Chapter 6 Laser Thermal Ablation
Historical Background
Bruising, transient pain, and induration of the thigh are common adverse events after endovenous laser (EVL) therapy and are most likely caused by laser-induced perforation of the vein wall with extravasation of blood into surrounding tissue.1–3 It is known that conversion of an incompetent vein into a fibrous cord, with subsequent sonographic disappearance, will guarantee permanent occlusion. At the onset of EVL therapy, little was known about the mechanism of action and durability of treatment after intervention with these devices. Studies have indicated that heat-related damage to the inner vein wall leads to thrombotic occlusion of the treated vein.4,5
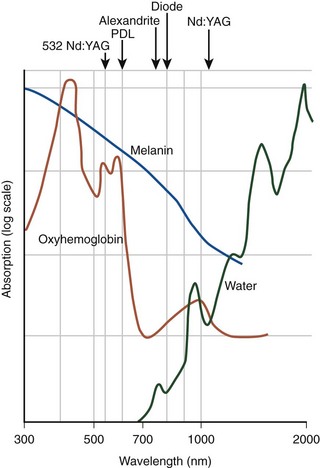
EVL can be classified into hemoglobin-specific laser wavelengths (HSLWs) and water-specific laser wavelengths (WSLWs) (Fig. 6-1). Wavelengths of 808, 810, 940, 980, 1064, 1319, and 1320 nm have been successfully used for great saphenous vein (GSV) ablation4,6–10 and for other superficial axial and perforating veins.11 Hemoglobin and, to a lesser extent, myoglobin in venous smooth muscle cells are the dominant chromophores at the lower end of this range, while at the 1320-nm wavelength range, water dominates as the energy-absorbing molecule.4,7
Published reports suggest that delivery of higher energy is required to effect secure vein closure; however, with increased energy delivery, pain and bruising after treatment are encountered more frequently. After EVL, studies have demonstrated 70% of limbs experience some degree of pain, and 50% require analgesics for pain management.12 Kabnick13 reported an average pain score of 2.6 on a scale of 0 to 5 after EVL. There is increasing focus on reducing perioperative pain and bruising in the field of EVL saphenous ablation. The most recent WSLW to become available is the 1470 nm, which requires less energy delivery for closure, and reports of less pain and bruising postprocedure are beginning to populate the literature.
The WSLWs were developed to target the interstitial water in the vein wall and minimize perforations.7 Two WSLW (1319 and 1320 nm) lasers are in widespread clinical use, and trends in the literature suggest that these longer wavelength lasers may produce fewer side effects than HSLW lasers at comparable linear endovenous energy density (LEED). Two comparisons of different wavelengths with similar delivered laser energy have been performed. One study compared 940-nm and 1320-nm wavelengths in a retrospective analysis, and another compared 810-nm and 980-nm wavelengths in a randomized prospective study.13,14 The two studies demonstrated equivalent safety and efficacy at similar energy dosing. However, EVL performed at comparable LEED with either the 940-nm (HSLW) or the 1320-nm (WSLW) lasers showed a reduction in postoperative pain and bruising with the 1320-nm device. Also, use of less power (5 W) demonstrated a lower rate of side effects than did 8 W with a laser operating at a wavelength of 1320 nm.14 A low rate of pain and bruising was reported after GSV treatment for GSV reflux with the 1470-nm wavelength at 5 W, 30 J/cm.15
Etiology and Natural History of Disease
There remains controversy over the mechanism of action of EVL and most of the investigations were performed with HSLWs. Proebstle found in vitro with an HSLW that the extensive heat damage of the endothelium and the intima came from steam bubble formation and induced full-length thrombotic occlusion of the vein.4 Bush et al.,16 in a histologic study, found that the mechanism of injury with HSLWs is secondary to steam bubbles caused by water evaporation of the blood followed by transmitted heat injury to the tissue. Acutely, there is complete loss of the endothelium and early thrombus formation, followed by the injury response with inflammatory cellular infiltration into the subintimal layers. Eventually, fibroblasts deposit collagen and represent the predominant histologic finding at 4 months postoperatively.
