Liver Transplantation in Children
Overview
The first liver transplantation was performed in 1963, with posttransplant survival reaching 1 year in 1968.1 Subsequent use of immunosuppressants such as cyclosporine and tacrolimus, coupled with improved surgical techniques, has resulted in 1-year survival rates exceeding 90%.2,3 The organ pool for children has been increased by reduced-size liver transplants, the sharing of organs between two recipients, and living related-donor transplantation.4–6
Liver transplantation is indicated in children with irreversible liver disease, liver failure, nonresectable liver tumors, vascular abnormalities, and some metabolic abnormalities (Box 94-1).7 Biliary atresia with development of cirrhosis and portal hypertension after a Kasai portoenterostomy is performed accounts for more than 60% of liver transplants in children younger than 5 years. More than two thirds of patients with biliary atresia eventually require liver transplantation, with about one third of these procedures performed in the first year of life.8
Other cholestatic causes of cirrhosis include bile duct hypoplasia syndromes (e.g., Alagille), cystic fibrosis, and primary sclerosing cholangitis.9–11 Children with cholestasis related to total parenteral nutrition may have underlying short-bowel syndrome and may require combined liver and small bowel transplantation.
Diffuse nonbiliary liver diseases necessitating transplantation include infectious and autoimmune hepatitis, neonatal hemochromatosis, graft-versus-host disease, and fulminant liver failure.12–14 Congenital hepatic fibrosis is associated with autosomal recessive polycystic kidney disease; patients with these diseases may require combined liver and kidney transplantation.15 Liver transplantation in persons with metabolic disorders is a well-established therapeutic option for glycogen storage disease, which involves a risk of liver adenomas and hepatocellular carcinoma; persons with tyrosinemia and Wilson disease have a risk of neurologic damage and cirrhosis.16–18
Most liver transplants performed in older children involve the use of a whole cadaveric liver (Fig. 94-1), whereas most young children receive reduced transplants. Liver segments are defined according to the segmental and vascular anatomy.19 Most living donor transplants use the donor’s left lateral segment or left lobe, although the right lobe also may be used. A cadaveric liver may be split between two recipients; however, both result in challenging vascular and biliary connections because of limited pedicle lengths.5
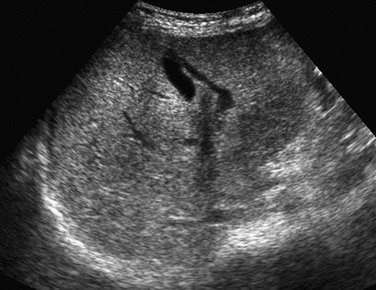
Figure 94-1 Transverse ultrasound image of a whole liver transplant.
Fluid along the falciform ligament demarcates the left lateral and medial segments. A right lobe is also present.
Left lobe and left lateral segment hila and their cut surfaces face to the right; however, they can be differentiated by the presence of the falciform ligament separating lateral segments two and three from medial segment four in the left lobe transplant, which is absent in left lateral segment grafts (Fig. 94-2). The small bowel, duodenum, colon, and right kidney often migrate into the vacated liver bed; a migrated cecum and right colon may imitate malrotation.
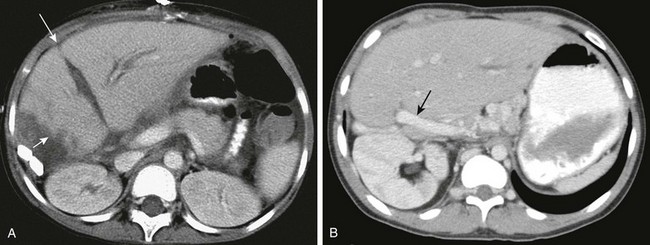
Figure 94-2 Segmental liver grafts.
A, Computed tomography (CT) of a left lobe liver graft. Note the ill-defined, irregular cut edge with adjacent fluid (short arrow). Fluid along the falciform ligament (long arrow) separates the left lateral segment (segments two and three) from the medial segment (segment four). B, CT of a left lateral segment graft. Note the course of the portal vein entering from the right (arrow) and the absence of the falciform ligament. Also note the position of the right kidney in the upper abdomen.
The portal vein usually is connected end-to-end to the recipient portal vein, with as much of the native portal vein preserved during hepatectomy as possible.20 Children with older transplants may have had extension grafts, which may still be used in patients with unsuitable native portal veins.
The donor hepatic artery usually is anastomosed to the recipient’s hepatic artery or celiac axis. The hepatic artery may be connected to the infrarenal aorta if technically required. In such cases the native hepatic artery is ligated and should not be confused with arterial occlusion on imaging. The bile duct typically is attached to a Roux-en-Y jejunal loop (Fig. 94-3).
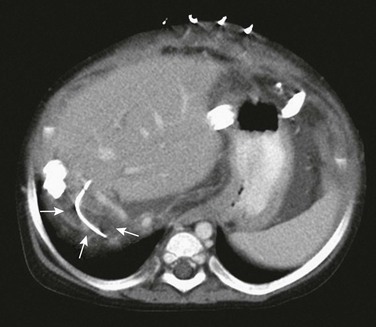
Figure 94-3 The Roux-en-Y biliary anastomosis and loop are marked early after transplantation by a small biliary stent (arrows).
Being an efferent loop from the liver, it frequently does not fill with intestinal contrast, making it difficult to differentiate from a pathologic fluid collection. Diffuse mesenteric edema and skin staples are noted.
