CHAPTER 44 Laser peripheral iridotomy and iridoplasty
Laser peripheral iridotomy
The continuous wave argon laser revolutionized the treatment of glaucoma. In 1973, Beckman and Sugar reported successful argon laser iridotomies in humans1. Others soon reported success in human eyes with angle closure2. The ease and convenience of the procedure for both patient and surgeon and the paucity of severe complications led to its rapid acceptance. ALPI was first described in 19823.
Indications
Acute primary angle closure
A recent randomized controlled trial reported that LPI may not be as effective as early phacoemulsification in reversing angle closure and preventing the subsequent IOP increase in acute angle closure eyes4. It was suggested that if LPI could be performed within 7 days after the onset of symptoms in acute angle closure the chance of successful post-iridotomy IOP control would be higher5. After LPI in acute angle closure eyes, up to 55.6% may still have appositionally closed angles6. It is therefore important to monitor both the drainage angle and IOP in acute angle closure patients following LPI.
Chronic angle closure
LPI alone may control IOP, or elevated IOP may persist as a result of prior trabecular damage, warranting continued medical treatment or filtration surgery. Failure of medications to control IOP, particularly if the angle is closed, does not mean necessarily that the IOP will be uncontrollable medically after iridotomy. It is often not possible to predict which eyes will respond favorably; in one series, LPI reduced the IOP in 44% of eyes in patients with chronic angle closure glaucoma7.
Other situations
Secondary angle closure glaucoma
In situations of angle closure secondary to other eye diseases, e.g. anterior uveitis or Behcet’s disease8, LPI may help relieve the pupillary block and reverse, partially or totally, the appositionally closed portions of the angle. This may improve IOP control.
Surgical techniques
The various techniques for LPI as developed in the late 1970s and early 1980s, with multiple variations, are detailed elsewhere9. We describe our current most commonly used techniques and settings.
Contact lenses for laser procedures
The Abraham lens (Fig. 44.1) consists of a fundus lens with an anterior +66 diopter planoconvex button. The button magnifies without loss of depth of focus. The effective size of a 50 µm spot is reduced to approximately 30 µm; this provides higher energy per unit area and permits the procedure to require a lower total energy. Posterior to the site of focus, the beam is more rapidly defocused, decreasing potential injury to structures behind.
Argon laser peripheral iridotomy
Contraction burn
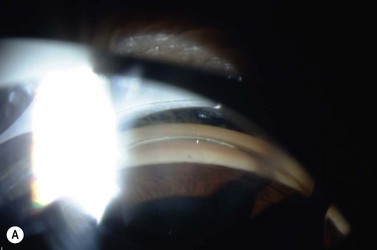
The linear incision technique involves placing a short line of circumferential burns instead of penetrating at a single spot (Fig. 44.2). When the stroma is fully incised in this manner, the iris dilator muscle assists in the separation of the iris pigment epithelium, reducing the amount of pigment epithelium which must be lasered and creating a larger opening.
The contraction burn bed technique helps in lightly pigmented irides without crypts. One to three contraction burns are placed in a circumferential line, partially superimposed (see Fig. 44.2). Punch or penetrating burns are then placed using the contraction burns as a bed for the site of laser peripheral iridotomy. In very light irides, this method combined with linear incision usually results in larger iridotomies than attainable with other argon laser techniques. For light brown irides, we place a single contraction burn on either side of the selected penetration site (modified drumhead). The area between them is tautened and an iridotomy created with penetrating burns.
Penetrating burn
This is a higher power, small spot size burn designed to vaporize iris tissue and create an opening; use a 50 µm spot size. In the late 1970s and early 1980s, burns of 0.1 or 0.2 second duration were common. This introduces far too much power especially with dark brown irides. Shorter duration, lower power burns were shown to be more effective10, with less complications and less total energy for penetration. Burns of 0.01 or 0.02 second duration were formerly termed ‘punch burns’. The optimal power is between 600 and 1200 mW; it varies depending on burn duration and iris consistency and pigmentation.
Cleanup burns
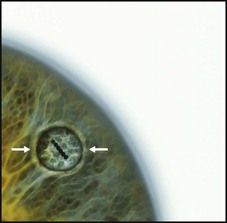
At the completion of the iridotomy, the lens capsule should be visible through the opening (Fig. 44.3). If the opening is small and the capsule is not visible, one can often get a sense of depth behind the opening or note a green reflex off the lens capsule when the beam is applied to the opening. Gonioscopy should be performed to ensure that the angle is open.
Elevated IOP associated with LPI typically occurs in the first 1–2 hours postoperatively11. One should monitor IOP during this time. Postoperative topical steroids for several days are recommended to control inflammation.
