Chapter 97B Liver transplantation
Anesthesia, perioperative management, and postoperative care
Overview
Approximately 20,000 liver transplantations are performed annually worldwide (Matesanz et al, 2009), but the number of patients on the waiting list continues to exceed the donor organ availability. Because of the continuous donor shortage, dramatic changes in recipient and donor selection have emerged in the past decade, leading to an amplification of clinical challenges for perioperative liver transplant physicians. In fact, United Network for Organ Sharing (UNOS) data from 2009 (UNOS/OTPN, 2010) show that older patients are now accepted to the waiting list and transplanted; combined kidney and liver transplants are performed more often than in prior years; and extended-criteria donors, including donors after cardiac death, are increasingly utilized (see Chapter 97A).
Despite the increasing medical complexity, overall graft and patient outcomes have continued to improve. According to the UNOS database, survival rates for patients transplanted with a cadaveric graft are around 87% at 1 year, 80% at 3 years, and 74% at 5 years. These encouraging figures, achieved by experienced transplant teams, have resulted in part from the progressive advancement and standardization of surgical and anesthetic techniques (Mandell et al, 2008). If the current trends continue, with transplantation of increasingly older and sicker recipients (Costa et al, 2009; Xia et al, 2008) and use of marginal donor organs, the outcomes will greatly depend on accurate preoperative assessment and on appropriate and timely intraoperative medical management of comorbidities.
Preoperative Anesthetic Assessment
Liver transplantation is the current gold standard treatment for acute and chronic irreversible liver failure (see Chapters 70A, 72, and 97A); for selected clinical syndromes not manifesting with end-stage liver disease (ESLD), including polycystic liver disease (see Chapter 69B) and other metabolic diseases; for some malignancies confined to the liver (see Chapters 97D and 97E); and for cholestatic liver diseases in children, such as biliary atresia (see Chapters 40 and 71). Four contraindications to liver transplantation are widely accepted: 1) unstable cardiopulmonary conditions, such as symptomatic coronary artery disease (CAD), severe systolic dysfunction, advanced cardiomyopathy, severe valvular heart disease, and severe pulmonary hypertension; 2) substance abuse; 3) morbid obesity (body mass index [BMI] >40); and 4) uncontrolled sepsis (Gallegos-Orozco & Vargas, 2009; Murray & Carithers, 2005).
As part of a multidisciplinary transplant team, anesthesiologists should participate in the evaluation of candidate recipients. Ideally, an anesthetic evaluation should precede the acceptance of patients to the waiting list. The goals of this assessment are to appraise suitable candidates based on functional status and comorbidities and to design a patient-specific preoperative and perioperative treatment plan. For patients in whom the burden of comorbidities presents a prohibitive operative risk, alternative treatments should be sought (Merion, 2004).
Cardiovascular System
Hyperdynamic circulation, cardiomyopathy, and CAD are common clinical problems that impact postoperative outcomes in orthotopic liver transplantation (OLT) candidates (Fouad et al, 2009).
Hyperdynamic Circulation
Patients with ESLD develop high cardiac output hemodynamics secondary to reduced systemic vascular resistance (SVR) and abnormal distribution of central, splanchnic, and peripheral circulation. This condition is due to humoral and autonomic dysregulation (Møller & Henriksen, 2008). When this situation is associated with cardiac valve dysfunction, especially mitral or tricuspid valve insufficiency, more hemodynamic support is needed in the perioperative period (Alper et al, 2009).
Coronary Artery Disease
Perioperative complications and mortality rates are high in patients with CAD: up to 50% of patients with significant CAD may die perioperatively from cardiac complications (Plotkin et al, 1996). Safadi and colleagues (2009) recently reported that history of stroke, CAD, postoperative sepsis, and increased interventricular septal thickness were markers of adverse perioperative cardiac outcomes, whereas use of perioperative β-blockers was significantly protective. Occult CAD is also common: 5% to 26% of OLT candidates have at least one critical coronary stenosis. Computed tomography (CT) scan detection of coronary calcification is a sign of possible ischemic heart disease in asymptomatic patients, but the sensitivity and specificity are low (Mandell & Tsou, 2008; Tiukinhoy-Laing et al, 2006).
Dobutamine stress echocardiography and dobutamine myocardial perfusion imaging are considered the most sensitive noninvasive tests to assess occult CAD in liver transplant candidates. Both tests have a higher negative than positive predictive value (Donovan et al, 1996; Mandell & Tsou, 2008; Murray & Carithers, 2005; Plotkin et al, 1998; Williams et al, 2000). Cardiac catheterization is indicated in patients with a positive noninvasive test to assess coronary lesions and plan further treatment. Left-heart catheterization is associated with higher risk of minor complications but can be performed safely in candidates for OLT (Sharma et al, 2009).
Cirrhotic Cardiomyopathy
Cirrhotic cardiomyopathy is characterized by systolic dysfunction more evident under conditions of hemodynamic stress, impaired diastolic relaxation, and electrophysiologic alterations often manifesting with a prolonged QT interval. Diagnosis is based on electrocardiograph (ECG), cardiac echo, and elevated levels of brain natriuretic factor. Physiopathologic hallmarks of this disease are altered β-adrenergic activity, myocardial fibrosis, myocyte hypertrophy, and ion-channel defects. Medical treatment is based on sodium restriction and administration of β-blockers and aldosterone antagonists; however, liver transplantation can reverse this condition (Fukazawa et al, 2009; Mandell & Tsou, 2008; Wong, 2009).
Nonischemic Cardiomyopathy
Nonischemic cardiomyopathy can complicate alcoholic cirrhosis, amyloidosis, hemochromatosis, and Wilson disease, and it must be considered in the anesthetic preoperative assessment and intraoperative management. Dilated and hypertrophic cardiomyopathy can also be present in OLT candidates, and these rarely revert to normal even after OLT (Mandell & Tsou, 2008).
Pulmonary System
As many as 70% of patients with chronic liver disease (CLD) suffer from respiratory problems, that manifest as impaired respiratory mechanics and gas exchange (Kim et al, 2009). Most pulmonary diseases are independent from the liver disease but are in some instances related to liver disease (e.g., α1-antitrypsin deficiency and cystic fibrosis) (Yeshua et al, 2009). Pulmonary evaluation includes history and physical examination, radiologic studies, arterial blood gas, and pulmonary function tests. Right-heart catheterization is indicated when clinical or ECG evidence suggests pulmonary hypertension.
Chronic Obstructive Pulmonary Disease and Smoking
Chronic obstructive pulmonary disease (COPD) is common and is often undiagnosed in candidates for liver transplantation. In a recent prospective study involving several academic centers in the United States, 18% of OLT candidates had COPD, and 80% of those patients had not been previously diagnosed. Older age and smoking were significant risk factors for this condition, although the impact of COPD on perioperative outcome in liver transplantation has not been well defined (Ryback et al, 2008).
Hepatic Hydrothorax
Hepatic hydrothorax is defined as a pleural effusion greater than 500 mL not caused by cardiac or pulmonary diseases in a patient with cirrhosis (Singh & Sager, 2009). A diaphragmatic defect allowing passage of ascites from the peritoneal to the pleural cavity is considered the main mechanism leading to this complication. Pleural effusions are symptomatic in 6% to 10% of patients with ESLD (Kiafar & Gilani, 2008), and the main respiratory impairment is hypoxemia secondary to atelectasis and shunting; chest radiograph confirms the diagnosis. Treatment is based on medical management of ascites with paracentesis and possible placement of a transjugular intrahepatic portosystemic shunt (TIPS). In cases of refractory hydrothorax, pleurodesis and diaphragmatic repair can be considered; thoracentesis is performed only in emergency situations, because avoidance of tube thoracostomy is preferred.
Portopulmonary Hypertension
Portopulmonary hypertension (PPHTN) is a specific type of pulmonary artery hypertension. It comprises increased pulmonary vascular resistance (PVR) and portal hypertension with or without advanced liver disease. From 2% to 10% of patients with cirrhosis are affected by PPHTN (Yeshua et al, 2009). The physiologic mechanism is multifactorial and incompletely understood, but hyperdynamic circulation, imbalance between pulmonary vasodilators (nitric oxide and prostacyclin) and vasoconstrictors (endothelin-1 and thromboxane), and proliferative pulmonary arteriopathy all play a role in the genesis of this syndrome (Singh & Sager, 2009).
Diagnosis requires presence of portal hypertension, mean pulmonary arterial pressure (mPAP) greater than 25 mm Hg, PVR greater than 240 dynes/sec/cm−5, and pulmonary artery occlusion pressure of less than 15 mm Hg (Rodriguez-Roisin et al, 2004); right-heart catheterization is indicated if pulmonary artery systolic pressure is higher than 50 mm Hg on ECG screening. The severity of PPHTN is then evaluated based on hemodynamic data obtained by right-heart catheterization at rest and is classified as mild (mPAP of 24 to 34 mm Hg), moderate (mPAP of 35 to 44 mm Hg), or severe (mPAP ≥45 mm Hg) (Singh & Sager, 2009).
Medical optimization is indicated before transplantation, and treatment is based on vasodilators. Prostaglandins (intravenous epoprostenol or inhaled iloprost), phospodiesterase inhibitors (sildenafil), and endothelin-1 antagonists (bosentan) are often used in combination therapy. Most liver transplant centers do not transplant patients with an mPAP greater than 50 mm Hg or a PVR greater than 240 dyne/sec/cm−5; however, 5-year survival is 14% without treatment, 45% with medical management, and 65% for those who had an OLT following pretreatment with prostacyclin (Swanson et al, 2008).
Hepatopulmonary Syndrome
Hepatopulmonary syndrome (HPS) is a vascular disorder characterized by hypoxemia secondary to pulmonary capillary vasodilation in patients with liver disease. The estimated incidence in patients with cirrhosis is between 5% and 32% (Singh & Sager, 2009). The natural course of HPS is characterized by poor survival, especially in patients with a Pao2 level of less than 50 mm Hg: median survival without liver transplantation is approximately 24 months (Schenk et al, 2002; Umeda & Kamath, 2009).
The pathologic feature of HPS is gross dilation of the pulmonary precapillary and capillary vessels (Rodriguez-Roisin & Krowka, 2008) leading to a ventilation-perfusion mismatch. The clinical symptoms are dyspnea with or without orthodeoxia (arterial oxygen desaturation while standing). The diagnosis is confirmed with arterial blood gas measurement and contrast-enhanced ECG or macroaggregated albumin lung perfusion scan. The ECG diagnostic sign is the visualization of saline bubbles in the left heart, three to six beats after the appearance of bubbles in the right heart, following the injection of agitated saline in a peripheral vein.
No effective medical therapy for HPS has been found, and liver transplantation is the only treatment. UNOS criteria consider a Pao2 level below 60 mm Hg an indication of high priority for OLT, even if the allocation of “exception points” to patients with HPS has now become controversial (Sulieman et al, 2008). About 85% of patients who underwent OLT for HPS showed resolution or improvement of hypoxemia. Still, the mortality after liver transplantation is significantly increased in patients with HPS (Fallon et al, 2008).
Hemostasis in End-Stage Liver Disease
The liver synthesizes most of the factors involved in clot formation and, equally importantly, factors opposing clot formation or mediating its lysis. The traditional concept that patients with advanced liver disease are hypocoagulable has been challenged. The current view emphasizes complex disturbances of platelet function, procoagulant, anticoagulant, and fibrinolytic pathways that result in rebalancing of the hemostatic system (Senzolo et al, 2006). The net effect may be a hypocoagulable or hypercoagulable state with a tendency for bleeding or thromboembolism (Warnaar et al, 2008b). Understanding the hemostatic system in ESLD is essential to perioperative coagulation management and optimization of blood product transfusion practices, which are directly related to morbidity and mortality in liver transplantation (de Boer et al, 2008; Massicotte et al, 2005).
