Chapter 10 Limb Apraxias and Related Disorders
This chapter is about the limb apraxias. The term apraxia occurs broadly in neurology and is usually interchangeable with dyspraxia. Apraxia describes non-learned motor dysfunctions including oculomotor movements, gait initiation (magnetic apraxia), and eyelid opening. It also describes skilled motor tasks that are dependent on visuospatial processing, including optic, constructional, and dressing apraxia. Apraxia is used as well for conditions that are more clearly consistent with the definition of disturbances in learned skilled movements but involve body parts other than the limbs, including orobucchal-facial and speech apraxias. These clinical entities are not included in this chapter, because they are either not limb apraxias or not disorders of “praxis” in the sense of disturbances in learned skilled movements (Zadikoff and Lang, 2005). This chapter focuses on the seven major limb apraxias of the upper extremities, where apraxia is most evident. They include ideomotor apraxia, parietal variant; ideomotor apraxia, disconnection variant; dissociation apraxia; ideational apraxia; and conceptual apraxia. Also included is limb-kinetic apraxia, a disorder that some argue is not a true apraxia, but instead a more basic disturbance in fine motor movements. Callosal apraxias comprise a separate category because of their unique unilateral and varied manifestations.
Historical Perspective
Many clinicians and investigators helped develop the current concept of limb apraxia. In 1866, John Hughlings Jackson probably recognized the clinical phenomenon of apraxia in a patient (Pearce, 2009). Jackson observed that the patient had “power in his muscles and in the centres for coordination of muscular groups, but he – the whole man, or the ‘will’ – cannot set them agoing.” In 1870, Carl Maria Finkelnburg used “asymbolia” to describe the clumsy and incomprehensible communicative gestures in aphasics, and in 1890, Meynert distinguished motor asymbolia from decreased motor “images” for movement. In 1899, D. De Buck used “parakinesia” to describe a patient who “though retaining the concepts for her actions, did not succeed in awakening the corresponding kinetic image.” By this time, the stage was set for Hugo Karl Liepmann’s seminal model of the limb apraxias.
In the early 1900s, Liepmann published a series of papers that led to the contemporary concept of limb apraxias. He proposed that the execution of purposeful movements could be divided into three steps (Goldenberg, 2003). First is the retrieval of the spatial and temporal representation or “movement formulas” of the intended action from the left hemisphere. Second is the transfer and association of these movement formulas via cortical connections with the “innervatory patterns” or motor programs located in the left “sensomotorium” (which includes premotor and supplementary motor areas). Third is the transmission of the information to the left primary motor cortex for performance of the intended actions in the right limb. Finally, in order for the left limb to perform the movements, the information traverses the corpus callosum to the right sensomotorium to activate the right primary motor cortex. Using Heymann Steinthal’s term of “apraxia,” Liepmann classified disturbances in these connections as “ideational, ideo-kinetic (melokinetic), and limb-kinetic apraxia.” Over the years, this classification nomenclature has evolved and the application of these terms has shifted, but Liepmann’s basic formulation of apraxia has persisted to the present day.
A Model for Praxis
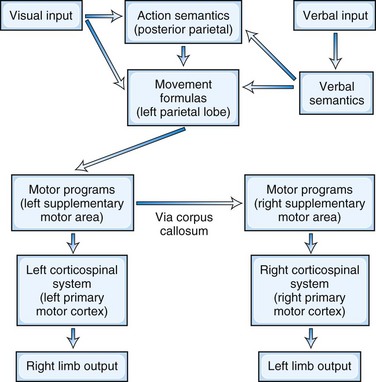
The left parietal region retains its central role of converting mental images of intended action into motor execution (Heilman and Rothi, 2003) (Fig. 10.1). The inferior parietal lobule contains the spatial and temporal movement programs (praxicons, visuokinesthetic motor engrams, or movement formulas) needed to carry out learned skilled movements. Multiple input modalities including visual, verbal-auditory, and tactile can activate these movement formulas. Cells in the inferior parietal lobule fire selectively in response to hand movements, visually presented information about object size and shape, or the actual manipulation of objects (Rizzolatti et al., 1998), and functional neuroimaging studies show activity of this region in response to recognition of actions associated with object or tool use (transitive actions) (Damasio et al., 2001). The right parietal lobe participates in the integration of visual information and upper-extremity movement. In addition to movement formulas, the left parietal region appears to contain action semantics and conceptual systems such as tool action, tool-object association information, and general principles of tool use (Goldenberg and Spatt, 2009; Ochipa et al., 1992). If a movement involves the use of a tool or object, action semantics specify knowledge of tool action (turning, pounding, etc.) and the knowledge of which tool or object to use to choose for a task (Leiguarda and Marsden, 2000).