In reduced-size transplants, the donor hepatic vein is connected to the recipient’s modified hepatic vein orifice. In full-size liver grafts, the donor liver is removed with the intrahepatic inferior vena cava (IVC), which is then interposed between the infraatrial and distal native IVC. The donor IVC may be sutured distally and connected proximally, piggyback fashion, to the preserved native IVC. In patients with polysplenia and biliary atresia, vascular connections may be modified as a result of the frequent absence of the hepatic segment of the IVC, azygous continuation, malrotation, a preduodenal portal vein, and other anomalies.5,20,21
Vascular, biliary, infectious, and immunosuppression-related complications may befall liver recipients postoperatively, including posttransplant lymphoproliferative disease (PTLD). The most common complication of liver transplant is infection, but vascular thrombosis and primary graft nonfunction account for most cases of graft loss leading to retransplantation. Death in most pediatric liver recipients is caused by infection, neurologic complications, or multisystem organ failure. Enteric complications include bowel perforation and obstruction, gastrointestinal hemorrhage, and infectious enteritis. Neurologic complications include cerebral hemorrhage and infarction, infection, and drug toxicity.22–24 Immunosuppressive drug-induced nephrotoxicity is common, but imaging features generally are nonspecific.
Other complications include hepatic parenchymal defects, pleural effusions, and splenic infarcts, which may occur after splenic artery ligation.25,26 Right adrenal hemorrhage and right phrenic nerve injury are potential complications seen after the standard transplant operation, but they rarely occur after a cava-sparing operation.
Imaging
Portable sonography may be used during surgery, mainly when vascular anastomoses are difficult or revised and their patency is uncertain. Vascular patency is evaluated daily with Doppler ultrasound for the first few posttransplant days because the fate of the transplant is much improved if vascular occlusions are diagnosed and managed early. The frequency of required ultrasound examinations then decreases, based on individual needs and clinical concerns.27,28
Early postoperative ultrasound may be challenging because of poor acoustic windows caused by dressings, open incisions, free intraabdominal air, and body wall edema. The flow patterns in all vessels vary in the early postoperative period because of edema of the anastomosis and surrounding tissues, as well as hemodynamic changes in the graft’s vascular bed. Vascular patency, flow direction, pulsatility pattern, and velocity can be evaluated using gray-scale, spectral, and color Doppler ultrasound techniques. The color velocity scale may need to be adjusted for each vessel separately to avoid aliasing in the faster flowing vessels, which can lead to ambiguity of flow direction. Efforts must be made to interrogate at the anastomoses, where stenosis, thrombosis, and occlusion are most likely.27
Early findings seen in otherwise normal liver grafts include increased periportal echogenicity (low attenuation on computed tomography [CT] and increased signal on water-sensitive magnetic resonance [MR] sequences) related to transient lymphatic engorgement (Fig. 94-4), right pleural effusion, subhepatic fluid collections, and biliary air in recipients with choledochojejunostomies.25,26
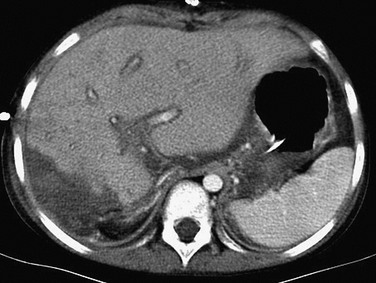
Figure 94-4 Early computed tomography findings in liver transplantation.
Diffuse periportal hypoattenuation, irregularity of the liver cut surface edge, and adjacent fluid are present.
Periodic long-term ultrasound screening of liver recipients is mandatory because vascular complications may be clinically silent. CT, CT angiography (CTA), magnetic resonance cholangiopancreatography (MRCP), and magnetic resonance angiography (MRA) are used to determine the need for biliary and vascular interventions in selected patients. CT remains the primary modality for evaluating many intraabdominal complications, such as infection and PTLD. New magnetic resonance imaging (MRI) sequences have increased the utility of MRI in these patients, with the obvious advantage of avoiding ionizing radiation.29
Pretransplant Imaging
Pertinent anatomic features for surgical planning include a small, absent, or occluded portal vein; IVC thrombosis; biliary atresia associated with polysplenia syndrome and absent intrahepatic IVC, with hepatic veins draining into the right atrium; systemic drainage of the splenic veins; congenital portosystemic shunts; and malrotation. Transplantation may be impossible when both the portal and superior mesenteric veins are occluded or in cases of nonresponsive metastatic hepatoblastoma. Detection of hepatocellular carcinoma in a patient with cirrhosis changes the transplant urgency and requires tumor staging and decisions about pretransplant therapy, which affect the timing and feasibility of transplantation.27
Ultrasound is the method used most often in the preoperative evaluation of pediatric transplant candidates. Doppler ultrasound assesses vascular patency and hemodynamics. CT with intravenous contrast is excellent for showing vascular and visceral anatomy, unimpeded by overlying bowel gas. MRI may supply hemodynamic information and is superior to ultrasound in depicting varices, collaterals, and spontaneous splenorenal shunts. Bone radiographs may detect rickets, osteopenia, and fractures, and chest radiographs may show cardiomegaly and vascular prominence in patients with hepatopulmonary disease (see Chapter 93).29,30 Angiography is seldom needed but is reserved for patients for whom additional information might be required.