Neodymium:YAG laser peripheral iridotomy
The Nd:YAG laser creates a plasma of free ions and electrons at the site of optical breakdown. This photodisruption releases shock waves that mechanically cause tissue rupture, as opposed to the thermal effect of the argon laser12. Iris color and density are much less important than with argon LPI.
Suggested settings for Nd:YAG LPI have varied from one to four pulses per burst and from 1 to 10 mJ per burst. One should begin with a single pulse at approximately 1.5–3 mJ to assess the response of both the patient and the iris to the laser application. An increase in laser energy to 4–6 mJ is often sufficient to create a patent iridotomy with one to three additional applications. We prefer a linear incision technique using lower power burns, of the order of 1 mJ. We also prefer a single pulse per burst. It is very important to focus on the anterior iris stroma to maximize photodisruption and to minimize the possibility of lens injury. Portable Nd:YAG systems may facilitate iridotomy in debilitated patients or in remote areas13.
Other wavelengths
Combining argon laser techniques to thin the iris stroma and coagulate blood vessels followed by Nd:YAG completion of the iridotomy has been advocated as an approach with few complications which takes advantage of the photothermal effects of the first and easier penetration of the latter14. This is particularly effective for light blue irides. The risk of operative hemorrhage is reduced. Laser iridotomies may also be created with the diode, krypton, and picosecond lasers.
Complications
Diplopia and glare
A rather common finding and a not uncommon source of complaint is the sensation of a horizontal line in the upper visual field, occasionally accompanied by glare. This occurs when the iridotomy is partially covered by the lid margin and is relieved when completely covered or completely exposed15. There is also a ‘shutter effect’ created by the lid crossing the iridotomy site during blinking. Patients eventually become used to it and often no longer notice it after a few months. Tinted soft contact lenses may be used to ameliorate diplopia. Opaque contact lenses to cover the iridotomy site may be used to reduce the amount of stray light entering through it.
Corneal damage
Endothelial burns from the argon laser are generally dense white with sharp margins and result from the thermal effects of iris photocoagulation. They require more time for resolution and may result in focal endothelial cell loss. Although endothelial cell loss following LPI has not been statistically significant during follow-up of up to 1 year, an increase in mean endothelial cell size and endothelial cell loss with greater laser power have been reported16. Both epithelial and endothelial burns have been rare since 0.1–0.2-second duration pulses have been abandoned.
Although endothelial cell analysis after Nd:YAG iridotomy may be unchanged17, focal endothelial cell loss has been documented when photodisruption is less than 1 mm from the corneal endothelium and at the site of treatment17. The area of focal loss is reduced in size with the use of an Abraham lens. Damage to Descemet’s membrane occurs when this distance is reduced to 0.1 mm. Progressive corneal edema requiring penetrating keratoplasty has been reported following Nd:YAG or argon LPI. Risk factors include pre-existing guttata and excessive laser energy.
Anterior uveitis
Post-laser anterior segment inflammation is typical, usually mild and ceases within a few days. It may last longer than 1 month and may be worse in individuals with heavily pigmented irides18. Topical corticosteroids are routinely prescribed.
Lens opacities
Prophylactic LPI was reported to be associated with cataract progression in fellow eyes of patients with acute angle closure19,20.
Retinal damage
Photocoagulation of the peripheral retina between the equator and the ora serrata during argon LPI usually occurs when an opening is being enlarged after iris penetration has been achieved. Inadvertent foveal photocoagulation can occur, and proper positioning of the laser beam is essential. Choroidal and retinal detachment and non-rhegmatogenous retinal detachment in nanophthalmic eyes have also been reported. Microperforations of the retina may occur with the Nd:YAG laser if the beam is inadvertently focused to within 2 to 3 mm of the retina. Decompression retinopathy has been reported after Nd:YAG LPI19.
Laser peripheral iridoplasty (ALPI)
Peripheral iridoplasty is a simple and effective means of opening an appositionally closed angle when LPI either cannot be performed or has not eliminated appositional angle closure because mechanisms other than pupillary block are present. Contraction burns (long duration, low power, and large spot size) are placed in the extreme iris periphery to contract the iris stroma and physically pull open the angle (Fig. 44.4)3.
Indications
Acute primary angle closure
ALPI can break an attack of acute primary angle closure which is unresponsive to medical therapy and where corneal edema, a shallow anterior chamber, or marked inflammation precludes immediate LPI. ALPI may also be useful for an attack which is unresponsive despite a patent iridotomy1,4. Circumferential treatment of the iris is virtually 100% successful at opening the angle in those areas in which there are no PAS. The effect lasts sufficiently long for the cornea and anterior chamber to clear so that iridotomy can be performed.