Opposed to the steam bubble theory, Fan and Rox-Anderson17 studied existing histologic reports from studies with excellent closure rates using tumescent anesthesia and found that HSLWs produce a transmural vein wall injury, typically associated with perforations and carbonization. The pattern of injury was eccentrically distributed, with maximum injury occurring along the path of laser contact. The authors concluded that steam production during EVL accounted for only 2% of applied energy dose (i.e., EVL causes permanent vein closure through a high-temperature photothermolytic process at the point of contact between the vein and the laser). Precise mechanism-of-action studies with ample histologic information are lacking with WSLWs.
Although it is not known exactly how much damage to the individual layers of the vein wall is required, it seems that at a minimum, intimal and medial coagulation are necessary for long-term closure. Technically, the depth of penetration of a 940-nm laser beam into blood is limited to approximately 0.3 mm.18 Qualitative analysis with optical coherence tomography in an ex vivo model matched with histologic cross sections showed a symmetric, complete, circular disintegration of intima and media structures, without any transmural tissue defects after radiofrequency ablation (RFA).19 However, with an HSLW laser, pronounced semicircular tissue ablations and complete vessel wall perforations were detected at 35 J/cm LEED. The quantitative analysis demonstrated a significant (p < .0001) increase in intima-media thickness after RF ablation (38% to 67%) and EVL (11% to 46%) and a significant (p < .0001) reduction in vessel lumen diameter (36% to 42%) after RF ablation. No linear correlation could be identified between laser energy level and effects on tissue such as ablation/perforation, media thickening, or vein lumen diameter.22
Weiss20 examined the gross tissue effects and tissue temperatures generated during EVL with an 810-nm diode laser in an in vivo goat model. Using thermal sensors mounted adjacent to the laser optical fiber, they determined the mean temperature at the firing tip was 729° C (peak 1334° C). The intense thermal heating zone appeared to be focally situated around the laser tip; the mean temperature decreased to 231° C and 307° C, 2 mm proximal and distal to the fiber tip, respectively. At 4 mm distal from the fiber tip, the mean temperature decreased further to 93° C. Recently, Disselhoff et al,10 with intravascular temperature measurements in an in vitro system, found that despite the intense heat at the laser tip, the thermal heating zone is predominantly contained within the venous lumen. Zimmet and Min21 demonstrated in a swine model that during EVL with an 810-nm diode laser, ear vein outer wall temperatures ranged from 40° to 49° C. In hind extremity veins, these investigators showed that with tumescent anesthesia, the external vein wall temperatures never exceeded 40° C.
These findings were corroborated in humans by Beale et al.22 when he inserted thermocouples percutaneously, positioned at 3, 5, and 10 mm from a small saphenous vein (SSV), after administration of tumescent anesthesia. He recorded temperatures during EVL with an 810-nm diode laser, using 1-second pulse application at 12 W, and found peak temperatures of 43°, 42°, and 36° C at 3, 5, and 10 mm, respectively, in perivenous tissues.
Pearls and Pitfalls
Dosing
Data on the dose-response relationship between laser energy and durability of vein occlusion began to be published in 2004, when parameters describing the LEED and endovenous fluence equivalent (EFE) were introduced.23 LEED is a linear energy density quantity measured in joules per centimeter, and EFE is an energy parameter that uses a cylindric approximation of the inner vein surface area expressed in joules per centimeter squared.
