Chapter 125 Leukopenia
Marked developmental changes in normal values for the total white blood cell (WBC) count occur during childhood (Chapter 708). The mean WBC count at birth is high, followed by a rapid fall beginning at 12 hr until the end of the 1st wk. Thereafter, values are stable until 1 yr of age. A slow, steady decline in the WBC count continues throughout childhood until reaching the adult value during adolescence. Leukopenia in adolescents and adults is defined as a total WBC count <4,000/µL. Evaluation of patients with leukopenia, neutropenia, or lymphopenia begins with a thorough history, physical examination, family history, and screening laboratory tests (Table 125-1).
Table 125-1 DIAGNOSTIC APPROACH FOR PATIENTS WITH LEUKOPENIA
| EVALUATION | ASSOCIATED CLINICAL DIAGNOSES |
|---|---|
| INITIAL EVALUATION | |
| • History of acute or chronic leukopenia | |
| • General medical history | Congenital syndromes (Shwachman-Diamond, Wiskott-Aldrich, Fanconi anemia, dyskeratosis congenita, glycogen storage disease type Ib, disorders of vesicular transport) |
| • Physical examination: stomatitis, gingivitis, dental defects, congenital anomalies | |
| • Spleen size | Hypersplenism |
| • History of drug exposure | Drug-associated neutropenia |
| • Complete blood count with differential and reticulocyte counts | Neutropenia, aplastic anemia, autoimmune cytopenias |
| IF ANC <1,000/µL | |
| EVALUATION OF ACUTE ONSET NEUTROPENIA | |
| • Repeat blood counts in 3-4 weeks | Transient myelosuppression (e.g., viral) |
| • Serology and cultures for infectious agents | Active or chronic infection with viruses (e.g., EBV, CMV), bacteria, mycobacteria, rickettsia |
| • Discontinue drug(s) associated with neutropenia | Drug-associated neutropenia |
| • Test for antineutrophil antibodies | Autoimmune neutropenia |
| • Measure quantitative immunoglobulins (G, A, and M), lymphocyte subsets | Neutropenia associated with disorders of immune function |
| IF ANC <500/µL ON 3 SEPARATE TESTS | |
| • Bone marrow aspiration and biopsy, with cytogenetics | Severe congenital neutropenia, Shwachman-Diamond syndrome, myelokathexis; chronic benign or idiopathic neutropenia |
| • Serial CBCs (3/week for 6 weeks) | Cyclic neutropenia |
| • Exocrine pancreatic function | Shwachman-Diamond syndrome |
| • Skeletal radiographs | Shwachman-Diamond syndrome, cartilage-hair hypoplasia, Fanconi anemia |
| IF ABSOLUTE LYMPHOCYTE COUNT <1000/µL | |
| • Repeat blood counts in 3-4 weeks | Transient leukopenia (e.g., viral) |
| IF ALC <1000/µL ON 3 SEPARATE TESTS | |
| • HIV-1 antibody test | HIV-1 infection, AIDS |
| • Quantitative immunoglobulins (G, A, and M), lymphocyte subsets | Congenital or acquired disorders of immune function |
| IF THERE IS PANCYTOPENIA | |
| • Bone marrow aspiration and biopsy | Bone marrow replacement by malignancy, fibrosis, granulomata, storage cells |
| • Bone marrow cytogenetics | Myelodysplasia, leukemia |
| • Vitamin B12 and folate levels | Vitamin deficiencies |
ANC, absolute neutrophil count; CBC, complete blood count; CMV, cytomegalovirus; EBV, Epstein-Barr virus.
Neutropenia
Etiology
Acute neutropenia evolving over a few days often occurs when neutrophil use is rapid and production is compromised. Chronic neutropenia lasting months or years can arise from reduced production, increased destruction, or excessive splenic sequestration of neutrophils. Neutropenia may be classified by whether it arises secondary to factors extrinsic to marrow myeloid cells (Table 125-2), which is common; as an acquired disorder of myeloid progenitor cells (Table 125-3), which is less common; or, more rarely, as an intrinsic defect affecting proliferation and maturation of myeloid progenitor cells (Table 125-4).
