Chapter 97D Liver transplantation for hepatocellular carcinoma
Overview
Cancer of a solid organ is an uncommon indication for organ transplantation, but hepatocellular carcinoma (HCC) is an important exception to that general rule (see Chapter 97A). HCC is the most common tumor treated with whole-organ transplantation, and it is listed as the primary indication in approximately 15% to 20% of liver transplantations. The existing treatment options for HCC include hepatic resection, local ablation, or liver transplantation, and in the last decade, transplantation has become the therapy of choice for early-stage disease. The literature lacks randomized controlled trials regarding the treatment of HCC; most centers therefore base their practice guidelines on local experience, retrospective data, theoretic decision analyses, and the availability of treatment options.
Ever since the first liver transplantation was performed in a patient with HCC in the 1960s, recurrence has been a concern. Prior to the late 1990s, liver transplantation for HCC carried high recurrence rates, and the 2-year survival was only about 30%. The poor outcomes were due to the consensus at that time to treat smaller tumors with liver resection and more advanced disease with liver transplantation. Discouraging results plagued liver transplantation for HCC, until it was noted that subgroups of patients with smaller tumors who underwent transplantation achieved better outcomes (Pichlmayer, 1993).
A group from Milan, Italy, conducted a prospective trial, published in 1996, in which patients with smaller tumors achieved 4-year survival rate of 75%, equivalent to the survival for non-HCC patients who underwent transplantation (Mazzaferro et al, 1996), and similar results were seen in the United States (Venook, 1993). Resultant changes in patient selection, dictated by these Milan criteria, allowed similar results to be reproduced at other centers (Jonas et al, 2001; Llovet et al, 1998), and the United Network of Organ Sharing (UNOS) adopted these criteria in 1998 and gave priority for transplantation to HCC patients. This priority, coupled with the rise in patients with hepatitis C virus (HCV), who frequently develop HCC, fueled the rapid expansion of this indication for transplantation. This chapter will discuss liver transplantation for HCC, concentrating on its unique pathophysiology and how it relates to diagnosis and dictates therapy.
Hepatocellular Carcinoma
HCC is the fifth most common cancer and the most common primary liver tumor (see Chapter 80). This disease develops in the setting of cirrhosis in more than 90% of cases (Fattovich et al, 2004). The risk of HCC development in patients with cirrhosis from any cause is about 2.0% to 6.6% per year versus 0.4% for patients with viral hepatitis without cirrhosis (Benvegnu et al, 2004; Degos et al, 2000; Ikeda et al, 1993a; Llovet et al, 2003; Sangiovanni et al, 2004; see Chapter 64, Chapter 70A, Chapter 70B ).
The incidence of HCC has been rising since the late 1990s (Bosch et al, 2004; Deuffic et al, 1998; El-Serag & Mason, 1999; Parkin et al, 2001; Stroffolini et al, 1998; Taylor-Robinson, et al, 1997), a trend that is expected to continue for at least the next 2 decades because patients who were infected with HCV 20 to 30 years ago are currently living with cirrhosis and are aging (El-Serag, 2004). In addition, the epidemic of obesity and resultant nonalcoholic fatty liver disease (NAFLD) can be expected to generate cirrhotic patients at risk for the foreseeable future (see Chapter 65). Currently, only the incidence of thyroid cancer is rising faster than the incidence of HCC in the United States (Fattovich et al, 2004). It is hoped that the introduction of vaccination for HBV will decrease the incidence of HCC, an effect that has been seen already in Taiwan (Chang et al, 2009).
Biology of Hepatocellular Carcinoma
An understanding of the biology of HCC is critical to assist in clinical decision making about surveillance and treatment. Although the molecular biology of HCC remains to be fully elucidated, the close association between cirrhosis and the development of HCC is clear (Miyazawa et al, 2003; see Chapter 8C). Viral hepatitis, chronic alcohol use, and NAFLD are the most common causes of cirrhosis in the Western world (see Chapters 64, 65, and 70A). NAFLD, an unfamiliar diagnosis as recently as the early 1980s, is now present in 17% to 30% of Americans (Farrell & Larter, 2006); that progresses to steatohepatitis in 32% to 37% (Fassio et al, 2004; Harrison et al, 2003; Hui et al, 2003) and then to cirrhosis in 5% to 24% (El-Zayadi, 2008; Hui et al, 2003). The development of cirrhosis in patients with NAFLD is driven by repetitive liver injury from lipid peroxidation, fatty acid toxicity, mitochondrial impairment, and oxidative stress.
Viral infection with HBV and HCV lead to cirrhosis by different mechanisms. HBV induces transgene activation, oncogene transcription, and loss of tumor suppressor genes as a result of viral DNA integration into the host genome (Brechot et al, 1980; Bressac et al, 1990; Di Bisceglie, 2000; Kim et al, 1991). As many as 30% of patients with chronic HBV without cirrhosis will develop HCC (Zhou et al, 2001). HCV does not integrate into the host genome but rather leads to chronic inflammation through continued viral replication (Sherlock, 1994). This rapid hepatocyte turnover in the presence of oxidative stress drives the development of multiple dysplastic nodules.
Unlike what has been found in some other cancers, no sequential progression of genetic defects has been identified that leads to the development of HCC. Instead, a variety of genetic alterations are commonly seen, and they have been broadly grouped into events that occur either early or late in the development of a tumor. Genetic alterations that lead to inhibition of the insulin-like growth factor II receptors are considered early mutations (Yamada et al, 1997). Loss of heterozygosity of a variety of genes, often of tumor suppressor genes such as TP53, is a late event in the development of neoplasia (Buetow et al, 1989; Fang et al, 2000).
Clinically Relevant Aspects of Hepatocellular Carcinoma Pathogenesis
First, HCC most commonly develops in the context of cirrhosis, and the biology of HCC is related directly to the environment found in the cirrhotic liver. Repeated insult causes chronic inflammation driven by tumor necrosis factor (TNF)-α; transforming growth factor (TGF)-β and interleukin (IL)-1 from Kupffer cells; endothelial cells; and hepatocytes. This inflammation stimulates nodular regeneration with bridging fibrosis. Rapid cellular turnover in this regenerative environment leads to low-grade dysplastic nodules that do not contain atypia and high-grade dysplastic nodules that do. Eventually, a small percentage of these high-grade dysplastic nodules will go on to develop microscopic foci of HCC before becoming frank carcinoma (Rocken & Carl-McGrath, 2001). Recent evidence suggests that tumor cells may even secrete IL-6 and TNF-α, directly contributing to the inflammatory microenvironment by activating Toll-like receptors (Kim et al, 2009).
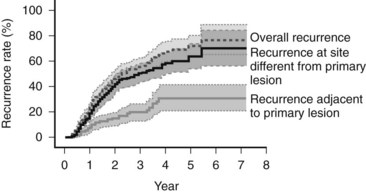
Because of the diffuse nature of the injury, multiple tumors can develop at the same time. If a focus of HCC is removed, recurrence most often happens at a distant site (Koike et al, 2000; Fig. 97D.1). This second tumor at the distant site is most likely a second primary tumor. As most of the second lesions are found within a few years of treatment of the primary lesion, the tumor was most likely present when the first tumor was treated but was too small to be detected. The occurrence of synchronous and metachronous lesions in the same organ suggests the entire cirrhotic organ has the potential for neoplastic conversion, much like the genetic defect in the colon in familial adenomatous polyposis, which leads to multiple cancers; this situation is referred to as a field defect.