Deficiency in primary hemostasis is due to quantitative and qualitative platelet changes (Pereboom et al, 2008). Mild to moderate thrombocytopenia is common. Causes are splenomegalic platelet sequestration in portal hypertension, impaired megakaryocytopoiesis as a result of reduced production of thrombopoietin by the cirrhotic liver, folic acid deficiency in alcoholic cirrhosis or acute hepatitis C infection, and reduced platelet half-life related to autoantibodies (Lisman & Leebeek, 2007; Senzolo et al, 2006). Evidence for impaired platelet aggregation and adhesion in cirrhosis is controversial. The proposed mechanisms are complex and multifactorial, including the important role of two potent endothelium-derived platelet inhibitors, nitric oxide and prostacyclin. Contrary to this, more recent work suggests that the functional capacity of platelets in cirrhotic patients may be normal when platelet number and hematocrit are normalized (Lisman & Leebeek, 2007; Tripodi et al, 2007), suggesting that the main defect in primary hemostasis is thrombocytopenia. An important compensatory mechanism for the overall deficient platelet function in cirrhosis is an increase in endothelium-synthesized von Willebrand factor (vWf). The markedly upregulated vWf promotes adhesion of platelets to injured vasculature and seems to be more effective than plasma from healthy volunteers (Lisman & Leebeek, 2007).
All proteins involved in fibrinolysis are synthesized by the liver except tissue plasminogen activator (tPA) and plasminogen activator inhibitor type 1 (PAI-1). Consequently, reduced levels of liver-synthesized factors—plasminogen, plasmin inhibitor, thrombin activatable fibrinolysis inhibitor (TAFI), and factor XIII—are found in both acute and chronic liver failure. Cirrhosis is classically associated with hyperfibrinolysis, whereas patients in ALF may have hypofibrinolysis as a result of increased production of PAI-1. The recent expert viewpoint is contrary to the traditionally held view and emphasizes that the existing in vitro evidence of hyperfibrinolysis in cirrhosis is weak (Lisman & Leebeek, 2007). Increased levels of fibrinogen degradation indicators—d-dimers, plasmin-antiplasmin and thrombin-antithrombin complexes, thrombin fragment F1+2—could be explained by their delayed clearance by the diseased liver.
Coagulation tests, such as the prothrombin time (PT) and activated partial thromboplastin time (aPTT), are used to assess the severity of synthetic dysfunction, and PT-INR is a part of the Child-Turcotte-Pugh (CTP) and Model for End-Stage Liver Disease (MELD) prognostic indexes (Ng, 2009). As tests of in vivo coagulation function, PT-INR and PTT are conceptually deficient because they measure only the procoagulant pathway and ignore the anticoagulant function. This inadequacy is best illustrated by the observation that when the (prolonged) PT is modified by including thrombomodulin to allow full activation of protein C, the thrombin generation in cirrhotic patients is close to normal (Tripodi et al, 2005). This may in part explain why PT-INR and PTT are poor predictors of bleeding in cirrhosis and in liver transplantation (Massicotte et al, 2008). Thromboelastogram (TEG; Haemoscope Corp., Niles, IL) and rotation thromboelastometry (ROTEM; Pentapharm, Munich, Germany) have the advantage of assaying whole-blood clot formation and lysis, including the platelet contribution, but these assays do not incorporate the endothelial component (see Intraoperative Monitoring, below). Importantly, these assays allow easy recognition of hypercoagulable states (Fig. 97B.1A).
Renal System
Renal failure confers increased risk of death to cirrhotic patients, both while on the transplant waiting list and after liver transplantation. Implementation of the MELD score in 2002 (Moreau et al, 2003) has prioritized patients with renal failure waiting for a liver transplant, resulting in reduced overall mortality on the transplant list (Ojo, 2009).
With progression of cirrhosis, sympathetic and rennin-angiotensin vasoconstrictor systems are activated that temporarily maintain arterial blood pressure, but they also cause renal vasoconstriction and hypoperfusion. Activation of nonosmotic hypersecretion of arginine vasopressin, aimed at preserving circulatory volume, results in retention of solute-free water with attendant hyponatremia, edema, and ascites. HRS is almost invariably associated with ascites and rarely develops in its absence (see Chapter 74). HRS is classified into one of two types: HRS 1 is characterized by a rapid decline in renal function reflected in doubling of serum creatinine (SCr) to above 2.5 mg/dL over a 2-week period; this type of HRS has a particularly ominous clinical course, leading to multiorgan dysfunction that affects the heart, brain, and other organs. HRS 2 has a more benign, slowly progressive course. It is manifested by a milder elevation of SCr (1.5 to 2.5 mg/dL) and refractory ascites. Onset of HRS is often triggered by a discrete event, such as spontaneous bacterial peritonitis, use of nonsteroidal antiinflammatory drugs (NSAIDs), or hypovolemia as a result of diuretic use, gastrointestinal (GI) bleeding, or fluid losses.
Measurement of SCr remains the primary test of renal function despite its well-known limitations. By consensus, SCr above 1.5 mg/dL (133 µmol/L) defines renal insufficiency in cirrhosis (Gines & Schrier, 2008; Salerno et al, 2007). GFR and creatinine clearance are more accurate but less practical tests when repeated assessments are needed. Other tests measure serum and urinary electrolytes, fractional sodium excretion (FENa), albumin, osmolarity, sediment, and biologic markers. Specific diagnostic tests for HRS do not exist, rather diagnosis is based on the presence of liver disease, a precipitating factor, and FENa less than 1%, signifying preserved tubular reabsorption; however, the latter test is invalidated by the use of diuretics.
The main pharmacologic approach utilizes vasoconstrictors, including a selective vasopressin V1 receptor agonist, such as terlipressin, or α-adrenergic agonists, such as norepinephrine and midodrine. Vasoconstrictors in conjunction with albumin are only modestly beneficial but are the best medical therapy currently available. Two recent studies showed a marked improvement in renal function in 40% of patients treated with terlipressin and albumin compared with controls treated with albumin alone (Martin-Liahi et al, 2008; Sanyal et al, 2008). Although the overall survival in the treatment group was not better than in the control group, the probability of 3-month survival in patients who responded with improved renal function was significantly higher (58% vs. 15%) than in nonresponders (Martin-Liahi et al, 2008); however, organ ischemia is a concerning side effect of vasoconstrictor administration.
Placement of a TIPS improves renal function and GFR and reduces sympathetic and renin-angiotensin-aldosterone axis activation in about 60% of patients (Cardenas & Gines, 2009; see Chapter 76E). TIPS is less applicable in HRS 1 as a result of severe liver disease, but it may delay progression of HRS 2 to HRS 1. No advantage in survival has been demonstrated in patients with refractory ascites treated by TIPS compared with repeated paracentesis and intravascular volume replacement with albumin.
Renal replacement therapy (RRT) offers a bridge to transplant, but the optimal RRT method in HRS and the benefit to patient outcomes are not known (Gines & Schrier, 2009). Continuous venovenous hemodialysis (CVVHD) seems to be hemodynamically the most favorable form of RRT (Davenport, 2009). CVVHD can be used to effectively control intravascular volume, pH levels, and solutes (Na, K, SCr, urea, ammonia); in addition, it corrects sodium in a time-controlled fashion, which is particularly relevant to minimize the risk of pontine demyelination in patients with hyponatremia.
Liver transplantation is the treatment of choice for patients with cirrhosis and HRS (Cardenas & Gines, 2009; see Chapter 97A). About 90% of patients with HRS recover kidney function after successful liver transplantation. The remaining 10% do not recover their kidney function and require prolonged RRT and eventual kidney transplantation. When the cause of renal failure in cirrhosis is HRS, the best predictor of need for a combined liver-kidney transplant is pretransplantation RRT for longer than 8 weeks.
Central Nervous System
Hepatic encephalopathy (HE) is a progressive but potentially reversible metabolic disorder of the central nervous system (CNS), and it is associated with variable degrees of brain edema. HE is an overt manifestation of cirrhosis in up to 45% of patients, and it is a defining sign and main cause of death in ALF (Eroglu et al, 2009). The World Congress of Gastroenterology recognizes three classifications of HE based on the etiology (Ferenci et al, 2002): type A is associated with ALF, type B with TIPS, and type C with cirrhosis. Based on the severity of neurologic impairment, HE ranges from normal (grade 0) to mildly impaired cognitive and neuromuscular function (grade 1), progressive lethargy (grade 2), confusion with muscle rigidity or clonus (grade 3), to coma and brain death (grade 4). Although presence and severity of HE has prognostic and therapeutic significance in cirrhosis and ALF, it is not a part of the MELD score.
The pathophysiology of HE is complex and incompletely understood. Multiple putative mediators have been implicated that include ammonia, endogenous benzodiazepine-like or γ-aminobutyric acidlike agonists, reactive oxygen species, inflammatory cytokines, hyponatremia, and manganese. The exact pathogenesis of cerebral edema remains controversial, but good evidence supports the role of ammonia, which promotes astrocyte and neuron swelling by increasing intracellular glutamine content. Transported to the mitochondria, glutamine contributes to production of reactive oxygen species and activation of mitochondrial transition permeability pore and various kinases, resulting in cellular swelling. Increased ammonia levels have been shown to alter cerebral blood flow and brain glucose utilization (Bjerring et al, 2009; Sundaram et al, 2009). High arterial and brain levels of ammonia in ALF correlate well with cerebral edema and increased ICP, with consequent high risk of mortality from brain herniation and hypoxia (Wendon et al, 2008).
HE in cirrhosis features slow onset, a progressive course, and only partial responsiveness to therapy. Significant cerebral edema typically does not develop, and a precipitating event—such as infection, excessive dietary protein, dehydration, or GI bleeding—can be often identified. TIPS is a known risk factor and is associated with 30% of new or worsening encephalopathy (see Chapter 76E). In addition to correction of any precipitating factors, treatment includes nonabsorbable disaccharides, which reduce the intestinal production and absorption of ammonia; antibiotics that target urease-producing bacteria; ornithine and acarbose; benzodiazepine receptor antagonists; and probiotics.
The hallmarks of HE in ALF are cerebral edema and increased intracranial pressure (ICP). Both are life-threatening complications of grade 3 and 4 encephalopathy. Treatment includes a number of general and specific interventions (Table 97B.1) aimed at promoting cerebral perfusion pressure (CPP) above 60 mm Hg; this is achieved by maintaining mean arterial pressure (MAP) and reducing ICP to below 20 to 25 mm Hg by reducing brain edema. Grade 3 or 4 HE requires endotracheal intubation for airway protection. Mild hyperventilation temporarily relieves brain hyperemia, but prolonged hyperventilation is not beneficial and may cause CNS ischemia. Mannitol is effective in reducing ICP in the short term, but its prophylactic use is not recommended. Recent evidence demonstrates that N-acetylcysteine treatment may also prolong transplant-free survival in ALF unrelated to paracetamol/acetaminophen toxicity (Lee et al, 2007).
Table 97B.1 Management of Encephalopathy and Elevated Intracranial Pressure in Acute Liver Failure
| General Measures | Specific Interventions |
|---|---|
| Head elevation to 30 degrees | Endotracheal intubation for grades 3 and 4 hepatic encephalopathy |
| Judicious (restrictive) fluid management | Hyperventilation: limited, temporary benefit |
| Judicious if any sedation | N-acetylcysteine: benefits all causes of acute liver failure |
| Cerebral perfusion pressure >60 mm Hg | Renal replacement therapy: CVVHD |
| Osmotic diuresis, hypertonic saline | ICP monitoring (>10% bleed); transcranial Doppler |
| Empiric: antibiotics, disaccharides | Hypothermia: mild, bridge to transplantation |
CVVHD, continuous venovenous hemodialysis; ICP, intracranial pressure
The role of ICP monitoring is controversial, as ICP monitor placement is associated with high risk of bleeding in this patient population, and no data exist to support its role in improving patient outcomes. The Acute Liver Failure Study Group recommends this intervention only for liver transplant candidates but recognizes regional practice differences (Stravitz et al, 2007). An attractive, noninvasive alternative to ICP monitors is transcranial Doppler (TCD) sonography. By measuring blood flow velocities in middle and anterior cerebral arteries and calculating resistance and pulsatility indexes, TCD allows serial estimates of cerebral blood flow in the setting of impaired autoregulation and developing brain edema (Bhatia & Gupta, 2007). In a small series of ALF patients, confirmation of adequate cerebral blood flow by TCD was successfully used as a basis to proceed with OLT (Bindi et al, 2008). Intraoperative use of TCD in OLT has not been reported.
