Chapter 45
Local Complications
Graft Thrombosis
David H. Stone, Daniel B. Walsh
Postoperative graft thrombosis remains a significant clinical challenge in contemporary vascular surgical practice. Whether early or delayed, graft thrombosis continues to account for significant morbidity, limb loss, and mortality in patients requiring vascular intervention. Specifically, at 1 year after infrageniculate bypass graft failure, more than 50% of patients will have undergone major amputations.1,2 Among the remaining patients, ischemic pain at rest or ulceration will have developed in 25%, and more than 15% will have died. Underlying risk factors associated with graft failure continue to be the focus of vascular surgical outcomes analyses and regional quality improvement groups alike.3–5
The causes of graft thrombosis are multifactorial and involve patient demographics, risk factors, and comorbid conditions, as well as technical issues associated with arterial reconstruction. Such risk factors and technical aspects of reconstruction have an impact on graft patency from the initial operation through the entire follow-up period. With this in mind, technical precision at initial reconstruction is imperative to achieve an optimal outcome because technical errors account for 4% to 25% of early failure after revascularization.6–8 Furthermore, optimal long-term graft durability remains, in part, predicated on lifetime surveillance, timely re-interventions, and vigilant risk factor modification.9–12 To facilitate intraoperative assessment of the technical adequacy of the reconstruction at the time of surgery, numerous diagnostic tools are available to the surgeon, which we review. Thereafter, the discussion focuses primarily on surgical revascularization, including autogenous and prosthetic conduits and factors associated with their failure. Understanding the etiology and clinical manifestations of graft thrombosis and current experience with available treatment options is crucial for achieving the best and most durable results after initial failure of revascularization.
Prevention: Techniques of Graft Assessment
To ensure optimal patency after revascularization, it is imperative that the surgeon determine the technical adequacy of the reconstruction before leaving the operating room.
Inspection, Palpation, and Measurement of Flow
The most convenient and readily available methods for graft assessment include inspection and palpation of pulses. These processes involve not only inspection of the graft itself for kinks, twists, and stenoses, but also examination of the distal target vessel and of the revascularized tissue, if possible. Intuitive to this process are the surgeon’s expectations. Is the foot pink and perfused? Has capillary refill time been shortened? Is a pulse now palpable in the foot?
The process is facilitated by having the target organ, as much as possible, included in the sterile field and available to the surgeon for intraoperative examination. For example, for aortobifemoral or more distal bypasses, covering the sterilely prepared feet with clear plastic bags permits rapid examination after completion of the bypass. However, inspection and palpation are subjective and thus susceptible to observer bias. After a complex reconstruction, the surgeon’s expectations may cloud the evaluation of capillary refill time in the feet. Calcified arteries secondary to long-standing diabetes may not adequately transmit an improved pulse. The effects of anesthesia combined with concomitant chronic occlusion of runoff arteries may delay the appearance of adequate lower extremity reperfusion. More importantly, false-negative results can occur when a graft has a strong pulse because of distal outflow obstruction.
Measurement of arterial inflow and outflow is often significantly affected by anesthesia. Furthermore, small, seemingly hemodynamically insignificant defects in the graft may also result in failure. Low-flow measurements may accurately predict graft failure, but this finding alone does not localize the specific defect, once discovered. Studies using an ultrasound flowmeter have confirmed the inability of graft flow as an isolated parameter to predict future graft function.13 Measurement of flow by ultrasonically measured transit times has been reported to be sensitive and specific for graft defects.14 Performance of these studies is cumbersome, however, and requires additional adjunctive measures to both localize and identify specific graft defects. Because of these problems and the ease and effectiveness of other techniques, these measures are used less frequently and as perfunctory diagnostic adjuncts.
Arteriography
Since its introduction, intraoperative completion arteriography has been the “gold standard” for anatomic evaluation of the technical adequacy of arterial reconstructions. One appealing feature of arteriography is its ability to assess anatomic arterial outflow—the “runoff.” This is particularly important in the clinical context of preoperative studies that fail to reveal adequate target vessels in diffusely diseased vascular systems. Although completion arteriography is an invasive procedure associated with potential complications because of arterial puncture (intimal injury, dissection), injection (air embolism), use of radiographic contrast agents (renal failure, anaphylaxis), and radiation exposure, the actual observed complication rate has been negligible in large series.15,16
The technique varies according to individual application but generally involves insertion of an 18- to 20-gauge plastic angiocatheter or 5- to 6-Fr sheath into the arterial graft to allow subsequent injection of 10 to 30 mL of radiographic contrast agent. Temporary occlusion of arterial inflow maximizes the concentration of contrast agent without the need for excessively rapid injection. Portable C-arm fluoroscopy units or fixed imaging operating room suites that permit sophisticated imaging after arterial reconstruction are now available in most centers performing vascular care. The presence of such technology in both the operating room and angiography suites will clearly facilitate more routine graft interrogation throughout the follow-up interval when needed (Fig. 45-1).
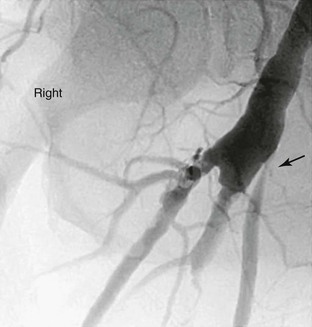
Figure 45-1 Follow-up surveillance angiogram of a femoral-to-popliteal bypass graft with a significant stenosis noted at the proximal anastomosis (arrow). This angiogram was performed after graft duplex ultrasound detected increased velocity at this location.
Several weaknesses are inherent in the technique of arteriography, however. In lower extremity bypass grafts, the proximal anastomosis is frequently not evaluated, which may obviate detection of a proximal technical defect. Air bubbles or overlying structures may lead to false-positive interpretations. A potential source of false-negative results is the use of a single plane of view to analyze a multidimensional target; this method can result in underestimation of the stenosis from a small defect, such as an intimal flap or platelet aggregate.17 Another problem associated with arteriography is the use of potentially nephrotoxic iodinated contrast agents.
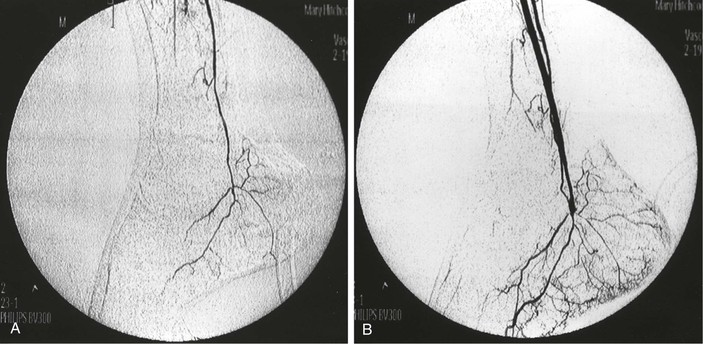
To avoid these problems, intra-arterial digital subtraction angiography (DSA) using a portable, axially rotatable imaging device can more easily obtain views from different angles.18 The small amounts of dye and the “cine” nature of modern fluoroscopic units enable visualization of particular areas of the graft, and thereby, allow more accurate interpretation of images. DSA also permits the use of smaller contrast volumes and real-time video replay. DSA is more applicable to localized areas, such as a distal anastomosis and pedal runoff, than to an entire extremity. An entire extremity can be filmed with this technology using repeated injections of small amounts of contrast agent to obtain sequential angiographic images, or the so-called pulse-chase technique, in which the DSA machine is moved along the extremity and “chases” the injected contrast material (Fig. 45-2).
Ultrasonography
The development of ultrasound technology has provided multiple noninvasive modalities for the intraoperative and subsequent longitudinal assessment of arterial reconstructions.
Continuous-Wave Doppler
The simplest and least expensive ultrasound device is continuous-wave Doppler with an 8- to l0-MHz pencil probe. The major advantages of these probes are that they are easy to sterilize with gas and are readily available for intraoperative use. Their small size allows insonation of arteries in areas less accessible to larger probes. With sterile saline used for acoustic coupling, the probe can be passed along the graft or reconstruction site, where localized increases in the audible sound frequency or audible turbulence indicates a potential defect. Patent residual vein branches in in-situ saphenous vein bypasses can be readily identified by locally increased frequency, continuous flow, and flow outside the graft boundary. Successively compressing the graft from the proximal to the distal end while listening for residual proximal flow with a Doppler device can also localize these arteriovenous fistulae.
