Listeria, Corynebacterium, and Similar Organisms
1. Describe the general characteristics of the Corynebacterium spp., including Gram stain morphology, culture media, and colonial appearance.
2. List two selective and differential media used for identification of Corynebacterium diphtheriae and describe the chemical principle for each.
3. Identify the clinically relevant indicators (e.g., signs, symptoms) associated with the need to identify Corynebacterium spp.
4. Describe four methods used to detect C. diphtheriae toxin, along with the chemical principle of each test.
5. Describe two methods used to observe motility in Listeria monocytogenes.
6. Explain how diphtheria is controlled by immunization and describe the course of treatment for individuals exposed to the disease.
7. Define “cold enrichment” and explain how it enhances the isolation of L. monocytogenes.
8. List the foods pregnant women and immunocompromised patients should avoid to reduce the risk of infection with L. monocytogenes.
9. Describe the clinical significance of identification of Corynebacterium pseudotuberculosis, Corynebacterium ulcerans, and Rhodococcus sp.
Epidemiology
Most of the organisms listed in Table 17-1 are part of the normal human flora and colonize various parts of the human body, are found in the environment, or are associated with various animals. The two most notable pathogens are Listeria monocytogenes and Corynebacterium diphtheriae. However, these two species differ markedly in epidemiology. L. monocytogenes is widely distributed in nature and occasionally colonizes the human gastrointestinal tract. Many foods are contaminated with L. monocytogenes, including milk, raw vegetables, cheese, and meats. C. diphtheriae is only carried by humans, but in rare cases it is isolated from healthy individuals. Primary transmission for C. diphtheriae is through respiratory secretions or exudates from skin lesions.
TABLE 17-1
| Organism | Habitat (Reservoir) | Mode of Transmission |
| Listeria monocytogenes | Colonizer: Animals, soil, and vegetable matter; widespread in these environments Human gastrointestinal tract |
Direct contact: Ingestion of contaminated food, such as meat and dairy products Endogenous strain: Colonized mothers may pass organism to fetus. Portal of entry is probably from gastrointestinal tract to blood and in some instances from blood to meninges. |
| Corynebacterium diphtheriae | Colonizer: Human nasopharynx but only in carrier state; not considered part of normal flora Isolation from healthy humans is not common. |
Direct contact: Person to person by exposure to contaminated respiratory droplets Contact with exudate from cutaneous lesions Exposure to contaminated objects |
| Corynebacterium jeikeium | Colonizer: Skin flora of hospitalized patients, most commonly in the inguinal, axillary, and rectal sites |
Uncertain Direct contact: May be person to person Endogenous strain: Selection during antimicrobial therapy Introduction during placement or improper care of intravenous catheters |
| Corynebacterium ulcerans | Normal flora: Humans and cattle |
Uncertain Zoonoses: Close animal contact, especially during summer |
| Corynebacterium pseudotuberculosis | Normal flora: Animals such as sheep, goats, and horses |
Uncertain Zoonoses: Close animal contact, but infections in humans are rare |
| Corynebacterium pseudodiphtheriticum | Normal flora: Human pharyngeal and occasionally skin flora |
Uncertain Endogenous strain: Access to normally sterile site |
| Corynebacterium minutissimum | Normal flora: Human skin |
Uncertain Endogenous strain: Access to normally sterile site |
| Corynebacterium urealyticum | Normal flora: Human skin |
Uncertain Endogenous strain: Access to normally sterile site |
| Leifsonia aquatica (formerly Corynebacterium aquaticum) | Environment: Fresh water |
Uncertain |
| Corynebacterium xerosis | Normal flora: Human conjunctiva Skin Nasopharynx |
Uncertain Endogenous strain: Access to normally sterile site |
| Corynebacterium striatum | Normal flora: Skin |
Uncertain Endogenous strain: Access to normally sterile site |
| Corynebacterium amycolatum | Normal flora: Human conjunctiva Skin Nasopharynx |
Uncertain Endogenous strain: Access to normally sterile site |
| Corynebacterium auris | Uncertain: Probably part of normal human flora |
Uncertain Rarely implicated in human infections |
| Kurthia spp. | Environment | Uncertain Rarely implicated in human infections |
| Brevibacterium spp. | Normal flora: Human Various foods |
Uncertain Rarely implicated in human infections |
| Dermabacter hominis | Normal flora: Human skin |
Uncertain Rarely implicated in human infections |
| Turicella otitidis | Uncertain: Probably part of normal human flora |
Uncertain Rarely implicated in human infections |
| Arthrobacter spp., Microbacterium spp., Cellulomonas spp., and Exiguobacterium sp. | Uncertain Probably environmental |
Uncertain Rarely implicated in human infections |
Pathogenesis and Spectrum of Disease
L. monocytogenes, by virtue of its ability to survive within phagocytes, and C. diphtheriae, by production of an extremely potent cytotoxic exotoxin, are the most virulent species listed in Table 17-2. Not all strains of C. diphtheriae are toxin-producing strains. The toxin gene is present in strains that have acquired the gene by viral transduction. The result is the incorporation of the toxin gene into the organisms’ genome. C. diphtheriae occurs in four biotypes: gravis, intermedius, belfanti, and mitis; C. gravis causes the most severe form of disease. The biotypes can be differentiated based on colonial morphology, biochemical reactions, and hemolytic patterns on blood agar.