Secondly, there is a clinically relevant difference in blood supply between foci of HCC and low-grade dysplastic nodules, which derive most of their blood supply from the portal vein. Histopathologic evaluation of high-grade dysplasia and HCC demonstrates the tumor being fed by branches of the hepatic artery that are not paired with the other structures of the portal tract, a process called arterial recruitment, or neoangiogenesis. The resulting hepatic artery dominance in blood supply produces the characteristically hyperdense appearance of HCC that is used to identify tumors during the arterial phase of contrast imaging (Matsui, 2004; Willatt et al, 2008), and it allows for treatment via selective hepatic artery embolization.
Lastly, HCC is known to invade vascular structures, most commonly the portal vein but also hepatic veins and, less commonly, the biliary tree. The development of vascular invasion appears to be a key step in the metastatic potential of the tumor, and the risk of portal venous invasion seems to be directly related to size and differentiation status of the tumor (Esnaola et al, 2002). Venous invasion is such a strong predictor of metastatic disease that tumor size and tumor number can be thought of as surrogate markers for vascular invasion.
Hepatocellular Carcinoma Screening
HCC presenting as symptomatic disease has a 0% to 10% 5-year survival rate (Llovet et al, 2003), so a screening program that identifies early disease can have a major impact on treatment outcomes. HCC meets the World Health Organization criteria for performing screening (Meissner et al, 2004), and a randomized, controlled clinical trial showed that screening for HCC reduces mortality (Zhang et al, 2004). An effective screening program evaluates patients at risk of developing disease, using a highly standardized and relatively inexpensive test with a high sensitivity. A screening program is used if the cost of screening a large population is less than the benefit of early detection of the disease in a small number of individuals in that population. Several decision-analysis and cost-efficiency models for HCC screening suggest the ability of a screening program to decrease mortality while remaining cost-effective depends on the inclusion of patients with an incidence of HCC of at least 1.4% to 1.5% (Arguedas et al, 2003; Lin OS, et al, 2004; Sarasin et al, 1996). Patients who should be included in a screening program can be found in Box 97D.1. Further information about the incidence of HCC in cirrhosis from nonalcoholic steatohepatitis (NASH), α1-antitrypsin deficiency, and autoimmune hepatitis is required, but most centers include these patients in their screening population.
Box 97D.1 Populations with Hepatocellular Carcinoma Incidence Sufficiently High for Inclusion in Surveillance Programs
Traditionally, the most commonly used serum tumor marker to detect HCC was α-fetoprotein (AFP), a protein produced by normal fetal hepatocytes, and by tumor cells, but not by healthy, mature hepatocytes. AFP has traditionally been used for diagnosing HCC, predicting outcome after resection, and screening for recurrence postoperatively (Tan et al, 2003). The normal range of AFP in the serum is 10 to 20 ng/mL; an absolute value of 20 ng/mL was used commonly as the screening cutoff; however, using this cutoff yields a sensitivity of only about 64%, making it a poor screening test (Sherman et al, 1995). Moreover, normal AFP levels vary widely across ethnic backgrounds (Soresi et al, 2003), and the test has a lower positive predictive value in patients with acute viral hepatitis or cirrhosis from a viral cause (Nguyen et al, 2002; Soresi et al, 2003).
In patients with a liver mass, a concomitant serum AFP greater than 200 ng/mL carries a very high positive predictive value (Sherman et al, 1995), but because of its unacceptably low sensitivity and specificity, the American Association for the Study of Liver Disease (AASLD) recommended that serum AFP no longer be used as a screening tool, although it still plays a diagnostic role (Bruix & Sherman, 2005). Using an absolute value greater than 400 ng/mL is considered diagnostic regardless of the patient’s ethnic background or the cause of their liver disease. Research is ongoing to identify better serum markers for screening, diagnosis, and prognostication, and possible candidate compounds include AFP-L3 and des-γ-carboxy prothrombin (Bruix & Sherman, 2005; Grazi et al, 1995; Izuno et al, 1995; Koike et al, 2001; Marrero et al, 2003; Tsai et al, 1990).
Ultrasound (US) is the most widely used radiologic screening tool. It has a sensitivity of 65% to 85% and specificity over 90% when used as a screening test (Bolondi et al, 2001). Although the performance of US in cirrhotic patients is not as well defined (Bruix et al, 2001; Chen et al, 2002; Larcos et al, 1998; Sherman, 1999), and the test is operator dependent, US remains superior to any widely available serologic screening test. A large, prospective, randomized controlled study done in China suggests that surveillance of high-risk individuals with US and serum AFP testing every 6 months reduces HCC-related mortality by close to 40%, even though patients in that trial were offered only resection but not transplantation. One of the limitations of this study is that screening compliance was only approximately 60%, which may have dampened the reduction in mortality (McMahon, 2009; Zhang et al, 2004). Combined AFP and US surveillance increases sensitivity, but it raises the cost, and using both tests increases the rate of false-positive results from 5% for AFP alone to 7.5% for combined screening (Zhang & Yang, 1999). It is clear that further randomized controlled trials are needed, but based on the available information, the AASLD recommends screening patients at risk for HCC with US alone every 6 to 12 months (Bruix & Sherman, 2005). Evaluation of serum AFP can be added to US in the event of concerns regarding operator influence, but the use of AFP as the lone surveillance tool is discouraged.
Most centers use a 6-month screening interval, but this remains controversial. No evidence suggests that a 6-month interval improves outcome compared with a 12-month interval (Santagostino et al, 2003). The surveillance interval was derived from tumor growth rates, so the interval does not need to be shortened for patients who have a very high risk of developing HCC.
A nodule that is 2 cm or larger and hypervascular with portal venous washout on a single dynamic imaging study, or a 1- to 2-cm nodule on two such confirmatory studies, is treated as HCC. If the results are contradictory, a biopsy of the lesion should be obtained (Bruix & Sherman, 2005). If the lesion is larger than 2 cm and has a typical appearance on a single dynamic imaging study, or if it is found in concert with a serum AFP level above 200 ng/mL, biopsy is not required. Biopsy should be performed on any 1- to 2-cm lesion in any noncirrhotic patient has who has risk factors for the development of HCC. Biopsies should be read by expert pathologists, and patients with negative biopsy results should undergo US or CT scanning every 3 to 6 months and should be rebiopsied if the characteristics of the lesion change (Bruix & Sherman, 2005). Biopsy results that suggest a high risk for progression to HCC include dysplastic nodules, both low and high grade (Hytiroglou et al, 2007); small and large cell change, formerly called small cell dysplasia and large cell dysplasia (International Working Party, 1995; International Consensus Group for Hepatocellular Neoplasia [ICGHN], 2009); and dysplastic foci, defined as atypia outside of a dysplastic nodule (ICGHN, 2009).
Another commonly used radiologic surveillance tool is CT scanning (Kobayashi et al, 1985; Miller et al, 1994; Takayasu et al, 1990). Although the use of CT scanning is becoming commonplace, the radiation exposure to a patient from screening every 6 to 12 months is considerable. The use of CT for diagnosis and staging has been defined, but the performance of CT as a screening test has not been described. Finally, CT scan is the diagnostic test used to confirm HCC, and the screening test should be different from the confirmatory test.