Use of the audible continuous-wave Doppler technique is subjective and operator dependent. Considerable experience is required for maximum accuracy. The presence of high-frequency sound waves within the graft or at the distal anastomosis is worrisome. However, some investigators have shown that the continuous-wave Doppler device is not highly sensitive.19 Most vascular surgeons use this modality as an easy, available, and inexpensive screening device to guide their use of a more precise evaluation technique. To improve the objectivity of this technique, a fast-Fourier transform spectral analysis can be used to quantify changes in frequency or velocity. A further potential refinement is the use of a high-frequency (20-MHz) pulsed Doppler device contained in a small probe that permits easy access to all operative sites.20 Considerable experience with pulsed Doppler probes is required to achieve accurate results, however, and the technique does not provide the anatomic images that are reassuring to most surgeons considering arterial re-exploration.
B-Mode Ultrasonography
B-mode ultrasonography has been used intraoperatively to obtain anatomic images noninvasively, although it is more commonly used in conjunction with duplex ultrasonography. Initial experimental studies established that its ability to detect small arteriographic defects in patients was comparable to that of arteriography.21 In an evaluation of arterial defects created in dogs, both arteriography and B-mode ultrasonography were nearly 100% specific in excluding arterial defects. However, ultrasonography has significantly greater sensitivity in detecting defects, 92% overall, than serial biplanar arteriography at 70% and portable arteriography at 50%. These techniques have comparable accuracy in detecting stenoses.
B-mode ultrasonography has utility in assessing lower extremity arterial reconstructions. Kresowik et al22 reported that in 106 patients, intraoperative B-mode ultrasonography detected defects in 20% of patients, and that half of these defects were deemed important enough to warrant correction. In follow-up, there were no early graft occlusions in the B-mode group, and no residual defects were discovered with duplex scanning follow-up in the postoperative period.
Intraoperative use of B-mode ultrasonography, however, is not without its problems. Because this modality does not evaluate blood flow, it cannot differentiate fresh thrombus from flowing blood, which has the same echogenicity. Compared with Doppler pencil probes, B-mode ultrasound probes are larger and cannot be sterilized. Thus, their use is more cumbersome. The probes require a sterile covering containing a gel to maintain an appropriate acoustic interface. Significant operator experience is needed to obtain optimal images and make accurate interpretations.
In clinical situations, one difficulty with the technique is determining the significance of the many defects identified, because most do not require repair. The lack of accompanying blood flow information makes this decision more difficult. In one study, B-mode ultrasonography failed to create technically adequate images for evaluation in nearly 25% of patients. Such inadequacy can frequently be encountered during graft interrogation in limited operative fields, and therefore, can confound the probe manipulation required for accurate imaging.23
Duplex Ultrasonography
With the addition of flow-measuring capability to B-mode ultrasonographic technology, duplex scanning brings a more powerful, although more expensive and complex tool, to the operating room. Like B-mode ultrasound probes, duplex scanning probes are large, cannot be sterilized, and require considerable operator skill, not only to obtain accurate images, but also to position the probe over the sample target appropriately so that accurate velocity measurements can be obtained. Duplex color-flow technology provides continuous Doppler signals along the graft and artery at multiple points. Color imaging facilitates identification of areas of higher velocity, albeit at a significant increase in equipment cost.24
Examination of outflow arteries with duplex scanning is less precise than with arteriography, although the information provided is physiologic rather than purely anatomic. Duplex scanning provides an easier mechanism for identifying defects in proximal arterial anastomoses than arteriography does, because contrast-enhanced imaging of the proximal anastomosis from a more distally placed catheter is cumbersome and often difficult.25 In addition, duplex scanning can identify low graft velocities that are undetectable by arteriography. Intraoperative experience with this imaging modality has shown greater sensitivity for detecting technical defects. Early results with intraoperative duplex scanning have demonstrated an association between these defects and suboptimal results in the postoperative period.26,27 It is noteworthy that duplex scanning is unable to access newly placed polytetrafluoroethylene and polyester (Dacron) grafts because the graft walls contain air, which prevents penetration of the ultrasound waves.
In a study from 2002, Rzucidlo et al28 reported intraoperative completion duplex scanning to be a useful tool after the completion of infrageniculate arterial reconstruction. Specifically, the authors documented that a 10-MHz, low-profile transducer could be used successfully to identify compromised grafts with a predilection for early failure. Moreover, it was determined that low end-diastolic velocity was both associated with and predictive of early graft failure. More recently, Scali et al29 validated the utility of intraoperative completion duplex scanning following distal bypass, documenting that end-diastolic velocity (EDV) measurements less than 5 cm/s predicted early graft failure, and more broadly, crural vein graft patency.
Angioscopy
Intraoperative angioscopy has become an attractive technique for evaluation of arterial reconstructions and thorough interrogation of autogenous conduit. Angioscopy requires irrigation with saline accompanied by inflow, and sometimes outflow, occlusion to provide a visually clear image. The presence of any red blood cells can completely obscure accurate visualization of the graft lumen. The use of a specifically designed infusion pump with high- and low-flow rates has greatly facilitated angioscopy techniques.30 As with duplex ultrasonography, experience is required to manipulate both the angioscope and the visual target to obtain adequate and clear visualization.
Angioscopy has been most widely used for inspection of in-situ saphenous vein grafts to ensure complete valve lysis,31 exclude unligated venous branches, and assess the quality of the venous conduit (Fig. 45-3). A 1.4-mm-diameter angioscope is most commonly used at our institution to interrogate lower extremity vein grafts. The angioscope is inserted through an introducer in the most proximal end of the vein or through the most proximal vein branch, which can be deliberately left unligated for this purpose. Saline irrigation is administered through a sheath. Before angioscopy, it is useful to identify and ligate as many venous side branches as possible to optimize distal visualization while minimizing the irrigation required to clear red blood cells. Angioscopy can be used at other sites if blood flow can be temporarily occluded. Such occlusion sometimes necessitates the use of balloon occlusion catheters if proximal control is not surgically accessible. Angioscopy appears to be particularly important in detecting abnormalities within an arm vein conduit that may not be evident by external visual inspection, including important evaluation of venovenostomy anastomoses.32–34
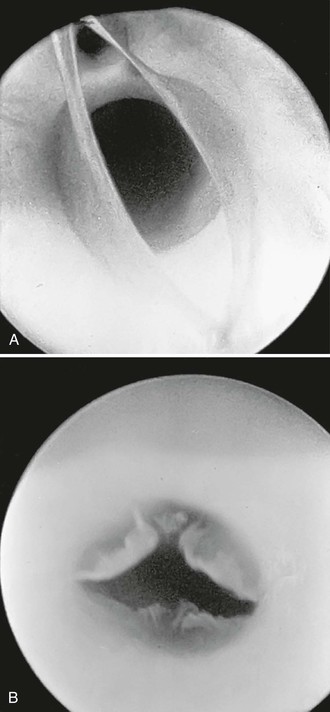
Figure 45-3 A, Photograph of a valve via an angioscope before lysis. B, Photograph of an angioscopic image of a valve after lysis. (B, From McCaughan JJ, Jr, et al: In vitro observations of greater saphenous vein valves during pulsatile and nonpulsatile flow and following lysis. J Vasc Surg 1:356, 1984.)
