Local and regional anaesthesia
Conduction of the nociceptive pain signal
Pharmacology of local anaesthetics
Classification of local anaesthetics
Benefits of local anaesthetics
Disadvantages and limitations of local anaesthesia
Nursing implications of procedures involving local anaesthetics
Introduction
This chapter will describe local and regional anaesthetics, the applicable aspects of the physiology of the conduction of the pain signal, and the pharmacology of the drugs used. (Other aspects of pain and pain management in the ED situation are discussed in Chapter 25.) This will be followed by the consideration of the advantages and disadvantages associated with local anaesthetics, a review of the various methods of use, and an outline of the principles of managing patients undergoing these procedures in the ED.
Conduction of the nociceptive pain signal
Local anaesthetics address the nociceptive pain signal in the first two of stages of nociception, which is the normal method of processing the sensation along the neuron. Nociceptive pain has been classified as tissue damage that triggers the release of inflammatory mediators that bathe and sensitize the nociceptors (Butcher 2004). The neuron has a range of receptors including the nociceptors for the sensation of pain, named from the Latin ‘noci’ to injure or harm. The process of nociception contains four stages, the first of which is transduction followed by transmission or conduction, perception, and modulation (National Pharmaceutical Council and Joint Commission on Accreditation of Healthcare Organizations 2001).
Before the process of transmitting the nerve impulse begins, the neuron is in the resting phase in which all the active ion channels and almost all voltage-gated sodium (Na+) and potassium (K+) gates are closed, with the exception of the leakage channels allowing some potassium ions to diffuse out and smaller quantities of sodium ions to diffuse in (the movement ratio is 3 Na+ out of the neuron to every 2 K+ in; Clark 2008). In this phase the neuron interior is negatively charged at −70 mV.
Transmission happens when this depolarization travels the length of the nociception fibres in milliseconds, causing the impulse to be experienced as a conscious sensation in the brain (McCaffery & Passero 1999). For this to transpire, a critical level of depolarization of the neuron membrane must be reached; this is known as the threshold potential. This is reached when the ion flow becomes self-generating and the membrane potential alters from −70 mV to a maximum +30 mV, with the process slowing at 0 mV as the membrane becomes less permeable to sodium ions and the electrical gradient begins to prevent the sodium ions from entering freely.
Pharmacology of local anaesthetics
Local anaesthetics act by blocking the conduction of the nerve impulses when applied either locally to the nerve fibre endings or regionally to the nerve trunks that convey the impulses from the affected area. They do this by binding to the sodium channels in the membrane to prevent changes to membrane permeability, creating a membrane-stabilizing effect that blocks the depolarization of the membrane (Fatovich & Brown 2004). The smaller the diameter of the neuron, the more sensitive it is to the effects of local anaesthetic. The clinical consequences following injection of a local anaesthetic are initial loss of autonomic function, followed by loss of pain sensation, then touch and pressure sensation, and finally motor function; recovery occurs in the reverse order (Rosenberg 2000).
All local anaesthetics, with the exception of cocaine, interfere with autonomic function, which means that they cause local or regional vasodilatation depending on the technique used. This vasodilatation can lead to rapid dispersal, thereby minimizing the duration of the local anaesthetic. To address this effect a vasoconstrictor such as adrenaline (epinephrine) can be added, which can delay absorption, prolonging anaesthesia and preventing flooding of the circulation (Fatovich & Brown 2004). For example, the normal duration of action for lignocaine of 15–45 minutes when infiltrated subcutaneously can be increased by 50 % by the addition of adrenaline (De Jong 2001). If adrenaline is used, the total dose should not exceed 0.5 mg/ml or the concentration 1:200 000 (British National Formulary 2011). The duration of anaesthetic action is dependent upon:
• the concentration and dose of local anaesthetic
• the mode of drug administration
• the rate of diffusion from the injection site to the axon.
This latter point is important as it emphasizes the need to allow sufficient time for the local anaesthetics to work before commencing the procedure.