MRI with gadolinium-based or iron oxide–based contrast has not been widely studied as a screening test, but there are reports that this modality is 81% sensitive and 85% specific for HCC compared with 68% and 93% for contrast-enhanced helical CT scanning (Colli et al, 2006). Moderate signal strength on T2-weighted images is specific for HCC, because dysplastic and regenerating nodules are not hyperintense on T2—unless they have infarcted, which can occur. Delayed venous phase hypointensity with concomitant delayed enhancement of the rim of the lesion are also features specific for HCC (Freeny et al, 2003). Similar to CT scanning, characterization of small nodules remains difficult, as MRI is only 50% to 80% sensitive for lesions 2 cm or smaller and only 4% to 33% sensitive for lesions 1 cm or smaller (Willatt et al, 2008); this is due in part to the fact that lesions 1 cm or smaller are frequently isointense on T2 (Krinsky, 2004). It remains to be seen whether contrast-enhanced MRI is cost-effective as a screening tool for HCC, but it may prove to be marginally superior to CT scanning for characterization of known hypervascular lesions.
Treatment Options
HCC is not responsive to traditional cytotoxic chemotherapeutic agents. Recently, a Phase III clinical trial showed that sorafenib, an oral multikinase inhibitor of the vascular endothelial growth factor receptor, the platelet-derived growth factor receptor, and Raf kinase prolonged the median survival (10.7 vs. 7.9 months) and the time to radiologic progression (5.9 vs 2.8 months) in patients with advanced HCC versus placebo (Llovet et al, 2008). Given this modest improvement, local ablation/embolization, hepatic resection, and liver transplantation are still the best options available for patients with early stage disease to achieve a cure.
Treatment decisions should be based on three factors: 1) extent of local disease, 2) underlying liver disease, and 3) local organ availability. Patients whose liver disease is Child-Turcotte-Pugh (CTP) class A who have early-stage HCC are candidates for treatment with the goal of long-term survival free of HCC. Unfortunately, because of the advanced biologic activity of the cancer, 80% to 90% of patients will present at a stage too advanced for surgical resection or transplantation (Llovet et al, 2004). Those patients with advanced disease or patients with poor functional status should be offered sorafenib, locoregional therapies, or palliative care.
In the rare patient without cirrhosis, no evidence of venous invasion or metastatic disease, and tumor burden limited to one lobe, resection should be considered. Conversely, resection of part of a cirrhotic liver invariably leaves scattered dysplastic nodules behind. The tumorigenic cytokine milieu that bathes the remaining dysplastic nodules puts the patient at risk for the development of a second primary tumor. In addition to these de novo tumors, some researchers have suggested recurrence is more frequently the result of microscopic dissemination of the primary tumor at the time of treatment (Chen et al, 2000; Morimoto et al, 2003). Astoundingly, the risk of developing recurrence after resection for HCC in cirrhotic patients is about 35% at 1 year (Lu et al, 2009), 40% to 50% over 3 years (Koike et al, 2000; Jaeck et al, 2004; Sakon et al, 2000), and as high as 70% at 5 years (Bismuth et al, 1999; Llovet et al, 1999; Mazzaferro et al, 1996; Minagawa et al, 2003), making the history of a previous HCC the greatest risk factor in the development of a new primary HCC.
It is this high risk of recurrence that has driven liver transplantation as a method to remove the precancerous liver to prevent the development of subsequent lesions. The results of liver transplantation have improved dramatically in the last 20 years, in large part because of better patient selection, and currently the recurrence rate ranges from 11% to 18%. The mortality rate after liver resection for HCC is 1% to 7%, and the major cause is decompensated liver failure (Fong et al, 1999; Grazi et al, 2001; Hanazaki et al, 2000; Hsia et al, 2000; Makuuchi et al, 1998; Poon et al, 2001; Takenaka et al, 1996; see Chapter 90F). Given the risks of HCC recurrence and postoperative liver failure following resection, some controversy exists regarding the best treatment strategy in patients with limited disease and adequate hepatic function. Although sufficient hepatic reserve can usually be determined, no method is currently available to determine whether a patient will be among the 60% to 70% to recur within 5 years, or if they will remain recurrence free.
A decision analysis from 2000 compared primary resection and salvage transplantation for recurrence versus primary liver transplantation in patients with small tumors (Majno et al, 2000). Theoretic decision models use multiple variables, so the weight of any single variable is difficult to determine, but the authors concluded that wait times shorter than 6 months favored liver transplantation, and those longer than 12 months favored primary liver resection.
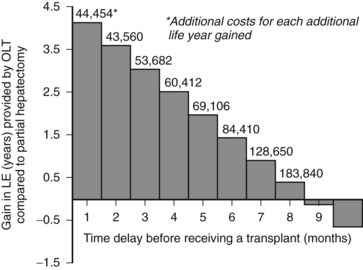
Another decision analysis compared the projected survival benefit from liver transplantation when compared to resection in patients with small HCCs (Sarasin et al, 1998). The authors noted that the magnitude of the survival benefit relied heavily on the amount of time spent on the waiting list. They concluded that if the waiting period exceeded 6 to 10 months, the survival benefit gained from removal of the field effect is overwhelmed by the risk of progression while waiting, and resection becomes the preferred therapy (Fig. 97D.2). Although this analysis did not include the costs or clinical effects of locoregional therapy, it offers insight into how extended waiting times increase the relative benefit of resection, transarterial chemoembolization (TACE), and radiofrequency thermal ablation (RFA; see Chapters 83 and 85A).
The possibility of mitigating the risk of progression with a locoregional therapy while awaiting a transplantation has been suggested. Whether locoregional therapy offers a measure of disease control while awaiting a liver transplantation is not clear. Maddala and colleagues (2004) examined 54 patients with HCC treated with an average of three TACE sessions prior to transplantation at a median of 211 days. Eight patients dropped off the waiting list, two for non-HCC reasons and six from HCC progression. Of the six with progression, two developed extrahepatic metastases, two developed portal vein invasion, and two had intrahepatic progression. Interestingly, these events all occurred within 4 months of listing. The short period of time between initiation of therapy and discovery of disease progression would suggest the disease had spread much earlier, and neither resection nor transplantation would have been helpful.
In a small study of 20 patients (median of two treatments, mean time to transplantation of 343 days), Hayashi and colleagues (2004a) found that seven patients whose tumors were within Milan guidelines dropped off the waiting list: two for causes unrelated to HCC, one for decompensation, and the other five for increased intrahepatic disease. The time to exclusion for HCC was less than 6 months in four of five patients, and no patients developed HCC after transplantation at a mean follow-up of 2.9 years.
RFA has also been reported to provide good results in patients who undergo subsequent transplantation. Mazzaferro and colleagues (2004) reported 50 patients who underwent a single ablation session and then waited an average of 9.5 months before transplantation. Apparently, there were no waiting list dropouts, and at a median follow-up of 22 months, only two patients had cancer recurrence. Lu and colleagues (2005) reported 52 patients who underwent RFA, most frequently in isolation (mean sessions 1.46). After 12 months, three patients had dropped out at a mean of 11 months, and two patients had extrahepatic disease; in all, 41 patients underwent transplantation, and none had HCC recurrence at a mean follow-up of 15 months. For small tumors, it has been suggested that RFA may be equivalent to resection for long-term disease control, with a lower risk of postoperative liver decompensation (Shiina, 2009), but further trials with long-term outcome data are required.
Indications for Transplantation (See Chapter 97A)
An unlimited supply of organs and money would allow for a liberal policy of transplantation for patients with HCC confined to the liver; but resources are limited, so efforts must be made to allocate organs to those recipients with a high likelihood of long-term survival. Recurrence risk is clearly higher in patients with more advanced disease, and the size and number of nodules in the patient’s explanted liver is directly correlated with the risk of HCC recurrence (Jonas et al, 2001; Mazzaferro et al, 1996).
