:180-182.
Lebrec D, et al. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis: a controlled study. N Engl J Med. 1981;305:1371-1374.
Lebrec D, et al. The effect of propranolol on portal hypertension in patients with cirrhosis: a hemodynamic study. Hepatology. 1982;2:523-527.
Le Moine O, et al. Factors related to early mortality in cirrhotic patients bleeding from varices and treated by urgent sclerotherapy. Gut. 1992;33:1381-1385.
Lo GH, et al. A prospective, randomized trial of sclerotherapy versus ligation in the management of bleeding esophageal varices. Hepatology. 1995;22:466-471.
Lo GH, et al. Emergency banding ligation versus sclerotherapy for the control of active bleeding from esophageal varices. Hepatology. 1997;25:1101-1104.
MacDougall BR, et al. Increased long-term survival in variceal haemorrhage using injection sclerotherapy: results of a controlled trial. Lancet. 1982;1:124-127.
Malisch T, et al. Life-table analysis of middle-term patency of transjugular intrahepatic portosystemic stent. Radiology. 1993;189(Suppl):227.
Martin L, et al. Is transjugular intrahepatic portosystemic shunt the treatment of choice for class C cirrhotic patients with uncontrolled hemorrhage? Radiology. 1993;189(Suppl):253.
Martin M, et al. Transjugular intrahepatic portosystemic shunt in the management of variceal bleeding: indications and clinical results. Surgery. 1993;114:719-727.
McCormick PA, et al. Emergency transjugular intrahepatic portasystemic stent shunting as salvage treatment for uncontrolled variceal bleeding. Br J Surg. 1994;81:1324-1327.
Menegaux F, et al. Comparison of transjugular and surgical portosystemic shunts on the outcome of liver transplantation. Arch Surg. 1994;129:1018-1024.
Merkel C, et al. Prognostic indicators of survival in patients with cirrhosis and esophageal varices, without previous bleeding. Am J Gastroenterol. 1989;84:717-722.
Millis M, et al. TIPS: impact on liver transplantation. Transplant Proc. 1995;27:1252-1253.
Monescillo A, et al. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004;40:793-801.
Moreau R, et al. Low dose of nitroglycerin failed to improve splanchnic hemodynamics in patients with cirrhosis: evidence for an impaired cardiopulmonary baroreflex function. Hepatology. 1989;10:93-97.
Odorico JS. Impact of transjugular intrahepatic portosystemic shunt on liver transplantation. In: Knechtle, SJ, editor. Portal Hypertension: A Multidisciplinary Approach to Current Clinical Management. Armonk, NY: Futura; 1998:253-263.
Orloff MJ, et al. Prospective randomized trial of emergency portacaval shunt and emergency medical therapy in unselected cirrhotic patients with bleeding varices. Hepatology. 1994;20:863-872.
Orozco H, et al. Elective treatment of bleeding varices with the Sugiura operation over ten years. Am J Surg. 1992;163:585-589.
Pagliaro L, et al. Therapeutic controversies and randomized controlled trials (RCTs): prevention of bleeding and rebleeding in cirrhosis. Gastroenterol Int. 1989;2:71-84.
Papatheodoridis GV, et al. Transjugular intrahepatic portosystemic shunt compared with endoscopic treatment for prevention of variceal rebleeding: a meta-analysis. Hepatology. 1999;30:612-622.
Planas R, et al. A prospective randomized trial comparing somatostatin and sclerotherapy in the treatment of acute variceal bleeding. Hepatology. 1994;20:370-375.
Poynard T, et al. Beta-adrenergic–antagonist drugs in the prevention of gastrointestinal bleeding in patients with cirrhosis and esophageal varices: an analysis of data and prognostic factors in 589 patients from four randomized clinical trials. Franco-Italian Multicenter Study Group. N Engl J Med. 1991;324:1532-1538.
PROVA Study Group. Prophylaxis of first hemorrhage from esophageal varices by sclerotherapy, propranolol or both in cirrhotic patients: a randomized multicenter trial. Hepatology. 1991;14:1016-1024.
Raia S, et al. Portal hypertension in mansonic schistosomiasis. World J Surg. 1991;15:176-187.
Resnick RH, et al. A controlled study of the therapeutic portacaval shunt. Gastroenterology. 1974;67:843-857.
Richter G, et al. Six-year results of transjugular intrahepatic portosystemic shunt stent placement: essentials for success. Radiology. 1994;193(Suppl):130.
Riggio O, et al. Pharmacological prophylaxis of hepatic encephalopathy after transjugular intrhepatic protosystemic shunt: a randomized controlled study. J Hepatol. 2005;42:674-679.
Rikkers LF. The changing spectrum of treatment for variceal bleeding. Ann Surg. 1998;228:536-546.
Rikkers LF, Jin G. Surgical management of acute variceal hemorrhage. World J Surg. 1994;18:193-199.
Rikkers LF, Jin G. Emergency shunt: role in the present management of variceal bleeding. Arch Surg. 1995;130:472-477.
Rikkers LF, et al. Which portosystemic shunt is best? Gastroenterol Clin North Am. 1992;21:179-196.