In the premotor region, the supplementary motor area (SMA) translates the movement formulas into motor programs before sending them on to primary motor cortex (Roy and Square, 1985). The SMA, which is involved in complex movements of the upper extremities, receives projections from parietal neurons and in turn projects axons to motor neurons in the primary motor cortex. The SMA programs a specific order of movements and is involved in bimanual coordination. It translates these time-space movement formulas to specific motor programs that activate the motor neurons such that the contralateral extremity moves in the proscribed spatial trajectory and timing. For movements in the opposite extremity, the brain further conveys these programs across the corpus callosum to the opposite premotor cortex and activates the motor neurons for the desired contralateral extremity movements.
Beyond this traditional model for praxis, apraxia may result from damage in other regions including the prefrontal cortex, right hemisphere, basal ganglia (putamen and globus pallidus), thalamus, and their white-matter connections. The prefrontal region participates in sequencing multiple arm, hand, and finger movements. The right parietal region participates in performing nonpurposeful movements. Although the left inferior parietal lobule is more active than the right during action imagery and actual discrimination of nonpurposeful gestures, the right parietal region is more active during imitation and when these gestures consist of finger postures (Buccino et al., 2001; Hermsdorfer et al., 2001). The role of basal ganglia and thalamus is less clear, but they function as part of cortical-subcortical motor loops. Apraxia could, theoretically, result from damage to any of these areas outside the traditional model of praxis.
Classification of Limb Apraxias
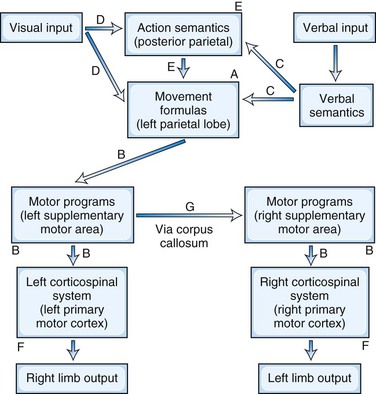
Beginning with Liepmann, there have been multiple attempts to classify and define the limb apraxias (Hanna-Pladdy and Rothi, 2001). The classification presented here is based on the seminal work of Heilman and associates, who have significantly contributed to the understanding of the limb apraxias (Heilman and Rothi, 2003). Depending on the location of the lesion, the patient has different patterns of ability to imitate and recognize gestures, perform sequential movements, and do fine motor activities (Fig. 10.2). The presence of production and content errors further characterize the subtypes of limb apraxia.
Ideomotor Apraxia, Parietal Variant
The parietal variant of ideomotor apraxia may be the most common and prototypical limb apraxia. Disruption of the movement formulas in the inferior parietal lobule impairs skilled movements on command and to imitation, as well as the recognition of gestures (see Fig. 10.2, A). Patients make spatial and temporal errors while producing movements. There is a failure to adopt the correct posture or orientation of the arm and hand or to move the limb correctly in space and at the correct speeds. Spatial errors involve the configuration of the hand and fingers, the proper orientation of the limb to the tool or object, and the spatial trajectory of the motion. A major distinguishing feature of the parietal variant of ideomotor apraxia is difficulty recognizing or identifying gestures, implicating damage to the praxicons, visuokinesthetic motor engrams, or movement formulas themselves.
Ideomotor Apraxia, Disconnection Variant
The disconnection variant of ideomotor apraxia results from disruptions of motor programs in the SMA or in their intra- and interhemispheric connections (Heilman and Watson, 2008). This form of ideomotor apraxia is a disconnection of an intact parietal region from the pathways to primary motor cortices. These lesions result in impaired pantomime to verbal commands, impaired imitation of gestures, and the presence of spatiotemporal production errors. The movement formulas themselves are preserved, but in contrast to the parietal variant of ideomotor apraxia, these patients can recognize and identify gestures. The lesions lie along the route from the left inferior parietal cortex to primary motor cortices (see Fig. 10.2, B). Although SMA lesions tend to affect both upper extremities, if the SMA lesion is limited to the right, apraxia may be limited to the left upper extremity.
