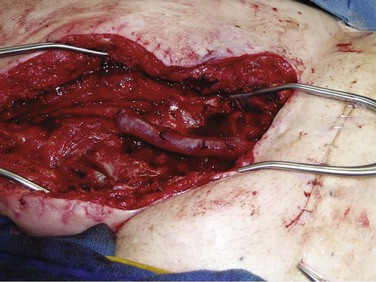
Chapter 42
Local Complications
Graft Infection
Martin R. Back
The use of prosthetic biomaterials (polymer grafts, metallic stents, endovascular stent-grafts) implanted endoluminally or during open reconstruction within the arterial or venous circulation has permitted palliation of a large number of disabling or potentially fatal vascular conditions. The overall incidence of infection involving vascular prostheses is relatively low because of routine antibiotic prophylaxis before surgical procedures, refinements in sterilization and packaging of devices, and careful adherence to aseptic procedural and surgical technique.
When infection does occur, detection and definitive therapy of the vascular prosthesis are often delayed, with potentially catastrophic consequences. Infection involving a vascular prosthesis is difficult to eradicate. If it is not recognized or treated promptly, implant failure will occur as a result of sepsis, hemorrhage, or thrombosis with end-organ ischemia.1–6
The clinical manifestations of prosthetic vascular infection vary depending on the anatomic location and the virulence of the pathogen.1,3,5,7–9 The resulting clinical spectrum of graft infection can allow surgeons to use a patient-specific treatment approach. However, widely varying treatment approaches, usually encompassing small patient cohorts, have resulted in reports of variable outcomes in the literature, making selection of optimal therapy difficult.
In general, surgical therapy is always required, often coupled with excision of the prosthesis, because antibiotics alone are insufficient to eradicate an established infectious process. Adherence to specific criteria in selecting an appropriate treatment care plan is imperative and is influenced by the clinical findings, anatomic location, time since initial implantation, type of graft/device material, extent of infection, virulence of infecting organism, and underlying patient comorbid conditions.
Keys to a successful outcome include use of accurate diagnostics to identify the infecting organism and extent of graft infection, administration of culture-specific antibiotic therapy, and well-planned surgical intervention(s) to excise or replace the infected graft and sterilize the local perigraft tissues. Appropriate management may involve graft excision alone, graft preservation within the implant wound, in situ graft replacement, or graft excision in conjunction with extra-anatomic bypass grafting.
Improved results have been reported in the past 20 years after both graft excision coupled with extra-anatomic bypass and in situ replacement procedures.10–18 Because most patients with late manifestations have a low-virulence graft infection, in situ replacement therapy with autogenous venous conduits, cryopreserved allografts, or antibiotic-impregnated prostheses to replace the infected grafts has evolved to be a preferred treatment strategy.*
Regardless of the technique used to eradicate the graft infection, success is measured by patient survival, freedom from recurrent infection, patency of the revascularization, and avoidance of major morbidity and amputation. Even when treatment is successful, the morbidity associated with vascular graft infections is considerable, with outcomes often worse than the natural history of the vascular condition that led to graft implantation.
Incidence
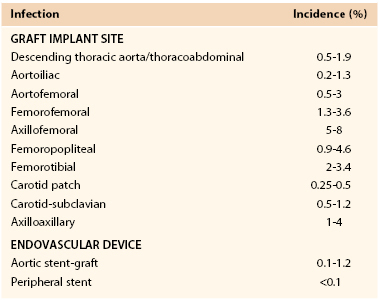
The reported incidence of infection involving a vascular prosthesis varies; infection occurs after 0.2% to 5% of operations and is influenced by the implant site, indication for the intervention, underlying disease, and host defense mechanisms (Table 42-1).† Vascular surgeons now realize that the potential for graft infection extends well beyond the perioperative period. Aortic graft infections can develop months to years after implantation, and thus the long-term incidence is higher. A population-based study from the Mayo Clinic estimated that during a 10-year period after prosthetic grafting of the aorta, the incidence of infection was 5%.34 Graft infection occurs much less frequently than wound infection, with the incidence of early (<30-day) graft infection being in the range of 1% of procedures. Infection is more likely to involve prosthetic grafts implanted during an emergency procedure (e.g., ruptured abdominal aortic aneurysm [AAA], acute arterial ischemia) and to occur when the prosthesis is anastomosed to the femoral artery or placed in a subcutaneous tunnel (e.g., axillofemoral or cross-femoral bypass). In a Canadian prospective multicenter trial of nonruptured AAA open repair, the incidence of graft infection was 0.2%,23 similar to that reported after endovascular stent-graft AAA repair.31,35–37 Infection can also develop after endoluminal stent deployment, but the incidence appears to be extremely low (<0.1%).
Classification
Prosthetic graft infections can be classified according to time of appearance after implantation, relationship to postoperative wound infection (Szilagyi’s classification), and the extent of graft involvement (Bunt’s classification) (Box 42-1).
Early (<4 months after graft implantation) infections correlate with Szilagyi grade III wound infections that involve vascular prostheses.33 These infections are caused by virulent hospital-acquired bacteria and are associated with sepsis, as evidenced by fever, leukocytosis, bacteremia, and advanced wound infection. There is evidence that even Szilagyi grade I and II wound infections increase the likelihood of a late-appearing graft infection. Late infections are the result of graft colonization by low-virulence organisms such as Staphylococcus epidermidis or, infrequently, Candida species.* The low titer of microorganisms on graft surfaces produces an indolent infection without signs of sepsis, and cultures of perigraft fluid or tissue may yield no growth.
Bunt2 proposed using standardized terminology to reflect the spectrum of graft infections and allow comparison of treatment outcomes. Categories of this terminology include perigraft infection (P0, P1, P2, and P3), graft-enteric erosion (GEE), graft-enteric fistula (GEF) (see Chapter 43), and aortic stump infection.
Most early graft infections occur after patients have been discharged from the hospital, typically within 1 to 3 months, and involve extracavitary grafts. Cavitary (i.e., aortic) graft infections are manifested as late (>4 months) infections with a mean time to appearance of more than 40 months.6,10,39,40 Both early and late infections can be accompanied by either total (P0, P1) or partial (P2, P3) graft involvement.
Pathogenesis
Etiology
The presence of a foreign body potentiates the infectivity of bacteria. In 1957, Elek and Conen41 demonstrated that a single braided silk suture significantly reduces the inoculum of staphylococci required to produce a local infection. The risk of foreign body infection is enhanced in the presence of a larger inoculum, more virulent bacterial strains, depressed host immune function, and invasion of sites more remote from host defenses.
Cellular and Biomolecular Events
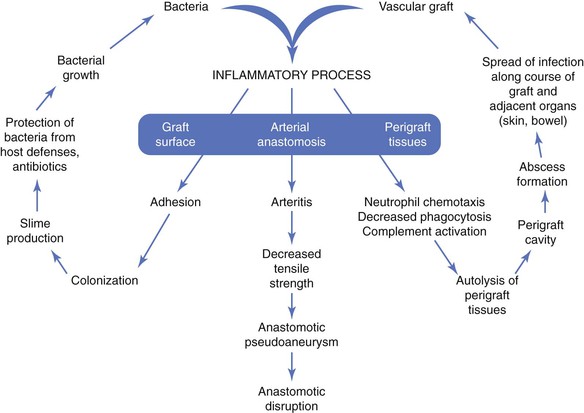
The pathogenesis of biomaterial-associated infection involves the following fundamental steps: (1) adhesion of bacteria to graft or stent surfaces, (2) formation of microcolonies within a bacterial biofilm, (3) activation of host defenses (neutrophil chemotaxis, complement activation), and (4) an inflammatory response involving perigraft tissues and the graft-artery anastomoses (Fig. 42-1).
The initiating event is adherence of bacteria to the biomaterial surface, followed by colonization and development of a bacteria-laden biofilm that resists host defenses and penetration of antibiotics. Both graft and bacterial characteristics influence the likelihood of colonization. Bacterial adherence to polyester grafts is 10 to 100 times greater than adherence to polytetrafluoroethylene (PTFE) grafts. Gram-positive bacteria, such as staphylococci, produce an extracellular glycocalyx, or mucin, that promotes adherence to biomaterials in greater numbers than are seen with gram-negative bacteria. The increased adhesion of staphylococci to biomaterials is due to specific capsular adhesions that mediate attachment and colonization of microorganisms.
The vascular prosthesis and adherent bacteria act together as a coinflammatory stimulus to activate the immune system, in particular, inflammatory cytokines. The local inflammatory response after implantation serves to establish connective tissue ingrowth (“incorporation”) to the outer surface of the graft material. This healing process can be impaired by early perigraft seroma, lymphocele, or hematoma formation, which increases the risk for bacterial adherence and colonization. The inflammatory response to an implanted prosthetic graft also creates an unfavorable environment characterized by local ischemia and an acidic pH that is potentially conducive to bacterial colonization. Local disruption of the fine balance between proinflammatory and antiinflammatory mediators may lead to excess production of matrix metalloproteinases (MMPs) by tumor necrosis factor–stimulated macrophages.42 Excessive degradation of secreted extracellular matrix and angiogenic growth factors by matrix metalloproteinases may hinder optimal graft healing by restricting capillary ingrowth, tissue incorporation, and potential luminal endothelialization. Lack of perigraft ingrowth and vascularity also favors greater exposure of the implanted biomaterial to bacteria and sequestration within graft pores/interstices away from activated phagocytic cells.
Neutrophil function can also be directly impaired in the presence of biomaterials. Decreased neutrophil opsonic, phagocytic, and bactericidal activity against Staphylococcus aureus has been observed in PTFE tissue cages implanted subcutaneously in guinea pigs.43 As local tissue autolysis occurs, the infectious process can spread along the length of the graft and into adjacent structures (e.g., adjacent artery, skin, bowel). The pathobiology is manifested clinically as a spectrum of signs, including graft sepsis, localized perigraft abscess, anastomotic pseudoaneurysm, graft-cutaneous sinus tract, or GEE/GEF (e.g., secondary aortoduodenal fistula). Frequently, the only features of a low-grade, late graft infection are the absence of graft incorporation into surrounding tissue and perigraft fluid containing large numbers of white blood cells (WBCs).
Clinical Sources of Infection
Exposure of a vascular prosthesis to microorganisms (bacteria or fungi) can result in clinical infection by any of four mechanisms: perioperative contamination via the surgical wound, seeding of the biomaterial by bacteremia, mechanical erosion into the bowel or genitourinary tract or through the skin, and involvement in a contiguous infectious process. Underlying impairment of host defenses can further increase the risk for infection.
Perioperative Contamination
Skin and lymph nodes are a major reservoir of bacteria. Biomaterial surfaces can contact microorganisms (1) by a direct route during implantation, (2) through the surgical wound (in the event of a healing complication), or (3) by hematogenous or lymphatic sources arising from remote sites of infection (e.g., urinary tract infection, pedal fungal infection, pneumonia, venous or arterial catheter sepsis, endocarditis, and ischemic foot lesions). Important potential sources of direct graft contamination include breaks in aseptic operative technique, such as contact with the patient’s endogenous flora harbored within sweat glands, lymph nodes, diseased arterial walls (e.g., atherosclerotic plaque or aneurysm thrombus), disrupted lymphatics, and intestinal bag effluents, and injury to or opening of the gastrointestinal or genitourinary tract.
