Chapter 95 Life-Threatening Viral Diseases and Their Treatment
Myocarditis
Background
Although many infectious and noninfectious causes have been identified, viruses account for most cases of myocarditis.1 The spectrum of disease ranges from asymptomatic, with only minimal changes on the electrocardiogram, to fulminant, with rapid onset of severe disease. Likewise, myocardial involvement may be focal or diffuse.2 Most patients have an indolent illness that may progress to dilated cardiomyopathy. Because of the varied presentations and the difficulty in the establishment of a definitive diagnosis, the true incidence of myocarditis is unknown. In a large series from Sweden, 1% of myocardial biopsies from autopsies conducted over a 10-year period fulfilled the Dallas criteria3 for myocarditis.4
Pathogenesis
Although the pathogenesis of viral myocarditis is not well understood, viruses appear to enter cardiac myocytes or macrophages through specific receptors and coreceptors.5 Viral virulence is likely modified by differential coreceptor binding6 and variations in the viral genome.7 Myocardial damage is thought to occur at least in part as a direct result of viral infection, with active viral replication leading to myocardial necrosis.8 Coxsackie virus protease 2A cleaves dystrophin in cultured myocytes and in infected mouse hearts. The results are impaired dystrophin function and poor myocyte contractility.9 In addition, both humoral and cellular immune responses contribute to the pathogenesis of myocarditis,10,11 through postinfectious autoimmune processes,11 cytotoxic T lymphocytes, and antibody-dependent cell-mediated cytotoxicity.12 Cytokines13 may also cause direct myocardial injury and affect cardiac function.
Cause
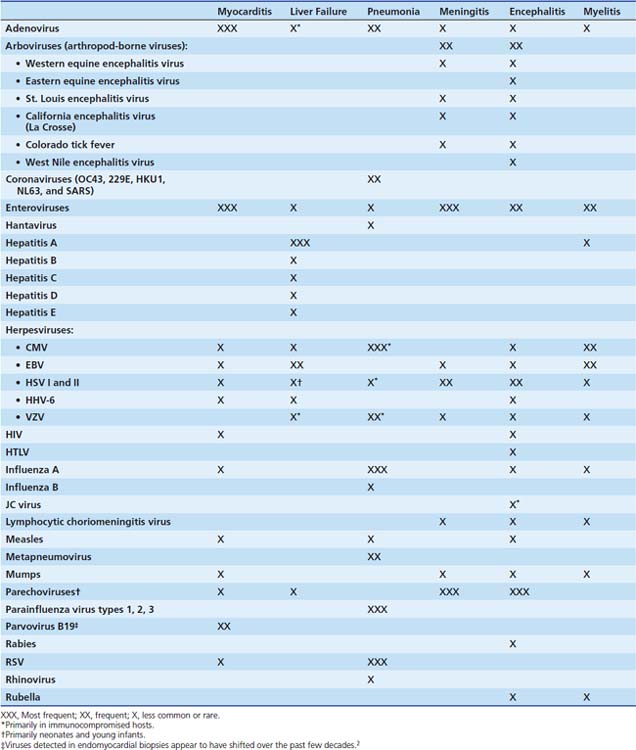
The viruses most frequently associated with myocarditis are enteroviruses, particularly Coxsackie virus B, and adenoviruses (serotypes 2 and 5).14 Many other viruses have caused myocarditis in children, including influenza A, herpes simplex virus (HSV); human immunodeficiency virus (HIV); cytomegalovirus (CMV); respiratory syncytial virus (RSV); and the mumps and measles viruses, before the widespread use of the measles-mumps-rubella (MMR) vaccine (Table 95-1). Polymerase chain reaction (PCR) of cardiac tissue from endomyocardial biopsy specimens in 34 children with a clinical diagnosis of myocarditis identified adenovirus in 44%, enterovirus in 24%, and HSV in 6%.15 In addition to enteroviruses, adenovirus,14 CMV, human herpesvirus 6 (HHV-6) and parvovirus B1916 are increasingly recognized as important causes of myocarditis in adolescents and adults. Adenoviruses and enteroviruses are the viruses most frequently identified in patients with dilated cardiomyopathy.
Clinical Presentation
Infants with myocarditis usually have symptoms that include poor feeding, fever, irritability, and listlessness. Physical findings are consistent with congestive heart failure. Enteroviral myocarditis in infancy frequently occurs in conjunction with severe hepatitis, pneumonitis, or both and can be difficult to distinguish from bacterial sepsis.17 The death rate may be as high as 75%. Severe dysrhythmias have been described in infants with myocardial involvement from RSV,18 and viral myocarditis has been implicated in some cases of sudden death.19,20 Older children and adolescents are more likely to appear for examination after a prodromal viral illness. Early symptoms include lethargy, low-grade temperature, and decreased appetite. They may have diaphoresis, dyspnea on exertion, malaise, chest pain, and palpitations. Resting tachycardia disproportionate to the amount of fever is common, and an apical systolic murmur may be heard. A subset of children and adults have fulminant myocarditis, characterized by rapid onset of symptoms, severe hemodynamic compromise, and fever.21
Laboratory abnormalities may include elevated white blood cell count and erythrocyte sedimentation rate.22 Serum aspartate aminotransferase (AST) levels are often elevated,23 as are creatinine kinase-MB levels. Cardiac troponin I may be a more sensitive measure of cardiac muscle injury in myocarditis.24
Electrocardiographic abnormalities are almost always present in acute myocarditis, with findings of low-voltage QRS complexes and nonspecific ST and T wave changes. Both atrial and ventricular arrhythmias may be present, including supraventricular and ventricular tachycardia, as well as conduction abnormalities. Echocardiography reveals left ventricular dysfunction, in most cases with either segmental wall motion abnormalities or global hypokinesis. Pericardial effusions are common. In one series, nondilated, thickened, and hypocontractile left ventricles (LVs) were seen in subjects with fulminant myocarditis compared with significant LV dilation and normal LV thickness in subjects with a more insidious onset. Subjects with fulminant myocarditis had more evidence of inflammation on endomyocardial biopsy and were more likely to recover ventricular function.25 Pulmonary edema, enlarged cardiac silhouette, and prominent pulmonary vasculature may be seen on a chest radiograph. Contrast-enhanced cardiac magnetic resonance imaging (MRI) can document the location and extent of inflammation and can be used to assist in diagnosis or to guide endomyocardial biopsy 26,27
Acute Liver Failure
Background
Acute liver failure (ALF) is a rare condition, which prior to the availability of orthotopic liver transplantation, carried a mortality of 70% to 80% (see Chapter 88). Early studies of ALF in children used the adult definition that required rapid development of severe hepatic dysfunction with the development of hepatic encephalopathy within 8 weeks of clinical jaundice in a person without a prior history of liver disease. However, due to the difficulty in assessing encephalopathy in young children and the recognition that terminal liver failure can occur in the absence of clinical encephalopathy, the Pediatric Acute Liver Failure Study (PALF) group has accepted the diagnosis of ALF in children with no history of chronic liver disease who present with biochemical evidence of acute liver injury and severe hepatic-based coagulopathy regardless of the presence or absence of hepatic encephalopathy.28 The causes of ALF in children can be metabolic, toxic, drug-related, immune-mediated or infectious. The percentage of ALF caused by viral infections varies significantly by age group and geographic location, with infections causing only 6% of ALF in a large pediatric series from North America and Great Britain28,29 but 50% in a series from South America.30
Cause
While less than 1% of infections with these viruses result in ALF, the hepatotropic viruses, hepatitis A and B, comprise the majority of cases with a definitive viral diagnosis, particularly in endemic areas.30–34 Infection with hepatitis C rarely causes ALF; however, there are case reports of ALF with both postnatally and perinatally acquired hepatitis C in children.28,35,36 In 2006, PALF published a report of the first 348 children presenting with ALF to the study sites throughout North America and Great Britain. Only 6% of the cases in this series had definitive viral causes, with the viruses identified being adenovirus, CMV, hepatitis A, enterovirus, HSV, Epstein-Barr virus (EBV), and hepatitis C. However, both in this series and in a later series of 703 children published by the same group, almost 50% of all the cases were indeterminate, due at least in part to lack of a complete diagnostic workup.29 It is likely that some of these indeterminate cases were also due to undiagnosed infectious causes. Additional viruses that have been implicated in ALF include hepatitis D and E, parvovirus,37,38 varicella-zoster virus (VZV),39 and HHV-6.40,41 ALF in infants is most likely to be associated with systemic illness due to enterovirus, echovirus, HSV, HHV-6, or CMV.28,42–44 While most perinatally acquired infections with hepatitis B are asymptomatic, infants born to women with both HBsAg and anti-HBeAb appear to be at greater risk for ALF due to perinatally acquired hepatitis B.45
Risk factors for HSV hepatitis outside of the neonatal period include pregnancy and immune suppression.46,47 Immune suppression is also a risk factor for CMV-, adenovirus-, and VZV-associated ALF. Hepatitis E virus (HEV) is an enterically transmitted virus that causes epidemic hepatitis in many areas of the world, particularly the Indian subcontinent and Southeast Asia.34 ALF can also occur in severe dengue48,49 and yellow fever infection.48–50 HEV, dengue, and yellow fever are not endemic in Western countries.
Clinical Presentation
Symptoms of acute hepatitis include jaundice, anorexia, fatigue, nausea, and vomiting.32,51,52 In fulminant disease, there is rapid progression to hepatic failure and encephalopathy. Physical examination may demonstrate fever, hepatosplenomegaly with liver tenderness, scleral or cutaneous icterus, and mucosal bleeding. Patients with severe vomiting may have significant dehydration. Laboratory studies include elevated hepatic enzymes (10- to 100-fold increases in AST and alanine aminotransferase), hyperbilirubinemia, prolonged prothrombin time, and elevated ammonia levels. As hepatocyte necrosis progresses, hepatic enzyme levels and liver size may decrease. Cerebral edema is common in patients with severe encephalopathy32,52 and renal failure is a common complication.51,52
Viral Pneumonia/Pneumonitis
Background
Influenza and pneumonia combined are a leading cause of death of children in developing countries and the eighth leading cause of death in the United States for patients of all ages. A greater burden of disease is present in infants, young children, and older individuals.53 Although only 20% to 50% of community-acquired pneumonias in adults are associated with viral pathogens,54–58 viruses account for the majority of the causes of lower respiratory infection in children. It is estimated that more than 500,000 hospitalizations occur each year in the United States for lower respiratory tract infections among children younger than 18 years old,59,60 and an estimated 80% of these hospitalizations are attributable to viral etiologies, with the majority in children aged less than 5 years.59,61 The peak season is from midwinter to early spring.
