Chapter 94 Liver transplantation
Liver transplantation has revolutionised the care of patients, with both acute and chronic end-stage liver disease becoming the treatment of choice in the absence of contraindications. It has become an almost routine procedure, with the majority of patients having a short postoperative intensive care unit (ICU) stay and 1-year survival > 90%.1–4 Indications have widened, and contraindications decreased. As a consequence, the number of patients awaiting transplantation continues to outstrip cadaveric donor rates; waiting times lengthen, hence patients become critically ill before receiving a transplant, increasing risk and perioperative complications, and impairing long-term outcome.5 Innovative strategies have evolved as possible solutions to the lack of cadaveric donor organs, including widening the donor pool to include previously unsuitable donors (so called marginal donors), paediatric and adult living-related donation, reduced size and splitting techniques and the use of ‘non-heart beating donation’.
PATIENT SELECTION
Portopulmonary and hepatopulmonary syndromes are now an active indication for transplantation as opposed to a contraindication.6 Such patients are likely to have a more complex postoperative course, especially if graft function is borderline or they develop sepsis. Monitoring of patients whilst on the waiting list is essential to ensure that their disease does not progress such that transplantation is no longer a feasible option. Patients must have the required cardiorespiratory reserve to tolerate the procedure. Much work has gone into the development of prognostic tools to allow accurate prediction of the need and timing for transplantation, and increasingly the model used is the model for end-stage liver disease (MELD) system. This system was initially developed for predicting survival following transjugular intrahepatic portosystemic stent (TIPS) shunt but has been shown to be equally useful in predicting survival in those awaiting liver transplantation. Once multiorgan failure has developed in a debilitated patient awaiting transplantation, survival rates decrease to 20–30% and these patients often require weeks to months of postoperative hospitalisation.2,7
PERIOPERATIVE ASPECTS
OPERATIVE TECHNIQUE
Two main techniques are used in adult liver transplantation – those with vena cava preservation (‘piggyback technique’) and those using portal bypass (either internal, temporary portocaval shunt or external, veno-venous bypass). The advantages of the piggyback technique include haemodynamic stability during the anhepatic phase, without large volume fluid administration, and the negation of the need for veno-venous bypass with its associated risks and complications. Decreased transfusion requirements, shorter anhepatic time and shorter total operating time are also observed. There is no observed difference in renal function between the two techniques.8–10 The donor hepatic artery is directly anastomosed, utilising an ‘end-to-end’ technique, or a conduit is constructed. Portal venous anastomosis must also be undertaken. In most patients this is an end-to-end anastomosis; however, portal venous thrombosis is no longer a contraindication to transplantation. These patients may under go a re-cannulisation procedure or require a jump graft technique. Such conduits and grafts are normally fashioned from donor vessels.
It is imperative that all those caring for the patients are aware of the surgical technique undertaken, as complications may vary. The radiologist must be aware of the technique used to allow appropriate interpretation of subsequent investigations and vascular imaging. This applies not just to the vascular anastomosis but also to the presence of a full graft, reduced size graft, right or left split graft or indeed an auxiliary graft. The biliary anastomosis is normally also undertaken as an end-to-end procedure, the donor bile duct being directly joined to the recipient duct. It is no longer standard for this to be undertaken over a T-tube, but this may be required where there is marked discrepancy between donor and recipient duct size. Some conditions (e.g. extrahepatic biliary atresia, primary sclerosing cholangitis) may preclude end-to-end anastomosis and formation of choledochojejunostomy may be required.
Split-liver grafts allow one liver to provide an organ for two recipients. Initially comprised of a child receiving the left lateral segment and an adult the remaining liver, nowadays two adults may receive grafts from one liver if the anatomy and size match allow. Such splits may be less than ideal when the recipient has a high MELD score, as is increasingly the case with the prioritisation of sick patients awaiting liver grafts. Such grafts are at increased risk of postoperative complications such as bile leaks from the cut surface and haematoma/collections at the cut surface.3,11
Non-heart beating transplantation (NHBD) has emerged in recent times as a potential way of increasing organs for transplantation.12,13 The success in renal transplantation has lead to exploration of its application in the fields of liver, pancreas and lung retrieval. Most retrievals are undertaken in the context of controlled NHBD, i.e. in the context of planned withdrawal of care. Warm ischaemia can be accurately assessed and cold ischaemia minimised. Early experience with NHBD was associated with inferior survival for patients and grafts but recent experience suggests that survival is approaching that for heart beating donation. There are, however, continuing concerns over biliary and vascular complications. Prolonged cold ischaemia is associated with poor graft function and biliary complications, as are warm ischaemia times of greater than 30 minutes.14 With regard to postoperative care, an understanding of the pre- and perioperative factors is essential in anticipating potential complications, initiation of monitoring and proactive management.