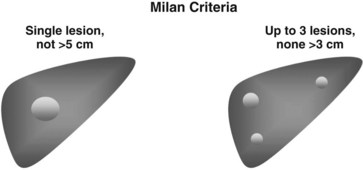
Based on these observations, selection criteria have been developed. The primary set of selection criteria are the Milan criteria, created to allocate organs to patients with HCC who would have a similar chance for long-term survival as patients without HCC (Mazzaferro et al, 1996; Fig. 97D.3). To allocate scarce organs in such a way that all recipients have an acceptable chance for long-term survival, patients with HCC are currently evaluated for suitability based on three factors: 1) tumor size in centimeters, 2) number of radiologically evident tumors, and 3) evidence of portal venous invasion or extrahepatic disease. These factors are a crude but somewhat effective method for predicting overall tumor biology and subsequent risk of recurrence.
Preoperative staging, as outlined by UNOS, includes a triple-phase CT scan (Otto et al, 2006) of the abdomen to quantify intrahepatic disease burden, and a scan should be done of the chest to look for extrahepatic disease. This can also be accomplished with an MRI of the abdomen and a noncontrast CT of the chest (Bruix et al, 2003; Maddala et al, 2004). Unfortunately, these imaging modalities—the gold standard for HCC imaging—understage about 20% of patients (Yao et al, 2005) and can sometimes also overestimate actual tumor burden. A review of the HCC imaging literature that included only studies with explant pathologic correlation reported that triple-phase CT scan is only 37% to 75% sensitive at detecting HCC in a cirrhotic liver (Krinsky, 2004), although it remains the gold standard for diagnosis and preoperative staging. Bone scintigraphy was used in the past to locate extrahepatic disease but is no longer used because of low sensitivity and specificity.
In the United States, the modified tumor-node-metastasis (TNM) staging system of the American Liver Tumor Study Group (ALTSG) is used to allocate livers from deceased donors to patients with HCC awaiting transplantation (UNOS, 2004; Table 97D.1) to ensure that all patients who receive an organ have a reasonable chance at long-term survival. The original transplantation criteria from the mid-1990s, the Milan criteria, are equivalent to stage I or II of this TNM classification system. Using these criteria, recurrence is between 4% and 16%, and 5-year survival is 71% to 75% (Hayashi et al, 2004b; Jonas et al, 2001; Llovet et al, 1998, 1999). Patients whose disease falls outside the ALTSG criteria for stage II disease, and thus outside the Milan criteria, may be at higher risk for recurrence.
Table 97D.1 American Liver Tumor Study Group Modified TNM Staging and the University of California–San Francisco Staging Criteria
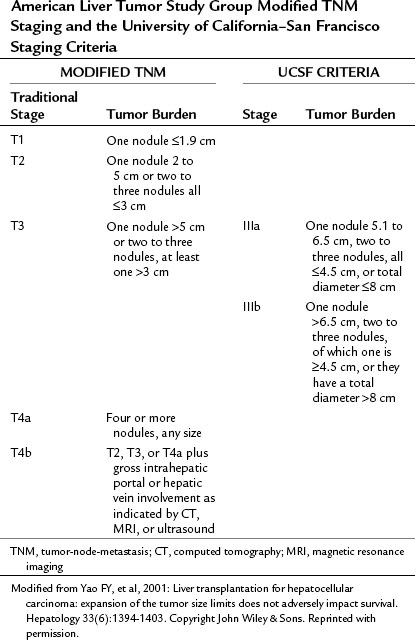
In February 2002, the Model for End-Stage Liver Disease (MELD) scoring system was implemented. As part of this system, priority was given to patients with TMN stage T1 and T2 HCC in an effort to combat the risk of tumor progression in patients awaiting a transplant. The prioritization was revised in 2004 to no longer include T1 tumors, so that only patients with T2 tumors get priority listing (Wiesner et al, 2004) to prevent overallocation of organs to patients with HCC, and because approximately 30% of patients with presumed HCC (a single lesion ≤2 cm with arterial enhancement) were found to have no tumor in their explant (Wiesner et al, 2004). Currently, the MELD points added to a patient with a T2 lesion are equivalent to a 15% 3-month probability of candidate mortality.
It has been suggested that the Milan criteria are too restrictive and that the size criteria are somewhat arbitrary. Several centers have extended their criteria to include highly selected stage III patients who are expected to have a tolerable rate of recurrence (Duffy et al, 2007; Herrero et al, 2001; Kneteman et al, 2004; Onaca et al, 2007; Roayaie et al, 2002; Yao et al, 2001, 2007). A recent retrospective analysis of 1556 patients undergoing transplantation at 36 centers included 1112 patients with tumor burden exceeding the Milan criteria on posttransplant pathologic review. Of these, 283 patients had no evidence of microvascular invasion with tumor burden up to 7 cm in largest diameter or up to seven nodules, and this subset of patients achieved a 5-year survival of 71%.
The Univerity of California–San Francisco (UCSF) criteria—single tumor smaller than 6.5 cm, maximum of three total tumors with none larger than 4.5 cm, and cumulative tumor size smaller than 8 cm (see Table 97D.1)—represent a modest expansion of the Milan criteria and have been validated at other centers. One such evaluation, a retrospective review of preoperative imaging and explant pathology, looked at 467 transplantations performed over a 20-year period and found similar 5-year survival in patients meeting the Milan criteria and the UCSF criteria by explant pathology (86% vs. 71%) and preoperative imaging (79% vs. 64%). Survival for patients with tumors beyond UCSF criteria was less than 50% at 5 years (Duffy et al, 2007).
Another consideration is the impact of MELD exception points for cancer patients or the selection of patients with liver failure as a result of benign disease. In regions with long waiting times, if patients with tumor burden beyond the Milan criteria are transplanted, organs are taken away from patients with liver failure from other causes. Volk and colleagues (2008) examined the issue of what is the acceptable outcome risk for transplantation of patients with HCC as compared to using the organ in a patient without HCC. On a national basis, the authors found that expansion of the Milan criteria would significantly increase waiting list mortality, unless 5-year posttransplant survival for the expanded criteria recipients exceeded 61%.
Pretransplant Management of Patients with Hepatocellular Carcinoma
Unfortunately, controlled trials to evaluate different treatment options for patients awaiting transplantation have not been completed. The sole published series followed patients undergoing surveillance without local therapy and showed a high rate of dropouts from the waiting list because of tumor progression or progression of liver failure over the period of the study (Llovet et al, 1999). This report, combined with the understandable anxiety felt by patients and caregivers observing unhindered tumor progression, compelled most centers to offer cancer-directed treatment for patients awaiting transplantation. Retrospective data would suggest that cancer-directed therapy should be used if the expected waiting time exceeds 6 months (Bruix & Sherman, 2005).
Options for pretransplant locoregional control include TACE, RFA, percutaneous ethanol injection (Hoshida et al, 2008), or some combination of these modalities (see Chapters 83 and 85A). TACE is used by many centers because it offers locally effective treatment with a low risk of complications. Three studies support the argument that TACE may help reduce attrition from the pretransplant waiting list (Maddala et al, 2004; Majno et al, 2007; Millonig et al, 2007). These data include patients with variable waiting times and follow-up periods, but they suggest that pretransplant TACE is associated with a dropout rate of only 2% to 14%, with the possible added benefit of a lower posttransplant recurrence rate (8% to 10%).
A recent decision analysis evaluated the waiting-list time interval associated with the greatest impact of neoadjuvant TACE (i.e., greatest reduction in the dropout rate) and showed the greatest benefit in patients transplanted within 4 to 9 months (Aloia et al, 2007). The authors reported that when waiting time was less than 4 months, waiting-list attrition was similar in treated and untreated patients (20% vs. 34%; P = .08), and when waiting times exceed 9 months, dropout rates reequilibrated (33% vs. 46%; P = .06).