In a retrospective study,24 recanalization was reported in 20% of cases treated with administration of less than 80 J/cm and was significantly reduced if laser energy exceeded 80 J/cm at a wavelength of 980 nm. However, in a follow-up prospective study by the same author, 9% of veins treated with LEEDs exceeding 80 J/cm unexpectedly recanalized at 6-month follow-up.25 Multiple regression analysis determined that EFE was the most significant predictor of recanalization events and, when exceeding 20 J/cm2, was associated with durable GSV occlusion after 1-year follow-up.26 Another study reported on 129 GSVs and found that 52 J/cm2 EFE was ideal to produce long-term occlusion; this author cautioned that recanalization can occur in patients treated with higher fluence.27
A sliding scale approach has been used at Miami Vein Center since 2002 and has yielded excellent results.11 High rates of vein occlusion and ultimate sonographic disappearance were noted when the thermal dose in each segment of the GSV was tailored to the diameter in that segment. The ranges of energies used fell between 50 J/cm for veins 5 mm in diameter and 120 J/cm for veins 10 mm in diameter at the saphenofemoral junction (SFJ). No increase in complications were seen with any of the higher energy strategies.28
Reports from John Hopkins University on “low-energy” EVL treatment of 34 consecutive GSVs with a 980-nm diode laser at 11 W in continuous mode (mean GSV diameter 12 mm) using mean LEED of 35 J/cm resulted in zero recanalization (100% success) at a mean follow-up of 1 year.29 Interestingly, the same author reported later in the same year that 60 consecutive GSVs demonstrated a reduced treatment success of 95%; the mean follow-up for this series was 6.8 months and mean LEED for successful and failed treatments was 33 J/cm. The mean maximum diameter of successfully treated GSVs was 11 mm, and that for failed treatments was 21 mm (p = .008). The investigators concluded that there were no significant differences in mean unit energy applied for successful, failed, and repeat treatments (p > .05); however, larger GSV diameter was associated with early treatment failures.30
A recent clinical trial indicated that energy density may not be the most important determinant of recanalization. In 471 segments, 11 failures were encountered, including 4 in a group treated with less than 60 J/cm (4%), 2 in a 60 to 80 J/cm group (3%), 4 in an 81 to 100 J/cm group (3%), and 1 in a group treated with more than 100 J/cm (1%). There were no statistically significant differences in failure rates among energy density ranges. The authors concluded that EVL has a low failure rate and that failure rate is not a function of energy density.31
Complications
Significant adverse events reported following EVL include skin burns, sensory nerve injuries, and deep vein thrombosis (DVT). Early experience32 reported skin burn rates as high as 4%; this decreased to almost zero as the use of tumescent anesthesia became the standard of practice. The overall rate for these complications has been shown to be higher in low-volume centers compared to high-volume centers; the rate of skin burns in one series using RFA was 1.7% before and 0.5% after the initiation of the tumescent technique.33 The nerves at highest risk include the saphenous nerve, located adjacent to the GSV below the midcalf perforating vein, and the sural nerve, adjacent to the SSV in the midcalf and lower calf. The most common manifestations of a nerve injury are paresthesias, which are usually transient. The nerve injuries can occur during venous access, the delivery of tumescent anesthesia, or lasering by transfer of thermal energy to perivenous tissues.
Patients treated with EVL, using extraordinarily high rates of energy delivery without tumescent anesthetic infiltrations, demonstrated a high rate of nerve injuries and skin burns.34 However, in a series using RF ablation, delivery of perivenous fluid was thought to be responsible for the low rate of cutaneous and neurologic thermal injuries, where the 1-week paresthesia rate was shown to decrease from 15% to 9% after the introduction of tumescent anesthesia.35 The addition of tumescent anesthesia has been demonstrated to reduce outer vein wall temperatures during EVL and RFA in animal models.36,37
Fortunately, thromboembolic events are uncommon after endovenous thermal ablation, probably because tumescent anesthesia has allowed for speedy procedures, which promotes ambulation immediately after surgery.11 The thrombus extensions at the SFJ seen occasionally after thermal ablation were discussed in Chapter 5.
Small Saphenous Vein
In a 2003 report38 on the treatment of SSV in 41 limbs using a 940-nm diode laser, 39 SSVs (95%) were successfully treated with EVL. During a median follow-up interval of 6 months, no recanalization was observed. Apart from one thrombosis of the popliteal vein in a patient with polycythemia vera, four transient sural nerve paresthesias were reported.