It should be noted that experimental studies have documented that mild intimal injury may occur with angioscopy. However, such injury occurs only after multiple repeated passages of larger diameter scopes.35 The long-term effects of this mild trauma have not been firmly established, but a few passes of a small-diameter (1.4 mm) angioscope in human vein grafts appear to have no significant late clinical consequences. Several studies have revealed that infusion of irrigation solution can be limited to 500 mL or less in most instances, an amount that has not been associated with complications, especially when planned as a part of the overall fluid administration during the procedure.30,36 In a 2002 study from Lund, Sweden, the authors documented successful application of intraoperative angioscopy in a group of patients who underwent below-knee in-situ saphenous vein graft bypass; they demonstrated its utility in this setting and concluded that angioscopy has an impact on primary graft patency rates, and therefore, reduces the need for subsequent re-intervention. Specifically, this adjunct was useful in identifying persistent saphenous vein branches, incomplete valve destruction, and partially occlusive intraluminal thrombus.37
Intravascular Ultrasonography
More frequently in contemporary practice, intravascular ultrasound (IVUS) has become a useful intraoperative imaging adjunct. This technology is based on a flexible catheter system and generates two-dimensional cross-sectional images through the circumferential rotation of a miniaturized (10-30 MHz) ultrasound crystal at the catheter tip. In experimental studies, IVUS has proved to be accurate in measuring luminal diameter and identifying stenoses caused by atherosclerosis or intimal hyperplasia.38–40 This technology has significant appeal as an imaging modality in the setting of complex aortic procedures, in particular for acute aortic dissection.41–43 Both IVUS and angioscopy were found to be 100% accurate in detecting 2-mm intimal flaps in canine femoral arteries compared with only 60% accuracy for single-plane arteriography.44
Few clinical studies assessing the role of IVUS have established its efficacy and potential role in evaluating the technical adequacy of vascular surgical reconstructions. However, its utility appears to be growing. Although IVUS appears to be useful in the evaluation of lesions appropriate for placement of devices such as bare-metal stents and stent-grafts,45 it is unclear whether IVUS will provide information that is different and useful enough to justify its current cost in comparison to alternative intraoperative techniques. Nevertheless, this real-time, radiation-free, contrast-free, dynamic imaging modality offers significant appeal.
Miscellaneous Modalities
In addition to direct methods of evaluating arterial reconstructions intraoperatively, several indirect methods can measure resistance within the graft or within the outflow bed, which may, in turn, help evaluate the adequacy of revascularization. This is most easily accomplished intraoperatively with a continuous-wave Doppler probe placed over a distal artery while the examiner listens for audible augmentation of the waveform after release of a temporary graft occlusion. Although frequently used intraoperatively, this modality provides little objective quantifiable measure of the adequacy of reconstruction.
A more quantitative assessment can be obtained by measuring distal extremity pressure with a sterile blood pressure cuff during surgery. In patients with more proximal reconstructions and residual outflow abnormalities, ankle pressure may not be maximal immediately after revascularization, but rather may increase only gradually in the postoperative period. Thus, this intraoperative pressure measurement does not provide absolute proof of the success of reconstruction, and therefore, must be interpreted on the basis of the preoperative anatomy of each patient. Other similar modalities, including pulse volume recording (plethysmography), strain-gauge plethysmography, photoplethysmography, and even transcutaneous oxygen tension measurement, can be used intraoperatively as clinical adjuncts to evaluate the restoration of distal blood flow. In each, the surgeon should assess for a significant difference in magnitude with and without graft occlusion.
Outflow resistance can be measured intraoperatively to predict subsequent graft failure in arterial reconstructions. This technique enables calculation of outflow resistance on the basis of the pressure measured while saline is injected into the distal end of a bypass graft at a known rate. Ascer et al46 found that grafts with an outflow resistance of greater than 1.2 resistant units (pressure in mm Hg divided by flow in milliliters per minute) all experienced failure within 30 days. Other groups, however, have not confirmed this observation and have reported long-term patency in grafts with high outflow resistance, especially when using a vein for a conduit.47,48 Like other indirect methods, this technique does not provide anatomic information sufficient to identify and isolate the cause of the high outflow resistance, and thus, adjunctive anatomic study of the graft and its outflow is necessary to identify potentially correctable problems. In most instances, high outflow resistance is due to severe distal disease that cannot be improved. In a few cases, this technique may lead to identification of distal anastomotic problems or the need for extension of a proximal graft to a more distal site for better outflow. In practice, most vascular surgeons have found these techniques somewhat complicated and cumbersome.
Pathogenesis of Graft Thrombosis
Despite meticulous attention to preoperative planning and to technical perfection in the operating room, revascularizations may be unsuccessful. Depending on the type of arterial reconstruction, between 0.3% and 10% fail in the early postoperative period, regardless of attempts by numerous groups to identify and abrogate factors associated with graft failure.1,4,49 Early graft thrombosis has significant prognostic implications, in that a failed reconstruction is associated with poor clinical outcomes, particularly when performed for limb salvage indications.50–52 Several studies have identified a variety of factors potentially contributing to graft failure, including patient demographics, risk factors, comorbid diseases, conduit characteristics, anesthesia type, adjuvant medical therapy, and technical precision.4,53–57 A study from the Washington Hospital Center and Georgetown University Medical Center prospectively collected and analyzed the National Surgical Quality Improvement Program (NSQIP) database from 1995 to 2003 in an attempt to elucidate risk factors predictive of graft failure in patients who underwent infrainguinal arterial bypass. Multivariate logistic regression revealed that younger age (<60 years), African American race, and crural target vessel were associated with graft failure.4 These findings were confirmed in a 2008 NSQIP study by Singh et al4 as well. Furthermore, other modifiable factors predictive of graft failure have been identified and should be incorporated into treatment paradigms. Giswold et al1 documented that dialysis dependency, a known hypercoagulable state, ongoing smoking, and failure to undergo routine graft duplex surveillance were all independently associated with reversed vein graft occlusion.
The role of hypercoagulability as a cause of graft failure has become increasingly recognized in contemporary practice. As the population ages, it has been hypothesized that the number of arterial reconstructions will increase.1,50,58 This, coupled with the introduction and evolution of myriad percutaneous technologies, will lead to an increasing number of interventions that a typical patient will undergo, thereby compounding the impact of hypercoagulability in light of the inherent multiple previous exposures to heparin.59–61
Long-term follow-up has demonstrated that nearly 50% of vascular reconstructions are subject to some degree of restenosis or eventual occlusion,49,62 and thus, has confirmed the efficacy of aggressive graft surveillance and subsequent endovascular or conventional surgical revision in an attempt to identify and prospectively repair deteriorating grafts and prevent graft occlusion52,62–65
When thrombosis does occur, therapeutic alternatives range from expectant supportive care to thrombectomy, thrombolysis, or placement of an entirely new arterial reconstruction. Optimal results in these most difficult circumstances require rapid decisions by the surgeon regarding the etiology of the graft failure and/or thrombosis, selection of either surgical or endovascular repair, timing of re-intervention, and assessment and modification of complex patient risk factors. This discussion summarizes therapeutic guidelines for the most frequently encountered scenarios after thrombosis of an arterial reconstruction.66
Therapeutic Approach
Documentation of Graft Thrombosis
It is incumbent on the clinician to document graft thrombosis in a timely fashion. It may be as easy as noting the absence of a previously palpable pulse combined with dramatic progression or return of the patient’s ischemic symptoms. In less obvious circumstances, noninvasive testing to measure the ankle-brachial index and the use of duplex ultrasonography to determine patency and the location of the occlusion or restenosis may obviate the need for administration of contrast material. This is particularly germane in light of the fact that the patient will probably require further contrast as part of the definitive revision and/or reconstruction.
Assessment of Neurologic Status
A primary determinant of the necessity for and urgency of aggressive intervention is the patient’s neurologic status at initial evaluation (see Chapters 48 and 160). As the level of neurologic dysfunction increases from dysesthesia to paralysis, the impetus for rapid resolution of the situation grows. Consequently, the time available for lengthy diagnostic or therapeutic measures outside the operating room decreases correspondingly. Considerable clinical judgment is required in these cases because delay in treatment may precipitate irreversible tissue injury and culminate in untoward outcomes, including limb loss or death. If there is no neurologic compromise and the degree of tissue ischemia appears minimal, diagnostic or alternative therapeutic maneuvers, including thrombolysis, can be contemplated. This is predicated on recognition of the delayed therapeutic efficacy associated with thrombolytic therapy. Nevertheless, adequate clinical outcomes with thrombolytic therapy can be anticipated when applied in select clinical circumstances67–69 (see Chapter 36). However, it should be emphasized that secondary patency after treatment of infrainguinal graft thrombosis is generally poor. If the initial reconstruction was performed for ongoing tissue loss, and the ulcer or amputation has subsequently healed, urgent revascularization may not be required. Likewise, graft failure in the setting of recurrent claudication may not warrant urgent re-intervention, particularly if a patient has extensive medical comorbidities.