TABLE 17-2
Pathogenesis and Spectrum of Diseases
| Organism | Virulence Factors | Spectrum of Diseases and Infections |
| Listeria monocytogenes | Listeriolysin O: A hemolytic and cytotoxic toxin that allows for survival within phagocytes Internalin: Cell surface protein that induces phagocytosis Act A: Induces actin polymerization on the surface of host cells, producing cellular extensions and facilitating cell-to-cell spread. Siderophores: Organisms capable of scavenging iron from human transferrin and of enhanced growth of organism.* |
Systemic: Bacteremia, without any other known site of infection CNS infections: Meningitis, encephalitis, bran abscess, spinal cord infections Neonatal: Early onset: Granulomatosis infantisepticum—in utero infection disseminated systemically that causes stillbirth Late onset: Bacterial meningitis Immunosuppressed patients |
| Corynebacterium diphtheriae | Diphtheria toxin: A potent exotoxin that destroys host cells by inhibiting protein synthesis. |
Respiratory diphtheria is a pharyngitis characterized by the development of an exudative membrane that covers the tonsils, uvula, palate, and pharyngeal wall; if untreated, life-threatening cardiac toxicity, neurologic toxicity, and other complications occur. Respiratory obstruction develops and release of toxin into the blood can damage various organs, including the heart. |
| Nontoxigenic strains: Uncertain |
Cutaneous diphtheria is characterized by nonhealing ulcers and membrane formation. Immunocompromised patients, drug addicts, and alcoholics. Invasive endocarditis, mycotic aneurysms, osteomyelitis, and septic arthritis* |
|
| Corynebacterium jeikeium | Unknown: Multiple antibiotic resistance allows survival in hospital setting |
Systemic: Septicemia Skin infections: Wounds, rashes and nodules Immunocompromised: Malignancies, neutropenia, AIDS patients. Associated with indwelling devices such as catheters, prosthetic valves, and CSF shunts* |
| Corynebacterium ulcerans | Unknown | Zoonoses: Bovine mastitis Has been associated with diphtheria-like sore throat, indistinguishable from C. diphtheriae Skin infections Pneumonia |
| Corynebacterium pseudotuberculosis | Unknown | Zoonoses: Suppurative granulomatous lymphadenitis |
| Corynebacterium pseudodiphtheriticum | Unknown Some stains have been identified that are resistant to macrolides* |
Systemic: Septicemia Endocarditis Pneumonia and lung abscesses; primarily in immunocompromised |
| Corynebacterium minutissimum | Unknown Probably of low virulence |
Superficial, pruritic skin infections known as erythrasma Immunocompromised: Septicemia Endocarditis Abscess formation |
| Corynebacterium urealyticum | Unknown Multiple antibiotic resistance allows survival in hospital setting. |
Immunocompromised and elderly: Urinary tract infections Wound infections Rarely: endocarditis, septicemia, osteomyelitis, and tissue infections |
| Leifsonia aquatica ( formerly Corynebacterium aquaticum) | Unknown | Immunocompromised: Bacteremia Septicemia |
| Corynebacterium xerosis | Unknown | Immunocompromised: Endocarditis Septicemia |
| Corynebacterium striatum | Unknown | Immunocompromised: Bacteremia Pneumonia and lung abscesses Osteomyelitis Meningitis |
| Corynebacterium amycolatum | Unknown Multiple antibiotic resistance patterns |
Immunocompromised: Endocarditis Septicemia Pneumonia Neonatal sepsis |
| Corynebacterium auris | Unknown Multiple antibiotic resistance patterns |
Uncertain disease association but has been linked to otitis media |
| Kurthia spp., Brevibacterium and Dermabacter sp. | Unknown | Immunocompromised: Rarely causes infections in humans Bacteremia in association with indwelling catheters or penetrating injuries |
| Turicella otitidis | Unknown | Uncertain disease association but has been linked to otitis media |
| Arthrobacter spp., Microbacterium spp., Aureobacterium spp., Cellulomonas spp., and Exiguobacterium sp. | Unknown | Uncertain disease association |
AIDS, Acquired immunodeficiency syndrome; CSF, cerebrospinal fluid; CNS, central nervous system.
Most of the remaining organisms in Table 17-2 are opportunistic, and infections are associated with immunocompromised patients. For this reason, whenever Corynebacterium spp. or the other genera of gram-positive rods are encountered, careful consideration must be given to their role as infectious agents or contaminants. Corynebacterium urealyticum is an up-and-coming cause of cystitis in hospitalized patients, in those who have undergone urologic manipulation, and in the elderly.
Laboratory Diagnosis
Specimen Collection and Transport
No special considerations are required for specimen collection and transport of the organisms discussed in this chapter. Refer to Table 5-1 for general information on specimen collection and transport.
Specimen Processing
No special considerations are required for processing of most of the organisms discussed in this chapter. (Refer to Table 5-1 for general information on specimen processing.) One exception is the isolation of L. monocytogenes from placental and other tissue. Because isolating Listeria organisms from these sources may be difficult, cold enrichment may be used to enhance the recovery of the organism. The specimen is inoculated into a nutrient broth and incubated at 4°C for several weeks to months. The broth is subcultured at frequent intervals to enhance recovery.