Almeida and Raines11 reported on 987 treated veins in 2006, 115 of which were SSVs. Most recanalizations occurred in the first 12 months and developed in the SSV proximal to May’s perforator. Five of 115 treated SSVs recanalized partially (4%), most likely as a result of cooler blood (37 °C) entering the treatment site from a midcalf perforating vein. No thrombus extensions into the popliteal vein or other thrombotic complications of the deep system were identified. Ravi et al.39 then reported the series in 2006, which consisted of 981 patients and included 101 SSV procedures. There were 9 (9.0%) failures among the 101 SSVs treated with EVL.
Gibson et al.40 published their series of 210 SSVs treated with EVL. Their data demonstrated that EVL of the SSV is feasible and safe and has excellent clinical outcomes in combination with concomitant therapies where indicated. All procedures were technically successful; 96% of SSVs remained closed at a mean follow-up of 4 months. The incidence of nerve injury is acceptably low and not clinically significant. Three patients (1.6%) had numbness at the lateral malleolus at the 6-week follow-up. Only three patients (1.6%) complained of numbness at the 6-week follow- up. In only one of these patients could EVL alone be implicated in causing numbness, because two of the patients also had microphlebectomy of large varicose vein branches at the lateral malleolus. None of the patients found the numbness to be a significant concern. The incidence of DVT, defined as a tail of thrombus protruding into the popliteal vein, was noted in 12 limbs (5.7%) at the 1-week follow-up examination. The incidence of DVT after treatment of the SSV was 5.7%, higher than in the other reported series. The reason for this difference is unclear; DVT was present in 11.4% of type A anatomy compared with 2.9% of limbs with type B anatomy and 0% of limbs with type C anatomy.
In a multicenter prospective study41 on 229 limbs, the feasibility, safety, and efficacy of endovenous laser ablation to treat SSVs were evaluated. Duplex ultrasound imaging showed immediate occlusion of the SSV with no thrombosis in the proximal veins. No complications occurred intraoperatively. All patients had postoperative ecchymosis, but it was minimal. Complete occlusion with absence of flow at less than 2 months of follow-up was detected in 226 SSVs (98.7%). It occurred in 22 patients with large SSV diameters. Recanalization was found in one patient at 12 months and in two patients at 24 months. After 1 year, eight limbs developed reflux in new locations and four underwent treatment. Symptoms resolved in most patients soon after the operation. The mean follow-up was 16 months. After 8 to 12 months postprocedurally, the laser-treated veins were fibrotic and almost indistinguishable from the surrounding tissues on duplex ultrasound imaging. In five patients (2.25%), postoperative paresthesia occurred more than 2 to 3 days postoperatively and persisted in the follow-up period.
Comparative Effectiveness of Existing Treatments
Nonrandomized Studies*
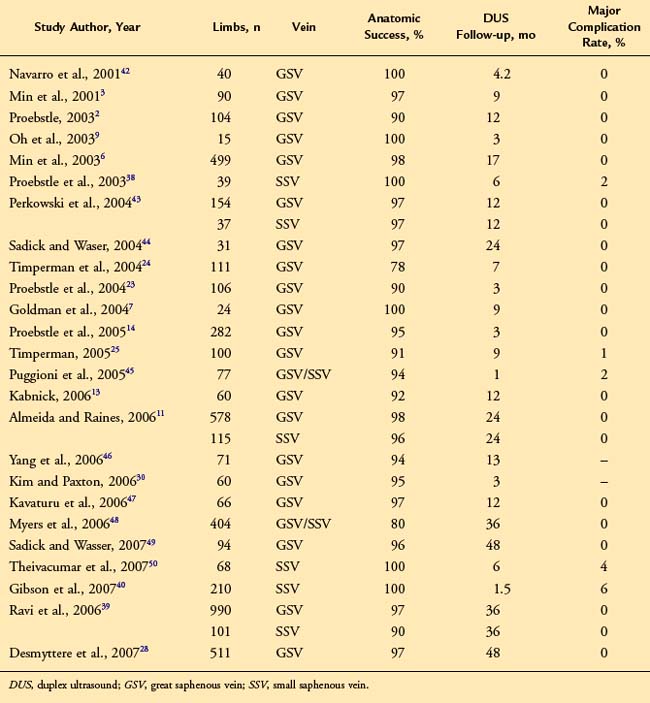
There have been several large case series describing outcomes for endovenous laser ablation. Most report GSV ablation rates of over 90% with associated improvement in symptoms and minimal complications (Table 6-1).