Dissociation Apraxia
Patients with dissociation apraxia only exhibit errors when the movement is evoked by stimuli in one specific modality, usually verbal. Dissociation apraxia is a special type of disconnection apraxia where the disconnection is between language areas and movement formulas in the inferior parietal lobule. Information, however, can reach the inferior parietal lobe via other input modalities than language. Patients with dissociation apraxia may be impaired when attempting to perform skilled movements in response to verbal commands, but they are able to imitate gestures and to indicate or use actual objects correctly. Their errors are often unrecognizable movements rather than spatiotemporal or content errors. In addition to verbal dissociation apraxia (see Fig. 10.2, C), there can be visual (see Fig. 10.2, D) and tactile dissociation apraxias as well.
Conceptual Apraxia
Conceptual apraxia results in errors in content of the action, such as in tool-selection errors or in tool-object knowledge. Whereas dysfunction of praxis production results in ideomotor apraxia, defects in the conceptual knowledge needed to successfully select tools and objects results in conceptual apraxia. Although conceptual apraxia often co-occurs with ideomotor apraxia, it can occur by itself, indicating that praxis production and praxis conceptual systems are independent. Patients with conceptual apraxia are unable to name or point to a tool when its function is discussed, or recall the type of actions associated with specific tools, utensils, or objects. They make content errors in which they substitute the action associated with the wrong tool for the requested tool. For example, when asked to demonstrate the use of a hammer or a saw either by pantomiming or using the tool, the patient with the loss of tool-object action knowledge may pantomime a screwing twisting movement as if using a screwdriver. Other terms used to describe these errors include disturbances in mechanical knowledge or in action semantics (see Fig. 10.2, E). Conceptual apraxia is most common in Alzheimer disease, other dementias (Ochipa et al., 1992), and in patients with diffuse posterior cerebral lesions, particularly involving the left hemisphere.
Limb-Kinetic Apraxia
Limb-kinetic apraxia is the inability to make finely graded, precise, coordinated individual finger movements. Limb-kinetic apraxia may not be a real apraxia in the traditional definition, but it is prominently considered in the differential diagnosis of the limb apraxias. Patients with limb-kinetic apraxia complain of a loss of dexterity or deftness that makes fine motor movements such as buttoning or tying shoes difficult. Weakness or changes in muscle tone do not account for this “clumsiness,” and limb-kinetic apraxia may be intermediate between paresis and other limb apraxias. Limb-kinetic apraxia is usually confined to the limb contralateral to a hemispheric lesion; however, when limb-kinetic apraxia occurs in the preferred hand, it may also be present in the non-preferred hand (Hanna-Pladdy et al., 2002). Clinicians need to distinguish limb-kinetic apraxia from right parietal functions such as nonsymbolic gestures (e.g., copying meaningless fine finger movements) and from optic ataxia, or decreased coordination of the hands under visual guidance. Limb-kinetic apraxia results from lesions in the primary motor cortex or corticospinal system (see Fig. 10.2, F). Liepmann (1920) also thought that limb-kinetic apraxia could result from lesions in the sensory motor cortex, and Kleist (1931) attributed it to damage in the premotor areas.
Callosal Apraxia
Several limb apraxia syndromes can result from callosal lesions (see Fig. 10.2, G). What distinguishes these patients is that their apraxia is confined to the non-dominant limb, usually the left arm or hand in right-handed individuals. The right limb may be affected in left-handed individuals, or they may have a similar lateralization as right-handers. Liepmann and others described left-sided disconnection-variant ideomotor apraxia due to callosal lesions and strokes (Heilman and Watson, 2008). These patients cannot pantomime with their left hand to verbal command or imitate but can recognize and identify gestures. Others described left-sided dissociative apraxia due to callosal lesions (Gazzaniga et al., 1967; Geschwind and Kaplan, 1962). Patients who have had surgical disconnection of the corpus callosum could not gesture normally to command with their left arm and hand but performed well with imitation and actual tools. Some patients have had a combination of both ideomotor and dissociative apraxia of their left arm and hand manifested by unrecognizable movements on verbal command and spatiotemporal errors on imitation. Other patients have a callosal “alien limb” with independent movements of the non-dominant limb, sometimes with “diagonistic apraxia” or the intermanual conflict of the hands acting in opposition to each other. The classic example of this is the split-brain patient who has undergone a corpus callosotomy who finds that his or her left hand is unbuttoning his shirt or blouse while the right one is trying to button it. Finally, there is a rare description of callosal lesions resulting in conceptual apraxia, indicating that conceptual knowledge as well as movement formulas have lateralized representations, and that such representations are contralateral to the preferred hand (Heilman et al., 1997).