ALPI may be used as primary therapy in eyes with acute primary angle closure, either with or without preliminary treatment with topical medications21–24. It was shown to be more effective than conventional systemic medications in lowering IOP in acute primary angle closure in a randomized controlled study23. Since ALPI does not eliminate pupillary block, LPI is still required in acute primary angle closure once IOP is controlled and the cornea has cleared sufficiently; otherwise the effect of ALPI may reverse.
Chronic angle closure
Eyes with chronic angle closure and a combination of PAS and appositional closure may respond to ALPI with opening of the appositionally closed portions of the angle, allowing filtration surgery to be avoided25. If extensive PAS are present after ALPI, goniosynechialysis (GSL) may be performed to surgically strip PAS from the angle wall to restore aqueous access to the trabecular meshwork.
Plateau iris syndrome
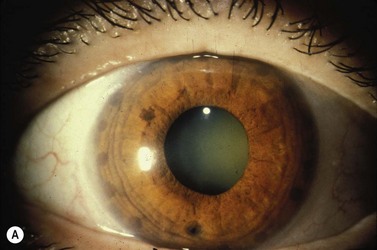
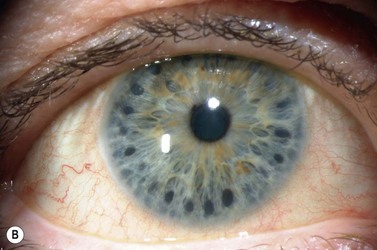
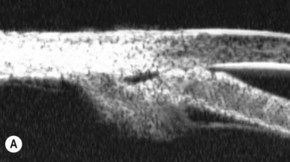
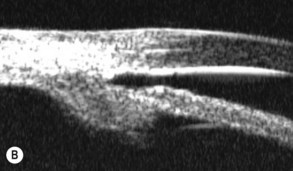
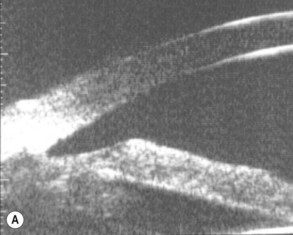
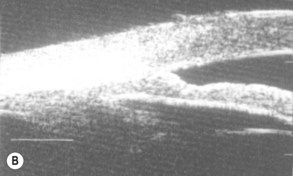
A large or anteriorly positioned ciliary body can push the iris root onto the trabecular meshwork, creating a configuration known as plateau iris. In plateau iris syndrome, the angle remains appositionally closed or occludable following LPI. ALPI provides successful long-term control in eyes with plateau iris syndrome (Figs 44.5–44.7)26.
Angle closure related to the size or position of the lens
Angle closure caused by an enlarged lens (phacomorphic) or pressure posterior to the lens (‘malignant’ or ciliary block) often fails to respond to iridotomy, although a component of pupillary block may be present and must be eliminated first by iridotomy. In suspected cases of ciliary block in which the angle remains appositionally closed after LPI, the apposition can be reduced or eliminated by ALPI. After the angle has been opened and IOP reduced, cycloplegics may be given cautiously to ascertain the mechanism of the angle closure. ALPI is effective as a primary treatment to break attacks of acute phacomorphic angle closure27,28. This may allow a week or more for the inflammation and Descemet’s folds to clear, facilitating cataract extraction. Any pupillary block can be treated subsequently with iridotomy as soon as possible (usually within 2–3 days).
1 Beckman H, Sugar HS. Laser iridectomy therapy of glaucoma. Arch Ophthalmol. 1973;90(6):453-455.
2 Abraham RK, Miller GL. Outpatient argon laser iridectomy for angle closure glaucoma: a two-year study. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1975;79(3 Part 2):OP529-OP537.
3 Ritch R. Argon laser treatment for medically unresponsive attacks of angle-closure glaucoma. Am J Ophthalmol. 1982;94(2):197-204.
4 Lam DS, Leung DY, Tham CC, et al. Randomized trial of early phacoemulsification versus peripheral iridotomy to prevent intraocular pressure rise after acute primary angle closure. Ophthalmology. 2008;115(7):1134-1140.
5 Lee JW, Lee JH, Lee KW. Prognostic factors for the success of laser iridotomy for acute primary angle closure glaucoma. Korean J Ophthalmol. 2009;23(4):286-290.
6 Yeung BY, Ng PW, Chiu TY, et al. Prevalence and mechanism of appositional angle closure in acute primary angle closure after iridotomy. Clin Exp Ophthalmol. 2005;33(5):478-482.
7 Quigley HA. Long-term follow-up of laser iridotomy. Ophthalmology. 1981;88(3):218-224.
8 Elgin U, Berker N, Batman A, et al. Nd:YAG laser iridotomy in the management of secondary glaucoma associated with Behcet’s disease. Eur J Ophthalmol. 2007;17(2):191-195.