No studies have described percutaneous ethanol injection (PEI) as a sole treatment in patients awaiting a transplantation, although several studies suggest it is a safe and reliable component of multimodal therapy for tumors smaller than 3 cm (Troisi et al, 1998; Veltri et al, 1998; Vilana et al, 1992). PEI uses a small needle that reduces the risk of tract seeding to 0.6%. Some authors have suggested that pretreatment with arterial embolization to disrupt intratumoral septae facilitate the ethanol diffusion and may therefore improve the efficacy of subsequent ethanol injection (Lencioni et al, 1994). PEI requires several sessions when used as primary treatment for unresectable HCC, and prospective evidence supporting it as a neoadjuvant therapy is lacking; as a result, the trend has been toward other treatment methods.
RFTA induces tissue necrosis by delivering heat at the tip of one or several electrodes. Major complications occur in less than 10% of patients, and mortality is less than 1% (Orlando et al, 2009). This strategy is at least as efficacious as PEI for tumors smaller than 2 cm but requires fewer treatments (Livraghi et al, 1999; Lencioni et al, 2003), and randomized controlled trials suggest it improves survival (Lencioni, et al, 2003; Lin SM, et al, 2004; Livraghi et al, 1999).
A recent meta-analysis reported that RFA is superior to PEI in the treatment of small HCC with respect to survival and cancer-free survival at 1, 2, and 3 years; tumor response; and risk of local recurrence (Orlando et al, 2009). Recurrence is more common in patients with tumors larger than 4 cm, and in 95%, it occurs within 6 months and at a site distant from the primary tumor (Curley et al, 2000). This again suggests that tumor size is only a surrogate marker for microscopic metastatic spread and neoplastic potential of the remaining dysplastic nodules.
Given these data, RFA has evolved into the preoperative treatment of choice for tumors larger than 2 cm (Bruix & Sherman, 2005). With the advent of sorafenib, a chemotherapeutic agent that has shown greater activity against HCC than older cytotoxic agents (see Chapter 88), the paradigm may shift to a combination of locoregional therapy and chemotherapy. Trials examining these combinations are underway.
Patients with small tumors that fall within the Milan criteria have about a 10% risk of recurrence, which corresponds reasonably well to the percentage of patients with tumors that have aggressive histologic characteristics (Jonas et al, 2001). Fast-tracking such patients likely would significantly increase recurrence rates. One series examining this issue showed that recurrence rates were significantly higher in patients who were fast-tracked and in patients who underwent living-donor liver transplantation (LDLT) compared with patients who had longer waiting times (Kulik & Abecassis, 2004).
Patients with small but aggressive tumors are infrequent, but fast-tracking yields unacceptably high recurrence rates in the more common patient, with tumor burden close to the limits of or beyond the Milan criteria (Bismuth et al, 1993; Jonas et al, 2001; Llovet et al, 1999; Mazzaferro et al, 1996). It has also been suggested that fast-track prioritization is not cost-effective (Sarasin et al, 2001). Unfortunately, no stated guidelines have been established, but it appears that even for LDLT, a waiting time of approximately 6 months is appropriate (Bruix & Llovet, 2002).
Downstaging
Two observations have fueled the current debate about the proper selection criteria for transplantation in patients with HCC. First, it is clear that not all tumors of the same size carry the same risk of recurrence. Secondly, it is well documented that some patients outside the Milan criteria will benefit from liver transplantation (Yao, 2008). This has led to efforts to select patients with more advanced disease, but with favorable tumor biology, who might benefit from transplantation (Otto et al, 2006). The process of downstaging disease to within Milan criteria may be a mechanism for identifying such patients. This approach allows treatment of the tumor, and concomitant selection of patients with favorable tumor biology, by excluding patients who do not respond to locoregional ablative therapy, and it allows time for unfavorable biology to manifest.
A prospective intention-to-treat analysis evaluated 61 highly selected patients with tumor stage exceeding T2 who underwent tumor-directed therapy prior to transplantation. The study included patients with one lesion larger than 5 cm but smaller than 8 cm; those with two to three lesions, with one lesion larger than 3 cm but smaller than 5 cm, with a total diameter less than 8 cm; and those with four to five lesions, all smaller than 3 cm with a total diameter less than 8 cm (Yao et al, 2008). An interval of at least 3 months was required between treatment and transplantation. Downstaging was successful in 70% of patients, with the remaining patients dropping out, usually from disease progression. The 1- and 4-year posttransplant survival rates were 96% and 92%, respectively, suggesting that carefully selected patients with disease exceeding the Milan criteria can be downstaged with excellent posttransplant outcome.
It is important to note that the median time between the first treatment and transplantation was 8.2 months, with a range of 3 to 25 months. It is this waiting time that appears to allow tumor biology to manifest; patients with more biologically aggressive tumors dropped out during the waiting period, allowing selection of lower risk patients for transplantation. There are no formal guidelines for downstaging, but the UCSF protocol for downstaging of patients initially exceeding the Milan criteria is included for reference (Box 97D.2).
Box 97D.2 University of California–San Francisco Downstaging Protocol for Patients Initially Outside the Milan Criteria
Additional Guidelines
A minimum follow-up period of 3 months is required before liver transplantation, with concomitant imaging studies that meet the “success” criteria.
Approval must be obtained by the regional United Network of Organ Sharing review board for priority listing for deceased-donor liver transplantation after successful downstaging.
Patients can undergo living-donor liver transplantation if a donor is available and imaging studies meet the selection criteria.
Patients with liver decompensation after downstaging are not eligible for liver transplantation unless the above criteria are met.
In patients treated with resection as a downstaging procedure, the presence of microvascular invasion in the resection specimen is a contraindication to liver transplantation.
Predicting and Screening for Recurrence
The propensity of HCC to recur after surgical therapy has spurred investigations of potential biomarkers that might help improve patient selection. Recently, formalin-fixed tissue was used for genome-wide expression profiling of the normal tissue surrounding a focus of HCC, and a reproducible expression signature was seen that correlated well with survival (Hoshida et al, 2008). Even with the diversity of genetic events that lead to HCC, specific regions of loss of heterozygosity have been identified that could be used to quantify a patient’s risk of HCC recurrence.
Efforts have been made to identify markers of extrahepatic tumor spread to avoid transplantation in the face of unrecognized extrahepatic disease. One attempt has been to find evidence of malignant cells in the bone marrow of patients with HCC as a method to predict recurrence or survival (Sutcliffe et al, 2005), although this practice is not routinely performed.
A recent multivariate analysis of 136 patients undergoing hepatectomy for HCC reported that low AFP mRNA levels identified by real-time quantitative polymerase chain reaction (PCR) correlated with patient survival and disease-free survival (Kamiyama et al, 2006; Lindemann et al, 1992; Pantel et al, 1996). Patients with negative AFP mRNA had 97% survival at 1 year and 91% survival at 3 years versus 86% and 56%, respectively, in patients with positive AFP mRNA (P < .0001). Similarly, 1- and 3-year disease-free survival were 73% and 45% in patients with negative AFP mRNA versus 55% and 26%. Although not widely used, these tests may help quantify risk of recurrence and could have implications for organ allocation and the effectiveness of transplantation for cure.
Unfortunately, evidence-based data regarding posttransplant screening protocols are lacking, and screening all patients with imaging and serum AFP for recurrence does not appear to be cost effective (Roberts, 2005). Alternatively, screening high-risk patients, as defined by adverse histopathologic characteristics in the explanted liver, is a more promising and potentially cost-effective strategy (Roberts, 2005; Ladabaum et al, 2011). Moreover, because 70% to 75% of recurrence occurs within the first 2 years after transplantation, screening during this time period is the most critical and is most likely to have the greatest yield (Regalia et al, 1998; Roayaie et al, 2004).