Classification of local anaesthetics
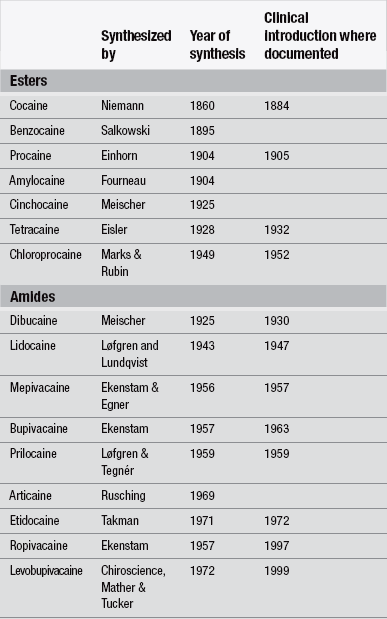
The oldest recorded method for local or regional analgesia for medical interventions is the use of cooling or freezing by Hippocrates (460−377 BC) (Trescot 2003). Ether spray was introduced for topical anaesthesia (Richardson 1866) followed by ethylchloride spray in 1891 which is still in use today alongside other variations (Trescot 2003). The first chemical local anaesthetic agent to be isolated was cocaine, isolated from the coco leaf by German chemist Albert Nieman in 1860; the first recorded clinical use was topically on a cornea by Köller in 1884 (Brown & Allen 2009, Walsh & Walsh 2011). Since then, the field of local and regional anaesthesia has advanced with the synthesis of other agents, particularly procaine in 1904 and lignocaine in 1947. These agents and the establishment of increasingly sophisticated techniques have led to a growth in the use of local anaesthetics to achieve pain-free procedures. Brown & Allen (2009) provide a detailed chronology of the development of local and regional anaesthesia and agents (Table 26.1).
Types of anaesthesia
Non-coolant local anaesthetics can be divided into two chemical groupings: ester caines and amide caines. Esters are readily metabolized in the plasma by pseudocholinesterase, and then by the liver to para-aminobenzoic acid. Amides are slowly metabolized by the liver and can therefore only be used in those patients whose liver function is uncompromised. The esters are less stable and more readily metabolized than the amides (Titcomb 2003). Table 26.1 lists some of these agents according to their chemical group.
The ideal local anaesthetic should be non-irritant, non-toxic, have a rapid onset of effect, have an action duration that is appropriate to the procedure, and leave no local after-effects. Butterworth (2009) lists three classifications for local anaesthetic agents for humans as:
Uses of local anaesthetics
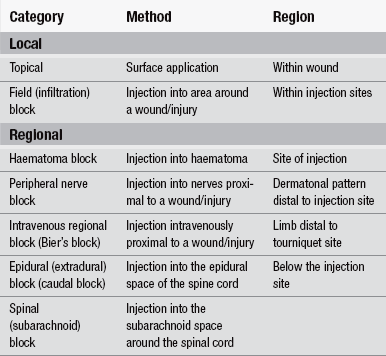
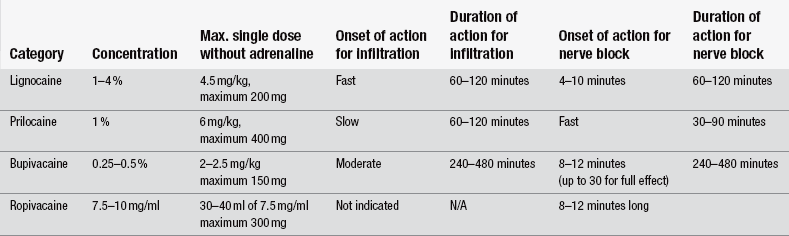
The pharmacological properties of four commonly used local anaesthetic agents for field blocks, haematoma blocks and peripheral nerve blocks are outlined in Table 26.2. Note: both generic and some brand names have been used, including those related to the combination preparations known by acronyms and based upon their contents.