Living donor-related transplantation (LDLT) is now a routine undertaking in paediatric liver transplantation. It is becoming increasingly utilised in adult liver transplantation although its application in countries with good cadaveric donor pools is less established. Adult LDRT using right lobe grafts is an effective procedure with good survival outcomes but is associated with significant complications. From a postoperative perspective the intensive care team may be responsible for the management of both the donor and the recipient. Morbidity rates for donors are significantly higher with use of a right lobe donation compared with a left lobe graft. Mortality for donors has been reported. Survival rates now reported for living related recipients are good, with rates of 80% at 12 months.15,16
Adequate function of undersized transplanted liver grafts is essential to successful outcome. Primary graft non-function is relatively rare and one of the main areas of concern is that of the so-called ‘small for size syndrome’.15,17 This was first recognised in the post transplant setting but also occurs following liver resection. It is still an area under discussion but the clinical entity is that of hyperbilirubinaemia, graft dysfunction, ascites, and portal hypertension with associated end-organ dysfunction/failure. The clinical picture is that of portal hyperaemia, with portal flow passing into a small liver remnant/graft with associated pathophysiological consequences, and at a histological level there is evidence of arteriolar constriction. In some patients consideration should also be given to the potential compounder of hepatic venous outflow limitation.18,19 Other factors that predispose to the syndrome are an inappropriate graft weight to recipient and steatotic grafts. In regard of management of this syndrome most trials have focused on optimising venous outflow and limiting/preventing portal hyperaemia and limiting portal hypertension.15,20 Animal studies have also examined the role intrahepatic vasodilators with good effect. Management of the syndrome remains controversial but its early consideration allows the clinical team time to consider therapeutic options and interventions.
BLOOD LOSS AND COAGULOPATHY
Orthotopic liver transplantation may be associated with massive blood loss. The causes of this are multifactorial and include preoperative coagulation disorders secondary to end-stage liver disease, portal hypertension, surgical technique, adhesions related to previous surgery and intraoperative changes in haemostasis. Activation of the fibrinolytic system, especially during the anhepatic and post-reperfusion phases, occurs in some recipients. Platelet dysfunction, both quantitative and qualitative, is also common. The consequences of massive bleeding and replacement are significant, not only in terms of postoperative morbidity and mortality, but also intraoperatively, when issues such as acute hypovolaemia, reduced ionised calcium due to citrate intoxication, hyperkalaemia, acidosis and hypothermia become important. Transfusion-related acute lung injury (TRALI) is a potentially devastating complication. It is believed to result from neutrophil antibodies preformed in donor serum. The immunosuppressive effects of large volume blood transfusions are well recognised and pertinent in a group of patients who are already functionally immunosuppressed. In addition to these immediate problems is the risk of transmission of, as yet, unidentified viral infections.
POSTOPERATIVE CARE
Straightforward recipients who return to the ICU in a stable condition with good graft function may be woken up and weaned immediately. The tracheal tube and some of the invasive monitoring lines should be removed as soon as no longer required to reduce the risk of infection and encourage mobility. Close monitoring of all physiological systems is important in the early postoperative period (Tables 94.1 and 94.2).
| Parameter | Comment | |
|---|---|---|
| General | Liver perfusion | Characteristics at surgery |
| Bile production | Quality ± volume if T-tube in situ | |
| Haemodynamics | Stabilisation, with cessation of vasopressor requirements | |
| Coagulation | INR/Prothrombin time (hours) | 8-hourly for the first 24 hours, thereafter daily unless indicated. The fall in PT is more important than the actual value. FFP should be withheld to assess graft function although platelet support should be provided as usual |
| Biochemistry | Glucose | Hypoglycaemia is an ominous sign. 4-hourly measurement in the first 24 hours. Euglycaemia or hyperglycaemia requiring insulin infusion is the norm |
| Arterial blood gases and lactate | 4–6-hourly depending on ventilatory requirement. Hyperlactataemia and acid–base disturbance should rapidly resolve. Other causes of base deficit such as renal tubular acidosis and hyperchloraemia should be excluded and managed appropriately | |
| AST | Should fall steadily (50% fall each day). The first measurement may reflect washout and thus the next may be higher. Daily measurements. The initial measurement reflects the degree of preservation injury. | |
| Bilirubin | Early increases may reflect absorption of haematoma and do not reflect graft function. Haemolysis should be considered if the graft is not blood group matched, termed passenger lymphocyte syndrome | |
| ALP/GGT | Usually normal; increases may reflect biliary complications or cholestasis of sepsis |
EARLY COMPLICATIONS
CARDIOVASCULAR
End-stage liver disease is characterised by a hyperdynamic circulation, with low systemic vascular resistance, high cardiac index and a relatively reduced circulating volume. The majority of patients can be managed with adequate volume loading with or without vasopressor inotropes to maintain adequate perfusion pressures. However, in some patients this state may compensate for degrees of cardiomyopathy (which may be difficult to detect with non-invasive preoperative investigation).21–23 The massive increase in the volume of liver transplants performed in the last decade has revealed cardiac failure as an important cause of morbidity and mortality in the transplant recipient. So-called cirrhotic cardiomyopathy, quite independent of the effects of alcohol, may be multifactorial in nature, possibly due to overproduction of nitric oxide, abnormal β-adrenoceptor structure and/or function, or the presence of some as yet unidentified myocardial depressant factor. Whatever the cause, OLT can impose severe stresses on the cardiovascular system: haemorrhage, third-space loss, impaired venous return due to caval clamping, hypocalcaemia and acidosis all impair myocardial contractility. Reperfusion can also be a time of profound circulatory instability, as discussed above. In addition, the impaired exercise tolerance of the pretransplant recipient may have limited the clinical importance of coronary ischaemia which becomes pertinent in the posttransplant period.