Direct Detection Methods
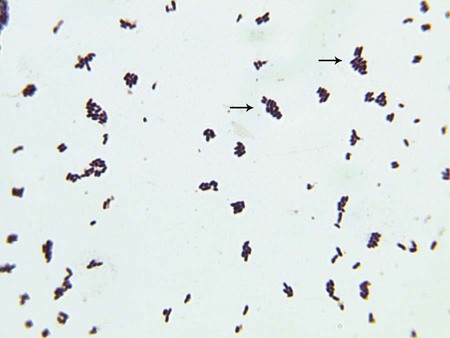
Gram stain of clinical specimens is the only procedure used for the direct detection of these organisms. Most of the genera in this chapter (except Listeria, Rothia, and Oerskovia spp.) are classified as coryneform bacteria; that is, they are gram-positive, short or slightly curved rods with rounded ends; some have rudimentary branching. Cells are arranged singly, in “palisades” of parallel cells, or in pairs of cells connected after cell division to form V or L shapes. Groups of these morphologies seen together resemble and are often referred to as Chinese letters (Figure 17-1). The Gram stain morphologies of clinically relevant species are described in Table 17-3. L. monocytogenes is a short, gram-positive rod that may occur singly or in short chains, resembling streptococci.
TABLE 17-3
Gram Stain Morphology, Colonial Appearance, and Other Distinguishing Characteristics
| Organism | Gram Stain | Appearance on 5% Sheep Blood Agar |
| Arthrobacter spp. | Typical coryneform gram-positive rods after 24 hr, with “jointed ends” giving L and V forms, and coccoid cells after 72 hr (i.e., rod-coccus cycle*) | Large colony; resembles Brevibacterium spp. |
| Brevibacterium spp. | Gram-positive rods; produce typical coryneform arrangements in young cultures (<24 hr) and coccoid-to-coccobacillary forms that decolorize easily in older cultures (i.e., rod-coccus cycle*) | Medium to large; gray to white, convex, opaque, smooth, shiny; nonhemolytic; cheeselike odor |
| Cellulomonas spp. | Irregular, short, thin, branching gram-positive rods | Small to medium; two colony types, one starts out white and turns yellow within 3 days and the other starts out yellow |
| CDC coryneform group F-1 | Typical coryneform gram-positive rods | Small, gray to white |
| CDC coryneform group G† | Typical coryneform gram-positive rods | Small, gray to white; nonhemolytic |
| Corynebacterium accolens | Resembles C. jeikeium | Resembles C. jeikeium |
| C. afermentans subsp. afermentans | Typical coryneform gram-positive rods | Medium; white; nonhemolytic; nonadherent |
| C. afermentans subsp. lipophilum | Typical coryneform gram-positive rods | Small; gray, glassy |
| C. amycolatum | Pleomorphic gram-positive rods with single cells, V forms, or Chinese letters | Small; white to gray, dry |
| C. argentoratense | Typical coryneform gram-positive rods | Medium; cream-colored; nonhemolytic |
| C. aurimucosum | Typical coryneform gram-positive rods | Slightly yellowish sticky colonies; some strains black-pigmented |
| C. auris | Typical coryneform gram-positive rods | Small to medium; dry, slightly adherent, become yellowish with time; nonhemolytic |
| C. coyleae | Typical coryneform gram-positive rods | Small, whitish and slightly glistening with entire edges; either creamy or sticky |
| C. diphtheriae group‡ | Irregularly staining, pleomorphic gram-positive rods | Various biotypes of C. diphtheriae produce colonies ranging from small, gray, and translucent (biotype intermedius) to medium, white, and opaque (biotypes mitis, belfanti, and gravis); C. diphtheriae biotype mitis may be beta-hemolytic; C. ulcerans and C. pseudotuberculosis resemble C. diphtheriae |
| C. falsenii | Typical coryneform gram-positive rods | Small; whitish, circular with entire edges, convex, glistening, creamy; yellow pigment after 72 hr |
| C. freneyi | Typical coryneform gram-positive rods | Whitish; dry; rough |
| C. glucuronolyticum | Typical coryneform gram-positive rods | Small; white to yellow, convex; nonhemolytic |
| C. jeikeium | Pleomorphic; occasionally, club-shaped gram-positive rods arranged in V forms or palisades | Small; gray to white, entire, convex; nonhemolytic |
| C. imitans | Typical coryneform gram-positive rods | Small, white to gray, glistening, circular, convex; creamy; entire edges |
| C. macginleyi | Typical coryneform gram-positive rods | Tiny colonies after 48 hr; nonhemolytic |
| C. matruchotii | Gram-positive rods with whip-handle shape and branching filaments | Small; opaque, adherent |
| C. minutissimum | Typical coryneform gram-positive rods with single cells, V forms, palisading and Chinese letters | Small; convex, circular, shiny, and moist |
| C. mucifaciens | Typical coryneform gram-positive rods | Small, slightly yellow and mucoid; circular, convex, glistening |
| C. propinquum | Typical coryneform gram-positive rods | Small to medium with matted surface; nonhemolytic |
| C. pseudodiphtheriticum | Typical coryneform gram-positive rods | Small to medium; slightly dry |
| C. pseudotuberculosis | Typical coryneform gram-positive rods | Small, yellowish white, opaque, convex; matted surface |
| C. riegelii | Typical coryneform gram-positive rods | Small, whitish, glistening, convex with entire edges; either creamy or sticky |
| C. simulans | Typical coryneform gram-positive rods | Grayish white; glistening; creamy |