Vascular Complications
Overview: Hepatic arterial complications include thrombosis, occlusion, stenosis, peripheral arteriovenous fistula, and aneurysm. Acute arterial thrombosis may be catastrophic and result in acidosis, sepsis, and organ necrosis. Early hepatic artery thrombosis is associated with a high incidence of transplant loss.31 If the problem is diagnosed within the first 2 to 3 days and the patient is still asymptomatic, most transplanted livers with hepatic artery thrombosis may be salvaged. However, if elevated liver function tests, bile leak, liver abscess, or sepsis have supervened, graft loss may reach 75%.32
Imaging: The hepatic artery is much more difficult to visualize by gray-scale ultrasound than the portal vein because of its small size. It is best evaluated by color and power Doppler ultrasound.25 It is easier to follow the artery to its anastomosis when it is attached to the native hepatic artery or celiac axis rather than to the infrarenal aorta, where much of its course might be obscured by bowel gas. Both CTA and MRA are useful in showing hepatic artery anatomy, course, and complications (Fig. 94-5).26
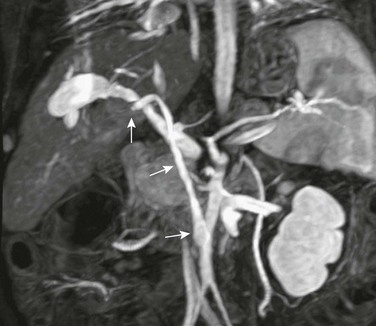
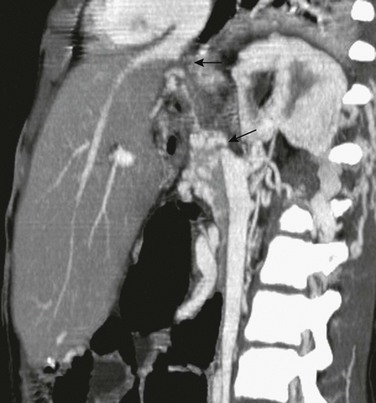
Figure 94-5 Hepatic artery occlusion.
Magnetic resonance angiography shows the origin of the transplant hepatic artery from the infrarenal aorta (arrows). It is occluded near the liver.
The diagnosis of hepatic artery thrombosis is made by the inability to detect hepatic artery signal on color and spectral Doppler ultrasound.25 Acute arterial thrombosis is treated by thrombectomy, revision, and occasionally retransplantation.33 High-grade narrowing is manifested by turbulence and severely attenuated velocity distally with a parvus-tardus pattern in intrahepatic branches, with narrow systolic peaks and absent diastolic flow beyond the anastomosis.31
Later onset hepatic artery occlusion may manifest with biliary leaks, strictures, and infection, including peribiliary abscess and sepsis because the biliary tree depends on hepatic arterial supply. In long-standing hepatic artery occlusion, hilar or transcapsular collaterals may result in a detectable arterial Doppler signal.31
Stenosis of the hepatic artery may be silent or may manifest with biliary complications and infection. High velocity and turbulence may be seen at the stenotic site. The flow pattern is abnormal, with a low-amplitude, parvus tardus representing a systolic acceleration time longer than 0.08 second and a resistive index lower than 0.5.31 Very high-grade stenosis manifests with narrow and low-amplitude systolic peaks and absence of diastolic flow. The stenosis itself is usually extrahepatic and difficult to demonstrate directly by Doppler ultrasound (Fig. 94-6).26,31 The hemodynamic changes of hepatic artery stenosis may be more dramatic than its appearance on CTA or MRA.
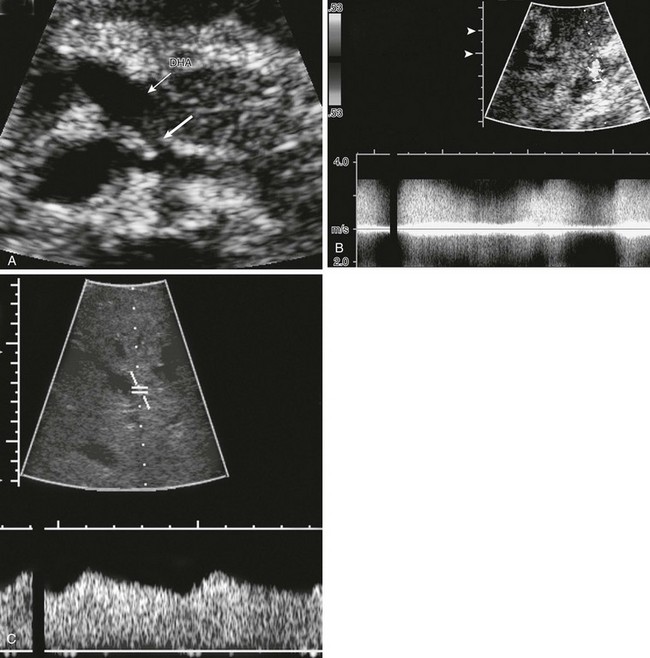
Figure 94-6 Ultrasound and Doppler characteristics of hepatic artery stenosis.
A, Transverse ultrasound shows stenosis immediately distal to the celiac axis (thick arrow). The donor hepatic artery (DHA) distal to the anastomotic stenosis is marked by a thin arrow. B, Spectral and color Doppler at the stenosis show extreme turbulence and high velocity. The normal arterial signal is difficult to recognize because of aliasing. C, Spectral and color Doppler in an intrahepatic hepatic artery branch show a slow systolic upstroke (tardus phenomenon), and relatively high diastolic flow. Resistive index was also abnormally low.