Haemodynamic changes after OLT are also common; hypertension with an increased systemic vascular resistance is frequent and may be due to the restoration of normal liver function and portal pressure, as well as the hypertensive effect of the calcineurin immunosuppressants. The increased afterload in the early posttransplant period may unmask cardiac dysfunction. Management of myocardial dysfunction post OLT is largely empirical; diuretics, afterload reduction and positive-pressure ventilation may all be required. In the longer term, control of cardiovascular risk factors is required and many of these patients may over the years return to the intensive care environs with other system failures and considerable burdens of hypertension, coronary ischaemia, diabetes, hyperlipidaemia and renal dysfunction.
PULMONARY
Specific management of portopulmonary syndrome may be required in the postoperative period if right-sided pressures are elevated, to ensure that liver congestion and graft dysfunction do not ensue.6,24,25 Control of pulmonary pressures may require a variety of therapeutic options, with the treatment options being similar to those utilised in primary pulmonary hypertension. Concern about potential hepatotoxicity needs to be balanced against the need to control right-sided pressures and provide optimal graft function. Similarly, hepatopulmonary syndrome may take a variable time to resolve and hypoxia during this period will require management and recognition.
NEUROLOGICAL
The quoted incidence of central nervous system (CNS) complications varies widely from 10 to 40% in the published series. Most neurological complications occur within the first month of transplant. The commonest causes relate to persistent encephalopathy post transplant in a patient with pre-existing encephalopathy.26,27 The causes are multiple, including hepatic, metabolic, infectious, vascular and pharmacological. A patient with acute liver failure will remain encephalopathic in the immediate posttransplant period, and is at risk for intracranial hypertension for 48 hours following transplantation, or longer in the face of graft dysfunction. De novo hepatic encephalopathy may develop in patients with severe graft dysfunction and/or primary graft non-function; again the patient is at risk of cerebral oedema. The effects of sepsis, rejection (and its treatment with high-dose steroids), drug therapy (especially the sedatives and analgesics used in the ICU setting) and the presence of renal failure may all contribute to the presence of altered conscious level. The calcineurin inhibitors are particularly associated with seizures and altered conscious level. All such patients will require brain imaging to further define the aetiology of their impaired neurology.
RENAL DYSFUNCTION
Despite intraoperative efforts, renal dysfunction can be exacerbated and acute renal dysfunction is a relatively common complication, with an incidence of between 12 and 50%28–30 and a multifactorial aetiology. Risk factors include the presence of pretransplant comorbidity (e.g. hypertension, diabetes mellitus, hepatorenal syndrome), severity of underlying liver disease, intraoperative instability, blood product requirement, drug toxicity and graft dysfunction. Mortality in those who require renal replacement is high, and graft survival is lower. To avoid exacerbation of existing renal impairment, agents with inherent nephrotoxicity, such as the calcineurin inhibitors, may be omitted or their dose significantly reduced in the early pretransplant period. There must be a balance between the risk of rejection and that of side-effects of drug therapies. Mycophenolate mofetil, a cytotoxic immunosuppressant, may be substituted in the posttransplant course to limit or mitigate against renal dysfunction, and increasingly agents such as interleukin-2 (IL-2) blockers are utilised to decrease renal dysfunction.31
Hepatorenal syndrome is a reversible entity following establishment of normal liver function post transplantation. There are considerable data in the literature to suggest that such patients do well with no prolongation of care or increase in mortality. Some data suggest that combined renal and liver transplant is associated with improved outcome. Patients with significant intrinsic renal dysfunction, as compared to those with functional hepatorenal dysfunction, may be offered combined liver–kidney transplantation.32,33 Interestingly, the risk of acute rejection for combined liver–kidney grafts is as for liver transplantation as compared to the higher rate of rejection that is seen for sole renal transplant.