Mild to moderate hypothermia (32° C to 35° C) is an emerging therapeutic modality. Hypothermia reduces brain ammonia and cytokine levels and in part restores cerebrovascular autoregulation and brain glucose metabolism. Although experimental evidence provides a strong rationale, only limited clinical data on efficacy and safety of hypothermia in humans in ALF are available, and future studies are warranted (Stravitz et al, 2009).
Intraoperative Monitoring
Hemodynamic Management
Hemodynamic management is a cornerstone in the perioperative care of liver transplant patients, but no consensus has been reached on the standards of hemodynamic monitoring (Claus-Georg & De Wolf, 2008). Continuous invasive arterial pressure monitoring may be the only ubiquitously used hemodynamic monitor in liver transplantation. When measured at the most common site, the radial artery, peripheral arterial pressure may underestimate the true aortic pressure, particularly during periods of hypotension. A femoral arterial line reflects central aortic pressure with higher fidelity and consistently records higher systolic pressures than the radial artery. The difference between the femoral and the arterial pressure waveform is particularly pronounced after graft reperfusion or when high doses of vasopressors are used (De Wolf, 2006; Shin et al, 2007). Because of this discrepancy, and because of the need for frequent arterial blood sampling, some centers routinely use both femoral and radial arterial lines.
The pulmonary artery catheter (PAC, see Chapter 22) has long been the mainstay of hemodynamic monitoring in liver transplantation (De Wolf et al, 1993); however, the clinical utility of PACs has recently been questioned because of criticisms that its invasiveness is not justified by improved outcomes (Sandham et al, 2003; Wheeler et al, 2006) and that measured pressures are not good estimates of ventricular preload (Kumar et al, 2004). Although a number of less invasive technologies have emerged, resulting in a decrease in their use, PACs are still advocated by many for OLT (Della Rocca et al, 2008; De Wolf, 2008; Pinsky, 2006), and the ability of PACs to continuously display pulmonary artery pressures is particularly advantageous when managing patients with portopulmonary hypertension. Transesophageal echocardiography (TEE) provides different but complementary information to that provided by PACs (see below), and concurrent use of both devices is considered superior (De Wolf & Aggarwal, 2008). A notable shortcoming of PACs in OLT is the inaccuracy of thermodilution cardiac output in the period following liver reperfusion because of postreperfusion hypothermia and subsequent instability of central temperature.
Newer, less invasive hemodynamic monitors for OLT have been introduced. The PiCCO system (Pulsion Medical Systems, Munich, Germany) combines transpulmonary thermodilution and arterial pressure contour analysis, and it provides continuous cardiac output in alignment with PAC thermodilution and a number of indices of preload that include global end-diastolic volume (GEDV) and intrathoracic blood volume (ITBV), which agree better with cardiac index than filling pressures (Della Rocca et al, 2002a, 2002b). PiCCO requires central venous and femoral or brachial arterial access, which arguably is not much less invasive than the PAC. The LiDCO device (LiDCO Group, London, UK) estimates cardiac output based on the peripheral arterial pressure waveform pulse power analysis after a lithium dilution calibration, and it has been used successfully in patients recovering from liver transplantation in the ICU (Costa et al, 2008). A device that estimates cardiac output based on the arterial pressure waveform without need for calibration, Flo-Trac/Vigileo (Edwards Lifesciences, Irvine, CA), did not perform well intraoperatively during OLT (Biais et al, 2008; Biancofiore et al, 2009).
Cardiac output determined by a novel suprasternal ultrasound cardiac output monitor (USCOM Pty, Coffs Harbour, Australia) correlates well with the PAC cardiac output (Wong et al, 2008; Su et al, 2008). Other USCOM measurements, such as Doppler estimation of peripheral artery pressures and left ventricular contractility, remain to be validated.
TEE has been gaining more popularity in liver transplantation (Burtenshaw & Isaac, 2006). Data from 2002 estimated use of TEE in only 21% of high-volume centers in the United States (Schuman, 2003), but a more recent survey showed that 86% of anesthesiologists in 40 large-volume U.S. centers use TEE in some or all liver transplantations (Wax et al, 2008).
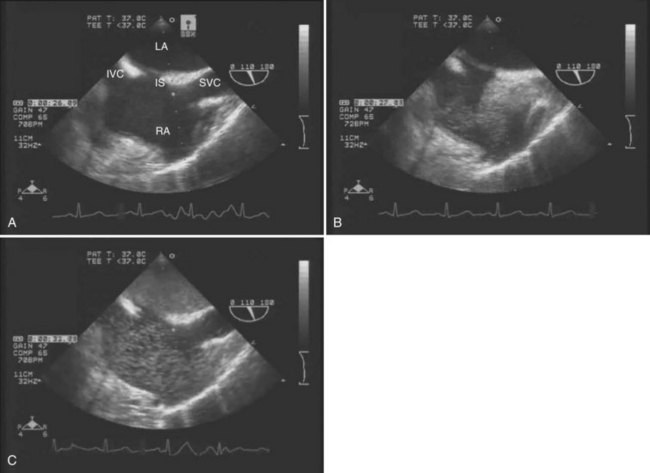
The main advantages of TEE are that it provides continuous, real-time visualization of cardiac structures and dynamic function. TEE is a superior modality for estimating ventricular volume status, global LV contractility and ejection fraction, right ventricular function, segmental wall motion abnormalities, and septal motion. Doppler modalities allow for interrogation of transvalvular flows, dynamic left ventricular outflow obstruction, intracardiac shunts, and diastolic function. In addition, TEE is the only intraoperative monitor that allows an instantaneous diagnosis of pulmonary or intracardiac thrombus (Ellenberger et al, 2006; Planinsic et al, 2004) and pericardial or pleural effusion. TEE detection of a transpulmonary shunt as a positive bubble test in a patient with HPS is presented in Figure 97B.1.
Monitoring of Hemostasis
Conventional tests of hemostasis, PT and aPTT, have several important limitations (Dalmau et al, 2009; Ng et al, 2009). First, these assays utilize plasma samples devoid of platelets. The activated platelet surface, the naturally occurring medium of the in vivo coagulation, is replaced by addition of phospholipids. The contribution of platelets is thus not considered by these tests. Second, PT and PTT measure only the activity of procoagulant factors and do not take into account the anticoagulant factors. When PT is modified to include anticoagulant factors, thrombin generation in cirrhotic patients is not different from normal controls (Tripodi et al, 2005). The reported higher intralaboratory variability of INR than of PT may be of more relevance for MELD score determination than for acute intraoperative management (Lisman & Leebeek, 2007).
An important shortcoming of the conventional PT and PTT is a long turnaround time, typically about 45 minutes—suboptimal for an ongoing transplant surgery. Point-of-care (POC) assays require only a minimal delay between blood sampling and attaining the results of the test. POC assays of PT and PPT are quick (5 to 15 minutes), cartridge-type, whole-blood tests offered by several manufacturers. An acceptable agreement of such assays with corresponding plasma-based tests has been reported in OLT (Herbstreit et al, 2010), suggesting that the POC tests can effectively replace the slower, conventional testing.
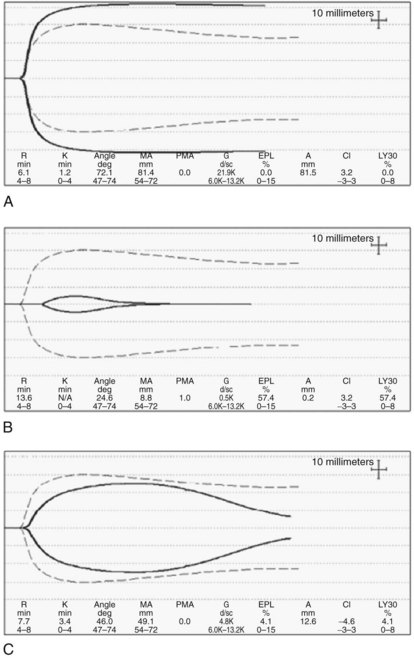
TEG and ROTEM are POC assays that measure viscoelastic properties of whole-blood clot formation and lysis. Both technologies have been used to drive transfusion algorithms (Kang et al, 1985; Coakley et al, 2006). TEG measures time to onset of clot, rate of clot formation, clot tensile strength, and rate of clot lysis (Fig. 97B.2). Treatment of samples with an intrinsic pathway activator, kaolin, shortens clot formation, whereas addition of heparinase dissects the effects of exogenous heparin from intrinsic heparinoids. TEG results are available more rapidly than the conventional laboratory tests. In our institution, progress of TEG clot formation and lysis is displayed on a dedicated operating room monitor and is available to the anesthesiologist in real time.
ROTEM generates a similar trace to that of TEG, although a ROTEM assay may be modified by a heparinase reagent (hep-TEM), an intrinsic pathway activator (in-TEM), and a tissue-factor activator (ex-TEM). In addition, ROTEM includes a qualitative test of fibrinogen activation and fibrin polymerization (fib-TEM), which is based on adding a blocker of platelet function. ROTEM is very useful in determination of poor tensile clot strength as a result of combined platelet and fibrinogen deficiency, and it is the most reliable method for detecting hypofibrinogenemia or dysfibrinogenemia, both common in liver disease (Kalina et al, 2008).
Both TEG and ROTEM are quick and superior monitors of hypercoagulability, fibrinolysis, and therapeutic effects of antifibrinolytics. The main conceptual deficiency of the two monitors is the inability to evaluate endothelial function. It is worth emphasizing that although both instruments plot clot formation in a similar fashion, TEG and ROTEM do not generate the same results when analyzing blood without exogenous activation, therefore they are not interchangeable (Coakley et al, 2006; Nielsen, 2007).
Monitoring and management of hemostasis remain great challenges of OLT, and it has been well established that blood loss and blood product transfusion are an important cause of morbidity and mortality (de Boer et al, 2008; Massicotte et al, 2005; Ramos et al, 2003). Adherence to an algorithm-driven transfusion practice may reduce unnecessary perioperative blood and blood-component transfusion (Avidan et al, 2004; Kang, 1997; Kang et al, 1985), but no consensus has been reached on what constitutes standard intraoperative monitoring of hemostasis or a standard hemostatic intervention.
Difficulties stem from the incomplete understanding of complex coagulation abnormalities in liver disease and OLT and from the limitations of currently available tests to emulate the in vivo conditions. For example, preoperative coagulation tests do not predict intraoperative bleeding (Massicotte et al, 2004; Steib et al, 2001), and transfusion requirements are actually lower when abnormal coagulation test results do not lead to routine correction by infusion of fresh frozen plasma (FFP) and other blood products (de Boer et al, 2005). Finally, bleeding in OLT is often related to factors other than coagulopathy. These findings emphasize the need for careful interpretation of coagulation tests within the clinical context rather than relying on interventions formulated solely on the basis of test results.
Metabolic Monitoring
Glucose Control
ESLD is associated with impaired glucose control, and hypoglycemia is common in ALF, whereas peripheral insulin resistance and metabolic syndrome are common in CLD. Factors that contribute to acute intraoperative hyperglycemia are routine administration of steroids and postoperative immunosuppressants and response to surgical stress. Two retrospective studies have shown association of a higher incidence of infections and 1-year mortality with poor perioperative glucose control (Ammori et al, 2007; Park et al, 2009). These studies, in general agreement with other perioperative outcome studies, emphasize the need for close POC perioperative monitoring and management of blood glucose levels, but the exact target range is unknown.
Electrolytes
Electrolyte monitoring is done as POC testing, usually on an hourly basis and when indicated, such as to optimize electrolyte balance immediately before graft reperfusion. Hyperkalemia is ominous and may cause fatal arrhythmias, particularly after reperfusion. Independent predictors of hyperkalemia are the number of red blood cells (RBCs) transfused and higher initial values, especially in acidemic patients with renal insufficiency (Naksuji & Bookallil, 2000). Ionized calcium levels predictably decrease with blood product and albumin administration. Low serum sodium is a hallmark of advanced cirrhosis and an independent predictor of mortality on the transplant waiting list (Kim et al, 2008; Yun et al, 2009), and intraoperative sodium levels are elevated by administration of normal saline-based fluids and particularly by sodium bicarbonate; in addition, rapid correction of serum sodium levels may cause pontine demyelination (Zhang et al, 2009).