Table 125-2 CAUSES OF NEUTROPENIA EXTRINSIC TO MARROW MYELOID CELLS
| CAUSE | ETIOLOGIC FACTORS/AGENTS | ASSOCIATED FINDINGS |
|---|---|---|
| Infection | Viruses, bacteria, protozoa, rickettsia, fungi | Redistribution from circulating to marginating pools, impaired production, accelerated destruction |
| Drug-induced | Phenothiazines, sulfonamides, anticonvulsants, penicillins, aminopyrine | Hypersensitivity reaction (fever, lymphadenopathy, rash, hepatitis, nephritis, pneumonitis, aplastic anemia), antineutrophil antibodies |
| Immune neutropenia | Alloimmune, autoimmune | Variable arrest from metamyelocyte to segmented neutrophils in bone marrow |
| Reticuloendothelial sequestration | Hypersplenism | Anemia, thrombocytopenia, neutropenia |
| Bone marrow replacement | Malignancy (lymphoma, metastatic solid tumor, etc.) | Presence of immature myeloid and erythroid precursors in peripheral blood |
| Cancer chemotherapy or radiation therapy to bone marrow | Suppression of myeloid cell production | Bone marrow hypoplasia, anemia, thrombocytosis |
Table 125-3 ACQUIRED DISORDERS OF MYELOID CELLS
| CAUSE | ETIOLOGIC FACTORS/AGENTS | ASSOCIATED FINDINGS |
|---|---|---|
| Aplastic anemia | Stem cell destruction and depletion | Pancytopenia |
| Vitamin B12 or folate deficiency | Malnutrition; congenital deficiency of B12 absorption, transport, and storage; vitamin avoidance | Megaloblastic anemia, hypersegmented neutrophils |
| Acute leukemia, chronic myelogenous leukemia | Bone marrow replacement with malignant cells | Pancytopenia, leukocytosis |
| Myelodysplasia | Dysplastic maturation of stem cells | Bone marrow hypoplasia with megaloblastoid red cell precursors, thrombocytopenia |
| Prematurity with birthweight <2 kg | Impaired regulation of myeloid proliferation and reduced size of postmitotic pool | Maternal preeclampsia |
| Chronic idiopathic neutropenia | Impaired myeloid proliferation and/or maturation | None |
| Paroxysmal nocturnal hemoglobinuria | Acquired stem cell defect secondary to mutation of PIG-A gene | Pancytopenia, thrombosis |
Table 125-4 INTRINSIC DISORDERS OF MYELOID PRECURSOR CELLS
| SYNDROME | INHERITANCE (GENE) | CLINICAL FEATURES (INCLUDING STATIC NEUTROPENIA UNLESS OTHERWISE NOTED) |
|---|---|---|
| PRIMARY DISORDERS OF MYELOPOIESIS | ||
| Cyclic neutropenia | AD (ELA2) | Periodic oscillation (21-day cycles) in ANC |
| Severe congenital neutropenia | AD (ELA2, GFI1, others) | Risk of MDS and AML |
| X-linked (WAS) | Neutropenic variant of Wiskott-Aldrich syndrome | |
| Kostmann syndrome | AR (HAX1) | Neurological abnormalities, risk of MDS and AML |
| DISORDERS OF RIBOSOMAL FUNCTION | ||
| Shwachman-Diamond syndrome | AR (SBDS) | Pancreatic insufficiency, variable neutropenia, other cytopenias, metaphysical dysostosis |
| Dyskeratosis congenita | Telomerase defects: XL (DKC1), AD (TERC), AR (TERT) | Nail dystrophy, leukoplakia, reticulated hyperpigmentation of the skin; 30-60% develop bone marrow failure |
| DISORDERS OF GRANULE SORTING | ||
| Chédiak-Higashi syndrome | AR (LYST) | Partial albinism, giant granules in myeloid cells, platelet storage pool defect, impaired natural killer cell function, hemophagocytic lymphohistiocytosis |
| Griscelli syndrome, type II | AR (RAB27a) | Partial albinism, impaired natural killer cell function, hemophagocytic lymphohistiocytosis |
| Cohen syndrome | AR (COH1) | Partial albinism |
| Hermansky-Pudlak syndrome, type II | AR (AP3P1) | Cyclic neutropenia, partial albinism |
| p14 deficiency | probable AR (MAPBPIP) | Partial albinism, decreased B and T cells |