If the surgical wound does not develop a fibrin seal or heal promptly after surgery, the underlying vascular prosthesis is susceptible to colonization from any superficial wound complications (e.g., cellulitis, dermal necrosis, lymphocele). Wounds with persistent drainage indicate the presence of ischemia or tissue injury that, if complicated by superficial infection, can extend to deeper tissue and involve the prosthesis. Diseased arterial walls and reoperative wounds are an unappreciated source of bacteria, with microbiologic culture recovering pathogenic strains of staphylococci in 10% to 20% of cases.1 Bacteria can be harbored in the scar tissue or lymphoceles of healed wounds and can contact prosthetic grafts undergoing revision or replacement for thrombosis or anastomotic aneurysm. Culture of explanted graft material from such procedures has isolated microorganisms, typically S. epidermidis, from 50% to 70% of thrombosed grafts and from more than 80% of grafts associated with anastomotic aneurysms.38
Bacteremia
Bacterial seeding of the prosthesis via a hematogenous route is an uncommon but important mechanism of graft and stent infection. Experimentally, intravenous infusion of 107 colony-forming units of S. aureus produces a clinical graft infection in nearly 100% of animals if administered within days of implantation.44 Thus, bacteremia arising from infected intravascular catheters, urinary tract infection, or other remote tissue infections (e.g., pneumonia, infected foot ulcer) increases the risk of graft infection and occurs not infrequently during the postoperative period in elderly patients undergoing vascular surgery.
Parenteral antibiotic therapy has been shown experimentally to significantly decrease the risk of graft colonization from bacteremia and is the rationale for both antibiotic prophylaxis and culture-specific antibiotic therapy in the patient with a known site of infection. As the prosthesis heals and becomes incorporated into surrounding tissue, susceptibility to bacteremic colonization decreases, but vulnerability has been documented more than 1 year after implantation, with infection developing as the result of dental and gastrointestinal diagnostic procedures. Transient bacteremia, in conjunction with altered immune status, may account for graft infections occurring years after the original operation. It is also possible for a low-grade graft infection to become secondarily infected by a more virulent organism. For example, Escherichia coli urosepsis might inoculate an unincorporated graft that already has a biofilm infection with S. epidermidis, thereby converting a low-grade infection to a more virulent graft infection.
Mechanical Erosion
Erosion of a prosthetic graft through the skin or into the gastrointestinal or genitourinary tract results in a perigraft infection that can spread along the length of the graft. GEE/GEF can develop as a result of pulsatile movement of an aortic graft against adjacent bowel, most commonly without adequate intervening retroperitoneal soft tissue. Enteric erosion may involve the graft body or anastomotic sites with intact suture lines or pseudoaneurysm formation. A low-grade underlying graft infection has been found in a fraction of cases (confirmed by operative findings and recovery of staphylococcal species) and may provide an additional inflammatory stimulus for bowel adhesion.45 The reported incidence of GEE/GEF after prosthetic aortic grafting is 0.4% to 2%. Graft erosion through intact skin is most commonly the result of a chronic, low-grade infection caused by S. epidermidis (see Chapter 43).
Involvement by a Contiguous Infectious Process
Prosthetic grafts can become colonized as a result of an adjacent infection. The most common clinical scenarios are an aortofemoral graft limb infection associated with diverticulitis and a peripheral graft infection secondary to an infected lymphocele. Frequently, the graft segment adjacent to the contiguous bowel or soft tissue infection may be involved. Initial treatment should be directed at drainage of the perigraft abscess and correction of the bowel abnormality if present.
Risk Factors
Multiple perioperative and patient-related factors can increase the risk for development of a vascular graft infection (Box 42-2). In addition to the contributing perioperative factors just discussed, the risk of infection is increased with emergency, extended-length, and reoperative46 reconstructions. A prolonged preoperative hospital stay allows conversion of normal skin flora to hospital-acquired strains that may be resistant to routinely administered antibiotics. Early graft infections are usually the result of wound complications, unplanned reoperation for hematoma or lymphocele, concomitant remote infection, and impaired immunocompetence. Patients with late-appearing graft infections often have a history of multiple operations for thrombosis or anastomotic aneurysm of previously placed prosthetic bypasses.
Impaired Host Defenses
Impaired host defenses from underlying systemic conditions can also predispose patients to prosthetic graft infection.47 The altered immune function associated with malnutrition, malignancy, lymphoproliferative disorders, autoimmune diseases, and drug administration (e.g., corticosteroids, antineoplastic agents, antirejection regimens) may potentiate graft infection with lower numbers of contaminating bacteria.
Although the concept is controversial, diabetic patients appear to have increased susceptibility to infection. Mean plasma glucose levels in diabetic patients before the development of infection and the prevalence of subsequent infections have been tightly correlated in clinical practice. At the cellular level, neutrophil chemotactic and intracellular bactericidal mechanisms are diminished in diabetic patients. Opsonization of S. aureus and E. coli is impaired in such patients. Hyperglycemia in diabetic patients also decreases adherence of neutrophils to foreign bodies.
In addition to the frequent hematogenous introduction of bacteria associated with repeated access to hemodialysis catheters, grafts, and fistulae, patients with chronic renal insufficiency also have higher susceptibility to infection as a result of immune suppression caused by uremia. The depressed neutrophil function in uremia appears to be multifactorial. Neutrophil chemotaxis is inhibited by an unknown agent in uremic serum that specifically blocks the synthesis of chemotactic factor. However, neutrophils from uremic patients placed in normal plasma also exhibit impaired chemotaxis. Adherence, phagocytosis, and bacterial killing by neutrophils and lymphocytes are also diminished in patients with acute or chronic renal failure.
Bacteriology
Although virtually any microorganism can infect a vascular prosthesis, S. aureus is the most prevalent pathogen and accounts for one fourth to one half of infections, depending on the implant site (Table 42-2). Graft infections with S. epidermidis or gram-negative bacteria have increased in frequency. This change in the microbiology of graft infection is the result of reporting of both early- and late-appearing graft infections, including aortic graft infections associated with GEE/GEF. Coagulase-negative staphylococci are present in normal skin flora but have the ability to adhere to and colonize biomaterials, where growth occurs within a biofilm on the surface of prostheses. Surgeons have also become aware of microbiologic sampling errors in late infections because of low numbers of bacteria present within the graft surface biofilm and their slow growth.48 Graft infections associated with negative culture results are caused by S. epidermidis or other coagulase-negative staphylococci and, on occasion, by Candida species. Infection with gram-negative bacteria such as E. coli and Pseudomonas, Klebsiella, Enterobacter, Serratia, and Proteus species can be particularly virulent. The incidence of anastomotic dehiscence and arterial rupture is high because of the ability of the organisms to produce destructive endotoxins (e.g., elastase and alkaline protease) that compromise the structural integrity of the vessel wall. Fungal (e.g., Candida and Aspergillus species) and mycobacterial (tuberculous) infections of grafts are rare, and most patients with such infections either are severely immunosuppressed or have an established fungal or opportunistic infection elsewhere.
Table 42-2
Bacteriology of Prosthetic Vascular Graft Infections from Collected Series
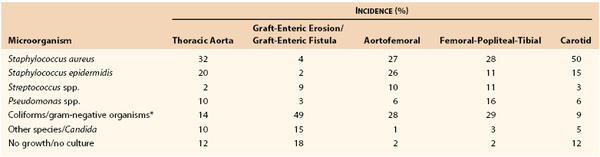
* Escherichia coli; Enterococcus, Bacteroides, Klebsiella, Enterobacter, Serratia, Proteus species.
Plasmid-mediated genetic mutations have afforded S. aureus resistance against penicillin, β-lactams, and other antibiotics (aminoglycosides, erythromycin, tetracycline). Nosocomial and community-acquired infections caused by methicillin-resistant S. aureus (MRSA) have rapidly increased in prevalence over the past decade. Although estimates in the general population have shown MRSA skin colonization (nares and wounds) in less than 2% of individuals, much higher prevalence is found in residents of long-term care facilities (23% to 49%).49–51 A study involving more than 13,000 surgical patients admitted in a University hospital in Switzerland found that the overall incidence of MRSA carriers was 4%, but of those carriers, 64% were newly identified. Previous hospitalization, age greater than 75 years and recent antibiotic treatment were each prognostic for unsuspected MRSA carriage.52 The combination of increased prevalence, harboring in high-risk populations, multiple staphylococcal virulence factors, and rapidly evolving antibiotic resistance mechanisms makes emergence of MRSA a daunting medical challenge. British reports have documented that MRSA is the most common pathogen involved in vascular wound and graft infections and that it is associated with higher morbidity and mortality rates than infection with other microbes.53,54 Our institution has witnessed the prevalence of MRSA arterial graft infections increase fourfold over the past 20 years (from 11% before 2000 to 49% after 2000), with more than half of early extracavitary graft infections being the result of MRSA. Early mortality, limb loss, and infection recurrence rates have not been appreciably higher for MRSA in our experience. However, the future possibility of a higher overall incidence of graft or vascular device infections caused by more prevalent MRSA colonization in the population is more concerning.
Prevention
Prevention of graft infection is imperative, and the surgical team must be cognizant of preoperative, operative, and postoperative prophylactic measures.
Principles
Vascular infections can be minimized if the following principles are applied:
Meticulous attention to sterile technique is imperative to avoid bacterial contact of vascular devices during implant procedures, especially emergency or prolonged reconstructive procedures. Careful handling of tissues, hemostatic technique to prevent hematoma formation, and closure of groin incisions in multiple layers to eliminate dead space are important technical measures that decrease wound complications and the risk of infection. Skin reapproximation without tension minimizes the development of dermal ischemia and wound edge necrosis.
Topical Antibiotics
The addition of topical antibiotics (e.g., bacitracin or cefazolin) to irrigating solutions allows soaking of grafts before implantation and cleansing of wounds before closure and may contribute to decreased wound infection rates. Randomized clinical trials using rifampin-soaked (1 mg/mL) gelatin-impregnated polyester aortofemoral grafts reported significantly reduced groin wound infection rates (4.4% without rifampin versus 2.7% with rifampin), although the rates of subsequent graft infections were similar (0.6% versus 0.3%).55,56 All graft infections were caused by S. aureus in this study. On the basis of the absence of a longer-term benefit, routine rifampin treatment of polyester grafts for primary aortic reconstruction cannot be currently recommended.
Prophylactic Antibiotics
Prophylactic antibiotics should be infused before skin incision and at regular intervals during long procedures to maintain tissue drug levels above the minimal bactericidal concentration for expected pathogens (Box 42-3). Additional dosing may be needed during the operation, depending on the rate of drug elimination and volume of distribution, with larger or more frequent dosing necessary during prolonged (>4-hour) procedures or excessive changes in blood volume, fluid administration, or renal blood flow during the procedure. Prophylaxis should also be instituted before percutaneous puncture of existing prosthetic grafts (e.g., peripheral or coronary arteriography accessed through existing femoral grafts) or before selected endovascular interventions involving stent/stent-graft implantation. Culture-specific antibiotics should be prescribed for patients undergoing vascular graft implantation who have coexisting infections of the leg or another remote site.
At some vascular centers, prophylactic antibiotics are continued for 2 to 3 days in patients deemed to be at high risk for infection from bacteremia, prolonged preprocedure hospitalization, or high (>10%) institutional wound infection rates. Because of the increasing risk of MRSA-related wound complications in these patients, our group advocates prophylactic use of daptomycin (4 mg/kg before and after surgery) as an alternative to vancomycin or cefazolin when prosthetic arterial grafts are implanted.