Temperature Monitoring
Monitoring of temperature is usually achieved via a PAC or an esophageal probe. Surprisingly little has been reported on temperature management in OLT, considering the fact that hypothermia is a key sign of liver failure and a significant problem during surgery. Well-known detrimental effects of hypothermia include impaired wound healing and impaired coagulation. An acute decrease in central temperature results from the cold perfusate returning to the heart following graft reperfusion. This precipitous hypothermia contributes to postreperfusion hemodynamic instability and bradyarrhythmias that may progress to cardiac arrest (Fig. 97B.3). The hypothermic insult can be partially mitigated by achieving upper-limit normothermia in the prereperfusion period, thus the value of continuous temperature monitoring and measures to assure normothermia cannot be overemphasized. A special circumstance is deliberate mild hypothermia, which acts by decreasing brain metabolism and mediators of oxidative stress; thus mild hypothermia is an accepted treatment of increased intracranial pressure in ALF (Stravitz & Larsen, 2009).
Intraoperative Management
Ventilatory Management
Mechanical ventilation should optimize respiratory gas exchange and maintain lung volumes and capacities to facilitate early postoperative extubation. Preexisting respiratory comorbidities, such as hydrothorax, have to be considered in setting the ventilatory parameters. Although not much is reported about the ventilator setting in OLT, it seems reasonable to adopt a protective ventilatory strategy and to avoid high airway peak pressures (below 30 to 35 cm H2O). Tidal volume of 6 to 8 mL/kg are a reasonable initial setting (Slinger, 2008; Shultz, 2008), but tidal volumes of least 8 mL/kg are necessary for reliable pulse pressure variation (PPV) or stroke volume variation (SVV) monitoring (Michard, 2005).
Multiple factors can cause increased pulmonary capillary permeability and predispose the lungs to interstitial edema. Positive end-expiratory pressure (PEEP) is a rational tool to prevent lung edema, and a PEEP of up to 10 mm Hg has been proven safe in maintaining hemodynamics and graft perfusion after cadaveric and living-donor liver transplant (LDLT) (Saner et al, 2006a, 2006b; 2008). Fio2 can be increased to modulate pulmonary resistance in case of pulmonary hypertension. It is not known whether high intraoperative values of inspiratory fraction of oxygen may have a role in modulating ischemia-reperfusion injury after reperfusion (Kabon & Kurz, 2006).
Intraoperative Hemodynamic, Fluid, and Transfusion Management
Hemodynamic management of fluids and blood-product transfusions are critically interconnected and should be considered together. Excessive fluid administration may result in increased pressure in the IVC, portal vein, and collateral veins, possibly contributing to blood loss during liver dissection, hemodilution of coagulation factors and platelets, and tissue edema (see Chapter 22; Westercamp et al, 2009). Conversely, inadequate intravascular volume repletion may result in hemodynamic instability and organ hypoperfusion.
Better understanding of pathophysiology in ESLD, improvement in surgical and anesthetic techniques, and availability of POC laboratories have resulted in marked reductions in blood-product administration, including those for transplantations without transfusion. This is of great significance, as reduced blood-product transfusion is associated with better long-term outcomes (Bennett-Guerrero et al, 2001; Massicotte et al, 2007; Pereboom et al, 2009).
A strategy of maintaining low central venous pressure (CVP ≤5 mm Hg) through restrictive fluid administration and phlebotomy has been advocated by Massicotte and colleagues (2006) as an effective and safe strategy for transfusion-free OLT (see Chapter 22). In contrast, Schroeder and colleagues (2004) reported increased mortality and renal failure associated with low-CVP management. Whereas the low-CVP strategy may be possible in select patients with normal kidney function and good cardiovascular reserve, targeting one specific low CVP (i.e., 5 mm Hg) may not be physiologically advantageous in patients with poor ventricular compliance and preexisting renal dysfunction.
A low-CVP regimen usually requires vasopressor administration to maintain arterial blood pressures (Ponnudurai et al, 2005; Schroeder et al, 2004) but with the possible side effect of end-organ hypoperfusion. As CVP is not a reliable index of preload, volumetric assessment with TEE or fluid-responsiveness indexes (dPP or SVV) may be a more physiologic approach to guide fluid management (Biais et al, 2008; Della Rocca, 2009). Fluid management strategy and interpretation of CVP values may vary based on the surgical technique; for example, during a complete IVC clamp, blood is sequestered below the clamp, and low CVP does not accurately reflect volume status. After unclamping of the IVC and portal vein, the CVP increases rapidly and may abolish the pressure gradient between the portal vein and the right atrium and thereby compromise liver perfusion. Hemodynamic changes during a piggyback technique rarely reach such magnitude.
Hypotension is common during all phases of OLT, but little is reported on the impact of intraoperative hypotension on long-term patient outcomes. Reich and colleagues (2003) reported the worst surgical outcomes early after liver transplantation in patients who had at least one episode of arterial hypotension with an MAP below 40 mm Hg.
Little evidence is available to provide guidance regarding the choice of intraoperative vasopressors. Agents are chosen based on their pharmacologic properties and side effects (Zhang et al, 2005). Commonly used agents are neosynephrine, norepinephrine, vasopressin, and dopamine. Of note, vasopressin 1 receptors and α1-adrenergic agonists are useful in preserving renal function in HRS (Gines & Schrier, 2009); vasopressin reduces splanchnic blood flow, which may reduce bleeding from portal collaterals during the dissection phase but may also impede portal vein flow during the neohepatic phase (Wagener et al, 2008).
Most centers use a combination of crystalloids and colloids for fluid replacement. A recent study showed that use of new-generation hetastarch (HES 130/0.4) may be a safe alternative to conventionally used 5% albumin (Mukhtar et al, 2009), but more studies are needed.
Severe pulmonary hypertension (PVR >240 dynes/sec/cm−5) is associated with high perioperative mortality and is a contraindication for proceeding with liver transplant. In moderate PPHTN with preserved right ventricular function, the goal of intraoperative management is to lower pulmonary vascular resistance and promote myocardial contractility. General measures include increasing Fio2, avoidance of hypercapnia and acidosis, and administration of nitrous oxide. Selective pulmonary vasodilators, inhaled nitric oxide, and prostacyclin are superior to intravenous vasodilators, which may cause systemic hypotension and compromise right-heart perfusion and preload. Milrinone and dobutamine are used as inotropes and pulmonary vasodilators but with caution, as they also cause systemic hypotension. Pulmonary pressure management is particularly challenging in the postreperfusion period, and pulmonary vasodilators are weaned gradually to avoid rebound pulmonary hypertension. Extubation should be delayed if inhalational vasodilators agents are needed (Mandell, 2004; Teo & Greenhalg, 2010).
With implementation of the MELD scoring system, more patients with renal insufficiency are being treated with OLT. Renal failure is associated with increased short- and long-term mortality after OLT, and perioperative renal failure often requires postoperative RRT. Intraoperative renal protection strategy is based on the maintenance of adequate intravascular volume and renal perfusion. No specific intraoperative renoprotective agents have been proven effective, but it is reasonable to maintain perfusion pressure with vasopressin and adrenergic agonists, which are effective in management of HRS. Surgical techniques that preserve vena caval flow, such as a piggyback technique, favor better renal outcomes. Intraoperative RRT is feasible and has been proven safe and successful in maintaining electrolyte and fluid balance (Ramsay & Garcia-Valdecasas, 2009; Townsend et al, 2009), although many transplant units try to avoid the need for it during the course of an OLT if at all possible. New markers of renal injury, such as neutrophil gelatinase–associated lipocalin, may be more useful than SCr in assessing acute kidney injury (AKI) (Niemann et al, 2009). The prognostic value of this rapid assay may prove useful in future studies to confirm the validity of intraoperative renoprotective strategies.
The administration of RBCs, FFP, and platelets has been associated with increased mortality (Bennett-Guerrero et al, 2001; de Boer et al, 2008; Massicotte et al, 2005; Pereboom et al, 2008; Ramos et al, 2003). Average blood-product requirements have decreased dramatically over the past decade, and now OLT can often be performed without transfusion (De Boer et al, 2005; Ozier & Tsou, 2008). Although it would be useful to prospectively identify patients at high risk of major bleeding, factors predictive of higher transfusion requirements are still debatable. Low pretransplant hemoglobin and a MELD score higher than 30 are better established predictors than previous abdominal surgery, prolonged INR, and low platelet counts (Massicotte et al, 2009; Ozier & Tsou, 2008; Xia et al, 2006).
No thresholds have been universally accepted for transfusing RBCs, FFP, and platelets, and practice differs among various centers and clinicians. POC testing, including TEG and ROTEM, have been used to define transfusion algorithms (see Monitoring of Hemostasis above).
The selective use of antifibrinolytic agents in the presence of persistent fibrinolysis is indicated, but routine prophylactic use of these agents is controversial. Aprotinin and tranexamic acid have been shown to reduce transfusion requirements in high-risk patients (Dalmau et al, 2000; Molenaar et al, 2007). Based on several case reports focused on the association of antifibrinolytics with vascular thrombosis (Dalmau et al, 2000; O’Connor et al, 2000) of either the arterial or venous circulation, and considering the current low transfusion rate, we question the validity of an indiscriminate prophylactic treatment in the absence of clinical and laboratory signs of hyperfibrinolysis. A recent meta-analysis (Molenaar et al, 2007) and a retrospective study of aprotinin by the same group (Warnaar et al, 2009) did not provide evidence for an increased risk of thromboembolic events associated with antifibrinolytic drugs in liver transplantation. It should be emphasized that in both of these studies, trends were observed that suggested a possible association between antifibrinolytics and thrombosis that did not reach statistical significance, perhaps because of the limited number of subjected enrolled and other confounding factors.
No consensus exists regarding the choice of any specific antifibrinolytic agent, dose, or time of administration; therefore monitoring of fibrinolysis and early detection of hypercoagulability are of critical importance. The prevailing view is that TEG/ROTEM may best provide such information, and TEE may be useful for detection of intracardiac clots adhering to the central lines. Use of activated factor VII as a prophylactic treatment has not been proven advantageous in a liver transplant setting, but it has been used successfully as a rescue treatment in a few individual cases (da Silva Viana, 2006). Cryoprecipitate, a source of factors I and VIIIc and of vWF, and prothrombin complex concentrates that contain vitamin K–dependent factors II, VII, IX, and X are increasingly used as an alternative to FFP. The advantage of these products over FFP is that a smaller volume is required, and risk of infectious complications is reduced (Ofosu et al, 2008; Samama et al, 2008).
Critical Intraoperative Events
Postreperfusion Syndrome and Refractory Hypotension
Postreperfusion syndrome (PRS), first described by Aggarwal and colleagues in 1987, was originally defined as a decrease in systemic arterial pressure to less than 70% of the prereperfusion value or a mean blood pressure (BP) below 60 mm Hg within 5 minutes of liver reperfusion and lasting at least 1 minute. The low systemic BP was associated with an increase in PVR and central pressures and occasionally with bradycardia, which could progress to asystole (Aggarawal et al, 1993). Other authors proposed similar definitions based on hemodynamics (Ayanoglu et al, 2003; Chui et al, 2000; Nanashima et al, 2001; Paugam-Burtz et al, 2009), and one proposed an expanded concept that, in addition to unstable hemodynamics, included postreperfusion fibrinolysis that required administration of antifibrinlolytics (Hilmi et al, 2008). Depending upon the reporting center, significant PRS is evident in 25% to 55% of patients, an acknowledgment that the incidence depends on the definition. In a significant percentage of patients undergoing liver transplant, reperfusion can be complicated by bradycardia that is resistant to chronotropic agents and asystole that requires cardiopulmonary resuscitation.
The mechanisms behind bradycardia and reperfusion syndrome are not yet fully understood. Several possible factors have been indicated, such as the release of vasoactive substances from the liver graft or the recipient gut—potassium, acids, prostanoids, bradykinin, interleukins—along with hypovolemia, hypothermia, or small air or thrombotic emboli (Chui et al, 2000; Ricciardi et al, 2002). In a multivariable logistic regression analysis, Paugam-Burtz and colleagues (2009) found that absence of portocaval shunt and duration of cold-ischemia time were independent predictors of PRS.