| C. singulare | Typical coryneform gram-positive rods | Circular; slightly convex with entire margins; creamy |
| C. striatum | Regular medium to large gram-positive rods; can show banding | Small to medium; white, moist and smooth (resembles colonies of coagulase-negative staphylococci) |
| C. sundsvallense | Gram-positive rods, some with terminal bulges or knobs; some branching | Buff to slight yellow, sticky, adherent to agar |
| C. thomssenii | Typical coryneform gram-positive rods | Tiny after 24 hr; whitish, circular, mucoid and sticky |
| C. ulcerans | Typical coryneform gram-positive rods | Small, dry, waxy, gray to white |
| C. urealyticum | Gram-positive coccobacilli arranged in V forms and palisades | Pinpoint (after 48 hr); white, smooth, convex; nonhemolytic |
| C. xerosis | Regular medium to large gram-positive rods can show banding; | Small to medium; dry, yellowish, granular |
| Dermabacter hominis | Coccoid to short gram-positive rods | Small; gray to white, convex; distinctive pungent odor |
| Exiguobacterium acetylicum | Irregular, short, gram-positive rods arranged singly, in pairs, or short chains; (i.e., rod-coccus cycle*) | Golden yellow |
| Kurthia spp. | Regular gram-positive rods with parallel sides; coccoid cells in cultures >3 days old | Large, creamy or tan-yellow; nonhemolytic |
| Leifsonia aquatica | Irregular, slender, short gram-positive rods | Yellow |
| Listeria monocytogenes | Regular, short, gram-positive rods or coccobacilli occurring in pairs (resembles streptococci) | Small; white, smooth, translucent, moist; beta-hemolytic |
| Microbacterium spp. | Irregular, short, thin, gram-positive rods | Small to medium; yellow |
| Oerskovia spp. | Extensive branching; hyphae break up into coccoid to rod-shaped elements | Yellow-pigmented; convex; creamy colony grows into the agar; dense centers |
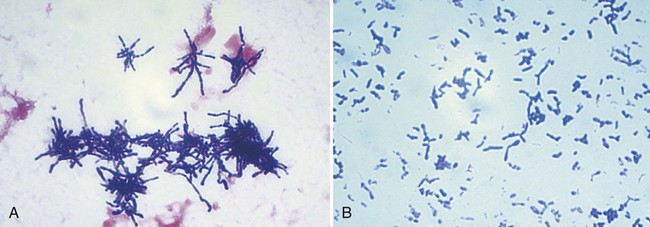
| Rothia spp. | Extremely pleomorphic; predominately coccoid and bacillary (broth, Figure 17-2, A) to branched filaments (solid media, Figure 17-2, B) | Small, smooth to rough colonies; dry; whitish; raised |
| Turicella otitidis | Irregular, long, gram-positive rods | Small to medium; white to cream, circular, convex |
†Includes strains G-1 and G-2.
‡Includes C. diphtheriae, C. ulcerans, and C. pseudotuberculosis.
Cultivation
Media of Choice
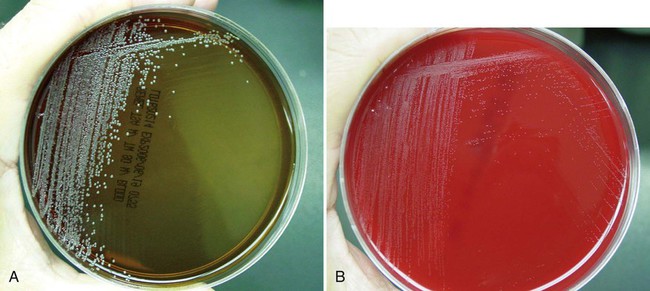
Corynebacterium spp. usually grow on 5% sheep blood and chocolate agars. Some coryneform bacteria do not grow on chocolate agar, and the lipophilic (lipid loving) species (e.g., C. jeikeium, C. urealyticum, C. afermentans subsp. lipophilum, C. accolens, and C. macginleyi) produce much larger colonies when cultured on 5% sheep blood agar supplemented with 1% Tween 80 (Figure 17-3).
Selective and differential media for C. diphtheriae should be used if diphtheria is suspected. The two media commonly used for this purpose are cystine-tellurite blood agar and modified Tinsdale agar (TIN). Tellurite blood agar maybe used with or without cystine. Cystine enhances the growth of fastidious organisms, including C. diphtheriae. Both media contain a high concentration of potassium tellurite that is inhibitory to normal flora. Organisms capable of growing on Tinsdale agar are differentiated based on the conversion of the tellurite to tellurium. This conversion results in color variations of grey to black colonies on the two media. C. diphtheriae also produces a halo on both media. C. diphtheriae can be presumptively identified by observing brown-black colonies with a gray-brown halo on Tinsdale agar (Figure 17-4). The brown halo is produced when the organism uses tellurite to produce hydrogen sulfide. The halo produced on cystine-tellurite blood agar appears brown as a result of the organism breaking down the cystine. In addition, Loeffler medium, which contains serum and egg, stimulates the growth of C. diphtheriae and the production of metachromatic granules in the cells. C. diphtheriae grows rapidly on the highly enriched agar and produces gray to white, translucent colonies within 12 to 18 hours. Primary inoculation of throat swabs to Loeffler serum slants is no longer recommended because of the inevitable overgrowth of normal oral flora.

Colonial Appearance
Table 17-3 describes the colonial appearance and other distinguishing characteristics (e.g., hemolysis and odor) of each clinically relevant genus or species of corynebacteria on blood agar. Colonies of C. diphtheriae on cystine-tellurite blood agar appear black or gray, whereas those on modified Tinsdale agar are black with dark brown halos (see Figure 17-4). C. diphtheriae colonies may be recognized by one of four varieties of colony morphologies. These colony types are referred to as gravis, intermedius, belfanti, and mitis, based on the phenotypic characteristics of size, texture, color, hemolysis, and the presence of metachromatic granules.