Intra-abdominal hypertension should always be considered in the posttransplant setting as a potential contributor to not only renal dysfunction but also cardiorespiratory dysfunction. Early consideration should be given to laparostomy in patients with elevated intra-abdominal pressures and associated organ dysfunction.34,35
PRIMARY NON-FUNCTION (PNF)/INITIAL POOR FUNCTION
A major reason for the initial graft dysfunction is ischaemic injury to the graft, which depends on the type of preservation fluid used, and the duration of cold and warm ischaemia time. The aetiology of primary non-function remains unclear.36
SURGICAL PROBLEMS
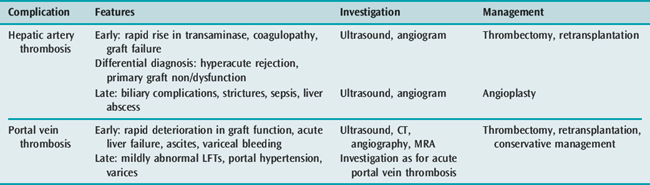
Anastomotic thromboses are uncommon complications of liver transplantation, but can cause significant morbidity, which may require further invasive procedures and even urgent retransplantation. Hepatic artery thrombosis occurring in the early postoperative period is associated with a similar picture to PNF. Small vessel calibre is a risk factor, and is more prevalent in the paediatric recipient where it has also been associated with prothrombotic states such as protein C deficiency. Ultrasound is the first-line screening test and is undertaken both routinely in the immediate postoperative period and if there is a sudden rise in transaminase measurements. If the vessel is not visualised, the patient should proceed to CT angiography. If diagnosed quickly, emergency intervention can be undertaken to re-establish arterial flow; however, emergency retransplantation may be required.
Biliary complications post liver transplant are relatively common. The bile duct normally receives two-thirds of its arterial supply from the gastroduodenal artery and one-third from the hepatic artery. Post transplant, the only supply is from the hepatic artery, making it vulnerable to ischaemic injury whether that be at the time of retrieval, reperfusion or postoperatively. The resulting complication depends on the type of biliary anastomosis and the timing of the insult. Strictures are more commonly observed than leaks. Management of biliary complications is in the first instance endoscopic, with stent placement and/or balloon dilatation. In patients with a T-tube in situ, cholangiography may be undertaken by that route. Open reconstruction in the early postoperative period is uncommon (Tables 94.3, 94.4 and 94.5). Bile leaks may also be seen in the postoperative period from the cut surface of a split graft. Bile leaks are associated with an increased risk of infection and potentially of pseudoaneurysm formation.
| Complication | Comment |
|---|---|
| Abdominal bleeding | |
| Anastomosis | Immediate |
| Graft surface (if cut down) | Immediate |
| General ooze secondary to coagulopathy | Immediate |
| Pseudo-aneurysm formation | Can present early or late and is usually associated with intra-abdominal sepsis and biliary leaks |
| Vascular complications | |
| Hepatic artery thrombosis | Early and late |
| Portal vein thrombosis | Early and late; there may also be a stenosis of the portal vein rather than thrombosis |
| Inferior vena caval obstruction | May be infra-, supra- or retrohepatic in site |
| Biliary complications | |
| Biliary leak | Usually early |
| Biliary stricture | Usually late |
| Papillary dysfunction | Late |
| Roux-en-Y dysfunction | Usually late |
Table 94.5 Differential diagnosis of graft dysfunction in ICU
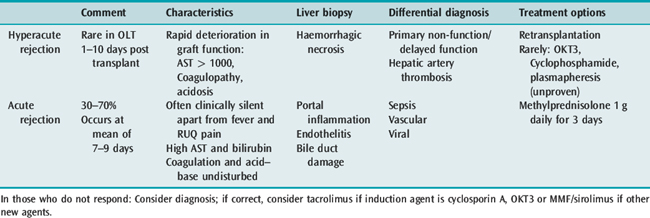
ACUTE REJECTION
Acute cellular rejection becomes a risk from approximately 5–7 days post transplant; the clinical signs of rejection are non-specific and include fever, deterioration in graft function, and a rapid rise in serum aminotransferase concentration. Liver biopsy is the only reliable diagnostic tool; however, biopsy may be relatively contraindicated due to coagulopathy. In some circumstances transjugular biopsy offers a solution to this problem. The normal management regime for an episode of acute rejection is that of pulsed methylprednisolone, 1 g for 3 days. The differential diagnosis may be that of sepsis, or problems with vascular integrity; there are some data to suggest that procalcitonin may be of use in the differentiation (Table 94.6).