PRS is associated with adverse outcomes, including an increase in blood loss with an attendant increase in blood-product requirements, higher incidence of postoperative allograft loss, kidney failure, and early mortality (Paugam-Burtz et al, 2009; Ramsay, 2008). No universally accepted preventative strategies have been developed, but common interventions include pretreatment with chronotropic and vasoconstrictor agents and optimization of temperature, pH, and electrolytes. The occurrence of ischemia-reperfusion injury is well recognized, but its exact relevance to PRS is not fully understood. Therapeutic approaches that use nitric oxide, prostaglandin E1 and various antioxidants aimed at mitigating the inflammatory response and cellular cascade triggered by the ischemia-reperfusion are undergoing clinical evaluation (Ramsay, 2008). Methylene blue has been reported in treatment of refractory hypotension after reperfusion, but its effectiveness has not been confirmed in larger studies (Cao et al, 2009; Fischer et al, 2010).
Pulmonary Embolism
Intravascular clotting may manifest with pulmonary embolism, and intracardiac thrombi may occur with or without venous thrombosis and are frequently adherent to central venous lines. The mechanism of intravascular clotting in OLT patients is not fully understood. Some ESLD diagnoses, such as primary biliary cirrhosis or primary sclerosing cholangitis, are associated with hypercoagulability. In other cases, intravascular clotting has been related to administration of antifibrinolytics or transfusion of blood products (Warnaar et al, 2008a). Use of aprotinin in patients undergoing OLT may be associated with an increased incidence of venous thrombosis (Warnaar et al, 2009). Still, in a single-center case series, four episodes of PE were reported in the absence of antifibrinolytic use (Lerner et al, 2005).
In a recent single-center series, reported incidence of pulmonary embolism was 3.3% (El-Baghdadi & Sakai, 2010); however, very high overall and intraoperative mortality were reported in a recent review focused on pulmonary embolism in an OLT setting (Warnaar et al, 2008a): hospital mortality was 68%, and intraoperative mortality rate 82%.
The most common initial indication of intraoperative pulmonary embolism is usually systemic hypotension with a simultaneous increase in pulmonary pressure (Ellenberger et al, 2006; Warnaar et al, 2008a). End-tidal CO2 may also decrease. A reasonable preventive strategy is based on prompt identification of hypercoagulable conditions with TEG/ROTEM and avoidance of unnecessary prophylactic administration of antifibrinolytics (Planinsic et al, 2006). TEE can be a diagnostic tool, but its role as a preventive tool is less clear. Treatment is based on hemodynamic support, and successful intraoperative administration of recombinant tissue plasminogen activator has been reported (Jackson et al, 2006).
Special Situations
Acute Liver Failure
Acute liver failure is defined as a severe liver dysfunction causing encephalopathy and coagulopathy (INR >1.5) within 24 weeks of the onset of symptoms in patients without previous liver disease (see Chapters 72 and 97C); it is considered fulminant ALF if it develops in less than 2 weeks. Overall transplant-free survival in ALF is dependent on etiology and is just above 40%; it is highest for acetaminophen/paracetamol overdose, which requires transplantation in less than 10% of patients (Trotter, 2009). Liver transplantation is the only proven therapeutic intervention in advanced ALF, accounting for just over 5% of all liver transplantations, and carries a 1-year survival of 60% to 80% (De Gasperi et al, 2008; Trotter, 2009; see Chapter 97C).
ICP typically arises during the dissection phase and peaks after reperfusion. A simultaneous and precipitous drop in MAP makes postreperfusion the most critical and challenging period for management of CPP. A protocol that included intraparenchymal ICP monitoring, maintenance of CPP greater than 60 mm Hg by administration of vasopressors, hyperventilation (Pco2 at 30 to 35 mm Hg), mannitol and 3% saline, mild hypothermia (33° to 34° C), and a high dose of pentobarbital titrated to ICP effect resulted in favorable neurologic outcomes in a small series of ICU and liver transplant patients (Raschke et al, 2008).
No randomized trials or consensus statements are available that stipulate the safety and efficacy of different types of ICP monitors or specific management protocols during liver transplantation, but it is increasingly recognized that clinically meaningful inferences may require multimodal approaches, including brain tissue oxygen monitoring and microdialysis (Stevens & Merritt, 2008). Interventions aimed at management of CPP are outlined in Table 97B.1.
Despite profound coagulation abnormalities in ALF (Box 97B.1), serious bleeding is rare during liver transplantation, because ALF patients have minimal or no portal hypertension (Muñoz et al, 2009). No evidence-based protocols have been outlined for correcting coagulopathy in ALF, but it is recognized that prophylactic administration of FFP and other products simply to correct the prothrombin time is not justified. Correction of coagulopathy is important before placement of an ICP transducer. Whole-blood assays (TEG, ROTEM) and clinical assessment are more useful guides for intraoperative treatment of coagulopathy. A major limitation of FFP is the potential for volume overload and transfusion-related acute lung injury (TRALI). Cryoprecipitate is typically indicated when the fibrinogen level falls below 100 mg/dL; activated recombinant factor VII (rFVIIa) and thrombin concentrates are beneficial, but optimal use of these last two remain to be determined. Antifibrinolytics should be considered if clinically significant fibrinolysis is proven.
Box 97B.1
Hemostatic Abnormalities in Liver Disease
Impaired platelet function (?)
Upregulated NO* and prostacyclin* inhibit platelets
Low levels of coagulation factors II, V, VII, IX, X, and XI
Hypofibrinogenemia, dysfibrinogenemia
Elevated levels of tPA,* decreased PAI-1*
Elevated factor VIII† and von Willebrand factor*
Lisman T, Leebeek WG, 2007: Hemostatic alterations in liver disease: a review on pathophysiology, clinical consequences, and treatment. Dig Surg 24:250-258.
Combined Liver-Kidney Transplantation
With the introduction of the MELD scoring system, the percentage of simultaneous liver-kidney transplantation doubled, to about 4.4% of all liver transplantations performed in the United States (Gines & Schrier, 2009). Although it is obvious that combined transplantation should be reserved only for patients with irreversible renal failure, reliable predictive factors for reversibility are sometimes difficult to fully define. Current criteria recommended by the International Liver Transplant Society expert panel (Gonwa & Davis, 2009) include patients who, in addition to having liver disease, are on dialysis because of end-stage renal disease; patients with AKI that requires dialysis for at least 6 weeks; and those not on dialysis but who have a low glomerular filtration rate (<30 mL/min) and proteinuria (3 g/day, 24-hour urine protein/creatinine ratio >3).
A matched case-control analysis of patients dialyzed for more than 3 months demonstrated a favorable impact of combined liver-kidney transplantation compared with liver transplantation alone. Patient survival was improved (87.2% vs. 74.5%), as was liver-graft survival (84.5% vs. 70.8%), 1 year after transplantation (Locke et al, 2008). In contrast, 1-year survival of the kidney graft was lower than after kidney transplantation alone (77.2% vs. 89.3%). The inferior renal results and the priority given to the kidney on the long waiting list point to a need for a standardized approach to combined liver-kidney allocation and for a better understanding of reversibility of renal dysfunction (Davis, 2008).
Intraoperative continuous RRT is possible, and its use is routine in some centers. In a series of 41 OLTs, this modality was well tolerated hemodynamically, and it allowed achievement of neutral fluid balance without a need for systemic anticoagulation (Townsend et al, 2009). Fluid strategies for the two parts of the combined procedure may seem conflicting; although the kidney transplantation part may prompt a somewhat more liberal fluid regimen, in our opinion, goals for both OLT and kidney transplantation should be maintenance of a normal range preload to allow optimal organ perfusion.
Retransplantation
Most common reasons for retransplantation are primary nonfunction, recurrence of viral hepatitis, acute or chronic rejection, and thrombosis of the hepatic artery (Ma et al, 2008; Pfitzmann et al, 2007). The time interval between the primary transplantation and retransplantation—that is, early versus delayed retransplantation—depends on the etiology of the graft failure.
The survival rate for retransplanted patients is lower than for primary transplant recipients: at 1 year, the survival is 67.2% versus 83.4%; at 5 years, it drops to 46% versus 67.4% (UNOS, 2010). With appropriate recipient selection, better outcomes are possible, and 1- and 5-year survival rates have been reported as high as 78% and 67%, respectively, with retransplantation (Ma et al, 2008; Pfitzmann et al, 2007). General agreement has been reached on two points: 1) that whole graft should be used in adults (Pfitzmann et al, 2007) and 2) that retransplantation should not be undertaken with extended-criteria donors (Zimmerman & Ghobrial, 2005).
The current modest results emphasize the need for strategies to improve retransplantation survival. In particular, a strong interest has been shown in the development of valid predictive criteria; for example, according to Zimmerman and Ghobrial (2005), patients who require mechanical ventilation, those who have advanced renal failure, and patients of advanced age have worse outcomes and should not be considered for retransplantation. MELD score has prognostic value in retransplantation: patients with of a score below 20 have better outcomes than those with higher scores (Ma et al, 2008); in another study, worse results were reported when MELD was more than 25 (Zimmerman & Ghobrial, 2005).
A few studies have also looked at survival as a function of the time interval between the primary transplantation and retransplantation; survival was better when retransplantation occurred less than 8 or more than 30 days after the initial transplantation (Ma et al, 2008).
Intraoperative anesthetic challenges depend on the overall patient condition on the day of surgery; factors that may impact transplantation include difficult vascular access, a need to support liver and coagulation function, increased blood losses, and a higher requirement for blood-product transfusions. The main causes of death were bacterial infection, multisystem organ failure, bleeding, and recurrence of disease (Pfitzmann et al, 2007).
Pediatric Transplantation
Indications for liver transplantation in the pediatric population include 1) primary liver disease, such as extrahepatic biliary atresia, and intrahepatic cholestasis, which includes sclerosing cholangitis, Alagille syndrome, and other similar syndromes; 2) metabolic diseases, such as Wilson disease, α1-antitrypsin deficiency, Crigler-Najjar syndrome, disorders of the urea cycle, oxaluria type 1, and disorders of carbohydrate metabolism; 3) ALF; and 4) liver disease brought about through other causes, including primary liver tumor and cystic fibrosis (Spada et al, 2009; Yudkowitz & Chietero, 2005; see Chapter 71).
Age at Transplantation
Age at transplantation mainly depends on the specific etiology of the liver disease. Historically, age at transplantation was one of the main factors that determined survival, and infants used to have poorer outcomes than older children; however, with recent improvement of surgical techniques and better overall perioperative care, infant survival is close to that of older children following OLT (Bennett & Bromley, 2006).
Survival
One-year survival for chronic liver disease is greater than 90%, and survival at 5 years is over 80% in most centers in the United States and Europe (Bennett & Bromley, 2006). Nephrotoxicity secondary to calcineurin inhibitors (CNIs) remains a frequent cause of posttransplant renal dysfunction (Bucuvalas & Alonso, 2008).
Recipient Selection
Organ allocation to pediatric recipients in the United States is based on the Pediatric End-Stage Liver Disease (PELD) score, developed based on data derived from a database of children with ESLD to predict mortality on the waiting list; it considers bilirubin, INR, serum albumin, age, and growth factors. Additional PELD points are awarded for specific risk factors not taken into account in the original PELD equation, such as presence of HPS, metabolic diseases, and liver tumors (Bennett & Bromley, 2006; Spada et al, 2009).
Intraoperative Anesthesia Challenges
Vascular Access
Thrombosis of the hepatic artery is a frequent complication that may lead to a graft loss and need for retransplantation. Keeping the hematocrit between 25% and 30% to prevent excessive blood viscosity and avoidance of overcorrecting the coagulation function are common protective strategies. Aspirin can be given (in adults) in the immediate postoperative period to blunt platelet activity (Bennett & Bromley, 2006; Yudkowitz & Chietero, 2005).