Hepatic artery pseudoaneurysm may occur at the anastomosis or within the liver at a biopsy site.26,31 Liver biopsy also is a cause of intrahepatic arteriovenous fistulas.33
Venous System
Overview: Obstructive lesions of the portal vein, hepatic vein, and IVC may manifest with progressive ascites, deterioration of liver function, gastrointestinal bleeding, decreased platelet count, and increased spleen size. Pulmonary hypertension and intrapulmonary shunting may develop. Doppler signal in the intrahepatic portions of the portal vein do not exclude stenosis or obstruction, which may be present in the extrahepatic segment, with intrahepatic flow reconstituted by collateral vessels.29 Portal vein, hepatic vein, and IVC stenosis result in flow acceleration at the stenosis and a jet of increased flow at the poststenotic segment, which appears as a contrasting color as a result of aliasing when the scale is adjusted to the velocity below the stenosis. The jet velocity is proportional to the pressure gradient across the stenosis.25,26
Portal Vein: Ultrasound of the transplant portal vein requires visualization of its entire course. One must measure the diameter at any site of narrowing and interrogate the portal vein by spectral and color Doppler along its extrahepatic and hilar course, as well as its major intrahepatic branches. The curved course of the portal vein in left lobe and left lateral segment transplants and the size discrepancy between donor and recipient veins frequently cause dilation at the neohilum because of altered flow dynamics (Fig. 94-7) with a “yin-and-yang” swirling pattern on color Doppler. This pattern has not been proved clinically important. Anastomotic and periportal edema causing relative narrowing with jet formation is very common in the early postoperative period; these instances of edema are most often transient but may progress or persist and should be monitored. Complications include early thrombosis, stenosis, and late occlusion (Fig. 94-8).25,26
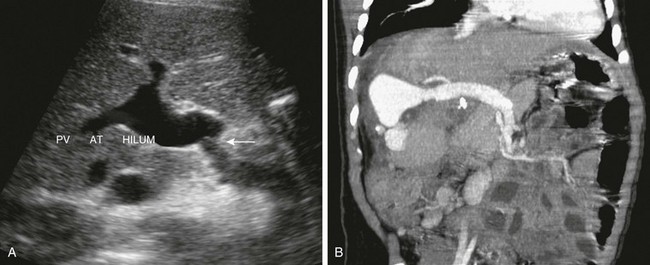
Figure 94-7 Portal vein in segmental transplantation.
A, An ultrasound image shows a size discrepancy (arrow) between the native and donor portal veins and dilation of the donor portal vein (PV AT HILUM). B, Coronal reconstruction from computed tomography angiography demonstrates the extrahepatic portal vein coursing from the left to the right edge of the transplant, near the right abdominal wall. The portal vein is dilated at the neohilum. Note slight narrowing at the anastomosis in this early transplant.
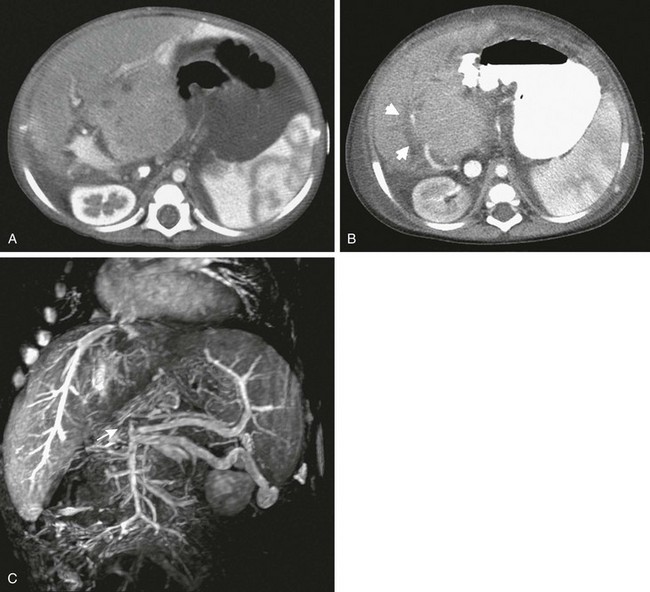
Figure 94-8 Portal vein occlusion.
A, Contrast-enhanced computed tomography (CT) in an infant after left lateral segment graft shows that the portal vein and the hepatic artery are well seen in the neohilum. B, At 4-month follow-up, contrast-enhanced CT shows a lucent band where the portal vein previously had been seen (arrows). The patient underwent successful thrombolysis. C, Long-standing portal vein occlusion. Magnetic resonance venography shows occlusion of the entire portal vein from the splenomesenteric confluence (arrow). A large venous collateral is seen from the confluence to the left flank.
A hypoechoic thrombus may not be recognized by gray-scale ultrasound but shows absence of signal on color Doppler imaging. Reversal of superior mesenteric or splenic vein flow also is seen. Early thrombosis usually is treated by surgical thrombectomy and revision. Early posttransplant reversal of entire portal vein flow may reflect extensive hepatic necrosis and arterioportal shunting—an ominous sign associated with frequent organ loss.26
Stenosis usually occurs at anastomoses and in extension grafts (Fig. 94-9).34 Because of the curved extrahepatic course of the portal vein, it may be difficult to demonstrate stenosis by ultrasound. The jet created at the stenotic site dissipates, such that flow velocity measured at the hilum is frequently normal despite a hemodynamically significant portal vein stenosis more proximally.26 The jet also may exaggerate the dilation of the portal vein at the hilum. Following successful angioplasty, the jet velocity decreases and the diameter at the stenotic site increases (Fig. 94-10). Less operator-dependent CT and MRI are highly useful and more consistent than ultrasound in the evaluation of portal vein occlusion and stenosis.29,31,35
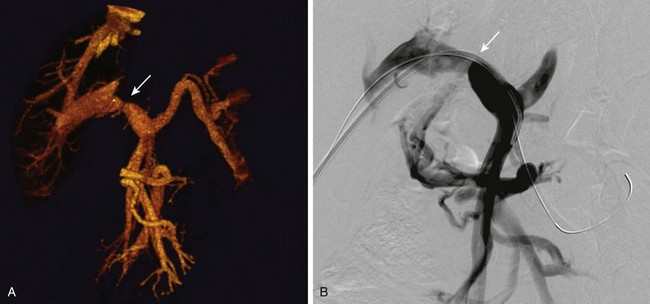
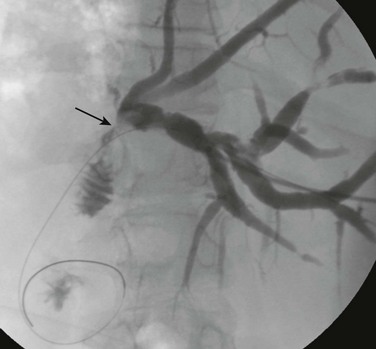
Figure 94-9 Portal vein stenosis.