Summary of Randomized Controlled Trials
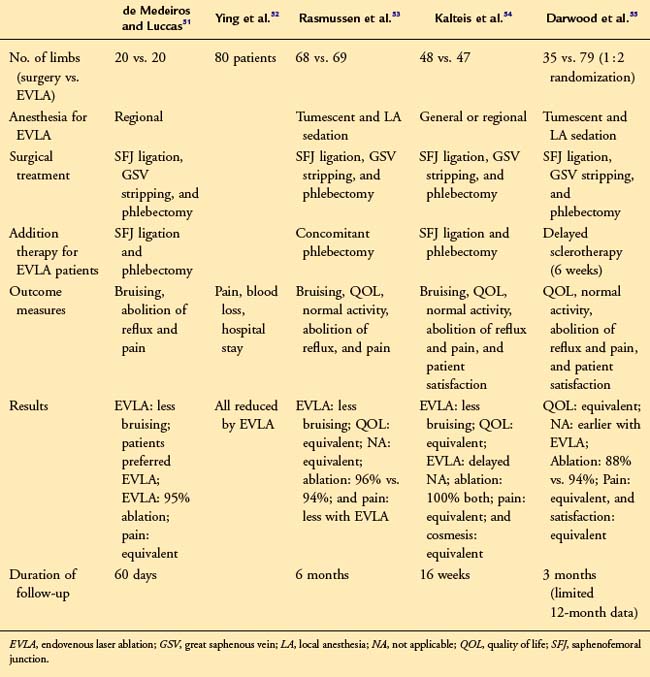
These randomized studies suggest that abolition of GSV reflux, improvements in quality of life, patient satisfaction, and cosmesis are similar for surgery and EVL51–55 (Table 6-2). Three studies also show that posttreatment discomfort was no different for either technique.
Although this may be surprising, it is likely to reflect GSV and adjacent soft tissue inflammation (“phlebitis”) following the thermal injury inflicted by EVL. Although pain levels appear similar for surgery and EVL, return to normal activity or work is variously reported as occurring earlier after EVL56 at the same time following either modality53 or delayed after laser therapy.54
It is evident from these trials that there is no consensus as to the optimum treatment protocol for EVL. Given the results reported by Rasmussen et al.53 and Darwood et al.,55 it seems that concomitant saphenofemoral ligation is unnecessary, thus allowing EVL to be performed without general or regional anaesthesia in an outpatient or office setting. The question of adjuvant treatment for the varicosities has not been answered by these studies. Only Darwood et al.55 used delayed sclerotherapy for persisting varicose veins after EVL. Provided that truncal vein ablation is commenced at the lowest point of reflux, it is still not known how many patients require additional treatment—thus, many operators support a policy of delayed sclerotherapy, while others prefer synchronous phlebectomy with EVL.
Meta-analysis
This meta-analysis of randomized controlled trials reviews the current evidence base, comparing open and endovascular treatment of varicose veins.56 Systematic review of studies reporting duplex scan follow-up after open surgical, laser (endovenous laser therapy [EVLT]), or radiofrequency (VNUS Closure device, VNUS Medical Technologies, San Jose, CA) treatment of refluxing great saphenous veins was completed. Primary outcome measures were occlusion and complication rates and time taken to resume work. Meta-analysis finds no significant difference in recurrence rates at 3 months between open surgery and endovenous laser ablation (EVLA) (relative risk [RR] 2.19, 95% confidence internal [CI] 0.99 to 4.85, p = .05) or VNUS (RR 7.57; 95% CI 0.42 to 136.02). Return to work is significantly faster following VNUS (by 8.24 days, 95% CI 10.50 to 5.97) or EVLA (by 5.02 days, 95% CI. 6.52 to 3.52). Endovascular treatment of varicose veins is safe and effective and offers the significant advantage of rapid recovery.