Living-Related Donor Liver Transplantation
Since 2006, fewer than 300 OLTs with a graft from a living relative donor have been performed annually in the United States—about 3% of adult and 10% of pediatric OLTs (see Chapter 98B). This is a marked decline with respect to the year 2001, when a record 524 OLTs came from living donors—about 9% of adult and 18% of pediatric OLTs. Although survival of the recipients of a living graft is superior to that of cadaveric graft recipients (UNOS, 2010), a justified concern for donor morbidity and mortality drives this trend. Challenges for the anesthesiologist depend more on the recipient’s characteristic and comorbidities than on the donor category (Table 97B.2).
| Type | Complication |
|---|---|
| Pulmonary | TRALI/ARDS, pneumonia, pleural effusions, persistent shunting |
| Cardiovascular | CHF, hypotension, myocardial ischemia, arrhythmias |
| Renal | ATN, hepatorenal syndrome, CNI nephrotoxicity |
| Metabolic | Hyperglycemia/diabetes mellitus, electrolyte abnormalities |
| Neurologic | Persistent encephalopathy, seizures, posterior leukoencephalopathy, central pontine myelinolysis |
| Infectious | Wound infections, seromas, intraabdominal abscess, sepsis |
| Abdominal | Surgical bleeding or bleeding as a result of coagulopathy |
| Vascular | Hepatic artery or portal vein stenosis/thrombosis, IVC obstruction |
| Biliary | Biliary stricture or leaks |
| Rejection | Cellular rejection |
| Immunosuppressant toxicity | Seizures, kidney failure |
ARDS, acute respiratory distress syndrome; ATN, acute tubular necrosis; CHF, congestive heart failure; CNI, calcineurin inhibitor; IVC, inferior vena cava; TRALI, transfusion-related acute lung injury
Postoperative Intensive Care
Early Extubation
Safe early or even immediate postoperative extubation of a majority of liver transplant patients has been reported by some centers; however, the exact criteria, the benefits of early extubation to liver recipients, and any cost benefits remain a matter of debate. A multicenter cohort study by a proponent group (Mandell et al, 2007) reported a lower rate (7.7%) of postoperative adverse events in the immediate extubation group than that commonly reported for ventilated liver transplant patients. This study showed great variability in rates of extubation (5% to 67%) and rates of complications between centers, suggesting a significant impact of the regional patterns of practice, intraoperative events, and physician experience. Multiple studies agree that encephalopathy, whether the result of acute or chronic liver failure, is a strong predictor of the need for prolonged ventilation. Other factors, such as volume of blood transfused and RRT, are less consistent predictors among studies and may be only indirect markers of poor graft function, severity of illness, and a lack of standardized intraoperative protocols (Mandell et al, 2009).
No randomized studies are currently available that demonstrate that early extubation improves patient outcomes or reduces cost (Ozier & Klinck, 2008). Although experienced clinicians can select appropriate patients for early extubation, no evidence-based criteria exist in support of this practice; more studies are needed to establish whether this intervention is beneficial and whether it should be a therapeutic goal in liver transplant recipients.
Medical Complications
Pulmonary complications that include acute respiratory distress syndrome (ARDS), TRALI, pneumonia, and pleural effusions are common in the immediate postoperative period. These complications are reported to occur in over 50% of patients after liver transplantation and lead to hypoxemia that often requires prolonged mechanical ventilation (Biais et al, 2009; Bozbas et al, 2008; Li et al, 2010). ARDS and TRALI may be related to blood-product transfusion and fluid overload. Pneumonia is the most frequent postoperative infection in patients following OLT, and it carries a high mortality. Pleural effusion is another common pulmonary complication; it promotes atelectasis and contributes to impaired respiratory exchange. The addition of PEEP monitoring may be effective in improving oxygenation in postoperative patients. PEEP of up to 10 cm of H2O was found not to affect the perfusion of the grafted liver, as assessed by Doppler (Saner et al, 2006b).
Cirrhotic patients continue to exhibit hyperdynamic circulation during the first hours or days after OLT. Arterial hypotension and the need for vasopressors are common. With the graft functioning and no evidence of surgical complications, the hemodynamics tend to normalize, which allows downgrading of invasive monitoring and of hemodynamic support. Maintenance of kidney perfusion pressure is paramount because AKI is common (17% to 95%) (Pham et al, 2009) and is associated with increased ICU length of stay and mortality (Smith et al, 2009). The causes of post-OLT acute renal failure are multifactorial and include preexisting renal dysfunction, HRS, perioperative acute tubular necrosis, and CNI nephrotoxicity. RRT is necessary in 5% to 35% among patients who develop postoperative AKI.
Endocrine and metabolic complications are common in the early posttransplant period. Surgical stress, corticosteroid administration, and CNIs contribute to early posttransplant hyperglycemia, which frequently evolves to diabetes mellitus, and hypertension. Diabetes occurs in up to 30% of posttransplant patients, and hypertension is seen in over 50%. Hyperglycemia requires an intravenous insulin regimen and improves as steroids are tapered; hypertension should be treated aggressively to prevent cardiovascular complications (Kallwitz & Cotler, 2008).
Neurologic complications may occur in up to 50% of liver transplant recipients (Saner et al, 2009). Such complications contribute to longer ICU and hospital stays and may result in long-term poor quality of life. The most common neurologic complications are encephalopathy and seizures, followed by psychotic disorders, stroke, and poorly understood posterior leukoencephalopathy. The etiology is multifactorial and includes poor graft function, use of steroids and possibly CNIs, infection and systemic inflammatory response, electrolyte imbalance, and embolic events.
Posttransplant rejection ranges from 10% to 30% (Mueller et al, 2004). Cellular rejection is suspected with increasing transaminases and normal hepatic vascular Doppler studies, and diagnosis is confirmed with liver biopsy; however, early episodes respond well to boluses of corticosteroids and rarely lead to graft failure.
Surgical Complications
Postoperative hemorrhage is primarily a clinical diagnosis that can be confirmed by abdominal imaging techniques, and it occurs in 10% to 15% of OLT patients. A priority is to distinguish whether hemorrhage is due to persisting coagulopathy or to surgical causes. Hemodynamic instability and transfusion of more than 4 to 6 U packed blood cells over a 24-hour period are usual indications for surgical reexploration (Mueller et al, 2004).
All vascular complications require urgent surgical intervention. If vascular repair is delayed, the ensuing graft failure may necessitate retransplantation. Many vascular complications have been successfully treated using various endovascular approaches, including percutaneous angioplasty, stent placement, or thrombolysis (Amesur & Zajko, 2006).
Biliary complications occur in about 10% to 15% of patients following liver transplantation. Stricture of the bilary tree accounts for most of the complications, but biliary leak is a more common early complication. The most severe manifestations of biliary leak are peritonitis and sepsis, but asymptomatic bilomas are also a common presentation. The diagnosis of the leak is based on percutaneous transhepatic cholangiography (PTC) or endoscopic retrograde cholangiography (ERCP). Ultrasound and CT scan are useful in diagnosis of biliary dilations due to obstruction, bile collections, or abscesses. Treatment of biliary obstruction includes percutaneous balloon dilation and drainage or surgical revision. Treatment of biliary leaks involves percutaneous transhepatic drainage (PTD), a technically difficult alternative to surgical repair. Nonanastomotic leaks and leaks secondary to hepatic artery thrombosis are particularly ominous, because they are less amenable to percutaneous repair and often require retransplantation (Amesur & Zajko, 2006; Mueller et al, 2004).
Recipients of grafts from donors after cardiac death (DCD) have worse graft and patient survival, a higher rate of postoperative complications, and higher resource utilization than the recipients of dead-brain donation (DBD) grafts (Reich et al, 2009). Primary graft nonfunction, nonanastomotic biliary strictures, and hepatic artery stenosis are the most significant postoperative complications of DCD liver transplantation. Higher immediate perioperative mortality in DCD transplants has been reported (Pine et al, 2009), but most complications lead to increased mortality or to the need for retransplantation in the weeks or months following the transplantation (Grewal et al, 2009). Strategies to improve DCD outcomes currently under development include a super-rapid recovery technique by experienced procurement teams, premortem cannulation techniques, ex vivo organ perfusion, and minimization of cold- and warm-ischemia time (Reich et al, 2009). Although DCD grafts are associated with inferior outcomes, DCD donation remains a valuable resource, and with selective and judicious use, these can be used to achieve acceptable results (Pine et al, 2009).
Aggarwal S, et al. Postreperfusion syndrome: cardiovascular collapse following hepatic reperfusion during liver transplantation. Transplant Proc. 1987;19(4 Suppl 3):54-55.
Aggarwal S, et al. Postreperfusion syndrome: hypotension after reperfusion of the transplanted liver. J Crit Care. 1993;8(3):154-160.
Alper I, et al. Effects of cardiac valve dysfunction on perioperative management of liver transplantation. Transplant Proc. 2009;41(5):1722-1726.
Amesur N, Zajko A. Interventional radiology in liver transplantation. Liver Transpl. 2006;12:330-351.
Ammori JB, et al. Effect of intraoperative hyperglycemia during liver transplantation. J Surg Res. 2007;15:227-233.
Avidan MS, et al. Comparison of structured use of routine laboratory tests or near-patient assessment with clinical judgment in the management of bleeding after cardiac surgery. Br J Anaesth. 2004;92:178-186.
Ayanoglu HO, et al. Causes of postreperfusion syndrome in living or cadaveric donor liver transplantations. Transplant Proc. 2003;35(4):1442-1444.
Bennett J, Bromley P. Perioperative issues in pediatric liver transplantation. Int Anesthesiol Clin. 2006;44(3):125-147.
Bennett-Guerrero E, et al. Preoperative and intraoperative predictors of postoperative morbidity, poor graft function, and early rejection in 190 patients undergoing liver transplantation. Arch Surg. 2001;136(10):1177-1183.
Bhatia A, Gupta AK. Neuromonitoring in the intensive care unit. I. Intracranial pressure and cerebral blood flow monitoring. Intensive Care Med. 2007;33:1263-1271.
Biais M, et al. Cardiac output measurement in patients undergoing liver transplantation: pulmonary artery catheter versus uncalibrated arterial pressure waveform analysis. Anesth Analg. 2008;106:1480-1486.
Biais M, et al. Uncalibrated stroke volume variations are able to predict the hemodynamic effects of positive end-expiratory pressure in patients with acute lung injury or acute respiratory distress syndrome after liver transplantation. Anesthesiology. 2009;111:855-862.
Biancofiore G, et al. Evaluation of an uncalibrated arterial pulse contour cardiac output monitoring system in cirrhotic patients undergoing liver surgery. Br J Anaesth. 2009;102:47-54.
Bindi ML, et al. Transcranial Doppler sonography is useful for the decision making at the point of care in patients with acute liver failure: a single centre’s experience. J Clin Monit Comput. 2008;22:449-452.
Bjerring PN, et al. The brain in acute liver failure: a tortuous path from hyperammonemia to cerebral edema. Metab Brain Dis. 2009;24:5-14.
Bozbas SS, et al. Pulmonary complications and mortality after liver transplant. Exp Clin Transplant. 2008;6(4):264-270.
Bucuvalas JC, Alonso E. Long-term outcomes after liver transplantation in children. Curr Opin Organ Transplant. 2008;13(3):247-251.
Burtenshaw AJ, Isaac JL. The role of transoesophageal echocardiography for perioperative cardiovascular monitoring during orthotopic liver transplantation. Liver Transpl. 2006;12:1577-1583.
Cao Z, et al. Vasoplegic syndrome during liver transplantation. Anesth Analg. 2009;108(6):1941-1943.
Cardenas A, Gines P. Hepatorenal syndrome: clinical features, management, and role of liver transplantation—report of the First International Liver Transplant Society Expert Panel Consensus Conference on Renal Insufficiency in Liver Transplantation. Liver Transpl. 2009;15(11):S6-S8.
Chui AK, et al. Postreperfusion syndrome in orthotopic liver transplantation. Transplant Proc. 2000;32(7):2116-2117.
Claus-Georg K, De Wolf AM. Current approach to intraoperative monitoring in liver transplantation. Curr Opin Organ Transplant. 2008;13:285-290.
Coakley M, et al. Transfusion triggers in orthotopic liver transplantation: a comparison of the thromboelastometry analyzer, the thromboelastogram, and conventional coagulation tests. J Cardiothorac Vasc Anesth. 2006;20(4):548-553.