SEPSIS AND FEVER
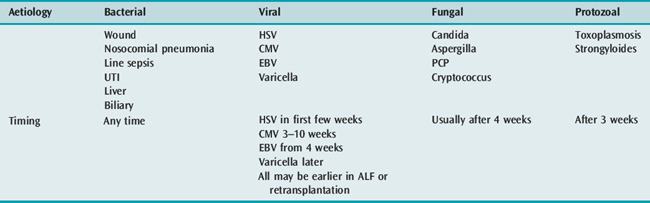
Transplant recipients are uniquely vulnerable to bacterial infection: preoperative colonisation, prolonged and technically difficult surgery, large wounds, urinary catheterisation and the frequent need for central venous access postoperatively all combine to make them at vastly increased risk. However, compared with a decade ago, the overall incidence is reduced, probably due to improved and patient-tailored immunosuppressive regimens. Sepsis remains an important and life-long complication of liver transplantation, which may require readmission to intensive care.37,38
The epidemiology of pathogens is evolving; the incidence of Gram-positive bacterial infection (enterococci and staphylococci) is now more common than Gram-negative sepsis. More concerning is the emergence of multiple antibiotic-resistant bacteria, in particular meticillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE) and extended-spectrum β-lactamase (ESBL) producing Gram-negative organisms. Mortality associated with infection caused by these multiply resistant organisms is significantly greater compared with other organisms.
A decline in the incidence of both Pneumocystis jiroveci (formerly P. carinii) and cytomegalovirus infection is probably a result of both modulating immunosuppressive regimens and more effective prophylaxis. Patients should be screened for viral infections, including herpes simplex virus (HSV) and cytomegalovirus (CMV) utilising polymerase chain reaction (PCR) techniques. Opportunistic fungal infection still remains problematic, especially in the context of environmental risk factors39,40 (Table 94.7).
MANAGEMENT OF CMV INFECTION AFTER LIVER TRANSPLANTATION
CMV infection is rarely associated with symptomatic illness in healthy hosts, but is a major cause of morbidity and mortality in transplant recipients; it is the single most common opportunistic infection after solid organ transplantation. In the absence of antiviral prophylaxis the overall incidence of CMV infection after OLT ranges from 23 to 85%, with approximately 50% of those developing clinical disease.
MANAGEMENT OF VIRAL HEPATITIS
Hepatitis C (HCV) related cirrhosis is the commonest indication for transplantation in both Europe and the USA. Post transplant, HCV viraemia is universal. Recurrent liver disease, with a more accelerated and aggressive course, is often observed; indeed, 20% are cirrhotic at 5 years post transplant.41 Those with histological evidence of recurrence also have a greater incidence of acute rejection. Immunosuppression, especially with steroids, directly increases the HCV RNA serum load. Most transplant programmes therefore convert to single- or double-agent immunosuppression regimens as soon as possible post transplant.42 Yet to be fully elucidated is the role of antiviral therapy (interferon and ribavirin) in both the pre- and posttransplant period. Provisional data are optimistic, although it requires considerable workload and supportive drug therapy. It would not normally be undertaken in the intensive care setting. Theoretically, early antiviral therapy is attractive as viral load is low, immunosuppressive therapy has just started and acute rejection necessitating pulsed steroids is relatively common. However, risk of infection and thrombocytopenia often contraindicates antiviral therapy in the early postoperative period.
IMMUNOSUPPRESSION
As the field of transplantation evolves, new immunosuppressive regimens and drugs become available. For all combinations, however, there is a balance to be struck between the optimal prevention of rejection and the toxicity and unwanted effects of the drugs. The incidence of acute rejection rises at about 1 week after OLT; it resembles a delayed-type hypersensitivity reaction, and immunosuppressive agents are highly effective at treating it. Chronic rejection occurs over months to years and is characterised by the ‘vanishing bile-duct’ syndrome, pathological mechanisms are poorly understood and immunosuppressant agents are largely ineffective.31 Currently, calcineurin inhibitors such as ciclosporin and tacrolimus, along with steroids, form the mainstay drugs after liver transplantation, certainly in the early stages. They have revolutionised the outcome of solid-organ transplantation, but both drugs are limited by their side-effects, predominantly nephro- and neurotoxicity, necessitating drug level monitoring.
Sirolimus is a novel immunosuppressant that has been used extensively in renal transplantation and more recently in liver transplant recipients in whom the calcineurin inhibitors are contraindicated.31 It resembles tacrolimus structurally, and binds to the same protein, but whereas ciclosporin and tacrolimus act by inhibiting IL-2 gene transcription, sirolimus acts by blocking postreceptor signal transduction and IL-2 dependent proliferation. In addition to its immunosuppressive actions, sirolimus is also an antifungal and antiproliferative agent. Sirolimus lacks neuro- and nephrotoxicity. However, it can raise the intracellular concentrations of cyclosporin A and tacrolimus, indirectly potentiating their toxicity. Hyperlipidaemia has also been noted although this may be a reflection of the often higher dose steroid regimens used in combination with sirolimus. Because of its antiproliferative effects, sirolimus can also cause thrombocytopenia, neutropenia and anaemia; there have also been concerns about its effects on wound healing. Sirolimus also requires therapeutic drug level monitoring, not only because serum concentrations have a high level of intra- and interindividual variability, but also because there are significant interactions with drugs that use the cytochrome P-450 3A system.