After implantation of a prosthetic graft, patients should be informed of the potential risk for graft colonization and infection from transient bacteremia, especially after interventional procedures such as dental work, colonoscopy, and cystoscopy. Antibiotic prophylaxis is recommended if these procedures are performed within 3 months of the vascular operation. Amoxicillin 2 g orally 1 hour before the procedure can be used; for the patient with penicillin allergy, clindamycin 600 mg orally 1 hour before the procedure is an alternative.
Diagnosis
Prompt diagnosis and treatment of prosthetic graft infections are essential to avoid major complications (e.g., sepsis and hemorrhage) and death. Clinical manifestations are varied and may be subtle, particularly when associated with cavitary graft infections. Operative exploration is the most accurate method for confirming infection and may be necessary when clinical suspicion of GEE/GEF exists (see Chapter 43). In equivocal cases, the vascular surgeon must prove that a graft infection is not present. The urgency of diagnostic evaluation depends on the clinical findings and the status of the patient.
Clinical Manifestations
Vascular surgeons should maintain a low threshold for proceeding with additional diagnostic testing when any symptom or sign suggests graft infection. In aortic grafts confined to the abdomen, unexplained sepsis, ileus, or abdominal distention might be the only clinical sign. If infection involves an extracavitary graft (i.e., in a limb, the groin, or the neck region), the initial sign of infection is usually overlying inflammation/cellulitis, cutaneous draining sinus tract, or anastomotic pseudoaneurysm. Any patient with gastrointestinal bleeding and an aortic graft should be presumed to have graft infection and GEE/GEF until either another source of bleeding is conclusively identified on endoscopy or no graft-bowel communication is verified at surgery.
In patients with vague suggestive symptoms and ultrasound or computed tomography (CT) evidence of perigraft fluid, careful review of the operative history and surgical notes may furnish clues that further support the diagnosis of graft infection and provide the rationale for invasive diagnostic testing. The patient should also be queried about recent medical illnesses that may have resulted in hematogenous or lymphatic seeding of the graft with bacteria. Early graft infections with S. aureus or other gram-negative bacteria typically manifest within weeks of the procedure as fever, leukocytosis, wound complications, and perigraft purulent drainage. Bacteremia is a sign of an advanced graft infection associated with arterial wall or mural thrombus infection or the secondary development of endocarditis. Patients with grafts infected by S. epidermidis are typically seen months to years after graft implantation with graft-healing complications (e.g., anastomotic aneurysm, perigraft fluid cavity, or graft-cutaneous sinus tract). Systemic signs of sepsis (e.g., fever, leukocytosis, and bacteremia) are frequently absent.
The clinician should carefully examine the site or sites of graft implantation for any signs of inflammation. Surgical incisions should be carefully inspected for erythema and draining sinuses. Masses near anastomotic sites can represent perigraft abscesses or anastomotic pseudoaneurysms. The extremities should be examined for signs of septic embolization (i.e., a cluster of petechiae downstream from the infected graft). Other sources of infection, such as infected foot lesions, osteomyelitis, and infected urinary calculi, should be sought because these conditions can predispose to hematogenous bacterial seeding and graft colonization.
An elevated WBC count with a left-shifted differential count and an increased erythrocyte sedimentation rate are common but nonspecific findings in patients with graft infection and fever. Routine laboratory testing should also include urinalysis, blood culture, and cultures of other clinical sites of infection, such as foot ulcers and surgical wound drainage. Positive blood culture results are uncommon (<5%) but, when present, indicate an advanced graft infection or virulent organisms (or both). All laboratory test results may be normal in patients with late-appearing perigraft infections with S. epidermidis.
Imaging Studies
Vascular imaging is essential for the diagnosis and treatment of graft infection and its sequelae. Anatomic signs of graft infection, such as perigraft abscess, anastomotic aneurysm, and GEE/GEF, can be accurately identified (with >90% sensitivity) thorugh a combination of ultrasonography, CT, magnetic resonance imaging (MRI), arteriography, and endoscopy. Functional radionuclide imaging (gallium Ga 67 citrate, indium In 111–labeled leukocytes, technetium Tc 99m hexametazime–labeled leukocytes) can confirm the presence of a clinically suspected graft infection when anatomic signs of perigraft infection are equivocal.
The combination of anatomic and functional vascular imaging techniques is highly accurate (sensitivity of 80% to 100%, specificity of 50% to 90%) in confirming the presence of infection, planning management, and assessing operative sites for residual or recurrent infection. Arteriography is used to develop an operative strategy for revascularization in the presence of distal ischemia, occlusive disease, or graft thrombosis. CT angiography (CTA) and three-dimensional vessel reconstructions provide improved resolution that may replace planning arteriography.
Imaging studies are performed (1) to identify the extent of infection and perigraft inflammation as well as safe routes of operative exposure and placement of vascular clamps and (2) to minimize the likelihood of injury or organ/limb ischemia secondary to anatomic anomalies or concomitant occlusive disease. Each imaging technique has unique diagnostic applications, and the extent of testing necessary is specific to the individual patient.
Contrast-Enhanced Computed Tomography and Computed Tomographic Angiography
CT is the preferred initial imaging technique for suspected aortofemoral, abdominal, or thoracic aorta and major cavitary branch vessel (P0, P2) graft infections and for extracavitary graft infections involving the neck, torso, and proximal part of the limbs (P1, P3). It should be performed with and without intravenous contrast and oral contrast agents (to detail the relationship of adjacent enteric structures) used only in patients in whom GEE/GEF is suspected. The exact location and extent of polyester and PTFE grafts can best be seen on initial non–contrast-enhanced imaging. Review of delayed arterial-phase images or the “bone” preset window for early arterial-phase imaging best separates the detail of prosthetic graft, arterial, and venous structures from perigraft tissues and aids in image interpretation.
Diagnostic criteria consistent with infection include the loss of normal tissue planes (e.g., fat density) of the retroperitoneal or subcutaneous perigraft structures (indicative of inflammation), collections of fluid or gas around the graft (Fig. 42-2), false aneurysm formation, hydronephrosis, and adjacent vertebral or bony osteomyelitis. Any gas in periprosthetic tissues beyond 2 or 3 months after implantation is an abnormal CT finding suggestive of graft infection. CT angiography provides assessment of continuity of the arterial lumen, associated distribution of occlusive disease, and the presence of thrombus at planned clamp sites and may enable operative planning for arterial reconstruction without invasive arteriography.
Ultrasonography
Ultrasonography is a readily available imaging technique that can be performed urgently as a portable bedside examination in severely ill patients. It should be the initial imaging study for extracavitary (P1, P2, P3) graft infections. Color duplex scanning can reliably differentiate a perigraft fluid collection from an anastomotic pseudoaneurysm, hematoma, and soft tissue masses (e.g., enlarged lymph nodes). Diagnostic accuracy depends on the skill of the examiner and the ability to adequately image the graft. Imaging of the graft within the abdominal cavity can be obscured by intestinal gas and obesity. Ultrasonography has the advantage of being the most accurate vascular imaging technique for verifying vessel or graft patency and assessing pulsatile masses adjacent to grafts in the groin and limbs.
Magnetic Resonance Imaging
MRI provides anatomic imaging equivalent to that of CT but is better able to distinguish between perigraft fluid and fibrosis on the basis of differences in signal intensity between T1- and T2-weighted images. A gadolinium contrast agent enhances vascular luminal resolution (magnetic resonance angiography) to allow planning of arterial reconstruction. Although less nephrotoxic than iodinated contrast agents, gadolinium has unfortunately been linked to a systemic fibrotic process in patients with renal insufficiency (via its potentially decreased/delayed renal clearance).
Functional White Blood Cell Scanning
All radionuclide imaging techniques aim to demonstrate abnormal accumulation of leukocytes in perigraft tissue for the diagnosis of graft infection but do not provide anatomic detail. However, they can be correlated with MRI and CT to delineate the anatomic extent of infection. The type of radionuclide used (gallium 67 citrate, indium 111–labeled leukocytes, technetium Tc 99m hexametazime–labeled leukocytes) can vary and affects diagnostic accuracy. The accuracy (positive predictive value) of indium 111–labeled WBC scans approaches 80% to 90% in detecting graft infection.
Functional WBC scans are not useful, however, during the early postoperative course (3 to 6 months) because of nonspecific radionuclide uptake in the healing, inflamed perigraft tissue (i.e., potential false-positive result). Normal scans (i.e., those showing no labeled WBC accumulation) have been reported in late-appearing aortic graft infection and documented cases of GEE/GEF (i.e., false-negative results). Immunoglobulin G scans are sometimes preferred over WBC scans because of ease of preparation, lack of exposure to the patient’s blood, absence of background red blood cell and platelet imaging, and the longer shelf life of immunoglobulin G.
Endoscopy
With use of upper endoscopy, an important diagnostic modality for suspected GEE/GEF, the lumen of the esophagus, stomach, entire duodenum, and ideally the proximal jejunum can be inspected for sources of bleeding (a pediatric colonoscope should be used) (see Chapter 43).
Arteriography
Contrast-enhanced arteriography can identify infection-associated vessel complications, such as graft rupture and anastomotic aneurysm, but has less overall accuracy than CT and MRI. Arteriography is currently recommended if CT or MR angiography is insufficient to allow reconstructive/revascularization planning. For cavitary graft infections, arteriography may aid in the evaluation of supra-aortic trunk or visceral/renal vessels and the proximal aorta and distal vessels as potential sites for extra-anatomic bypass grafts. Infected extracavitary or distal limb bypasses may require arteriography to define inflow and outflow targets for new bypass in the presence of extensive occlusive disease or graft thrombosis.
Computed Tomography–Guided Aspiration versus Operative Findings
CT-guided aspiration of cavitary perigraft fluid collections is increasingly being used to differentiate uninfected seroma from abscess formation. Sampling error (e.g., limited sampling in septated perigraft fluid collections) is possible with percutaneous aspiration techniques, as is misinterpretation of negative culture results as indicating no graft infection in some cases of S. epidermidis biofilm infection. The definitive diagnostic test for suspected graft infection is operative exploration, especially with equivocal anatomic imaging results and suspected GEE/GEF. Direct exposure of the outer surface of the biomaterial through the typically thick fibrous capsule encompassing a polyester graft allows detection of late indolent biofilm infections. Exploration, graft excision, and broth culture of the graft material may be the only reliable method of identifying the causative bacteria and selecting appropriate antibiotic therapy.1,48
Culture Techniques
Standard swab cultures of material taken directly from the graft surfaces and perigraft fluid are usually sufficient in patients with fever, leukocytosis, perigraft abscess, or cellulitis. Virulent organisms such as S. aureus, streptococci, and gram-negative bacteria produce systemic signs of graft sepsis, are present in high numbers in perigraft exudate, and are readily identified with standard culture techniques. However, surface swabs may not recover less virulent pathogens that do not invade perigraft tissues. An apparent negative result of Gram staining of tissue or fluid from around an unincorporated prosthetic graft showing no organisms (but typically the presence of white blood cells) is not sufficient to exclude a low-grade graft infection because low numbers of slow-growing bacteria can reside within a surface biofilm.
For reliable recovery of microorganisms from within the biofilm, mechanical (tissue grinding) or ultrasound disruption of the biofilm of an explanted graft segment must be performed before incubation in a broth culture medium. Placement of the graft in a trypticase soy broth medium is believed to maximize bacterial growth and recovery because the liquid medium allows submersion of the graft and optimal exposure of any adherent bacteria to the nutrient medium. The use of mechanical disruption and broth cultures is particularly helpful in confirming graft infection with S. epidermidis. Culture tubes should be maintained for 5 to 7 days to exclude growth.