Cause
The etiologic agents of viral pneumonia are varied (see Table 95-1), and recent studies using PCR for diagnosis have considerably improved the ability to detect viruses. RSV is the primary cause of hospitalization for respiratory tract illness in young children, with average annual hospitalization rates in the United States of 17 per 1000 children under 6 months of age and 3 per 1000 children younger than 5 years.62 In a recent national surveillance study, most children with RSV infection had no coexisting medical conditions or characteristics that significantly identified them as being at greater risk for severe RSV disease, except for being younger than 2 years.62 Among children, RSV infection is the cause of 50% to 90% of hospitalizations for bronchiolitis, 5% to 40% of those for pneumonia, and 10% to 30% of those for tracheobronchitis.63 Repeat infections are common; in the healthy host, they are localized to the upper respiratory tract. Among immunocompromised patients, RSV upper respiratory infections can progress to fatal pneumonia, with the greatest mortality risk in patients with severe lower tract disease and in those in whom treatment is significantly delayed.64,65
Influenza epidemics occur annually with significant morbidity and death in young children and older individuals. In a national surveillance study, an average of 0.9 per 1000 children aged 0 to 59 months were hospitalized with laboratory-confirmed influenza, with the highest rate of 4.5 per 1000 children in the 0 to 5-month age group.66 During the four influenza seasons from 2003 to 2007, the total number of reported pediatric influenza deaths ranged from 46 to 153, with an average of 82 deaths each year.67 Infected infants younger than 2 months may have symptoms mimicking bacterial sepsis, commonly including apnea. In children younger than 5 years, influenza can cause symptoms of laryngotracheobronchitis, whereas pneumonia occurs in 10% to 15% of those younger than 3 years. Finally, older children generally exhibit the classic flulike symptoms of fever, headache, myalgia, and malaise with upper respiratory tract symptoms. Bacterial superinfection is a common and potentially severe complication of influenza. Among immunocompromised patients, risk factors for more severe disease and progression to the lower respiratory tract include lymphopenia and infection early after hematopoietic stem cell transplant.65, 68 With earlier antiviral treatment, progression to the lower tract and mortality have been markedly reduced.
In 2009, the emergence of a pandemic strain of influenza A, termed novel H1N1, led to a greatly increased burden of influenza disease worldwide. Children and young adults were at a disproportionate risk for infection and hospitalization, with 60% of infections occurring among those 18 years of age or younger, and 95% in those younger than 50 years.69–71 The overall attack rate was highest among children aged 5 to 14 years (147 per 100,000 population),69 but infants had the highest hospitalization rates and persons aged 50 years or older had the highest mortality rates once hospitalized.69,71 This differs from seasonal influenza, where about 60 percent of influenza-related hospitalizations and 90 percent of influenza-related deaths occur in people 65 years and older. From April to October of 2009, the Centers for Disease Control and Prevention (CDC) received reports of 145 pediatric deaths associated with influenza infection.67 Correcting for under-ascertainment, the CDC estimates the actual count may be three to four times greater than reported (http://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm). The majority of hospitalized cases had underlying medical conditions associated with severe seasonal influenza; secondary bacterial infections were observed in 15% to 30% of fatal cases.71,72
In a typical influenza season, parainfluenza viruses (PIVs) are generally second only to RSV as important viral causes of lower respiratory infection in children and immunocompromised patients.73–77 The average annual PIV hospitalization rate is 1.02 per 1000 children 0 to 59 months of age, with the highest rate of 3.01 per 1000 in children 0 to 5 months of age.77 PIVs account for 50% of hospitalizations for acute laryngotracheitis (croup) and at least 15% of cases of bronchiolitis and pneumonia. PIVs types 1 and 2 cause more cases of croup whereas PIV type 3 is more likely to infect the small air passages and cause pneumonia or bronchiolitis. However, any PIV can cause lower respiratory tract disease, particularly during primary infection or in immunosuppressed patients. In immunocompromised hosts, virologically confirmed PIV pneumonia has a 30-day attributable mortality rate of at least 30% to 35%.75,78,79 Unlike RSV lower tract disease, in which RSV is usually the single pathogen, copathogens may be identified with PIV pneumonia more than 50% of the time,75 and management of PIV pneumonia should include workup and treatment for copathogens.
Other respiratory viruses that can cause pneumonia, particularly in young children and immunocompromised hosts, include human metapneumovirus, adenovirus, human rhinoviruses (HRVs), and human coronaviruses (HCoVs). Since it was first described in 2001,80 human metapneumovirus has been shown to be a common cause of croup, bronchiolitis, and pneumonia in children, the elderly, and immunocompromised patients.81–89 The clinical manifestations of human metapneumovirus are indistinguishable from RSV. Adenovirus pneumonia can occur as an isolated event or as part of disseminated disease. Risk factors for adenovirus pneumonia include a compromised immune function, chronic underlying respiratory or cardiac disease, and age younger than 7 years.90 Adenovirus pneumonia due to specific serotypes has been associated with outbreaks among young children (types 3 and 7),91 high school students (type 11),92and a recent community-based outbreak that included children (type 14).93 HRVs occasionally cause lower respiratory tract disease requiring admission to intensive care among pediatric and immunosuppressed patients, although a causative role is sometimes difficult to define because HRVs frequently occur in association with copathogens.94–98 Among pediatric patients, a new species of rhinovirus (HRV-C) has been discovered that may cause more severe disease than HRV-A or HRV-B, although this association is not yet clearly elucidated.99–104
The HCoV family currently includes five viruses that are known to infect humans: HCoV-229E, HCoV-OC43, the severe acute respiratory syndrome-associated CoV (SARS-CoV), and the recently described HCoV-NL63 and HCoV-HKU1. The SARS outbreak originated in Guangdong Province in China in the fall of 2002 and was characterized by a life-threatening, atypical pneumonia caused by a novel coronavirus most likely derived from masked palm civets.105 SARS-CoV was spread by close contact with infected humans, mostly to household contacts and health care workers. Death from progressive respiratory failure occurred in approximately 10% to 15% of adult patients106–110; in children, morbidity was less and no deaths occurred.111–113 SARS-CoV is not currently circulating in the world. The most recent human cases of SARS-CoV infection were reported in China in April 2004 in an outbreak resulting from laboratory-acquired infections.114 Should SARS-CoV re-emerge, updates to the case definition, including clinical criteria for moderate or severe respiratory illness of unknown cause and epidemiologic criteria for exposure, can be found at www.cdc.gov/SARS. Other HCoVs have been reported to cause pneumonia in children and immunocompromised patients treated for hematologic malignancies.115–119
Additional information about respiratory viruses including RSV, influenza, PIV, and adenovirus is available in Chapter 47.
Although CMV usually causes relatively benign disease in immunocompetent hosts, it is frequently severe and often fatal in immunocompromised hosts, including patients with acquired immunodeficiency syndrome (AIDS), malignancy, congenital immune deficiencies, and transplant recipients. CMV is a herpesvirus and, like other herpesviruses, it can cause latent infection in vascular endothelial cells, monocytes and macrophages, polymorphonuclear neutrophils, and renal and pulmonary epithelial cells. Cellular damage is caused directly by the viral lytic infection or indirectly by the immune response of the host. Among allogeneic stem cell transplant recipients, the risk of CMV pneumonia is high; historically, treatment of CMV pneumonia with ganciclovir and intravenous immunoglobulin (IVIG) in the late 1980s dramatically reduced mortality from 80% to 100% without therapy to approximately 50%.120 The spectrum of CMV pneumonia has changed with the introduction of routine antiviral prophylaxis and preemptive therapy strategies.121 As a result, CMV disease during the first 3 months after hematopoietic cell transplantation has been reduced from 20% to 30% to less than 5% in most studies.122 CMV disease now primarily occurs late after transplant. Risk factors for late CMV disease include reactivation of (or primary infection with) CMV during the early period after transplant, and therapy for acute or chronic graft-versus-host disease.120 Among solid organ transplant recipients, the risk of CMV disease is greatest for lung transplant recipients, followed by liver, heart, and renal transplant recipients.120,123
Hantaviruses are known for causing hemorrhagic fevers and acute severe respiratory infection in young adults. Hantaviruses can spread from mammal to mammal, including humans, by exposure to aerosolized feces, infected urine, or other secretions. In the United States, the Sin Nombre virus, which causes the pulmonary syndrome, is found in 10% to 80% of deer mice in rural areas of North America, although the overall seroprevalence rates in the western United States are less than 0.1%.124 In the United States, hantaviral infections are rare in children under the age of 10 years; however, severe cases resulting in death have been described in children as young as 5 years old in South America.124,125 Hantaviruses cause disease by creating leakage of plasma and erythrocytes through the vascular endothelium in the lung (hantavirus pulmonary syndrome [HPS]) or the kidneys (hemorrhagic fever with renal syndrome). The differential diagnosis includes influenza A, rickettsial disease, borreliosis, tularemia, Legionella pneumophila, Chlamydia pneumoniae, Mycoplasma pneumoniae and Pneumocystis jiroveci. HPS is fatal in 35% to 40% of cases across the Americas.124,126 Supportive care, including early consideration of extracorporeal membrane oxygenation, is key to treatment of patients with HPS.
Central Nervous System Infections
Background
Aseptic meningitis, encephalitis, and myelitis are inflammatory conditions of the central nervous system (CNS), involving meninges, brain, and spinal cord, respectively. Disease is caused by a variety of infectious pathogens, but viruses cause most disease. Viruses gain entry to the CNS via the bloodstream (enteroviruses and arboviruses) or by direct neuronal spread (HSV and rabies). Pathogenesis may involve direct viral invasion or a vigorous virus-specific immune response resulting in damage to the neurons and supporting cells. Alternatively, infection may trigger activation of an immune response specific for the oligodendroglia or the myelin components themselves. In the latter case, disease may follow an upper respiratory tract or other infection and primarily take the form of a demyelinating process. This disease is commonly termed postinfectious encephalomyelitis or acute disseminated encephalomyelitis.