LIVER TRANSPLANTATION FOR ACUTE LIVER FAILURE
Acute liver failure is a syndrome associated with an acute onset coagulopathy, jaundice and encephalopathy; the causes are many and the syndrome is notable for its high morbidity and mortality. The acceptance of emergency liver transplantation in selected cases has revolutionised the clinical course, but outcome is sometimes disappointingly poor, often due to the rapid development of uncontrollable cerebral oedema, sepsis and multiorgan failure. There is also a short window of opportunity in listing these patients; despite highest priority listing they may receive ‘marginal’ organs or even ABO blood group incompatible organs. Early determination of prognosis and appropriate listing for transplant are clearly important. The King’s College Hospital prognostic criteria for non-survival among patients with acute liver failure is a tool used to identify those at high risk while sparing those in whom spontaneous recovery will otherwise occur. It has been validated both in Europe and the USA (Table 94.8). Several advances in the supportive management of these patients have occurred since the original criteria were developed but their prognostic value holds true.
Table 94.8 King’s College Hospital prognostic criteria for non-survival among patients with acute liver failure
| Paracetamol induced | Non-paracetamol induced |
|---|---|
| pH < 7.3 (irrespective of grade of encephalopathy), following volume resuscitation and > 24 hours post ingestion | PT > 100 seconds (INR > 6.5) irrespective of grade of encephalopathy |
| or | |
| pH < 7.3 following volume resuscitation | |
| or | or |
| PT > 100 seconds (INR > 6.5) and creatinine > 300 μmol/l in patients with grade III–IV encephalopathy, occurring within a 24-hour timeframe | any three of the following variables (in association with encephalopathy): |
| Age < 10 years or > 40 years | |
| Aetiology: non-A, non-B or drug induced | |
| Jaundice to encephalopathy > 7 days | |
| PT > 50 seconds (INR > 3.5) | |
| Serum bilirubin > 300 μmol/l |
EXTRACORPOREAL HEPATIC SUPPORT
The liver has metabolic, excretory and synthetic functions, all of which need to be maintained in a patient who has liver failure until either an organ becomes available or regeneration takes place. The major components of a bioartificial liver include hepatocytes able to perform some of the functions of normal liver, a delivery system to bring blood to and from the patient, and a membrane designed to allow adequate exchange between blood and hepatocytes.
Clinical trials are in progress to evaluate the place for these devices and there has been some success with using them as a bridge to transplantation, and in acute liver failure in particular an improvement in neurological status and reduced cerebral oedema.43 Still to be elucidated is the timescale on which these devices can be used and whether they can bridge the gap to regeneration, thus sparing the patient from transplantation.
HEPATOPULMONARY SYNDROMES
Changes in the cardiovascular system associated with chronic liver disease may contribute to the spectrum of cardiopulmonary disease associated with chronic liver disease and portal hypertension. A hyperdynamic state with high cardiac output, long-standing portal hypertension with the development of collateral flows, together with an imbalance of vasoactive mediators either synthesised or metabolised by the liver, may lead to characteristic changes in both flow and pressure through the pulmonary vasculature. This may be associated with hypoxia and orthodeoxia. Two ends of the spectrum are hepatopulmonary syndrome (HPS) and portopulmonary hypertension (PPH). The two conditions are rare but important, as they have vastly different impacts on risk associated with liver transplantation and long-term outcome (Table 94.9). The role of agents used in the management of primary pulmonary hypertension is yet to be examined in a controlled manner in liver disease but data thus far suggest benefit.
Table 94.9 Diagnostic criteria for hepatopulmonary syndrome and portopulmonary hypertension
| Hepatopulmonary syndrome | Portopulmonary hypertension |
|---|---|
| Chronic liver disease(± cirrhosis) | Portal hypertension |
| Arterial hypoxaemia | Mean pulmonary artery pressure > 25 mmHg |
| PaO2 < 75 mmHg (10 kPa) or A–ao2 gradient > 20 mmHg | Pulmonary artery occlusion pressure < 15 mmHg |
| Intrapulmonary vascular dilatation | Pulmonary vascular resistance > 120 dynes/s per cm−5 |
Diagnostic criteria for both conditions are summarised in the table.
HEPATOPULMONARY SYNDROME
It can be seen from Table 94.9 that hypoxia is a characteristic finding in this condition. It results from intrapulmonary vascular dilatation at the pre- and postcapillary level, leading to decreased ventilation/perfusion ratios; more uncommonly, anatomical shunt is present with atrioventricular communication. One of the postulated mechanisms of this vasodilatation is overactivity of pulmonary vasculature nitric oxide synthetase; pretransplant patients have raised levels of exhaled nitric oxide that decrease post transplant with resolution of the syndrome. Medical treatment of the syndrome has been disappointing; indeed, most transplant centres agree that the syndrome is an indication for transplantation in itself, as resolution is reported in up to 80% after transplantation.