Testing for Limb Apraxias
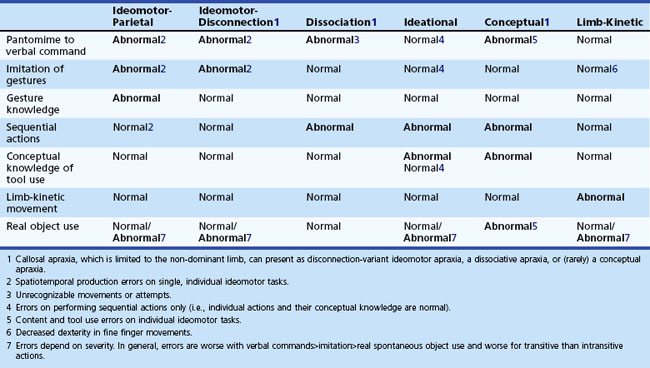
Apraxia testing involves a systematic approach (Box 10.1). Prior to testing of praxis, a neurological examination excludes the presence of significant motor, sensory, or cognitive disorders that could explain the inability to perform learned skilled movements. First, the testing of praxis itself begins with asking the patient to pantomime to command. The movements are transitive (associated with tool or instrument use) and intransitive (associated with communicative gestures such as waving goodbye). For transitive movements, the examiner asks the patient to demonstrate how to comb their hair, brush their teeth, or use a pair of scissors. For intransitive movements, the examiner asks the patient to demonstrate how to wave goodbye, beckon somebody to come, or hitchhike. The testing involves the right and left limb independently. The examiner observes the patient for the presence of temporal-spatial or content errors. Second, if the patient has difficulty pantomiming movements, the examiner tests their ability to imitate gestures. For gesture imitation, the examiner performs both transitive and intransitive movements and asks the patient to copy the movements. Third, for gesture knowledge, the examiner performs the same transitive and intransitive gestures and asks the patient to identify the gesture. The patient must identify the gesture and discriminate between those that are well and poorly performed. Fourth, the patient must perform tasks that require several motor acts in sequence, such as making a sandwich or preparing a letter for mailing. Fifth, the examiner shows the patient pictures of tools or objects or the actual tools or objects themselves. The examiner then requests that the patient pantomime the action associated with the tool or object. Finally, the examiner checks for fine finger movements by asking the patient to do repetitive tapping, picking up a coin with a pincer grasp, and twirling the coin. Additional impairment in the patient’s ability to use real objects indicates marked severity of the limb apraxia. The pattern of deficits will determine the types of apraxia (Table 10.1). Specialists in occupational therapy, physical therapy, speech pathology, and neuropsychology can further assess and quantify the deficits in limb apraxia using instruments like the Apraxia Battery for Adults-2 and the Florida Apraxia Battery (Power et al., 2010).
Box 10.1 The Examination for Limb Apraxias
The examiner demonstrates the same actions without naming them and asks the patient to copy them.
Alternate touching each finger tip with thumb
II. NON-DOMINANT UPPER EXTREMITY
The examiner repeats the same procedures as for the dominant upper extremity.
Testing for Ideomotor Apraxia, Parietal and Disconnection Variants
On attempting to pantomime, patients with ideomotor apraxia may substitute a body part for the tool or object (Raymer et al., 1997). For example, when attempting to pantomime combing their hair or brushing their teeth, they substitute their fingers for the comb or toothbrush. Normal subjects may make the same errors, so the examiner should ask patients not to substitute their fingers or other body parts but to pantomime using a “pretend tool.” Patients with ideomotor apraxia may not improve with these instructions and continue to make body-part substitution errors. The substitution of a body part for a tool or object activates the right inferior parietal lobe, hence patients with ideomotor apraxia with left parietal injury appear to be using their normal right parietal lobe in order to pantomime gestures (Ohgami et al., 2004).
Testing for Conceptual Apraxia
Patients with conceptual apraxia make content errors and demonstrate the actions of tools or objects other than the one they were asked to pantomime. For example, the examiner shows the patient either pictures or the actual tools or objects and asks the patient to pantomime or demonstrate their use or function. Patients with conceptual apraxia pantomime the wrong use or function, but they are able to imitate gestures without spatiotemporal errors (see Table 10.2).