Approach to Identification
Table 17-4 shows the key tests needed to separate the genera discussed in this chapter. In addition to the features shown, the Gram stain and colonial morphology should be carefully noted.
TABLE 17-4
Catalase-Positive, Non–Acid-Fast, Gram-Positive Rodsa
| Organism | Metabolismb | Motility | Pigmentc | Nitrate Reduction | Esculin | Glucose Fermentation | CAMPd | Mycolic Acide | Cell Wall Diamino Acidsf | Other Comments |
| Corynebacterium | F/O | – | n, w, y, bl | v | –g | v | v | +h | meso-DAP | |
| Arthrobacter | O | vi | w, g | v | v | –j | – | – | L-lys | Gelatin-positive |
| Brevibacterium | O | – | w, g, sl y, t | v | – | –j | – | – | meso-DAP | Gelatin- and casein-positive; cheese odor |
| Microbacteriumk | Fl/Om | vn | y, o, y-o | v | vo | v | –p | – | L-lys, D-orn | Gelatin and casein variable |
| Turicella otitidis | O | – | w | – | – | – | + | – | meso-DAP | Isolated from ears |
| Dermabacter hominis | F | – | n, w | – | + | + | – | – | meso-DAP | Pungent odor; decarboxylates lysine and ornithine; gelatin positive |
| Cellulomonas | Fl | v | sl y, y | + | + | + | – | – | L-orn | Gelatin-positive; casein-negative |
| Leifsonia aquatica | O | + | y | v | v | –q | – | – | DAB | Gelatin- and casein-negative |
| Rhodococcus equi | O | – | p | v | – | – | + | + | meso-DAP | Usually mucoid; can be acid-fast; urease-positive |
| Cellulosimicrobium cellulans (formerly Oerskovia xanthineolytica) | F | – | y | + | + | + | NT | – | L-lys | Hydrolyzes xanthine; colonies pit agar |
| Oerskovia turbata | F | v | y | + | + | + | NT | – | L-lys | Does not hydrolyze xanthine |
| Listeria monocytogenes | F | +r | w | – | + | + | + | – | meso-DAP | Narrow zone of beta hemolysis on sheep blood agar; hippurate-positive |
| Kurthia | O | +r | n, c | – | – | – | NT | – | L-lys | Large, “Medusa-head” colony with rhizoid growth on yeast nutrient agar; may be H2S-positive in TSI butt; gelatin-negative |
| Exiguobacterium acetylicum | F | + | Golden | v | + | + | NT | – | L-lys | Most are oxidase positive; casein and gelatin positive |
| Rothia dentocariosa | F | – | w | + | + | + | – | – | L-lys | If sticky, probably R. mucilaginosa; some strains are black pigmented |
| Actinomyces neuii | F | – | n | v | – | + | + | – | NT | Nonhemolytic |
| Actinomyces viscosus | F | – | n | + | – | + | – | – | NT | |
| Propionibacterium avidum/granulosum | F | – | w, n | – | v | v | + | – | NT | Beta-hemolytic, branching |
NT, Not tested; TSI, triple sugar iron agar; v, variable reactions; +, ≥90% of species or strains positive; –, ≥90% of species or strains negative.
aThe aerotolerant catalase-positive Propionibacterium spp. and Actinomyces spp. are also included in Table 23-4.
bF, Fermentative; O, oxidative.
cc, Cream; g, gray; n, nonpigmented; o, orange; sl, slightly; t, tan; w, white; y, yellow; y-o, yellowish-orange; p, pink; bl, black.
dCAMP test using a beta-lysin–producing strain of Staphylococcus aureus.
eMycolic acids of various lengths are also present in the partially acid-fast Nocardia, Gordona, Rhodococcus, and Tsukamurella and the completely acid-fast Mycobacterium genera.
fDAB, diaminobutyric acid; D-orn, d-ornithine; L-lys, L-lysine; L-orn, L-ornithine; meso-DAP, meso-diaminopimelic acid.
gOf the significant clinical Corynebacterium isolates, only C. matruchotii and C. glucuronolyticum are esculin-positive.
hOf the significant Corynebacterium isolates, Corynebacterium amycolatum does not have mycolic acid as a lipid in the cell wall, as determined by high-performance liquid chromatography (HPLC) profiling methods.
iRod forms of some species are motile.
jGlucose may be variably oxidized, but it is not fermented.
kMicrobacterium spp. now include the former Aureobacterium spp.
lSome grow poorly anaerobically.
mSlow and weak oxidative production of acid from some carbohydrates.
nOnly the orange-pigmented species M. imperiale and M. arborescens are motile at 28°C.
oPositive reaction may be delayed.
pSome strains of M. arborescens are CAMP-positive.