Risk stratification based on severity is important, as vastly increased peritransplant mortality is associated with severe hypoxia and high levels of vascular shunt. Mortality overall is 16% at 90 days and 38% at 1 year. Refractory hypoxia is the indirect cause of death, which may be due to multiorgan failure, intracerebral haemorrhage and sepsis due to bile leaks. Resolution of the syndrome can take months, lending support to the theory that it is vascular remodelling rather than just acute reversal of vasodilatation that reverses hypoxia.25,44
PORTOPULMONARY HYPERTENSION
In comparison to HPS, there are several differences in terms of response to medical treatment and outcome after transplantation. The response to epoprostenol, a PGI2 analogue, is encouraging. Decreases in pulmonary artery pressure but more importantly transpulmonary gradient (TPG) have been noted, although at least 3 months’ treatment seems necessary, suggesting remodelling rather than vasodilatation is the important mechanism. A limiting factor in the treatment may also be progressive thrombocytopenia and splenomegaly. Another difference is the perioperative risk and posttransplant prognosis, Resolution is not associated with transplantation and progression can be a feature. Perioperatively, the higher the mean pulmonary artery pressure (MPAP), pulmonary vascular resistance (PVR) and TPG the greater the risk of death, usually due to acute right ventricular decompensation. If the MPAP is >35 mmHg or the PVR is >250 dyne/s per cm, mortality reaches 40%. If MPAP is >50 mm Hg some have even suggested delisting or even intraoperative cancellation as the mortality may be as high as 100%.24
1 Roberts MS, Angus DC, Bryce CL, et al. Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transpl. 2004;10:886-897.
2 Habib S, Berk B, Chang CC, et al. MELD and prediction of post-liver transplantation survival. Liver Transpl. 2006;12:440-447.
3 Futagawa Y, Terasaki PI. An analysis of the OPTN/UNOS Liver Transplant Registry. Clin Transpl. 2004:315-329.
4 Burroughs AK, Sabin CA, Rolles K, et al. 3-month and 12-month mortality after first liver transplant in adults in Europe: predictive models for outcome. Lancet. 2006;367:225-232.
5 Fink MA, Berry SR, Gow PJ, et al. Risk factors for liver transplantation waiting list mortality. J Gastroenterol Hepatol. 2007;22:119-124.
6 Krowka MJ. Hepatopulmonary syndrome and portopulmonary hypertension: implications for liver transplantation. Clin Chest Med. 2005;26:587-597.
7 Fusai G, Dhaliwal P, Rolando N, et al. Incidence and risk factors for the development of prolonged and severe intrahepatic cholestasis after liver transplantation. Liver Transpl. 2006;12:1626-1633.
8 Reddy KS, Johnston TD, Putnam LA, et al. Piggyback technique and selective use of veno-venous bypass in adult orthotopic liver transplantation. Clin Transplant. 2000;14:370-374.
9 Parrilla P, Sanchez-Bueno F, Figueras J, et al. Analysis of the complications of the piggy-back technique in 1112 liver transplants. Transplant Proc. 1999;31:2388-2389.
10 Cabezuelo JB, Ramirez P, Acosta F, et al. Does the standard vs piggyback surgical technique affect the development of early acute renal failure after orthotopic liver transplantation? Transplant Proc. 2003;35:1913-1914.
11 Heaton N. Small-for-size liver syndrome after auxiliary and split liver transplantation: donor selection. Liver Transpl. 2003;9:S26-8.
12 Monbaliu D, Van Gelder F, Troisi R, et al. Liver transplantation using non-heart-beating donors: Belgian experience. Transplant Proc. 2007;39:1481-1484.
13 Muiesan P, Girlanda R, Jassem W, et al. Single-center experience with liver transplantation from controlled non-heartbeating donors: a viable source of grafts. Ann Surg. 2005;242:732-738.
14 Deshpande R, Heaton N. Can non-heart-beating donors replace cadaveric heart-beating liver donors? J Hepatol. 2006;45:499-503.
15 Chan SC, Fan ST, Lo CM, et al. Effect of side and size of graft on surgical outcomes of adult-to-adult live donor liver transplantation. Liver Transpl. 2007;13:91-98.
16 Polido WTJr, Lee KH, Tay KH, et al. Adult living donor liver transplantation in Singapore: the Asian centre for liver diseases and transplantation experience. Ann Acad Med Singapore. 2007;36:623-630.
17 Tucker ON, Heaton N. The ‘small for size’ liver syndrome. Curr Opin Crit Care. 2005;11:150-155.
18 Chan SC, Lo CM, Liu CL, et al. Tailoring donor hepatectomy per segment 4 venous drainage in right lobe live donor liver transplantation. Liver Transpl. 2004;10:755-762.
