Chapter 34 Liver and biliary tract
Pharmacokinetic changes in liver disease
Hepatic blood flow and metabolism
• Drugs metabolised rapidly with high extraction in a single pass through the liver. Clearance is limited normally by hepatic blood flow, but in severe liver disease less drug is extracted from blood passing through the liver because of poor hepatocyte function and portosystemic shunts that allow blood to bypass the liver. The predominant kinetic change for drugs given orally is increased systemic availability. The initial and maintenance doses of such drugs should be reduced. With severe liver impairment the t½ of drugs in this class may lengthen.
• Drugs metabolised slowly with poor extraction in a single pass through the liver. The rate-limiting factor for elimination of this type of drug is metabolic capacity and the major effect of liver disease is prolongation of t½. Consequently, the interval between doses may need to be lengthened and the time to reach steady-state concentration in the plasma (5 × t½) is increased.
Prescribing for patients with liver disease
• Impaired hepatic synthetic function (hypoalbuminaemia, increased INR).
• Current/recent hepatic encephalopathy.
For drugs with significant hepatic metabolism, a reasonable approach is to reduce the dose to 25–50% of normal and monitor responses. Specific examples include:
Drug-induced liver damage
The spectrum of hepatic abnormalities caused by drugs is broad (Table 34.1). Drugs tend to injure specific hepatocyte components, e.g. plasma membrane, biliary canaliculi, cytochrome P450 enzymes or mitochondria.
Table 34.1 Idiosyncratic drug reactions and the cell components that are affected
| Type of reaction | Effect on cells | Examples of drugs |
|---|---|---|
| Hepatocellular | Direct effect on production by enzyme–drug combination leads to cell and membrane dysfunction | Isoniazid, trazodone, diclofenac, nefazodone, venlafaxine, lovastatin |
| Immune mediated | Cytotoxic lymphocyte response directed at hepatocyte membranes altered by drug metabolite ± additional autoimmune component | Nitrofurantoin, methyldopa, lovastatin, minocycline, halothane |
| Cholestasis | Injury to canalicular membrane and transporters | Chlorpromazine, oestrogen, erythromycin and its derivatives |
| Granulomatous | Macrophages, lymphocytes infiltrate hepatic lobule | Diltiazem, sulfa drugs, quinidine |
| Microvesicular fat | Altered mitochondrial respiration, β-oxidation leads to lactic acidosis and triglyceride accumulation | Didanosine, tetracyclines, acetylsalicylic acid, valproic acid |
| Steatohepatitis (fatty liver) | Multifactorial | Amiodarone, tamoxifen |
| Fibrosis | Activation of stellate cells | Methotrexate, excess vitamin A |
| Vascular collapse | Causes ischaemic or hypoxic injury | Nicotinic acid, cocaine, methylenedioxymethylamfetamine (MDMA) |
| Oncogenesis | Encourages tumour formation | Oral contraceptives, androgens |
| Mixed | Cytoplasmic and canalicular injury, direct damage to bile ducts | Amoxicillin–clavulanic acid, carbamazepine, herbs, ciclosporin, methimazole, troglitazone |
• C-17α-substituted steroids impair bilirubin excretion into hepatic canaliculi. These include synthetic anabolic steroids and oestrogens in oral contraceptives; jaundice due to the latter is rare with current formulations. Many have genetic susceptibility with mutations in ABC cassette biliary transporter proteins.
• Rifampicin impairs hepatic uptake and excretion of bilirubin; unconjugated and conjugated bilirubin levels may be raised within 3 weeks.
• Fusidic acid interferes with hepatic bilirubin excretion, causing conjugated hyperbilirubinaemia, particularly with sepsis.
Diagnosis and management of drug-induced liver injury
• Always consider the possibility. Take careful drug histories, including over-the-counter, complementary, illicit and alternative medicines.
• Consider a viral aetiology with hepatitis.
• Consider other causes of cholestatic disease.
• Underlying liver disease can cause diagnostic confusion.
• Liver biopsy is often helpful, but eosinophil infiltration, often thought to be specific for drug reaction, has many causes.
• Diagnostic challenge is dangerous, may precipitate life-threatening liver disease and is never justified.
• Monitoring liver function in the early weeks of therapy is wise in detecting early reactions to drugs with hepatotoxic potential, e.g. isoniazid. Minor abnormalities (serum transaminase levels less than twice normal) are often self-limiting and progress should be monitored. For increases exceeding three-fold, consider drug withdrawal, even in asymptomatic patients.
Aspects of therapy
Complications of cirrhosis
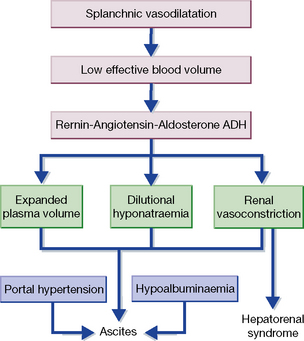
Ascites
Hepatorenal syndrome
This occurs in 10% of patients with advanced cirrhosis and ascites and is attributed to intense renal vasoconstriction (Fig. 34.1). It is defined by the urinary sodium under 5 mmol/L with euvolaemia. Three-month mortality is high. Some patients respond to vasoconstrictor agents, particularly terlipressin, while maintaining volume with albumin, over a 14-day interval. Dopamine is ineffective.
Immune-mediated liver disease
Viral hepatitis
Pancreas
Acute pancreatitis
• To provide adequate analgesia. Opioids are generally satisfactory; analgesic efficacy outweighs the potential disadvantage of contracting the sphincter of Oddi (and retarding the flow of pancreatic secretion); buprenorphine is often preferred.
• To correct hypovolaemia due to the exudation of large amounts of fluid around the inflamed pancreas. Plasma may be required, or blood if the haematocrit falls; in addition, large volumes of electrolyte solution may be needed to preserve renal function.
• To achieve biliary drainage (by endoscopic retrograde cannulation of the pancreas) early in the illness if gallstones are suspected.
• The value of additional interventions, including nutritional support and antibiotic prophylaxis, is as yet unproven.
Liver disease, Adams D.H., Haydon G., eds., Clin. Med. (Northfield Il). 2006;6. 19–46
American Association for the Study of Liver Disease practice guidelines Available online at: http://www.aasld.org/practice guidelines/
European Association for the Study of the Liver. Available online at: http://www.easl.eu/_clinicalpractice-guidelines/
Johnson C.D. UK guidelines for the management of acute pancreatitis. Gut. 2005;54(Suppl. iii):1–9.
Kingsnorth A., O’Reilly D. Acute pancreatitis. Br. Med. J.. 2006;332:1072–1076.
Krawitt E.L. Autoimmune hepatitis. N. Engl. J. Med.. 2006;354:54–66.
Lee W.M. Drug-induced hepatotoxicity. N. Engl. J. Med.. 2003;349:474–485.
Navarro V.J., Senior J.R. Drug-related hepatotoxicity. N. Engl. J. Med.. 2006;354:731–739.
NHS evidence for gastroenterology and liver disease Available online at: http://www.library.nhs.uk/gastroliver/