Once patients experience recurrence after transplantation, therapeutic options are somewhat limited. In approximately 40% of patients, HCC recurs at extrahepatic sites, and chemotherapy is generally ineffective, leading to a median survival of only 8.7 months (Mazzaferro et al, 1996). A subset of roughly 10% of patients with recurrence can be expected to achieve long-term survival after resection (Roberts, 2005). The use of sorafenib in this situation is currently being studied.
Salvage Transplantation
As a way to better maintain the pool of donor organs, some have argued that primary liver resection should be first-line therapy for patients with preserved liver function and solitary HCCs smaller than 5 cm, and salvage transplantation can be offered to patients who experience recurrence that falls within the Milan criteria. In 2003, Poon and Wong evaluated survival and recurrence after resection of potentially transplantable small HCCs. They concluded that in patients with preserved liver function (CTP class A) with small solitary HCCs, primary resection achieved 5-year survival of 70%, comparable to reported survival after primary transplantation for similar size tumors. In order to apply salvage transplantation, screening must be diligent to find those who recur. Screening after resection with US every 3 months would be an important aspect of any treatment strategy using salvage transplantation for treatment of recurrence. The hope is that if patients recur, they will do so with transplantable disease within the Milan criteria.
Two studies have addressed the issue of outcome after salvage transplantation but reached opposing conclusions. The first was a retrospective analysis of 358 patients with cirrhosis and HCC who underwent either transplantation (n = 195) or resection (n = 163, of whom 98 were transplant candidates at the time of therapy); those patients with recurrence after resection underwent salvage transplantation (n = 17). The authors reported lower operative mortality rate (2% vs. 28%), lower recurrence rate (18% vs. 54%), and higher 5-year posttransplant survival (61% vs. 41%) and disease-free survival rates (58% vs. 29%) for patients who underwent transplantation compared with patients who underwent resection and then had salvage transplantation for recurrence (Adam et al, 2003).
The results of this study suggest that resection and salvage transplantation produced worse outcomes, but this was not supported by the second study of 88 patients with disease meeting the Milan criteria who underwent liver transplantation versus 18 who underwent resection followed by salvage transplantation for recurrence (Belghiti et al, 2003). The authors reported no difference in morbidity (51% vs. 56%), 30-day mortality (5.7% vs. 5.6%), 3-year survival (82% vs. 82%), 5-year survival (59% vs. 61%), or recurrence after primary liver transplantation versus salvage transplantation (n = 3 vs. 1).
Reasons for the discordant results could be the differences in time to salvage transplantation, patient selection, or operative technique of liver resection between the studies (27% underwent a transthoracic resection in the latter study). Another issue limiting the comparability of these two studies is that 7 of the 18 patients who underwent “salvage transplantation” in the latter study did not have recurrence but were transplanted for deterioration of liver function or because of pathologic findings in the resection specimen. Also of note, as Adam and Azoulay pointed out in a letter to the editors of the Annals of Surgery, the theoretical transplantability of patients with recurrence after resection seems to be different than the actual transplantability documented by many centers (Adam & Azoulay, 2005). Although Poon and colleagues describe an optimistic rate of patients with recurrence who would theoretically be transplantable after resection (79%), the percentage of patients who have undergone salvage transplantation, documented in the literature, is 1% to 30% (Belghiti et al, 2002; Llovet et al, 1999; Poon et al, 2002).
Reports of recurrence after salvage vary widely, from 5% to 54% (Adam et al, 2003; Belghiti et al, 2003; Lo & Fan, 2004). Currently, the efficacy of resection followed by salvage transplantation for recurrence is limited by the difficulties that attend screening for recurrence, prolonged waiting times, and a shortage of donor organs, but it may be a viable option for patients with hepatitis B without cirrhosis who have a single lesion.
Future Perspectives
One strategy would be to use the well-established histopathologic findings of microvascular invasion or multiple nodules unseen on preoperative imaging (Castells et al, 1993; Ikeda et al, 1993b; Izumi et al, 1994; Llovet et al, 1999; Nagasue et al, 1993; Okada et al, 1994) to select patients who are high risk for recurrence after resection. Identification of these findings in the resection specimen could initiate listing for liver transplantation (Sala et al, 2004). This strategy may select for patients who are at risk for early recurrence to undergo transplantation (Colella et al, 1997; Klintmalm, 1998), thus selecting a population that is likely to recur, even if they do undergo transplantation. The concern with this strategy is that patients whose explant specimens after transplantation demonstrate vascular invasion are at increased risk for development of extrahepatic disease.
Another possible strategy takes advantage of recent advances in gene-expression profiling of fixed liver tissue, from the liver parenchyma away from the tumor, to reliably predict risk of late (de novo) recurrence by quantifying the field defect (Hoshida et al, 2008). Using this technology, gene-expression profiling could theoretically select the population with a field defect that places them at high risk for late recurrence; this group would perhaps be targeted for transplantation, while allowing those with low risk for late recurrence to have resection and be followed (Sherman, 2008). Although this strategy offers some promise, it remains investigational.
Adam R, Azoulay D. Is primary resection and salvage transplantation for hepatocellular carcinoma a reasonable strategy? Ann Surg. 2005;241(4):671-672.
Adam R, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg. 2003;238(4):508-518. discussion 518-519
Aloia TA, et al. A decision analysis model identifies the interval of efficacy for transarterial chemoembolization (TACE) in cirrhotic patients with hepatocellular carcinoma awaiting liver transplantation. J Gastrointest Surg. 2007;11(10):1328-1332.
Arguedas MR, et al. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: a cost-utility analysis. Am J Gastroenterol. 2003;98(3):679-690.
Belghiti J, et al. Resection of hepatocellular carcinoma: a European experience on 328 cases. Hepatogastroenterology. 2002;49(43):41-46.
Belghiti J, et al. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg. 2003;238(6):885-892. discussion 892-893
Benvegnu L, et al. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53(5):744-749.
Bismuth H, et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218(2):145-151.
Bismuth H, et al. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 1999;19(3):311-322.
Bolondi L, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48(2):251-259.
Bosch FX, et al. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127(5 Suppl 1):S5-S16.
Brechot C, et al. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature. 1980;286(5772):533-535.
Bressac B, et al. Abnormal structure and expression of p53 gene in human hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1990;87(5):1973-1977.
Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35(3):519-524.
Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208-1236.
Bruix J, et al. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35(3):421-430.
Bruix J, et al. Liver transplantation for hepatocellular carcinoma: Foucault pendulum versus evidence-based decision. Liver Transpl. 2003;9(7):700-702.
Buetow KH, et al. Loss of heterozygosity suggests tumor suppressor gene responsible for primary hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1989;86(22):8852-8856.
Castells A, et al. Treatment of small hepatocellular carcinoma in cirrhotic patients: a cohort study comparing surgical resection and percutaneous ethanol injection. Hepatology. 1993;18(5):1121-1126.
Chang MH, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101(19):1348-1355.
Chen TH, et al. Ultrasound screening and risk factors for death from hepatocellular carcinoma in a high risk group in Taiwan. Int J Cancer. 2002;98(2):257-261.
Chen YJ, et al. Chromosomal changes and clonality relationship between primary and recurrent hepatocellular carcinoma. Gastroenterology. 2000;119(2):431-440.