Cause
The potential viral causes are multiple; however, enteroviruses, herpesviruses, and arboviruses are responsible for most disease (see Table 95-1). Enteroviruses account for up to 99% of cases of aseptic meningitis when a cause is identified.127 Enterovirus meningitis in older children and adults is typically self-limited and associated with few complications. In contrast, enteroviral infections in neonates may mimic bacterial sepsis, and CNS involvement is often manifested as encephalitis. Parechovirus, another cause of meningoencephalitis in neonates, is a close relative of enteroviruses and clinically very similar.128
HSV is a common cause of CNS infection in individuals of all ages. During the neonatal period, HSV type 2, and to a lesser extent type 1, cause encephalitis because of the vertical transmission of the virus.129 In contrast, in older children and adults, most HSV encephalitis is caused by type 1. HSV-2, however, can cause benign aseptic meningitis in association with primary and recurrent genital infections.130 Other members of the herpesvirus family (CMV, EBV, VZV, and HHV-6) can also cause aseptic meningitis and encephalitis, albeit less commonly. CMV encephalitis occurs mostly in immunosuppressed individuals but may occasionally appear in otherwise healthy individuals.131,132 EBV aseptic meningitis and encephalitis present with or without the classic findings of infectious mononucleosis.133 Acute cerebellar ataxia is a common and usually benign complication of chickenpox. VZV encephalitis can sometimes occur in immunocompetent individuals,134,135 but more frequently occurs in immunocompromised individuals following days, weeks, or months after a case of varicella or zoster. Zoster encephalitis can be complicated by small- or large-vessel vasculitis (granulomatous arteritis), which carries the potentially serious consequences of infarction.134,136 HHV-6 has only rarely been reported to cause encephalitis in healthy children during primary infection,137 whereas it appears to be a more common problem for immunosuppressed patients, such as those receiving stem cell transplants.138,139
Arboviruses (arthropod-spread viruses) are important causes of aseptic meningitis and encephalitis. The specific arbovirus determines the epidemiology, morbidity, and risk of death of associated disease. The La Crosse and St. Louis encephalitis viruses account for most arboviral CNS infections in the United States. The La Crosse virus is found mainly in the Midwest, typically occurs in the summer and early fall, and is associated with a relatively low death rate. The St. Louis encephalitis virus occurs in every state but is more common in the Midwest, Florida, and Texas and has been responsible for large urban outbreaks.140,141 Eastern equine virus occurs less frequently, and mainly in the Northeast and Southeast, but carries a high rate of morbidity (70% to 80%) and death (20% to 80%).142,143 West Nile virus encephalitis first appeared in the United States in the summer of 1999 in New York state.144 Over the following summers, the West Nile virus moved southward and westward across the United States, infecting both animals and humans. Most individuals infected with the West Nile virus are symptom free or experience flulike illness; however, older individuals and those with underlying immune deficiency can experience encephalitis that may result in death. In addition to the more typical presentation of encephalitis, an acute flaccid paralysis has also been associated with West Nile virus infection.145
A number of viruses are infrequent causes of encephalitis, including mumps, influenza, and lymphocytic choriomeningitis viruses (LCMVs). Historically, mumps virus accounted for a large proportion of aseptic meningitis and encephalitis cases in the United States.146 Currently, because of the widespread use of the trivalent MMR vaccine, meningitis and encephalitis due to mumps are extremely rare.147 Influenza has been associated with encephalitis/encephalopathy, especially in Japan. In a national survey representing the 1998-1999 season, 142 cases, most occurring in children younger than 5 years, are reported.148 LCMV is an infrequently recognized cause of meningoencephalitis. This virus is found in the urine, droppings, and saliva of infected mice, guinea pigs, and hamsters, and disease in humans arises after exposure to these substances.
Postinfectious encephalomyelitis refers to an acute self-limited demyelinating process most commonly following viral respiratory infections and varicella. In contrast, subacute sclerosing panencephalitis (SSPE) and progressive multifocal leukoencephalopathy (PML) are two chronic, usually fatal, demyelinating diseases due to measles and JC virus, respectively. SSPE most commonly follows 5 to 10 years after natural measles infection. SSPE is extremely rare in the United States; however, it may occur as often as one case per a population of 10,000 in areas of the world where the MMR vaccine is not widely used.149 PML is also rare, usually affecting those with AIDS or, rarely, those with other serious immunodeficiencies. Transverse myelitis has been most frequently associated with enteroviruses; however, VZV,150,151 CMV, influenza A,152 and hepatitis A153 have been reported causes, even in immunologically normal individuals.
Clinical Presentation
Historic clues and physical findings can be helpful in focusing the search for an etiologic agent. Travel or residence in areas where arboviruses are endemic during the appropriate season for arthropod transmission (typically summer months) and a history or evidence of insect bites should raise suspicion for arboviruses. Seasonality also plays a role in enteroviral diseases, because in temperate climates enteroviruses are more prevalent during summer and fall months. History of a mother with recent symptoms consistent with viral illness (fever, sore throat, gastroenteritis, rash) should raise suspicion of enterovirus in a neonate with encephalitis or sepsis-like illness. VZV encephalitis and myelitis typically follow chickenpox or zoster by weeks to months and commonly occur in older individuals or those with immunosuppression, such as transplant recipients.154 VZV encephalitis may be complicated by CNS vasculopathy and resulting infarctions. Chronic encephalitis/meningitis due to enteroviruses occurs in individuals with agammaglobulinemia. Chronic or relapsing encephalitis may also be due to VZV, measles (SSPE), or rubella (progressive rubella panencephalitis), though the latter two are extremely rare with the current widespread use of the MMR vaccine. HIV itself may cause encephalopathy/encephalitis or may also be associated with certain opportunistic infections such as PML. Significant exposure to rodent droppings should raise concern for LCMV. Finally, history of exposure to a bat should raise the concern for rabies.
Cerebrospinal fluid (CSF) findings in aseptic meningitis typically include a normal glucose level, a normal to slightly elevated protein level, and a pleocytosis of up to 1000 cells/μL. The pleocytosis is classically monocytic (>80%); however, there can be an initial predominance of polymorphonuclear cells in the first 48 hours of illness.155 CSF findings in encephalitis can be normal, or there may be pleocytosis and elevated protein levels. The results of brain computed tomography (CT) and MRI studies are usually normal in viral meningitis, whereas disease is often seen in the setting of viral encephalitides. In general, CT scan is relatively insensitive for detecting acute encephalitis. MRI is the more sensitive study for detecting disease because of its ability to detect altered water content (see Chapter 56).156 In acute viral encephalitis, early findings include edema with minimal contrast enhancement. As disease progresses, edema and enhancement become more obvious and may be accompanied by mass effect, hemorrhagic changes, and necrosis. As the inflammation resolves, atrophy may become prominent. In HSV, imaging studies may reveal edema and enhancement, often first involving the temporal lobes with subsequent spread to other areas of the brain. Changes can ultimately progress to atrophy, multicystic encephalomalacia, and gyriform high attenuation, especially in children.157,158 In postinfectious encephalomyelitis, the lesions may be seen throughout the CNS. The lesions are more readily elucidated by MRI and primarily involve the white matter, although gray matter may also be involved.
Exotic Viral Diseases
With both the increase in foreign travel and the threat of bioterrorism, the potential to treat a child with an exotic viral disease exists. Although discussion of these infections, which include Andes virus, B virus, monkeypox, and the hemorrhagic fever viruses (Ebola virus, Marburg virus, Lassa virus, Crimean-Congo hemorrhagic fever virus, Argentine hemorrhagic fever virus, Bolivian hemorrhagic fever virus) is beyond the scope of this chapter, these infections should be kept in mind. If one of these agents is suspected, then the patient and patient garments should be contained in a single room, and infection control, infectious disease specialists, or the public health department should be called immediately.159–161
Diagnosing Viral Disease
If a viral cause is suspected, a few diagnostic studies can be performed immediately. Acute-phase serum should be held for later interpretation. It is critical that this specimen is drawn before administration of IVIG or blood products. Samples for viral cultures and PCR testing should be collected from the appropriate sites with Dacron or rayon swabs with plastic shafts (or other specific swabs appropriate for testing by PCR). Both cotton and wood inhibit viral growth and may contain substances that inhibit the enzymes used in PCR. The virology laboratory should be informed of the diagnosis or suspected pathogens because the cell lines chosen for inoculation vary by what virus is suspected. Nasal washes/swabs and swabs of the base of a vesicle or ulcer (for VZV, HSV) should include good cellular content, because fluorescent antibody assays stain cells and the more cells available, the more sensitive the assay. Table 95-2 outlines appropriate samples and testing for a number of specific viral pathogens.
Table 95–2 Potential Diagnostic Tests and Corresponding Specimens for Diagnosis of Viral Pathogens
| Viral Agent | Specimen | Recommended Diagnostic Tests∗ |
|---|---|---|
| Adenovirus |
Choice of test depends on clinical setting, including organ system involved and immune status of host.
NP, Nasopharyngeal secretions; OP, oropharyngeal; CSF, cerebrospinal fluid; BAL, bronchoalveolar lavage; FA, fluorescence assay; EIA, enzyme immunoassay; IA, immunoassay; ID, infectious disease; CPE, cytopathic effect; EEE, Eastern equine encephalitis; SLE, St. Louis encephalitis; WEE, Western equine encephalitis.
∗ Multiple diagnostic tests are available for each pathogen. Commonly recommended diagnostic tests are listed; however, if results are negative or specimens are not available, infectious disease consultation may be helpful for additional or special testing.
† PCR is usually run on plasma, though some laboratories may run serum samples.
‡ Pathogen may have significant public health implications and testing should be performed in consultation with infectious disease and/or local public health department (for enteroviruses, if enterovirus 71 suspected). Testing is often not available without assistance of the Public Health Department, and recommended specimens and tests are frequently evolving; see www.cdc.gov/ for updates.
§ IgM and IgG antibody may also be referred to as “serology” on laboratory request forms. For all viral pathogens, when testing for IgG it is optimal to collect acute and convalescent sera approximately 4 weeks apart.
‖ IgM antibody does not cross the blood-brain barrier. If found in CSF, IgM antibody denotes central nervous system infection.
¶ For suspected SARS, patients should also be evaluated with respiratory FA for influenza A, B, and RSV, sputum Gram stain and culture, blood culture, and urine for Legionella and pneumococcal antigen. Blood, serum, and respiratory specimens should be saved. See www.cdc.gov/ncidod/sars/diagnosis.htm for updated diagnostic information.
∗∗ Enteroviruses are shed in the stool for weeks and may not be diagnostic.
‡‡ FA and rapid testing have low sensitivity for 2009 novel influenza A (H1N1); PCR testing is the most sensitive and is necessary for subtyping.