Topical or surface application
This involves direct application of the local anaesthetic to mucous membranes, intact skin, or open wounds, such as grazes. Suitable sites for topical anaesthesia include the cornea, conjunctiva, upper airway, epidermal and dermal layers of the skin, and urethra. Local anaesthetics for this purpose come in various forms, including refrigerant sprays, aerosol sprays, liquid solutions, creams, jellies, balms and ointments. One local anaesthetic used as a topical agent is cocaine. The initial formulation of tetracaine, adrenaline and cocaine (TAC) gained widespread acceptance in North America and largely supplanted infiltration anaesthesia (Grant & Hoffman 1992). Unlike other local anaesthetics, cocaine potentiates the action of the sympathetic nervous system, thus causing local vasoconstriction. It is highly effective on very vascular areas, such as the nasal membranes. It also causes dilatation of the pupil when used on the cornea. Cocaine, however, has powerful central effects, making it too dangerous to inject. It also has complex administrative and financial issues resulting from it being a drug of addiction, which has led to the development of cocaine-free alternatives in the last decade as seen in Table 26.3 (Eidelman et al. 2005).
Table 26.3
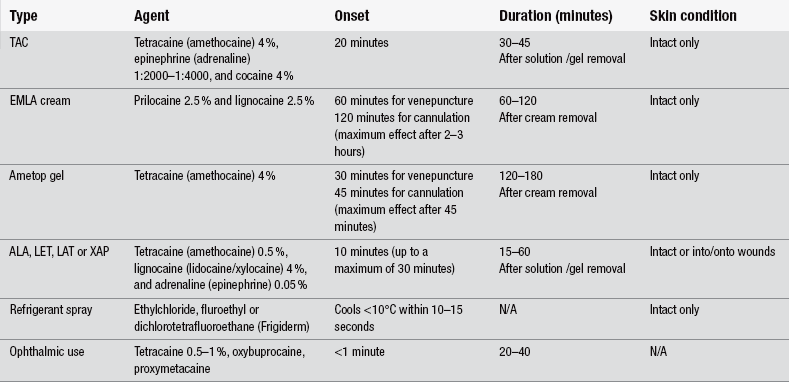
(Sources: McCaffery & Passero 1999, Lander & Weltman 2002, Butcher 2004, Dunn et al. 2003.)
These alternatives include creams and jellies containing a combination of lignocaine and prilocaine that can be used prior to venepuncture or cannulation in children. One example of such a topical anaesthetic is the Eutectic Mixture of Local Anesthetics (EMLA©) cream. The ‘eutectic’ property refers to the liquefaction of the constituents (McCaffery & Passero 1999). EMLA cream is applied only to intact skin (British National Formulary 2011) for cutaneous anaesthesia. To do this a thick layer is applied under an occlusive dressing and left for 60–120 minutes, dependent on the indication for its use. Numbing of the skin should occur one hour after application, reaching a maximum at two to three hours, and one hour for children less than three months old. The effect lasts for one to two hours after removal of the cream (Lander & Weltman 2002). However, this time lag sometimes makes it impractical for use in the ED especially when a painful activity needs to be performed before that time. An alternative option is amethocaine gel (Ametop©), which is similarly indicated for local topical anaesthesia prior to venepuncture or venous cannulation. As with EMLA, this gel is applied to the site required and sealed with an occlusive dressing; however, it is ready for the procedure to occur after 30 minutes for venepuncture and after 45 minutes for venous cannulation as it has a faster action. Like EMLA, it should only be applied to intact skin as it is rapidly absorbed and it should not be applied to inflamed, traumatized, highly vascular surfaces, or mucous membranes (British National Formulary 2011).
A further option is a solution or gel containing tetracaine (amethocaine/pontacaine), lignocaine (lidocaine/xylocaine) and adrenaline (epinephrine), known as ALA, LET, LAT or XAP (according to country of use), which can be made by the hospital pharmacy (McCaffery & Passero 1999). It is a faster-acting option and can be applied directly into or onto a wound in conjunction with the application of a piece of gauze soaked in the solution and left on the wound with a clear non-absorbent dressing used to hold it in place for 10 minutes (up to a maximum of 30 minutes). This can provide sufficient anaesthesia lasting up to 15 minutes to enable wound cleansing and closure with sutures if required (McCaffery & Passero 1999). Care should be taken to avoid the mucous membranes to avoid systemic absorption and possible toxicity, and the eyes should be avoided also due to the risk of causing corneal abrasions (McCaffery & Passero 1999). The benefit of these topical treatments is that they are painless to use.