Surgical Treatment and Outcomes
Treatment Considerations
The goals of managing vascular graft infections involve initial and long-term eradication of the local and systemic septic process and maintenance of normal arterial perfusion to involved end-organ and limb tissues. Unfortunately, varied approaches have been advocated, with many experienced vascular centers using a single preferred approach for most arterial graft infections, especially aortoiliofemoral conduits. Our group has instead advocated a patient-specific treatment algorithm involving the use of conventional (total graft excision and extra-anatomic/remote bypass) or in situ replacement modalities and, occasionally, graft preservation techniques.39
Selection criteria for specific treatment modalities are based primarily on the clinical findings, extent of graft involvement, and microbiology (Table 42-3). Important adjuncts to attain wound sterilization for a vascular surgical site infection (VSSI, encompassing the graft/device + perigraft tissue + wound), especially when in situ reconstruction or graft preservation is under consideration, include the use of multiple, staged débridement/“washout” operative procedures (every 3 to 4 days) to minimize residual bacterial counts with more virulent infections; aggressive excision of involved arterial wall and perigraft tissues to healthy tissue planes; intraoperative mechanical and passive wound irrigation with cytotoxic agents (pulsed oxychlorosene [Clorpactin]/bleach, passive dilute povidone-iodine plus peroxide); temporary placement of antibiotic-loaded beads; soft tissue coverage of arterial repairs with noninfected, well-vascularized rotational muscle or fasciocutaneous flaps or omental pedicles; negative-pressure wound “sponge” therapy; closed suction drains and continuous povidone-iodine irrigation in grossly infected tissue beds; and culture-specific parenteral antibiotics.
Table 42-3
Selection Criteria for Appropriate Operative Management of Prosthetic Vascular Graft Infection
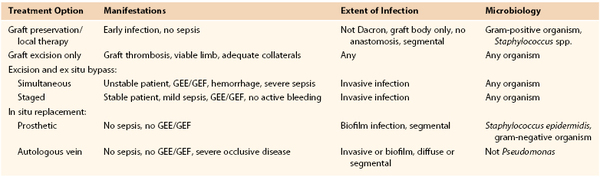
GEE, Graft-enteric erosion; GEF, graft-enteric fistula.
Several general treatment tenets are imperative. Infection involving prosthetic biomaterials frequently requires partial or complete graft removal to eradicate the local septic process. Attempts at preservation of prosthetic grafts are appropriate only in limited circumstances. However, graft preservation is possible for most arterial infections involving autologous tissue reconstruction (vein bypass or patch). The exception is invasive infections (i.e., those producing local or systemic sepsis) caused by Pseudomonas species or other potentially virulent gram-negative bacteria (e.g., Klebsiella species, Proteus species, E. coli). These bacterial strains possess autolytic properties that can cause disruption of the graft body or anastomosis and hemorrhage. Autologous graft excision with ex situ revascularization is indicated in this scenario.
Microbial species involved in late-appearing graft infections (more than 4 months after implantation) are typically less virulent (S. epidermidis, S. aureus), but the infections are more established and more difficult to eradicate locally. Graft excision is generally required for late-appearing infections. Late biofilm infections of aortic grafts occur an average of 40 months after implantation.39 Early infections (within 4 months of implantation) are usually associated with wound complications (more frequent after extracavitary reconstructions) and can involve more virulent bacterial strains. Like Calligaro and colleagues,57 we have seen more frequent successful graft preservation attempts with early-appearing extracavitary infections and overall equivalent outcomes (operative mortality or limb loss) in early and late graft infections with use of a selected treatment regimen.
Cavitary graft infections are less amenable than extracavitary infections to wound sterilization techniques involving multiple débridement procedures. Instead, a definitive procedure (simultaneous débridements) or two (staged) procedures that include total graft excision and in situ or ex situ reconstruction are required for cavitary infections. Because of the added invasiveness and physiologic stress of repeated cavitary exposure for the treatment of abdominal or thoracic graft infection, potential catastrophic cavitary graft disruption, and risk of development of enteric erosion or fistulization in the infected graft, preservation techniques should be attempted only rarely for cavitary graft infections.58
Graft Preservation
Attempts at graft preservation with just local treatment measures is possible in limited circumstances. Patent grafts with infection involving a shorter length (segmental) and sparing anastomoses (graft body only) can be considered for preservation. Results have been better with PTFE than with polyester conduits, with early (<4 months after implantation) rather than late (>4 months) infectious manifestations, with single gram-positive organisms than with polymicrobial or gram-negative infections, and with extracavitary rather than cavitary locations. Graft preservation should be attempted only for infections that are limited to the immediate perigraft region, caused by bacteria with limited virulence (not Pseudomonas species), and not associated with systemic sepsis.
Serial wound débridement in the operating room, use of cytotoxic irrigating solutions, and placement of antibiotic-loaded polymethyl methacrylate (PMMA) beads are necessary to minimize residual bacterial counts. Residual negative wound culture results are necessary for eventual rotational muscle flap coverage of the exposed graft segment or definitive skin closure, or both. More aggressive attempts at graft salvage by others, not necessarily adhering to the aforementioned criteria, have achieved complete graft preservation and wound healing in roughly 70% of patients with early aortofemoral graft limb59 or infrainguinal prosthetic bypass infections.21 However, initial treatment failures as a result of fatal graft disruption, persistent graft infection, and nonhealing wounds in 30% of cases limit broader application of this approach and emphasize the need to perform graft excision if local sepsis persists during serial treatments.11 Advocating use of débridement and negative-pressure wound therapy applied directly to exposed reconstructions for Szilagyi grade III (deep) infections, Mayer et al60 achieved 91% wound healing and 84% freedom from reinfection. These results were diluted by the fact that only 54% of cohort cases involved prosthetic graft infection and by the number of cases that were autologous reconstructions without mention of a treatment selection criterion. By comparison, with use of our stricter selection criteria, only 15% to 20% of our patients with early extracavitary prosthetic graft infections were deemed candidates, and they were successfully treated (avoidance of recurrent graft infection) by preservation techniques.
Graft Excision and Extra-Anatomic Bypass
Need for Revascularization
Graft excision without revascularization may be possible in some cases, including those in which claudication was the initial indication for intervention and when thrombosis of an infected graft does not result in critical limb ischemia. Arterial collaterals around a thrombosed or ligated bypass may take several weeks to develop, and inflow may be optimized by endovascular intervention if occlusive lesions are present in the native vasculature. Such an approach may be possible for a localized/segmental groin infection and aortofemoral graft via ligation of the affected limb through a clean retroperitoneal incision, segmental graft excision through the groin wound, endovascular recanalization of residual aortoiliac stenoses/occlusions, and femoral artery reconstruction (Fig. 42-3).
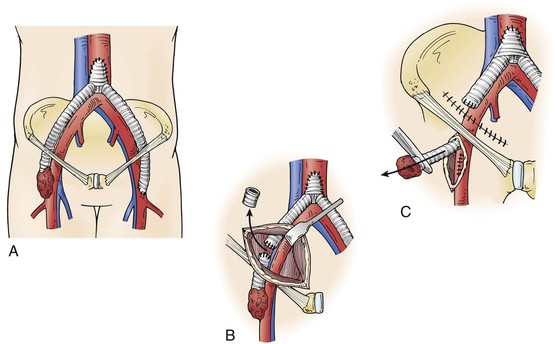
Figure 42-3 Excision of an infected distal aortofemoral graft segment. A, Localized/segmental distal graft groin infection. B, Retroperitoneal exposure of a noninfected graft limb through clean tissue planes. C, Excision of an infected graft segment. In situ prosthetic or autologous vein replacement is performed if needed.
Intraoperative decisions regarding the need for immediate revascularization in patients with patent, infected grafts can be made with the use of temporary bypass occlusion, a sterile blood pressure cuff, and continuous-wave Doppler assessment of pedal outflow. Persistence of a pulsatile pedal arterial signal and ankle pressure greater than 40 mm Hg with bypass occlusion may allow initial graft excision and consideration of delayed revascularization after eradication of local infection. Absence of pulsatile pedal flow during temporary bypass occlusion would probably result in critical ischemic symptoms and the potential for limb loss and thus mandates concomitant limb revascularization.
Graft Excision
Conventional management of an infected prosthetic arterial bypass involves total graft excision and revascularization via extra-anatomic/remote routes (ex situ) through uninfected tissue planes. This approach is generally required in patients with GEE/GEF and for more invasive infections associated with septic signs and symptoms and extensive perigraft inflammation (e.g., hydronephrosis with aortic graft infection). Complete removal of diffusely infected aortoiliofemoral grafts (including anastomotic suture lines) is accomplished by “repeat” celiotomy or a left retroperitoneal exposure and ideally without systemic heparinization. The explanted graft material should always be cultured.
Proximal control of the supraceliac aorta may be necessary before exposure of an infrarenal anastomosis with significant retroperitoneal inflammation. Although intraluminal balloon occlusion of the proximal iliac arteries may be possible, there is greater potential for large-volume blood loss, so direct dissection and clamping of uninvolved iliac vessels distal to the infected segments may be safer. Ureters should be identified and protected during pelvic dissection, and preoperative placement of ureteral stents may be helpful to avoid injury when hydronephrosis and extensive retroperitoneal inflammation are present. The infrarenal aorta should be débrided back to normal-appearing wall. Pledgets of prosthetic material should be avoided, and a double layer of interrupted monofilament suture should be used for aortic ligation. A similar closure technique for the distal aortic or iliac arteries should be performed to maintain retrograde flow from extra-anatomic bypasses at the femoral level into at least one pelvic artery system (external iliac and hypogastric) to avoid distal colonic, pelvic, and lower spinal cord ischemia.
Aggressive excisional débridement of infected perigraft tissue along with irrigation with cytotoxic agents and tunneling and placement of an omental pedicle to cover the aortic stump and retroperitoneal wound may reduce the risk of residual infection and catastrophic “stump blowout.” Temporary closed-suction drains and continuous povidone-iodine irrigation in the retroperitoneal wound bed can be considered in patients with severe inflammation and extensive local infection.
Timing of Limb Revascularization
In patients with aortic graft infection, timing of limb revascularization depends on the clinical findings. Simultaneous ex situ bypass with graft excision is necessary in hemodynamically unstable patients with systemic sepsis, hemorrhage from dehiscence of the anastomosis, or GEE/GEF. However, staged management with initial axillofemoral PTFE bypass followed in 1 to 2 days by excision of the aortic graft has been associated with lower perioperative mortality than a simultaneous approach in stable patients.15
Several decades of experience with conventional management of aortic graft infection has led to minimal changes in operative mortality but significant reductions in early limb loss, residual aortic stump infection and blowout, and recurrent infections involving ex situ prosthetic bypasses, as well as improved midterm patient survival61,62 (Table 42-4). The highest mortality rates continue to occur in patients with GEE/GEF or systemic sepsis, and conventional management is preferred over in situ replacement techniques for such patients.