Comments on Specific Organisms
• Guinea pig lethality test to ascertain whether diphtheria antitoxin neutralizes the lethal effect of a cell-free suspension of the suspect organism
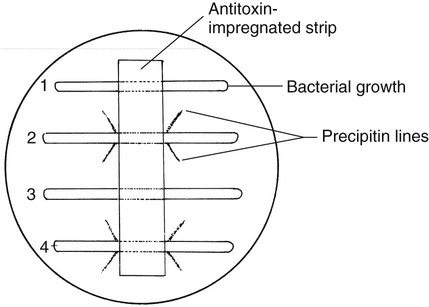
• Immunodiffusion test originally described by Elek (Figure 17-5)
• Tissue culture cell test to demonstrate toxicity of a cell-free suspension of the suspect organism in tissue culture cells and the neutralization of the cytopathic effect by diphtheria antitoxin
Identification criteria for Corynebacterium spp. (including C. diphtheriae) are shown in Tables 17-5 through 17-9. Most clinically relevant strains are catalase positive, nonmotile, nonpigmented, and esculin and gelatin negative. Therefore, isolation of an organism failing to demonstrate any of these characteristics provides a significant clue that another genus shown in Table 17-4 should be considered. In addition, an irregular, gram-positive rod isolate that is strictly aerobic, nonlipophilic and oxidizes or does not utilize glucose, will likely be Leifsonia aquatica, or Arthrobacter, Brevibacterium, or Microbacterium spp.
TABLE 17-5
Fermentative, Nonlipophilic, Tinsdale-Positive Corynebacterium spp.*
| Organism | Urease† | Nitrate Reduction† | Esculin Hydrolysis† | Fermentation of Glycogen† | Lipophilic |
| C. diphtheriae subsp. gravis | – | + | – | + | – |
| C. diphtheriae subsp. mitis | – | + | – | – | – |
| C. diphtheriae subsp. belfanti | – | – | – | – | – |
| C. diphtheriae subsp. intermedius | – | + | – | – | + |
| C. ulceransठ| + | Р| Р| + | Р|
| C. pseudotuberculosisठ| + | v | Р| Р| Р|
+, ≥90% of species or strains positive; –, ≥90% of species or strains are negative; v, variable reactions.
*Separation of lipophilic and nonlipophilic species can be determined by comparing growth on sheep blood agar and sheep blood agar with 1% Tween 80 or growth in brain-heart infusion broth with and without 1 drop of Tween 80 or rabbit serum.
‡Propionic acid produced as a product of glucose metabolism.
Data compiled from Coyle MB, Lipsky BA: Coryneform bacteria in infectious diseases: clinical and laboratory aspects, Clin Microbiol Rev 3:227, 1990; Funke G, Carlotti A: Differentiation of Brevibacterium spp. encountered in clinical specimens, J Clin Microbiol 32:1729, 1994; and Gruner E, Steigerwalt AG, Hollis DG et al: Human infections caused by Brevibacterium casei, formerly CDC groups B-1 and B-3, J Clin Microbiol 32:1511, 1994.
TABLE 17-6
Fermentative, Nonlipophilic, Tinsdale-Negative Clinically Relevant Corynebacterium spp.*†
| Organism | Urea‡ | Nitrate Reduction‡ | Propionic Acid§ | Motility | Esculin Hydrolysis‡ | Fermentation of Glucose‡ | Maltose‡ | Sucrose‡ | Xylose‡ | CAMP¶ |
| C. amycolatuma | v | v | + | – | – | + | v | v | – | – |
| C. argentoratense | – | – | + | – | – | + | – | – | – | – |
| C. aurimucosumb,c | – | – | – | – | – | + | + | + | – | – |
| C. coyleae | – | – | ND | – | – | (+) | – | – | – | + |
| C. falseniid | (+) | v | ND | – | v | (+) | v | – | – | – |
| C. freneyie | – | v | – | – | – | + | + | + | – | ND |
| C. glucuronolyticum | v | v | + | – | v | + | v | + | v | + |
| C. imitans | – | v | ND | – | – | + | + | (+) | – | + |
| C. matruchotii | – | + | + | – | v + | + | + | + | – | – |
| C. minutissimumf | – | – | – | – | – | + | + | v | – | – |
| C. riegelii | + | – | ND | – | – | – | (+) | – | – | – |
| C. simulansg | – | + | – | – | – | + | – | + | – | – |
| C. singulare | + | – | – | – | – | + | + | + | – | – |
| C. striatum | – | + | – | – | – | + | – | v | – | v |
| C. sundsvallenseb | + | – | ND | – | – | (+) | + | + | – | – |
| C. thomsseniib | + | – | ND | – | – | (+) | + | + | – | – |
| C. xerosis | – | v | – | – | – | + | + | + | – | – |
ND, No data; v, variable reactions; +, ≥90% of species or strains are positive; +W, (+), delayed positive reaction; –, ≥90% of species or strains are negative.
*Consider also Dermabacter, Cellulomonas, Exiguobacterium, and Microbacterium spp. if the isolate is pigmented, motile, or esculin or gelatin positive (see Table 17-4). The aerotolerant catalase-positive Propionibacterium spp. and Actinomyces spp. (see Table 18-4) should also be considered in the differential with the organisms in this table.
†Separation of lipophilic and nonlipophilic species can be determined by comparing growth on sheep blood agar and sheep blood agar with 1% Tween 80 or growth in brain-heart infusion broth with and without 1 drop of Tween 80 or rabbit serum.
§Propionic acid as an end-product of glucose metabolism.
¶CAMP data using a beta-lysin-producing strain of Staphylococcus aureus.
aMost frequently encountered species in human clinical material; frequently misidentified as C. xerosis.
cYellow or black-pigmented; black-pigmented strains have been previously listed as C. nigricans; may be pathogenic from female genital tract.