Anticoagulation
Once the diagnosis of graft thrombosis has been confirmed, immediate anticoagulation becomes imperative to minimize or halt thrombus propagation in the setting of recurrent symptoms. A short interval to permit blood collection for determination of hypercoagulability is permissible. If regional anesthesia is preferred for a surgical attempt at graft salvage, systemic anticoagulation can be delayed until anesthesia is achieved. If immediate surgery is not required or regional anesthesia is contraindicated, either systemic heparinization or anticoagulation with an alternative agent, when appropriate, should be instituted to inhibit ongoing thrombosis.
Etiologic Assessment
Several factors that may contribute to any graft failure should be investigated. If not previously established, the patient’s coagulation status should be determined. As noted earlier, graft thrombosis at any time after placement can be the consequence of a previously unrecognized hypercoagulable state (see Chapter 38).60,70–72 A blood specimen should be collected and sent immediately (before anticoagulation) for measurement of standard coagulation parameters, including platelet count, functional activated protein C resistance, anticardiolipin antibodies, antithrombin III, and protein S. Previous exposure to heparin, with consequent heparin-associated antibodies causing platelet aggregation, should be investigated and routinely considered in current practice scenarios because previous exposure to heparin is becoming more common, perhaps secondary to the advent and application of less invasive percutaneous interventions.60,73 Failure to recognize this insidious condition can lead to poor clinical outcomes and recurrent graft thrombosis.70,74 As the incidence of heparin-induced thrombocytopenia becomes more prevalent, so too will the untoward effects of administration of unfractionated and low-molecular-weight heparin.74 Alternative, less likely causes of hypercoagulable complications leading to thrombosis include increased blood viscosity from dehydration, polycythemia, or sepsis, which can easily be diagnosed by physical examination and routine hematologic screening. Moreover, a recent multivariate analysis from NSQIP implicated thrombocytosis as being independently associated with early graft failure, although this has not been widely studied in other analyses.3 Acute or chronic cardiac decompensation is an uncommon but real cause of failure of arterial revascularization; these conditions can be rapidly detected at initial patient evaluation with electrocardiography or echocardiography, as indicated. Rapid cardiac assessment is also critical in assessing the risk associated with various possible therapeutic options.
The first element fundamental to achieving successful treatment of a failed revascularization is accurate determination of the cause of the thrombosis. As noted previously, the incidence of technical errors as a cause of early graft failure has significantly decreased over the last 40 years. Technical errors now account for 20% or less of thromboses in arterial reconstructions in the early postoperative period, despite the historical supposition to the contrary. Other probable causes include inherent thrombogenicity of the graft conduit, underappreciated or unrecognized patient-related hypercoagulable states, and poor target artery selection with disadvantaged runoff.
In a review of 45 primary infrainguinal vein grafts placed for critical ischemia, Rzucidlo et al28 found the rate of graft failure within 12 months to be 44%. Closer examination of this cohort demonstrated that 87% of the patients had only single-vessel runoff. Nine bypasses (20%) were performed to compromised distal arteries that required thrombectomy before bypass, did not admit a 1-mm dilator, or were confined to the extremity, with only discontinuous tarsal or plantar arteries noted on completion arteriography. Fourteen vein conduits (31%) were considered “disadvantaged” because of compromised selective segments and the requirement for venovenostomy, vein patch angioplasty, or prosthetic interposition. Only two bypasses were found to have retained valve leaflets, which were considered to be the technical errors responsible for graft failure (10% of the graft failures).
A recent analysis of roughly 2500 patients from the Vascular Study Group of Northern New England, a regional quality improvement initiative including 11 community and academic medical centers, revealed several predictors of both early and late graft occlusion. Specifically, both a below-knee target and a tarsal distal target were predictive of diminished graft patency, as was a secondary reconstruction or “redo” revascularization in the early postoperative period. Analysis also determined that diabetes, preoperative tissue loss, greater body mass index (>35 kg/m2), and reconstructions requiring early revision (primary-assisted patency) were predictive of diminished long-term graft patency.75 In another study by Singh et al,4 early age, African American race, and diabetes were all independently associated with early graft failure as well.
Sauvage et al76 suggested that there is a critical minimum threshold velocity for sustaining early graft patency. This threshold velocity is a function of the inflow, the bypass conduit itself, and the condition of the runoff bed or outflow. An alternative approach to better determine the etiology of an unsuccessful arterial revascularization is to examine each of the elements required for effective durable graft patency. The five elements critical for sustained function of an arterial reconstruction are inflow, outflow, conduit, operative technique, and coagulation profile.
For any revascularization to function, blood pressure and blood flow at the origin of the reconstruction must be the same as the systemic parameters. Compromised inflow noted by a gradient between the proximal reconstruction and central systemic arterial pressure demonstrates the existence of a significant proximal arterial stenosis. If uncorrected, suboptimal clinical outcomes and graft durability can be expected.
The quality of outflow for a revascularization can be extremely difficult to characterize. For example, in the setting of lower extremity revascularization, only a few small vessels may constitute the outflow runoff, which at arteriography appears too sparse to support durable graft function (Fig. 45-4). Despite attempts to correlate the radiographic features depicted on angiograms with impending graft thrombosis or failure, no consistent angiographic criteria have been determined to be reliable predictors of graft patency. In the limb shown in Figure 45-4, the runoff bed is based on the lateral plantar artery. Despite conventional wisdom suggesting short-lived patency, this graft has remained patent for more than 5 years. Interestingly, although some data suggest that measurement of outflow bed resistance can predict graft failure, most surgeons find such methods cumbersome and labor intensive, and lacking the level of accuracy needed for a confident recommendation that an attempt at revascularization be changed to a primary amputation.46
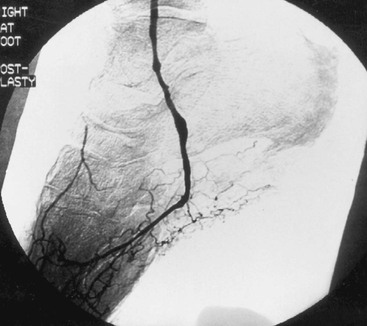
Figure 45-4 Intraoperative completion arteriogram demonstrating sparse runoff from a lateral plantar artery.
A pressure gradient detected at the distal end of an arterial reconstruction can successfully document a significant abnormality within the graft that may lead to early graft thrombosis.77 When decreased pressure is detected at the distal end of a reconstruction, the cause of the compromised pressure must be both identified and rectified if long-term patency is to be achieved.
When considering both the timing and the etiology of failed arterial reconstructions, two temporal categories should be taken into account: early (within 30 days of placement), and late (after 30 days) (Table 45-1). This discussion focuses primarily on outcomes of infrainguinal revascularization, which forms the largest cohort of failed revascularizations.
Table 45-1
Classification of Graft Failure by Time Interval with Associated Causes and Treatments
| Time | Etiology | Treatment |
| Early/mid-term failure | Technical Graft thrombogenicity Low flow Poor runoff |
Thrombectomy Thrombolysis Patch angioplasty |
| Late failure | Graft abnormality Metachronous disease |
Thrombolysis PTA Patch angioplasty Interposition graft |
PTA, Percutaneous transluminal angioplasty.