Table 42-4
Results of Treatment of Prosthetic Graft Infections Involving the Infrarenal Aorta or Aortoiliofemoral Bypass*
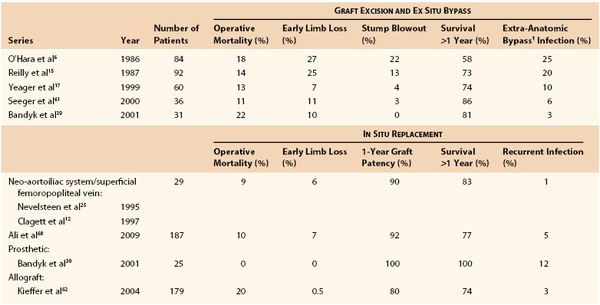
* Some series include graft-enteric erosion/graft-enteric fistula cases, which may influence the reported operative mortality.
† Cross-femoral, axillofemoral.
Diffuse aortobifemoral graft infections involving the groin region and with significant associated femoropopliteal occlusive disease can make ex situ approaches to revascularization challenging (Fig. 42-4). Limb loss is more frequent after aortofemoral graft infection than after aortoiliac reconstructions because of the higher risk for ex situ bypass thrombosis or recurrent infection. Unilateral axillofemoral bypasses to the profunda femoris or superficial femoral artery through an uninfected tissue plane have acceptable patency rates (94% at 6 months), but distal anastomoses to the popliteal artery are prone to early failure (42% at 6 months).63 Preservation of retrograde flow into the common femoral artery by vein patching (and endarterectomy for occlusive disease) after excision of the infected aortofemoral graft is also important for maintaining pelvic, colonic, and potentially lumbosacral neural viability.
Compromised patency of ex situ bypasses has led to increased use of in situ replacement with lower limb deep vein conduits to better facilitate a durable in-line reconstruction in patients with significant iliofemoral or distal occlusive lesions. A further option for managing aortofemoral graft infection with bilateral groin involvement is a combined unilateral axillofemoral PTFE bypass, autogenous deep vein cross-femoral bypass, and total graft excision.
In Situ Graft Replacement
Autologous Graft Replacement
The great saphenous vein (GSV) or a superficial upper extremity vein can be used for reconstruction of infrainguinal, visceral, cervical cerebrovascular, and upper extremity arteries after excision of an infected graft. For pseudomonal or other virulent gram-negative infections causing disruption of the anastomosis or graft body, ex situ tunneling of the new autologous vein graft out of infected fields is recommended.
However, use of GSV grafts for cross-femoral or iliofemoral bypass or supra-aortic trunk reconstruction (common carotid, subclavian) has resulted in limited patency because of a mismatch in the diameter (>6 mm) of available saphenous conduits and repair site vessels and subsequent progressive intimal proliferation, stenosis, and thrombosis.64 Use of appropriate lengths of a femoral vein segment has allowed larger-diameter, autologous arterial reconstruction without causing significant morbidity in the donor limb (e.g., acute deep venous thrombosis, limb edema, compartment syndrome) (Fig. 42-5). Preoperative ultrasonographic vein mapping of the GSV, an arm vein, and the femoral vein can be done in nonurgent scenarios to best match available autologous conduit and repair site diameters. Adequacy of the femoral vein (absence of acute/chronic thrombus, diameter >5 to 6 mm) can be confirmed by ultrasound when a small-diameter GSV or arm vein (<4 mm) is seen.
Neo-Aortoiliac System Reconstruction
Construction of an in situ neo-aortoiliac system (NAIS) from the femoral vein after removal of an infected aortoiliofemoral graft was first described by Clagett et al65 and Nevelsteen et al.25 Preoperative ultrasound mapping of the femoral vein is recommended because long vessel replacement lengths necessitate bilateral harvest. Longitudinal thigh incisions lateral to the sartorius muscle proximally and division of the adductor hiatus are needed with double-ligation techniques to secure side branch origins. The vein is harvested from the profunda femoris origin of the common femoral vein and into the popliteal vein, although numerous branches require careful division distally. Thigh wounds are closed in layers over closed-suction drains. Prophylactic low-dose anticoagulants (low-molecular-weight heparin) are used early, and sequential compression devices applied to the calf regions to augment venous outflow and limit propagation of thrombus in the ligated segments. Warfarin anticoagulation for 2 to 3 months can be considered to lower risk of additional deep venous thrombosis. Patients with prior GSV harvest who are undergoing femoral vein harvest are at higher risk for the development of calf compartment syndrome (up to 12% incidence), so a low threshold for decompressive fasciotomy should be maintained. Late venous insufficiency and chronic limb edema affects 15% of patients.
Deep vein segments are used in a nonreversed configuration after vessel eversion and direct excision of valve cusps. In situ replacement after graft excision and perigraft débridement can be tailored depending on the length of reconstruction needed and available femoral vein (Fig. 42-6). If a wide infrarenal aorta is present (18-26 mm), a “pantaloon” technique can be used, in which two deep vein segments are sutured together over half of their circumferences proximally to widen the conduit for end-to-end anastomosis. Excessively large aortic necks (>28 mm) may require ligation and ex situ bypass. If bilateral aortofemoral reconstruction is needed in patients in whom limited lengths of femoral vein are available, a unilateral aortofemoral femoral vein segment can be used with a shorter cross-femoral femoral vein segment between the distal aortofemoral limb and the contralateral groin.
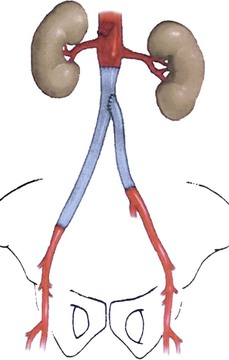
Figure 42-6 Schematic of deep vein replacement (neo-aortoiliac system) after excision of an infected aortobi-iliac prosthetic graft. Shortening of the total bypass length (and necessary bilateral femoral vein harvest) is possible because the contralateral femoral vein segment (to the left leg) originates off the mid or distal region of the ipsilateral aortoiliofemoral graft (to the right leg). (From Jackson MR, et al: Excision and autogenous revascularization of an infected aortic stent graft resulting from a urinary tract infection. J Vasc Surg 36:622, 2002.)
When compared with excision/ex situ bypass, in situ deep vein replacement for diffuse aorto-ilio-femoral graft infection has been associated with lower mortality and amputation rates, improved graft patency rates, and a lower incidence of recurrent infection (see Table 42-4).12 Longer-term audits of deep vein replacement for aortic graft infection by Clagett et al66–68 show a primary graft patency rate of 81%, a secondary/assisted patency rate of 91%, and a freedom from amputation rate of 89% at 6 years. This treatment approach has an associated operative mortality rate of 10% and a 5-year survival of 52%. Early mortality and/or recurrent/residual vein conduit infection and disruption occurred after treatment of GEE/GEF or identification of virulent gram-negative organisms. In situ deep vein replacement is contraindicated under these circumstances. Late vein conduit reinfection is rare. Vein conduit restenosis has been more common with a smaller-diameter femoral vein (<7 mm) and in patients with coronary artery disease or a history of smoking.66 Invasive infection confined to a single limb of an aortofemoral graft (i.e., localized/segmental infection) is also best treated by in situ deep vein replacement (from a single leg harvest).
Antibiotic-Treated Prosthetic Grafts
Use of a prosthetic conduit for in situ replacement of infected grafts is a treatment option in selected circumstances. For aortoiliofemoral and extracavitary (infrainguinal, axillofemoral, cross-femoral) graft infections, in situ prosthetic replacement with PTFE or polyester conduit has been associated with recurrent infection in 10% to 20% of cases, primarily with gram-negative bacteria and MRSA involvement. These results have increased interest in antibiotic-bonded prosthetic grafts to potentially improve results.18,69,70
We have advocated graft excision and in situ prosthetic replacement for localized/segmental, low-grade biofilm infections caused primarily by coagulase-negative staphylococci (S. epidermidis) (see Table 42-3). More than 50% of late extracavitary graft infections met these criteria and were treated with in situ PTFE graft replacement, with a resulting graft reinfection rate of less than 5%. Limited morbidity has also been demonstrated with conduit replacement using PTFE and rifampin-soaked (60 mg/mL), gelatin-sealed Dacron (Vascutek, Glasgow, United Kingdom) for low-grade aortoiliofemoral graft infections (see Table 42-4).39
Recurrent infections were due to rifampin-resistant S. epidermidis, MRSA, or more extensive graft involvement (i.e., bilateral graft limbs or aortic graft body), thus emphasizing that optimal results appear to be confined to limited biofilm infections (e.g., distal aortofemoral graft). Despite the risk for recurrent/residual infection, in situ prosthetic replacement of a distal aortofemoral graft limb through a groin incision may be preferred over total graft excision and deep vein replacement or ex situ bypass in frail, debilitated elderly patients with low-grade biofilm infection, groin region symptomatology, but more diffuse (cavitary) graft involvement. Selection criteria and treatment specifics for in situ prosthetic graft replacement are detailed in Box 42-4. In contradistinction, Oderich et al71 used in situ rifampin-soaked grafts with omental pedicle coverage to treat limited-involvement GEE/GEF (segmental enteric erosion onto body of aortic graft or limb, no anastomosis/aneurysm, no retroperitoneal sepsis), reporting 9% early mortality and 4% reinfection rates. Partial excision of the existing aortic graft was possible in 43% of these selected cases. More diffuse aortic graft infection, retroperitoneal inflammation/sepsis, and/or GEE/GEF involvement have dominated presentations at our institution and have limited the use of in situ prosthetic replacement therapy.
Cryopreserved Arterial Allografts
A third option for in situ replacement is the use of aortic and iliofemoral arterial segments harvested from transplant donors and rendered nonantigenic by cryopreservation. Fresh arterial allografts (stored up to 37 days) were used in a large-volume center in France until 1996 but were associated with a higher graft-related complication rate than were subsequent cryopreserved conduits.62 Cryopreserved arterial allografts/homografts (CAHs) are now commercially available, and modest-sized clinical experiences have been published.
Specific operative details for allograft implantation are provided in Box 42-5. Allograft replacement may have a primary role in mycotic or prosthetic graft infections of the thoracic or visceral aorta when in situ reconstruction is mandatory. Kieffer and colleagues reported an initial series in 1993 (36 patients)24 and a more comprehensive experience in 2004 (179 patients)62 with allograft replacement for infrarenal aortic graft infection. The higher operative mortality was influenced by adverse outcomes in the 30% of patients with GEE/GEF (see Table 42-4). The incidence of limb loss, recurrent infection, and midterm patient survival compares favorably with that for other methods of in situ replacement. However, allograft dilatation or aneurysm formation (17%) or stenosis/occlusion (20%) occurred during late follow-up (mean of 40 months), especially with the earlier fresh, iliofemoral allograft segments. Three later studies document in situ cryopreserved arterial allograft/homograft replacements performed in 151 total patients for arterial infectious pathologies (96 with infected prosthetic aortic grafts) with an overall operative mortality of 9.9% and a 5% cryopreserved arterial allograft/homograft-related complication rate (graft occlusion/stenosis, reinfection, degeneration) during follow-up ranging from 12 to 36 months.72–74 These encouraging results bear further comparative evaluation against alternative treatment methods.
Treatment Adjuncts
Antimicrobial Agents
Although antibiotics are a necessary adjunct in the treatment of graft infection, they are only part of the multimodality effort directed at sterilization of VSSIs. Parenteral antibiotics should be selected according to specific perigraft/graft culture results. Antibiotic adjustment should be based on results of cultures performed at each staged procedure to document the success of sterilization or to identify the development of bacterial resistance or change in microbial flora. Patients with more invasive/virulent graft infections, cavitary involvement, or more extensive soft tissue wounds may require additional enteral or parenteral nutritional support to combat the catabolic physiology associated with the infectious process.