| DISORDERS OF METABOLISM | ||
| Glycogen storage disease, type 1b | AR (G6PT1) | Hepatic enlargement, growth retardation, impaired neutrophil motility |
| G6Pase, catalytic subunit 3, deficiency | AR (G6PC3) | Structural heart defects, urogenital abnormalities, venous angiectasia |
| Barth syndrome | XL (TAZ1) | Episodic neutropenia, dilated cardiomyopathy, methylglutaconic aciduria |
| Pearson’s syndrome | Mitochondrial (DNA deletions) | Episodic neutropenia, pancytopenia; defects in exocrine pancreas, liver, and kidneys |
| NEUTROPENIA IN DISORDERS OF IMMUNE FUNCTION | ||
| Common variable immunodeficiency | Familial, sporadic (TNFRSF13B) | Hypogammaglobulinemia, other immune system defects |
| IgA deficiency | Unknown (Unknown or TNFRSF13B) | Decreased IgA |
| Severe combined immunodeficiency | AR, XL (multiple loci) | Absent humoral and cellular immune function |
| Hyper-IgM syndrome | XL (HIGM1) | Absent IgG, elevated IgM, autoimmune cytopenia |
| WHIM syndrome | AD (CXCR4) | Warts, hypogammaglobulinemia, infections, myelokathexis |
| Cartilage-hair hyperplasia | AR (RMKP) | Lymphopenia, short-limbed dwarfism, metaphysical chondrodysplasia, fine sparse hair |
| Schimke immuno-osseous dysplasia | probable AR (SMARCAL1) | Lymphopenia, pancytopenia, spondyloepiphyseal dysplasia, growth retardation, renal failure |
AD, autosomal dominant; AML, acute myelogenous leukemia; ANC, absolute neutrophil count; AR, autosomal recessive; MDS, myelodysplasia; XL, X-linked.
Infectious Causes
Transient neutropenia often accompanies or follows viral infections (Table 125-5). Neutropenia associated with common childhood viral disease occurs during the 1st 1-2 days of illness and may persist for 3-8 days. It usually corresponds to a period of acute viremia and is related to virus-induced redistribution of neutrophils from the circulating to the marginating pool. Neutrophil sequestration possibly occurs after virus-induced tissue damage. Moderate to severe neutropenia may also be associated with a wide variety of other infectious causes. Bacterial sepsis is a particularly serious cause of neutropenia and neonates are particularly vulnerable to developing neutropenia because of a deficient pool of reserve neutrophils in the bone marrow.
Table 125-5 INFECTIONS THAT ARE ASSOCIATED WITH NEUTROPENIA
VIRAL
Respiratory syncytial virus
Dengue fever
Colorado tick fever
Mumps
Viral hepatitis
Infectious mononucleosis (Epstein-Barr virus)
Influenza
Measles
Rubella
Varicella
Cytomegalovirus
Human immunodeficiency virus
Sandfly fever
BACTERIAL
Pertussis
Typhoid fever
Paratyphoid fever
Tuberculosis (disseminated)
Brucellosis
Tularemia
Gram-negative sepsis
Psittacosis
FUNGAL
Histoplasmosis (disseminated)
PROTOZOA
Malaria
Leishmaniasis (kala-azar)
RICKETTISIAL
Rocky Mountain spotted fever
Typhus fever
Rickettsialpox
From Boxer LA, Blackwood RA: Leukocyte disorders: quantitative and qualitative disorders of the neutrophil, part 1, Pediatr Rev 17:19–28, 1996.
Drug-Induced Neutropenia
Drugs constitute one of the most common causes of neutropenia (Table 125-6). The incidence of drug-induced neutropenia increases dramatically with age; only 10% of cases occur among children and young adults, and the majority of cases among adults over age 65 yr. Drug-induced neutropenia has several underlying mechanisms (immune-mediated, toxic, idiosyncratic, hypersensitivity reactions) that are distinct from the severe neutropenia that predictably occurs after administration of cytoreductive cancer drugs or radiotherapy.