Early Graft Failure (0-30 Days)
Despite improvement in infrainguinal vein graft revascularization techniques, 5% to 10% of grafts fail within 30 days of placement.49 The time elapsed from initial revascularization is the single most important characteristic aiding in determination of the cause of the graft failure (Fig. 45-5). Early (<30 days) thrombosis of vascular reconstructions has historically been attributed to technical error.6 In a review of the Dartmouth-Hitchcock experience, it was found that technical errors accounted for roughly 25% of early graft failures.7 Since that time, the number of graft failures referable to technical error has declined sharply (10%).66 This improvement can be directly attributed to the routine use of angioscopy, duplex scanning, and DSA to confirm the technical adequacy of the arterial reconstruction. Such confirmation also permits a prospective, qualitative determination of the likelihood that graft failure will occur, and a recognition that it is most likely related to the conduit or the quality of the runoff bed. Additionally, thorough graft interrogation greatly simplifies further therapeutic clinical decision making. If a failed graft was constructed with the only available autogenous vein conduit to the only possible target runoff vessel, and it showed no evidence of an anastomotic or an intrinsic conduit problem at the time of placement, early amputation usually sped the patient toward the best possible outcome. In other words, if such a patient undergoes further attempts at thrombectomy, revision of the anastomosis, replacement of the conduit, or substitution of the runoff are only likely to increase morbidity, mortality, and expense, with little improvement in the chance for limb salvage.50
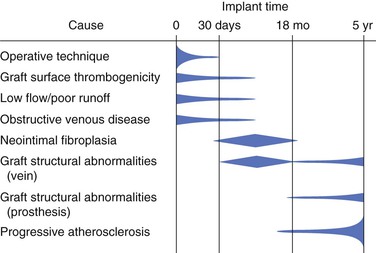
Figure 45-5 Factors contributing to graft occlusion with time. (Modified from Rutherford RB: The prevention and management of graft thrombosis. In: Kempczinski RF, ed. The Ischemic Leg. Chicago, IL: Year Book Medical; 1985.)
After diagnosis of a graft that failed early in the postoperative period, the surgeon must first determine whether to proceed with attempted salvage of the initial bypass graft. Commonly used options include either surgical thrombectomy or thrombolysis. The results of both techniques remain discouraging in the early postoperative time period, irrespective of the conduit used or the procedure performed.
After thrombolysis is attempted in the early postoperative period, the extended patency of thrombosed vein grafts ranges between 15% and 20% at 1 year.69,78,79 In one series, 3% of patients died, 14% experienced bleeding complications requiring transfusion, and 13% developed thromboembolic complications. Despite these associated complications, 75% of the treated limbs had been salvaged at 1 year. Results with thrombolysis of prosthetic grafts are slightly better, although this difference is probably related to the better runoff that usually exists when prosthetic graft material is used as the conduit for the initial bypass procedure.80 Vein graft longevity after thrombolytic intervention has been documented to correlate with the time interval since the vein graft was placed.81 Specifically, a shorter interval to graft failure will have a diminished likelihood for success with thrombolytic therapy, particularly in patients with diabetes. Review of the Dartmouth experience found that no patient with diabetes and a recently placed graft achieved reasonable secondary graft patency with thrombolysis.79 Of patients treated successfully with lytic therapy, 44% ultimately required early amputation, whereas in patients in whom thrombolysis failed, the amputation rate approached 69%.
Like lytic therapy, the results of surgical thrombectomy for early graft failure are also relatively disappointing, even with an adjunctive procedure such as patch angioplasty of the responsible lesion or lesions. Among 36 vein bypasses in which patency was re-established within 1 month after surgery via mechanical thrombectomy, graft stenosis was noted in as many as 39% of arterial reconstructions. Such stenosis is possibly due to rapid degeneration of normal cellular function in the thrombosed vein wall.82 Late bypass revisions, performed in 35% of grafts, did little to improve 1-year patency rates, which approached 38%.83 Robinson et al50 reported a cumulative secondary patency rate of 47% at 1 year. In this series, 26% of the patients required amputation within 1 month of graft thrombectomy, and 41% required amputation by 1 year. The results of surgical thrombectomy were significantly improved if technical problems (e.g., a twist in the graft or a retained valve cusp in an in-situ saphenous vein bypass graft) were identified at exploration. Review of the Dartmouth experience was again similar, with long-term graft patency rates for grafts in which thrombosis occurred because of correctable, underlying technical problems approaching that for grafts without complications.
It should be re-emphasized, however, that the incidence of graft failure referable to technical error has substantially decreased.50 This observation is true not only for vein graft reconstructions but perhaps more so for prosthetic bypasses. Surgical thrombectomy of prosthetic grafts is technically straightforward, significantly less time consuming, and less expensive than thrombolytic therapy. At the time of graft thrombectomy, determination of pressure gradients at the inflow and outflow sites, as well as angiography, can be performed to better ascertain the cause of the graft failure. Surgical correction can subsequently be undertaken at that time to correct the underlying problem.
It is our practice to perform immediate re-exploration in any patient with perioperative graft failure, irrespective of the conduit, when at initial surgery all components of the revascularization were judged technically optimal and in no way disadvantaged. In this setting, thrombolysis poses an additional risk of bleeding, delayed efficacy, and overall poor long-term results. Therefore, in such patients, thrombectomy, anticoagulation, and repair of any underlying potential technical problems yield superior results.84–86 In as many as 50% of patients who undergo re-exploration for early graft failure, no underlying cause of thrombosis is found.6 In such circumstances, when a correctable problem has not been detected, and poor conduit or disadvantaged runoff does not appear to be the cause of thrombosis, a 20-gauge polyethylene catheter can be placed in a proximal side branch of the vein graft after thrombectomy. Through this catheter, nitroglycerin (0.05 µχg/min) and heparin (10 U/min) are infused while the patient is held in the postanesthesia recovery unit or the intensive care unit. This measure is an attempt to counteract potential underlying undocumented inherent thrombogenicity within the revascularization, whether related to runoff spasm or to graft harvest trauma. The catheter is removed at the bedside or in the operating room after 24 to 36 hours of infusion therapy. Although not routinely used, this adjunct has been documented to confer reasonable patency in 8 of 10 patients at a mean follow-up of 17 months.7
If attempts to salvage a thrombosed infrainguinal graft remain unsuccessful in the early postoperative period, two options remain. One management strategy includes expectant therapy and anticoagulation. Frequently, amputation will ensue, although limb loss is not inevitable.87,88 An alternative strategy includes performing a second bypass procedure with the best available autogenous vein, assuming that there is adequate residual conduit length and an additional good quality target vessel. Although seemingly difficult, this aggressive posture often leads to improved results.89 The advent and evolution of stem cell therapy for patients without additional reconstruction options may play a growing role in clinical practice in this circumstance; however, definitive results of clinical trials are still largely pending.90–92
Late Graft Failure (>30 Days)
Many of the aforementioned diagnostic and therapeutic decisions remain germane in the setting of delayed or late graft failure. There are, however, several distinctions. First, technical errors no longer constitute a significant cause of graft pathology. Second, thrombolytic therapy offers greater therapeutic utility in this time interval. Third, a greater magnitude of technical difficulty can be anticipated in the surgical dissection of previously operated vessels or “redo” or “hostile” anatomy.
The time interval since arterial reconstruction can act as a positive predictor of the successful application of thrombolytic therapy. Specifically, the longer a graft has been in place, the greater the likelihood that thrombolytic therapy will confer graft patency. Two factors have been noted in the Dartmouth experience to be critical in predicting success after lytic therapy: graft age (since time of placement) of approximately 1 year or older, and the absence of diabetes. It was noted that although thrombolysis was successful in 15 failed grafts in patients with diabetes, only 1 graft was patent at 1 year. Conversely, in nondiabetic patients with at least 12 months of documented graft patency before thrombosis, 44% of patients achieved documented graft patency at 2 years.
After restoration of graft patency, it has been well documented that further endovascular or surgical therapy may be required in up to 85% of cases to achieve sustained patency. Available therapeutic options in this setting include (1) balloon angioplasty of an intragraft or juxta-anastomotic stenosis, (2) open surgical vein patch angioplasty, and (3) interposition bypass reconstruction. Although the results of such techniques when applied to failed grafts successfully treated with lytic therapy (secondary patency) are not comparable to the documented outcomes observed when applied to maintain the patency of threatened grafts (assisted primary patency), the “threatened” group constitutes a larger cohort and provides a significant body of data from which the surgeon may guide clinical decision making.