1 Mundy L, Merlin TL, Fitridge RA, et al. Systematic review of endovenous laser treatment for varicose veins. Br J Surg. 2005;92:1189-1194.
2 Proebstle TM, Gul D, Lehr HA, et al. Infrequent early recanalization of greater saphenous vein after endovenous laser treatment. J Vasc Surg. 2003;38:511-516.
3 Min RJ, Zimmet SE, Isaacs MN, et al. Endovenous laser treatment of the incompetent greater saphenous vein. J Vasc Interv Radiol. 2001;12:1167-1171.
4 Proebstle TM, Lehr HA, Kargl A, et al. Endovenous treatment of the greater saphenous vein with a 940 nm diode laser: thrombotic occlusion after endoluminal thermal damage by laser generated steam bubbles. J Vasc Surg. 2002;35:729-736.
5 Proebstle TM, Sandhofer M, Kargl A, et al. Thermal damage of the inner vein wall during endovenous treatment: key role of energy absorption by intravascular blood. Dermatol Surg. 2002;28:596-600.
6 Min RJ, Khilnani N, Zimmet S. Endovenous laser treatment of saphenous vein reflux: long-term results. J Vasc Interv Radiol. 2003;14:991-996.
7 Goldman MP, Mauricio M, Rao J. Intravascular 1320-nm laser closure of the great saphenous vein: a 6- to 12-month follow-up study. Dermatol Surg. 2004;30:1380-1385.
8 Goldman MP. Intravascular lasers in the treatment of varicose veins. J Cosmetic Dermatol. 2004;3:162-166.
9 Oh CK, Jung DS, Jang HS, et al. Endovenous laser surgery of the incompetent greater saphenous vein with a 980-nm diode laser. Dermatol Surg. 2003;29:1135-1140.
10 Disselhoff B, Rem AI, Verdaasdonk R, et al. Endovenous laser ablation: an experimental study on the mechanism of action. Phlebology. 2008;23:69-76.
11 Almeida JI, Raines JK. Radiofrequency ablation and laser ablation in the treatment of varicose veins. Ann Vasc Surg. 2006;20:547-552.
12 Lurie F, Creton D, Eklof B, et al. Prospective randomised study of endovenous radiofrequency obliteration (closure) versus ligation and vein stripping (EVOLVeS): two-year follow-up. Eur J Vasc Endovasc Surg. 2005;29:67-73.
13 Kabnick LS. Outcome of different endovenous laser wavelengths for great saphenous vein ablation. J Vasc Surg. 2006 Jan;43:88-93.
14 Proebstle TM, Moehler T, Gul D, et al. Endovenous treatment of the great saphenous vein using a1,320 nm Nd:YAG laser causes fewer side effects than using a 940 nm diode laser. Dermatol Surg. 2005;31:1678-1683.
15 Almeida JI, Mackay EG, Javier JJ, et al. Saphenous laser ablation at 1470 nm targets the vein wall, not blood. Vasc Endovascular Surg. 2009;43:467-472.
16 Bush RG, Shamma HN, Hammond K. Histological changes occurring after endoluminal ablation with two diode lasers (940 and 1319 nm) from acute changes to 4 months. Lasers Surg Med. 2008;40:676-679.
17 Fan CM, Rox-Anderson R. Endovenous laser ablation: mechanism of action. Phlebology. 2008;23:206-213.
18 Roggan A, Friebel M, Dorschel K, et al. Optical properties of circulating human blood in the wavelength range 400-2500 nm. J Biomed Opt. 1999;4:36-46.