When the diagnosis of vascular graft infection is first made, empirical broad-spectrum parenteral therapy should be instituted rapidly before the initial surgical exploration or planned reconstruction. Because of the emergence of MRSA, we use vancomycin 1 g every 12 hours as coverage for gram-positive organisms coupled with piperacillin/tazobactam 3.375 g every 6 hours, cefepime 2 g every 8 to 12 hours (for penicillin allergy), or levofloxacin 500 mg daily (for penicillin and cephalosporin allergy) for coverage of gram-negative organisms. Daptomycin 4 mg/kg daily is an alternative for coverage against MRSA when suspicion of this organism is high because of clinical outcomes equivalent to those for vancomycin and an observed more rapid bactericidal effect in vitro. On the basis of culture and Gram stain results, biofilm (S. epidermidis or S. aureus) infections are treated with parenteral daptomycin or vancomycin, and we have abandoned adjunctive oral or intravenous rifampicin (potential emergence of resistant S. epidermidis with continued use). Invasive and persistent MRSA soft tissue infection should be treated with higher-dose daptomycin, 6 mg/kg daily. Pseudomonal infections should be “double-covered” with combinations of third- or fourth-generation cephalosporins, piperacillin/tazobactam, aminoglycosides, fluoroquinolones (levofloxacin), and carbapenem/monobactams (imipenem, meropenem, aztreonam).
Parenteral antibiotics should be continued for a minimum of 2 weeks. Patients with invasive or cavitary infections or who underwent in situ replacement (prosthetic or allograft) or graft preservation techniques require 4 to 6 weeks of parenteral antibiotics. Oral linezolid 600 mg twice daily can be used for MRSA, staphylococcal, and biofilm infections for up to 3 weeks (myelosuppression occurs with longer duration) after hospital discharge and can shorten the duration of parenteral therapy and thus simplify outpatient management. Oral amoxicillin/clavulanate or an oral fluoroquinolone (levofloxacin or ciprofloxacin) can be used for less invasive, non-MRSA infections after discharge. We use indefinite oral antibiotic therapy as prophylaxis against reinfection only in rare circumstances.
Antibiotic-Loaded Beads
We have used antibiotic-loaded PMMA beads in our wound sterilization algorithm to aid in the treatment of early and late extracavitary prosthetic graft infection.75 Beads are made in the operating room out of PMMA powder by polymerizing the methacrylate with either vancomycin (1 g), daptomycin (1-1.5 g), tobramycin (1.2 g), or gentamicin (1 g) according to initial culture results. The hardening cement mix is molded into 5-mm beads on twisted stainless steel wire mandrels and implanted in the depth of the wound with loose skin closure (Fig. 42-7).
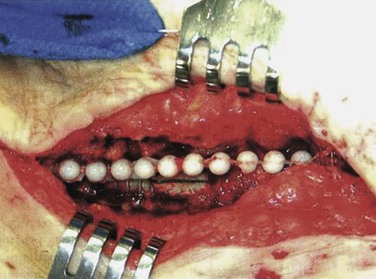
Figure 42-7 Antibiotic-loaded polymethyl methacrylate beads implanted adjacent to an infected polytetrafluoroethylene graft segment and polyester patch. Graft preservation will require further aggressive resection of the skin edges, subcutaneous abscess cavity, and perigraft tissue. (From Stone PA, et al: Use of antibiotic-loaded polymethylmethacrylate beads for the treatment of extra-cavitary prosthetic graft infection. J Vasc Surg 44:757, 2006.)
MRSA was found in 44% of initial cultures in our series. After an average of 2.5 bead replacements (staged débridement/washout/beads every 3 to 4 days) per patient, culture-proven wound sterilization allowed graft preservation or subsequent in situ reconstruction in all cases. Wound healing and avoidance of recurrent wound or graft infection was achieved in 90% of cases during an average follow-up of 2 years. This technique has been extrapolated from favorable orthopedic experiences and may augment therapies for vascular graft infection.
Local Muscle Coverage
Well-vascularized, sterile/noninfected surrounding soft tissue must exist at sites of vascular graft infection after débridement of skin, subcutaneous fat, and perigraft tissue and other treatment adjuncts to allow definitive wound closure. Transfer or rotation of local soft tissue structures to provide coverage of preserved or new in situ grafts may be needed when surrounding tissues are inadequate or an excessive skin defect exists. Pedicles of omentum mobilized within the abdominal or lower thoracic cavity are useful for minimizing recurrence after treatment of aortic graft infections. Multiple options exist for rotation or transfer of muscle flaps for cavitary and extracavitary graft infections.
Sartorius Muscle Flaps.
Because of the prevalence of VSSIs in the groin region, use of sartorius muscle flaps has been the most commonly reported technique.76 The muscle is typically exposed lateral to the femoral vessels, divided proximally from its attachment to the iliac spine, and mobilized medially without tension (Fig. 42-8). An attempt is made to interrupt as few segmental feeding vessels as possible to minimize flap ischemia (e.g., division of only lateral vessels and medial rotation of the flap over graft sites). However, longer-length mobilization of sartorius muscle into the upper medial groin region requires division of more lateral and medial feeding vessels and increases risk of flap ischemia. Timing of flap application for treating VSSI has varied but depends most on the indications for use and the absence of active gross infection.
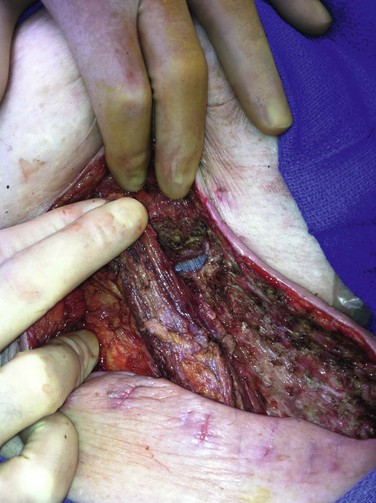
Figure 42-8 Sartorius muscle flap divided superiorly and mobilized toward the upper medial groin wound to cover exposed prosthetic cross-femoral graft after serial débridements of the abscess bed and adjunctive use of polymethyl methacrylate beads loaded with daptomycin + tobramycin . Skin closure was possible over the sartorius flap, and a bulb-suction drain was placed in the lateral harvested sartorius bed. The patient’s head is to the left of photo and the perineum is at the upper edge of the photo.
We have used sartorius flaps in 21% (89/422) of groin-related, early and late VSSI cases, primarily as part of graft preservation or in situ replacement therapies.77 Initial graft bed microbiology revealed 65% gram-positive, 20% gram-negative, and 15% mixed infections. An attempt was made to sterilize groin wounds with staged débridement and other adjuncts (e.g., antibiotic-loaded beads) to allow delayed application (after a mean 9 days) of sartorius flaps. However, immediate flap construction was required in 10% of patients because of extensive loss of local skin and soft tissues (exposed graft/repair). Overall, flap use was utilized in wounds with recurrent/persistent lymphocele/seroma formation (7% of cases) or extensive local tissue defects (48%). Selective flap application was used in the remaining patients (45%) for VSSI that was improving clinically after staged débridement and no gross infection or exudate but with residual positive wound culture results.
During a mean follow-up of 52 months, six (7%) recurrent infections occurred as a result of MRSA, recurrent biofilm bacteria (S. epidermidis), and one gram-negative species. Sartorius flaps may not completely eliminate the risk of reinfection for incompletely sterilized graft beds, but muscle coverage can salvage complex groin wounds associated with VSSI.
Femoral Rotational Muscle Flap.
Another strategy for treating localized graft infection in the groin is performance of a femoral rotational muscle flap procedure in which a muscle is mobilized from a clean bed, separate from the site of infection, and based on a pedicled blood supply that is independent of the site of infection. There is some evidence that this option may be better than local sartorius coverage.76 Because the muscle is mobilized from a separate bed, it is sterile at implantation. Because it is based on a pedicled blood supply that is independent of the site of infection, vascularity should be excellent. The specific muscle used depends on the patient’s arterial anatomy as well as the size and anatomic requirements of the wound that is being treated.76,78 A number of muscles may be mobilized to cover a graft in an infected groin, including the rectus abdominis, rectus femoris, tensor fasciae latae, and gracilis.79
Other Vascular Prosthetic Infections
Endovascular Device Infection
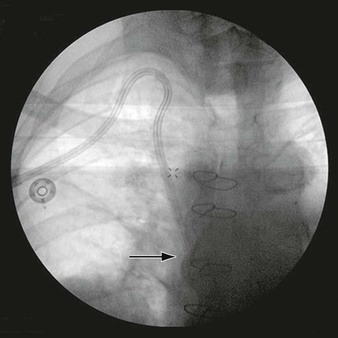
The incidence of infection involving endoluminal devices (endografts, stents) is lower than that involving prosthetic grafts placed during open surgical procedures (see Table 42-1). If the incidence is gauged by comparing numbers of case reports against estimated procedures performed annually, infection of peripherally placed stents appears to be extremely rare (<0.1%). Reported cases implicate periprocedural bacteremia from remote sources or during secondary endovascular interventions as the cause of stent infection.80–82 Use of stent-graft devices for arterial disease can involve a more invasive implantation procedure (open vessel exposure for endovascular aortic repair) that increases the risk for infection (wound and device) over that for percutaneous interventions. The incidence of aortic endograft infection in large institutional experiences has been low (0.1% to 1.2%),83–85 and isolated cases continue to be reported infrequently (Fig. 42-9).31,35–37,67,86–88
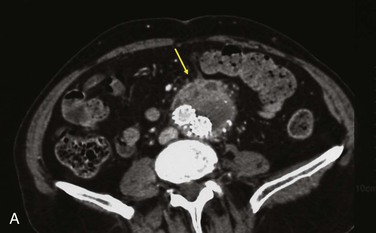
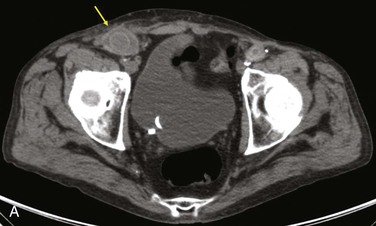
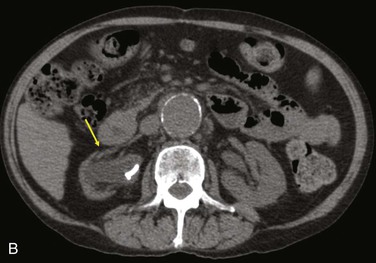
Figure 42-9 Infection associated with an abdominal aortic aneurysm endograft manifested as staphylococcal bacteremia and back pain. A, Inflammation in the anterior region of the distal infrarenal sac with patent aortoiliac stent-graft limbs (arrow). B, Resolved inflammation/infection 16 months later, after a 3-month course of parenteral antibiotics (arrow).
One third of abdominal and thoracic aortic endograft infections manifest as aorto-enteric or bronchial erosions/fistulae, with speculation that residual inflammation results from large intact aneurysms with pulsatility, persistent endoleak, or sac pressurization.86–88 As with bare-stent infections, other reports cite bacteremic seeding as being responsible for AAA endograft infection, especially at the time of catheter-based endoleak embolization.67,89,90
Corollary modeling of vascular prosthetic/device infection in animals has provided further insight into etiologic mechanisms. Both arterial stents and stent-grafts show decreasing susceptibility by 1 to 4 weeks to bacteremic (S. aureus) challenge as a result of early protective pseudointimal healing.91,92 Prophylactic antibiotics given before bacterial challenge significantly reduced luminal biomaterial infection at the susceptible early time points. Because luminal surface healing is more limited in humans, prophylactic antibiotics are recommended to prevent bacteremia before any additional open or percutaneous vascular procedure, and a low threshold should be maintained for early administration of antibiotics when remote infections are found. Additional animal work suggests that intra-arterial stent-grafts may have greater susceptibility to and less ability to clear infection than prosthetic interposition grafts when S. aureus is applied to the outer biomaterial surface.93 This situation could put endografts at added risk when embolization techniques are used to treat persistent endoleaks within the AAA sac and suggests the need for prophylactic antibiotics before these procedures.