In a series reported by Sanchez et al,93 graft patency was achieved in 86% of patients at a 21-month follow-up after surgical revision of vein graft lesions. By comparison, the patency rate for lesions treated with percutaneous angioplasty was 42%.93 This disparity is particularly noteworthy because the surgical group manifested more extensive disease than did the patient population treated percutaneously. Other groups, however, have reported substantially better results for angioplasty of documented underlying graft stenoses.94 Our current policy reserves percutaneous transluminal angioplasty for maintenance of graft patency until operative repair can be undertaken, for the treatment of straightforward, short stenoses that are difficult to approach surgically, or for dilatation of critical stenoses in patients with medical contraindications to anesthesia or surgery. In a patient with a failed prosthetic bypass graft secondary to progressive advanced atherosclerotic disease compromising outflow circulation, percutaneous treatment with angioplasty or other endovascular adjuncts is justified when no reasonable surgical alternatives are available.95,96
Selective application of patch angioplasty versus interposition grafting to address a vein graft lesion noted after successful thrombolysis should best be decided on the basis of lesion location and appearance, availability of adequate autogenous conduit, and surgeon preference. Review of both techniques demonstrates that similar results and outcomes can be expected.40 Although some suggest superior outcomes with a vein interposition graft to treat an intimal hyperplastic lesion, the requisite additional anastomoses carry their own potential associated complications.
The optimal autogenous vein conduit for replacement of a short segment of vein graft is ideally derived from either the remaining ipsilateral saphenous vein or the lesser saphenous vein. Insertion of a segment of contralateral great saphenous or arm vein as a short interposition graft segment should be avoided to preserve these intact longer conduits for alternative or metachronous uses. In patients who have older grafts with acquired diffuse degeneration or in patients with diabetes whose grafts have failed in less than 1 year after implantation, thrombolysis is unlikely to confer any significant secondary graft patency. Rather, a repeat bypass is more likely to offer successful long-term patency. This supposition is predicated, however, on the availability of sufficient quality distal target vessels and adequate autogenous conduit. When these are available, there does appear to be an enhanced sustained benefit associated with repeat bypass when applicable.
However, review of our own practice with failed vein grafts that underwent thrombolysis revealed that repeat bypass was possible in only a low percentage of patients (1 of 44; 2.3%). This fact not only underscores the gravity of a thrombosed graft but also highlights the inherent complexity associated with these cases and the necessity for understanding the elements required for successful bypass both at the time of initial reconstruction and at the time of graft thrombosis.
The choice of conduit constitutes yet another example of the associated clinical decision-making complexity when confronted with a failed graft. Historically, the contralateral great saphenous vein has been thought to be the preferred source of conduit for bypass in the setting of an inadequate ipsilateral great saphenous vein because of either disease or previous use. Appropriate concern that the donor extremity will potentially require future bypass or that the saphenous vein might better be used for coronary artery bypass has traditionally been outweighed by the more immediate need and clinical gravity. However, there is reason to question this conduit selection algorithm. Review of the infrainguinal bypass experience at Dartmouth-Hitchcock Medical Center revealed that 20% of patients required contralateral lower extremity revascularization at a mean of 31 months after initial ipsilateral bypass.97 The intervention rate in our patients, as calculated by life-table analysis, was nearly linear at 6% per year. Among these interventions, 83% were contralateral infrainguinal vein bypasses. Factors predictive of the need for future intervention at the time of initial ipsilateral revascularization included younger age, diabetes, coronary artery disease, and diminished contralateral ankle-brachial index.
By comparison, in patients with only unilateral lower extremity atherosclerosis warranting surgery, the likelihood of future contralateral intervention remained less than 10% during the following 5 years. Based on these findings, it could be argued that the contralateral saphenous vein should be reserved for revascularization of the leg in elderly patients in whom atherosclerosis remains isolated to one limb. In these clinical settings, the surgeon may proceed with confidence that the potential future requirement for use of this vein will be unlikely. Unfortunately, only 8% of patients who require infrainguinal revascularization have isolated unilateral lower extremity atherosclerosis. In actuality, 32% of our patients who underwent infrainguinal bypass had both diabetes and coronary artery disease. Among these patients, 31% required intervention for ischemia of the contralateral leg within 5 years of the initial infrainguinal arterial reconstruction. In patients treated at Dartmouth-Hitchcock, 22% had diabetes, coronary artery disease, and diminished ankle-brachial indices in the contralateral leg. Furthermore, half of these patients required subsequent contralateral arterial intervention during follow-up.
In light of these findings, selection of the most appropriate bypass conduit, when the ipsilateral great saphenous vein is not available, remains a complex decision confronting the vascular surgeon. Arm veins, when deemed acceptable on preoperative duplex evaluation and intraoperative angioscopic examination, remain an appropriate alternative conduit for graft revision or secondary bypass. Although the need for graft revision remains greater in reconstructions in which an arm vein was used as the conduit, documented assisted primary patency rates approach 72% over 5 years.98 Although alternative vein grafts spliced together from the lesser saphenous vein and the remaining ipsilateral saphenous vein have been documented to have equivalent patency to that of an arm vein, the associated added morbidity of distal incisions in an ischemic, previously operated limb should not be underestimated. Use of an arm vein conduit may also minimize the number of venovenostomies required in the scenario of small segments of a viable lower extremity conduit. Regular use of the profunda femoris artery and endarterectomy of the superficial femoral artery to lessen the requisite conduit length for bypass are important surgical adjuncts that should be incorporated into treatment paradigms. Although some advocate repeat infragenicular bypass with either cadaveric vein or, more commonly, prosthetic conduit with or without a distal vein cuff or associated arteriovenous fistula, we seldom use these techniques unless confronted with dire limb salvage circumstances without other therapeutic alternatives.99–102 In a comparative study of alternative graft conduits, Faries et al103,104 documented that autogenous arm vein grafts demonstrated better patency than did prosthetic conduit when applied to below-knee popliteal and tibial configurations. Despite optimistic reports from other centers regarding the use of prosthetic conduit, results at Dartmouth-Hitchcock have not been comparable in similar cases to those of autogenous vein. In this setting, it is our continued preference to use autogenous vein when available and prosthetic grafts as a reasonable alternative to primary amputation.
Selected Key References
Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, Namini H, Hamdan AD, Roddy SP, Belkin M, Berceli SA, DeMasi RJ, Samson RH, Berman SS, PREVENT III Investigators. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–751.
This recent paper documents the results of the PREVENT III multicenter randomized trial in determining whether edifoligide prevents vein graft failure. It is the basis for multiple recent publications that shed light on outcomes of lower extremity revascularization.
Lancaster RT, Conrad MF, Patel VI, Cambria RP, LaMuraglia GM. Predictors of early graft failure after infrainguinal bypass surgery: a risk-adjusted analysis from the NSQIP. Eur J Vasc Endovasc Surg. 2012;43(5):549–555.
This study is a recent National Surgical Quality Improvement Program analysis of a large cohort of patients that identifies predictors associated with early graft failure.
Scali ST, Beck AW, Nolan BW, Stone DH, De Martino RR, Chang CK, Rzucidlo EM, Walsh DB. Completion duplex ultrasound predicts early graft thrombosis after crural bypass in patients with critical limb ischemia. J Vasc Surg. 2011;54(4):1006–1010.
This study highlights the value of completion duplex ultrasonography as a useful intraoperative tool for identification of predictors of early graft failure.
Schanzer A, Hevelone N, Owens CD, Belkin M, Bandyk DF, Clowes AW, Moneta GL, Conte MS. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg. 2007;46:1180–1190.
This recent study uses the PREVENT III multicenter trial to draw conclusions regarding autogenous vein graft failure.
Singh N, Sidawy AN, DeZee KJ, Neville RF, Akbari C, Henderson W. Factors associated with early failure of infrainguinal lower extremity arterial bypass. J Vasc Surg. 2008;47:556–561.
This study documents independent predictors of early graft failure in a large cohort of bypass patients using the NSQIP.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Giswold ME, et al. Modifiable patient factors are associated with reverse vein graft occlusion in the era of duplex scan surveillance. J Vasc Surg. 2003;37(1):47–53.
2. Watson HR, et al. Relationship of femorodistal bypass patency to clinical outcome. Iloprost Bypass International Study Group. Eur J Vasc Endovasc Surg. 1999;17(1):77–83.
3. Lancaster RT, et al. Predictors of early graft failure after infrainguinal bypass surgery: a risk-adjusted analysis from the NSQIP. Eur J Vasc Endovasc Surg. 2012;43(5):549–555.
4. Singh N, et al. Factors associated with early failure of infrainguinal lower extremity arterial bypass. J Vasc Surg. 2008;47(3):556–561.
5. Goodney PP, et al. Risk Factors for Graft Failure and Amputation After Lower Extremity Bypass in Patients with Critical Limb Ischemia. Vascular Study Group of Northern New England. ME: Portland; 2008.