19 Schmedt CG, Meissner OA, Hunger K, et al. Evaluation of endovenous radiofrequency ablation and laser therapy with endoluminal optical coherence tomography in an ex vivo model. J Vasc Surg. 2007;45:1047-1058.
20 Weiss RA. Comparison of endovenous radiofrequency versus 810 nm diode laser occlusion of the large veins in an animal model. Dermatol Surg. 2002;28:56-61.
21 Zimmet SE, Min RJ. Temperature changes in perivenous tissue during endovenous laser treatment in a swine model. J Vasc Interv Radiol. 2003;14:911-915.
22 Beale RJ, Mavor AID, Gough MJ. Heat dissipation during endovenous laser treatment of varicose veins—is there a risk of nerve injury? Phlebology. 2006;21:32-35.
23 Proebstle TM, Krummenauer F, Gül D, et al. Non-occlusion and early reopening of the great saphenous vein after endovenous laser treatment is fluence dependent. Dermatol Surg. 2004;30(2 Pt 1):174-178.
24 Timperman PE, Sichlau M, Ryu RK. Greater energy delivery improves treatment success of endovenous laser treatment of incompetent saphenous veins. J Vasc Interv Radiol. 2004;15:1061-1063.
25 Timperman PE. Prospective evaluation of higher energy great saphenous vein endovenous laser treatment. J Vasc Interv Radiol. 2005;16:791-794.
26 Proebstle TM, Moehler T, Herdemann S. Reduced recanalization rates of the great saphenous vein after endovenous laser treatment with increased energy dosing: definition of a threshold for the endovenous fluence equivalent. J Vasc Surg. 2006;44:834-839.
27 Vuylsteke M, Liekens K, Moons P, et al. Endovenous laser treatment of saphenous vein reflux: how much energy do we need to prevent recanalizations. Vasc Endovasc Surg. 2008;42:141-149.
28 Desmyttere J, Grard C, Wassmer B, et al. Endovenous 980-nm laser treatment of saphenous veins in a series of 500 patients. J Vasc Surg. 2007;46:1242-1247.
29 Kim HS, Nwankwo IJ, Hong K, et al. Lower energy endovenous laser ablation of the great saphenous vein with 980 nm diode laser in continuous mode. Cardiovasc Intervent Radiol. 2006;29:64-69.
30 Kim HS, Paxton BE. Endovenous laser ablation of the great saphenous vein with a 980-nm diode laser in continuous mode: early treatment failures and successful repeat treatments. J Vasc Interv Radiol. 2006;17:1449-1455.
31 Prince EA, Ahn SH, Dubel GJ, et al. An investigation of the relationship between energy density and endovenous laser ablation success: does energy density matter? J Vasc Interv Radiol. 2008;19:1449-1453.
32 Merchant RF, dePalma RG, Kabnick LS. Endovascular obliteration of saphenous reflux: a muticenter study. J Vasc Surg. 2002;35:1180-1186.
33 Merchant RF, Pichot O, Meyers KA. Four-year follow-up on endovascular radiofrequency obliteration of great saphenous reflux. Dermatol Surg. 2005;31:129-134.
34 Chang C, Chua J. Endovenous laser photocoagulation (EVLP) for varicose veins. Lasers Surg Med. 2002;31:257-262.
35 Merchant RF, Pichot O. Long-term outcomes of endovenous radiofrequency obliteration of saphenous reflux as a treatment of superficial venous insufficiency, for the Closure Study Group. J Vasc Surg. 2005;42:502-509.
36 Zikorus AW, Mirizzi MS. Evaluation of setpoint temperature and pullback speed on vein adventitial temperature during endovenous radiofrequency energy delivery in an in-vitro model. Vasc Endovascular Surg. 2004;38:167-174.
37 Dunn CW, Kabnick LS, Merchant RF, et al. Endovascular radiofrequency obliteration using 90°C for treatment of great saphenous vein. Ann Vasc Surg. 2006;20:625-629.