Definitive treatment of stent and stent-graft infections, including aortoenteric erosions, has generally been managed by explantation of the device and ex situ bypass36,37,87,88 or in situ replacement with a femoroal vein67 or prosthetic graft.86 The operative mortality risk appears to be significant (20%-30%) for these definitive interventions. The potential use of endovascular techniques to treat endograft-related infected pseudoaneurysms or enteric erosions is extrapolated from limited experience with endograft treatment of mycotic aneurysms or aortoenteric/bronchial fistulae.94,95 Endovascular repair of primary and graft-related aortoenteric fistulae may be associated with lower morbidity but has shown limited durability and represents only a bridge to definitive open repair.96–99
Several reports have shown successful treatment of stent and stent-graft infections without device explantation. This approach should be offered only to extremely high-risk patients without aortoenteric erosions who have a rapid favorable clinical response to parenteral antibiotics (6 weeks’ minimum administration). Recurrent or persistent sepsis mandates explantation.
Thoracic Aortic and Supra-Aortic Branch Prosthetic Infections
Infections involving prosthetic grafts in the ascending or descending thoracic aorta, thoracoabdominal aorta, and brachiocephalic branch vessels have a prevalence pattern similar to that of graft reconstructions within the abdominal cavity (see Table 42-1).100–102 Graft excision and extra-anatomic bypass are not possible for most cases of ascending, arch, or descending thoracic aortic graft infection. The only recognized ex situ reconstruction option requires prosthetic grafting from the ascending aorta, tunneling through the diaphragm, and distal anastomosis to the abdominal aorta with subsequent excision of an infected descending thoracic graft through a left thoracotomy. In situ replacement with large-diameter polyester conduits coupled with wide débridement and antibacterial irrigation of infected tissues, coverage with rotated muscle (pectoralis major, rectus abdominis, latissimus dorsi) or tissue (pedicled omentum) flaps, and long-term antibiotics have most commonly been used.103 In situ replacement with arterial allografts has also been used.100,104 Early mortality has been 10% to 24%, with recurrent/residual infection complicating up to 20% of cases.100,103 Cryopreserved ascending aortic homografts have been prone to aneurysmal degeneration, necessitating secondary intervention for anastomotic disruption or aortic valve dysfunction, in 42% of surviving patients.104
Infected intrathoracic and cervical prosthetic bypasses are best managed by graft excision, and multiple ex situ prosthetic revascularization options are typically available from uninvolved ascending aorta or the carotid, subclavian, or femoral artery. In situ bypass with autologous femoral vein provides an optimal diameter match for supra-aortic trunk vessel replacement and allows shorter-length reconstruction than with some ex situ bypasses.
Infection of prosthetic carotid patch or graft reconstructions occurs in 0.25% to 0.5% of patients undergoing endarterectomy or bypass.30,32,105,106 Ligation of the common or internal carotid artery is associated with a high risk of cerebral ischemia and stroke, and the only ex situ bypass option above the carotid bifurcation is an extracranial-to-intracranial bypass. In situ replacement (from the common to the internal carotid) with larger-diameter GSV (>4 mm) or vein patch angioplasty is possible in most cases, can be performed with shunt or neuromonitoring protection, and is best covered with local rotation of the sternocleidomastoid muscle.107 Procedural complications are uncommon (<5% rate of stroke/death), and longer-term outcomes acceptable (12% rate of late stroke, reinfection, pseudoaneurysm formation).108
Acknowledgements
The author would like to acknowledge the inspirational efforts and diligent clinical assistance of Sunhee Moon, ARNP.
Selected Key References
Bandyk DF, Bergamini TM, Kinney EV, Seabrook GR, Towne JB. In situ replacement of vascular prostheses infected by bacterial biofilms. J Vasc Surg. 1991;13:575.
Represents an important culmination of work in the understanding of late graft infection, recognition of low-grade biofilm infection, identification of S. epidermidis as a causative organism, and appropriate use of in situ prosthetic replacement.
Bunt TJ. Synthetic vascular graft infections. Surgery. 1988;93:733.
Clinically useful classification scheme for the varied manifestations of vascular graft infection.
Calligaro KD, Veith FJ, Schwartz ML, Goldsmith J, Savarese RP, Dougherty MJ, DeLaurentis DA. Selective preservation of infected prosthetic grafts: analysis of a 20-year experience with 120 extra-cavitary infected grafts. Ann Surg. 1994;220:461.
Important proponent of graft preservation techniques contributing to the understanding of impaired prosthetic healing in variably infected beds. There was/is no substitute for the human model.
Clagett GP, Valentine RJ, Hagino RT. Autogenous aortoiliac/femoral reconstruction from superficial femoral-popliteal veins: feasibility and durability. J Vasc Surg. 1997;25:25.
Novel use of deep leg veins as large-artery replacement for aortic graft infection.
Kieffer E, Gomes D, Chiche L, Fléron MH, Koskus F, Bahnini A. Allograft replacement for infrarenal aortic graft infection: early and late results in 179 patients. J Vasc Surg. 2004;39:1009.
Description of outcomes after in situ allograft replacement for aortic graft infection in a large patient cohort in which potential failure modes of the technique are detailed.
Reilly LM, Stoney RJ, Goldstone J, Ehrenfeld WK. Improved management of aortic graft infection: the influence of operation sequence and staging. J Vasc Surg. 1987;5:421.
Largest experience with conventional management of infected infrarenal prostheses, in which the benefits of staging extra-anatomic bypass construction and aortic graft excision are recognized.
Szilagyi DE, Smith RF, Elliott JP, Vrandecic MP. Infection in arterial reconstruction with synthetic grafts. Ann Surg. 1972;176:321.
Seminal description of vascular biomaterial infection published within 10 to 15 years after the first polyester arterial reconstructions.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Bandyk DF. Vascular graft infections: epidemiology, microbiology, pathogenesis, and prevention. Bernhard VM, Towne JB. Complications in vascular surgery. Quality Medical: St Louis; 1991:223.
2. Bunt TJ. Synthetic vascular graft infections. Surgery. 1988;93:733.
3. Dean RH, et al. Aortoduodenal fistula: an uncommon but correctable cause of upper gastrointestinal bleeding. Am Surg. 1978;44:37.
4. Fry WJ, et al. Infection complicating the use of plastic arterial implants. Arch Surg. 1966;94:600.
5. Liekweg WG Jr, Greenfield LJ. Vascular prosthetic infections: collected experience and results of treatment. Surgery. 1977;81:335.
6. O’Hara PJ, et al. Surgical management of infected abdominal aortic grafts: review of a 25-year experience. J Vasc Surg. 1986;3:725.
7. Calligaro KD, et al. Are gram-negative bacteria a contraindication to selective preservation of infected prosthetic arterial grafts? J Vasc Surg. 1992;16:337.
8. Campbell WB, et al. Local complications after arterial bypass grafting. Ann R Coll Surg Engl. 1994;76:127.
9. Geary KJ, et al. Differential effects of a gram-negative and a gram-positive infection on autogenous and prosthetic grafts. J Vasc Surg. 1990;11:339.
10. Bandyk DF, et al. In situ replacement of vascular prostheses infected by bacterial biofilms. J Vasc Surg. 1991;13:575.
11. Calligaro KD, et al. Selective preservation of infected prosthetic grafts: analysis of a 20-year experience with 120 extra-cavitary infected grafts. Ann Surg. 1994;220:461.
12. Clagett GP, et al. Autogenous aortoiliac/femoral reconstruction from superficial femoral-popliteal veins: feasibility and durability. J Vasc Surg. 1997;25:25.
13. Daenens K, et al. Ten-year experience in autogenous reconstruction with the femoral vein in the treatment of aortofemoral prosthetic infection. Eur J Vasc Surg. 2003;25:240.
14. Fujitani RM, et al. Cryopreserved saphenous vein allogenic homografts: an alternative conduit in lower extremity arterial reconstruction in infected fields. J Vasc Surg. 1992;15:519.
15. Reilly LM, et al. Improved management of aortic graft infection: the influence of operation sequence and staging. J Vasc Surg. 1987;5:421.
16. Schmitt DD, et al. Graft excision and extra-anatomic revascularization: the treatment of choice for the septic aortic prosthesis. J Cardiovasc Surg. 1990;31:327.
17. Yeager RA, et al. Improved results with conventional management of infrarenal aortic infection. J Vasc Surg. 1999;30:76.
18. Young RM, et al. The results of in situ prosthetic replacement for infected aortic grafts. Am J Surg. 1999;178:136.
19. Bandyk DF, et al. Aortofemoral graft infection due to Staphylococcus epidermidis. Arch Surg. 1984;119:102.
20. Batt M, et al. In situ revascularization with silver coated polyester grafts to treat aortic infection: early and midterm results. J Vasc Surg. 2003;38:983.
21. Cherry KJ, et al. Infected femorodistal bypass: is graft removal mandatory? J Vasc Surg. 1992;15:295.
22. Ehrenfeld WK, et al. Autogenous tissue reconstruction in the management of infected prosthetic grafts. Surgery. 1979;85:82.
23. Johnston KW. Multicenter prospective study of nonruptured abdominal aortic aneurysm. II: Variables predicting morbidity and mortality. J Vasc Surg. 1989;9:437.
24. Kieffer E, et al. In situ allograft replacement of infected infrarenal aortic prosthetic grafts: results in 43 patients. J Vasc Surg. 1993;17:349.
25. Nevelsteen A, et al. Autogenous reconstruction of the lower extremity deep veins: an alternative treatment of prosthetic infection after reconstructive surgery of aortoiliac disease. J Vasc Surg. 1995;22:129.
26. Vogt PR, et al. Technical details with the use of cryopreserved arterial allografts for aortic infection: influence on early and midterm mortality. J Vasc Surg. 2002;35:80.
27. Durham JR, et al. Management of infected infrainguinal bypass grafts. Bergan JJ, Yao JST. Reoperative arterial surgery. Grune & Stratton: Orlando, FL; 1986:359.
28. Goldstone J, et al. Infection in vascular prostheses: clinical manifestations and surgical management. Am J Surg. 1974;128:225.
29. Lorentzen JE, et al. Vascular graft infection: an analysis of sixty-two graft infections in 2411 consecutively implanted synthetic vascular grafts. Surgery. 1985;98:81.
30. Naylor AR, et al. Prosthetic patch infection after carotid endarterectomy. Eur J Vasc Surg. 2002;23:11.
31. Ohki T, et al. Increasing incidence of midterm and long-term complications after endovascular graft repair of abdominal aortic aneurysms: a note of caution based on a 9-year experience. Ann Surg. 2001;234:323.
32. Rosenthal D, et al. Carotid patch angioplasty: intermediate and long-term results. J Vasc Surg. 1990;12:326.
33. Szilagyi DE, et al. Infection in arterial reconstruction with synthetic grafts. Ann Surg. 1972;176:321.
34. Hallett JW, et al. Graft-related complications after abdominal aortic aneurysm repair: population-based experience. J Vasc Surg. 1977;25:277.