6. Stept LL, et al. Technical defects as a cause of early graft failure after femorodistal bypass. Arch Surg. 1987;122(5):599–604.
7. Walsh DB, et al. Intragraft drug infusion as an adjunct to balloon catheter thrombectomy for salvage of thrombosed infragenicular vein grafts: a preliminary report. J Vasc Surg. 1990;11(6):753–759.
8. Wolfle KD, et al. [Follow-up of infra-inguinal bypass operations: value of the peak systolic velocity and arm-ankle index for evaluation of femorodistal reconstructions.]. Vasa. 1994;23(4):349–356.
9. Mills JL, et al. The characteristics and anatomic distribution of lesions that cause reversed vein graft failure: a five-year prospective study. J Vasc Surg. 1993;17(1):195–204.
10. O’Mara CS, et al. Recognition and surgical management of patent but hemodynamically failed arterial grafts. Ann Surg. 1981;193(4):467–476.
11. Turnipseed WD, et al. Postoperative surveillance. An effective means of detecting correctable lesions that threaten graft patency. Arch Surg. 1985;120(3):324–328.
12. Veith FJ, et al. Diagnosis and management of failing lower extremity arterial reconstructions prior to graft occlusion. J Cardiovasc Surg (Torino). 1984;25(5):381–384.
13. Beard JD, et al. Operative assessment of femorodistal bypass grafts using a new Doppler flowmeter. Br J Surg. 1989;76(9):925–928.
14. Schwartz LB, et al. Validation of a new and specific intraoperative measurement of vein graft resistance. J Vasc Surg. 1997;25(6):1033–1041.
15. Liebman PR, et al. Intraoperative arteriography in femoropopliteal and femorotibial bypass grafts. Arch Surg. 1981;116(8):1019–1021.
16. Courbier R, et al. Detecting complications of direct arterial surgery: the role of intraoperative arteriography. Arch Surg. 1977;112(9):1115–1118.
17. Bandyk DF, et al. Intraoperative duplex scanning of arterial reconstructions: fate of repaired and unrepaired defects. J Vasc Surg. 1994;20(3):426–432.
18. Bredenberg CE, et al. Operative angiography by intraarterial digital subtraction angiography: a new technique for quality control of carotid endarterectomy. J Vasc Surg. 1989;9(4):530–534.
19. Mills JL, et al. Contribution of routine intraoperative completion arteriography to early infrainguinal bypass patency. Am J Surg. 1992;164(5):506–510.
20. Bandyk DF, et al. Detection of technical error during arterial surgery by pulsed Doppler spectral analysis. Arch Surg. 1984;119(4):421–428.
21. Coelho JC, et al. An experimental evaluation of arteriography and imaging ultrasonography in detecting arterial defects at operation. J Surg Res. 1982;32(2):130–137.
22. Kresowik TF, et al. Intraoperative B-mode ultrasonography is a useful adjunct to peripheral arterial reconstruction. Ann Vasc Surg. 1993;7(1):33–38.
23. Gaunt ME, et al. A comparison of quality control methods applied to carotid endarterectomy. Eur J Vasc Endovasc Surg. 1996;11(1):4–11.
24. Machi J, et al. Operative color Doppler imaging for vascular surgery. J Ultrasound Med. 1992;11(2):65–71.
25. Cull DL, et al. Duplex scanning for the intraoperative assessment of infrainguinal arterial reconstruction: a useful tool? Ann Vasc Surg. 1992;6(1):20–24.
26. Jackson MR, et al. The fate of residual defects following carotid endarterectomy detected by early postoperative duplex ultrasound. Am J Surg. 1996;172(2):184–187.
27. Yu A, et al. The role of intra-operative duplex imaging in arterial reconstructions. Am J Surg. 1996;171(5):500–501.
28. Rzucidlo EM, et al. Prediction of early graft failure with intraoperative completion duplex ultrasound scan. J Vasc Surg. 2002;36(5):975–981.
29. Scali ST, et al. Completion duplex ultrasound predicts early graft thrombosis after crural bypass in patients with critical limb ischemia. J Vasc Surg. 2011;54(4):1006–1010.
30. Miller A, et al. Continued experience with intraoperative angioscopy for monitoring infrainguinal bypass grafting. Surgery. 1991;109(3 Pt 1):286–293.
31. Wilson YG. Vein quality in infrainguinal revascularisation: assessment by angioscopy and histology. Ann R Coll Surg Engl. 1998;80(1):3–15.
32. Marcaccio EJ, et al. Angioscopically directed interventions improve arm vein bypass grafts. J Vasc Surg. 1993;17(6):994–1002.
33. Sales CM, et al. Saphenous vein angioscopy: a valuable method to detect unsuspected venous disease. J Vasc Surg. 1993;18(2):198–204.
34. Stonebridge PA, et al. Angioscopy of arm vein infrainguinal bypass grafts. Ann Vasc Surg. 1991;5(2):170–175.
35. Lee G, et al. Hazards of angioscopic examination: documentation of damage to the arterial intima. Am Heart J. 1988;116(6 Pt 1):1530–1536.
36. Hashizume M, et al. Intimal response of saphenous vein to intraluminal trauma by stimulated angioscopic insertion. J Vasc Surg. 1987;5(6):862–868.
37. Thorne J, et al. Intraoperative angioscopy may improve the outcome of in situ saphenous vein bypass grafting: a prospective study. J Vasc Surg. 2002;35(4):759–765.
38. Neville RF Jr, et al. Endovascular management of arterial intimal defects: an experimental comparison by arteriography, angioscopy, and intravascular ultrasonography. J Vasc Surg. 1991;13(4):496–502.
39. Siegel RJ, et al. Histopathologic validation of angioscopy and intravascular ultrasound. Circulation. 1991;84(1):109–117.
40. Tabbara MR, et al. Sequential intraluminal ultrasound evaluation of balloon angioplasty of an iliac artery lesion. Ann Vasc Surg. 1992;6(2):179–184.
41. Song TK, et al. Endograft exclusion of acute and chronic descending thoracic aortic dissections. J Vasc Surg. 2006;43(2):247–258.
42. Koschyk DH, et al. How to guide stent-graft implantation in type B aortic dissection? Comparison of angiography, transesophageal echocardiography, and intravascular ultrasound. Circulation. 2005;112(9 Suppl):I260–I264.
43. Saket RR, et al. Novel intravascular ultrasound-guided method to create transintimal arterial communications: initial experience in peripheral occlusive disease and aortic dissection. J Endovasc Ther. 2004;11(3):274–280.
44. Neville RF, et al. Intravascular ultrasonography: validation studies and preliminary intraoperative observations. J Vasc Surg. 1991;13(2):274–282.
45. Diethrich EB. Endovascular treatment of abdominal aortic occlusive disease: the impact of stents and intravascular ultrasound imaging. Euro J Vasc Surg. 1993;7(3):228–236.
46. Ascer E, et al. Components of outflow resistance and their correlation with graft patency in lower extremity arterial reconstructions. J Vasc Surg. 1984;1(6):817–828.
47. Peterkin GA, et al. Runoff resistance and early graft failure in infrainguinal bypass surgery. Arch Surg. 1988;123(10):1199–1201.
48. Wolfle KD, et al. The importance of graft blood flow and peripheral outflow resistance for early patency in infrainguinal arterial reconstructions. Vasa. 1999;28(1):34–41.
50. Robinson KD, et al. Long-term outcome after early infrainguinal graft failure. J Vasc Surg. 1997;26(3):425–437.
51. Seeger JM, et al. Potential predictors of outcome in patients with tissue loss who undergo infrainguinal vein bypass grafting. J Vasc Surg. 1999;30(3):427–435.
52. Baldwin ZK, et al. Limb salvage after infrainguinal bypass graft failure. J Vasc Surg. 2004;39(5):951–957.
53. Singh N, et al. The effects of the type of anesthesia on outcomes of lower extremity infrainguinal bypass. J Vasc Surg. 2006;44(5):964–968.
54. Gibson KD, et al. Identification of factors predictive of lower extremity vein graft thrombosis. J Vasc Surg. 2001;33(1):24–31.