A, Reconstructed images from contrast-enhanced computed tomography shows anastomotic stenosis (arrow) in a patient with a whole liver transplant. B, A portal venogram in the same patient confirms the portal vein stenosis (arrow).
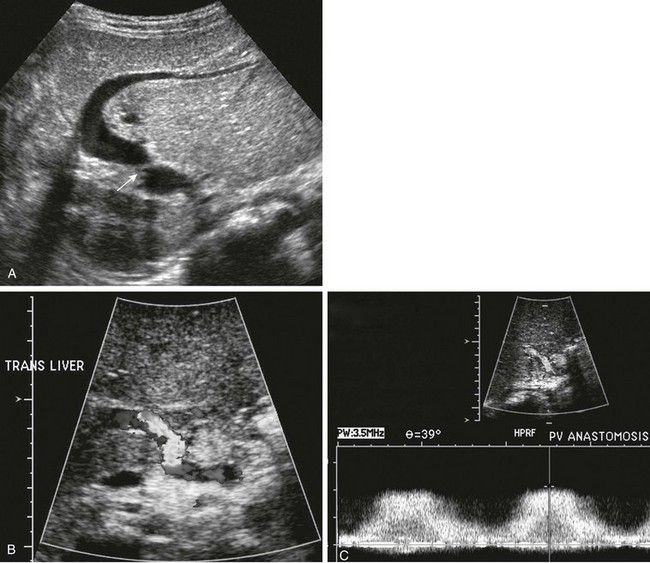
Figure 94-10 Ultrasound and Doppler characteristics of portal vein stenosis.
A, Ultrasound of a child with a left lateral segment graft. The stenosis (arrow) is outside the liver hilum, beyond the portal vein curvature. B, On color Doppler, the velocity scale is adjusted such that aliasing demonstrates the higher velocity jet outlining the stenotic site. C, On spectral and color Doppler with sampling at the jet, maximal flow velocity is obtained to assess the hemodynamic impact of the stenosis. The maximal jet velocity exceeds 2 m/sec.
Hepatic Vein: Hepatic vein stenosis occurs mainly in reduced-size transplants at the anastomosis to the recipient IVC. In a posttransplant patient with the onset of portal hypertension, special efforts must be made to evaluate the hepatic veins and IVC if a portal vein complication is not seen. Stenosis is near the diaphragm, making it difficult to demonstrate by ultrasound (Fig. 94-11). Doppler ultrasound demonstrates increased flow velocity and a jet with loss of triphasic flow.31,36 A caveat is that triphasic flow may be absent without obstruction. CTA and MRA are excellent to demonstrate or confirm the diagnosis.26
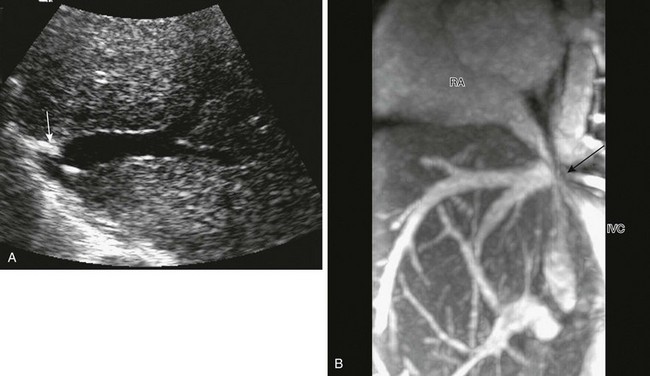
Figure 94-11 Hepatic vein stenosis.
A, Ultrasound imaging demonstrates stenosis (arrow) at the typical high location near the diaphragm, where it may be very difficult to identify. B, Sagittal plane magnetic resonance venography confirms tight stenosis (arrow) and retraction of both the hepatic veins and the inferior vena cava (IVC). RA, Right atrium.
Inferior Vena Cava: IVC stenosis and obstruction may occur at the anastomosis. Anastomotic stenosis and obstruction may be short or long segment and may result from compression or graft torsion (Fig. 94-12). The clinical manifestations of IVC stenosis or obstruction depend on the location. If the problem is at or above the hepatic veins, it manifests with ascites and portal hypertension. If it is at a lower level, it may be clinically silent.26
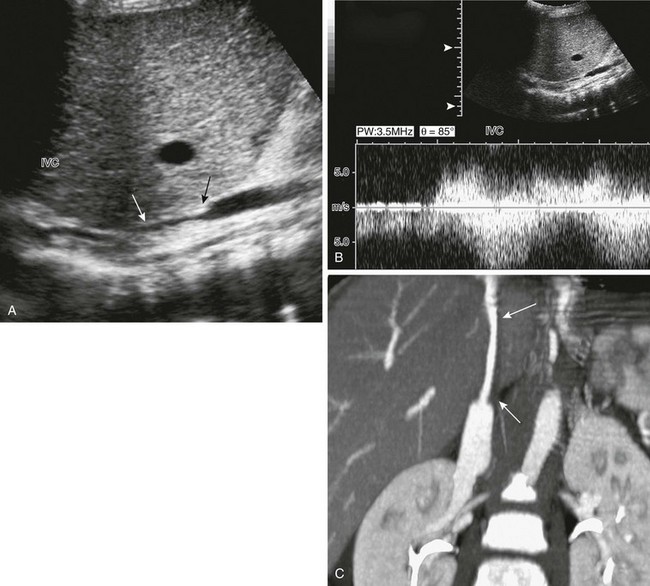
Figure 94-12 Long-segment inferior vena cava (IVC) stenosis.