§§ Because shedding of CMV occurs in the lungs of seropositive stem cell transplant recipients without overt CMV disease, the recovery of CMV DNA by PCR from BAL fluid (which is considerably more sensitive than culture) without shell vial or culture positivity is of uncertain significance. PCR for CMV DNA in BAL or biopsy fluid should thus not be ordered routinely.
Myocarditis
Isolation of virus from the myocardium provides a definite viral diagnosis of myocarditis; however, recovery of viruses from the myocardium by culture is rarely possible, even in cases of histologically proven myocarditis. Controversy now exists as to whether endomyocardial biopsy and histologic confirmation will continue to be recommended in myocarditis given the availability of cardiac MRI and the risks associated with biopsy.162 Viral culture of peripheral specimens such as stool and nasopharyngeal secretions or the demonstration of a fourfold rise in specific viral antibody titers provides an indirect determination of causality; however, the sensitivity is also low, 16% to 26%15,163 and 30% to 40%, respectively. Molecular biologic techniques such as PCR and in situ hybridization have expanded the number of viruses implicated in the etiology of myocarditis. In addition, because of the increased sensitivity of PCR, the application of PCR for viral nucleic acid in myocardial tissue provides a virologic diagnosis in up to 60% of cases.15
Acute Liver Failure
Viral diagnosis relies on serology, detection of viral nucleic acid in serum, and detection of viral antigens or nucleic acids in tissue obtained from liver biopsy. Hepatitis A infection is confirmed by demonstrating anti-HAV IgM antibodies. In patients with acute hepatitis A, anti-HAV IgM antibodies are detectable in the serum at the onset of symptoms, peak 1 week after onset of symptoms, and become undetectable by 3 to 6 months postinfection. The presence of HBsAg (hepatitis B surface antigen) in serum indicates active HBV replication and is present in acute and chronic HBV infection. Due to the destruction of actively infected hepatocytes, HBsAg may be absent in ALF and the only marker of acute HBV infection may be anti-HBcAb (anti-HBV core) IgM antibodies. Hepatitis B DNA can also be demonstrated in serum and liver tissue by PCR. Absence of HBsAg or HBV-DNA in the serum does not rule out HBV as the cause of ALF, as HBV DNA has been demonstrated in liver tissue of patients with non-A and non-B ALF in whom serologic markers did not suggest HBV infection.164 Hepatitis D, a hepatotropic virus that causes infection only in the presence of active hepatitis B infection, should be looked for in patients with acute HBV hepatitis, as coinfection or superinfection with HDV may result in more severe disease.165 Hepatitis D coinfection can be determined by demonstrating anti-HDV antibodies or HDV-RNA in serum.166
Although the newer generation antibody assays for hepatitis C are more sensitive than past assays, anti-HCV antibodies may not be detectable early in disease. Therefore, when the epidemiology suggests possible infection with HCV, serum and liver tissue should be analyzed for HCV-RNA by PCR.
Pneumonia/Pneumonitis
If available, fluorescence assays or PCR testing on nasal wash specimens are the initial diagnostic tests of choice for respiratory viruses, because most of these pathogens are concentrated in the nasopharynx. However, by the time the patient has developed lower respiratory tract disease, a lower respiratory sample by bronchoscopy may provide the best yield and may be positive even with a negative nasopharyngeal sample. The sensitivity of indirect immunofluorescence assays is generally as high as 90% to 95% for RSV, influenza A and B, and PIV 1, 2, and 3; if a rapid result is needed, this may be the best approach. However, PCR may be used if the results can be provided in an expedited fashion, as this is a more sensitive assay albeit more expensive. A rapid antigen test for RSV and influenza is also available. However, for the pandemic 2009 influenza A (H1N1) virus, in particular, FA and rapid testing have shown low sensitivity and a negative test result is not reliable.167–170 PCR testing is most sensitive and necessary for correct virus subtype identification. Adenovirus immunofluorescence assays are available in some laboratories, but sensitivity is generally lower (around 50% to 70%). Shell vial assays can increase sensitivity, and although they are usually performed for CMV, they can also be performed for RSV and adenovirus. The same samples can be sent for culture in addition to immunofluorescence studies; this should be considered for immunocompromised and severely ill children with respiratory distress/failure of unclear etiology if PCR testing is not available. The diagnosis of hantavirus can be made by culture of the virus (which is difficult), PCR for viral RNA from serum, plasma, or tissues, or serologic testing. Antibodies to hantaviruses are usually detectable in serum on the first day of symptoms.124 A review of diagnostic testing options for etiologies of viral pneumonia is provided in Table 95-2.
Meningitis/Encephalitis
CSF, blood, and throat swabs should be collected for evaluation. One can make a diagnosis of enterovirus by culturing the virus from CSF or by detecting virus in CSF using reverse transcriptase PCR. Because of the greater sensitivity of PCR, compared with culture,171,172 it should be used whenever possible. Viral culture of a throat swab may also reveal enterovirus and is indicative of a current or recent infection. Rectal or stool viral cultures are less helpful because enteroviruses may be shed in the stool for weeks after infection. DNA PCR of CSF offers relatively sensitive and specific diagnosis of herpesviruses.132 Detection of viral-specific antibodies in the CSF can add supporting evidence. Additionally, the detection of HHV-6 DNA in plasma or serum by PCR confirms active systemic viral replication. Arboviruses are typically diagnosed through detection of antibodies in acute and convalescent serum specimens. CSF may also be tested for antibodies. PCR and immunohistochemistry have also been used to diagnose arboviral infections and are available in some settings. Diagnosis of LCMV is made through serologic testing. The JC virus can be detected in CSF with PCR, and this appears to be a relatively sensitive and specific method for diagnosing PML.173,174 Definitive diagnosis, however, is usually made with brain biopsy. Diagnosis of SSPE is made with the evaluation of CSF for oligoclonal bands, IgG level, and specific measles antibody titer.
Treatment for Viral Infections
In general, for most life-threatening viral infections the primary treatment is supportive. Because of improvements in intensive medical care, death from these illnesses has decreased even without the availability of specific antiviral therapy. Despite recent advances, there are no effective antiviral medications for many viral infections. There are, however, antivirals for most of the herpes group viruses and many of the respiratory viruses. For most infections, the efficacy of antiviral therapy is decreased if therapy is delayed, so early diagnosis and rapid initiation of therapy are essential. Consultation with an infectious disease specialist is recommended because some antiviral agents are not commercially available and new treatment modalities continue to be identified. A listing of antiviral agents, indications, and dosages is provided in Table 95-3.
| Virus | Drug of Choice/Dose† | Alternate Agents/Dose |
|---|---|---|
| Adenovirus | There is no currently approved therapy for the treatment of adenoviral infections. | Both ribavirin and cidofovir have in vitro activity against adenovirus, and cidofovir appears to be more clinically active.201 Antiviral therapy may be considered for immunocompromised patients with severe adenoviral pneumonia. Small case series in immunocompromised children have suggested potential efficacy with intravenous ribavirin (25 mg/kg loading dose then 10 mg/kg/day; available on compassionate use basis)202 or cidofovir (5 mg/kg once weekly203,204 or 1 mg/kg three times a week).205 |
| Coronavirus | There is no currently approved therapy for the treatment of coronaviral infections. | The sudden and severe nature of the SARS outbreak in 2002–2003 necessitated the use of empiric treatment strategies. A number of agents have been used to treat SARS-CoV, including ribavirin, lopinavir-ritonavir, oseltamivir, and corticosteroids; however, none was given in a controlled fashion, and the efficacy of these drugs has not been established.201,206 |
| Enterovirus | There is no currently approved therapy for the treatment of enteroviral infections. | |
| Hantavirus | There is no currently approved therapy for the treatment of hantaviral infections. | Intravenous ribavirin has shown benefit in hantavirus renal syndrome,207,208 but not in hantavirus pulmonary syndrome at the cardiopulmonary stage.209,210 There is an ongoing NIH/NIAID-sponsored controlled trial of intravenous methylprednisolone for hantavirus pulmonary syndrome in Chile.126 |
| HERPES VIRUSES | ||
| • CMV | Ganciclovir (5 mg/kg q12h × 2–3 weeks, then 5 mg/kg q24h) is primary therapy for CMV disease. IVIG (500 mg/kg qod × 2 wk then once weekly) or CMV-IG (150 mg/kg, same schedule) should be given concurrently for CMV pneumonia in immunocompromised patients. | Foscarnet (90 mg/kg q12h × 2–3 weeks, then 90 mg/kg q24h), cidofovir (5 mg/kg/wk; high risk of renal toxicity, use with probenecid and saline hydration). Increased efficacy of cidofovir as second-line therapy suggested in allogeneic stem cell transplant recipients with CMV pneumonia in one small study.211 |
| • HSV | Acyclovir (20 mg/kg/dose IV q8h) for encephalitis in neonates and children <12 years and for neonates with disseminated disease; 10 mg/kg/dose IV q8h for children >12 years. | No specific dosing recommendations are available for HSV-associated hepatitis and pneumonitis. At least 10 mg/kg/dose should be considered outside the neonatal period. |
| • HHV-6 | There is no currently approved therapy for the treatment of HHV-6 infections. | Foscarnet and ganciclovir have in vitro activity. Case reports and series show variable clinical response with one or both drugs in combination. |
| • VZV | Acyclovir (10-12 mg/kg/dose IV q8h); high-dose acyclovir (20 mg/kg/dose) should be used for VZV encephalitis or for disease in immunocompromised children. | |
| Influenza A/B |
Oseltamivir (2 mg/kg q12h × 5 days; max 75 mg bid)∗ or zanamivir (≥7 years) 10 mg (2 oral inhalations) q12h × 5 days.
|
Local circulating influenza viruses must be considered. Combination therapy as empiric treatment may be indicated during periods of concomitant circulating viruses or for severely ill immunosuppressed patients.65,194 The use of investigational agents such as IV zanamivir or IV peramivir may also be considered.195–198
|
| JCV | No effective therapy. | In HIV infection, treatment with combination antiretroviral therapy may improve survival. Potential role for cidofovir.213 |
| Metapneumovirus | There is no currently approved therapy for the treatment of metapneumoviral infections. | Ribavirin has in vitro activity against human metapneumovirus and has been shown to decrease viral load and lung inflammation in mouse models.214,215 Case reports suggest ribavirin and IVIG may be used successfully in immunosuppressed patients.216–218 |
| Parechoviruses | There is no currently approved therapy for the treatment of parechoviral infections. | |
| Parainfluenza virus | There is no currently approved therapy for the treatment of parainfluenza viral infections. |
Ribavirin is active in vitro and in animal models and has thus been used for treatment of parainfluenza pneumonia in immunocompromised hosts. Anecdotal reports of the benefit of aerosolized or systemic ribavirin have shown responses to be highly variable, and a retrospective series of stem cell transplant recipients showed no benefit.75,201
|
| RSV | Aerosolized ribavirin (6 g reconstituted in 100 mL tid or 6 g in 300 mL administered over 12–18 hours daily) × 5 days has been used with modest efficacy in patients with severe RSV pneumonia and in immunocompromised patients201,219; not recommended for uncomplicated disease. | Combination therapy with aerosolized ribavirin and palivizumab (RSV monoclonal antibody, 15 mg/kg given once) may improve outcome of RSV pneumonia in immunocompromised and high-risk patients and may be used in combination for treatment of documented RSV pneumonia in these populations.65,219,220 |
IVIG, Intravenous immunoglobulin.