55. Gentile AT, et al. Identification of predictors for lower extremity vein graft stenosis. Am J Surg. 1997;174(2):218–221.
56. Schanzer A, et al. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg. 2007;46(6):1180–1190.
57. Schanzer A, et al. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg. 2008;47(4):774–781.
58. Bartlett ST, et al. The reoperative potential of infrainguinal bypass: long-term limb and patient survival. J Vasc Surg. 1987;5(1):170–179.
59. Assadian A, et al. Safety and efficacy of intravenous enoxaparin for carotid endarterectomy: a prospective randomized pilot trial. J Vasc Surg. 2008;47(3):537–542.
60. Curi MA, et al. Long-term outcome of infrainguinal bypass grafting in patients with serologically proven hypercoagulability. J Vasc Surg. 2003;37(2):301–306.
61. Donaldson MC, et al. Screening for hypercoagulable states in vascular surgical practice: a preliminary study. J Vasc Surg. 1990;11(6):825–831.
62. Desiron Q, et al. Comparison of results of carotid artery surgery after either direct closure or use of a vein patch. Cardiovasc Surg. 1997;5(3):295–303.
63. Berceli SA, et al. Surgical and endovascular revision of infrainguinal vein bypass grafts: analysis of midterm outcomes from the PREVENT III trial. J Vasc Surg. 2007;46(6):1173–1179.
64. Bandyk DF. Surveillance after lower extremity arterial bypass. Perspect Vasc Surg Endovasc Ther. 2007;19(4):376–383.
65. Brumberg RS, et al. The relative importance of graft surveillance and warfarin therapy in infrainguinal prosthetic bypass failure. J Vasc Surg. 2007;46(6):1160–1166.
66. Walsh DB. Management of the thrombosed infrainguinal vein graft. Advances in Vascular Surgery. Mosby Year Book; 1998:181. Whittemore AD, et al. Advances in Vascular Surgery. Vol 6.
67. Greenberg RK, et al. A multi-modal approach to the management of bypass graft failure. Vasc Med. 1998;3(3):215–220.
68. Madhavan P, et al. Low dose intraarterial thrombolysis with tissue plasminogen activator: does it deliver as promised? Vasc Endovascular Surg. 2002;36(5):351–356.
69. Nehler MR, et al. Outcome of catheter-directed thrombolysis for lower extremity arterial bypass occlusion. J Vasc Surg. 2003;37(1):72–78.
70. Donaldson MC, et al. Impact of activated protein C resistance on general vascular surgical patients. J Vasc Surg. 1997;25(6):1054–1060.
71. Fisher CM, et al. Prevalence and outcome of activated protein C resistance in patients after peripheral arterial bypass grafts. Cardiovasc Surg. 1999;7(5):519–525.
72. Nielsen TG, et al. Antibodies to cardiolipin may increase the risk of failure of peripheral vein bypasses. Eur J Vasc Endovasc Surg. 1997;14(3):177–184.
73. Prechel M, et al. The laboratory diagnosis and clinical management of patients with heparin-induced thrombocytopenia: an update. Semin Thromb Hemost. 2008;34(1):86–96.
74. Bartholomew JR. Heparin-induced thrombocytopenia: 2008 update. Curr Treat Options Cardiovasc Med. 2008;10(2):117–127.
75. Goodney PP, et al. Factors associated with death 1 year after lower extremity bypass in Northern New England. J Vasc Surg. 2010;51(1):71–78.
76. Sauvage LR, et al. Future directions in the development of arterial prostheses for small and medium caliber arteries. Surg Clin North Am. 1974;54(1):213–228.
77. Schmitt DD, et al. Early patency of in situ saphenous vein bypasses as determined by intraoperative velocity waveform analysis. Ann Vasc Surg. 1990;4(3):270–275.
78. Berkowitz HD, et al. Occluded infrainguinal grafts: when to choose lytic therapy versus a new bypass graft. Am J Surg. 1995;170(2):136–139.
79. Nackman GB, et al. Thrombolysis of occluded infrainguinal vein grafts: predictors of outcome. J Vasc Surg. 1997;25(6):1023–1031.
80. Rickard MJ, et al. Limitations of intra-arterial thrombolysis. Cardiovasc Surg. 1997;5(6):634–640.
81. Schwierz T, et al. Indications for directed thrombolysis or new bypass in treatment of occlusion of lower extremity arterial bypass reconstruction. Ann Vasc Surg. 2001;15(6):644–652.
82. Kawai S, et al. Biologic degeneration of vein grafts after thrombotic occlusion: thrombectomy within 3 days results in better indices of viability. J Vasc Surg. 2003;38(2):305–312.
83. Nielsen TG, et al. Early vein bypass thrombectomy is associated with an increased risk of graft related stenoses. Eur J Vasc Endovasc Surg. 1997;13(2):134–138.
84. McMillan WD, et al. Perioperative low molecular weight heparin for infrageniculate bypass. J Vasc Surg. 1997;25(5):796–801.
85. Rutherford RB, et al. The efficacy of dextran 40 in preventing early postoperative thrombosis following difficult lower extremity bypass. J Vasc Surg. 1984;1(6):765–773.
86. Sarac TP, et al. Warfarin improves the outcome of infrainguinal vein bypass grafting at high risk for failure. J Vasc Surg. 1998;28(3):446–457.
87. Rivers SP, et al. Successful conservative therapy of severe limb-threatening ischemia: the value of nonsympathectomy. Surgery. 1986;99(6):759–762.
88. Sullivan TR Jr, et al. Clinical results of common strategies used to revise infrainguinal vein grafts. J Vasc Surg. 1996;24(6):909–917.
89. De Frang RD, et al. Repeat leg bypass after multiple prior bypass failures. J Vasc Surg. 1994;19(2):268–276.
90. Powell RJ. Update on clinical trials evaluating the effect of biologic therapy in patients with critical limb ischemia. J Vasc Surg. 2012;56(1):264–266.
91. Powell RJ, et al. Cellular therapy with Ixmyelocel-T to treat critical limb ischemia: the randomized, double-blind, placebo-controlled RESTORE-CLI trial. Mol Ther. 2012;20(6):1280–1286.
92. Benoit E, et al. Improved amputation-free survival in unreconstructable critical limb ischemia and its implications for clinical trial design and quality measurement. J Vasc Surg. 2012;55(3):781–789.
93. Sanchez LA, et al. Is percutaneous balloon angioplasty appropriate in the treatment of graft and anastomotic lesions responsible for failing vein bypasses? Am J Surg. 1994;168(2):97–101.
94. Houghton AD, et al. Percutaneous angioplasty for infrainguinal graft-related stenoses. Eur J Vasc Endovasc Surg. 1997;14(5):380–385.
95. Gray BH, et al. Limitations of percutaneous transluminal angioplasty with stenting for femoropopliteal arterial occlusive disease. Semin Vasc Surg. 1997;10(1):8–16.
96. Stanley B, et al. Efficacy of balloon angioplasty of the superficial femoral artery and popliteal artery in the relief of leg ischemia. J Vasc Surg. 1996;23(4):679–685.
97. Tarry WC, et al. Fate of the contralateral leg after infrainguinal bypass. J Vasc Surg. 1998;27(6):1039–1047.
98. Gentile AT, et al. Results of bypass to the popliteal and tibial arteries with alternative sources of autogenous vein. J Vasc Surg. 1996;23(2):272–279.
100. Farber A, et al. Cryopreserved saphenous vein allografts in infrainguinal revascularization: analysis of 240 grafts. J Vasc Surg. 2003;38(1):15–21.
101. Parsons RE, et al. Comparison of endovascular and conventional vascular prostheses in an experimental infection model. J Vasc Surg. 1996;24(6):920–925.
102. Raptis S, et al. Influence of a vein cuff on polytetrafluoroethylene grafts for primary femoropopliteal bypass. Br J Surg. 1995;82(4):487–491.
103. Faries PL, et al. A comparative study of alternative conduits for lower extremity revascularization: all-autogenous conduit versus prosthetic grafts. J Vasc Surg. 2000;32(6):1080–1090.
104. Faries PL, et al. Arm vein conduit is superior to composite prosthetic-autogenous grafts in lower extremity revascularization. J Vasc Surg. 2000;31(6):1119–1127.