9 Ritch R, Solomon IS. Glaucoma surgery. In: L’Esperance FA, editor. Ophthalmic Lasers. 3rd edn. St. Louis: CV Mosby Co; 1988:650-748.
10 Ritch R, Palmberg P. Argon laser iridectomy in densely pigmented irides. Am J Ophthalmol. 1982;93(6):800-801.
11 Krupin T, Stone RA, Cohen BH, et al. Acute intraocular pressure response to argon laser iridotomy. Ophthalmology. 1985;92(7):922-926.
12 Goldberg MF, Tso MO, Mirolovich M. Histopathological characteristics of neodymium-YAG laser iridotomy in the human eye. Br J Ophthalmol. 1987;71(8):623-628.
13 Robin AL, Pollack IP. Q-switched neodymium-YAG laser iridotomy in patients in whom the argon laser fails. Arch Ophthalmol. 1986;104(4):531-535.
14 de Silva DJ, Gazzard G, Foster P. Laser iridotomy in dark irides. Br J Ophthalmol. 2007;91(2):222-225.
15 Murphy PH, Trope GE. Monocular blurring. A complication of YAG laser iridotomy. Ophthalmology. 1991;98(10):1539-1542.
16 Hong C, Kitazawa Y, Tanishima T. Influence of argon laser treatment of glaucoma on corneal endothelium. Jpn J Ophthalmol. 1983;27(4):567-574.
17 Panek WC, Lee DA, Christensen RE. The effects of Nd:YAG laser iridotomy on the corneal endothelium. Am J Ophthalmol. 1991;111(4):505-507.
18 Siam GA, de Barros DS, Gheith ME, et al. Post-peripheral iridotomy inflammation in patients with dark pigmentation. Ophthalmic Surg Lasers Imaging. 2008;39(1):49-53.
19 Landers J, Craig J. Decompression retinopathy and corneal oedema following Nd:YAG laser peripheral iridotomy. Clin Exp Ophthalmol. 2006;34(2):182-184.
20 Lim LS, Husain R, Gazzard G, et al. Cataract progression after prophylactic laser peripheral iridotomy: potential implications for the prevention of glaucoma blindness. Ophthalmology. 2005;112(8):1355-1359.
21 Lai JS, Tham CC, Chua JK, et al. To compare argon laser peripheral iridoplasty (ALPI) against systemic medications in treatment of acute primary angle-closure: mid-term results. Eye. 2006;20(3):309-314.
22 Lam DS, Lai JS, Tham CC. Immediate argon laser peripheral iridoplasty as treatment for acute attack of primary angle-closure glaucoma: a preliminary study. Ophthalmology. 1998;105(12):2231-2236.
23 Lam DS, Lai JS, Tham CC, et al. Argon laser peripheral iridoplasty versus conventional systemic medical therapy in treatment of acute primary angle-closure glaucoma: a prospective, randomized, controlled trial. Ophthalmology. 2002;109(9):1591-1596.
24 Tham CC, Lai JS, Lam DS. Immediate argon laser peripheral iridoplasty for acute attack of PACG (addendum to previous report). Ophthalmology. 1999;106(6):1042-1043.
25 Chew PT, Yeo LM. Argon laser iridoplasty in chronic angle closure glaucoma. Int Ophthalmol. 1995;19(2):67-70.
26 Ritch R, Tham CC, Lam DS. Long-term success of argon laser peripheral iridoplasty in the management of plateau iris syndrome. Ophthalmology. 2004;111(1):104-108.
27 Tham CC, Lai JS, Poon AS, et al. Immediate argon laser peripheral iridoplasty (ALPI) as initial treatment for acute phacomorphic angle-closure (phacomorphic glaucoma) before cataract extraction: a preliminary study. Eye. 2005;19(7):778-783.
28 Yip PP, Leung WY, Hon CY, et al. Argon laser peripheral iridoplasty in the management of phacomorphic glaucoma. Ophthalmic Surg Lasers Imaging. 2005;36(4):286-291.
29 Ispa-Callen MC, Lara-Medina J, Zarco-Tejada JM, et al. [Argon laser iridoplasty as treatment of plateau-like iris configuration secondary to multiple ciliary body cysts: long-term follow-up by ultrasound biomicroscopy]. Arch Soc Esp Oftalmol. 2009;84(11):569-572.
30 Sbeity Z, Gvozdyuk N, Amde W, et al. Argon laser peripheral iridoplasty for topiramate-induced bilateral acute angle closure. J Glaucoma. 2009;18(4):269-271.
31 Mansouri K, Ravinet E. Argon-laser iridoplasty in the management of uveitis-induced acute angle-closure glaucoma. Eur J Ophthalmol. 2009;19(2):304-306.