TABLE 17-7
Strictly Aerobic, Nonlipophilic, Nonfermentative, Clinically Relevant Corynebacterium spp.a,b
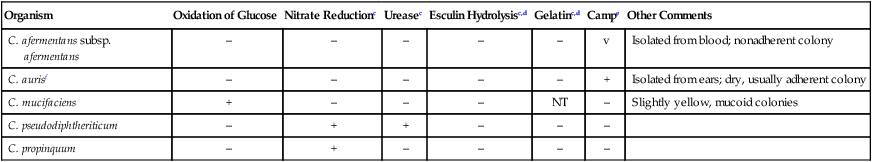
| Organism | Oxidation of Glucose | Nitrate Reductionc | Ureasec | Esculin Hydrolysisc,d | Gelatinc,d | Campe | Other Comments |
| C. afermentans subsp. afermentans | – | – | – | – | – | v | Isolated from blood; nonadherent colony |
| C. aurisf | – | – | – | – | – | + | Isolated from ears; dry, usually adherent colony |
| C. mucifaciens | + | – | – | – | NT | – | Slightly yellow, mucoid colonies |
| C. pseudodiphtheriticum | – | + | + | – | – | – | |
| C. propinquum | – | + | – | – | – | – |
NT, Not tested; v, variable reactions; +, ≥90% of species or strains are positive; –, ≥90% of species or strains are negative.
aKurthia sp. is also a strictly aerobic, nonlipophilic, nonfermentative organism. However, as described in Table 17-3, the colonial and cellular morphology of Kurthia organisms should easily distinguish it from the organisms in this table.
bSeparation of lipophilic and nonlipophilic species can be determined by comparing growth on sheep blood agar and sheep blood agar with 1% Tween 80 or growth in brain-heart infusion broth with and without 1 drop of Tween 80 or rabbit serum.
dConsider also Brevibacterium and Microbacterium spp., Leifsonia aquatica, and Arthrobacter sp. in the differential if the isolate is gelatin or esculin positive (see Table 17-4).
eCAMP test using a beta-lysin–producing strain of Staphylococcus aureus.
fFor isolates from the ear, also consider Turicella otitidis, which is nitrate and urease positive, in the differential (see Table 17-4).
Data compiled from Coyle MB, Lipsky BA: Coryneform bacteria in infectious diseases: clinical and laboratory aspects, Clin Microbiol Rev 3:227, 1990; Funke G, Carlotti A: Differentiation of Brevibacterium spp. encountered in clinical specimens, J Clin Microbiol 32:1729, 1994; and Mandell GL, Bennett JE, Dolin R: Principles and practices of infectious diseases, 2010, Churchill Stone and Livingston, Elsevier.
TABLE 17-8
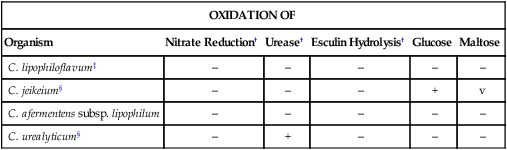
Strictly Aerobic, Lipophilic, Nonfermentative, Clinically Relevant Corynebacterium spp.*
| OXIDATION OF | |||||
| Organism | Nitrate Reduction† | Urease† | Esculin Hydrolysis† | Glucose | Maltose |
| C. lipophiloflavum‡ | – | – | – | – | – |
| C. jeikeium§ | – | – | – | + | v |
| C. afermentens subsp. lipophilum | – | – | – | – | – |
| C. urealyticum§ | – | + | – | – | – |
+, ≥90% of species or strains positive; –, ≥90% of species or strains negative; v, variable reactions.
*Separation of lipophilic and nonlipophilic species can be determined by comparing growth on sheep blood agar and sheep blood agar with 1% Tween 80 or growth in brain-heart infusion broth with and without one drop of Tween 80 or rabbit serum.
§Isolates are usually multiply antimicrobial resistant.
Data compiled from Coyle MB, Lipsky BA: Coryneform bacteria in infectious diseases: clinical and laboratory aspects, Clin Microbiol Rev 3:227, 1990; Funke G, Carlotti A: Differentiation of Brevibacterium spp. encountered in clinical specimens, J Clin Microbiol 32:1729, 1994; Mandell GL, Bennett JE, Dolin R: Principles and practices of infectious diseases, 2010, Churchill Stone and Livingston, Elsevier; and Riegel P, de Briel D, Prévost G et al: Genomic diversity among Corynebacterium jeikeium strains and comparison with biochemical characteristics, J Clin Microbiol 32:1860, 1994.
TABLE 17-9
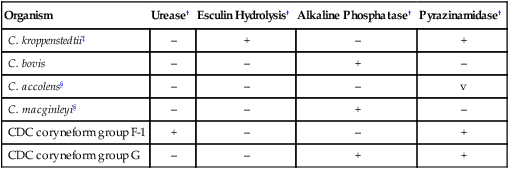
Lipophilic, Fermentative, Clinically Relevant Corynebacterium spp.*
| Organism | Urease† | Esculin Hydrolysis† | Alkaline Phosphatase† | Pyrazinamidase† |
| C. kroppenstedtii‡ | – | + | – | + |
| C. bovis | – | – | + | – |
| C. accolens§ | – | – | – | v |
| C. macginleyi§ | – | – | + | – |
| CDC coryneform group F-1 | + | – | – | + |
| CDC coryneform group G | – | – | + | + |
+, ≥90% of species or strains positive; –, ≥90% of species or strains negative; v, variable reactions.