∗ Care should be taken when dosing children <1 year, with doses of 3 to 3.5 mg/kg recommended. In an influenza pandemic or some outbreak situations, treatment should not wait for laboratory confirmation of influenza because laboratory testing can delay treatment and because a negative rapid test for influenza does not rule out influenza. The sensitivity of rapid tests in detecting 2009 H1N1 ranged from 10% to 70%.
† These agents are generally recommended with infectious disease consultation for the infection listed. However, please note that not all these agents are FDA approved for the indicated use.
Myocarditis
Mechanical circulatory support should be considered for children with fulminant myocarditis unresponsive to standard management (see Chapter 27). Aggressive therapy is warranted because both adults and children who survive their illness have a good prognosis for return to normal ventricular function.175–177 Cardiac transplantation may be necessary for those children refractory to other management. Current recommendations do not support the use of immunosuppressive therapy22,178 or nonsteroidal antiinflammatory agents,179,180 particularly early in the course of myocarditis. Treatment with high-dose IVIG has been associated with improved left ventricular function in several small studies in children181,182and warrants investigation in larger, controlled studies. In adults, a controlled study found IVIG to be no better than placebo for acute dilated cardiomyopathy182 and a Cochrane Review found no role for the routine use of IVIG in presumed viral myocarditis.183 Several immune modulators are being investigated for use in treatment of myocarditis.179,184 Specific antiviral therapy is indicated when the inciting viral agent has been identified.
Acute Liver Failure
The role of antiviral therapy in ALF is limited. Acyclovir should be initiated if HSV is suspected or confirmed, and there are reports of the successful use of lamivudine for treatment of severe acute hepatitis B.185,186
Trials using plasma exchange and plasmapheresis have demonstrated improved hemodynamic parameters, decreased intracranial pressure, and improved survival.187,188 Experimental therapies such as N-acetylcysteine for non–acetaminophen-induced ALF, hepatocyte transplantation, and artificial hepatic support systems have shown promise in early studies.188–190 For a detailed discussion of the management of ALF,52 see Chapter 88.
Pneumonitis
The cornerstone of treatment remains supportive with supplemental oxygen, fluids, bronchodilators, and mechanical ventilation. Corticosteroids are generally of no proven benefit in viral-mediated pneumonia, and the data regarding systemic glucocorticoids among infants and young children with bronchiolitis show no benefit compared with placebo.191,192 The use of empiric broad-spectrum antibiotics may be important until a diagnosis can be established, and because some viral infections such as PIVs, HRVs, and coronaviruses may occur in the context of a bacterial coinfection. For certain immunocompromised patients, fungal copathogens must also be considered. Early isolation and infection control measures for suspected viral infections should be implemented.
A number of antiviral strategies have been investigated or employed on an anecdotal basis to treat viral pneumonia, but the only FDA-approved agents include ribavirin for RSV and antivirals for influenza (oseltamivir, zanamavir, amantadine, and rimantadine). For influenza, the strain of local circulating viruses must be considered when planning a treatment regimen. Seasonal H3N2 viruses have shown almost universal resistance to adamantanes since the 2005–2006 season, and seasonal H1N1 viruses have shown rising oseltamivir resistance since 2008–2009.193 Seasonal H1N1 viruses typically remain sensitive to adamantanes and zanamavir. The reader is encouraged to consult http://www.cdc.gov/flu/ for national surveillance data on influenza viruses circulating in the United States. Combination therapy as empiric treatment may be indicated during periods of concomitant circulating viruses or for severely ill immunosuppressed patients.65,194 The use of investigational agents such as IV zanamivir or IV peramivir may also be considered.195–198 Table 95-3 provides a detailed summary of antiviral agents for various causes of viral pneumonia.
Encephalitis
Untreated, HSV encephalitis carries a death rate in excess of 70%199 and, even treated, death and complications for those who survive remain on the order of 15% and 20%, respectively.200 Similarly, despite treatment, neonatal HSV CNS disease carries significant risk of death and morbidity, ranging from 0% to 15% and 43% to 68%, respectively.129 Early identification of patients and rapid initiation of acyclovir have been associated with better outcome.199,200 Unless an alternative cause is clear, high-dose acyclovir should be initiated in all children with encephalitis until HSV can be ruled out. Other specific antiviral therapy may be directed as outlined in Table 95-3.
References are available online at http://www.expertconsult.com.
1. Pisani B., Taylor D.O., Mason J.W. Inflammatory myocardial diseases and cardiomyopathies. Am J Med. 1997;102(5):459-469.
2. Cooper L.T.Jr. Myocarditis. N Engl J Med. 2009;360(15):1526-1538.
3. Aretz H.T., Billingham M.E., Edwards W.D., et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1(1):3-14.
4. Gravanis M.B., Sternby N.H. Incidence of myocarditis. A 10-year autopsy study from Malmo. Sweden, Arch Pathol Lab Med. 1991;115(4):390-392.
5. Bergelson J.M., Cunningham J.A., Droguett G., et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275(5304):1320-1323.
6. Martino T.A., Petric M., Brown M., et al. Cardiovirulent coxsackieviruses and the decay-accelerating factor (CD55) receptor. Virology. 1998;244(2):302-314.
7. Tracy S., Hofling K., Pirruccello S., et al. Group B coxsackievirus myocarditis and pancreatitis: connection between viral virulence phenotypes in mice. J Med Virol. 2000;62(1):70-81.
8. Khatib R., Chason J.L., Lerner A.M. A mouse model of transmural myocardial necrosis due to coxsackievirus B4: observations over 12 months. Intervirology. 1982;18(4):197-202.
9. Badorff C., Lee G.H., Lamphear B.J., et al. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999;5(3):320-326.
10. Liu P.P., Mason J.W. Advances in the understanding of myocarditis. Circulation. 2001;104(9):1076-1082.
11. Maisch B., Ristic A.D., Hufnagel G., Pankuweit S. Pathophysiology of viral myocarditis: the role of humoral immune response. Cardiovasc Pathol. 2002;11(2):112-122.
12. Wong C.Y., Woodruff J.J., Woodruff J.F. Generation of cytotoxic T lymphocytes during coxsackievirus tb-3 infection. II. Characterization of effector cells and demonstration cytotoxicity against viral-infected myofibers. J Immunol. 1977;118(4):1165-1169.
13. Fairweather D., Frisancho-Kiss S., Rose N.R. Viruses as adjuvants for autoimmunity: evidence from Coxsackievirus-induced myocarditis. Rev Med Virol. 2005;15(1):17-27.
14. Bowles N.E., Ni J., Kearney D.L., et al. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42(3):466-472.
15. Martin A.B., Webber S., Fricker F.J., et al. Acute myocarditis. Rapid diagnosis by PCR in children. Circulation. 1994;90(1):330-339.
16. Mahrholdt H., Goedecke C., Wagner A., et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109(10):1250-1258.
17. Kaplan M.H., Klein S.W., McPhee J., et al. Group B coxsackievirus infections in infants younger than three months of age: a serious childhood illness. Rev Infect Dis. 1983;5(6):1019-1032.
18. Thomas J.A., Raroque S., Scott W.A. Successful treatment of severe dysrhythmias in infants with respiratory syncytial virus infections: two cases and a literature review. Crit Care Med. 1997;25(5):880-886.
19. Maron B.J., Doerer J.J., Haas T.S. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009;119(8):1085-1092.
20. Weber M.A., Ashworth M.T., Risdon R.A., et al. Clinicopathological features of paediatric deaths due to myocarditis: an autopsy series. Arch Dis Child. 2008;93(7):594-598.
21. Lieberman E.B., Hutchins G.M., Herskowitz A. Clinicopathologic description of myocarditis. J Am Coll Cardiol. 1991;18(7):1617-1626.
22. Mason J.W., O’Connell J.B., Herskowitz A., et al. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333(5):269-275.
23. Helin M., Savola J., Lapinleimu K. Cardiac manifestations during a Coxsackie B5 epidemic. Br Med J. 1968;3(5610):97-99.
24. Smith S.C., Ladenson J.H., Mason J.W., Jaffe A.S. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation. 1997;95(1):163-168.
25. Felker G.M., Boehmer J.P., Hruban R.H., et al. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36(1):227-232.
26. Friedrich M.G., Strohm O., Schulz-Menger J., et al. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97(18):1802-1809.
27. Laissy J.P., Messin B., Varenne O., et al. MRI of acute myocarditis: a comprehensive approach based on various imaging sequences. Chest. 2002;122(5):1638-1648.
28. Squires R.H.Jr., Shneider B.L., Bucuvalas J., et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148(5):652-658.
29. Narkewicz M.R., Dell Olio D., Karpen S.J., et al. Pattern of diagnostic evaluation for the causes of pediatric acute liver failure: An opportunity for quality improvement. J Pediatr. 2009;155(6):801-806.
30. Ciocca M., Moreira-Silva S.F., Alegria S., et al. Hepatitis A as an etiologic agent of acute liver failure in Latin America. Pediatr Infect Dis J. 2007;26(8):711-715.
31. Ciocca M., Ramonet M., Cuarterolo M. Prognostic factors in paediatric acute liver failure. Arch Dis Child. 2008;93(1):48-51.
32. Cochran J.B., Losek J.D. Acute liver failure in children. Pediatr Emerg Care. 2007;23(2):129-135.
33. Lee W.S., McKiernan P., Kelly D.A. Etiology, outcome and prognostic indicators of childhood fulminant hepatic failure in the United kingdom. J Pediatr Gastroenterol Nutr. 2005;40(5):575-581.
34. Samanta T., Ganguly S. Aetiology, clinical profile and prognostic indicators for children with acute liver failure admitted in a teaching hospital in Kolkata. Trop Gastroenterol. 2007;28(3):135-139.