Testing for Limb-Kinetic Apraxia
For limb-kinetic apraxia testing, the examiner asks the patient to perform fine finger movements and looks for evidence of incoordination. For example, the examiner asks the patient to pick up a small coin such as a dime from the table with the thumb and the index finger only. Normally, people use the pincer grasp to pick up a dime by putting a forefinger on one edge of the coin and the thumb on the opposite edge. Patients with limb-kinetic apraxia will have trouble doing this without sliding the coin to the edge of the table or using multiple fingers. Another test involves the patient rotating a nickel between the thumb, index, and middle fingers 10 times as rapidly as they can. Patients with limb-kinetic apraxia are slow and clumsy at these tasks (Hanna-Pladdy et al., 2002).
Testing for Callosal Apraxia
The examination for callosal apraxias is the same as for the other limb apraxias except that the abnormalities are limited to the non-dominant hand in most cases. The testing for callosal apraxia may reveal a disconnection-variant ideomotor apraxia, a dissociative apraxia, or even a conceptual apraxia in the non-dominant limb (Heilman et al., 1997).
Pathophysiology of Limb Apraxias
Ideomotor apraxia is associated with lesions in a variety of structures including the inferior parietal lobe, the frontal lobe, and the premotor areas, particularly the SMA. There are reports of ideomotor apraxia due to subcortical lesions in the basal ganglia (caudate-putamen), thalamus (pulvinar), and associated white-matter tracts including the corpus callosum. Limb apraxias can be caused by a variety of central nervous system disorders that affect these regions. The different forms of limb apraxia result from cerebrovascular lesions, especially right middle cerebral artery strokes with right hemiparesis. There may be left-sided limb apraxia in these patients. Right anterior cerebral artery strokes and paramedian lesions could produce ideomotor apraxia, disconnection variant. Ideomotor apraxia and limb-kinetic apraxia can be the initial or presenting manifestation of disorders such as corticobasal syndrome, primary progressive aphasia, or parietal-variant Alzheimer disease (Rohrer et al., 2010). Tumors, traumatic brain injury, infections, and other pathologies can also lead to limb apraxias.
Rehabilitation for Limb Apraxias
Because many instrumental and routine ADLs depend on learned skilled movements, patients with limb apraxia usually have impaired functional abilities. The presence of limb apraxia, more than any other neuropsychological disorder, correlates with the level of caregiver assistance required 6 months after a stroke, whereas the absence of apraxia is a significant predictor of return to work after a stroke (Saeki et al., 1995). The treatment of limb apraxia is therefore important for improving the quality of life of the patient.
Even though many treatments have been studied, none has emerged as the standard. There are no effective pharmacotherapies for limb apraxia, and treatments primarily involve rehabilitation strategies. Buxbaum and associates (2008) surveyed the literature on the rehabilitation of limb apraxia and identified 10 studies with 10 treatment strategies: multiple cues, error type reduction, 6-stage task hierarchy, conductive education, strategy training, transitive/intransitive gesture training, rehabilitative treatment, error completion, exploration training, and combined error completion and exploration training. Most of these approaches emphasize cueing with multiple modalities, with verbal, visual, and tactile inputs, repetitive learning, and feedback and correction of errors. Patients with post-stroke apraxia have had generalization of cognitive strategy training to other activities of daily living (Geusgens et al., 2006), but others have not (Bickerton et al., 2006). Although most studies have shown positive treatment effects, fewer studies have demonstrated the effects to be generalizable compared to those that have not. It is also unclear whether the benefits can be sustained long term. In sum, patients can learn and produce new gestures, but the newly learned gestures may not generalize well to contexts outside the rehabilitation setting. Nevertheless, some patients with ideomotor apraxia have improved with gesture-production exercises (Smania et al., 2000), and patients with apraxia would benefit from referral to a rehabilitation specialist with experience in treating apraxias.
Summary
Limb apraxia, or the disturbance of learned skilled movements, is an important but often missed or unrecognized impairment. Clinicians may misattribute limb apraxia to weakness, hemiparesis, clumsiness, or other motor or cognitive disturbance. Apraxia may only be evident in natural or real situations, with actual tool or object use, or on fine, sequential, or specific movements of the upper extremities (Zadikoff and Lang, 2005). Apraxia is an important cognitive disturbance and a salient sign in patients with strokes, Alzheimer disease, corticobasal syndrome, and other conditions. The model of left parietal movement formulas and disconnection syndromes introduced by Liepmann over 100 years ago continues to be compelling today. This model, in the context of a dedicated apraxia examination and analysis for spatiotemporal or content errors, clarifies and classifies the limb apraxias. Although more effective treatments need to be developed, rehabilitation strategies can be helpful interventions for these disturbances. Fortunately, recent advances in technology and rehabilitation promise to enhance our understanding and management of the limb apraxias.