Other uses of anaesthesia include those for ophthalmic procedures in the ED such as irrigation or examination, in which case tetracaine is often chosen due to its profound effect although oxybuprocaine and proxymetacaine are options recommended by the College of Optometrists since they are less irritating than tetracaine (Titcomb 2003). Tetracaine, oxybuprocaine and proxymetacaine have a similar duration of action, approximately 30 minutes, that of lignocaine is somewhat longer (45 minutes).
An alternative to chemical topical agents is the use of cryoanaesthesia via a refrigerant spray, in which the rapid cooling of the skin causes superficial anaesthesia suitable for immediate pinpoint pain relief (Brown 2004). Examples of these sprays are ethylchloride, fluroethyl and dichlorotetrafluoroethane (Frigiderm®). Refrigerant sprays work by extremely rapid cooling of the skin (<10°C within 10–15 seconds of application); however, their duration of action is briefer than that provided by EMLA, amethocaine gel or ALA/LET/LAT/XAP and this sudden cooling can be distressing and children particularly may object to it (McCaffery & Passero 1999). Refrigerant sprays should be used with caution as prolonged application can cause frostbite.
Local infiltration and field block
Local infiltration and field block are ideal methods of anaesthesia for the suturing and extensive cleansing of minor wounds or small abscesses (although local anaesthetic may be less effective when used for infiltrating an abscess due to the altered pH of the infected tissue/fluid). It is recommended that infiltration is not used for inflamed or infected tissues (British National Formulary 2011). It is also imperative to avoid introducing lignocaine directly into a vein due to its cardiac arrhythmia properties.
The difference between these two methods of drug administration is that local infiltration involves the injection of the anaesthetic drug directly into the subcutaneous tissue involved, while a field block involves injecting the chosen drug into the surrounding area for treatment (Chan et al. 2002). The latter has the advantage of avoiding distortion of the wound edges. Care needs to be exercised to avoid injection of large volumes of local anaesthetic as this can lead to localized oedema, causing distortion of wound edges and tissue hypoxia, which makes the apposition and healing of the wound edges difficult.
While these methods provide excellent levels of pain relief, the act of infiltration or injection can be painful. Warming the vial between the hands prior to drawing up and use can relieve this discomfort to some extent. Buffering the lignocaine with sodium bicarbonate 8.4 % has been seen to significantly decrease the pain perceived by the patient on infiltration by raising the pH of the solution (Achar & Kundu 2002, Chan et al. 2002). However, this latter option significantly decreases the solution’s shelf-life, limiting the efficacy of the anaesthetic to one week post combination with the sodium bicarbonate, and it is recommended to only mix the two drugs together immediately prior to the procedure. Lignocaine 1 % or 2 % with or without adrenaline (dependent on the wound location) is the drug of choice for these methods of administration due to its rapid onset. Toxicity can be avoided if the dose of lignocaine does not exceed 3 mg/kg body weight, which is approximately 20 ml of a 1 % solution in an adult, increased to a maximum of 500 mg if the lignocaine solution contains adrenaline. Table 26.4 details the pharmacological properties of the drugs involved in these blocks.
Haematoma blocks
Haematoma blocks are principally used for the reduction of wrist fractures. They involve injecting the anaesthetic drug directly into the haematoma surrounding the fracture (Chan et al. 2002). The benefit of this type of block is that it is easy and quick to give, and is sufficiently effective to enable the reduction and immobilization of the fracture; however, a peripheral nerve block is more selective in that the local anaesthetic drug is injected around nerve trunks supplying the injury/operation site.
Peripheral nerve blocks
Peripheral nerve blocks are occasionally mistakenly referred to as haematoma blocks; however, a peripheral nerve block affects a larger area and in a dermatonal pattern (McCaffery & Passero 1999). Various peripheral nerve blocks using local anaesthetic agents have been described in order to reduce pain and facilitate treatments; these nerve blocks are used to administer an anaesthetic agent locally to nerves serving particular parts of the body. The techniques for administering peripheral nerve blocks are also more difficult than that of haematoma blocks. They provide excellent analgesia over limited fields with minimal systemic effects, and are generally easy to perform, inexpensive and safe (Summers 2011). They also act as muscle relaxants since the motor fibres are also blocked. Care should be taken not to exceed maximum local anaesthetic doses (shown in Table 26.4).
