Chapter 26 Lasers
Principles
The increasing surgical use of lasers, with their inherent potential hazards to patients and operating room staff, mandates an understanding of their physical principles by anaesthetists.1
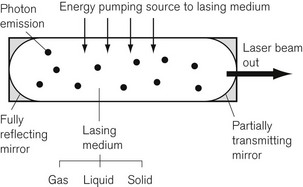
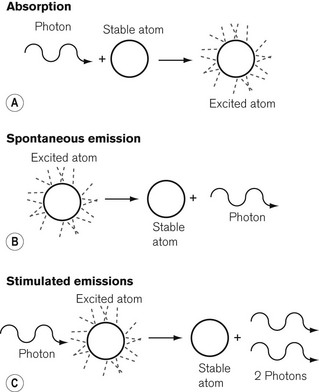
Although there are many more complex laser systems,2 the basic components of a laser are shown in Fig. 26.1. The lasing medium may be a solid, liquid or gas. The atoms of a lasing medium are excited to high energy levels by a ‘pumping’ source, which may be a high-voltage discharge in the case of a gas, an intense flash of light from a flashtube or the energy from a radio frequency power source. Fig. 26.2 shows the excitation and emission process possible in a gaseous lasing medium. A photon of energy from the pumping source may be absorbed by a stable atom in its so called ‘ground state’, which then becomes an atom in an excited state, with an electron or electrons in an orbital shell at a higher energy level. Spontaneous emission of a photon of energy occurs as the electrons fall back to shells of a lower energy state and the excited atom reverts to the ground state.
If a further photon of pumping energy, at the correct wavelength, is applied to an atom in its excited state, it will fall to its ground state and two photons of energy will be emitted instead of one. This is known as stimulated emission, originally described by Einstein in 1917 as the basis for laser technology3 and the inversion of the energy states is referred to as population inversion. The emitted photons thus produced are in phase with, have the same polarization, and travel in the same direction, as the stimulating radiation. This mechanism is amplified by many of the escaping photons being reflected back into the lasing medium by the mirrors. Thus a chain reaction occurs, and this can be thought of as a positive feedback system. The process produces an intense source of light energy, some of which is allowed to escape through the partially reflecting mirror at the output end of the lasing medium. The output beam of the laser is usually directed to the tissues through a fibre-optic light guide. However, the wavelength of the carbon dioxide laser is so long, at 10.6 µm, that there is no fibre currently available to transmit energy, so that it has to be directed by a series of mirrors instead.
Clinical applications
The clinical use of lasers depends on a compromise between:
The extent of the effect of a laser on human tissues depends primarily on the intensity and the frequency of the light being used.5 At low light intensity, stimulation occurs within the cell and this is exploited in physiotherapy applications. At slightly higher intensity, attenuation of cellular activity occurs. At still higher intensity levels, about 40 J cm−2, sensitizing agents in tissue become activated (this is the basis of protecting the skin from the sun’s ultraviolet rays using suntan lotions). By the time the light intensity has risen to 400 J cm−2, the tissue temperature has risen to 60oC and protein denaturation and photocoagulation predominate. Further large increases in light intensity result in a tissue temperature rise to 100oC, vaporization of tissue fluids and destruction of cell structures. In order to control the destructive power of a laser, most systems can be pulsed; the light is emitted in short bursts, to allow heat dissipation between bursts and reduction of thermal damage to neighbouring tissues. ‘Q switching’ of a laser refers to a device which allows aliquots of laser light to be stored and released in bursts of even higher energy and shorter duration. Such a technique is used in ophthalmology to cause photoablation and to minimize thermal damage to the eye. With Q switching, while collateral thermal damage may be reduced, the frequency of switching is such that tissue vibration and, therefore, mechanical damage may predominate.
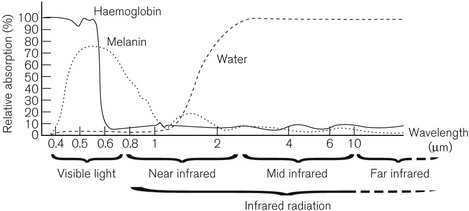
The graph in Fig. 26.3 shows the spectrum of absorption of light at different wavelengths by haemoglobin, melanin and water. Monochromatic light energy is absorbed by tissue of complementary colour (opposite colour) and reflected by substances of the same colour.
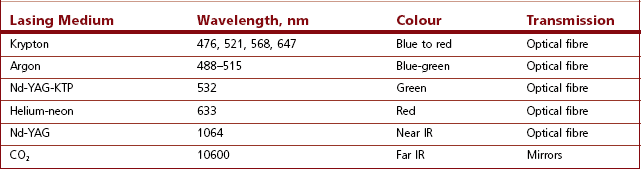
Carbon dioxide laser energy at 10.6 µm is absorbed by water within 1 mm depth, causing rapid vaporization of intracellular water. The main use of the carbon dioxide laser is, therefore, as a bloodless cutter and vaporizer. The blue-green argon laser beam penetrates to about 2 mm depth and is maximally absorbed in the 500 nm waveband by substances of a complementary colour (red), such as haemoglobin. Thus, the argon laser is used to coagulate blood in small vessels with very little effect on other more transparent tissues, for example the retina. The Nd:YAG (neodymium: yttrium-aluminium garnet) laser has a solid lasing medium and produces energy in the near infrared region of the spectrum, which has maximum penetration, being absorbed at 3–5 mm depth, by haemoglobin, melanin and water. When invisible infrared lasers are used (e.g. CO2 laser), it is common practice to make use of a low powered, visible-light laser, such as a helium:neon laser at the same time, in order to aid in aiming the therapeutic laser accurately. Table 26.1 shows the currently available lasers in medical use.