A, A longitudinal ultrasound image shows a long-segment IVC narrowing (arrows) along the posterior aspect of the transplanted liver graft. B, Spectral Doppler identifies extremely high-velocity and turbulence at the stenotic site. C, Sagittal reconstruction of computed tomographic angiography shows the long-segment IVC narrowing (arrows).
IVC obstruction can be diagnosed by all cross-sectional modalities; however, several pitfalls may be encountered with Doppler ultrasound. Flow in the hepatic veins may be normal and triphasic if the IVC obstruction is distal to their insertion (Fig. 94-13). On the other hand, when the obstruction is proximal to the hepatic vein insertion, hepatic venous blood may enter the IVC and flow retrograde to decompress into systemic collaterals. In patients with a surgical portosystemic shunt, IVC obstruction prevents adequate decompression, sometimes reversing flow in the shunt and increasing preexistent varices.31,34
Biliary Complications
Bile leaks and strictures occurring early after transplantation are related to technical surgical problems, such as anastomotic leak and narrowing or kinking. Biliary complications with a later onset commonly are associated with ischemia and infection as a result of arterial occlusion or stenosis.31 Biliary strictures are more common in reduced-size liver transplants than in full-sized ones.37 Inspissated bile and biliary compression by a mucocele or cystic duct remnant have been described.29 Chronic rejection may be associated with loss of bile ducts and mild chronic dilation.
Ultrasound identifies biliary dilation and is the primary screening modality. When bile duct dilation is found, further imaging is indicated.25 MRCP increasingly is used to assess biliary obstruction and the need for intervention.38–40 Direct transhepatic cholangiogram usually is performed as part of the therapeutic approach (Fig. 94-14).
Fluid Collections
Early postoperative bleeding from leaking anastomoses or inadequate hemostasis leading to visible fluid collections occurs in as many as 15% of recipients. Up to half of these patients undergo operative exploration. Most fluid collections occur at the cut edge, contain serous fluid, blood, or bile, and are transient and clinically insignificant. Other collections may result from an anastomotic bile leak, abscess, or bowel perforation. The imaging appearance of perihepatic fluid collections is nonspecific; most contain some debris.25 Specific diagnosis may require fluid aspiration or surgery (Fig. 94-15).
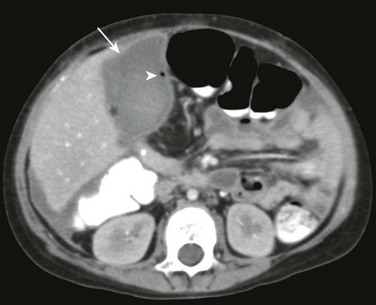
Figure 94-15 Infected bile collection.
Computed tomography shows a fluid collection (arrow) containing a small gas bubble (arrowhead). Image-guided aspiration confirmed an infectious etiology.
When a fluid collection is suspected, it is important to realize that although the blind end of the Roux-en-Y loop may dilate, the normal loop at the hilum of the transplanted liver may mimic a fluid collection on ultrasound and may not fill with oral contrast during CT scanning.28,34
Mycotic arterial pseudoaneurysms at anastomoses may rupture, producing abrupt exsanguinations.25 Thus fluid collections near an artery should be interrogated carefully.
Infection
Postoperative infection develops in most liver recipients at some time, although consequent mortality is less than 10%. Bacterial and fungal infections are most common during the first postoperative month. Risk factors include hepatic artery occlusion, immunosuppression, central venous catheters, and nosocomial exposure. Viral and opportunistic infections usually occur between 30 and 180 days after transplantation.41 Ultrasound and CT are the mainstay for assessing intraabdominal infections, particularly abscesses.42 Cholangitis may prompt evaluation with MRCP or percutaneous transhepatic cholangiography.38
Rejection
Imaging plays a relatively minor role in the diagnosis of rejection, which is established mainly by liver biopsy. Rejection may be associated with mild degrees of nonobstructive bile duct dilation and an increase in hepatic arterial resistive indices. However, neither hepatic arterial waveforms nor flow pattern in the hepatic veins have proved to be predictive of rejection.35
Posttransplantation Lymphoproliferative Disease
PTLD represents a spectrum of abnormalities of lymphoid proliferation linked with Epstein-Barr virus (in 98% of pediatric patients) and immune cell proliferation that ranges from polyclonal reversible B cell proliferation to aggressive monoclonal B cell lymphoma. T cell, Burkitt lymphoma, and Hodgkin disease are less common.43 Risk factors for the development of PTLD are seronegativity for Epstein-Barr virus at transplantation, young age, intensity and type of immunosuppression (especially antilymphocyte antibody), cytomegalovirus infection, and type of transplant.39 PTLD develops in children about three times as often as in adults, reported at 9.7% versus 2.9%, respectively.44 The time from transplantation to the development of PTLD averages about 8 months in children, although it may be shorter in patients treated with tacrolimus, occurring as early as within a few weeks.45 Survival is better in patients with polyclonal PTLD and those with limited disease. In persons with polyclonal disease, PTLD may reverse completely with modifications of immune suppression.46
Sites of involvement in children include lymphoid tissue (particularly Waldeyer ring, pericardial, and mesenteric nodes), the gastrointestinal tract, the spleen, and the transplanted liver (Fig. 94-16). Newly enlarged tonsils and adenoids in a child with a transplanted liver should raise suspicion of PTLD. Unusually large mesenteric nodes are common in children with PTLD, although lack of normal standards makes it difficult to set a threshold size for diagnosis. Central nervous system involvement is uncommon.40 The appearance of PTLD in the transplanted liver consists of multiple nonspecific focal lesions.47
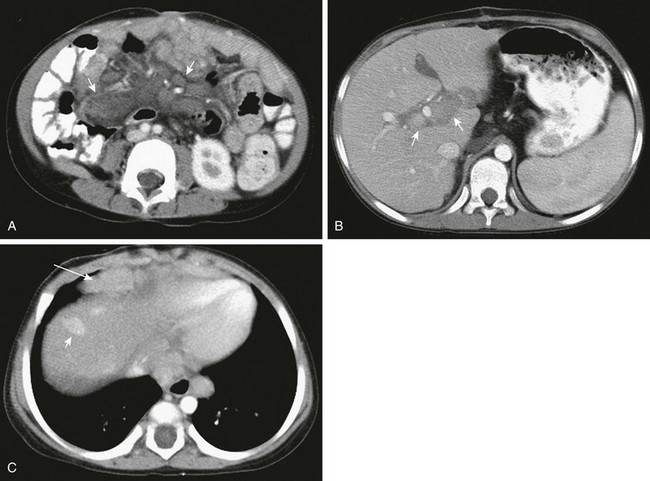
Figure 94-16 Posttransplantation lymphoproliferative disease.