The most frequent uses of regional nerve blocks in the ED setting are ring blocks to achieve anaesthesia of a digit and femoral blocks for pain relief of a hip or femur fracture, although others are used according to the skill of the clinician. A ring block involves injection into the base of the finger via the interdigital web; bilateral infiltration is required to ensure blockage of all four digital nerves. Brachial plexus or axillary blocks can be used to achieve anaesthesia of the forearm and hand, for which the local anaesthetic is injected into the neurovascular sheath surrounding the axillary artery and the median, radial and ulnar nerves. A number of options are available for femoral blocks, which differ according to the experience and skill of the practitioner and include the lateral cutaneous nerve of thigh, subcostal nerve, femoral nerve, sciatic nerve, triple nerve block (femoral, obturator and sciatic nerves), psoas (lumbar plexus) block, or continuous epidural block (Parker et al. 2002). The triple nerve femoral block using a three-in-one injection method has been found to effectively reduce pain and the need for opiates, and can be given by all grades of medical staff trained in the procedure (Fletcher et al. 2003). The main advantage of the triple nerve block is it requires fewer injections and avoids the pain involved in injection directly into already sensitive tissue.
Potential complications, as with all nerve blocks, relate to the possibility of damage to surrounding structures (Rivellini 1993). It is imperative that adrenaline is not added to the local anaesthetic solution for any procedure where it may compromise the flow of blood to an area that has an end-arteriole blood supply, as the adrenaline leads to vasoconstriction and can cause the occlusion of the supplying arteries: these include fingers or toes, skin flaps with marginal viability, penis and tips of the ears, or the nose-tip (McCaffery & Passero 1999, Achar & Kundu 2002). It should also be noted that the onset and duration of action of the various drugs differ according to the site of injection: for example, there has been seen a difference of up to 25 minutes for onset time and 6 hours difference of duration time of bupivacaine when comparing an intercostal nerve block with a brachial plexus block (Chan et al. 2002) (see Table 26.4).
Intravenous regional anaesthesia (Bier’s block)
The technique of intravenous regional anaesthesia (IVRA) was first described by Karl Bier in 1908 and the technique later became known as a Bier’s block (Checketts 2010). It is used primarily to achieve anaesthesia below the elbow, although it can also be used for procedures below the knee and in ED the main uses of this technique are for the manipulation of Colles’ and Smith fractures. The use of IVRA has been found to provide better relief from pain and it facilitates better limb reduction with a reduced risk of re-dislocation or the need for re-plastering in comparison to the use of haematoma blocks (Handoll 2005).
IVRA can be a useful option in the ED, although it requires training for safe use. Contraindications for the use of IVRA are outlined in Box 26.1.
In preparation for an IVRA procedure, the patient’s blood pressure, ECG and weight must be recorded. A cuffed tourniquet underlaid by wool is placed around the proximal limb – a double-cuffed tourniquet is recommended. Two points of intravenous access are required; the first for the IVRA should ideally be placed distal to the injury (Dunn et al. 2003) or at the antecubital fossa (ACF) (Blyth et al. 1995), although there is a greater risk of leakage under the inflated tourniquet with the latter location (Kamming 2010). The second is to enable the administration of other drugs as required. The injured limb needs to be elevated for between 30 seconds and 2 minutes following insertion of the intravenous cannula to drain some of the blood from the veins in order to reduce the dilatation of the local anaesthetic (Dunn et al. 2003). When the procedure is ready to begin, the proximal cuff is inflated to a pressure at least 100 mmHg above the patient’s systolic blood pressure and the anaesthetic, e.g., 40 ml prilocaine 0.5 %, can then be injected into the collapsed veins of the limb, which will cause the limb to develop a blue, mottled appearance. If a single-cuff tourniquet is used the pain from the direct compression of nerve and muscle tissue will be evident after 30 minutes and may be unbearable after an hour. Where a double-cuff is used, once the anaesthetic has taken effect the distal cuff is inflated to the same pressure and the proximal cuff is then deflated reducing the patient’s discomfort as the inflated cuff is now over an anaesthetized area.