Bickerton W.L., Humphreys G.W., Riddoch J.M. The use of memorised verbal scripts in the rehabilitation of action disorganisation syndrome. Neuropsychol Rehabil. 2006;16:155-177.
Buccino G., Binkofski F., Fink G.R., et al. Action observation activates premoter and parietal areas in somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13:400-404.
Buxbaum L.J., Haal K.Y., Hallett M., et al. Treatment of limb apraxia. Am J Physical Med & Rehab. 2008;87:149-161.
Damasio H., Grabowski T.J., Tranel D., et al. Neural correlates of naming actions and of naming spatial relations. Neuroimage. 2001;13:1053-1064.
Gazzaniga M., Bogen J., Sperry R. Dyspraxia following diversion of the cerebral commissures. Arch Neurol. 1967;16:606-612.
Geschwind N., Kaplan E. A human cerebral disconnection syndrome. A preliminary report. Neurology. 1962;12:675-685.
Geusgens C., van Heugten C., Donkervoort M., et al. Transfer of training effects in stroke patients with apraxia: an exploratory study. Neuropsychol Rehabil. 2006;16:213-229.
Goldenberg G. Apraxia and beyond: life and work of Hugo Liepmann. Cortex. 2003;39:509-524.
Goldenberg G., Spatt J. The neural basis of tool use. Brain. 2009;132:1645-1655.
Hanna-Pladdy B., Mendoza J.E., Apostolos G.T., et al. Lateralized motor control: hemispheric damage and the loss of deftness. J Neurol Neurosurg Psychiatry. 2002;73:574-577.
Hanna-Pladdy B., Rothi L.J.G. Ideational apraxia: Confusion that began with Liepmann. Neuropsychol Rehabil. 2001;11:539-547.
Heilman K.M., Maher L.M., Greenwald M.L., et al. Conceptual apraxia from lateralized lesions. Neurology. 1997;49:457-464.
Heilman K.M., Rothi L.J.G. Apraxia. In: Heilman K.M., Valenstein F. Clinical Neuropsychology. New York: Oxford University Press; 2003:215-235.
Heilman K.M., Watson R.T. The disconnection apraxias. Cortex. 2008;44:975-982.
Hermsdorfer J., Goldenberg G., Wachsmuth C., et al. Cortical correlates of gesture processing: Clues to the cerebral mechanisms underlying apraxia during the imitation of meaningless gestures. Neuroimage. 2001;14:149-161.
Kleist K. Gehirnpathologische und lokalisatorische Ergebnisse: das Stirnhirn im engeren Sinne und seine Störungen. Z ges Neurol Psychiat. 1931;131:442-448.
Liepmann H. Apraxie. In: Brugsch H., editor. Ergebnisse der gesamten medizin. Wien Berlin: Urban & Schwarzenberg; 1920:516-543.
Ochipa C., Rothi L.J., Heilman K.M. Conceptual apraxia in Alzheimer’s disease. Brain. 1992;115:1061-1071.
Ohgami Y., Matsuo K., Uchida N., et al. An fMRI study of tool-use gestures: body part as object and pantomime. Neuroreport. 2004;15:1903-1906.
Pearce J.M.S. Hugo Karl Liepmann and apraxia. Clin Med. 2009;9:466-470.
Power E., Code C., Croot K., et al. Florida Apraxia Battery-Extended and Revised Sydney (FABERS): Design, description, and a healthy control sample. J Clin Exp Neuropsychol. 2010;32:1-18.
Raymer A.M., Maher L.M., Foundas A.L., et al. The significance of body part as tool errors in limb apraxia. Brain Cogn. 1997;34:287-292.
Rizzolatti G., Luppino G., Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol. 1998;106:283-296.
Rohrer J.D., Rossor M.N., Warren J.D. Apraxia in progressive nonfluent aphasia. J Neurol. 2010;257:569-574.
Roy E.A., Square P.A. Common considerations in the study of limb, verbal, and oral apraxia. In: Roy E.A., editor. Neuropsychological Studies of Apraxia and Related Disorders. Amsterdam: North-Holland; 1985:111-161.
Saeki S., Ogata H., Okubo T., et al. Return to work after stroke. A follow-up study. Stroke. 1995;26:399-401.
Smania N., Girardi F., Domenicali C., et al. The rehabilitation of limb apraxia: a study in left-brain-damaged patients. Arch Phys Med Rehabil. 2000;81:379-388.
Zadikoff C., Lang A.E. Apraxia in movement disorders. Brain. 2005;128:1480-1497.