Rikkers LF, et al. Shunt surgery versus endoscopic sclerotherapy for variceal hemorrhage: late results of a randomized trial. Am J Surg. 1993;165:27-33.
Rikkers LF, et al. Shunt surgery during the era of liver transplantation. Ann Surg. 1997;226:51-57.
Ring EJ, et al. Using transjugular intrahepatic portosystemic shunts to control variceal bleeding before liver transplantation. Ann Intern Med. 1992;116:304-309.
Rosemurgy AS, et al. Transjugular intrahepatic portosystemic shunt vs. small-diameter prosthetic H-graft portacaval shunt: extended follow-up of an expanded randomized prospective trial. J Gastrointest Surg. 2000;4:589-597.
Rosemurgy A, et al. Survival and variceal rehemorrhage after shunting support small-diameter prosthetic H-graft portacaval shunt. J Gastrointest Surg. 2007;11(3):325-332.
Rössle M, et al. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994;330:165-171.
Salerno F, et al. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology. 2004;40:629-635.
Sarfeh IJ, Rypins EB. Partial versus total portacaval shunt in alcoholic cirrhosis: results of a prospective, randomized clinical trial. Ann Surg. 1994;219:353-361.
Sarfeh IJ, et al. A systematic appraisal of portacaval H-graft diameters: clinical and hemodynamic perspectives. Ann Surg. 1986;204:356-363.
Sauerbruch T, et al. Prophylactic sclerotherapy before the first episode of variceal hemorrhage in patients with cirrhosis. N Engl J Med. 1988;319:8-15.
Saxon R, et al. Transjugular intrahepatic portosystemic shunt: middle-term shunt patency. Radiology. 1993;189(Suppl):227.
Shaked A, Busuttil RW. Liver transplantation in patients with portal vein thrombosis and central portacaval shunts. Ann Surg. 1991;214:696-702.
Siringo S, et al. Timing of the first variceal hemorrhage in cirrhotic patients: prospective evaluation of Doppler flowmetry, endoscopy and clinical parameters. Hepatology. 1994;20:66-73.
Smith JL, Graham DY. Variceal hemorrhage: a critical evaluation of survival analysis. Gastroenterology. 1982;82:968-973.
Sterling K, Darcy M. Transjugular intrahepatic portosystemic shunts stenosis: incidence and management. AJR Am J Roentgenol. 1995;164(Suppl):96A.
Stiegmann GV, et al. Endoscopic sclerotherapy as compared with endoscopic ligation for bleeding esophageal varices. N Engl J Med. 1992;326:1527-1532.
Stipa S, et al. A randomized controlled trial of mesentericocaval shunt with autologous jugular vein. Surg Gynecol Obstet. 1981;153:353-356.
Suc B, et al. Intrahepatic portocaval shunt in patients waiting for transplantation. Transplant Proc. 1995;27:1715-1716.
Sugiura M, Futagawa S. A new technique for treating esophageal varices. J Thorac Cardiovasc Surg. 1973;66:677-685.
Superina R, et al. Surgical guidelines for the management of extra-hepatic portal vein obstruction. Pediatr Transplant. 2006;10:908-913.
Thulin L, et al. Treatment of bleeding oesophageal varices with somatostatin. Acta Chir Scand. 1979;145:395-398.
Triger DR, et al. Prophylactic sclerotherapy for esophageal varices: long-term results of a single-center trial. Hepatology. 1991;13:117-123.
Tyden G, et al. Treatment of bleeding esophageal varices with somatostatin. N Engl J Med. 1978;299:1466-1467.
United States Department of Health and Human Services (USDHHS), United Network for Organ Sharing [UNOS], University Renal Research and Education Association. Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1993-2002. Richmond, VA, USDHHS. 2003.
Van Ruiswyk J, Byrd JC. Efficacy of prophylactic sclerotherapy for prevention of a first variceal hemorrhage. Gastroenterology. 1992;102:587-597.
Veterans Affairs Cooperative Variceal Sclerotherapy Group. Prophylactic sclerotherapy for esophageal varices in men with alcoholic liver disease: a randomized, single-blind, multicenter clinical trial. N Engl J Med. 1991;324:1779-1784.
Veterans Affairs Cooperative Variceal Sclerotherapy Group. Sclerotherapy for male alcoholic cirrhotic patients who have bled from esophageal varices: results of a randomized, multicenter clinical trial. Hepatology. 1994;20:618-625.
Villanueva C, et al. Nadolol plus isosorbide mononitrate compared with sclerotherapy for the prevention of variceal bleeding. N Engl J Med. 1996;334:1624-1629.
Vorobioff J, et al. Propranolol compared with propranolol plus isosorbide dinitrate in portal-hypertensive patients: long-term hemodynamic and renal effects. Hepatology. 1993;18:477-484.
Warren WD, et al. Selective trans-splenic decompression of gastroesophageal varices by distal splenorenal shunt. Ann Surg. 1967;166:437-455.
Warren WD, et al. Management of variceal bleeding in patients with non-cirrhotic portal vein thrombosis. Ann Surg. 1988;207:623-634.
Westaby D, et al. Controlled clinical trial of injection sclerotherapy for active variceal bleeding. Hepatology. 1989;9:274-277.