The maximum length of time for which the cuff can remain inflated is 11⁄2 hours as the tourniquet is preventing blood from entering the limb and extended use can lead to ischaemia of the area. The minimum time for constant inflation is at least 30 minutes to permit fixation of the local anaesthetic to the tissues of the limb (Rivellini 1993). Release or leakage of the anaesthetic into the systemic circulation prior to this time can lead to cardiovascular and CNS depression and therefore the presence of resuscitation facilities and circulatory access are mandatory for this procedure. It is recommended that lipid therapy is always available wherever IVRA is undertaken. (For signs of toxicity, see Box 26.2.)
Disadvantages and limitations of local anaesthesia
The first major disadvantage with local anaesthesia is that, while patients may not feel pain, they will often experience other sensations associated with the procedure, such as pressure and movement. Because they are conscious they will be able to see, hear and smell all that is going on. There will be many patients who simply do not wish to be awake because they would rather not know anything; they may find the feelings of numbness, paraesthesia, paralysis and the sense of being detached from the affected part of the body disconcerting (Rivellini 1993). For this reason, many, although not all, children and adults with learning difficulties are not suitable candidates for treatment under local or regional anaesthesia. The use of local anaesthetic requires the active cooperation of the patient and so may not be suitable for confused, aggressive and agitated people. Children and patients with learning difficulties may pose a challenge for the nurse, but they should by no means automatically be excluded from the use of local anaesthesia.
A second disadvantage is that the use of local anaesthetic may be limited by the suitability of the surgical site. In addition, some of the procedures involved for regional anaesthesia may be perceived as technically difficult and require a high level of expertise beyond that of the majority of ED medical and nursing staff. However, standardized training in the use of each technique could and should be available and local anaesthesia has been recommended as a practical alternative to general anaesthesia (Graham et al. 1997).
It is important to remember that while the term ‘local’ is used, local anaesthetics are only minimally metabolized at the site of injection; most pass ultimately into the bloodstream and, potentially, may produce systemic toxic effects. These result from the membrane-stabilizing effects on other cells, notably those of the cardiovascular and central nervous systems.
Allergic reactions to local anaesthetic drugs are uncommon; those seen are generally caused by the preservative added (e.g., methylparaben) rather than by the anaesthetic drug (Chan et al. 2002). Also allergic reactions have been confused with systemic toxicity; however, the amino-ester drugs have a higher frequency of allergic reactions than the amino-amide group (Fatovich & Brown 2004) and adverse side-effects have been reported (British National Formulary 2011). The potential effects of toxicity are listed in Box 26.2.
Other potential toxic effects include hypotension due to loss of vascular tone, respiratory depression, and allergic reactions (British National Formulary 2011), although the latter two are uncommon.
Nursing implications of procedures involving local anaesthetics
The patient’s past and current medical history should be assessed and factors that might contraindicate the use of local anaesthetics should be noted, in particular previous allergic reactions. A baseline of the current neurovascular status of the proposed surgical site should be established. When intravenous regional anaesthesia is proposed, the patient’s baseline cardiovascular status in the form of their haemodynamic observations and their weight is necessary. The nurse needs to be familiar with the technique to be used, the length of time the block will last, and whether any vasoconstrictive agent has been added (Rivellini 1993).
Intra-operatively
Special consideration should be given to the fact that patients are awake. Careless remarks should be avoided and technical terms, when used, even between staff, need to be explained. The focus of conversation should be the patient rather than the procedure. Where conscious awareness of the procedure causes distress, social conversation can be used to distract the patient (Edwards 1994).
Constant vigilance is required to assess for signs of toxicity and allergic reaction. Should there be a risk of systemic absorption, it is recommended that a reversal agent, i.e., an intravenous lipid emulsion, is available for use. The administration of such a reversal agent can restore haemodynamic stability after local anaesthetic-induced cardiac arrest (Clark 2008) and may be used to pre-empt complete cardiovascular collapse when toxicity has been identified. Examples of lipid emulsions are Intralipid® and Liposyn®.