Safety aspects
Apart from the danger to the patient from the beam of laser energy if it is misused, there is a risk to the operator and other persons in the operating environment.6 This is because of the long range of laser light due to the virtual non-divergence of the beam; thus, in contrast to a collimated X-ray beam for example, increased distance from the source has very little safety benefit. Even reflected laser light may be very dangerous to the eyes. Visible laser light transmitted to the retina of the eye may burn it irreparably, leaving a blind spot in the field of vision. A similar lesion over the optic nerve may result in total blindness of that eye. The cornea, lens and aqueous and vitreous humours partially or totally absorb far-infrared laser radiation; these tissues, therefore, are more susceptible to damage than the retina.
In terms of the danger that lasers pose to humans, there is a complex relationship between power, frequency and time of exposure. There is an international classification for lasers, shown in Table 26.2, where Class I lasers are inherently safe and Class IV lasers are, broadly speaking, hazardous if misused. Most lasers in medical use are in Class IV. No one should use a laser who is not trained to do so, and everyone who is working in the vicinity of a laser should be trained in the safety aspects of its use. This includes the anaesthetist, who is often standing in the line of fire of the laser.
Table 26.2 International classification of continuously working lasers
| Class I | Powers not to exceed maximum permissible exposure for the eye |
| Class II | Visible laser beams only; powers up to 1 mW; eye protected blink-reflex time of 0.25 s |
| Class IIIa | Relaxation of class II to 5 mW for radiation, provided beam is expanded so that the eye is still protected by the blink-reflex |
| Class IIIb | Powers up to 500 mW; direct viewing hazardous |
| Class IV | Powers over 500 mW; extremely hazardous |
Anaesthetic-related risks
• No flammable anaesthetic agents or nitrous oxide should be used; nitrous oxide supports combustion better than oxygen under some circumstances.7
• Non-reflective (matt-black) instruments should be used, since the reflected laser beam is almost as powerful as the main beam.
• The oxygen concentration in the immediate vicinity of the laser beam should not exceed 25% where possible.
• Non-flammable endotracheal tubes constructed from special materials (see Chapter 6) should be used. Plastic endotracheal tubes should be avoided. The cuff of a LMA is more vulnerable to laser damage than the shaft and should be filled with saline as a protective measure.8 Other tissues should be protected with wet swabs.
• Protective goggles fitted with specific lenses that absorb the wavelength of the laser in use and should be worn by everybody in the operating theatre, including the patient. Goggles give better protection than spectacles. They should also be kept with the laser for which they are relevant.
• Doors to the operating area should be locked and all windows should be covered to protect those outside the operating area. Warning signs should be posted outside doors.
Safety codes
In 1995 in the UK, the Medical Devices Agency (MDA)9 published guidance on laser safety, both in medical and, increasingly, in dental practice. With recent incorporation of the MDA into the Medical and Healthcare Regulatory Agency (MHRA), a new set of guidance principles has been issued.10 This guidance should form the basis of a set of local rules and a Safety Code. It specifically recommends the appointment of a laser protection supervisor, who should not be the laser operator, ensure all staff are wearing correct eye protection, and be available in every area where a laser is in use. Additionally, the guidelines recommend that a laser protection adviser be identified, who advises on the risk management aspects of a laser to be installed, and on the drawing up of local safety policy and rules.
1 van der Speck AFL, Spargo PM, Norton ML. The physics of lasers and implications for their use during airway surgery. Br J Anaesth. 1988;60:709–729.
2 Houssin M, Courteille P, Champenois C, Herbane M, Knoop M, Vedel M, et al. Linewidth reduction by 6 orders of magnitude of a broad area 729 nm diode laser. Appl Opt. 2003;42:4871–4876.
3 Graudenz K Raulin C. From Einstein’s quantum theory to modern laser therapy: a history of lasers in dermatology and aesthetic medicine. Appl Opt. 2003;54:575–582.
4 Jacques SL. Laser-tissue interactions. Surg Clin North Am. 1992;72:531–558.
5 Parsons R. Basic principles of lasers. In: Anaesthesia and Intensive Care Medicine. Abingdon: The Medicine Publishing Co.; 2002:419–421.
6 Sliney DH. Laser safety. Lasers Surg Med. 1995;16:215–225.
7 MacDonald AG. A short history of fires and explosions caused by anaesthetic agents. Br J Anaesth. 1994;72:710–722.
8 Pandit JJ, Chambers P, O’Malley S. KTP laser resistant properties of the reinforced LMA. Br J Anaesth. 1997;78:594–600.
9 Medical Devices Agency. Guidance on the safe use of lasers in medical and dental practice. London: Department of Health; 1995.
10 Medical and Healthcare Regulatory Agency. Device Bulletin. Guidance on the safe use of lasers, intense light source systems and LEDs in medical, surgical, dental and aesthetic practices DB2008(03). London: Medical and Healthcare Regulatory Agency; April 2008.