Neutropenia commonly and predictably follows the use of anticancer drugs or radiation therapy, especially radiation therapy directed at the pelvis or vertebrae, secondary to cytotoxic effects on rapidly replicating myeloid precursors. A decline in the WBC count typically occurs 7-10 days after administration of the anticancer drug and may persist for 1-2 wk. The neutropenia accompanying malignancy or following cancer chemotherapy is frequently associated with compromised cellular immunity, thereby predisposing patients to a much greater risk of infection (Chapter 171) than found in disorders associated with isolated neutropenia.
Ineffective Myelopoiesis
Intrinsic Disorders of Myeloid Precursors
The isolated disorders of proliferation and maturation of myeloid precursor cells are rare. Table 125-4 presents a classification based on genetics and molecular mechanisms; selected disorders are discussed in the next sections.
Granule Sorting Disorders
This constellation of very rare autosomal recessive disorders combine neutropenia with partial oculocutaneous albinism, immunodeficiencies, and other features, all derived from defects in formation or trafficking of lysosome-related organelles (see Table 125-4). Treatment usually includes hematopoietic stem cell transplantation.
Disorders of Metabolism
Recurrent infections with neutropenia are a distinctive feature of glycogen storage disease (GSD) type Ib. Both classic von Gierke glycogen storage disease (GSDIa) and GSDIb cause massive enlargement of liver and severe growth retardation (Chapter 81.1). In contrast to GSDIa, glucose-6-phosphatase (G6Pase) enzyme activity is normal, but mutations in the G6P transporter 1, G6PT1, inhibit glucose transport in GSDIb, resulting in both defective neutrophil motility and increased apoptosis associated with neutropenia and recurrent bacterial infections. Treatment with rhG-CSF can correct the neutropenia.
Neutropenia in Disorders of Immune Function
Congenital immunologic disorders that have severe neutropenia as a clinical feature include common variable immunodeficiency, the severe combined immunodeficiencies, hyper-IgM syndrome, WHIM syndrome, and a number of even rarer immunodeficiency syndromes (see Table 125-4).
Unclassified Disorders
Acquired idiopathic chronic neutropenia is characterized by onset of neutropenia after 2 yr of age. Patients with an ANC persistently <500/µL are afflicted with recurrent pyogenic infections involving the skin, mucous membranes, lungs, and lymph nodes. Bone marrow examination reveals variable patterns of myeloid formation with arrest generally occurring between the myelocyte and band forms (see Table 125-3). Often there is overlap with the diagnoses of chronic benign or autoimmune neutropenias.
Laboratory Findings
Isolated absolute neutropenia has a limited number of causes (see Tables 125-1 through 125-4). The duration and severity of the neutropenia greatly influence the extent of laboratory evaluation. Patients with chronic neutropenia since infancy and a history of recurrent fevers and chronic gingivitis should have WBC counts and differential counts determined 3 times weekly for 6 wk to evaluate the periodicity suggestive of cyclic neutropenia. Bone marrow aspiration and biopsy should be performed on selected patients to assess cellularity. Additional marrow studies such as cytogenetic analysis and special stains for detecting leukemia and other malignant disorders should be obtained for patients with suspected intrinsic defects in the myeloid progenitors and for patients with suspected malignancy. Selection of further laboratory tests is determined by the duration and severity of the neutropenia and the associated findings on physical examination (see Table 125-1).
Treatment
The management of acquired transient neutropenia associated with malignancies, myelosuppressive chemotherapy, or immunosuppressive chemotherapy differs from that of congenital or chronic forms of neutropenia. In the former situation, infections sometimes are heralded only by fever, and sepsis is a major cause of death. Early recognition and treatment of infections may be lifesaving (Chapter 171).
Lymphopenia
Inherited Causes of Lymphocytopenia
Inherited immunodeficiency disorders may have a quantitative or qualitative stem cell abnormality resulting in ineffective lymphocytopoiesis (Table 125-7). Other disorders such as Wiskott-Aldrich syndrome may have associated lymphocytopenia arising from accelerated destruction of T cells. A similar mechanism is present in patients with adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency. Lymphocyte counts may also be decreased in some forms of inherited bone marrow failure, such as reticular dysgenesis, severe congenital neutropenia secondary to GFI1 mutation, or dyskeratosis congenita.