A, A computed tomography (CT) scan of the abdomen shows multiple enlarged mesenteric lymph nodes (arrows). B, A high CT slice reveals lymphadenopathy in the hilum of the transplanted liver (arrows). C, CT slice at the level of the diaphragm demonstrates an enhancing liver nodule (short arrow) as well as pericardial (long arrow) and posterior mediastinal lymphadenopathy.
References
1. Otte, JB. History of pediatric liver transplantation. Where are we coming from? Where do we stand? Pediatr Transplant. 2002;6(5):378–387.
2. Rook, M, Rand, E. Predictors of long-term outcome after liver transplant. Curr Opin Organ Transplant. 2011;16(5):499–504.
3. Ryckman, FC, Bucuvalas, JC, Nathan, J, et al. Outcomes following liver transplantation. Semin Pediatr Surg. 2008;17(2):123–130.
4. Bourdeaux, C, Darwish, A, Jamart, J, et al. Living-related versus deceased donor pediatric liver transplantation: a multivariate analysis of technical and immunological complications in 235 recipients. Am J Transplant. 2007;7(2):440–447.
5. Mehrabi, A, Fonouni, H, Müller, SA, et al. Current concepts in transplant surgery: liver transplantation today. Langenbecks Arch Surg. 2008;393(3):245–260.
6. Hong, JC, Yersiz, H, Busuttil, RW. Where are we today in split liver transplantation? Curr Opin Organ Transplant. 2011;16(3):269–273.
7. O’Leary, JG, Lepe, R, Davis, GL. Indications for liver transplantation. Gastroenterology. 2008;134(6):1764–1776.
8. Baerg, J, Zuppan, C, Klooster, M. Biliary atresia—a fifteen-year review of clinical and pathologic factors associated with liver transplantation. J Pediatr Surg. 2004;39(6):800–803.
9. Kamath, BM, Schwarz, KB, Hadzic, N, et al. Alagille syndrome and liver transplantation. J Pediatr Gastroenterol Nutr. 2010;50(1):11–15.
10. Aydogdu, S, Cakir, M, Arikan, C, et al. Liver transplantation for progressive familial intrahepatic cholestasis: clinical and histopathological findings, outcome and impact on growth. Pediatr Transplant. 2007;11(6):634–640.
11. Lu, BR, Esquivel, CO. A review of abdominal organ transplantation in cystic fibrosis. Pediatr Transplant. 2010;14(8):954–960.
12. Mieli-Vergani, G, Vergani, D. Autoimmune paediatric liver disease. World J Gastroenterol. 2008;14(21):3360–3367.
13. Heffron, T, Pillen, T, Welch, D, et al. Medical and surgical treatment of neonatal hemochromatosis: single center experience. Pediatr Transplant. 2007;11(4):374–378.
14. Barshes, NR, Myers, GD, Lee, D, et al. Liver transplantation for severe hepatic graft-versus-host disease: an analysis of aggregate survival data. Liver Transpl. 2005;11(5):525–531.
15. Chapal, M, Debout, A, Dufay, A, et al. Kidney and liver transplantation in patients with autosomal recessive polycystic kidney disease: a multicentric study. Nephrol Dial Transplant. 2012;27(5):2083–2088.
16. Arnon, R, Annunziato, R, Schilsky, M, et al. Liver transplantation for children with Wilson disease: comparison of outcomes between children and adults. Clin Transplant. 2011;25(1):E52–E60.
17. Büyükpamukçu, M, Varan, A, Haberal, M, et al. The efficacy of liver transplantation in malignant liver tumors associated with tyrosinemia: clinical and laboratory findings of five cases. Pediatr Transplant. 2006;10(4):517–520.
18. Stevenson, T, Millan, MT, Wayman, K, et al. Long-term outcome following pediatric liver transplantation for metabolic disorders. Pediatr Transplant. 2010;14(2):268–275.
19. Oliveira, DA, Feitosa, RQ, Correia, MM. Segmentation of liver, its vessels and lesions from CT images for surgical planning. Biomed Eng Online. 2011;10:30.