*Separation of lipophilic and nonlipophilic species can be determined by comparing growth on sheep blood agar and sheep blood agar with 1% Tween 80 or growth in brain-heart infusion broth with and without one drop of Tween 80 or rabbit serum.
‡Propionic acid produced as a product of glucose metabolism.
Data compiled from Coyle MB, Lipsky BA: Coryneform bacteria in infectious diseases: clinical and laboratory aspects, Clin Microbiol Rev 3:227, 1990; Funke G, Carlotti A: Differentiation of Brevibacterium spp. encountered in clinical specimens, J Clin Microbiol 32:1729, 1994; Mandell GL, Bennett JE, Dolin R: Principles and practices of infectious diseases, 2010, Churchill Stone and Livingston, Elsevier; and Riegel P, Ruimy R, de Briel D et al: Genomic diversity and phylogenetic relationships among lipid-requiring diphtheroids from humans and characterization of Corynebacterium macginleyi sp nov, Int J Syst Bacteriol 45:128, 1995.
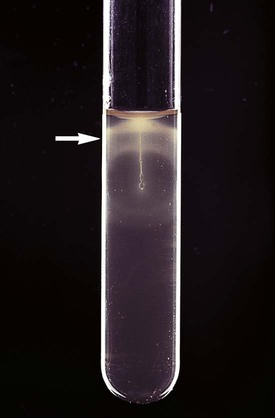
L. monocytogenes can be presumptively identified by observation of motility by direct wet mount. The organism exhibits characteristic end-over-end tumbling motility when incubated in nutrient broth at room temperature for 1 to 2 hours. Alternatively, characteristic motility can be seen by an umbrella-shaped pattern (Figure 17-6) that develops after overnight incubation at room temperature of a culture stabbed into a tube of semisolid agar. L. monocytogenes ferments glucose and is Voges-Proskauer positive and esculin positive. Isolation of a small, gram-positive, catalase-positive rod with a narrow zone of beta-hemolysis from blood or CSF should be considered strong presumptive evidence for listeriosis. L. monocytogenes can be differentiated from other Listeria spp. by a positive result on the Christie, Atkins, Munch-Petersen (CAMP) test, as described in Chapter 15 for the identification of Streptococcus agalactiae. A reverse CAMP reaction (i.e., an arrow of no hemolysis formed at the junction of the test organism with the staphylococci) is used to identify C. pseudotuberculosis and C. ulcerans. C. urealyticum is rapidly urea positive.
Antimicrobial Susceptibility Testing and Therapy
Definitive guidelines have been established for antimicrobial therapy for L. monocytogenes against certain antimicrobial agents. Because there is no resistance to the therapeutic agents of choice, antimicrobial susceptibility testing is not routinely necessary (Table 17-10).
TABLE 17-10
Antimicrobial Therapy and Susceptibility Testing
| Organism | Therapeutic Options | Resistance to Therapeutic Options | Validated Testing Methods* |
| Listeria monocytogenes | Ampicillin, or penicillin (MIC ≤2 µg/mL), with or without an aminoglycoside | Occasional resistance to tetracyclines | Yes, but testing is rarely needed to guide therapy; typically treated empirically. |
| Corynebacterium diphtheriae | Antitoxin to neutralize diphtheria toxin plus penicillin or erythromycin to eradicate organism | Not to recommended agents; rare instances of penicillin or macrolide resistance | See CLSI document M45-A: Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria. |
| Other Corynebacterium spp. | No definitive guidelines. All are susceptible to vancomycin and teicoplanin. | Multiple resistance to penicillins, macrolides, aminoglycosides, fluoroquinolones, tetracyclines, clindamycin and cephalosporins | See CLSI document M45-A: Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria. |
| Kurthia spp., Brevibacterium spp., Dermabacter sp., Arthrobacter spp., Microbacterium spp., Cellulomonas spp., and Exiguobacterium sp. | No definitive guidelines | Unknown | Not available |
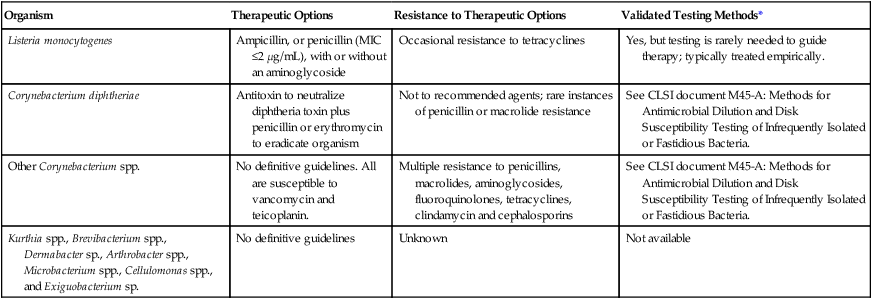
CLISI, Clinical and Laboratory Standards Institute; MIC, minimum inhibitory concentration.
*Validated testing methods include the standard methods recommended by CLSI and commercial methods approved by the U.S. Food and Drug Administration (FDA).
As shown in Table 17-10, Clinical and Laboratory Standards Institute (CLSI) document M45 provides some guidelines for testing of Corynebacterium spp. Chapter 12 should be reviewed for strategies that can be used to provide susceptibility information and data when warranted. It is important to note that some strains of Corynebacterium spp. may require 48 hours of incubation for growth. If growth is insufficient or if the isolate appears susceptible to β-lactams at 24 hours, the medium should be incubated for a total of 48 hours before the result is reported.