38 Proebstle TM, Gul D, Kargl A, et al. Endovenous laser treatment of the lesser saphenous vein with a 940-nm diode laser: early results. Dermatol Surg. 2003;29:357-361.
39 Ravi R, Rodriguez-Lopez JA, Traylor EA, et al. Endovenous ablation of incompetent saphenous veins: a large single-center experience. J Endovasc Ther. 2006;13:244-248.
40 Gibson KD, Ferris BL, Polissar N, et al. Endovenous laser treatment of the small saphenous vein: efficacy and complications. J Vasc Surg. 2007;45:795-801.
41 Kontothanassis D, Di Mitri R, Ferrari Ruffino S, et al. Endovenous laser treatment of the small saphenous vein. J Vasc Surg. 2009;49:973-979.
42 Navarro L, Min RJ, Bone C. Endovenous laser: a new minimally invasive method of treatment of varicose veins—preliminary observations using an 810 nm diode laser. Dermatol Surg. 2001;27:117-122.
43 Perkowski P, Ravi R, Gowda RCN, et al. Endovenous laser ablation of the saphenous vein for treatment of venous insufficiency and varicose veins: early results from a large single-center experience. J Endovasc Ther. 2004;11:132-138.
44 Sadick NS, Waser S. Combined endovascular laser with ambulatory phlebectomy for the treatment of superficial venous incompetence: a 2-year perspective. J Cosmet Laser Ther. 2004;24:149-153.
45 Puggioni A, Kalra M, Carmo M, et al. Endovenous laser therapy and radiofrequency ablation of the great saphenous vein: analysis of early efficacy and complications. J Vasc Surg. 2005;42:488-493.
46 Yang CH, Chou HS, Lo YF. Incompetent great saphenous veins treated with endovenous 1,320-nm laser: results for 71 legs and morphologic evolvement study. Dermatol Surg. 2006;32:1453-1457.
47 Kavuturu S, Girishkumar H, Ehrlich F. Endovenous laser ablation of saphenous veins is an effective treatment modality for lower extremity varicose veins. Am Surg. 2006;72:672-675.
48 Myers K, Fris R, Jolley D. Treatment of varicose veins by endovenous laser therapy: assessment of results by ultrasound surveillance. Med J Austral. 2006;185:199-202.
49 Sadick NS, Wasser S. Combined endovascular laser plus ambulatory phlebectomy for the treatment of superficial venous incompetence: a 4-year perspective. J Cosmet Laser Ther. 2007;9:9-13.
50 Theivacumar NS, Beale RJ, Mavor AI, et al. Initial experience in endovenous laser ablation (EVLA) of varicose veins due to small saphenous vein reflux. Eur J Vasc Endovasc Surg. 2007;33:614-618.
51 de Medeiros C, Luccas G. Comparison of endovenous treatment with an 810 nm laser versus conventional stripping of the great saphenous vein in patients with primary varicose veins. Dermatol Surg. 2005;31:1685-1694.
52 Ying L, Sheng Y, Ling H, et al. Random, comparative study on endovenous laser therapy and saphenous veins stripping for the treatment of great saphenous vein incompetence. Zhonghua-Yi-Xue-Za-Zhi. 2007;87:3043-3046.
53 Rasmussen LH, Bjoern L, Lawaetz M, et al. Trial comparing endovenous laser ablation of the great saphenous vein with high ligation and stripping in patients with varicose veins: short term results. J Vasc Surg. 2007;46:308-315.
54 Kalteis M, Berger I, Messie-Werndl S, et al. High ligation combined with stripping and endovenous laser ablation of the great saphenous vein: early results of a randomised controlled study. J Vasc Surg. 2008;47:822-829.
55 Darwood R, Theivacumar N, Dellagrammaticus D, et al. Randomised clinical trial comparing endovenous laser ablation with surgery for the treatment of primary great saphenous varicose veins. Br J Surg. 2008;95:294-301.
56 Brar R, Nordon IM, Hinchliffe RJ, et al. Management of varicose veins: meta-analysis. Vascular. 2010;18:205-220.