Costa MG, et al. Continuous intermittent cardiac output measurement in hyperdynamic conditions: pulmonary artery catheter vs. lithium dilution technique. Intensive Care Med. 2008;34:257-263.
Costa MG, et al. Perioperative considerations in HIV-infected liver transplanted patients. Transplant Proc. 2009;41(4):1249-1252.
da Silva Viana J. Recombinant factor VIIa in major abdominal surgery and liver transplantation. Transplant Proc. 2006;38(3):818-819.
Dalmau A, et al. Tranexamic acid reduces red cell transfusions better than epsilon-aminocaproic acid or placebo in liver transplantation. Anesth Analg. 2000;91:29-34.
Dalmau A, et al. Hemostasis and coagulation monitoring and management during liver transplantation. Curr Opin Organ Transplant. 2009;14:286-290.
Davenport A. Continuous renal replacement therapies in patients with liver disease. Semin Dialysis. 2009;22:169-172.
Davis CL. Controversies in combined liver-kidney transplantation: indications and outcomes. Transplant Rev (Orlando). 2008;22(1):82-88.
de Boer MT, et al. Minimizing blood loss in liver transplantation: progress through research and evolution of techniques. Dig Surg. 2005;22:265-275.
de Boer MT, et al. The impact of intraoperative transfusion of platelets and red blood cells on survival after liver transplantation. Anesth Analg. 2008;106:32-44.
De Gasperi A, et al. Acute liver failure: managing coagulopathy and the bleeding diathesis. Transplant Proc. 2008;41:1256-1259.
Della Rocca G, et al. Preload and hemodynamic assessment during liver transplantation: a comparison between pulmonary artery catheter and transpulmonary indicator dilution technique. Eur J Anesth. 2002;19:868-875.
Della Rocca G, et al. Preload index: pulmonary artery occlusion pressure versus intrathoracic blood volume monitoring during liver transplantation. Anesth Analg. 2002;95:835-843.
Della Rocca G, et al. Continuous right ventricular end diastolic volume and right ventricular ejection fraction during liver transplantation: a multicenter study. Liver Transpl. 2008;14:327-332.
Della Rocca G, Brondani A, Costa MG. Intraoperative hemodynamic monitoring during organ transplantation: what is new? Curr Opin Organ Transplant. 2009;14(3):291-296.
De Boer MT, et al. Minimizing blood loss in liver transplantation: progress through research and evolution of techniques. Dig Surg. 2005;22(4):265-275.
De Wolf AM. Perioperative assessment of the cardiovascular system in end-stage liver disease and transplantation. Int Anesthesiol Clin. 2006;44:59-78.
De Wolf AM. Pulmonary artery catheter: rest in peace? Not quite yet. Liver Transpl. 2008;14:917-918.
De Wolf AM, Aggarwal S. Monitoring of preload during liver transplantation. Liver Transpl. 2008;14:268-269.
De Wolf AM, et al. Right ventricular function during orthotopic liver transplantation. Anesth Analg. 1993;76(3):562-568.
Donovan CL, et al. Two-dimensional and dobutamine stress echocardiography in the preoperative assessment of patients with end-stage liver disease prior to orthotopic liver transplantation. Transplantation. 1996;61:1180-1188.
El-Baghdadi MM, Sakai T. Fatal pulmonary embolism during liver transplantation in a patient with fulminant hepatic failure: a diagnostic challenge of the “flat line” thromboelastograms. J Cardiothorac Vasc Anesth. 2010;24(4):641-643. Epub Oct 12 2009
Ellenberger C, et al. Cardiovascular collapse due to massive pulmonary thromboembolism during orthotopic liver transplantation. J Clin Anesth. 2006;18(5):367-371.
Eroglu Y, et al. Hepatic encephalopathy. Emerg Med Clin N Am. 2009;27:401-414.
Fallon MB, et al. Pulmonary Vascular Complications of Liver Disease Study Group: impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology. 2008;135(4):1168-1175.
Ferenci P, et al. Hepatic encephalopathy: definition, nomenclature, diagnosis and quantification—final report of the working party at the 11th World Congress of Gastroenterology. Hepatology. 2002;35:716-721.
Fischer GW, et al. Methylene blue for vasopressor-resistant vasoplegia syndrome during liver transplantation. J Cardiothorac Vasc Anesth. 2010;24(3):463-466. Epub Oct 4 2008
Fouad TR, et al. Prediction of cardiac complications after liver transplantation. Transplantation. 2009;87(5):763-770.
Fukazawa K, et al. Is the immediate reversal of diastolic dysfunction of cirrhotic cardiomyopathy after liver transplantation a sign of the metabolic etiology? Liver Transpl. 2009;15(11):1417-1419.
Gallegos-Orozco JF, Vargas HE. Liver transplantation: from Child to MELD. Med Clin North Am. 2009;93(4):931-950. ix
Gines P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279-1290.
Gonwa TA, Davis C. Evaluation and management of pretransplant renal insufficiency and criteria for simultaneous liver-kidney transplantation. Liver Transpl. 2009;15:S1-S34.
Grewal HP, et al. Liver transplantation using controlled donation after cardiac death donors: an analysis of a large single-center experience. Liver Transpl. 2009;15:1028-1035.
Herbstreit F, et al. Monitoring of haemostasis in liver transplantation: comparison of laboratory based and point of care tests. Anaesthesia. 2010;65(1):44-49. Epub November 4 2009
Hilmi I, et al. The impact of postreperfusion syndrome on short-term patient and liver allograft outcome in patients undergoing orthotopic liver transplantation. Liver Transpl. 2008;14(4):504-508.
Jackson D, et al. Successful intraoperative use of recombinant tissue plasminogen activator during liver transplantation complicated by massive intracardiac/pulmonary thrombosis. Anesth Analg. 2006;102(3):724-728.
Kabon B, Kurz A. Optimal perioperative oxygen administration. Curr Opin Anaesthesiol. 2006;19(1):11-18.
Kalina U, et al. Rotational thromboelastography for monitoring of fibrinogen concentrate therapy in fibrinogen deficiency. Blood Coagul Fibrinolysis. 2008;19:777-783.
Kallwitz ER, Cotler SJ. Care of the liver transplant patient. Dis Mon. 2008;54:486-507.
Kang YG. Does transfusion that is based on clinical coagulation monitors reduce hemorrhage during liver transplantation? J Hepatol. 1997;3:655-699.
Kang YG, et al. Intraoperative changes in blood coagulation and thromboelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888-896.
Kiafar C, Gilani N. Hepatic hydrothorax: current concepts of pathophysiology and treatment options. Ann Hepatol. 2008;7(4):313-320.
Kim RW, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018-1026.
Kim YK, et al. Thoracic complications of liver cirrhosis: radiologic findings. Radiographics. 2009;29(3):825-837.
Kumar A, et al. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med. 2004;32:691-699.
Lee WM, et al. Intravenous N-acetyl cysteine improves spontaneous survival in early stage non-acetaminophen acute liver failure. Hepatology. 2007;46:71A.
Lerner AB, et al. Four cases of cardiopulmonary thromboembolism during liver transplantation without the use of antifibrinolytic drugs. Anesth Analg. 2005;101(6):1608-1612.
Li Q, et al. Relevant risk factors affecting time of ventilation during early postoperative period after orthotopic liver transplantation. J Crit Care. 2010;25(2):221-224.
Lisman T, Leebeek WG. Hemostatic alterations in liver disease: a review on pathophysiology, clinical consequences, and treatment. Dig Surg. 2007;24:250-258.
Locke JE, et al. Declining outcomes in simultaneous liver-kidney transplantation in MELD era: ineffective usage of renal allografts. Transplantation. 2008;85:935-942.
Ma Y, et al. Liver retransplantation: a single-centre experience. Chin Med J (Engl). 2008;121(20):1987-1991.
Mandell MS, Tsou MY. The development of perioperative practices for liver transplantation: advances and current trends. J Chin Med Assoc. 2008;71(9):435-441.
Mandell MS, et al. A multicenter evaluation of safety of early extubation in liver transplant patients. Liver Transpl. 2007;13:1557-1563.
Mandell MS, et al. Cardiac evaluation of liver transplant candidates. World J Gastroenterol. 2008;14(22):3445-3451.
Mandell MS, et al. The clinical value of early extubation. Curr Opin Organ Transplant. 2009;14:297-302.
Mandell S. Hepatopulmonary syndrome and portopulmonary hypertension in the model for end-stage liver disease (MELD) era. Liver Transpl. 2004;10(10 Suppl 2):S54-S58.
Martin-Liahi M, et al. Randomized comparative study of terlipressin and albumin vs. albumin alone in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134(5):1352-1359.
Massicotte L, et al. Transfusion predictors in liver transplant. Anesth Analg. 2004;98:1245-1251.
Massicotte L, et al. Survival rate changes with transfusion of blood products during liver transplantation. Can J Anesth. 2005;52:148-155.
Massicotte L, et al. Effect of low central venous pressure and phlebotomy on blood product transfusion requirements during liver transplantations. Liver Transpl. 2006;12(1):117-123.
Massicotte L, et al. Evaluation of cell salvage autotransfusion utility during liver transplantation. HPB (Oxford). 2007;9(1):52-57.
Massicotte L, et al. Coagulation defects do not predict blood product requirements during liver transplantation. Transplantation. 2008;85(7):956-962.
Massicotte L, et al. Independent validation of a model predicting the need for packed red blood cell transfusion at liver transplantation. Transplantation. 2009;88(3):386-391.
Matesanz R, et al. Global observatory and database on donation and transplantation: world overview on transplantation activities. Transplant Proc. 2009;41(6):2297-2301.
Merion RM. When is a patient too well and when is a patient too sick for a liver transplant? Liver Transpl. 2004;10(Suppl 2):S69-S73.
Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005;103(2):419-428.
Molenaar IQ, et al. Efficacy and safety of antifibrinolytic drugs in liver transplantation: a systematic review and meta-analysis. Am J Transplant. 2007;7(1):185-194.
Møller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut. 2008;57(2):268-278.
Moreau R, et al. Acute renal failure in patients with cirrhosis: perspectives in the age of MELD. Hepatology. 2003;37:1179-1185.
Mueller AR, et al. Early postoperative complications following liver transplantation. Best Pract Res Clin Gastroenterology. 2004;18:881-900.
Mukhtar A, et al. The safety of modern hydroxyethyl starch in living donor liver transplantation: a comparison with human albumin. Anesth Analg. 2009;109(3):924-930.
Muñoz SJ, Stravitz RT, Gabriel DA. Coagulopathy of acute liver failure. Clin Liver Dis. 2009;13:95-107.
Murray KF, Carithers RLJr. AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology. 2005;41(6):1407-1432.
Naksuji M, Bookallil M. Pathophysiological mechanism of postrevascularization hyperkalemia in orthotopic liver transplantation. Anesth Analg. 2000;91:464-470.
Nanashima A, et al. Analysis of postrevascularization syndrome after orthotopic liver transplantation: the experience of an Australian liver transplant center. J Hepatobiliary Pancreat Surg. 2001;8:557-563.
Ng VL, et al. Liver disease, coagulation testing and hemostasis. Clin Lab Med. 2009;29:265-282.
Nielsen VG. A comparison of the thromboelastogram and the ROTEM. Blood Coagul Fibrinolysis. 2007;18:247-252.
Niemann CU, et al. Acute kidney injury during liver transplantation as determined by neutrophil gelatinase-associated lipocalin. Liver Transpl. 2009;15:1852-1860.
O’Connor CJ, et al. Pulmonary thromboembolism during liver transplantation: possible association with antifibrinolytic drugs and novel treatment options. Anesth Analg. 2000;91:296-299.
Ofosu FA, et al. Plasma-derived biological medicines used to promote haemostasis. Thromb Haemost. 2008;99(5):851-862.
Ojo OA. Scope of the problem and impact on outcomes: report of the First International Liver Transplant Society Expert Panel Consensus Conference on Renal Insufficiency in Liver Transplantation. Liver Transpl. 2009;15(11):S2-S4.
Organ Procurement and Transplantation Network. Data. Available at optn.transplant.hrsa.gov/data, 2010.
Ozier Y, Klinck JR. Anesthetic management of hepatic transplantation. Curr Opin Anaesthesiol. 2008;21(3):391-400.