35. Baker M, et al. Stent-graft infection after abdominal aortic aneurysm repair: a case report. J Vasc Surg. 2002;36:180.
36. Sharif MA, et al. Prosthetic stent-graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2007;46:442.
37. Jimenez JC, et al. Acute and chronic open conversion after endovascular aortic aneurysm repair. J Vasc Surg. 2007;46:642.
38. Kaebnick HW, et al. The microbiology of explanted vascular prostheses. Surgery. 1987;102:756.
39. Bandyk DF, et al. Expanded application of in situ replacement for prosthetic graft infection. J Vasc Surg. 2001;34:411.
40. Quinones-Baldrich WJ, et al. Long-term results following surgical management of aortic graft infection. Arch Surg. 1991;126:507.
41. Elek SD, et al. The virulence of Staphylococcus pyogenes in man: a study of the problems of wound infection. Br J Exp Pathol. 1957;38:573.
42. Back MR, et al. Arterial prosthetic grafts stimulate local production of tumor necrosis factor and matrix metalloproteases. ACS Surg Forum. 1997;158:395.
43. Zimmerl W, et al. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982;146:487.
44. Moore WS, et al. Prosthetic arterial graft material: influence on neointimal healing and bacteremic infectibility. Arch Surg. 1980;115:1379.
45. Armstrong PA, et al. Improved outcomes in the recent management of secondary aortoenteric fistula. J Vasc Surg. 2005;42:660.
46. Durham JR, et al. The impact of multiple operations on the importance of arterial wall cultures. J Vasc Surg. 1987;5:160.
47. Back MR. Infections and antibiotics in vascular surgery. White RA, Hollier LH. Vascular surgery: basic science and clinical correlations. ed 2. Blackwell: Malden, MA; 2005:477.
48. Bergamini TM, et al. Identification of Staphylococcus epidermidis vascular graft infections: a comparison of culture techniques. J Vasc Surg. 1989;9:665.
49. Salgado CD, et al. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis. 2003;36:131.
50. Bradley SF, et al. MRSA. Colonization and infection in a long-term care facility. Ann Intern Med. 1991;115:417.
51. Hsu CCS, et al. High rate of MRSA isolated from hospitalized nursing home patients. Arch Intern Med. 1988;148:569.
52. Harbarth S, et al. A predictive model for identifying surgical patients at risk of methicillin-resistant Staphylococcus aureus carriage on admission. J Am Coll Surg. 2008;207:638.
53. Nasim A, et al. The impact of MRSA on vascular surgery. Eur J Vasc Endovasc Surg. 2001;22:211.
54. Naylor AR, et al. A prospective audit of complex wound and graft infections in Great Britain and Ireland: the emergence of MRSA. Eur J Vasc Endovasc Surg. 2001;21:289.
55. D’Addato M, et al. Prophylaxis of graft infection with rifampicin-bonded Gelseal graft: 2-year follow-up of a prospective clinical trial. Cardiovasc Surg. 1996;4:200.
56. Braithwaite BD, et al. Early results of a randomized trial of rifampicin-bonded Dacron grafts for extra-anatomic vascular reconstruction. Joint Vascular Research Group. Br J Surg. 1998;85:1378.
58. Calligaro KD, et al. Intra-abdominal aortic graft infection: complete or partial graft preservation in patients at very high risk. J Vasc Surg. 2003;38:1199.
59. Calligaro KD, et al. Infrainguinal anastomotic arterial graft infections treated by selective graft preservation. Ann Surg. 1993;216:74.
60. Mayer D, et al. Long-term results of vascular graft and artery preserving treatment with negative pressure wound therapy in Szilagyi grade III infections justify a paradigm shift. Ann Surg. 2011;254:754.
61. Seeger JM, et al. Long-term outcome after treatment of aortic graft infection with staged extra-anatomic bypass and aortic graft removal. J Vasc Surg. 2000;32:451.
62. Kieffer E, et al. Allograft replacement for infrarenal aortic graft infection: early and late results in 179 patients. J Vasc Surg. 2004;39:1009.
63. Seeger JM, et al. Influence of patient characteristics and treatment options on outcome of patients with prosthetic aortic graft infection. Ann Vasc Surg. 1999;13:413.
64. Seeger JM, et al. Autogenous graft replacement of infected prosthetic grafts in the femoral position. Surgery. 1983;93:39.
65. Clagett GP, et al. Creation of a neo-aortoiliac system from lower extremity deep and superficial veins. Ann Surg. 1993;218:239.
66. Beck AW, et al. Aortic reconstruction with femoro-popliteal vein: graft stenosis incidence, risk and reintervention. J Vasc Surg. 2008;47:36.
67. Jackson MR, et al. Excision and autogenous revascularization of an infected aortic stent graft resulting from a urinary tract infection. J Vasc Surg. 2002;36:622.
68. Ali AT, et al. Long-term results of the treatment of aortic graft infection by in situ replacement with femoral popliteal vein grafts. J Vasc Surg. 2009;50:30.
69. Hayes PD, et al. In situ replacement of infected aortic grafts with rifampicin bonded prostheses: the Leicester experience (1992-1998). J Vasc Surg. 1999;30:92.
70. Koshiko S, et al. Limitations in the use of rifampicin-gelatin grafts against virulent organisms. J Vasc Surg. 2002;35:779.
71. Oderich GS, et al. In situ rifampin-soaked grafts with omental coverage and antibiotic suppression are durable with low reinfection rates in patients with aortic graft enteric erosion or fistula. J Vasc Surg. 2011;53:99.
72. Bisdas T, et al. Eight-year experience with cryopreserved arterial homografts for the in situ reconstruction of abdominal aortic infections. J Vasc Surg. 2010;52:323.
73. Zhou W, et al. In situ reconstruction with cryopreserved arterial allografts for management of mycotic aneurysms or aortic prosthetic graft infections: a multi-institutional experience. Tex heart Inst J. 2006;33:14.
74. Brown KE, et al. Arterial reconstruction with cryopreserved human allografts in the setting of infection: a single-center experience with mid-term follow-up. J Vasc Surg. 2009;49:660.
75. Stone PA, et al. Use of antibiotic-loaded polymethylmethacrylate beads for the treatment of extra-cavitary prosthetic graft infection. J Vasc Surg. 2006;44:757.
76. Perler BA, et al. Rotational muscle flaps to treat localized prosthetic graft infection: long-term follow-up. J Vasc Surg. 1993;18:358.
77. Armstrong PA, et al. Selective application of sartorius muscle flaps and aggressive staged surgical debridement can influence long-term outcomes of complex prosthetic graft infections. J Vasc Surg. 2007;46:71.
78. Budney PG, et al. Salvage of prosthetic grafts and joints in the lower extremity. Clin Plast Surg. 1991;18:583.
79. Mathes SSJ, et al. Muscle transposition flaps for coverage of lower extremity deficits: anatomic considerations. Surg Clin North Am. 1954;54:1337.
80. Deiparine MK, et al. Endovascular stent infection. J Vasc Surg. 1996;23:529.
81. Dosluoglu HH, et al. Stent-related iliac artery and iliac vein infections: two unreported presentations and review of the literature. J Endovasc Ther. 2001;8:202.
82. Pruitt A, et al. Distal septic emboli and fatal brachiocephalic artery mycotic pseudoaneurysm as a complication of stenting. J Vasc Surg. 2002;36:625.
83. Laser A, et al. Graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2011;54:58.
84. Cernohorsky P, et al. The relevance of aortic endograft prosthetic infection. J Vasc Surg. 2011;54:327.
85. Heyer KS, et al. Secondary infections of thoracic and abdominal aortic endografts. J Vasc Interv Radiol. 2009;20:173.
86. Riesenman PJ, et al. Aortoesophageal fistula after thoracic endovascular aortic repair and transthoracic embolization. J Vasc Surg. 2007;46:789.
87. Abou-Zamzam AM Jr, et al. Aortoenteric fistula development following endovascular abdominal aortic aneurysm repair: a case report. Ann Vasc Surg. 2003;17:119.
88. Alankar S, et al. Aortoduodenal fistula and associated rupture of abdominal aortic aneurysm after endoluminal stent-graft repair. J Vasc Surg. 2003;37:465.
89. Eliason JL, et al. Infected endovascular graft secondary to coil embolization of endoleak; a demonstration of the importance of operative sterility. Ann Vasc Surg. 2002;16:562.
90. Hulin SJ, et al. Aortic endograft infection: open surgical management with endograft preservation. Eur J Vasc Endovasc Surg. 2007;34:191.
91. Kirksey L, et al. Prophylactic antibiotics prior to bacteremia decrease endovascular graft infection in dogs. Vasc Endovasc Surg. 2002;36:171.
92. Paget DS, et al. Infectibility of endovascular stents following antibiotic prophylaxis or after arterial wall incorporation. Am J Surg. 1999;178:219.
93. Parsons RE, et al. Comparison of endovascular and conventional vascular prostheses in an experimental infection model. J Vasc Surg. 1996;24:920.
94. Kan CD, et al. Outcome after endovascular stent-graft treatment for mycotic aortic aneurysm: a systematic review. J Vasc Surg. 2007;46:906.
95. Wheatley GH 3rd, et al. Have we gone too far? Endovascular stent-graft repair of aortobronchial fistulas. J Thorac Cardiovasc Surg. 2007;133:1277.
96. Deshpande A, et al. Endovascular repair of an aortoenteric fistula in a high-risk patient. J Endovasc Surg. 1999;6:379.
97. Eskandari MK, et al. Endovascular repair of an aortoduodenal fistula. J Endovasc Ther. 2000;7:328.
98. Curti T, et al. Endovascular treatment of an ilioenteric fistula: a bridge to aortic homograft. Eur J Vasc Endovasc Surg. 2000;20:204.
99. Chuter TAM, et al. Endovascular repair of a presumed aortoenteric fistula: late failure due to recurrent infection. J Endovasc Ther. 2000;7:240.
100. Kieffer E, et al. Prosthetic graft infection after descending thoracic/thoracoabdominal aortic aneurysmectomy: management with in situ arterial grafts. J Vasc Surg. 2001;33:671.
101. Svensson LG, et al. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17:357.
102. Svensson LG, et al. Variables predictive of outcome in 832 patients undergoing repairs of the descending thoracic aorta. Chest. 1993;104:1248.
103. Coselli JS, et al. Treatment of postoperative infection of ascending aorta and transverse aortic arch, including use of viable omentum and muscle flaps. Ann Thorac Surg. 1990;50:868.
104. Khaladj N, et al. Cryopreserved human allografts/homografts for the management of graft infections in the ascending aortic position extending to the arch. Eur J Cardiothorac Surg. 2012;34:25.
105. Rockman CB, et al. Postoperative infection associated with polyester patch angioplasty after carotid endarterectomy. J Vasc Surg. 2003;38:251.
106. Knight BC, et al. Dacron patch infection following carotid endarterectomy: a systematic review of the literature. Eur J Vasc Endovasc Surg. 2009;37:176.
107. Zacharoulis DC, et al. Use of muscle flap to cover infections of the carotid artery after carotid endarterectomy. J Vasc Surg. 1997;25:769.
108. Bergamini TM, et al. Symptomatic recurrent carotid stenosis and aneurysmal degeneration following endarterectomy. Surgery. 1993;113:580.