35. Kong M.S., Chung J.L. Fatal hepatitis C in an infant born to a hepatitis C positive mother. J Pediatr Gastroenterol Nutr. 1994;19(4):460-463.
36. Taga T., Ikeda M., Suzuki K. Fulminant hepatitis caused by hepatitis C virus. Pediatr Infect Dis J. 1998;17(12):1174-1176.
37. Dwivedi M., Manocha H., Tiwari S. Coinfection of Parvovirus B19 with Other Hepatitis Viruses Leading to Fulminant Hepatitis of Unfavorable Outcome in Children. Pediatr Infect Dis J. 2009;28(7):649-650.
38. Sokal E.M., Melchior M., Cornu C., et al. Acute parvovirus B19 infection associated with fulminant hepatitis of favourable prognosis in young children. Lancet. 1998;352(9142):1739-1741.
39. Matsuzaki A., Suminoe A., Koga Y., et al. Fatal visceral varicella-zoster virus infection without skin involvement in a child with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2008;25(3):237-242.
40. Chevret L., Boutolleau D., Halimi-Idri N., et al. Human herpesvirus-6 infection: a prospective study evaluating HHV-6 DNA levels in liver from children with acute liver failure. J Med Virol. 2008;80(6):1051-1057.
41. Harma M., Hockerstedt K., Lautenschlager I. Human herpesvirus-6 and acute liver failure. Transplantation. 2003;76(3):536-539.
42. Dhawan A. Etiology and prognosis of acute liver failure in children. Liver Transpl. 2008;14(Suppl 2):S80-S84.
43. Durand P., Debray D., Mandel R., et al. Acute liver failure in infancy: a 14-year experience of a pediatric liver transplantation center. J Pediatr. 2001;139(6):871-876.
44. Verma A., Dhawan A., Zuckerman M. Neonatal herpes simplex virus infection presenting as acute liver failure: prevalent role of herpes simplex virus type I. J Pediatr Gastroenterol Nutr. 2006;42(3):282-286.
45. Chen H.L., Chang C.J., Kong M.S., et al. Pediatric fulminant hepatic failure in endemic areas of hepatitis B infection: 15 years after universal hepatitis B vaccination. Hepatology. 2004;39(1):58-63.
46. Peters D.J., Greene W.H., Ruggiero F., McGarrity T.J. Herpes simplex-induced fulminant hepatitis in adults: a call for empiric therapy. Dig Dis Sci. 2000;45(12):2399-2404.
47. Sevilla J., Fernandez-Plaza S., Gonzalez-Vicent M., et al. Fatal hepatic failure secondary to acute herpes simplex virus infection. J Pediatr Hematol Oncol. 2004;26(10):686-688.
48. Kumar R., Tripathi P., Tripathi S., et al. Prevalence of dengue infection in north Indian children with acute hepatic failure. Ann Hepatol. 2008;7(1):59-62.
49. Poovorawan Y., Hutagalung Y., Chongsrisawat V. Dengue virus infection: a major cause of acute hepatic failure in Thai children. Ann Trop Paediatr. 2006;26(1):17-23.
50. Dias L.B.Jr., Alves V.A., Kanamura C. Fulminant hepatic failure in northern Brazil: morphological, immunohistochemical and pathogenic aspects of Labrea hepatitis and yellow fever. Trans R Soc Trop Med Hyg. 2007;101(8):831-839.
51. Shakil A.O., Kramer D., Mazariegos G.V., et al. Acute liver failure: clinical features, outcome analysis, and applicability of prognostic criteria. Liver Transpl. 2000;6(2):163-169.
52. Squires R.H.Jr. Acute liver failure in children. Semin Liver Dis. 2008;28(2):153-166.
53. National Vital Statistics Report, Vol. 57, No. 14. Hyattsville, MD, 2009. National Center for Health Statistics, Centers for Disease Control and Prevention. U.S. Department of Health and Human Services.
54. Diederen B.M., Van Der Eerden M.M., Vlaspolder F. Detection of respiratory viruses and Legionella spp. by real-time polymerase chain reaction in patients with community acquired pneumonia. Scand J Infect Dis. 2009;41(1):45-50.
55. Falsey A.R. Community-acquired viral pneumonia. Clin Geriatr Med. 2007;23(3):535-552.
56. Johnstone J., Majumdar S.R., Fox J.D., Marrie T.J. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest. 2008;134(6):1141-1148.
57. Oosterheert J.J., van Loon A.M., Schuurman R., et al. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin Infect Dis. 2005;41(10):1438-1444.
58. Templeton K.E., Scheltinga S.A., van den Eeden W.C., et al. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41(3):345-351.
59. Henrickson K.J., Hoover S., Kehl K.S., Hua W. National disease burden of respiratory viruses detected in children by polymerase chain reaction. Pediatr Infect Dis J. 2004;23(1 Suppl):S11-S18.
60. Peck A.J., Holman R.C., Curns A.T., et al. Lower respiratory tract infections among american Indian and Alaska Native children and the general population of U.S. Children. Pediatr Infect Dis J. 2005;24(4):342-351.
61. Sinaniotis C.A. Viral pneumoniae in children: incidence and aetiology. Paediatr Respir Rev.. 2004;5(Suppl A):S197-S200.
62. Hall C.B., Weinberg G.A., Iwane M.K., et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588-598.
63. Hall C.B. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917-1928.
64. Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol. 2008;143(4):455-467.
65. Ison M.G. Respiratory syncytial virus and other respiratory viruses in the setting of bone marrow transplantation. Curr Opin Oncol. 2009;21(2):171-176.
66. Poehling K.A., Edwards K.M., Weinberg G.A., et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355(1):31-40.
67. Update: influenza activity—United States, August 30-October 31, 2009. MMWR Morb Mortal Wkly Rep 58(44):1236–41, 2009.
68. Nichols W.G., Guthrie K.A., Corey L., Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39(9):1300-1306.
69. Update: influenza activity—United States, April-August 2009, MMWR Morb Mortal Wkly Rep 58(36):1009–12, 2009.
70. Dawood F.S., Jain S., Finelli L., et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360(25):2605-2615.
71. Louie J.K., Acosta M., Winter K., et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302(17):1896-1902.
72. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May-August 2009. MMWR Morb Mortal Wkly Rep 58(38):1071–4, 2009.
73. Englund J.A.Diagnosis and epidemiology of community-acquired respiratory virus infections in the immunocompromised host Biol Blood Marrow Transplant 2001:Suppl 7;2S-4S
74. Iwane M.K., Edwards K.M., Szilagyi P.G., et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113(6):1758-1764.
75. Nichols W.G., Corey L., Gooley T. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98(3):573-578.
76. Schanzer D.L., Langley J.M., Tam T.W. Hospitalization attributable to influenza and other viral respiratory illnesses in Canadian children. Pediatr Infect Dis J. 2006;25(9):795-800.
77. Weinberg G.A., Hall C.B., Iwane M.K., et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr. 2009;154(5):694-699.
78. Lewis V.A., Champlin R., Englund J., et al. Respiratory disease due to parainfluenza virus in adult bone marrow transplant recipients. Clin Infect Dis. 1996;23(5):1033-1037.
79. Wendt C.H., Weisdorf D.J., Jordan M.C. Parainfluenza virus respiratory infection after bone marrow transplantation. N Engl J Med. 1992;326(14):921-926.
80. van den Hoogen B.G., de Jong J.C., Groen J., et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7(6):719-724.
81. Boivin G., Abed Y., Pelletier G., et al. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186(9):1330-1334.
82. Englund J.A., Boeckh M., Kuypers J., et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med. 2006;144(5):344-349.
83. Esper F., Boucher D., Weibel C. Human metapneumovirus infection in the United States: clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics. 2003;111(6 Pt 1):1407-1410.
84. Kahn J.S. Epidemiology of human metapneumovirus. Clin Microbiol Rev.. 2006;19(3):546-557.
85. Oliveira R., Machado A., Tateno A. Frequency of human metapneumovirus infection in hematopoietic SCT recipients during 3 consecutive years. Bone Marrow Transplant. 2008;42(4):265-269.
86. Peck A.J., Englund J.A., Kuypers J., et al. Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood. 2007;110(5):1681-1688.
87. Stempel H.E., Martin E.T., Kuypers J. Multiple viral respiratory pathogens in children with bronchiolitis. Acta Paediatr. 2009;98(1):123-126.
88. Stockton J., Stephenson I., Fleming D., Zambon M. Human metapneumovirus as a cause of community-acquired respiratory illness. Emerg Infect Dis. 2002;8(9):897-901.
89. Williams J.V., Harris P.A., Tollefson S.J., et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350(5):443-450.
90. Gray G.C., McCarthy T., Lebeck M.G., et al. Genotype prevalence and risk factors for severe clinical adenovirus infection, United States 2004-2006. Clin Infect Dis. 2007;45(9):1120-1131.
91. Hong J.Y., Lee H.J., Piedra P.A., et al. Lower respiratory tract infections due to adenovirus in hospitalized Korean children: epidemiology, clinical features, and prognosis. Clin Infect Dis. 2001;32(10):1423-1429.
92. Centers for Disease Control and Prevention:. Civilian outbreak of adenovirus acute respiratory disease–South Dakota, 1997. JAMA. 1998;280(7):596.
93. Lewis P.F., Schmidt M.A., Lu X. A community-based outbreak of severe respiratory illness caused by human adenovirus serotype 14. J Infect Dis. 2009;199(10):1427-1434.
94. Gerna G., Piralla A., Rovida F., et al. Correlation of rhinovirus load in the respiratory tract and clinical symptoms in hospitalized immunocompetent and immunocompromised patients. J Med Virol. 2009;81(8):1498-1507.
95. Ghosh S., Champlin R., Couch R., et al. Rhinovirus infections in myelosuppressed adult blood and marrow transplant recipients. Clin Infect Dis. 1999;29(3):528-532.
96. Ison M.G., Hayden F.G., Kaiser L., et al. Rhinovirus infections in hematopoietic stem cell transplant recipients with pneumonia. Clin Infect Dis. 2003;36(9):1139-1143.
97. Kusel M.M., de Klerk N.H., Holt P.G. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25(8):680-686.
98. Louie J.K., Roy-Burman A., Guardia-Labar L., et al. Rhinovirus associated with severe lower respiratory tract infections in children. Pediatr Infect Dis J. 2009;28(4):337-339.