Ozier Y, Tsou MY. Changing trends in transfusion practice in liver transplantation. Curr Opin Organ Transplant. 2008;13(3):304-309.
Park C, et al. Severe intraoperative hyperglycemia is independently associated with surgical site infection after liver transplantation. Transplant. 2009:1031-1036.
Paugam-Burtz C, et al. Postreperfusion syndrome during liver transplantation for cirrhosis: outcome and predictors. Liver Transpl. 2009;15(5):522-529.
Pereboom IT, Lisman T, Porte RJ. Platelets in liver transplantation: friend or Foe? Liver Transpl. 2008;14:923-931.
Pereboom IT, et al. Platelet transfusion during liver transplantation is associated with increased postoperative mortality due to acute lung injury. Anesth Analg. 2009;108(4):1083-1091.
Pfitzmann R, et al. Trends and experiences in liver retransplantation over 15 years. Liver Transpl. 2007;13(2):248-257.
Pham PT, Slavov C, Pham PC. Acute kidney injury after liver, heart, and lung transplants: dialysis modality, predictors of renal function recovery and impact on survival. Adv Chronic Kidney Dis. 2009;16(4):256-267.
Pine JK, et al. Liver transplantation following donation after cardiac death: an analysis using matched pairs. Liver Transpl. 2009;15(9):1072-1082.
Pinsky MR. Hemodynamic monitoring over the past 10 years. Crit Care. 2006;10:117-119.
Planinsic RM, et al. Diagnosis and treatment of intracardiac thrombosis during orthotopic liver transplantation. Anesth Analg. 2004;99(2):353-356.
Planinsic RM, et al. Prevention of intracardiac/pulmonary thrombosis during liver transplantation. Anesth Analg. 2006;103(5):1329.
Plotkin JS, et al. Morbidity and mortality in patients with coronary artery disease undergoing orthotopic liver transplantation. Liver Transpl Surg. 1996;2:426-430.
Plotkin JS, et al. Dobutamine stress echocardiography for preoperative cardiac risk stratification of patients undergoing orthotopic liver transplantation. Liver Transpl Surg. 1998;4:253-257.
Ponnudurai RN, et al. Vasopressor administration during liver transplant surgery and its effect on endotracheal reintubation rate in the postoperative period: a prospective, randomized, double-blind, placebo-controlled trial. Clin Ther. 2005;27(2):192-198.
Ramos E, et al. Intraoperative red blood cell transfusion in liver transplantation: influence on patient outcome, prediction of requirements, and measures to reduce them. Liver Transpl. 2003;9:1320-1327.
Ramsay M. The reperfusion syndrome: have we made any progress? Liver Transpl. 2008;14:412-414.
Ramsay MAE, Garcia-Valdecasas JC. Intraoperative renal protection: anesthesia approaches. Liver Transpl. 2009;15:S23-S24.
Raschke RA, et al. Results of a protocol for the management of patients with fulminant liver failure. Crit Care Med. 2008;36:2244-2248.
Reich DL, et al. Association of intraoperative hypotension and pulmonary hypertension with adverse outcomes after orthotopic liver transplantation. J Cardiothorac Vasc Anesth. 2003;17(6):699-702.
Reich DJ, et al. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transpl. 2009;9:2004-2011.
Ricciardi R, et al. Donor hepatic function: a factor in postreperfusion syndrome. J Gastrointest Surg. 2002;6(2):248-254.
Rodriguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome: a liver-induced lung vascular disorder. N Engl J Med. 2008;358(22):2378-2387.
Rodriguez-Roisin R, et al. ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee: pulmonary-hepatic vascular disorders (PHD). Eur Respir J. 2004;24(5):861-880.
Rybak D, et al. Pulmonary Vascular Complications of Liver Disease Study Group: risk factors and impact of chronic obstructive pulmonary disease in candidates for liver transplantation. Liver Transpl. 2008;14(9):1357-1365.
Ryback D, et al. Risk factors and impact of chronic obstructive pulmonary disease in candidates for liver transplantation. Pulmonary Vascular Complications of Liver Disease Study Group. Liver Transpl. 2008;14(9):1357-1365.
Safadi A, et al. Perioperative risk predictors of cardiac outcomes in patients undergoing liver transplantation surgery. Circulation. 2009;120(13):1189-1194.
Salerno F, et al. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-1318.
Samama CM. Prothrombin complex concentrates: a brief review. Eur J Anaesthesiol. 2008;25(10):784-789.
Sandham JD, et al. A randomized controlled trial of the use of pulmonary artery catheter in high-risk surgical patients. N Engl J Med. 2003;348:5-14.
Saner FH, et al. Does PEEP impair the hepatic outflow in patients following liver transplantation? Intensive Care Med. 2006;32:1584-1590.
Saner FH, et al. Effects of positive end-expiratory pressure on systemic haemodynamics, with special interest to central venous and common iliac venous pressure in liver transplanted patients. Eur J Anaesthesiol. 2006;23(9):766-771.
Saner FH, et al. Positive end-expiratory pressure induces liver congestion in living donor liver transplant patients: myth or fact. Transplantation. 2008;85(12):1863-1866.
Saner FH, et al. Liver transplantation and neurological side effects. Metab Brain Dis. 2009;24(1):183-187.
Sanyal A, et al. A prospective, randomized, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome (HRS). Gastroenterology. 2008;134:1360-1368.
Schenk P, et al. Hepatopulmonary syndrome: prevalence and predictive value of various cutoffs for arterial oxygenation and their clinical consequences. Gut. 2002;51(6):853-859.
Schroeder RA, et al. Intraoperative fluid management during orthotopic liver transplantation. Cardiothorac Vasc Anesth. 2004;18(4):438-441.
Schuman R. Intraoperative resource utilization in anesthesia for liver transplantation in the United States: a survey. Anest Analg. 2003;97:21-28.
Senzolo M, et al. New insights into the coagulopathy of liver disease and liver transplantation. World J Gastroenterol. 2006;12(48):7725-7736.
Sharma M, et al. Safety of cardiac catheterization in patients with end-stage liver disease awaiting liver transplantation. Am J Cardiol. 2009;103(5):742-746.
Shin BS, et al. Comparison of femoral arterial blood pressure with radial arterial blood pressure and noninvasive upper arm blood pressure in the reperfusion period during liver transplantation. Transplant Proc. 2007;39(5):1326-1328.
Shultz MJ. Lung-protective mechanical ventilation with lower tidal volumes in patients not suffering from acute lung injury: a review of clinical studies. Med Sci Monit. 2008;14(2):RA22-RA26.
Singh C, Sager JS. Pulmonary complications in cirrhosis. Med Clin North Am. 2009;93(4):871-883. viii
Slinger P. Perioperative lung injury. Best Pract Res Clin Anaesthesiol. 2008;22(1):177-191.
Smith JO, et al. Incidence of prolonged length of stay after orthotopic liver transplantation and its influence on outcomes. Liver Transpl. 2009;15(3):273-279.
Spada M, et al. Pediatric liver transplantation. World J Gastroenterol. 2009;15(6):648-674.
Steib A, et al. Intraoperative blood loss and transfusion requirements during adult liver transplantation remain difficult to predict. Can J Anaesth. 2001;48:1075-1079.
Stevens RD, Merritt WT. Rescuing the brain in acute liver failure. Crit Care Med. 2008;36(7):2212-2213.
Stravitz RT, et al. Intensive care of patients with acute liver failure: recommendations of the US Acute Liver Failure Study Group. Crit Care Med. 2007;35:2498-2508.
Stravitz RT, et al. Therapeutic hypothermia for acute liver failure. Crit Care Med. 2009;37:S258-S264.
Stravitz RT, Larsen FS. Therapeutic hypothermia for acute liver failure. Crit Care Med. 2009;37(7 Suppl):S258-S264.
Su B-B, et al. Reliability of a new ultrasonic cardiac output monitor in recipients of living donor liver transplantation. Liver Transpl. 2008;14:1029-1037.
Sulieman BM, et al. OPTN policy regarding prioritization of patients with hepatopulmonary syndrome: does it provide equitable organ allocation? Am J Transplant. 2008;8(5):954-964.
Sundaram V, Shaikh OS. Hepatic encephalopathy: pathophysiology and emerging therapies. Med Clin North Am. 2009;93(4):819-836.
Swanson KL, et al. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008;8(11):2445-2453.
Teo YW, Greenhalg DL. Update on anesthetic approach to pulmonary hypertension. Eur J Anaesthiol. 2010;27(4):317-323.
Tiukinhoy-Laing SD, et al. Cardiac hemodynamic and coronary angiographic characteristics of patients being evaluated for liver transplantation. Am J Cardiol. 2006;98:178-181.
Townsend DR, et al. Intraoperative renal support during liver transplantation. Liver Transpl. 2009;15(1):73-78.
Tripodi A, et al. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology. 2005;41:553-558.
Tripodi A, et al. Abnormalities of hemostasis in chronic liver disease: reappraisal of their significance and need for clinical and laboratory research. J Hepatol. 2007;56:727-733.
Trotter JF. Practical management of acute liver failure in the Intensive Care Unit. Curr Opin Crit Care. 2009;15(2):163-167.
Umeda N, Kamath PS. Hepatopulmonary syndrome and portopulmonary hypertension. Hepatol Res. 2009;39(10):1020-1022.
Wagener G, et al. Vasopressin decreases portal vein pressure and flow in the native liver during liver transplantation. Liver Transpl. 2008;14(11):1664-1670.
Warnaar N, et al. Intraoperative pulmonary embolism and intracardiac thrombosis complicating liver transplantation: a systematic review. J Thromb Haemost. 2008;6(2):297-302.
Warnaar N, et al. The two tales of coagulation in liver transplantation. Curr Opin Org Transplant. 2008;13:298-303.
Warnaar N, et al. Aprotinin and the risk of thrombotic complications after liver transplantation: a retrospective analysis of 1492 patients. Liver Transpl. 2009;15(7):747-753.
Wax DB, et al. Transesophageal echocardiography utilization in high-volume liver transplantation centers in the United States. J Cardiothor Vasc Anest. 2008;22(6):811-813.
Wendon J, et al. Encephalopathy and cerebral edema in the setting of acute liver failure: pathogenesis and management. Neurocrit Care. 2008;9:97-102.
Westerkamp AC, et al. How to minimize blood loss during liver surgery in patients with cirrhosis. HPB (Oxford). 2009;11(6):453-458.
Wheeler AP, et al. Pulmonary artery vs. central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213-2224.
Williams K, et al. Dobutamine stress echocardiography in patients undergoing liver transplantation evaluation. Transplantation. 2000;69:2354-2356.
Wong F. Cirrhotic cardiomyopathy. Hepatol Int. 2009;3(1):294-304.
Wong L-SG, et al. Comparison of the USCOM ultrasound cardiac output monitor with pulmonary artery catheter thermodilution in patients undergoing liver transplantation. Liver Transpl. 2008;14:1038-1043.
Xia VW, et al. Preoperative characteristics and intraoperative transfusion and vasopressor requirements in patients with low vs. high MELD scores. Liver Transpl. 2006;12(4):614-620.
Xia VW, et al. The changing face of patients presenting for liver transplantation. Curr Opin Organ Transplant. 2008;13(3):280-284.
Yeshua H, et al. Pulmonary manifestations of liver diseases. Semin Cardiothorac Vasc Anesth. 2009;13(1):60-69.
Yudkowitz FS, Chietero M. Anesthetic issues in pediatric liver transplantation. Pediatr Transplant. 2005;9(5):666-672.
Yun BC, et al. Impact of pretransplant hyponatremia on outcome following liver transplantation. Hepatology. 2009;49(5):1610-1615.
Zhang LP, et al. Effects of different vasopressors on hemodynamics in patients undergoing orthotopic liver transplantation. Chin Med J (Engl). 2005;118(23):1952-1958.
Zhang ZW, et al. Therapy of central pontine myelinolysis following living donor liver transplantation: report of three cases. World J Gastroenterol. 2009;15(31):3960-3963.
Zimmerman MA, Ghobrial RM. When shouldn’t we retransplant? Liver Transpl. 2005;11(Suppl 2):S14-S20.