19 Cheaito A, Craig B, Abouljoud M, et al. Sonographic differences in venous return between piggyback versus caval interposition in adult liver transplantations. Transplant Proc. 2006;38:3588-3590.
20 Chan SC, Fan ST, Lo CM, et al. Toward current standards of donor right hepatectomy for adult-to-adult live donor liver transplantation through the experience of 200 cases. Ann Surg. 2007;245:110-117.
21 Cirrhotic cardiomyopathy: multiple reviews. Liver Transpl. 2007;13:1060-1061.
22 Lee RF, Glenn TK, Lee SS. Cardiac dysfunction in cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21:125-140.
23 Milani A, Zaccaria R, Bombardieri G, et al. Cirrhotic cardiomyopathy. Dig Liver Dis. 2007;39:507-515.
24 Krowka MJ. Evolving dilemmas and management of portopulmonary hypertension. Semin Liver Dis. 2006;26:265-272.
25 Krowka MJ, Plevak D. The distinct concepts and implications of hepatopulmonary syndrome and portopulmonary hypertension. Crit Care Med. 2005;33:470.
26 Dhar R, Young GB, Marotta P. Perioperative neurological complications after liver transplantation are best predicted by pre-transplant hepatic encephalopathy. Neurocrit Care. 2008;8:253-258.
27 Saner F, Gu Y, Minouchehr S, et al. Neurological complications after cadaveric and living donor liver transplantation. J Neurol. 2006;253:612-617.
28 Farmer DG, Venick RS, McDiarmid SV, et al. Predictors of outcomes after pediatric liver transplantation: an analysis of more than 800 cases performed at a single institution. J Am Coll Surg. 2007;204:904-914. discussion 914–6
29 Gonwa TA, McBride MA, Anderson K, et al. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006;6:2651-2659.
30 O’Riordan A, Wong V, McQuillan R, et al. Acute renal disease, as defined by the RIFLE criteria, post-liver transplantation. Am J Transplant. 2007;7:168-176.
31 Perry I, Neuberger J. Immunosuppression: towards a logical approach in liver transplantation. Clin Exp Immunol. 2005;139:2-10.
32 Ruiz R, Barri YM, Jennings LW, et al. Hepatorenal syndrome: a proposal for kidney after liver transplantation (KALT). Liver Transpl. 2007;13:838-843.
33 Ruiz R, Kunitake H, Wilkinson AH, et al. Long-term analysis of combined liver and kidney transplantation at a single center. Arch Surg. 2006;141:735-741. discussion 741–2
34 Biancofiore G, Bindi L, Romanelli AM, et al. Renal failure and abdominal hypertension after liver transplantation: determination of critical intra-abdominal pressure. Liver Transpl. 2002;8:1175-1181.
35 Biancofiore G, Bindi ML, Romanelli AM, et al. Postoperative intra-abdominal pressure and renal function after liver transplantation. Arch Surg. 2003;138:703-706.
36 Fischer-Frohlich CL, Lauchart W. Expanded criteria liver donors (ECD): effect of cumulative risks. Ann Transplant. 2006;11:38-42.
37 Philpott-Howard J, Burroughs A, Fisher N, et al. Piperacillin-tazobactam versus ciprofloxacin plus amoxicillin in the treatment of infective episodes after liver transplantation. J Antimicrob Chemother. 2003;52:993-1000.
38 Safdar N, Said A, Lucey MR. The role of selective digestive decontamination for reducing infection in patients undergoing liver transplantation: a systematic review and meta-analysis. Liver Transpl. 2004;10:817-827.
39 Cruciani M, Mengoli C, Malena M, et al. Antifungal prophylaxis in liver transplant patients: a systematic review and meta-analysis. Liver Transpl. 2006;12:850-858.
40 Limaye AP, Bakthavatsalam R, Kim HW, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. 2006;81:1645-1652.
41 Gringeri E, Vitale A, Brolese A, et al. Hepatitis C virus-related cirrhosis as a significant mortality factor in intention-to-treat analysis in liver transplantation. Transplant Proc. 2007;39:1901-1903.
42 Llado L, Xiol X, Figueras J, et al. Immunosuppression without steroids in liver transplantation is safe and reduces infection and metabolic complications: results from a prospective multicenter randomized study. J Hepatol. 2006;44:710-716.
43 Wai CT, Lim SG, Aung MO, et al. MARS: a futile tool in centres without active liver transplant support. Liver Int. 2007;27:69-75.
44 Schiffer E, Majno P, Mentha G, et al. Hepatopulmonary syndrome increases the postoperative mortality rate following liver transplantation: a prospective study in 90 patients. Am J Transplant. 2006;6:1430-1437.