99. Han T.H., Chung J.Y., Hwang E.S., Koo J.W. Detection of human rhinovirus C in children with acute lower respiratory tract infections in South Korea. Arch Virol. 2009;154(6):987-991.
100. Lau S.K., Yip C.C., Tsoi H.W., et al. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. 2007;45(11):3655-3664.
101. Linsuwanon P., Payungporn S., Samransamruajkit R., et al. High prevalence of human rhinovirus C infection in Thai children with acute lower respiratory tract disease. J Infect. 2009;59(2):115-121.
102. Piralla A., Rovida F., Campanini G., et al. Clinical severity and molecular typing of human rhinovirus C strains during a fall outbreak affecting hospitalized patients. J Clin Virol. 2009;45(4):311-317.
103. Tapparel C., L’Huillier A.G., Rougemont A.L., et al. Pneumonia and pericarditis in a child with HRV-C infection: a case report. J Clin Virol. 2009;45(2):157-160.
104. Xiang Z., Gonzalez R., Xie Z., et al. Human rhinovirus group C infection in children with lower respiratory tract infection. Emerg Infect Dis. 2008;14(10):1665-1667.
105. Shi Z., Hu Z. A review of studies on animal reservoirs of the SARS coronavirus. Virus Res. 2008;133(1):74-87.
106. Lee N., Hui D., Wu A., et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986-1994.
107. Peiris J.S., Chu C.M., Cheng V.C., et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767-1772.
108. Poutanen S.M., Low D.E., Henry B., et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348(20):1995-2005.
109. Schrag S.J., Brooks J.T., Van Beneden C., et al. SARS surveillance during emergency public health response, United States, March-July 2003. Emerg Infect Dis. 2004;10(2):185-194.
110. Tsang K.W., Ho P.L., Ooi G.C., et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1977-1985.
111. Hon K.L., Leung C.W., Cheng W.T., et al. Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet. 2003;361(9370):1701-1703.
112. Leung T.F., Wong G.W., Hon K.L., Fok T.F. Severe acute respiratory syndrome (SARS) in children: epidemiology, presentation and management. Paediatr Respir Rev. 2003;4(4):334-339.
113. Stockman L.J., Massoudi M.S., Helfand R., et al. Severe acute respiratory syndrome in children. Pediatr Infect Dis J. 2007;26(1):68-74.
114. Liang W.N., Zhao T., Liu Z.J., et al. Severe acute respiratory syndrome–retrospect and lessons of 2004 outbreak in China. Biomed Environ Sci. 2006;19(6):445-451.
115. Heugel J., Martin E.T., Kuypers J., Englund J.A. Coronavirus-associated pneumonia in previously healthy children. Pediatr Infect Dis J. 2007;26(8):753-755.
116. Kuypers J., Martin E.T., Heugel J., et al. Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics. 2007;119(1):e70-e76.
117. Oosterhof L., Christensen C.B., Sengelov H. Fatal lower respiratory tract disease with human corona virus NL63 in an adult haematopoietic cell transplant recipient. Bone Marrow Transplant. 2009.
118. Pene F., Merlat A., Vabret A., et al. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin Infect Dis. 2003;37(7):929-932.
119. Simon A., Volz S., Fleischhack G., et al. Human coronavirus OC43 pneumonia in a pediatric cancer patient with down syndrome and acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2007;29(6):432-434.
120. Ison M.G., Fishman J.A. Cytomegalovirus pneumonia in transplant recipients. Clin Chest Med. 2005;26(4):691-705.
121. Boeckh M., Nichols W.G., Papanicolaou G., et al. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9(9):543-558.
122. Boeckh M., Nichols W.G. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004;103(6):2003-2008.
123. Kotloff R.M., Ahya V.N., Crawford S.W. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2004;170(1):22-48.
124. Chang B., Crowley M., Campen M., Koster F. Hantavirus cardiopulmonary syndrome. Semin Respir Crit Care Med. 2007;28(2):193-200.
125. Overturf G.D. Clinical sin nombre hantaviral infections in children. Pediatr Infect Dis J. 2005;24(4):373-374.
126. Jonsson C.B., Hooper J., Mertz G. Treatment of hantavirus pulmonary syndrome. Antiviral Res. 2008;78(1):162-169.
127. Berlin L.E., Rorabaugh M., Heldrich F. Aseptic meningitis in infants <2 years of age: diagnosis and etiology. J Infect Dis. 1993;168:888-892.
128. Verboon-Maciolek M.A., Krediet T.G., Gerards L.J. Severe neonatal parechovirus infection and similarity with enterovirus infection. Pediatr Infect Dis J. 2008;27(3):241-245.
129. Kimberlin D.W., Lin C., Jacobs R.F., et al. Natural history of neonatal herpes simplex virus infections in the Acyclovir era. Pediatrics. 2001;108:223-229.
130. Tedder D.G., Ashley R., Tyler K.L., Levin M.J. Herpes simplex virus infection as a cause of benign recurrent lymphocytic meningitis. Ann Intern Med. 1994;121:334-338.
131. Arribas J.R., Storch G.A., Clifford D.B., Tselis A.C. Cytomegalovirus encephalitis. Ann Intern Med. 1996;125:577-587.
132. Prosch S., Schielke E., Reip A., et al. Human cytomegalovirus (HCMV) encephalitis in an immunocompetent young person and diagnostic reliability of HCMV DNA PCR using cerebrospinal fluid of nonimmunosuppressed patients. J Clin Microbiol. 1998;36:3636-3640.
133. Domachowske J.B., Cunningham C.K., Cummings D.L. Acute manifestations and neurologic sequelae of Epstein-Barr virus encephalitis in children. Pediatr Infect Dis J. 1996;15:871-875.
134. Caruso J.T., Tung G.A., Brown W.D. Central nervous system and renal vasculitis associated with primary varicella infection in a child. Pediatrics. 2001;107:E9.
135. Hausler M., Schaade L., Kemeny S. Encephalitis related to primary varicella-zoster virus infection in immunocompetent children. J Neurol Sci. 2002;195:111-116.
136. Kuroiwa Y., Furukawa T. Hemispheric infarction after herpes zoster ophthalmicus: computed tomography and angiography. Neurology. 1981;31:1030-1032.
137. Ahtiluoto S., Mannonen L., Paetau A., et al. In situ hybridization detection of human herpesvirus 6 in brain tissue from fatal encephalitis. Pediatrics. 2000;105:431-433.
138. Tiacci E., Luppi M., Barozzi P., et al. Fatal herpesvirus-6 encephalitis in a recipient of a T-cell depleted peripheral blood stem cell transplant from a 3-loci mismatched related donor. Haematologica. 2000;85:94-97.
139. Zerr D.M., Gupta D., Huang M.L. Effect of antivirals on human herpesvirus 6 replication in hematopoietic stem cell transplant patients. Clin Infect Dis. 2002;34:309-314.
140. Arboviral disease–United States, 1994. MMWR Morb Mortal Wkly Rep. 1995;44(35):641-644.
141. Arboviral infections of the central nervous system–United States, 1996-1997. MMWR Morb Mortal Wkly Rep. 1998;47(25):517-522.
142. Lowry P.W. Arbovirus ecephalitis in the United States and Asia. J Lab Clin Med. 1997;129:405-411.
143. Whitley R.J. Viral encephalitis. N Engl J Med. 1990;323:242-250.
144. Nash D., Mostashari F., Fine A., et al. The outbreak of West Nile virus infection in the New York area in 1999. N Engl J Med. 2001;344:1807-1814.
145. Acute flaccid paralysis syndrome associated with West Nile virus infection–Mississippi and Louisiana, July-August 2002. MMWR Morb Mortal Wkly Rep 51(37):825–8, 2002.
146. Forsey T. Mumps vaccines–current status. J Med Microbiol. 1994;41(1):1-2.
147. Mumps prevention. MMWR Morb Mortal Wkly Rep. 1989;38(22):388-392.
148. Morishima T., Togashi T., Yokota S., et al. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis. 2001;35:512-517.
149. Kondo K., Takasu T., Ahmed A. Neurological diseases in Karachi, Pakistan–elevated occurrence of subacute sclerosing panencephalitis. Neuroepidemiology. 1988;7(2):66-80.
150. Celik Y., Tabak F., Mert A. Transverse myelitis caused by varicella. Clin Neurol Neurosurg. 2001;103:260-261.
151. Gilden D.H., Beinlich B.R., Rubinstien E.M., et al. Varicella-zoster virus myelitis: an expanding spectrum. Neurology. 1994;44:1818-1823.
152. Salonen O., Koshkiniemi M., Saari A., et al. Myelitis associated with influenza A virus infection. J Neurovirol. 1997;3:83-85.
153. Tyler K.L., Gross R.A., Cascino G.D. Unusual viral causes of transverse myelitis: Hepatitis A virus and cytomegalovirus. Neurology. 1986;36:855-858.
154. Koc Y., Miller K.B., Schenkein D.P., et al. Varicella zoster virus infections following allogenic bone marrow transplantation: frequency, risk factors, and clinical outcome. Biol Blood Bone Marrow Trans. 2000;6:44-49.
155. Feigin R.D., Shackelford P.G. Value of repeat lumbar puncture in the differential diagnosis of meningitis. N Engl J Med. 1973;289(11):571-574.
156. Tarr R.W., Edwards K.M., Kessler R.M., Kulkarni M.V. MRI of mumps encephalitis: comparison with CT evaluation. Pediatr Radiol. 1987;17:59-62.
157. Noorbehesht B., Enzmann D.R., Sullender W. Neonatal herpes simplex encephalitis: correlation of clinical and CT findings. Radiology. 1987;162:813-819.
158. Shaw D.W., Cohen W.A. Viral infections of the CNS in children: imaging features. AJR. 1993;160:125-133.
159. Management of patients with suspected viral hemorrhagic fever. MMWR Morb Mortal Wkly Rep. 1988;37(Suppl 3):1-16.
160. Update management of patients with suspected viral hemorrhagic fever–United States. MMWR Morb Mortal Wkly Rep. 1995;44(25):475-479.
161. Weber D.J., Rutala W.A. Risks and prevention of nosocomial transmission of rare zoonotic diseases. Clin Infect Dis. 2001;32(3):446-456.
162. Vashist S., Singh G.K. Acute myocarditis in children: current concepts and management. Curr Treat Options Cardiovasc Med. 2009;11(5):383-391.
163. Sainani G.S., Dekate M.P., Rao C.P. Heart disease caused by Coxsackie virus B infection. Br Heart J. 1975;37(8):819-823.