Post-operatively and advice for discharge
Pain provides a protective function both as an initiator of reflex mechanisms and by alerting the person when a body part has sustained trauma. In eliminating pain, local anaesthetics eliminate these protective mechanisms. Nurses will need to consider measures to ensure the safety of the patient and the body part involved; for example, the patient’s limb should not be allowed to rest on or become trapped in cot sides. The transient loss of motor function necessitates that affected extremities will need to be supported in anatomically neutral positions. A sling will serve both to protect and to support the weight of an arm. Any attempt at using the affected area until full sensation has returned should be discouraged. The post-anaesthesia levels of sensory and motor function should be continually assessed and delays in expected dissipation times reported. It is vital that care is taken to differentiate between paraesthesiae related to dissipation of the block and those of neurovascular compromise (Jasinski & Snyder 1996). These observations should be documented at least once prior to discharge.
Conclusion
This chapter has highlighted both the rationale for the use of local anaesthetics and the nursing implications. Local anaesthetics are a valuable tool in the options available in ED care. Used with vigilance they can provide an alternative to the torture of the ‘one quick pull’ or the fear of ‘being put to sleep’, and improve the experiences of the patients and their supporters while in ED.
References
Achar, S., Kundu, S. Principles of office anesthesia: Part 1. Infiltrative anesthesia. American Family Physician. 2002;66(1):91–95.
Blyth, M.J.G., Kinninmonth, A.W.G., Asante, D.K. Biers block: a change of injection site. Journal of Trauma-Injury & Critical Care. 1995;39(4):726–728.
British National Formulary. British National Formulary No. 62. London: British Medical Association and the Royal Pharmaceutical Society of Great Britain; 2011.
Brown, A. Pain relief. In Cameron P., Jelinick G., Kelly A.-M., Murray L., Brown A., Heyworth J., eds.: Textbook of Adult Emergency Medicine, second ed, Edinburgh: Churchill Livingstone, 2004.
Brown, D.L., Allen, R.F. The history of regional anesthesia. In Cousins M.J., Carr D.B., Horlocker T.T., Bridenbaugh P.O., eds.: Cousins & Bridenhaugh’s Neural Blockade in Clinical Anesthesia and Pain Medicine, fourth ed, Wolters Philadelphia: Kluwer/Lippincott Williams and Wilkins, 2009.
Butcher, D. Pharmacological techniques in managing acute pain in emergency departments. Emergency Nurse. 2004;12(1):26–36.
Butterworth, J.F. Clinical pharmacology of local anesthetics. In Cousins M.J., Carr D.B., Horlocker T.T., Bridenbaugh P.O., eds.: Cousins & Bridenhaugh’s Neural Blockade in Clinical Anesthesia and Pain Medicine, fourth ed, Philadelphia: Wolters Kluwer/Lippincott Williams and Wilkins, 2009.
Cartwright, P.D., Fyhr, P. The manufacture and storage of local anesthetics. Regional Anesthesia & Pain Medicine. 1988;13(1):1–12.
Chan, S.K., Karmakar, M.K., Chui, P.T. Local anesthesia outside the operating room. Hong Kong Medical Journal. 2002;8(2):106–113.
Checketts, M.R. Intravenous regional anaesthesia. Anaesthesia and Intensive Care Medicine. 2010;11(3):111–112.
Clark, M.K. Lipid emulsion as rescue for local anesthetic-related cardiotoxicity. Journal of PeriAnesthesia Nursing. 2008;23(2):111–121.
De Jong, R.H. Local anesthetics in clinical practice. In Waldman S.D., ed.: Interventional Pain Management, second ed, Philadelphia: WB Saunders Company, 2001.
Dunn, R., Dilley, S., Brookes, J., et al. The Emergency Medicine Manual, third ed. Adelaide: Venom Publishing; 2003.
Edwards, B. Local and regional anesthesia. Emergency Nurse. 1994;2(2):10–15.
Eidelman, A., Weiss, J.M., Enu, I.K., McNicol, E., Lau, J., Carr, D.B. Topical anesthetics for repair of dermal lacerations (Protocol). In: The Cochrane Database of Systematic Reviews. London: John Wiley & Sons; 2005.