Colella G, et al. Is hepatocellular carcinoma in cirrhosis an actual indication for liver transplantation? Transplant. Proc Natl Acad Sci U S A. 1997;29:492-494.
Colli A, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101(3):513-523.
Curley SA, et al. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232(3):381-391.
Degos F, et al. Hepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and death. Gut. 2000;47(1):131-136.
Deuffic S, et al. Trends in primary liver cancer. Lancet. 1998;351(9097):214-215.
Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology. 2000;31(4):1014-1018.
Duffy JP, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246(3):502-509. discussion 509-511
El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5 Suppl 1):S27-S34.
El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340(10):745-750.
El-Zayadi AR. Hepatic steatosis: a benign disease or a silent killer. World J Gastroenterol. 2008;14(26):4120-4126.
Esnaola NF, et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg. 2002;6(2):224-232. discussion 232
Fang W, et al. Mapping of a minimal deleted region in human hepatocellular carcinoma to 1p36.13-p36.23 and mutational analysis of the RIZ (PRDM2) gene localized to the region. Genes Chromosomes Cancer. 2000;28(3):269-275.
Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1):S99-S112.
Fassio E, et al. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40(4):820-826.
Fattovich G, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35-S50.
Fong Y, et al. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229(6):790-799. discussion 799-800
Freeny PC, et al. Significance of hyperattenuating and contrast-enhancing hepatic nodules detected in the cirrhotic liver during arterial phase helical CT in pre-liver transplant patients: radiologic-histopathologic correlation of explanted livers. Abdom Imaging. 2003;28(3):333-346.
Grazi GL, et al. The role of tumor markers in the diagnosis of hepatocellular carcinoma, with special reference to the des-gamma-carboxy prothrombin. Liver Transpl Surg. 1995;1(4):249-255.
Grazi GL, et al. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2001;234(1):71-78.
Hanazaki K, et al. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. 2000;191(4):381-388.
Harrison SA, et al. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98(9):2042-2047.
Hayashi PH, et al. Hepatic artery chemoembolization for hepatocellular carcinoma in patients listed for liver transplantation. Am J Transplant. 2004;4(5):782-787.
Hayashi PH, et al. Impact of pretransplant diagnosis of hepatocellular carcinoma on cadaveric liver allocation in the era of MELD. Liver Transpl. 2004;10(1):42-48.
Herrero JI, et al. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transpl. 2001;7(7):631-636.
Hoshida Y, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359(19):1995-2004.
Hsia CY, et al. Perioperative safety and prognosis in hepatocellular carcinoma patients with impaired liver function. J Am Coll Surg. 2000;190(5):574-579.
Hui JM, et al. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology. 2003;38(2):420-427.
Hytiroglou P, et al. Hepatic precancerous lesions and small hepatocellular carcinoma. Gastroenterol Clin North Am. 2007;36(4):867-887. vii
Ikeda K, et al. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology. 1993;18(1):47-53.
Ikeda K, et al. Risk factors for tumor recurrence and prognosis after curative resection of hepatocellular carcinoma. Cancer. 1993;71(1):19-25.
International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the International Consensus Group for Hepatocellular Neoplasia. Hepatology. 2009;49(2):658-664.
International Working Party. Terminology of nodular hepatocellular lesions. Hepatology. 1995;22(3):983-993.
Izumi R, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology. 1994;106(3):720-727.
Izuno K, et al. Early detection of hepatocellular carcinoma associated with cirrhosis by combined assay of des-gamma-carboxy prothrombin and alpha-fetoprotein: a prospective study. Hepatogastroenterology. 1995;42(4):387-393.
Jaeck D, et al. Surgical resection of hepatocellular carcinoma: post-operative outcome and long-term results in Europe: an overview. Liver Transpl. 2004;10(2 Suppl 1):S58-S63.
Jonas S, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33(5):1080-1086.
Kamiyama T, et al. AFP mRNA detected in bone marrow by real-time quantitative RT-PCR analysis predicts survival and recurrence after curative hepatectomy for hepatocellular carcinoma. Ann Surg. 2006;244(3):451-463.
Kim CM, et al. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351(6324):317-320.
Kim S, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102-106.
Klintmalm GB. Liver transplantation for hepatocellular carcinoma: a registry report of the impact of tumor characteristics on outcome. Ann Surg. 1998;228(4):479-490.
Kneteman NM, et al. Sirolimus-based immunosuppression for liver transplantation in the presence of extended criteria for hepatocellular carcinoma. Liver Transpl. 2004;10(10):1301-1311.
Kobayashi K, et al. Screening methods for early detection of hepatocellular carcinoma. Hepatology. 1985;5(6):1100-1105.
Koike Y, et al. Risk factors for recurring hepatocellular carcinoma differ according to infected hepatitis virus: an analysis of 236 consecutive patients with a single lesion. Hepatology. 2000;32(6):1216-1223.
Koike Y, et al. Des-gamma-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: a prospective analysis of 227 patients. Cancer. 2001;91(3):561-569.
Krinsky G. Imaging of dysplastic nodules and small hepatocellular carcinomas: experience with explanted livers. Intervirology. 2004;47(3-5):191-198.
Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S277-S282.
Ladabaum M, et al. Cost-effectiveness of screening for recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2011;25(2):283-291.
Larcos G, et al. Sonographic screening for hepatocellular carcinoma in patients with chronic hepatitis or cirrhosis: an evaluation. AJR Am J Roentgenol. 1998;171(2):433-435.
Lencioni R, et al. Transcatheter arterial embolization followed by percutaneous ethanol injection in the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1994;17(2):70-75.
Lencioni RA, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228(1):235-240.
Lin OS, et al. Cost-effectiveness of screening for hepatocellular carcinoma in patients with cirrhosis due to chronic hepatitis C. Aliment Pharmacol Ther. 2004;19(11):1159-1172.
Lin SM, et al. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or = 4 cm. Gastroenterology. 2004;127(6):1714-1723.
Lindemann F, et al. Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet. 1992;340(8821):685-689.
Livraghi T, et al. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210(3):655-661.
Llovet JM, et al. Liver transplantation for small hepatocellular carcinoma: the tumor-node-metastasis classification does not have prognostic power. Hepatology. 1998;27(6):1572-1577.
Llovet JM, et al. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30(6):1434-1440.
Llovet JM, et al. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907-1917.
Llovet JM, et al. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10(2 Suppl 1):S115-S120.
Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390.
Lo CM, Fan ST. Liver transplantation for hepatocellular carcinoma. Br J Surg. 2004;91(2):131-133.
Lu DS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41(5):1130-1137.
Lu X, et al. A prospective clinical study on early recurrence of hepatocellular carcinoma after hepatectomy. J Surg Oncol. 2009;100(6):488-493.
Maddala YK, et al. Drop-out rates of patients with hepatocellular cancer listed for liver transplantation: outcome with chemoembolization. Liver Transpl. 2004;10(3):449-455.
Majno P, et al. Management of hepatocellular carcinoma on the waiting list before liver transplantation: time for controlled trials? Liver Transpl. 2007;13(11 Suppl 2):S27-S35.
Majno PE, et al. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology. 2000;31(4):899-906.
Makuuchi M, et al. Hepatic resection for hepatocellular carcinoma: Japanese experience. Hepatogastroenterology. 1998;45(Suppl 3):1267-1274.
Marrero JA, et al. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in American patients. Hepatology. 2003;37(5):1114-1121.
Matsui O. Imaging of multistep human hepatocarcinogenesis by CT during intra-arterial contrast injection. Intervirology. 2004;47(3-5):271-276.
Mazzaferro V, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693-699.
Mazzaferro V, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240(5):900-909.
McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49(5 Suppl):S45-S55.
Meissner HI, et al. Promoting cancer screening: learning from experience. Cancer. 2004;101(5 Suppl):1107-1117.
Miller WJ, et al. Malignancies in patients with cirrhosis: CT sensitivity and specificity in 200 consecutive transplant patients. Radiology. 1994;193(3):645-650.
Millonig G, et al. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2007;13(2):272-279.
Minagawa M, et al. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003;238(5):703-710.
Miyazawa K, et al. Analysis of background factors and evaluation of a population at high risk of hepatocellular carcinoma. Intervirology. 2003;46(3):150-156.
Morimoto O, et al. Diagnosis of intrahepatic metastasis and multicentric carcinogenesis by microsatellite loss of heterozygosity in patients with multiple and recurrent hepatocellular carcinomas. J Hepatol. 2003;39(2):215-221.
Nagasue N, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 1993;105(2):488-494.
Nguyen MH, et al. Racial differences in effectiveness of alpha-fetoprotein for diagnosis of hepatocellular carcinoma in hepatitis C virus cirrhosis. Hepatology. 2002;36(2):410-417.
Okada S, et al. Predictive factors for postoperative recurrence of hepatocellular carcinoma. Gastroenterology. 1994;106(6):1618-1624.
Onaca N, et al. Expanded criteria for liver transplantation in patients with hepatocellular carcinoma: a report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl. 2007;13(3):391-399.
Orlando A, et al. Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009;104(2):514-524.
Otto G, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12(8):1260-1267.
Pantel K, et al. Frequency and prognostic significance of isolated tumour cells in bone marrow of patients with non–small-cell lung cancer without overt metastases. Lancet. 1996;347(9002):649-653.
Parkin DM, et al. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94(2):153-156.
Pichlmayer R. Liver transplantation in primary hepatocellular carcinoma. J Hepatol. 1993;18(2):151-153.
Poon RT, Wong J. Long-term disease-free survival after resection of hepatocellular carcinoma: both tumor behavior and surgeon’s performance are important determinants. Ann Surg Oncol. 2003;10(8):834-836.
Poon RT, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234(1):63-70.
Poon RT, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235(3):373-382.
Regalia E, et al. Pattern and management of recurrent hepatocellular carcinoma after liver transplantation. J Hepatobiliary Pancreat Surg. 1998;5(1):29-34.
Roayaie S, et al. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235(4):533-539.
Roayaie S, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10(4):534-540.
Roberts JP. Tumor surveillance: what can and should be done? Screening for recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2005;11(11 Suppl 2):S45-S46.
Rocken C, Carl-McGrath S. Pathology and pathogenesis of hepatocellular carcinoma. Dig Dis. 2001;19(4):269-278.
Sakon M, et al. Clinical significance of hepatic resection in hepatocellular carcinoma: analysis by disease-free survival curves. Arch Surg. 2000;135(12):1456-1459.
Sala MJ, et al. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl. 2004;10(10):1294-1300.
Sangiovanni A, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126(4):1005-1014.
Santagostino E, et al. A 6-month versus a 12-month surveillance for hepatocellular carcinoma in 559 hemophiliacs infected with the hepatitis C virus. Blood. 2003;102(1):78-82.
Sarasin FP, et al. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in Western patients with Child-Pugh class A cirrhosis. Am J Med. 1996;101(4):422-434.
Sarasin FP, et al. Partial hepatectomy or orthotopic liver transplantation for the treatment of resectable hepatocellular carcinoma? A cost-effectiveness perspective. Hepatology. 1998;28(2):436-442.
Sarasin FP, et al. Living donor liver transplantation for early hepatocellular carcinoma: a life-expectancy and cost-effectiveness perspective. Hepatology. 2001;33(5):1073-1079.
Sherlock S. Viruses and hepatocellular carcinoma. Gut. 1994;35(6):828-832.
Sherman M. Screening for hepatocellular carcinoma. Baillieres Best Pract Res Clin Gastroenterol. 1999;13(4):623-635.
Sherman M. Recurrence of hepatocellular carcinoma. N Engl J Med. 2008;359(19):2045-2047.
Sherman M, et al. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22(2):432-438.
Shiina S. Image-guided percutaneous ablation therapies for hepatocellular carcinoma. J Gastroenterol. 2009;44(Suppl 19):122-131.
Soresi M, et al. Usefulness of alpha-fetoprotein in the diagnosis of hepatocellular carcinoma. Anticancer Res. 2003;23(2C):1747-1753.
Stroffolini T, et al. Characteristics of hepatocellular carcinoma in Italy. J Hepatol. 1998;29(6):944-952.
Sutcliffe R, et al. Detection and clinical significance of bone marrow micrometastases in patients undergoing liver transplantation for hepatocellular carcinoma. Transplantation. 2005;80(1):88-94.
Takayasu K, et al. The diagnosis of small hepatocellular carcinomas: efficacy of various imaging procedures in 100 patients. AJR Am J Roentgenol. 1990;155(1):49-54.
Takenaka K, et al. Results of 280 liver resections for hepatocellular carcinoma. Arch Surg. 1996;131(1):71-76.
Tan CK, et al. Simple clinical prognostic model for hepatocellular carcinoma in developing countries and its validation. J Clin Oncol. 2003;21(12):2294-2298.
Taylor-Robinson SD, et al. Increase in primary liver cancer in the UK, 1979-94. Lancet. 1997;350(9085):1142-1143.
Troisi R, et al. Multimodal treatment for hepatocellular carcinoma on cirrhosis: the role of chemoembolization and alcoholization before liver transplantation. Clin Transplant. 1998;12(4):313-319.
Tsai SL, et al. Plasma des-gamma-carboxyprothrombin in the early stage of hepatocellular carcinoma. Hepatology. 1990;11(3):481-488.
United Network of Organ Sharing, http://www.unos.org/policiesandbylaws/policies.asp?resources, 2004. UNOS Policy 3.6 organ distribution: allocation of livers. Available at
Veltri A, et al. Effect of preoperative radiological treatment of hepatocellular carcinoma before liver transplantation: a retrospective study. Cardiovasc Intervent Radiol. 1998;21(5):393-398.
Venook AP. Liver transplantation for hepatocellular carcinoma. Hepatology. 1993;18(1):218-219.
Vilana R, et al. Tumor size determines the efficacy of percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Hepatology. 1992;16(2):353-357.
Volk ML, et al. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am J Transplant. 2008;8(4):839-846.
Wiesner RH, et al. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127(5 Suppl 1):S261-S267.
Willatt JM, et al. MR Imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology. 2008;247(2):311-330.
Yamada T, et al. Loss of the gene encoding mannose 6-phosphate/insulin-like growth factor II receptor is an early event in liver carcinogenesis. Proc Natl Acad Sci U S A. 1997;94(19):10351-10355.
Yao FY. Liver transplantation for hepatocellular carcinoma: beyond the Milan criteria. Am J Transplant. 2008;8(10):1982-1989.
Yao FY, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394-1403.
Yao FY, et al. The impact of pre-operative loco-regional therapy on outcome after liver transplantation for hepatocellular carcinoma. Am J Transplant. 2005;5(4 Pt 1):795-804.
Yao FY, et al. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7(11):2587-2596.
Yao FY, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48(3):819-827.
Zhang B, Yang B. Combined alpha fetoprotein testing and ultrasonography as a screening test for primary liver cancer. J Med Screen. 1999;6(2):108-110.
Zhang BH, et al. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130(7):417-422.
Zhou XD, et al. Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer. 2001;91(8):1479-1486.