164. Fukai K., Yokosuka O., Fujiwara K., et al. Etiologic considerations of fulminant non-A, non-B viral hepatitis in Japan: analyses by nucleic acid amplification method. J Infect Dis. 1998;178(2):325-333.
165. Hoofnagle J.H. Type D (delta) hepatitis. JAMA. 1989;261(9):1321-1325.
166. Tang J.R., Cova L., Lamelin J.P., et al. Clinical relevance of the detection of hepatitis delta virus RNA in serum by RNA hybridization and polymerase chain reaction. J Hepatol. 1994;21(6):953-960.
167. Performance of rapid influenza diagnostic tests during two school outbreaks of 2009 pandemic influenza A (H1N1) virus infection—Connecticut, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(37):1029-1032.
168. Drexler J.F., Helmer A., Kirberg H., et al. Poor clinical sensitivity of rapid antigen test for influenza A pandemic (H1N1) 2009 virus. Emerg Infect Dis. 2009;15(10):1662-1664.
169. Ginocchio C.C., Zhang F., Manji R., et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol. 2009;45(3):191-195.
170. Vasoo S., Stevens J., Singh K. Rapid antigen tests for diagnosis of pandemic (swine) influenza A/H1N1. Clin Infect Dis. 2009;49(7):1090-1093.
171. Carroll K.C., Taggart B., Robison J., et al. Evaluation of the Roche AMPLICOR enterovirus PCR assay in the diagnosis of enteroviral central nervous system infections. J Clin Virol. 2000;19:149-156.
172. Sawyer M.H., Holland D., Aintablian N. Diagnosis of enteroviral central nervous system infection by polymerase chain reaction during a large community outbreak. Pediatr Infect Dis J. 1994;13:177-182.
173. Bogdanovic G., Priftakis P., Hammarin A.L., et al. Detection of JC virus in cerebrospinal fluid (CSF) samples from patients with progressive multifoccal leukoencephalopathy but not in CSF samples from patients with herpes simples encephalitis, enteroviral meningitis, or multiple sclerosis. J Clin Microbiol. 1998;36:157-163.
174. Koralnik I.J., Boden D., Mai V.X., et al. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999;52:253-260.
175. Amabile N., Fraisse A., Bouvenot J., et al. Outcome of acute fulminant myocarditis in children. Heart. 2006;92(9):1269-1273.
176. Duncan B.W., Bohn D.J., Atz A.M., et al. Mechanical circulatory support for the treatment of children with acute fulminant myocarditis. J Thorac Cardiovasc Surg. 2001;122(3):440-448.
177. McCarthy R.E.3rd, Boehmer J.P., Hruban R.H., et al. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342(10):690-695.
178. O’Connell J.B., Reap E.A., Robinson J.A. The effects of cyclosporine on acute murine Coxsackie B3 myocarditis. Circulation. 1986;73(2):353-359.
179. Frishman W.H., O’Brien M., Naseer N., Anandasabapathy S. Innovative drug treatments for viral and autoimmune myocarditis. Heart Dis. 2002;4(3):171-183.
180. Khatib R., Reyes M.P., Smith F., et al. Enhancement of coxsackievirus B4 virulence by indomethacin. J Lab Clin Med. 1990;116(1):116-120.
181. Drucker N.A., Colan S.D., Lewis A.B., et al. Gamma-globulin treatment of acute myocarditis in the pediatric population. Circulation. 1994;89(1):252-257.
182. McNamara D.M., Holubkov R., Starling R.C., et al. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation. 2001;103(18):2254-2259.
183. Robinson J., Hartling L., Vandermeer B. Intravenous immunoglobulin for presumed viral myocarditis in children and adults. Cochrane Database Syst Rev. (1):2005. CD004370
184. Zhang A., Zhang H., Wu S. Immunomodulation by atorvastatin upregulates expression of gap junction proteins in coxsackievirus B3 (CVB3)-induced myocarditis. Inflamm Res. 2010;59(4):255-262.
185. Laubscher B., Gehri M., Roulet M., et al. Survival of infantile fulminant hepatitis B and treatment with lamivudine. J Pediatr Gastroenterol Nutr. 2005;40(4):518-520.
186. Roussos A., Koilakou S., Kalafatas I., et al. Lamivudine treatment for acute severe hepatitis B: report of a case and review of the literature. Acta Gastroenterol Belg. 2008;71(1):30-32.
187. De Silvestro G., Marson P., Brandolese R. A single institution’s experience (1982-1999) with plasma-exchange therapy in patients with fulminant hepatic failure. Int J Artif Organs. 2000;23(7):454-461.
188. Lee W.M., Squires R.H.Jr., Nyberg S.L. Acute liver failure: summary of a workshop. Hepatology. 2008;47(4):1401-1415.
189. Kortsalioudaki C., Taylor R.M., Cheeseman P. Safety and efficacy of N-acetylcysteine in children with non-acetaminophen-induced acute liver failure. Liver Transpl. 2008;14(1):25-30.
190. Puppi J., Dhawan A. Human hepatocyte transplantation overview. Methods Mol Biol. 2009;481:1-16.
191. King V.J., Viswanathan M., Bordley W.C., et al. Pharmacologic treatment of bronchiolitis in infants and children: a systematic review. Arch Pediatr Adolesc Med. 2004;158(2):127-137.
192. Patel H., Platt R., Lozano J.M., et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. (3):2004. CD004878
193. Weinstock D.M., Zuccotti G. The evolution of influenza resistance and treatment. JAMA. 2009;301(10):1066-1069.
194. Poland G.A., Jacobson R.M., Ovsyannikova I.G. Influenza virus resistance to antiviral agents: a plea for rational use. Clin Infect Dis. 2009;48(9):1254-1256.
195. Beigel J., Bray M. Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral Res. 2008;78(1):91-102.
196. Birnkrant D., Cox E. The emergency use authorization of peramivir for treatment of 2009 H1N1 influenza. N Engl J Med. 2010;361(23):2204-2207.
197. Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold? Clin Infect Dis. 2009;48(Suppl 1):S3-13.
198. Kidd I.M., Down J., Nastouli E., et al. H1N1 pneumonitis treated with intravenous zanamivir. Lancet. 2009;374(9694):1036.
199. Whitley R.J., Corey L., Arvin A.M., et al. Changing presentation of herpes simplex virus infection in neonates. J Infect Dis. 1988;158:109-116.
200. Raschilas F., Wolff M., Delatour F., et al. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis. 2002;35:254-260.
201. Nichols W.G., Peck Campbell A.J., Boeckh M. Respiratory viruses other than influenza virus: impact and therapeutic advances. Clin Microbiol Rev. 2008;21(2):274-290.
202. Gavin P.J., Katz B.Z. Intravenous ribavirin treatment for severe adenovirus disease in immunocompromised children. Pediatrics. (1 Pt 1):, 2002.
203. Hoffman J.A., Shah A.J., Ross L.A., Kapoor N. Adenoviral infections and a prospective trial of cidofovir in pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7(7):388-394.
204. Yusuf U., Hale G.A., Carr J., et al. Cidofovir for the treatment of adenoviral infection in pediatric hematopoietic stem cell transplant patients. Transplantation. 2006;81(10):1398-1404.
205. Doan M.L., Mallory G.B., Kaplan S.L., et al. Treatment of adenovirus pneumonia with cidofovir in pediatric lung transplant recipients. J Heart Lung Transplant. 2007;26(9):883-889.
206. Stockman L.J., Bellamy R., Garner P.SARS systematic review of treatment effects PLoS 2006:Med 9;3-e343
207. Huggins J.W., Hsiang C.M., Cosgriff T.M., et al. Prospective, double-blind, concurrent, placebo-controlled clinical trial of intravenous ribavirin therapy of hemorrhagic fever with renal syndrome. J Infect Dis. 1991;164(6):1119-1127.
208. Rusnak J.M., Byrne W.R., Chung K.N., et al. Experience with intravenous ribavirin in the treatment of hemorrhagic fever with renal syndrome in Korea. Antiviral Res. 2009;81(1):68-76.
209. Chapman L.E., Mertz G.J., Peters C.J., et al. Intravenous ribavirin for hantavirus pulmonary syndrome: safety and tolerance during 1 year of open-label experience. Ribavirin Study Group. Antivir Ther. 1999;4(4):211-219.
210. Mertz G.J., Miedzinski L., Goade D., et al. Placebo-controlled, double-blind trial of intravenous ribavirin for the treatment of hantavirus cardiopulmonary syndrome in North America. Clin Infect Dis. 2004;39(9):1307-1313.
211. Ljungman P., Deliliers G.L., Platzbecker U., et al. Cidofovir for cytomegalovirus infection and disease in allogeneic stem cell transplant recipients. The Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2001;97(2):388-392.
212. Treanor J.J., Hayden F.G., Vrooman P.S., et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283(8):1016-1024.
213. De Luca A., Giancola M.L., Ammassari A., et al. Potent antiviral therapy with or without cidofovir for AIDS-associated progressive multifocal leukoencephalopathy: extended follow-up of an observational study. J Neurovirol. 2001;7:364-368.
214. Hamelin M.E., Prince G.A., Boivin G. Effect of ribavirin and glucocorticoid treatment in a mouse model of human metapneumovirus infection. Antimicrob Agents Chemother. 2006;50(2):774-777.
215. Wyde P.R., Chetty S.N., Jewell A.M., et al. Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by ribavirin and immune serum globulin in vitro. Antiviral Res. 2003;60(1):51-59.
216. Bonney D., Razali H., Turner A., Will A. Successful treatment of human metapneumovirus pneumonia using combination therapy with intravenous ribavirin and immune globulin. Br J Haematol. 2009;145(5):667-669.
217. Kamble R.T., Bollard C., Demmler G. Human metapneumovirus infection in a hematopoietic transplant recipient. Bone Marrow Transplant. 2007;40(7):699-700.
218. Raza K., Ismailjee S.B., Crespo M., et al. Successful outcome of human metapneumovirus (hMPV) pneumonia in a lung transplant recipient treated with intravenous ribavirin. J Heart Lung Transplant. 2007;26(8):862-864.
219. Boeckh M., Berrey M.M., Bowden R.A., et al. Phase 1 evaluation of the respiratory syncytial virus-specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants. J Infect Dis. 2001;184(3):350-354.
220. Chavez-Bueno S., Mejias A., Merryman R.A. Intravenous palivizumab and ribavirin combination for respiratory syncytial virus disease in high-risk pediatric patients. Pediatr Infect Dis J. 2007;26(12):1089-1093.