Fatovich, D.M., Brown, A.F.T. Pain relief in emergency medicine. In Cameron P., Jelinick G., Kelly A.-M., Murray L., Brown A., Heyworth J., eds.: Textbook of Adult Emergency Medicine, second ed, Edinburgh: Churchill Livingstone, 2004.
Fletcher, A.K., Rigby, A.S., Heyes, F.L. Three-in-one femoral nerve block as analgesia for fractured neck of femur in the emergency department: a randomized control trial. Annals of Emergency Medicine. 2003;42(4):596–597.
Graham, C.A., Gibson, A.J., Goutcher, C.M., et al. Anesthesia for the management of distal radius fracture in adults in Scottish hospitals. European Journal of Emergency Medicine. 1997;4(4):210–212.
Grant, S., Hoffman, R. Use of tetracaine, epinephrine, and cocaine as a topical anesthetic in the emergency department. Annals of Emergency Medicine. 1992;21:987–997.
Handoll, H.H.G. Anesthesia for treating distal radial fracture in adults. In: Cochrane Review, Cochrane Collaboration. London: John Wiley & Sons; 2005.
Horlocker, T.T., Wedel, D.J. Local anesthetic toxicity – does product labeling reflect actual risk? Regional Anesthesia and Pain Medicine. 27(6), 2002. [562–267].
Jasinski, D., Snyder, C. Invasive interventions. In: Sallerno E., Williams J., eds. Pain Management Handbook: An Interdisciplinary Approach. St Louis: Mosby, 1996.
Johansen, Ø. Comparison of articaine and lidocaine used as dental local anaesthetics. University of Oslo; 2004. [Thesis paper.].
Kamming, D. Day Surgery Analgesia. In: Bromley L., Brander B., eds. Acute Pain. Oxford: Oxford University Press, 2010.
Lander, J.A., Weltman, B.J. Topical anesthetics (EMLA and AMETOP creams) for reduction of pain during needle insertion in children (Protocol). In: The Cochrane Database of Systematic Reviews. London: Issue 4. John Wiley & Sons; 2002.
Mather, L.E., Tucker, G.T. Properties, absorbtion and disposition of local anesthetic agents. In Cousins M.J., Carr D.B., Horlocker T.T., Bridenbaugh P.O., eds.: Cousins & Bridenhaugh’s Neural Blockade in Clinical Anesthesia and Pain Medicine, fourth ed, Philadelphia: Wolters Kluwer/Lippincott Williams and Wilkins, 2009.
McCaffery, M., Passero, C. Pain: Clinical Manual, second ed. St Louis: Mosby; 1999.
National Pharmaceutical Council and Joint Commission on Accreditation of Healthcare Organizations. Pain: Current Understanding of Assessment, Management, and Treatments. Reston, VA: National Pharmaceutical Council; 2001.
Parker, M.J., Griffths, R., Appadu, B.N. Nerve blocks (subcostal, lateral cutaneous, femoral, triple, psoas) for hip fractures. In: The Cochrane Database of Systematic Reviews. London: Issue 4. John Wiley & Sons; 2002.
Rayner-Klein, J., Rowe, C.A. Analgesia and anesthesia. In: O’Shea R.A., ed. Principles and Practice of Trauma Nursing. Edinburgh: Elsevier Churchill Livingstone, 2005.
Richardson, B.W. On a new and ready mode of producing local anesthesia. Medical Times and Gazette. 1866:1–115.
Rivellini, D. Local and regional anesthesia. Nursing Clinics of North America. 1993;28(3):547–572.
Rosenberg, P.H. Local and Regional Anesthesia. Oxford: Blackwell Publishing; 2000.
Summers, A. Use of digital nerve blocks to provide anaesthetic relief. Emergency Nursing. 2011;19(5):25–29.
Titcomb, L.C. Drugs used in optometric practice. Optometry Today. 2003;46(13):26–33.
Trescot, A.M. Cryoanalgesia in Interventional Pain Management. Pain Physician. 2003;6(3):345–360.
Walsh, A., Walsh, S. Local anaesthesia and the dermatologist. Clinical and Experimental Dermatology. 2011;36(4):337–343.