20. Fung, J, Kelly, D, Kadry, Z, et al. Immunosuppression in liver transplantation: beyond calcineurin inhibitors. Liver Transpl. 2005;11(3):267–280.
21. Hernanz-Schulman, M, Ambrosino, MM, Genieser, NB, et al. Current evaluation of the patient with abnormal visceroatrial situs. AJR Am J Roentgenol. 1990;154(4):797–802.
22. Welling, TH, Heidt, DG, Englesbe, MJ, et al. Biliary complications following liver transplantation in the model for end-stage liver disease era: effect of donor, recipient, and technical factors. Liver Transpl. 2008;14(1):73–80.
23. Berrocal, T, Parrón, M, Alvarez-Luque, A, et al. Pediatric liver transplantation: a pictorial essay of early and late complications. Radiographics. 2006;26(4):1187–1209.
24. Diamond, IR, Fecteau, A, Millis, JM, et al. Impact of graft type on outcome in pediatric liver transplantation: a report from Studies of Pediatric Liver Transplantation (SPLIT). Ann Surg. 2007;246(2):301–310.
25. O’Brien, J, Buckley, AR, Browne, R. Comprehensive ultrasound assessment of complications post-liver transplantation. Eur J Radiol. 2010;74(1):206–213.
26. Zamboni, GA, Pedrosa, I, Kruskal, JB, et al. Multimodality postoperative imaging of liver transplantation. Eur Radiol. 2008;18(5):882–891.
27. Huang, TL. The role of color Doppler ultrasound in living donor liver transplantation. J Med Ultrasound. 2008;16:177–187.
28. Andronikou, S, Millar, AJ, Kader, E, et al. Anatomical considerations in the imaging of ‘reduced-size’ liver transplantation in children. Pediatr Radiol. 2002;32(11):793–797.
29. Caruso, S, Miraglia, R, Maruzzelli, L, et al. Imaging in liver transplantation. World J Gastroenterol. 2009;15(6):675–683.
30. Babyn, PS. Imaging of the transplant liver. Pediatr Radiol. 2010;40(4):442–446.
31. Caiado, AH, Blasbalg, R, Marcelino, AS, et al. Complications of liver transplantation: multimodality imaging approach. Radiographics. 2007;27(5):1401–4017.
32. Duffy, JP, Hong, JC, Farmer, DG, et al. Vascular complications of orthotopic liver transplantation: experience in more than 4,200 patients. J Am Coll Surg. 2009;208(5):896–903. [discussion 903-905].
33. Miraglia, R, Maruzzelli, L, Caruso, S, et al. Interventional radiology procedures in pediatric patients with complications after liver transplantation. Radiographics. 2009;29(2):567–584.
34. Singh, AK, Nachiappan, AC, Verma, HA, et al. Postoperative imaging in liver transplantation: what radiologists should know. Radiographics. 2010;30(2):339–351.
35. Cheng, YF, Chen, CL, Huang, TL, et al. 3DCT angiography for detection of vascular complications in pediatric liver transplantation. Liver Transpl. 2004;10(2):248–252.
36. Suzuki, L, de Oliveira, IR, Widman, A, et al. Real-time and Doppler US after pediatric segmental liver transplantation: II. Hepatic vein stenosis. Pediatr Radiol. 2008;38(4):409–414.
37. Anderson, CD, Turmelle, YP, Darcy, M, et al. Biliary strictures in pediatric liver transplant recipients —early diagnosis and treatment results in excellent graft outcomes. Pediatr Transplant. 2010;14(3):358–363.
38. Rozel, C, Garel, L, Rypens, F, et al. Imaging of biliary disorders in children. Pediatr Radiol. 2011;41(2):208–220.
39. Fernández, MC, Bes, D, De Dávila, M, et al. Post-transplant lymphoproliferative disorder after pediatric liver transplantation: characteristics and outcome. Pediatr Transplant. 2009;13(3):307–310.
40. Sevmis, S, Pehlivan, S, Shabazov, R, et al. Posttransplant lymphoproliferative disease in pediatric liver transplant recipients. Transplant Proc. 2009;41(7):2881–2883.
41. Shepherd, RW, Turmelle, Y, Nadler, M, et al. Risk factors for rejection and infection in pediatric liver transplantation. Am J Transplant. 2008;8(2):396–403.
42. Almusa, O, Federle, MP. Abdominal imaging and intervention in liver transplantation. Liver Transpl. 2006;12(2):184–193.
43. Pasquale, MA, Weppler, D, Smith, J, et al. Burkitt s lymphoma variant of post-transplant lymphoproliferative disease (PTLD). Pathol Oncol Res. 2002;8(2):105–108.
44. Jain, A, Nalesnik, M, Reyes, J, et al. Posttransplant lymphoproliferative disorders in liver transplantation: a 20-year experience. Ann Surg. 2002;236(4):429–436. [discussion 436-437].
45. George, TI, Jeng, M, Berquist, W, et al. Epstein-Barr virus-associated peripheral T-cell lymphoma and hemophagocytic syndrome arising after liver transplantation: case report and review of the literature. Pediatr Blood Cancer. 2005;44(3):270–276.
46. Koh, BY, Rosenthal, P, Medeiros, LJ, et al. Posttransplantation lymphoproliferative disorders in pediatric patients undergoing liver transplantation. Arch Pathol Lab Med. 2001;125(3):337–343.
47. Borhani, AA, Hosseinzadeh, K, Almusa, O, et al. Imaging of posttransplantation lymphoproliferative disorder after solid organ transplantation. Radiographics. 2009;29(4):981–1000. [discussion 1000-1002].