Table 125-7 CAUSES OF LYMPHOCYTOPENIA
ACQUIRED CAUSES
Infectious Diseases
AIDS
Viral hepatitis
Influenza
Tuberculosis
Typhoid fever
Sepsis
Iatrogenic
Immunosuppressive therapy
Corticosteroids
High-dose PUVA therapy
Cytotoxic chemotherapy
Radiation
Thoracic duct drainage
Systemic and Other Diseases
Systemic lupus erythematosus
Myasthenia gravis
Hodgkin disease
Protein-losing enteropathy
Renal failure
Sarcoidosis
Thermal injury
Aplastic anemia
Dietary Deficiency
Dietary deficiency associated with ethanol abuse
INHERITED CAUSES
Aplasia of lymphopoietic stem cells
Severe combined immunodeficiency
Ataxia-telangiectasia
Wiskott-Aldrich syndrome
Immunodeficiency with thymoma
Cartilage-hair hypoplasia
Idiopathic CD4 T lymphocytopenia
ADA, adenosine deaminase; IL-2, interleukin 2; PNP, purine nucleoside phosphorylase; PUVA, psoralen and ultraviolet A irradiation.
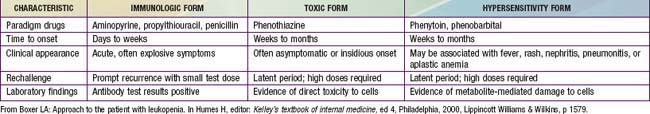
From Boxer LA: Approach to the patient with leukopenia. In Humes HD, editor: Kelley’s textbook of internal medicine, ed 4, Philadelphia, 2000, Lippincott Williams & Wilkins, p 1580.
Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007;146:657-665.
Berliner N. Lessons from congenital neutropenia: 50 years of progress in understanding myelopoiesis. Blood. 2008;111:5427-5432.
Boxer LA, Newburger PE. A molecular classification of congenital neutropenia syndromes. Pediatr Blood Cancer. 2007;49:609-614.
Bruin M, Dassen A, Pajkrt D, et al. Primary autoimmune neutropenia in children: a study of neutrophil antibodies and clinical course. Vox Sang. 2005;88:52-59.
Donini M, Fontana S, Savoldi S, et al. G-CSF treatment of severe congenital neutropenia reverses neutropenia but does not correct the underlying functional deficiency of the neutrophil in defending against microorganisms. Blood. 2007;109:4716-4723.
Hsieh MM, Everhart JE, Byrd-Holt DD, et al. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146:486-492.
Klein C, Grudzien M, Appaswamy G, et al. HAX1 deficiency causes autosomal recessive severe congenital neutropenia (Kostmann disease). Nat Genet. 2007;39:86-92.
Latger-Cannard V, Bensoussan D, Bordigoni P. The WHIM syndrome shows a peculiar dysgranulopoiesis: myelokathexis. Br J Haematol. 2006;132:669.
Melis D, Fulceri R, Parenti G, et al. Genotype/phenotype correlation in glycogen storage disease type 1b: a multicentre study and review of the literature. Eur J Pediatr. 2005;164:501-508.
Pannicke U, Hönig M, Hess I, et al. Reticular dysgenesis (aleukocytosis) is caused by mutations in the gene encoding mitochondrial adenylate kinase 2. Nat Genet. 2009;41:101-105.
Patel S, de la Fuente J, Atra A, et al. Where have all the neutrophils gone? Arch Dis Child Educ Pract Ed. 2009;94:74-77.
Rosenberg PS, Alter BP, Bolyard AA, et al. The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy. Blood. 2006;107:4628-4635.
Shimamura A. Shwachman-Diamond syndrome. Semin Hematol. 2006;43:178-188.
Townshend J, Clark J, Cant A, et al. Congenital neutropenia. Arch Dis Child Educ Pract Ed. 2008;93:14-18.